Summary
Integrins are heterodimeric transmembrane cell adhesion receptors that are essential for a wide range of biological functions via cell-matrix and cell-cell interactions. Recent studies have provided evidence that some of the subunits in the integrin family are involved in synaptic and behavioral plasticity. To further understand the role of integrins in the mammalian CNS, we generated a postnatal forebrain and excitatory neuron-specific knockout of α8-integrin in the mouse. Behavioral studies demonstrated that the mutant mice are normal in multiple hippocampal-dependent learning tasks, including a T-maze, non-match-to-place working memory task for which other integrin subunits like α3- and β1-integrin are required. In contrast, mice mutant for α8-integrin exhibited a specific impairment of long-term potentiation (LTP) at Schaffer collateral-CA1 synapses, whereas basal synaptic transmission, paired-pulse facilitation and long-term depression (LTD) remained unaffected. Since LTP is also impaired in the absence of α3-integrin, our results indicate that multiple integrin molecules are required for the normal expression of LTP, and different integrins display distinct roles in behavioral and neurophysiological processes like synaptic plasticity.
Keywords: integrins, long-term potentiation, learning, memory
Introduction
Integrins are transmembrane cell adhesion receptors that mediate cell-matrix and cell-cell interactions. To date, 18 α and 8 β subunits have been characterized in mammals that together form at least 24 integrin heterodimers (for review, see Chan and Davis, 2008). Although the cytoplasmic domains of either subunit lack enzymatic activity, they interact with a large number of signaling molecules, thereby transducing information bi-directionally through the “inside-out” and “outside-in” signal transduction pathways. By physically interacting with the extracellular factors and the intracellular cytoskeletal elements, integrins also serve as pivotal molecules that dynamically link the external environment of the cell to its internal cytoarchitectural components.
Accumulating evidence has implicated integrin function in the central nervous system physiology underlying synaptic and behavioral plasticity. For instance, mutations of Drosophila volado, the gene encoding the αPS3-integrin, produce a short-term olfactory memory deficit (Grotewiel et al., 1998). In vertebrates, mice with reduced expression of the α3, α5, and α8 integrin subunits (triple heterozygotes) are defective in hippocampus-dependent spatial reference memory in the Morris water maze (Chan et al., 2003). Moreover, forebrain and excitatory neuron-specific deletion of α3- or β1-integrin impairs working memory in the hippocampal-dependent, non-match-to-place T-maze task (Chan et al., 2006; Chan et al., 2007).
Physiologically, the volado mutants exhibit significant defects in two forms of Ca2+-dependent short-term facilitation, paired-pulse facilitation and frequency-dependent short-term facilitation, as well as in impaired post-tetanic potentiation at the neuromuscular junction (Rohrbough et al., 2000). In vertebrates, deletion of α3- or β1-integrin subunits through genetic means impairs hippocampal long-term potentiation (LTP) in the mutant animals (Chan et al., 2003; Chan et al., 2006; Huang et al., 2006; Chan et al., 2007). Moreover, treatment of hippocampal slices with function blocking antibodies against α3- and α5-integrins or disintegrins, which are small, high binding affinity, RGD-containing integrin inhibitors found in various snake venoms, destabilize LTP (Chun et al., 2001; Kramár et al., 2002). More recently, Kramár et al (2006) have shown that function-blocking antibodies against β1 integrins applied shortly after stimulation eliminates not only the stabilization of LTP but actin reassembly as well. In addition to participating in hippocampal LTP, evidence indicating a function for integrins in other forms of synaptic plasticity has been obtained from studies focusing on different brain regions. For example, overexpression of the α3 subunit or activation of integrins by extracellular Mn2+ suppresses rebound potentiation induction at the GABAergic synapses between inhibitory interneurons and a Purkinje neuron in the cerebellum, and such suppression is blocked by function blocking antibodies against the α3 or β1 subunit, suggesting a critical role of α3β1 integrin in negatively regulating the long-term plasticity at inhibitory synapses on cerebellar Purkinje neurons (Kawaguchi and Hirano, 2006). Together, these results indicate that integrins play diverse roles in synaptic and behavioral plasticity.
Studies on the expression of integrins have revealed that with the exception of α2, β2 and β3, RNA transcripts for all of the surveyed integrin subunits are expressed in multiple brain regions and overlap extensively (Pinkstaff et al, 1999; www.stjudebgem.org, www.genepaint.org and www.brain-map.org). Although prior studies have clearly confirmed the requirement of a handful of integrin subunits in synaptic plasticity and behavioral function, the complexity of the integrin family and their expression patterns suggest that other subunits are likely to be involved in important synaptic and behavioral processes as well. One such candidate is α8-integrin. Using electron miscroscopy, Einheber et al. (1996) demonstrated that α8-integrin immunolabeling is localized to the dendritic spines of pyramidal neurons where it is associated with the postsynaptic density. Its only known binding partner, β1-integrin, has been shown to be required for normal LTP and working memory. To further understand the roles for this integrin in the CNS, we generated a floxed allele of α8-integrin and examined the functional consequences of deleting α8-integrin in the excitatory neurons of the mouse forebrain.
