Abstract
Ethnopharmacological relevance
HLXL is a traditional Chinese medicine that has long been used in folk medicine for the treatment of chronic inflammatory diseases. However, the precise immunological mechanisms by which HLXL mediates its anti-inflammatory activity are not fully defined.
Aim of the study
To determine the effects of HLXL on antigen-specific immune parameters in adjuvant-induced inflammation model in the Lewis rat.
Materials and Methods
Rats were fed daily with either HLXL (2.3 g/kg) or vehicle (water) beginning 3 d before subcutaneous injection of heat-killed M. tuberculosis H37Ra (Mtb), and then continued for another 6 d. After 9 d of Mtb injection, the draining lymph node cells were tested for T cell proliferative and cytokine responses against mycobacterial heat-shock protein 65 (Bhsp65). Moreover, sera were tested for anti-Bhsp65 antibodies and nitric oxide (NO).
Results
HLXL-treated rats showed reduced T cell proliferative response to Bhsp65 compared to control rats. Furthermore, HLXL suppressed IL-17 response but enhanced IL-10 response without much effect on IFN-γ. HLXL treatment also reduced the levels of anti-Bhsp65 antibodies but not that of NO.
Conclusions
HLXL feeding modulated both the cellular and the humoral immune response to Bhsp65 favoring an anti-inflammatory milieu for suppression of adjuvant-induced inflammation.
Keywords: Huo-Luo-Xiao-Ling Dan, Immune modulation, Cytokines, Antibodies, T cells, Inflammation
Introduction
Inflammation is an integral component of immune pathology induced following infection or autoimmunity. Uncontrolled inflammation can lead to tissue damage along with metabolic or other functional impairments. Unlike many infections in which antigens targeted by the immune system are defined, the precise antigens that trigger the anti-self response in the major human autoimmune diseases are not fully defined (Ben-Yedidia T. and Arnon R., 2007, Imboden J.B., 2008, Knip M. and Siljander H., 2008, Lin C.L. and Kao J.H., 2008). However, evidence from genetic and immunological studies suggest the involvement of antigen-induced immune response in disorders involving autoimmune inflammation, for example, rheumatoid arthritis (RA) and type 1 diabetes. Accordingly, both general anti-inflammatory agents (Simon and Yocum, 2000) as well as experimental antigen-based approaches (Satpute et al., 2008) are being employed for the management of some of the inflammatory autoimmune diseases. However, despite significant advances in the development and use of conventional anti-inflammatory drugs, the significant adverse effects associated with such drugs remain a major obstacle in designing an optimal and safe therapeutic strategy for such diseases (Stiel, 2000; Weir, 2002; Fitzgerald, 2004). The increasing success and scope of the products of complementary and alternative (CAM) medicine as an adjunct or alternative to conventional drugs (Eisenberg et al., 1998; Barnes et al., 2004) highlight the increasing need to identify new natural products for the treatment of disorders involving inflammation of autoimmune or other etiologies.
Natural herbal products have been used in folk medicine in traditional Chinese medicine (TCM) for centuries. Huo-Luo-Xiao-Ling Dan (HLXL) represents an herbal mixture comprised of 11 components that has been used in TCM for the treatment of chronic inflammatory diseases (Lao et al., 2006). However, the immunological basis of the anti-inflammatory activity of HLXL remains to be determined. In this study, using a rat model of adjuvant-induced inflammation initiated by subcutaneous injection of heat-killed M. tuberculosis H37Ra (Mtb) in the Lewis rat (RT.1l), we have examined the influence of HLXL on defined antigen specific immunological events.
Methods and Materials
Animals
Inbred Lewis (LEW/Hsd) (RT.1l) male (5-6 wk, 180-210 g) rats were purchased from Harlan Sprague Dawley (Indianapolis, IN), and maintained in our animal facility at the University of Maryland School of Medicine, Baltimore. Rats were kept under controlled environmental conditions: temperature 22 ± 0.5 °C, relative humidity 40–60%, alternate light–dark cycles from 7 am to 7 pm, and food and water provided ad libitum. Rats were placed in plastic cages (3-4 rats/cage) with their floor covered with paper pellets to avoid discomfort to animals during walking in the case. The animals were rested for acclimatization before using them for experiments. All experimental procedures performed on these rats were conducted following the guidelines of the institutional animal care and use committee (IACUC) and the research protocol was approved by IACUC, which enforces compliance with the U.S. laboratory animal use and care guidelines.
