Abstract
It is well known that the anxiolytic potential of ethanol is maintained during chronic exposure. We have confirmed this using a light-dark box paradigm following chronic ethanol ingestion via a liquid diet. However, cessation from chronic ethanol exposure is known to cause severe withdrawal anxiety. These opposing effects on anxiety likely result from neuro-adaptations of neurotransmitter systems within the brain regions regulating anxiety. Recent work highlights the importance of amygdala ligand-gated chloride channels in the expression of anxiety. We have therefore examined the effects of chronic ethanol exposure on GABAA and strychnine-sensitive glycine receptors expressed by acutely isolated adult rat lateral/basolateral amygdala neurons. Chronic ethanol exposure increased the functional expression of GABAA receptors in acutely isolated basolateral amygdala neurons without altering strychnine-sensitive glycine receptors. Neither the acute ethanol nor benzodiazepine sensitivity of either receptor system was affected. We explored the likelihood that subunit composition might influence each receptor’s response to chronic ethanol. Importantly, when expressed in a mammalian heterologous system, GABAA receptors composed of unique α subunits were differentially sensitive to acute ethanol. Likewise, the presence of the β subunit appeared to influence the acute ethanol sensitivity of glycine receptors containing the α2 subunit. Our results suggest that the facilitation of GABAA receptors during chronic ethanol exposure may help explain the maintenance of ethanol’s anti-anxiety effects during chronic ethanol exposure. Furthermore, the subunit composition of GABAA and strychnine-sensitive glycine receptors may ultimately influence the response of each system to chronic ethanol exposure.
Classification Terms: Theme – Neural Basis of Behavior; Drugs of Abuse – Alcohol, barbiturates, and benzodiazepines
Keywords: electrophysiology, heterologous expression, light-dark box, midazolam
1. Introduction
It has long been accepted that anxiety and drug abuse are intimately associated. For example, acute ethanol reduces anxiety in both experimental systems as well as humans. Likewise, pronounced anxiety is a common symptom associated with withdrawal from chronic ethanol exposure. This ethanol-anxiety association persists throughout the lifetime of human alcoholics, with increased anxiety being reported as a common, underlying cause of relapse [4]. More intriguing still, anxiety disorders and alcoholism are often co-morbid, with first-order relatives of individuals suffering from anxiety disorders being 4–5 times more likely to abuse alcohol than the general population [37]. Finally, stress or anxiety disorders are frequently associated with earlier age of drinking onset, higher numbers of detoxifications, and larger amounts of alcohol consumed [23,41,45], suggesting that anxious temperament may play some causative role in the development of alcohol abuse. Although the neural substrates regulating the ethanol-anxiety interaction are not well established, measures of heightened neuronal activity in discrete brain regions are evident during ethanol withdrawal (see below); and, many of these appear in turn to belong to the sensory, memory, and cognitive pathways regulating anxiety.
The amygdala can be divided into separate anatomically and functionally distinct sub regions. The lateral and basolateral subdivisions act as primary input regions for processed sensory and cognitive information that in turn process and project to additional amygdala subdivisions and other forebrain areas that ultimately mediate the physical and psychological manifestations of anxiety. In human alcoholics, chronic ethanol exposure dramatically decreases dopamine D2/D3 receptor binding in the amygdala [48]. Alvarez and colleagues [2] have also shown prominent loss of amygdala neurons in a side-by-side comparison of human alcoholics or alcoholized rats with controls, suggesting that the pathology related to chronic exposure is similar between species. While the role of the amygdala in human or non-human primates is not yet understood with regard to alcohol abuse, the amygdala’s functional roles are well conserved between divergent species; and, experimental lesions of the rodent central amygdala concurrently reduce ethanol consumption and basal anxiety [35]. Furthermore, both acute ethanol exposure and withdrawal from chronic ethanol increase c-fos expression in the central amygdala [36], a major target of projections from the lateral/basolateral subdivisions. Together, these findings strongly implicate the amygdala as one potential brain region associated with the ethanol/anxiety relationship.
While numerous neurotransmitter systems are found within the amygdala, ligand-gated chloride channels have definitive roles in regulating the expression of fear/anxiety. For example, direct injection of bicuculline or picrotoxin [43] into the basolateral amygdala increases apparent anxiety. Conversely, treatments that increase GABAA receptor activity in the lateral/basolateral amygdala reduce anxiety [17] or inhibit fear learning [53]. More importantly for the ethanol-anxiety interaction, direct administration of the GABAA agonist muscimol, via chronic amygdala cannulae, to ethanol-dependent rats can diminish direct ethanol self-administration via these same cannulae [40]. Finally, a recent study by Papadeas et al. [38] has demonstrated that chronic ethanol exposure enhances muscimol-dependent Cl− flux in an 'extended amygdala' preparation while decreasing GABAA subunit protein levels. Together these studies serve to highlight the role of amygdala GABAA channels in the interaction between ethanol and anxiety and serve as rationale for our more specific examination of the neurophysiologic response to chronic ethanol in lateral/basolateral amygdala neurons.
In addition to GABAA channels, strychnine-sensitive glycine receptors are also chloride channels that exhibit some sensitivity to the acute and chronic effects of ethanol. In the spinal cord, glycine receptor-mediated currents are consistently facilitated by acute ethanol treatment in both isolated cell preparations [1] and synaptic responses [12]. These acute effects in the spinal cord are apparently responsible for some of the more stereotypical intoxication-related behaviors like impaired motor coordination [13]. Chronic ethanol exposure of cultured spinal cord neurons can decrease the zinc and picrotoxin sensitivity glycinergic responses in cultured spinal neurons [51], suggesting an alteration in subunit expression following ethanol exposure. However, the sensitivity of forebrain glycine receptors to the acute effects of ethanol is more controversial. For example, acute ethanol can either facilitate or inhibit glycine currents in neonatal neurons isolated from ventral tegmental area [55,56] and can depress glycine-enhanced acetylcholine release in neonatal rat caudate [8]. The influence exerted by chronic ethanol on forebrain strychnine-sensitive glycine receptors is even less well studied. However, strychnine-sensitive glycine receptors are indeed expressed by isolated adult rat basolateral amygdala neurons [29], indicating that forebrain glycine receptors in brain regions regulating anxiety are a potential target for neuro-adaptations to chronic ethanol exposure. Indeed, we examine here the effects of chronic ethanol ingestion on both GABAA and strychnine-sensitive glycine receptors expressed by isolated amygdala neurons.
2. Materials & Methods
Chronic ethanol administration
All animal procedures were approved by the Texas A&M ULACC in accordance with the guidelines set forth by NIH animal care and use policy. Adult male Sprague-Dawley rats (Harlan, Inc.; Houston, TX) were housed individually in an AAALAC-accredited facility and were kept on a 12-hr light/dark cycle. Animals were randomly placed into two treatment groups, those receiving a non-ethanol containing liquid diet (“Control” group) and those chronically exposed to alcohol via an ethanol-containing liquid diet. Over the course of our studies, two different nutritionally complete liquid diets were utilized: an ‘in-house’ diet that was manufactured on-site as described elsewhere [16]; and, a commercially available diet (Bio-Serv Inc.; Frenchtown, NJ) that is similar to that reported by Lieber & DeCarli [24]. Data collected using both diets were not statistically different from one another and were pooled for analysis. ‘Chronic ethanol’ rats received the ethanol diet (4–6% v/v) for a total of 10–12 days. ‘Control’ rats were pair-fed with the same diet except isocaloric dextrose substituted for ethanol. Both ‘control’ and ‘chronic ethanol’ rats gained weight during the liquid diet treatments: start weights were 127.9±9.3g for controls and 127.1±7.2g for chronic ethanol rats; and, finishing weights were 192.5±11.4g and 199.2±10.3g for control and chronic ethanol rats, respectively. These values were not significantly different from one another. Water was given ad libitum and diet intake monitored daily. In the ‘chronic ethanol’ rats, blood ethanol concentrations at the time of sacrifice were determined using a commercially-available alcohol dehydrogenase/NADH (SIGMA Chemical Co.; St. Louis, MO) end-point spectrophotometric measurement; these BEC values were 147±11mg/dl (n=28).