Results
Generation of forebrain-specific α8-integrin mutant mice
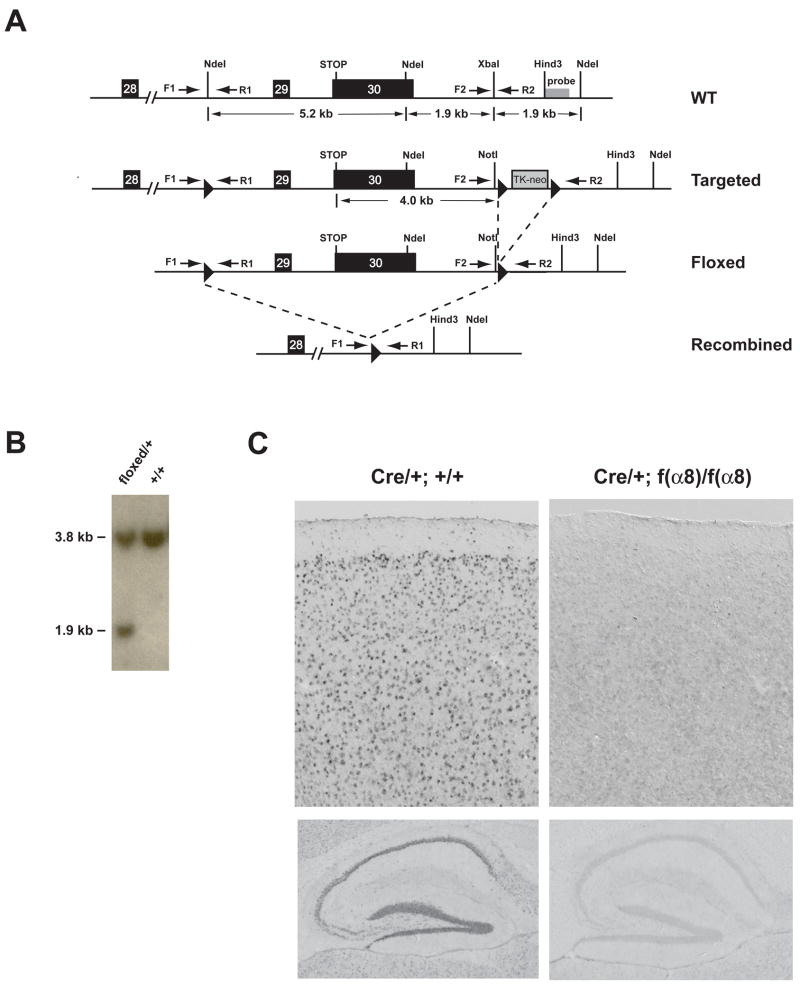
To generate mice with floxed allele of α8-integrin, we created a construct in which a loxP site was inserted into intron 28 and a cassette with the second and third loxP sites flanking a neomycin-thymidine kinase gene was inserted 4 kb downstream of the STOP codon in the 3′ flanking region of the gene (Figure 1A). Targeted ES cell clones produced via homologous recombination with the construct and missing the TK-neo cassette produced by Cre-mediated recombination between the second and the third loxP sites were selected (Figure 1B) to generate germline-transmitting chimeras. Heterozygous mice carrying the floxed [f(α8)] allele were outcrossed to wild type C57BL/6J mice for more than six generations to minimize the potential effects of genetic background on physiological and behavioral testing described below.
Figure 1. Generation of the f(α8) allele and forebrain KOs of integrin-α8.
(A) Schematic diagram of a portion of the α8-integrin genomic region, the targeting vector, the targeted locus, and the recombined allele. The solid dark boxes with numbers mark the positions of exons. The 34 bp loxP sequence is indicated by arrowheads. F1, R1 and F2, R2 represent primer pairs used to detect the presence of the 5′ and 3′ loxP sequence, respectively. The probe used for Southern blot analysis of the ES cell DNA in (B) is indicated. Dotted lines indicate Cre-mediated recombination events.
(B) Southern blot analysis of genomic DNA isolated from Cre-transfected ES cells doubly-digested with NotI and NdeI and probed with an external probe as indicated in (A). The probe detected a 3.8 kb NdeI fragment in wild-type (+/+) and heterozygous (+/−) cells, and a 1.9 kb NotI-NdeI fragment only in the heterozygous cells.
(C) In situ hybridization of α8-integrin mRNA expression in the neocortex (upper panels) and hippocampus (lower panels) of Cre/+ control (left) and f(α8)KO animals (right). The expression of the α8-integrin gene was undetectable in the neocortical neurons and in the hippocampal CA1, CA3 and dentate gyrus cells in α8-integrin knockouts.
To study the role of α8-integrin in synaptic plasticity and behavior, we inactivated the α8-integrin gene specifically in the forebrain excitatory neurons by introducing a transgene carrying Cre recombinase driven by the α-CaMKII promoter (Dragatsis and Zeitlin, 2000) into the genome of f(α8) homozygous mice. Cre-mediated recombination between the first two loxP sites removes exon 29 and exon 30, the last two exons of the α8-integrin transcript. Any resulting mRNA, if made, is expected to encode a protein that lacks the transmembrane and the cytoplasmic domains. Using a probe whose sequence was specific to the deleted region, we confirmed by in situ hybridization that the mRNA expression of α8-integrin was markedly reduced or undetectable in the neocortex and hippocampus of the α8-integrin knockout mice [f(α8)KO] (Figure 1C).