Antigens
Recombinant mycobacterial heat-shock protein 65 (Bhsp65) was expressed from genetically transformed E. coli as described in detail elsewhere (Durai et al., 2004). The histidine-tagged Bhsp65 was purified using a nickel column, and then rendered endotoxin free by using an Etox column (Durai et al., 2004). Hen eggwhite lysozyme (HEL) and ovalbumin (OVA) were obtained from Sigma-Aldrich (St. Louis, MO).
Composition of HLXL and preparation of the herbal extract
The HLXL formula consists of a defined mixture of 11 herbs as follows (Lao et al., 2006, Zhang et al., 2008): Ruxiang (Boswellia carterii Birdw.), Qianghuo (Notopterygium incisum Ting ex H.T. Chang), Danggui (Angelica sinensis (Oliv.) Diels.), Baishao (Paeonia lactiflora Pall.), Gancao (Glycyrrhiza uralensis Fisch.) Yanhusuo (Corydalis yanhusuo W.T. Wang.) Danshen (Salvia miltiorrhiza Bge.) Chuanxiong (Ligusticum chuanxiong S.H. Qiu.) Qinjiao (Gentiana macrophylla Pall.) Guizhi (Cinnamomum cassia Presl.) and Duhuo (Angelica pubescens Maxim.). Unprocessed component herbs of HLXL were acquired through the Institute of Medicinal Plant Development (IMPLAD), Chinese Academy of Medical Sciences, Beijing, China, and the identities authenticated by Prof. Chen Shi-lin (IMPLAD). The herbs were processed and extracted with aqueous acetone as previously described (Lao et al., 2006, Zhang et al., 2008) at Phytoway Inc., Changsha, China, under GMP conditions. Each extract was monitored for the absence of contaminants (heavy metals, pesticides, and mycotoxins) prior to formulation. The detailed composition of HLXL, the Chinese, botanical and family names of each component plant, the pharmacopoeia used for reference, the clinical dosages of each component plant, the description of HPLC peaks of HLXL mixture, the weight and yield of the product, and the information about voucher samples and pharmacopoeia are given in our previously published reports (Lao et al., 2006, Zhang et al., 2008). The formulated HLXL was subjected to HPLC finger printing analysis in which major peaks were identified as the marker compounds to their originating individual herbs (i.e. swertiamarin, paeoniflorin, liquiritin, liquiritigenin, bergapten, senkyunolide A, phenethyl trans-ferulate, isoimperotorin, falcarindiol, crytotanshinone, tanshinone IIA, ostruthin, and anhydronotoptol) (unpublished data).
Adjuvant-induced inflammation model in the Lewis rat
Lewis rats were immunized subcutaneously (s.c.) with heat-killed M. tuberculosis H37Ra (Mtb) (Difco Laboratories) at the base of the tail. Mtb was first ground to a fine powder in a mortar and pestle and then suspended in mineral oil (Sigma-Aldrich) to the desired concentration (10 mg/ml). Each rat received 100 ul of this adjuvant suspension.
Protocol for HLXL feeding to Lewis rats
Lewis rats were fed HLXL (2.3 g/kg) in water by gavage daily, starting 3 d before injection of Mtb as described above, and then continued for another 6 days for a total of 9 d. This HLXL-feeding protocol was designed to assess the effect of HLXL on suppressing the development of inflammation induced by Mtb. Rats were fed for 3 d so as to ‘condition’ the physiological systems of the rat to the herbal preparation before giving them an inflammation-triggering stimulus (Mtb injection). Thereafter, rats were fed for 6 more days so as to provide herbal treatment during the initial period of the development phase of the inflammatory response. In pilot experiments, 6 d-treatment post-Mtb injection had similar results on the T cell response as 10 d-treatment, so we selected 6 d for our study. The control rats were given water (vehicle) on the days corresponding to HLXL feeding.
Collection of test specimen from HLXL-treated rats
1) Lymph node cells (LNC)
Groups of Lewis rats were fed either HLXL (test group) or vehicle (control group) and immunized with Mtb as described above. After 9 d of Mtb challenge, the rats were sacrificed and their draining lymph nodes were harvested. The LNC thus obtained were tested in a proliferation assay (Durai et al., 2004). In addition, total RNA extracted from LNC was tested for cytokine message by quantitative real-time PCR (Kim et al., 2008).