Behavioral measurements
The two-compartment light/dark box [6] was used to measure effects of chronic ethanol exposure on anxiety in the rat. The apparatus consisted of an open top wooden box (27 × 58 × 30 cm) lined into 9 cm squares. An area of 24 × 27 cm was painted black and illuminated by a 60W red light bulb. The remaining area was white and illuminated by a 40W incandescent light located 45 cm from the bottom of the box. The two chambers were connected by a 10 × 10 cm doorway in the center of the partition to allow free access to the adjacent chambers. Rats were moved to the test room immediately adjacent to the test apparatus at 1800h on the evening prior to the test, maintained on their standard 12h light/dark cycle, and allowed free access to the liquid diet and water. All tests are conducted between 0800h and 0900h. Animals were placed initially in the white chamber facing away from the door leading to the adjacent dark chamber; and, behavior was recorded for 300seconds (5min) using a video camera. At a later date, videos were viewed the following behaviors were documented: latency (in seconds) to enter the dark-side after initial placement in the light-side; latency (sec) to re-enter the light-side following the 1st cross into the dark-side; the number of chamber crossings, defined as at least partial passage between chambers with extension of at least ½ of the animal’s body from one chamber to the next; rearing & line crossing as measures of locomotor function; and, total time (sec) in each chamber. Animals subjected to behavioral measures were typically sacrificed within 1 hour of the test; and their brain used for electrophysiological analysis.
Brain slice preparation and neuronal isolation
To prepare brain slices, rats were anesthetized with isoflurane and decapitated. Tissue slices containing the basolateral amygdala were prepared as previously reported [29]. Briefly, the brain was rapidly removed and chilled with oxygenated, ice-cold artificial cerebro-spinal fluid (aCSF). Coronal sections containing the lateral/basolateral amygdala were held in aCSF bubbled with 95%O2 / 5%CO2 at room temperature until use (typically 1–6h). In some cases, 150mg/dl ethanol was added to brain slices from chronic ethanol exposed rats during isolation and storage to help prevent the onset of ‘withdrawal’. In a separate set of experiments, we determined that a ~2.5 hour in vitro ethanol exposure of a brain slice does not significantly affect either maximal GABA or maximal glycine responses (not shown). Neurons were isolated from coronal brain slices by digestion with 0.5–1mg/ml Pronase (CalBiochem) in aCSF at 37EC for 20min with constant oxygenation as previously described [30]. Following digestion, regions containing the lateral/basolateral amygdala were carefully dissected away from the remaining tissue. Individual neurons were isolated from these tissue pieces by mechanical separation using fire-polished Pasteur pipettes. The morphology of neurons in the preparation was consistent with lateral/basolateral amygdala neurons both in vitro [29] and in vivo [31].
Heterologous Expression of GABAA and Strychnine-sensitive Glycine receptors
Expression constructs containing cDNAs encoding the human GABAA α1, α2, and γ2S and the rat β2 subunits were a generous gift of Dr. Neil Harrison (Dept. Anesthesiology, Cornell University, N.Y., USA). Subunits corresponding to the rat α2 and β strychnine-sensitive glycine receptor cDNAs and their introduction into L-cell fibroblasts (NCTC 929 cells; American Type Culture Collection, Manassas, V.A., USA) using liposomes (Superfect; Qiagen Inc., Valencia C.A., USA) have been previously described [30]. Transfected cells were used for electrophysiology 18 to 24hr following exposure to the liposome/DNA mixture. Cells expressing GABAA of glycine receptor subunits were identified by co-expression of a GFP marker (pEGFP-N1; CloneTech, Palo Alto C.A., USA).
Electrophysiology
The whole cell patch clamp technique was performed on acutely dissociated neurons or L-cells that were continuously perfused with a HEPES-buffered saline (HBS) solution (containing in mM: 150 NaCl, 10 HEPES, 2.5 KCl, 2.5 CaCl2, 1.0 MgCl2, 10 D-glucose; pH 7.4 with NaOH, osmolality 320mmol/kg adjusted with sucrose). When recording from isolated neurons, tetrodotoxin (1µM) was added. Drugs were diluted from concentrated stocks into HBS and applied within 100µm of the cell using a linear array of fused silica tubes (150mm I.D., Hewlett Packard) mounted on a manipulator. For isolated neurons, a Cs+-based internal solution (in mM: 130 CsCl, 10 HEPES, 10 EGTA, 1 CaCl2, 4 Mg-ATP; pH 7.2 with CsOH, osmolality 305mmol/kg adjusted with sucrose) was used in the patch electrode. In experiments using L cells, this internal was modified so that the 130mM CsCl was replaced with 110mM CsOH and 20mM CsCl and the pH adjusted with methane sulfonic acid.
Recordings were done at room temperature according to published procedures using an Axopatch ID amplifier (Axon Inst.) in voltage-clamp mode as previously described [18,30]. Whole-cell capacitance and series resistance were determined by fits of the capacitive transients during square-wave voltage steps using standard software procedures contained within pClamp 7.0 software (Axon Inst.) and monitored throughout the recordings. For control amygdala neurons, these values were 21±1pF and 22±2MΩ (n=30). For chronic ethanol amygdala neurons, whole-cell capacitance was 19±1pF while series resistance was 22±1MΩ (n=53). Neither measure was significantly different between these two treatment groups. For comparison, L cells had values of 17±1pF and 21±1MΩ (n=47). Series resistance and capacitance were compensated manually. Current amplitudes were measured from the apparent peak of the response and are reported as values standardized to the whole cell capacitance (in pF). Summarized data in the text is reported as the mean ± SEM.
3. Results
Chronic ethanol reduces anxiety measured in a two-compartment light/dark box
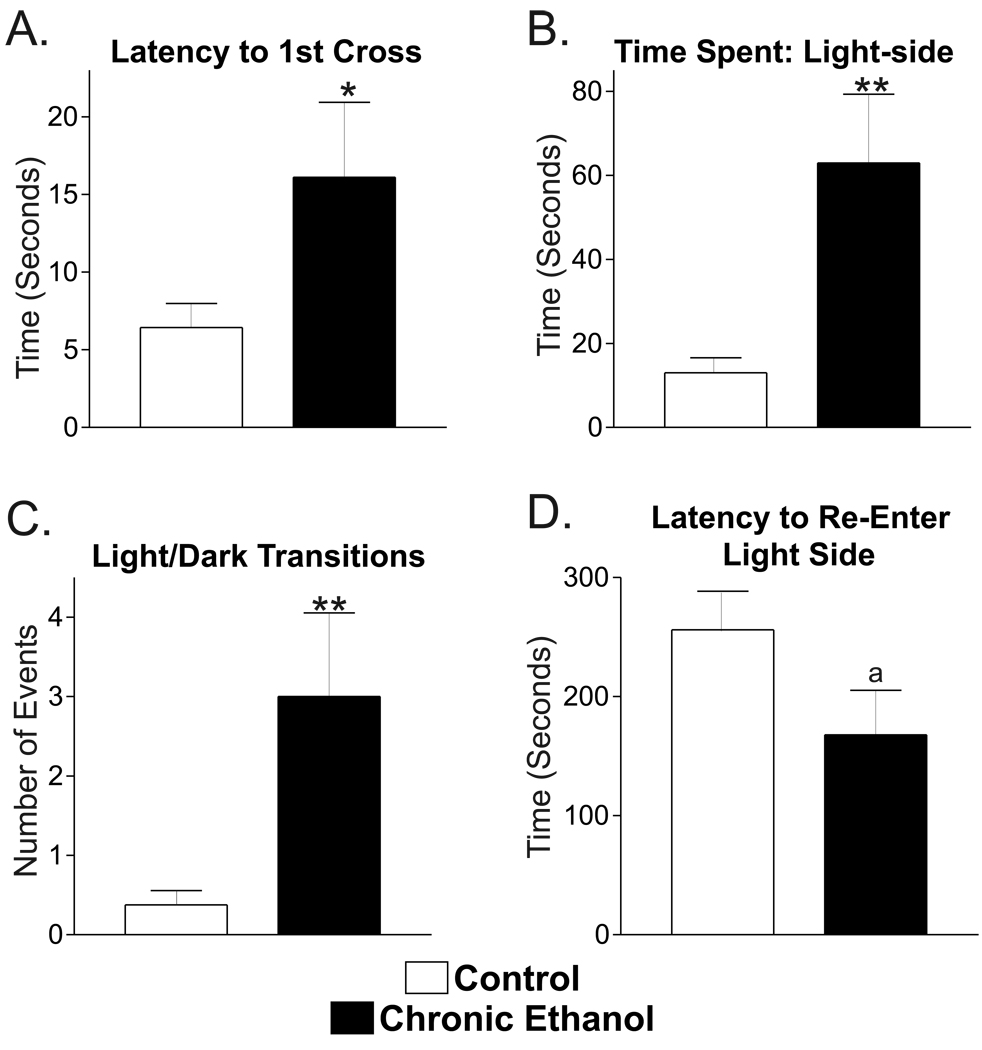
While it is well established that the anti-anxiety potential of ethanol is maintained during chronic exposure in mice [5], it is unclear whether this ethanol-anxiety interaction is intact in rats using chronic ethanol ingestion. Using a two-compartment light/dark box behavioral test, we found that chronic ethanol-treated rats (n=10) remained significantly longer in the light compartment following their initial placement (Fig. 1A, 15.3±4.5 seconds) compared to control rats (n=8; 6.4±1.6 seconds; P<0.1, t-test with Welch’s correction for unequal variances). During the course of their exposure to the test apparatus, both the control and ethanol treated rats generally preferred the dark area. However, chronic ethanol rats spent significantly more time in the light compartment (62.9±16.4 seconds out of 300 seconds, Fig. 1B) compared to control rats (13.0±3.6 seconds; P<0.05, t-test with Welch’s correction). This increase was due to a greater number of transitions between the light and dark sides of the apparatus by the chronic ethanol rats (3.0±1.0, Fig. 1C) compared to the control rats (0.4±0.2; P<0.05, t-test with Welch’s correction) since the average time spent on the light side following a particular dark-to-light transition was not significantly different between these groups (23.0±4.9 sec/transition in controls that made a transition vs. 21.8±4.6 sec/transition in chronic ethanol rats). Chronic ethanol rats also tended to re-enter the light compartment after their initial egress more quickly than did controls, with latency to re-enter being 167±37.5sec for ethanol rats (Fig. 1D) and 256±32.5sec for control rats (P#0.1, t-test). Thus, chronic ethanol ingestion in rats maintains ethanol’s anxiolytic potential, consistent with findings using chronic ethanol ingestion in mice [5].