f(α8)KO mice exhibit normal motor learning, habituation, anxiety, and hippocampal-dependent spatial learning and memory
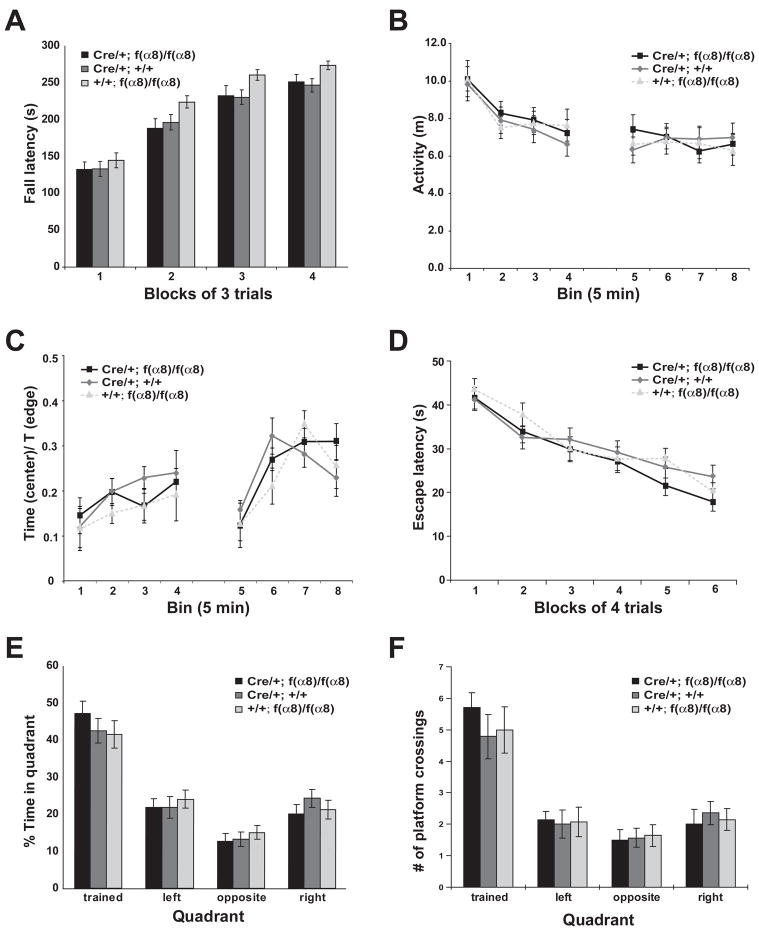
We began our studies on the role α8-integrin in learning and memory by examining the performance of the f(α8)KOs in a battery of behavioral tasks, using their littermates that carry either only the Cre transgene or homozygous f(α8) alleles but without the Cre transgene as experimental controls. We first evaluated the animal’s sensorimotor coordination and motor skill learning ability by using the rotarod test. In both initial performance and the rate of improvement over trials during the course of the experiment, the f(α8) KO mice performed indistinguishably from the control groups (Figure 2A).
Figure 2. α8-integrin knockouts exhibited normal behavioral performance in rotarod, open field and water maze tasks.
(A) Latency to fall as a function of trial block in the rotarod task. All three groups of animals showed significant improvement in their latency to fall in this task over 4 days of training (F[2, 47] = 146; p < 0.0001). No significant difference was detected in the performance between the genotypes on any day (F[2, 47] = 1.01; p > 0.37).
(B) Locomotor activity in the open field. The f(α8)KO mice showed no difference in activity from the controls in each 5-minute bin (F[2,47] = 0.281; p = 0.76). Like the controls, the f(α8)KO mice showed a significant reduction in activity 5 minutes after entering the open field in the first session, indicating that the knockout animals showed normal habituation (bin 1 vs bin 2, F[2,47] = 22.2; p = 0.0003). In addition, all groups retained a habituated level of activity across the 30 min interval between the two sessions, suggesting no difference in the memory of habituation between the genotypes (bin 4 vs bin 5, F[2,47] = 0.623; p = 0.433).
(C) Relative activity in the open field. The f(α8)KO mice showed no difference from the controls in the ratio of time spent in the center of the open field arena compared to near the edge of the arena for each 5-minute bin of both sessions (F[2,47] = 0.063; p = 0.94), indicating that the knockout animals were not significantly different from controls in this measure of anxiety.
(D) Acquisition of spatial memory plotted as escape latency as a function of training day in the hidden version of the water maze. No significant difference was observed between the f(α8)KO (Cre/+; f(α8)/f(α8), n = 14) and the control groups (Cre/+; +/+, n = 14 and +/+; f(α8)/f(α8), n = 14; F[2,39] = 0.859; p = 0.43) in escape latency across trials. (ANOVA with repeated measures).
(E) Percentage of time spent in each quadrant during a probe trial at 24 hr after the last training trial. The α8-integrin knockout and the control groups spent significantly more time in the trained quadrant than in the other three quadrants (p ≤ 0.001 for each genotype; Scheffé’s post hoc comparison). No significant difference was found between the knockout and the control groups (F[2,39] = 0.72; p > 0.93).
(F) Number of platform crossings during the probe trial at 24 hours after the last training trial. The α8-integrin knockout and the control groups passed through the platform location in the trained quadrant significantly more times than in the corresponding location in the other quadrants (≤ 0.001 for each genotype; Scheffé’s post hoc comparison). However, no significant difference was found between the knockout and the control groups (F[2,39] = 0.22; p > 0.79).