2) Blood specimen
Blood samples were collected from HLXL-fed and control rats at different time points before or after Mtb injection. The serum was separated and then tested in an ELISA for Bhsp65-specific antibodies (Mia et al., 2005) or in a colorimetric assay for nitric oxide (NO) products, nitrites/nitrates.
Lymph node cell (LNC) proliferation assay
The LNC (5×105/well) were tested in a proliferation assay using the appropriate recall antigens as described elsewhere (Durai et al., 2004). Purified protein derivative (PPD) (Mycos Research, Fort Collins, CO) or concanavalin A (Con A) (Sigma) was used as a positive control. The incorporation of radioactivity (3[H]-thymidine) was assayed by liquid scintillation counting. The results were expressed either as cpm or as a stimulation index (S.I. = cpm with recall antigen/cpm with cells in medium alone).
Measurement of the cytokine levels by quantitative real-time PCR
The LNC of antigen-primed rats were restimulated with antigen in vitro for 48 h. Thereafter, total RNA was extracted from the LNC and then tested by real-time PCR using specific primers for the cytokines IFN-γ, IL-17 and IL-10 (Kim et al., 2008). The results were expressed as fold increase over medium after normalizing the results with hypoxanthine-guanine phosphoribosyl transferase (HPRT). The Th1/Th2 ratio was derived from the levels of IFN-γ/IL-10, respectively.
Determination of serum levels of antigen-specific antibodies and nitric oxide (NO)
Total immunoglobulins (total Ig) and Ig isotypes IgG1 and IgG2a in sera were detected by ELISA using Bhsp65 (test antigen) and Ova (control antigen) following the method described elsewhere (Mia et al., 2005). The results were expressed as O.D. 450 nm. In another set of experiments, serum nitrites/nitrates that are products of NO were measured by using a colorimetric assay (Biovision Research Products, Mountain view, CA). The concentration of the test products was derived from the standard curve.
Statistical analysis
Differences between the ‘mean’ values of two groups under consideration were compared by using the Student t test. Differences with p value <0.05 were considered statistically significant.
Results
HLXL inhibited antigen-specific T cell proliferative response
HLXL-fed rats gave a reduced T cell proliferative response to Bhsp65 compared to that of the control rats, and the difference between the two groups was statistically significant (p<0.001) (Figure 1). The specificity of the T cells response to Bhsp65 was evident by the relative lack of response to the control antigen, Ova.
Figure 1. The T cell proliferative response of the draining LNC of HLXL-fed versus control Lewis rats immunized with Mtb.
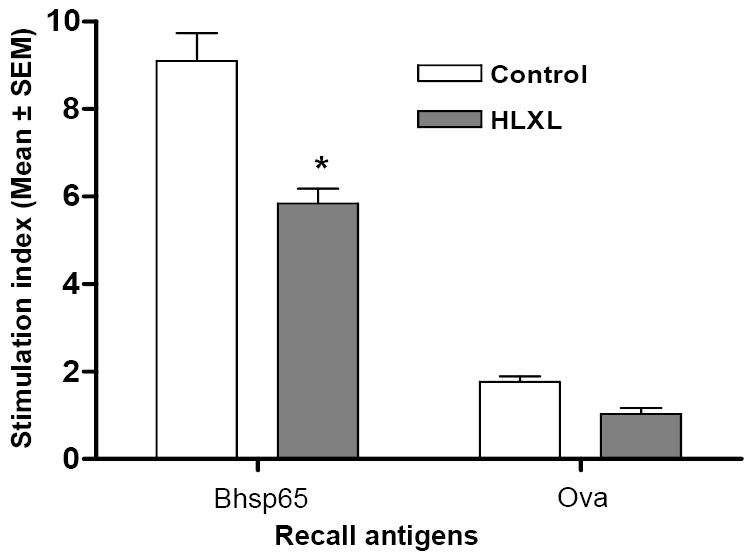
Lewis rats (n= 7 per group) that were fed either HLXL or vehicle (water) were sacrificed after 9 d of Mtb injection. The draining LNC of these rats were then harvested and tested in a proliferation assay using Bhsp65 as the test antigen and Ova as the control antigen. The results are expressed as a stimulation index (S.I.) (*, p< 0.001).