Figure 1.
Chronic ethanol exposure does not reduce ethanol’s anxiolytic effects measured in the light-dark box. Adult male rats chronically exposed to control liquid diet (‘Control’, n=8) or ethanol liquid diet (‘Chronic Ethanol’, n=10) were placed in the light-dark box and behavior monitored for 300 seconds. (A) The time for rats initially placed in the light compartment to cross to the dark compartment was increased from 6.4±1.6 seconds in control rats to 15.3±4.5 seconds in chronic ethanol rats. ‘*’, P<0.1, t-test with Welch’s correction. (B) Chronic ethanol rats (right) spent more time in the light compartment (62.9±16.4 seconds) than did rats fed the control liquid diet (left, 13.0±3.6 seconds). ‘**’, P<0.05, t-test with Welch’s correction. (C) The number of transitions between the light and dark compartments was increased in the chronic ethanol rats (right, 3.0±1.0) compared to controls (left, 0.4±0.2). ‘**’, P<0.05, t-test with Welch’s correction. (D) The time to re-enter the light compartment following the first transition to the dark compartment was decreased in chronic ethanol rats (right, 167.9±37.5 seconds) compared to control rats (left, 256.0±32.5 seconds). ‘a’, P≤0.1, t-test.
Unlike anxiety, the depression of locomotor behavior in rats following acute ethanol administration [15] tends to adapt during chronic exposure [44]. In our paradigm, locomotor activity was represented by the total number of rears and the total number of line crosses. The total number of line-crosses in both compartments during the 300s test was not significantly different (P=0.17, un-paired t-test) between control (64.5±6.7 crosses) and chronic ethanol exposed animals (77.3±8.3 crosses). The non-significant trend for increased line-crossing in chronic ethanol rats was predominantly due their exploration of the light side of the apparatus since the number of dark-side line crosses was similar for controls (57.1±5.0) and chronic ethanol rats (52.3±3.8; P=0.44, un-paired t-test). In contrast, the line crosses on the light-side were 7.4±1.8 and 25.0±6.8 (P<0.05, un-paired t-test) for control and chronic ethanol rats, respectively. Similar to line-crosses, the total number of rears (light-side + dark-side) was not significantly different between treatment groups, with controls having 21.5±2.6 rears and chronic ethanol rats having 20.1±2.6 (P=0.71, un-paired t-test). Our results suggest that, as demonstrated by other laboratories, ethanol’s effects on locomotor behavior adapt during our chronic ethanol treatment.
Chronic ethanol differentially effects amygdala GABAA and strychnine-sensitive glycine receptors
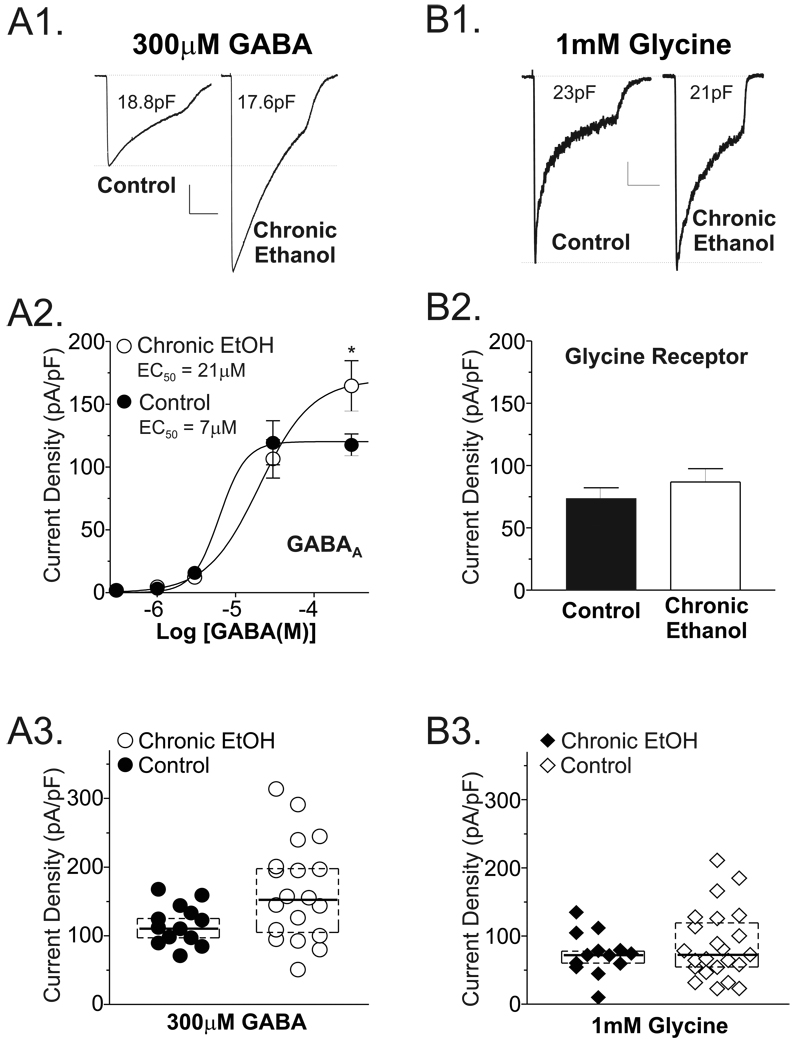
To better understand the neural mechanisms responsible for the selective maintenance of ethanol’s anti-anxiety potential during chronic exposure, we examined the effects of chronic ethanol on amygdala ligand-gated chloride channels since these neurotransmitter systems are either known or postulated to regulate anxiety. BLA neurons isolated from control or chronic ethanol brain slices expressed substantial GABA-evoked currents (Fig. 2A1). Importantly, the current density-response to a maximally efficacious GABA concentration (300µM) was significantly larger in neurons isolated from chronic ethanol rats (165.0±16.6 pA/pF, n = 19; Fig. 2A2 compared GABA responses in neurons from control rats (116.6±8.1 pA/pF, n = 13; P<0.05, t-test with Welch’s correction). This trend was also present when considering current amplitude (2.7±0.3 nA in control neurons vs. 3.3±0.3 nA in chronic ethanol neurons), although these measures only approached statistical significance due to the substantial variability in this absolute measure. Concentration-response relationships established in both treatment groups further indicated that the average log (EC50 [M]) from control neurons (−5.304±0.6, n=6) was significantly smaller (P<0.01, unpaired t-test) than that from chronic ethanol neurons (−4.828±0.1, n=8). This difference is also reflected in the ensemble EC50 values established by fits to averaged data for each concentration normalized to cell capacitance (Fig. 2A2). Together, these results suggest that chronic ethanol exposure both increases GABA efficacy, presumably by increasing receptor function or expression, as well as decreases GABA potency as evidenced by the left-ward shift in GABA concentration-response relationship.
Figure 2.