Next, we tested the animals in the open field test, a hippocampus- and basal ganglia-dependent task that measures locomotor and exploratory activity of the animal in a novel environment (Herrero et al., 2002; Gray et al., 1983; Pouzet et al., 1999). The location of each animal was recorded continuously during the test for 2 twenty-minute trials that were separated by a 30-minute inter-trial interval (ITI). As shown in Figure 2B, no detectable difference was observed between the f(α8)KO group and the control groups in the total distance traveled by the animals during each 5-minute time bin within the 20-minute test. The similar decrease in activity with time observed during the first trial indicated that the f(α8)KO animals were able to habituate to a novel environment as well as the control animals. Furthermore, the f(α8)KO animals showed the same reduced level of activity as the control animals at the start of the second trial, indicating that their memory of the habituation was maintained. In addition, no significant difference was found in the ratio of time spent at the center of the open field arena versus near the edges between the f(α8)KO animals and the control animals (Figure 2C), indicating that the f(α8)KOs exhibited a similar level of anxiety as the controls as measured by this parameter.
We next examined the ability of the knockout animals to acquire a hippocampal-dependent spatial reference memory using the watermaze assay, in which animals were trained to escape to a hidden platform using multiple distal spatial cues. As shown in Figure 2D, the f(α8)KO animals showed a similar ability as the control animals to acquire the spatial task across trials, as indicated by the rate of improvement in their escape latency and path lengths taken (data not shown) over 5 days of training. To measure the spatial reference memory of the knockout and control animals, a probe trial was conducted 24 hours after the last training trial (Figure 2E and 2F). The results showed that spatial memory in the f(α8)KO animals, as measured by time spent in the trained quadrant or the number of virtual platform crossings in the trained quadrant, was indistinguishable from the controls.
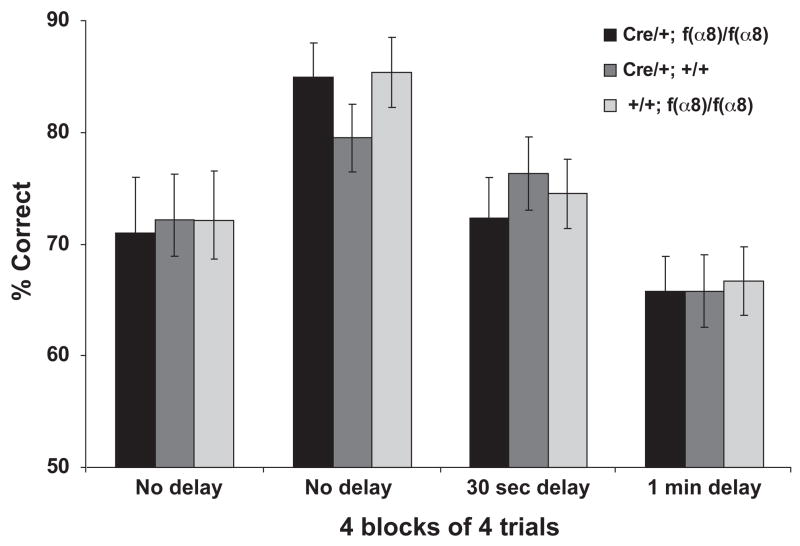
Our prior results have shown that forebrain KOs of α3- and β1-integrin exhibit impaired working memory when tested in a delayed, non-match-to-place T-maze assay (Chan et al., 2006; Chan et al., 2007). This prompted the question of whether this phenotype was a general one associated with integrin KOs or whether the phenotype is specific to the α3-and β1-integrins. We thus examined the hippocampal-dependent working memory of the f(α8)KO animals using the same assay. Our results showed that the f(α8)KO animals were able to acquire and perform the task as well as the controls by reaching criterion at a similar rate (75% correct choices over 16 consecutive trials in the choice run; see Materials and Methods). After reaching the criterion, we tested their performance with different delay intervals imposed between the sample and the choice runs. As shown in Figure 3, the performance of the f(α8)KO animals remained indistinguishable from the control animals when tested with 0, 20 or 60 seconds delay. Therefore, these results indicate that α8-integrin is not required for working memory under these experimental conditions.
Figure 3.
The α8-integrin knockouts exhibited normal working memory in a non-match-to-place T-maze assay. Working memory was expressed as the percentage of correct choices made during the choice run when performed immediately (no delay), 30 seconds or 1 minute after the sample run. All three groups acquired the task and reached criterion of 75% correct at a similar rate (36 trials). The first “No delay” entries represent acquisition performance prior to reaching the criterion of 75% correct. Although the performance of each group was significantly reduced with increasing delay (F[2,39] = 22.95; p < 0.0001), no significant difference was detected between any two genotypes at each delay time (F[2,39] = 0.147; p = 0.86). No significant interactions were found between genotypes and delay.