HLXL modulated the balance of pro- and anti-inflammatory cytokine levels
HLXL-fed rats showed reduced levels of the pro-inflammatory cytokine IL-17, but enhanced levels of the anti-inflammatory cytokine IL-10 (Figure 2). The difference for each of these two cytokines in HLXL-fed versus control rats was statistically significant (p< 0.001 for IL-17 and p<0.01 for IL-10). However, there was no significant change in the level of IFN-γ following HLXL feeding. Nevertheless, HLXL treatment induced deviation of the overall balance (ratio) between pro-inflammatory and anti-inflammatory cytokines (IFN-γ/IL10 ratio; Th1/Th2 ratio, respectively) in favor of the anti-inflammatory response.
Figure 2. Cytokines produced by LNC of HLXL-/vehicle-treated Mtb-immunized Lewis rats.
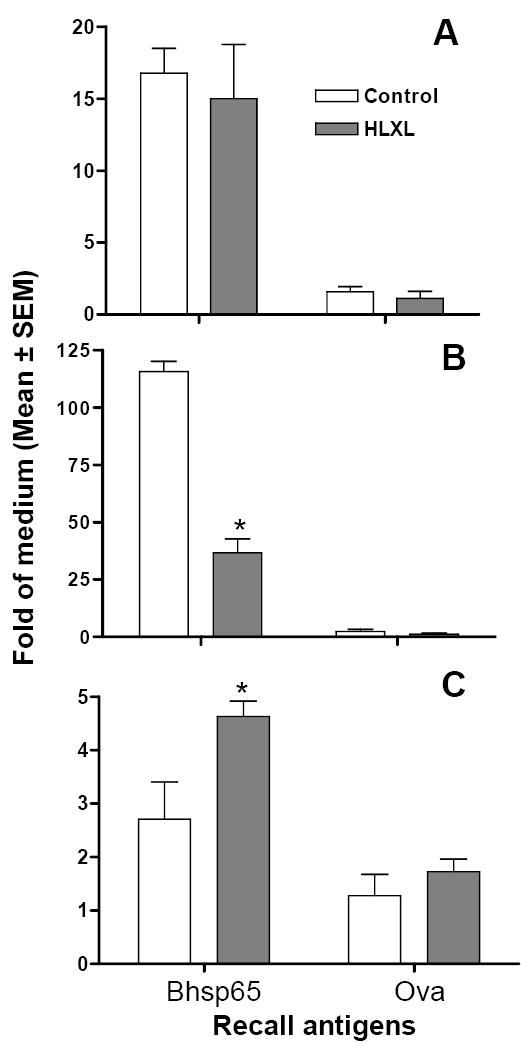
Total RNA extracted from the draining LNC of Lewis rats (n= 5-7 per group) injected with Mtb and fed either HLXL or water was tested by quantitative real-time PCR for mRNA for IFN-γ (A), IL-17 (B), and IL-10 (C) produced in response to the test antigen (Bhsp65) or the control antigen (Ova). The results are expressed as ‘Fold Increase’ over cells in medium. (*, p< 0.01- 0.001)
HLXL suppressed the production of antigen-specific antibodies
Sera of HLXL-fed rats had lower levels of anti-Bhsp65 antibodies compared to that of the control rats (Figure 3). Furthermore, most of this antibody reactivity was comprised of the IgG2a isotype. Beginning d 9 onwards, the difference in the antibody titers for total Ig as well as IgG2a of HLXL-fed rats was significantly (p< 0.001) lower than that of the control rats, indicating the HLXL-induced suppression of the humoral immune response to Bhsp65.
Figure 3. The influence of HLXL feeding on antibody response to Bhsp65 in Mtb-immunized Lewis rats.