Chronic ethanol ingestion differentially influences whole-cell GABAA and strychnine-sensitive glycine currents expressed by isolated lateral/basolateral amygdala neurons. Effects of chronic ethanol consumption on GABAA receptors (A) and strychnine-sensitive glycine receptors (B) expressed by lateral/basolateral amygdala neurons. (A1 & B1) Examples of GABA (300µM) or glycine (1mM) currents at in neurons from control (left trace) and chronic ethanol-exposed rats (right trace). Current traces are from different cells with current densities similar to the average for each respective group. Calibration bars: for GABA traces, y = 0.45nA, x = 2 seconds; for glycine traces, y = 0.2nA, x = 2 seconds. (A2) Summary of GABA concentration-response data. Note that the GABA current density at 300µM was larger in neurons isolated from chronic ethanol rats (169±14 pA/pF, n=26 neurons from 13 individual rats) compared to current densities in neurons from control rats (120±7 pA/pF, n=18 neurons from 9 individual rats; * = P<0.5, unpaired t-test). In neurons where complete concentration-response data was established, GABA also had a significantly higher apparent affinity (P<0.01, unpaired t-test using the Log [EC50 (M)] from individual neurons) in control (Log [EC50 (M)] = −5.3±0.1; ensemble EC50 = 7µM) compared to chronic ethanol rats (Log [EC50 (M)] = −4.8±0.1, ensemble EC50 = 21µM). (B2) Summary of glycine current density measures from control and chronic ethanol neurons. Whole-cell glycine current densities were comparable in neurons from control rats (left, 73.6±8.7 pA/pF, n=13 neurons from 6 individuals) and from chronic ethanol rats (right,86.9±10.7 pA/pF, n=23 neurons from 10 individuals. P>0.3, t-test with Welch’s correction. (A3&B3) GABA (●,◆) and glycine (○,◇) current density data for individual neurons isolated from control (circles) and chronic ethanol (diamonds) rats. Horizontal lines represent the average current density in each population. Dashed boxes enclose those data points containing the middle 50% of the population. Note that ethanol-sensitive and –insensitive cells are apparent for both glycine and GABA agonist, although only the mean GABA current density from the ethanol-exposed cells is significantly different from control.
In contrast to the pronounced effect of chronic ethanol on GABAA receptors, there was essentially no chronic ethanol-dependent perturbation of strychnine-sensitive glycine receptors (Fig. 2B1). Glycine-gated current densities were only slighted elevated in chronic ethanol neurons (86.9±10.7 pA/pF; Fig. 2B2) compared to control neurons (73.6±8.7 pA/pF); and this non-significant trend was not reproduced when current amplitudes were directly compared (1.8±0.3 nA in control neurons vs. 1.7±0.2 nA in chronic ethanol neurons). Importantly, for both the GABA and glycine studies, whole-cell capacitance was not significantly different between neurons from the different treatment groups (P>0.2, t-test; control = 23.7±1.9 pF, chronic ethanol = 20.8±1.3 pF).
Impact of chronic ethanol exposure on acute ethanol sensitivity
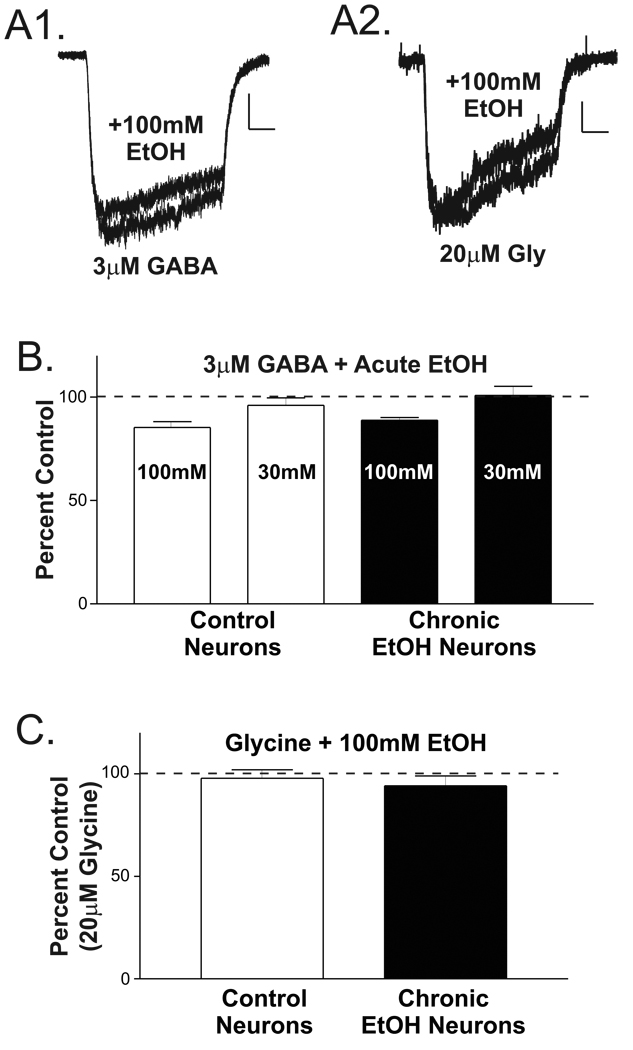
Both GABAA and strychnine-sensitive glycine receptors are sensitive to acute ethanol exposure in numerous central nervous system regions (for reviews, see [7,19]). The increased functional expression of GABAA receptors in response to chronic ethanol exposure may therefore involve alterations in their acute sensitivity. To directly test this, an EC10–15 GABA concentration (3µM) was applied to either control or chronic ethanol neurons both with and without different concentrations of ethanol (Fig. 3A1). The relative sensitivity of GABA currents to either 30mM or 100mM ethanol was not significantly different between treatment groups (Fig. 3B). Similar results were obtained using EC5–10 concentrations of GABA (not shown). Likewise, the relative sensitivity of glycine receptors to 100mM ethanol was also not significantly different between treatment groups. While, the effects of 100mM acute ethanol on glycine-gated currents was not significant (17.2±3.0 pA/pF for ‘glycine alone currents and 16.4±2.8 pA/pF for ‘glycine + ethanol’ currents; n = 18 for combined ‘control’ and ‘chronic ethanol’ cells; P>0.1, paired t-test), GABA currents from both treatment groups were modestly, but significantly, inhibited by co-application of 100mM ethanol (27.2±3.0 pA/pF for ‘GABA alone’ vs. 24.1±2.8 pA/pF for ‘GABA + ethanol’, 13.9±2.1% inhibition; P<0.001, paired t-test). 30mM acute ethanol did not significantly alter GABA-gated currents. Thus, while only the GABAA receptors appeared to be inhibited by acute ethanol, the acute sensitivity of neither GABAA nor strychnine-sensitive glycine receptors appeared to be altered by chronic ethanol exposure.
Figure 3.
Chronic ethanol exposure does not influence the acute effects of ethanol on whole-cell GABAA and strychnine-sensitive glycine currents expressed by isolated basolateral amygdala neurons. (A) Examples of acute ethanol actions on GABA-induced currents (A1, 3µM) and glycine currents (A2, 20µM). Calibration bars: y = 100pA, x = 1seconds for GABA currents; and, y = 50pA, x = 1sec for glycine currents. (B) Summary of acute ethanol inhibition of GABA currents in control and chronic ethanol exposed rats. 100mM ethanol inhibited currents in neurons isolated from both control (85±3% of 3µM GABA alone, n=8 cells from 3 individuals) and chronic ethanol rats (88±2%, n=11 cells from 5 individuals) to a similar extent (P>0.4 two-tailed unpaired t-test). Similarly, the effect of 30mM ethanol was not different between treatment groups (P>0.4, two-tailed unpaired t-test) and, unlike 100mM ethanol, did not substantially affect the GABA currents (96±4% in control, n = 5, versus 101±4% in chronic ethanol, n = 6). (C) Chronic ethanol exposure did not appear to alter the acute ethanol sensitivity of glycine currents. Glycine responses in the presence of ethanol were 98±4% of control (20µM glycine alone) in neurons isolated from control rats (left, n=8 cells from 3 individuals) compared to 94±5% in neurons from chronic ethanol rats (right, n=10 cells from 5 individuals; P>0.5 two-tailed unpaired t-test).