f(α8)KO mice have impaired synaptic plasticity of the hippocampus
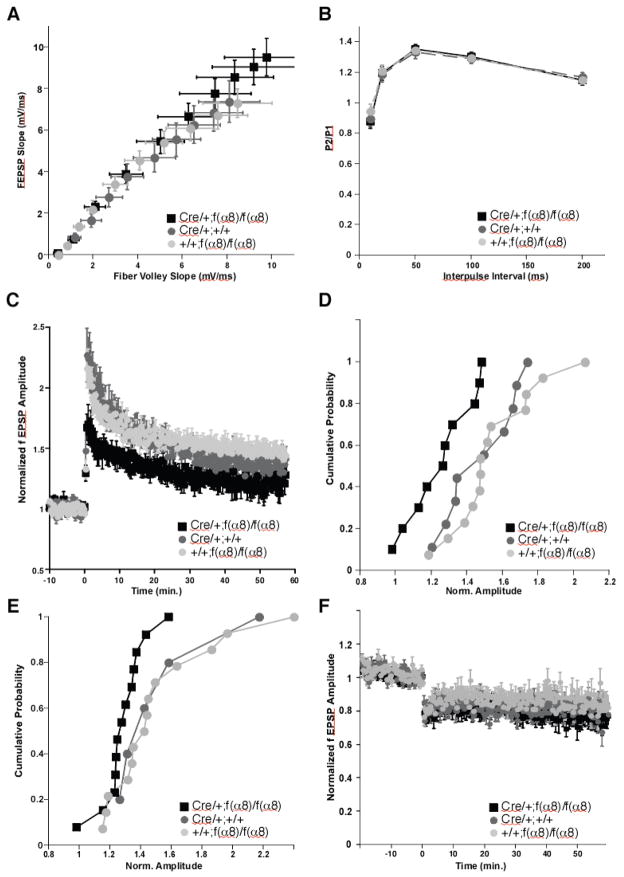
We surveyed the electrophysiological properties of the f(α8)KO animals to evaluate the importance of α8-integrin to synaptic transmission and plasticity. We first examined the basal synaptic transmission in the Schaffer collateral pathway in hippocampal slices. No significant difference was observed in the field excitatory postsynaptic potentials (fEPSPs) as a function of stimulation intensity or fiber volley amplitude between the f(α8)KO animals and their control littermates (Figure 4A). We used approximately 35 % of the maximal response as our baseline stimulus for subsequent experiments related to short-and long-term plasticity.
Figure 4. α8-integrin is required for long-term potentiation of the hippocampus. The effect of α8-integrin KO was determined on Schaffer-collateral-CA1 synapses. Electrophysiological recordings were performed on f(α8)KO [Cre/+; f(α8)/f(α8)] and control [Cre/+; +/+ and +/+; f(α8)/f(α8)] animals.
(A) Input/output plot revealed no significant difference in basal synaptic transmission between the experimental group and the two sibling control groups at all stimulus intensities examined (1–10 V, 1 V intervals). f(α8)KO, n=11; Cre/+, n=9; f(α8)/f(α8), n=14. n.s., 3-way ANOVA.
(B) No significant differences due to genotype were observed in the magnitude of paired-pulse facilitation measured by the ratio of the second to the first stimulus response (P2/P1) as a function of interpulse interval. f(α8)KO, n=11; Cre/+, n=9; f(α8)/f(α8), n=14. n.s., 2-way ANOVA.
(C) Induction of LTP by HFS (two 1-sec stimuli at 100 Hz separated by 20 sec) revealed a significant deficit in the f(α8)KO mice relative to the Cre/+; +/+ and +/+; f(α8)/f(α8) littermates. f(α8)KO, n=11; Cre/+, n=9; f(α8)/f(α8), n=14. f(α8)KO to Cre/+, p <0.05, f(α8)KO to f(α8)/f(α8), p<0.05, Cre/+ vs. f(α8)/f(α8), n.s.
(D) Cumulative Probability plot of the degree of HFS LTP induction from panel C. Average amplitudes were measured 30 ± 1 min after induction and normalized to the response −3 to 0 minutes before induction.
(E) Cumulative Probability plot of the degree of theta-burst induced LTP. Induction protocol was 15 trains of 4 pulses at 100 Hz, intertrain interval 200 ms. f(a8)KO, n=14; Cre/+, n=7; f(a8)/f(a8), n=14. f(a8)KO to Cre/+, p <0.05, f(a8)KO to f(a8)/f(a8), p<0.05, Cre/+ vs. f(a8)/f(a8), n.s.
(F) Induction of LTD in the f(α8)KO mice was not significantly different from the littermate control genotypes, indicating that loss of α8-integrin at Schaffer-collateral-CA1 synapses does not inhibit the induction and/or expression of all forms of synaptic plasticity. f(α8)KO, n=14; Cre/+, n=9; f(α8)/f(α8), n=14. f(α8)KO to Cre/+, n.s.; f(α8)KO to f(α8)/f(α8), n.s.; Cre/+ vs. f(α8)/f(α8), n.s.
We next used paired-pulse facilitation (PPF) to study the effects of α8-integrin on presynaptic release probability and short–term synaptic plasticity. When synapses are stimulated in a paired-pulse fashion within a short time interval, the ratio of the amplitude of the second to the first response is generally inversely proportional to the initial release probability. We found no significant differences in paired-pulse ratio over the test interpulse interval of 10–200 ms (Figure 4B). Together these results suggest that removal of α8-integrin does not alter basal synaptic transmission or the efficiency of neurotransmitter release.
In contrast, the magnitude of NMDA receptor-dependent LTP induced by three trains of high-frequency stimulation (100 Hz) was significantly lower in the f(α8)KO mice compared to the controls (Figure 4C, D). A similar significant but more modest reduction was observed in the experimental animals when theta burst stimulation was used to induce LTP (Figure 4E). Cumulative plots of LTP magnitudes did not reveal any specific changes in the distribution of LTP; both control groups were very well matched, while the f(α8)KO slices showed a globally reduced LTP amplitude. These results suggest that the presence of α8– integrin modulates the degree of long-term potentiation. To evaluate whether the absence of α8– integrin also affects long-term depression, we stimulated Schaffer collateral synapses to form long-term depression using a low frequency stimulation protocol (1 Hz, 900 times). No significant differences were detected between α8-integrin deficient slices and their controls(Figure 4F), indicating that the loss of α8-integrin does not impair all forms of synaptic plasticity.
Discussion
The current study was undertaken to broaden our understanding of the functions of integrins in the mammalian CNS by the continued effort to generate and characterize integrin mutants. Our results reveal that mice deficient in α8–integrin in the forebrain are impaired specifically in the expression of hippocampal LTP, whereas short-term plasticity such as PPF and spatial reference memory and hippocampus-dependent working remain intact. In contrast, our previous studies have shown that both β1- and α3-integrin subunits are required not only for normal hippocampal LTP expression but for hippocampus-dependent working memory as well (Chan et al, 2006; Chan et al., 2007). Since both α3 and α8 are known to have β1 as their only binding partner, these results suggest that the α3β1 heterodimer is required for both LTP and working memory, whereas the α8β1 heterodimer is required only for LTP. It is unclear whether these phenotypic differences observed for the two integrin heterodimers are due to the differential expression pattern of individual α subunits, different cellular functions, or both factors.
However, our results are important in several different ways. First, these studies provide evidence for alternative behavioral roles for integrin isoforms. The fact that working memory deficits are produced by the loss of β1- or α3-integrin but not α8 using identical training and testing conditions indicates that members of the integrin family of protein do not have a generalized function in working memory. Prior to this study, all forms of integrin tested genetically (α3 and β1) were shown to cause working memory deficits and thus a general role for all integrins in working memory was a formal possibility. Second, and in contrast, all isoforms of integrin tested genetically to date do have a role in LTP. Loss of α3-, β1-, or α8-integrin produces an LTP deficit. Although it seems unlikely that future studies will show that all members of the integrin protein family contribute to LTP, the fact that the first three tested have been shown to participate indicates that these proteins are mechanistically involved in the molecular and cellular processes underlying this important cellular model for learning.
Our studies of integrin isoforms in memory formation and LTP prompt two major questions. Where and how? Where in the forebrain is α3- and β1- integrin required for normal working memory, and where in the forebrain is α3-, β1- and α8-integrin required for normal LTP? The latter answer is presumably the hippocampus, although this has not been demonstrated. These “where” questions remain difficult to answer until reproducible and effective methodology is developed to impair gene function in discrete regions of the brain or in specific cell types. The questions of how integrins are mechanistically involved in working memory and LTP are also difficult to address, given the enormous complexity of integrin function and signaling. Nevertheless, the first level approach to this will be to determine whether their participation is via their roles in dynamic cell adhesion processes or through intracellular signaling.
Materials and Methods
Generation of knockout mice
The α8-integrin gene was isolated from a BAC library prepared from the 129S/SvEv strain. The targeting vector contained 12.5 kb of α8-integrin sequence containing the last two exons (exons 29 and 30) subcloned into the Bluescript vector(Figure 1A). The 34 bp loxP sequence was inserted at the unique NdeI site in intron 28–29, and a 4.9 kb loxP-TK-neo-loxP cassette was inserted at the XbaI site in the 3′ flanking sequence. The targeting construct DNA was linearized with PvuI and electroporated into 129S6/SvEvembryonic stem (ES) cells. ES cell clones were selected for neomycin resistance and homologous recombination was screened by Southern blotting using a 0.5 kb HindIII-SacI fragment 3′ of the α8-integrin gene. The selected clones were then electroporated with Cre-expressing plasmid and TK-negative clones were screened by Southern blotting. Two clones were chosen to produce chimeras by microinjection into C57BL/6J blastocysts and implantation into pseudopregnant foster mothers. Chimeric male mice were mated to C57BL/6J females, and offspring that carried the floxed α8-integrin [f(α8)] allele were identified by PCR genotyping of tail DNA using the following primer pairs: a) F1 (see Figure 1A): 5′-TCCTTGTCTTCCTGTGTATTTGAC-3′ and R1: 5′-TGTCTGAGAAGATTCAGCAGTGGG-3′ for the 5′ loxP insertion, and b) F2: 5′-GATCTCCAGGTTATATTTACAAGG-3′ and R2: 5′-CAGTGACCGTTTTCTCCAGTAAGG-3′ for the 3′ loxP insertion in the gene. The f(α3) animals were continuously backcrossed to C57BL/6J mice for more than 6 generations. To generate the forebrain-specific α8-integrin knockout f(α8)KO, the f(α8) animals were crossed to α-CaMKII-CRE mice. The f(α8)KO animals and the control littermates were obtained by crossing Cre/+; f(α8)/+ to +/+; f(α8)/+ animals. The presence of the Cre transgene was confirmed by PCR genotyping of tail DNA utilizing the following primers: 5′-GGCGTTTTCTGAGCATACCTGGAA-3′ and 5′-CACCATTGCCCCTGTTTCACTATC-3′. Mice were housed in conventional animal cages and maintained on a 12 hr light/dark cycle. All animals were handled and treated during the experiments in ways approved by the Baylor College of Medicine Institutional Animal Care and Use Committee and according to national regulations and policies.