Influence of subunit composition on acute sensitivity to ethanol
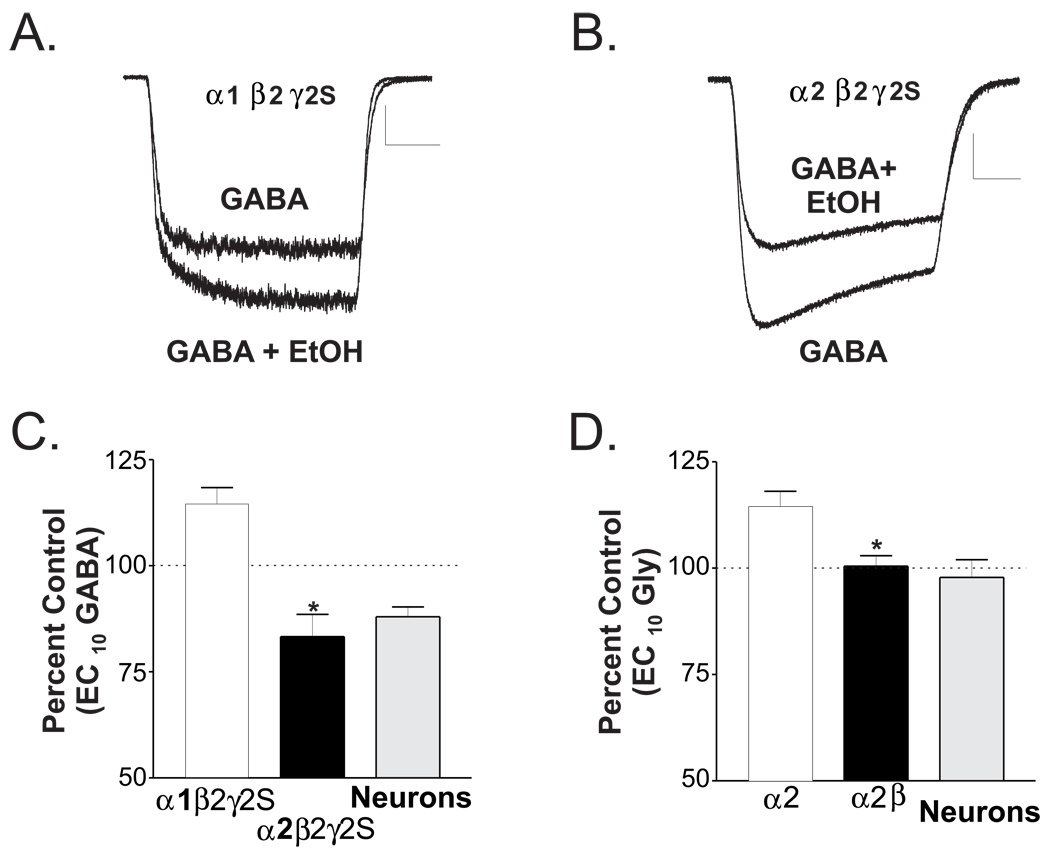
The results reported here are at odds with some reports in the literature demonstrating the potentiation of both GABAA and glycine receptor function by acute ethanol exposure. We postulated that the subunit composition of these receptors as they are expressed in the amygdala may ultimately influence their acute response to ethanol. We therefore expressed GABAA or glycine receptors in L-cell fibroblasts using specific subunits known to be expressed in the lateral/basolateral amygdala. For GABAA receptors, we used the α1β2γ2S or α2β2γ2S subunit combinations since these complexes are likely representative of GABAA receptors in this brain region [14,54]. Using an EC10 GABA concentration (12.1µM) for α1-containing receptors (Fig. 4A), co-application of 100mM ethanol along with GABA significantly increased (15±4%; Fig. 4C) the currents mediated by α1-containing receptors, from 41±7pA/pF to 48±7pA/pF (n=11, P<0.0002, paired t-test). In contrast, ethanol significantly reduced GABA (3.1µM) currents mediated by α2-containing receptors from 31±8 pA/pF to 24±6 pA/pF (n=11, P<0.02, paired t-test), a 17±5% inhibition. A similar dependence on subunit composition was noted for strychnine-sensitive glycine receptors (Fig. 4D). That is, glycine (EC10=148µM; not shown) currents mediated by α2-homomeric channels was significantly enhanced (14±4% above control) during exposure to 100mM acute ethanol (n=5, P<0.05, paired t-test) while 100mM acute ethanol did not appear to affect glycine (214µM) current mediated by α2/β-heteromeric glycine receptors (n=6, P>0.5, paired t-test).
Figure 4.
GABAA α1 and α2 subunits, major isoforms expressed in the amygdala, are differentially sensitive to acute ethanol exposure when expressed in a heterologous system. (A) In an L-cell expressing GABAA α1β2γ2S subunits, co-incident exposure to an EC10 concentration of GABA (12µM) along with 100mM ethanol resulted in substantial facilitation (bottom trace) of current amplitude compared to GABA alone (top trace). For this cell, ethanol increased the absolute current amplitude 28% above control. Calibration bars: y = 0.2nA, x = 1 second. (B) For an L-cell expressing GABAA receptors composed of α2β2γ2S subunits, GABA responses to 3µM GABA (EC10, bottom trace) were inhibited by co-application of 100mM ethanol (top trace). In this cell, ethanol reduced current amplitude to 70% of control. Calibration bars: y = 0.2nA, x = 1 second. (C) Summary of the acute effects of ethanol on L-cells expressing either α1β2γ2S or α2β2γ2S GABAA receptors. GABA currents in cells expressing α1-containing receptors were 115±4% larger when GABA was co-applied with ethanol (open box, n = 15). Conversely, ‘GABA + ethanol’ currents in cells expressing α2-containing receptors were only 83±5% of GABA-alone currents (closed box, n = 13). The ethanol effects on α1 receptors and α2 receptor were significantly different from one another (*, P<0.001, t-test). The effects of 100mM ethanol on native amygdala GABAA currents were taken from Figure 3 and are shown here for comparison. (D) For strychnine-sensitive glycine receptors in L-cells, α2-homomeric channels were facilitated by 100mM ethanol (114±4% of control, n=5) while α2β-heteromeric channels appeared unaffected (100±2%, n=6). The acute ethanol sensitivity for glycine responses in isolated basolateral amygdala neurons is taken from Figure 3 (94±5%, n=10) and is shown here for comparison.
Chronic ethanol effects on benzodiazepine sensitivity
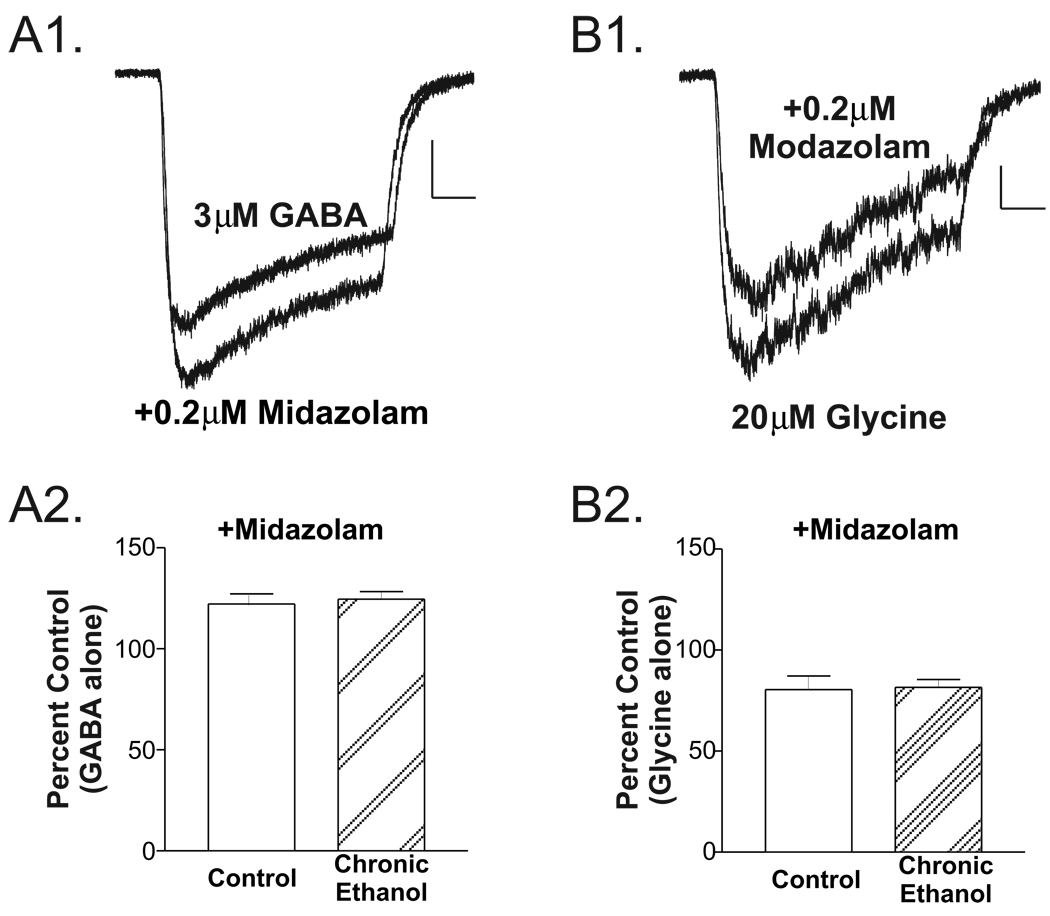
Chronic ethanol is known to alter GABAA receptor subunit composition in the ‘extended amygdala’ [38]. To provide some functional measure of chronic ethanol’s impact on subunit composition, we examined the sensitivity of GABA-mediated responses to the benzodiazepine, midazolam (0.2µM). In the presence of midazolam, GABA-induced currents (Fig. 5A1) were 122.2±4.9% of control (3µM GABA alone) in neurons isolated from control rats (n=8 cells from 3 individuals; Fig. 5A2) compared to 124.6±3.7% in neurons from chronic ethanol rats (n=12 cells from 5 individuals; P>0.7 two-tailed unpaired t-test). Unlike the positive effects of midazolam on GABAA receptors, the interaction between midazolam and strychnine-sensitive glycine receptors expressed by BLA neurons was inhibitory. Glycine currents in the presence of midazolam were 80.5±6.7% of control (e.g. 20µM glycine alone) in neurons isolated from control rats (n=7 cells from 3 individuals; Fig. 5A2) compared to 81.6±3.7% in neurons from chronic ethanol rats (n=9 cells from 5 individuals; P>0.8 two-tailed unpaired t-test). Thus, while both GABA and glycine currents were acutely sensitive to the benzodiazepine midazolam, this sensitivity was not influenced by chronic ethanol exposure.
Figure 5.
Chronic ethanol exposure does not influence the acute effects of midazolam on whole-cell GABAA and strychnine-sensitive glycine currents expressed by isolated basolateral amygdala neurons. (A) GABA-induced currents (A1; 3µM GABA alone, top trace) were modestly facilitated by co-application of 0.2µM midazolam (bottom trace). (A2) Chronic ethanol exposure did not appear to alter this acute sensitivity. GABA currents in the presence of midazolam were 122±5% of control (3µM GABA alone) in neurons isolated from control rats (left, n=8 cells from 3 individuals) compared to 125±4% in neurons from chronic ethanol rats (right, n=12 cells from 5 individuals; P>0.7 two-tailed unpaired t-test). (B) Glycine-induced currents (B1, 20µM glycine alone, bottom trace) were modestly inhibited by co-application of 0.2µM midazolam (top trace). (B2) Chronic ethanol exposure did not appear to alter this acute sensitivity. Glycine currents in the presence of midazolam were 81±7% of control (20µM glycine alone) in neurons isolated from control rats (left, n=7 cells from 3 individuals) compared to 82±4% in neurons from chronic ethanol rats (right, n=9 cells from 5 individuals; P>0.8 two-tailed unpaired t-test).
4. Discussion
While the ethanol-anxiety relationship appears to be substantial, the cellular/molecular mechanisms that potentially underlie this interaction are not well defined. In order to utilize a model system where this relationship is intact, we used a chronic liquid diet model to establish ethanol exposure and used the light/dark box behavioral test as a measure of anxiety. Here, we demonstrate that rats exposed to chronic ethanol had reduced levels of anxiety during exposure to this novel environment, as indicated by their longer latencies to move initially from the light to the dark compartment, shorter latencies to re-enter the light compartment following this first cross-over, more inter-compartment cross-overs, and larger amount of time spent in the light compartment. Similar findings have been reported for rats exposed to acute ethanol [3] and for mice chronically exposed to ethanol [5]. This is the first report to our knowledge using rats with these particular exposure and behavioral paradigms. Because rats are intoxicated at the time of testing, our results also indicate that chronic ethanol delivered by liquid diet does not alter the anxiolytic potential of ethanol itself. While this possibly means that the systems regulating anxiety-related behaviors do not adapt during chronic exposure, increased anxiety during withdrawal from chronic ethanol has been measured using the light-dark box [5]. Therefore, the maintenance of ethanol’s anxiolytic potential during chronic exposure is most likely the result of persistent adaptations in brain regions regulating this behavior. Similarly, our studies also indicate that acute ethanol’s well-established suppression locomotor activity in most rat strains normalizes during chronic treatment. For example, the number of exploratory rears and total number of line crosses was not significantly different between chronic ethanol and control rats. The modest but non-significant trend towards increased total line crosses in chronic ethanol rats is most likely a reflection of their increased activity within the ‘light’ compartment or perhaps unveils the stimulant properties of ethanol that are common in some strains of mice but typically not seen in Sprague Dawley rats [15]. Regardless, these findings are significant considering that the average blood-ethanol concentration for our chronic ethanol studies was similar to the acute ethanol levels needed to suppress locomotor activity [15].
We next examined the effects of chronic ethanol on ligand-gated chloride channels in the amygdala to appreciate the cellular and molecular basis for the persistence of ethanol’s anxiolytic potential during chronic exposure. Importantly, GABAA receptors in this brain region play a fundamental regulatory role during the expression of fear/anxiety; we demonstrate here that the maximal current density and absolute current amplitude mediated by GABA is significantly larger in neurons from chronic ethanol-exposed rats compared to controls. Importantly, these results strongly support a recent report of increased muscimol-dependent 36Cl− influx in synaptoneurosomes prepared from ‘extended amygdala’ following chronic ethanol [38]. Furthermore, the chronic ethanol-induced alteration in GABA potency described here also suggests that this treatment may have complex effects on receptor subunit composition as this is known to directly influence the agonist pharmacology of GABAA receptors [10,11]. Indeed, chronic ethanol is known to alter GABAA receptor α subunit protein levels in this brain region [38]. Importantly, chronic exposure did not significantly influence strychnine-sensitive glycine receptors expressed by these neurons [29,30], suggesting that the effects of chronic ethanol on amygdala ligand-gated chloride channels in our study were specific for GABAA receptors. While it is clear that the regulation of fear/anxiety is complex and involves numerous brain regions, increased GABAA receptor function in the lateral/basolateral amygdala following chronic exposure is potentially responsible for the maintenance of ethanol's anti-anxiety potential. However, we cannot easily predict the ultimate behavioral consequences of GABAA adaptations in the amygdala until we investigate other neurotransmitter receptor systems. Along these lines, the effects of chronic ethanol on functional GABAA receptor expression were not equivalent in all neurons tested (see Fig. 2A3 &2B3), suggesting the existence of chronic ethanol-sensitive and -insensitive populations. We are currently interested in determining if these different populations represent distinct phenotypic classes of lateral/basolateral amygdala neurons.
To establish a possible mechanism responsible for the apparent up-regulation of amygdala GABAA receptors following chronic ethanol exposure, we examined the acute ethanol sensitivity of receptors in native neurons and found that GABA-gated currents in both control and chronic ethanol-exposed rats were modestly but significantly inhibited by 100mM ethanol. Descriptions of acute ethanol effects on GABA- and glycine-gated currents are abundant in the literature although ethanol's effects vary from preparation to preparation and even within a particular brain region. For example, in rat cortex, acute ethanol has been shown both to facilitate [39] and have no effect on [34] GABAA receptor function. Regardless, ethanol's acute inhibition of lateral/basolateral amygdala GABAA receptors could participate in the adaptive facilitation of these responses during chronic exposure. Acute ethanol’s modest inhibition of amygdala GABAA receptors might also contribute to the ‘excitatory’ nature of ethanol seen at low-to-moderate doses in some systems [15]. However, our average blood ethanol levels (148mg/dl or about 30mM) would likely produce only a minor acute inhibition of amygdala receptors. It is therefore possible that additional mechanisms may also contribute to the increased GABAA expression during chronic ethanol exposure.