Brain slice electrophysiology
Adult mice (8–10 month) were sacrificed by cervical dislocation and used for all experiments except for the LTD experiments, for which 4- to 6-week old animals were used. The brain was immersed in ice-cold cutting saline (CS [in mM]: 220 Sucrose, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 7 Glucose) prior to isolation of the caudal portion containing the hippocampus and entorhinal cortex. Transverse slices (400 μm) were prepared with a Vibratome (Vibratome, St. Louis, MO). During isolation, slices were stored in ice-cold CS. After isolation, cortical tissue was removed and hippocampal slices were equilibrated in a mixture of 50% CS and 50% artificial cerebrospinal fluid (ACSF [in mM]: 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, 10 glucose) at room temperature (RT). Slices were further equilibrated in 100% ACSF for 45 min at RT, followed by a final incubation in 100% ACSF at 31° C for 1 h. All solutions were saturated with 95%/5% O2/CO2.
Electrophysiology was performed in an interface chamber (Fine Science Tools, Foster City, CA). Oxygenated ACSF (95%/5% O2/CO2) was warmed (31° C, TC-324B temperature controller, Warner Instruments, Hamden, CT) and perfused into the recording chamber at a rate of 1 ml/min. Electrophysiological traces were amplified (Model 1800 amplifier, A-M Systems, Sequim, WA), digitized and stored (Digidata models 1200 and 1320A with Clampex software, Molecular Devices, Sunnyvale, CA). Extracellular stimuli were administered (Model 2200 stimulus isolator, A-M Systems) on the border of Area CA3 and CA1 along the Schaffer-collaterals using enameled, bipolar platinum-tungsten (92%:8%) electrodes. fEPSPs were recorded in stratum radiatum with an ACSF-filled glass recording electrode (1–3 MΩ). The relationship between fiber volley and fEPSP slopes over various stimulus intensities (1–10 V) was used to assess baseline synaptic transmission. All subsequent experimental stimuli were set to an intensity that evoked a fEPSP that had a slope of 30–40 % of the maximum fEPSP slope.
Paired-pulse facilitation was measured at various interstimulus intervals (10, 20, 50, 100, 200 ms). Long-term potentiation was induced by administering 2, 100 Hz tetani (1 sec duration, 20 s apart). Theta-burst type stimulation (TBS) consisted of 15 trains, each containing four pulses at 100 Hz, with an inter-train interval of 200 ms. Long-term depression (LTD) was induced using low frequency stimulation (1 Hz, 15 min.). Synaptic efficacy was monitored 20 min prior to and 1 h following induction of LTP or LTD by recording fEPSPs every 20 sec. The experimenter performing the electrophysiology was blind to genotype or treatment. Statistical Analysis. Paired pulse facilitation, long-term potentiation, and long-term depression were analyzed using a two-way ANOVA with repeated measures, with post-hoc comparisons performed using the method of Bonferroni. Significance for all tests was set at p ≤ 0.05. Data represents mean ± S.E.M.
Behavioral assays
Rotarod
The rotarod apparatus (Type 7650; Ugo Basile, Milan, Italy) was used to evaluate coordination and motor learning skill of the animals. Groups of 5 mice (5 to 6-month old) were trained on the accelerating rod (3 cm diameter, 30 cm long; 4 to 40 rpm in 5 min), 3 trials per day with an inter-trial interval of 30 min over a period of 4 days or until they reached plateau performance. Each trial lasted 5 min and the time the mice spent on the rod without falling was recorded.
Open Field
Mice were placed in the center of a 60 × 60 cm open arena made of opaque Plexiglass and allowed to move freely under indirect illumination for two 30 min sessions separated by an inter-session of 30 min in the home cage. The surface of the arena was cleaned with 70% ethanol and air-dried between mice. The activity of the animals in the arena was tracked with a ceiling mounted video camera connected to a digital tracking device (VP200, HVS Image, San Diego, CA). Data were analyzed by the HVS open field software.
Water maze
The pool was 1.3 m in diameter and made of white polypropylene. The hiddened platform (10 cm × 10 cm) was made of Plexiglass and kept 2 cm below the surface of the water. The water was made opaque using non-toxic paint and maintained at room temperature (24 °C). Briefly, mice (5 to 6-month old) were handled extensively for 2 weeks prior to training in the water maze. The animals were kept in individual cages during training and allowed to acclimatize to the water maze room for 1 hr prior to the start of the experiment on each day. Each trial began by placing the mouse into the water facing the wall of the maze and the animal was allowed to search for the platform until the animal climbed onto the platform or when a maximum of 60 shad elapsed. The animal was allowed to remain on the platform for 20 s before it was returned to its cage. Four trials were performed each day with an inter-trial interval (ITI) of 60 min. Trials were balanced for starting positions and began at either the 12, 3, 6, or 9 o’clock position of the pool. The platform location remained constant for any individual mouse for the duration of the training, but different animals were trained with the platform in different positions to avoid quadrant bias. Animals were trained for 5 days at the same time of each day. Twenty-four hours after the last training trial, a probe trial (or transfer test) was administered in which the platform was removed from the pool and animals were placed in a quadrant opposite to the location of the training platform and allowed to swim for 60 s. The time the mice spent searching for the platform in each quadrant and the number of times the mice crossed the virtual platform location was measured. In all trials, the swimming pattern of the animals was tracked with a ceiling mounted video camera connected to a digital tracking device (VP200, HVS Image, San Diego, CA) and data were analyzed by the HVS water maze software.
T-maze non-match-to-place working memory task
The working memory task was performed as described by Reisel et al (2002). The T-maze was made of wood and was elevated to a height of 1 m from the floor. It consisted of a start arm (47 × 10 cm) and two identical goal arms (35 × 10 cm), each covered with the bedding used in the animals’ home cage environment. Animals (5 to 6-month old) were restricted to 1 hr of food access per day, which resulted in animals maintaining 85% of their free-feeding weight. The animals were habituated to the maze by allowing group exploration (with cage-mates) for 5 min, followed by individual exploration for another 5 min with the food reward (50 μl of 50% sweetened, condensed milk in a 2-cm dish) placed 2 cm from the ends of the goal arms.
Each test trial consisted of a sample run followed by a choice run. On the sample run, food reward was placed about 3 cm from the end on both goal arms in depression wells, but access to one arm was blocked by a wooden block. A mouse was placed inside an opaque cylindrical tube and transported from the home cage to the end of the start arm where it was released facing the end wall of the start arm. The animal was allowed to find and drink the food reward in the unblocked goal arm. The animal was then collected into the transfer tube and returned to the end of the start arm, with access into the start arm blocked by a wooden block. The time required for this transfer was less than 5 sec and represented the minimum time lapse between the sample and the choice runs, but was not included in the actual timing of the delay. For the choice run, the wooden block was removed with or without delay and the animal was released to choose either goal arm. The animal was allowed to drink the food reward if it chose the previously unvisited arm. The animal was then returned to the home cage. Four trials with a 20 min ITI were performed on each animal per day for 17 days (only data from the last 16 days were included in Figure 3). The reward arm for each trial was assigned pseudorandomly, i.e. two times for each goal arm each day but with a different order for each animal. Working memory was expressed as the ratio of correct choices over total choices binned for 16 trials.
Statistical Analysis
All the behavioral results were analyzed by two-way ANOVA with repeated measures, with post-hoc comparisons performed using Scheffé’s test. Significance for all tests was set at p ≤ 0.05. Data represent the mean ± S.E.M.
Acknowledgments
This work was supported by grants MH60420 (R.L.D.) from the National Institutes of Mental Health and from the Baylor College of Medicine Mental Retardation Research Center grant HD24064. We thank Hsiao-Tuan Chao for statistical support. R. L. D. is the recipient of the R. P. Doherty-Welch Chair in Science at the Baylor College of Medicine.
References
- Chan CS, Weeber EJ, Kurup S, Sweatt JD, Davis RL. Integrin requirement for hippocampal synaptic plasticity and spatial memory. Journal of Neuroscience. 2003;23:7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Zong L, Fuchs E, Sweatt JD, Davis RL. Beta 1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. Journal of Neuroscience. 2006;26:223–232. doi: 10.1523/JNEUROSCI.4110-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C-S, Levenson JM, Mukhopadhyay PS, Zong L, Bradley A, Sweatt JD, Davis RL. Alpha3-integrins are required for hippocampal long-term potentiation and working memory. Learn Mem. 2007;14(9):606–15. doi: 10.1101/lm.648607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C-S, Davis RL. Integrins and Cadherins – Extracellular Matrix in Memory Formation. In: Byrne John H., editor. Learning and Memory: A Comprehensive Reference. 35. Vol. 4. Elsevier; 2008. pp. 721–740. Chapter 4. [Google Scholar]
- Chun D, Gall CM, Bi X, Lynch G. Evidence that integrins contribute to multiple stages in the consolidation of long term potentiation in rat hippocampus. Neuroscience. 2001;105:815–829. doi: 10.1016/s0306-4522(01)00173-7. [DOI] [PubMed] [Google Scholar]
- Dragatsis I, Zeitlin S. CamKIIalphaCre transgene expression and recombination patterns in the mouse brain. Genesis. 2000;26:133–135. doi: 10.1002/(sici)1526-968x(200002)26:2<133::aid-gene10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Einheber S, Schnapp LM, Salzer JL, Cappiello ZB, Milner TA. Regional and ultrastructural distribution of the α8 integrin subunit in developing and adult rat brain suggests a role in synaptic function. J Comp Neurol. 1996;370:105–134. doi: 10.1002/(SICI)1096-9861(19960617)370:1<105::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev. 1983;7:119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nervous System. 2002;18(8):386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Huang Z, Shimazu K, Woo N, Zang K, Müller U, Lu B, Reichardt L. Distinct Roles of the β1-Class Integrins at the Developing and the Mature Hippocampal Excitatory Synapse. Journal of Neuroscience. 2006;26:11208–11219. doi: 10.1523/JNEUROSCI.3526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi SY, Hirano T. Integrin alpha3beta1 suppresses long-term potentiation at inhibitory synapses on the cerebellar Purkinje neuron. Molecular Cell Neuroscience. 2006;31:416–426. doi: 10.1016/j.mcn.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Bernard JA, Gall CM, Lynch G. Alpha3 integrin receptors contribute to the consolidation of long-term potentiation. Neuroscience. 2002;110:29–39. doi: 10.1016/s0306-4522(01)00540-1. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proceeding of the National Academy of Science U S A. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. Journal of Neuroscience. 1999;19:1541–1556. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouzet B, Feldon J, Veenman CL, Yee BK, Richmond M, Rawlins JNP, Weiner I. The effects of hippocampal and fimbria-fornix lesions on prepulse inhibition. Behavioral Neuroscience. 1999;113:968–981. doi: 10.1037//0735-7044.113.5.968. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Grotewiel MS, Davis RL, Broadie K. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. Journal of Neuroscience. 2000;20:6868–6878. doi: 10.1523/JNEUROSCI.20-18-06868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]