Because GABAA subunit composition has been proposed to influence the acute sensitivity of these receptors to ethanol or other alcohols ([49,52]; but see [33]) and could ultimately be responsible for at least some of the adaptations we found during chronic exposure, we examined whether the acute ethanol inhibition of GABAA currents or the apparent lack of acute ethanol effect on strychnine-sensitive glycine currents in isolated amygdala neurons was somehow due to the subunit composition of these receptors. The GABAA α1, α2, β2/3, and γ2 subunits [14,54] are prominently expressed in the lateral/basolateral amygdala and were presumed to contribute to native receptors. Currents mediated by α1/β2/γ2S receptors expressed in L-cells were in fact facilitated by acute ethanol; conversely, receptors consisting of α2/β2/γ2S were inhibited by ethanol. Foremost, these findings suggest that acute ethanol inhibition of GABA currents in isolated amygdala neurons may reflect a substantial contribution of α2-containing receptors, similar to the α2/β2/γ2S channels expressed in L-cells. This is consistent with the findings that α2- containing GABAA receptors are the primary mediators of benzodiazepine’s anti-anxiety effects [25,32] and that these effects are at least partially focused in the amygdala [42]. In contrast to the acute ethanol inhibition of the α2/β2/γ2 subunit combination used in this study, recent reports have shown that α2/β1/γ2 receptors are potentiated by acute ethanol [49] when expressed in Xenopus oocytes. Importantly, Ueno and colleagues show that the mutation of a single serine residue (S280) in the second transmembrane helix of the β1 subunit [49] obliterates acute ethanol facilitation of the α2/β1/γ2 combination. It is noteworthy that the equivalent residue in the β2 subunit used in our studies is an asparagine (N279, B. McCool unpublished observations), suggesting that the divergent effects of ethanol on α2/β1/γ2 receptors (facilitation; [49]) and α2/β2/γ2 receptors (inhibition; this work) may be heavily influenced by a single amino acid in TM2 of the β subunit. Since the α1β2γ2S receptors expressed in our studies were facilitated by acute ethanol, our results could also suggest that multiple subunits contribute the molecular determinates for acute ethanol sensitivity and that contributions among different subunits are not equivalent.
Our heterologous expression studies with the strychnine-sensitive glycine receptor subunits may also provide some insight into the mechanisms underlying the apparent lack of sensitivity of native amygdala receptors to acute ethanol. Previous studies have shown that the glycine α2 and β subunits are likely to be the major subunits expressed in the lateral/basolateral amygdala [30]. This work demonstrates that glycine currents both in isolated amygdala neurons and in L-cells expressing the α2/β heteromeric channels are relatively insensitive to modulation by acute ethanol. Importantly, the ethanol sensitivity of native glycine receptors in cultured spinal cord neurons evolves from inhibition/no effect in young cultures to moderate potentiation in older neurons [47]. This shift during in vitro development parallels a developmental increase in the apparent ethanol sensitivity of glycinergic synaptic responses in medullary slice preparations [12]. It is therefore possible that the shift from α2-containing glycine receptors in neonatal animals to α1-containing receptors in adults, as denoted by mRNA and biochemical studies [20,26], may also result in a shift in ethanol’s efficacy at this receptor. While studies in heterologous systems have shown that both α1- and α2-homomeric glycine receptors are sensitive to ethanol, these differ in their absolute ethanol sensitivity [27,28,50], supporting the possibility that the presence of the α2 subunit may reduce the ethanol sensitivity of amygdala glycine receptors. However, among the most significant findings of the present study, glycine receptors composed of both α2 and β subunits appear resistant to acute ethanol compared to α2 homomeric channels, suggesting that the β subunit may in some cases ‘silence’ or at least attenuate the modulation of glycine receptors by ethanol. The presence of the β subunit in amygdala glycine receptors has been suggested by their relative insensitivity to picrotoxin [30]; and, this may be the largest contributing factor for determining the ethanol sensitivity of these native channels. The suppression of ethanol modulation by the β subunit may be a characteristic of glycine receptors in general since α1/β channels appear less sensitive to ethanol than their α1-homomeric counterparts [50].
Finally, it has recently been shown that the anti-anxiety effects of benzodiazepines like diazepam are due to positive actions at GABAA receptors containing α2/γ, but not α1/γ, subunits [25,32]. Because amygdala receptors are not facilitated by acute ethanol but nonetheless are likely to contain α2 subunits [14,54], the acute anxiolytic effects of ethanol may rely upon mechanisms distinct from postsynaptic actions at amygdala GABAA receptors. Our results also demonstrate that the apparent efficacy of benzodiazepine agonists on amygdala GABAA receptors is not altered by chronic ethanol exposure. When coupled with the apparent increase in functional GABAA expression in the lateral/basolateral amygdala following chronic ethanol, this result may in fact provide the cellular basis for the pronounced clinical efficacy of benzodiazepine therapies during ethanol withdrawal.
While it must be recognized that numerous brain regions and neurotransmitter systems are likely to contribute to the ethanol-anxiety interaction, there are several lines of evidence suggesting that the amygdala is likely to profoundly influence these processes. For example, central amygdala lesions both enhance ethanol consumption in a two bottle choice paradigm and decrease anxiety following a stressor [35], while basolateral amygdala lesions did not affect either measure. Similarly, neurochemical disruption of amygdala serotonergic function, which targets primarily the basolateral amygdala due to the prevalence of serotonin in this area compared to the central nucleus, does not influence ethanol preference in the same two bottle choice [46]. While these studies could be interpreted to indicate that the basolateral amygdala may not influence ‘basal’ ethanol drinking per se, serotonin type-3 receptors are highly expressed in the basolateral amygdala [22]; and, 5-HT3 antagonists injected into the amygdala disrupt ethanol drinking in a learned-limited access procedure [9]. Due to the distinct but overlapping roles of the basolateral and central amygdala in fear-learning [21], it is equally possible that distinct measures of ethanol consumption may be differentially influenced by basolateral or central amygdala. We conclude therefore that adaptation of lateral/basolateral amygdala neurotransmitter systems following chronic ethanol, especially increased GABAA receptor function, may represent cellular substrates regulating ethanol's anxiolytic potential during prolonged exposure.
Acknowledgements
This work supported by NIAAA (AA13120) and by the Texas A&M University Center for Environmental & Rural Public Health Pilot Project Program (B.A.M.). We would also like to acknowledge the technical assistance of Shailendra Das and Matthew Pesek.
References
- 1.Aguayo LG, Pancetti FC. Ethanol modulation of the gamma-aminobutyric acidA- and glycine- activated Cl- current in cultured mouse neurons. J Pharmacol Exp Ther. 1994;270:61–69. [PubMed] [Google Scholar]
- 2.Alvarez I, Gonzalo LM, Llor J. Effects of chronic alcoholism on the amygdaloid complex. A study in human and rats. Histol Histopathol. 1989;4:183–192. [PubMed] [Google Scholar]
- 3.Bilkei-Gorzo A, Gyertyan I, Levay G. mCPP-induced anxiety in the light-dark box in rats--a new method for screening anxiolytic activity. Psychopharmacology (Berl) 1998;136:291–298. doi: 10.1007/s002130050568. [DOI] [PubMed] [Google Scholar]
- 4.Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- 5.Costall B, Kelly ME, Onaivi ES, Naylor RJ. The effect of ketotifen in rodent models of anxiety and on the behavioural consequences of withdrawing from treatment with drugs of abuse. Naunyn Schmiedebergs Arch Pharmacol. 1990;341:547–551. doi: 10.1007/BF00171735. [DOI] [PubMed] [Google Scholar]
- 6.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 7.Crews FT, Morrow AL, Criswell H, Breese G. Effects of ethanol on ion channels. Int Rev Neurobiol. 1996;39:283–367. doi: 10.1016/s0074-7742(08)60670-4. [DOI] [PubMed] [Google Scholar]
- 8.Darstein M, Loschmann PA, Knorle R, Feuerstein TJ. Strychnine-sensitive glycine receptors inducing [3H]-acetylcholine release in rat caudatoputamen: a new site of action of ethanol? Naunyn Schmiedebergs Arch Pharmacol. 1997;356:738–745. doi: 10.1007/pl00005112. [DOI] [PubMed] [Google Scholar]
- 9.Dyr W, Kostowski W. Evidence that the amygdala is involved in the inhibitory effects of 5-HT3 receptor antagonists on alcohol drinking in rats. Alcohol. 1995;12:387–391. doi: 10.1016/0741-8329(95)00023-k. [DOI] [PubMed] [Google Scholar]
- 10.Ebert B, Thompson SA, Saounatsou K, McKernan R, Krogsgaard-Larsen P, Wafford KA. Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1997;52:1150–1156. [PubMed] [Google Scholar]
- 11.Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of gamma-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different alpha, beta, and gamma receptor subunit combinations. Mol Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- 12.Eggers ED, O'Brien JA, Berger AJ. Developmental changes in the modulation of synaptic glycine receptors by ethanol. J Neurophysiol. 2000;84:2409–2416. doi: 10.1152/jn.2000.84.5.2409. [DOI] [PubMed] [Google Scholar]
- 13.Findlay GS, Wick MJ, Mascia MP, Wallace D, Miller GW, Harris RA, Blednov YA. Transgenic expression of a mutant glycine receptor decreases alcohol sensitivity of mice. J Pharmacol Exp Ther. 2002;300:526–534. doi: 10.1124/jpet.300.2.526. [DOI] [PubMed] [Google Scholar]
- 14.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 15.Frye GD, Breese GR. An evaluation of the locomotor stimulating action of ethanol in rats and mice. Psychopharmacol. 1981;75:372–379. doi: 10.1007/BF00435856. [DOI] [PubMed] [Google Scholar]
- 16.Frye GD, Chapin RE, Vogel RA, Mailman RB, Kilts CD, Mueller RA, Breese GR. Effects of acute and chronic 1,3-butanediol treatment on central nervous system function: a comparison with ethanol. J Pharmacol Exp Ther. 1981;216:306–314. [PubMed] [Google Scholar]
- 17.Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- 18.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 19.Harris RA. Ethanol actions on multiple ion channels: which are important? Alcohol Clin Exper Res. 1999;23:1563–1570. [PubMed] [Google Scholar]
- 20.Hoch W, Betz H, Becker CM. Primary cultures of mouse spinal cord express the neonatal isoform of the inhibitory glycine receptor. Neuron. 1989;3:339–348. doi: 10.1016/0896-6273(89)90258-4. [DOI] [PubMed] [Google Scholar]
- 21.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 22.Kilpatrick GJ, Jones BJ, Tyers MB. The distribution of specific binding of the 5-HT3 receptor ligand [3H]GR65630 in rat brain using quantitative autoradiography. Neurosci Lett. 1988;94:156–160. doi: 10.1016/0304-3940(88)90287-x. [DOI] [PubMed] [Google Scholar]
- 23.Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- 24.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989;24:197–211. [PubMed] [Google Scholar]
- 25.Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 26.Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. Embo J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascia MP, Machu TK, Harris RA. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br J Pharmacol. 1996;119:1331–1336. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996;50:402–406. [PubMed] [Google Scholar]
- 29.McCool BA, Botting SK. Characterization of strychnine-sensitive glycine receptors in acutely isolated adult rat basolateral amygdala neurons. Brain Res. 2000;859:341–351. doi: 10.1016/s0006-8993(00)02026-6. [DOI] [PubMed] [Google Scholar]
- 30.McCool BA, Farroni JS. Subunit composition of strychnine-sensitive glycine receptors expressed by adult rat basolateral amygdala neurons. Eur J Neurosci. 2001;14:1082–1090. doi: 10.1046/j.0953-816x.2001.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- 32.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 33.Mihic SJ, Whiting PJ, Harris RA. Anaesthetic concentrations of alcohols potentiate GABAA receptor- mediated currents: lack of subunit specificity. Eur J Pharmacol. 1994;268:209–214. doi: 10.1016/0922-4106(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 34.Mihic SJ, Wu PH, Kalant H. Potentiation of gamma-aminobutyric acid-mediated chloride flux by pentobarbital and diazepam but not ethanol. J Neurochem. 1992;58:745–751. doi: 10.1111/j.1471-4159.1992.tb09781.x. [DOI] [PubMed] [Google Scholar]
- 35.Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- 36.Morales M, Criado JR, Sanna PP, Henriksen SJ, Bloom FE. Acute ethanol induces c-fos immunoreactivity in GABAergic neurons of the central nucleus of the amygdala. Brain Res. 1998;798:333–336. doi: 10.1016/s0006-8993(98)00457-0. [DOI] [PubMed] [Google Scholar]
- 37.Munjack DJ, Moss HB. Affective disorder and alcoholism in families of agoraphobics. Arch Gen Psychiatry. 1981;38:869–871. doi: 10.1001/archpsyc.1981.01780330027002. [DOI] [PubMed] [Google Scholar]
- 38.Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor- mediated 36 Cl(−) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–1275. [PubMed] [Google Scholar]
- 39.Proctor WR, Soldo BL, Allan AM, Dunwiddie TV. Ethanol enhances synaptically evoked GABAA receptor-mediated responses in cerebral cortical neurons in rat brain slices. Brain Res. 1992;595:220–227. doi: 10.1016/0006-8993(92)91053-h. [DOI] [PubMed] [Google Scholar]
- 40.Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers B, Korten AE, Jorm AF, Jacomb PA, Christensen H, Henderson AS. Non-linear relationships in associations of depression and anxiety with alcohol use. Psychol Med. 2000;30:421–432. doi: 10.1017/s0033291799001865. [DOI] [PubMed] [Google Scholar]
- 42.Sanders SK, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry. 1995;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- 43.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- 44.Schaefer GJ, Michael RP. Effects of an alcohol diet on locomotor activity and the acquisition of brain self-stimulation in rats. Alcohol. 1991;8:71–75. doi: 10.1016/0741-8329(91)91296-e. [DOI] [PubMed] [Google Scholar]
- 45.Schneider U, Altmann A, Baumann M, Bernzen J, Bertz B, Bimber U, Broese T, Broocks A, Burtscheidt W, Cimander KF, Degkwitz P, Driessen M, Ehrenreich H, Fischbach E, Folkerts H, Frank H, Gurth D, Havemann-Reinecke U, Heber W, Heuer J, Hingsammer A, Jacobs S, Krampe H, Lange W, Lay T, Leimbach M, Lemke MR, Leweke M, Mangholz A, Massing W, Meyenberg R, Porzig J, Quattert T, Redner C, Ritzel G, Rollnik JD, Sauvageoll R, Schlafke D, Schmid G, Schroder H, Schwichtenberg U, Schwoon D, Seifert J, Sickelmann I, Sieveking CF, Spiess C, Stiegemann HH, Stracke R, Straetgen HD, Subkowski P, Thomasius R, Tretzel H, Verner LJ, Vitens J, Wagner T, Weirich S, Weiss I, Wendorff T, Wetterling T, Wiese B, Wittfoot J. Comorbid anxiety and affective disorder in alcohol-dependent patients seeking treatment: the first Multicentre Study in Germany. Alcohol Alcohol. 2001;36:219–223. doi: 10.1093/alcalc/36.3.219. [DOI] [PubMed] [Google Scholar]
- 46.Sommer W, Moller C, Wiklund L, Thorsell A, Rimondini R, Nissbrandt H, Heilig M. Local 5,7-dihydroxytryptamine lesions of rat amygdala: release of punished drinking, unaffected plus-maze behavior and ethanol consumption. Neuropsychopharmacology. 2001;24:430–440. doi: 10.1016/S0893-133X(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 47.Tapia JC, Aguayo LG. Changes in the properties of developing glycine receptors in cultured mouse spinal neurons. Synapse. 1998;28:185–194. doi: 10.1002/(SICI)1098-2396(199803)28:3<185::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 48.Tupala E, Hall H, Bergstrom K, Sarkioja T, Rasanen P, Mantere T, Callaway J, Hiltunen J, Tiihonen J. Dopamine D(2)/D(3)-receptor and transporter densities in nucleus accumbens and amygdala of type 1 and 2 alcoholics. Mol Psychiatry. 2001;6:261–267. doi: 10.1038/sj.mp.4000859. [DOI] [PubMed] [Google Scholar]
- 49.Ueno S, Wick MJ, Ye Q, Harrison NL, Harris RA. Subunit mutations affect ethanol actions on GABA(A) receptors expressed in Xenopus oocytes. Br J Pharmacol. 1999;127:377–382. doi: 10.1038/sj.bjp.0702563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valenzuela CF, Cardoso RA, Wick MJ, Weiner JL, Dunwiddie TV, Harris RA. Effects of ethanol on recombinant glycine receptors expressed in mammalian cell lines. Alcohol Clin Exp Res. 1998;22:1132–1136. [PubMed] [Google Scholar]
- 51.van Zundert B, Albarran FA, Aguayo LG. Effects of chronic ethanol treatment on gamma-aminobutyric acid(A) and glycine receptors in mouse glycinergic spinal neurons. J Pharmacol Exp Ther. 2000;295:423–429. [PubMed] [Google Scholar]
- 52.Whitten RJ, Maitra R, Reynolds JN. Modulation of GABAA receptor function by alcohols: effects of subunit composition and differential effects of ethanol. Alcohol Clin Exp Res. 1996;20:1313–1319. doi: 10.1111/j.1530-0277.1996.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 53.Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye JH, Tao L, Ren J, Schaefer R, Krnjevic K, Liu PL, Schiller DA, McArdle JJ. Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. J Pharmacol Exp Ther. 2001;296:77–83. [PubMed] [Google Scholar]
- 56.Ye JH, Tao L, Zhu L, Krnjevic K, McArdle JJ. Ethanol inhibition of glycine-activated responses in neurons of ventral tegmental area of neonatal rats. J Neurophysiol. 2001;86:2426–2434. doi: 10.1152/jn.2001.86.5.2426. [DOI] [PubMed] [Google Scholar]