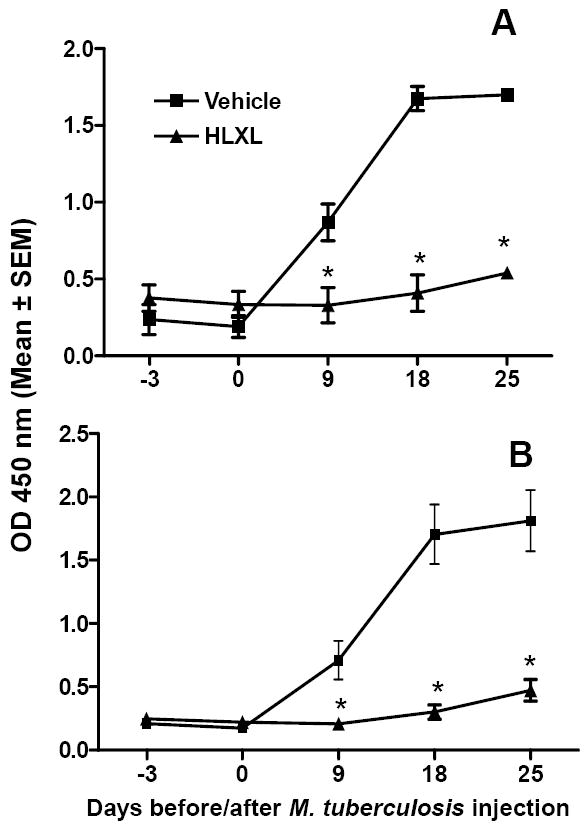
Sera of test (HLXL-treated) and control (vehicle-treated) Lewis rats (n= 4 per group) that were injected with Mtb were tested for anti-Bhsp65 antibodies by ELISA. The results are expressed as O.D. 450 nm. The results of total Ig (A) and IgG2a (B) in sera collected over the entire course of observation are shown (*, p<0.001 between groups)
Serum nitric oxide levels remained unchanged following HLXL treatment
We tested whether HLXL had any effect on a well-known biochemical mediator of inflammation, nitric oxide (NO). Sera of HLXL-fed and control rats revealed comparable levels of nitrites/nitrates that are generated from NO (Figure 4).
Figure 4. The levels of serum nitrites/nitrates in HLXL-fed and control rats.
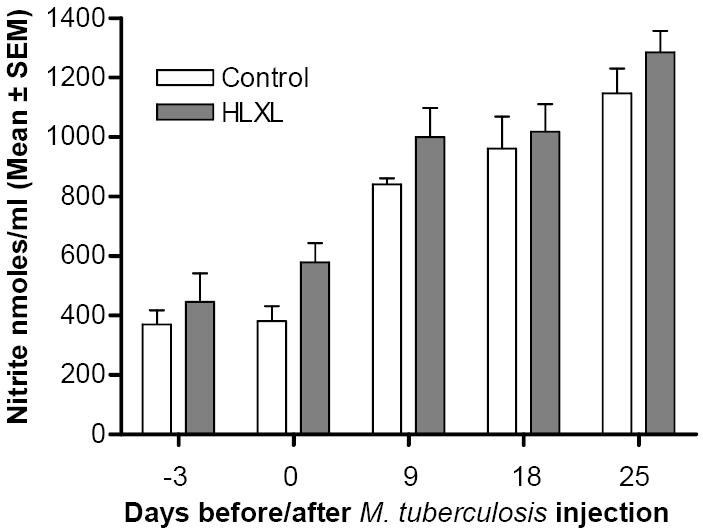
Sera collected at different time points before and after Mtb injection of HLXL-treated and control rats (n= 3 per group) were tested in a colorimetric assay for the nitric oxide (NO)-derived product, nitrite/nitrate. The difference between the two groups at each of the different time points tested was not significant (p> 0.05).
Taken together, our results (Figures 1-4) show that the anti-inflammatory response of HLXL involves modulation of both the cell-mediated and the humoral arms of the immune response, but without a detectable change in serum NO.
Discussion
Anti-inflammatory drugs are the mainstay of the therapeutic arsenal against chronic inflammatory diseases such as RA. However, despite their efficacy, the considerations of severe adverse effects following long-term use of such drugs (Stiel, 2000; Weir, 2002; Fitzgerald, 2004) warrant the search for new and safe anti-inflammatory agents. Natural plant products represent a major group of promising therapeutic agents and TCM has offered a large variety of such herbal products that belong to the realm of CAM (Eisenberg et al., 1998; Barnes et al., 2004). In this study, we have unraveled the immunological changes induced by a Chinese herbal mixture (HLXL) that possesses anti-inflammatory attributes (Lao et al., 2006). Our results of testing HLXL in an experimental model of inflammation show that this herbal mixture suppresses antigen (Bhsp65)-specific T cell proliferative response, induces a shift in the cytokine balance favoring an anti-inflammatory activity, and inhibits the production of antigen-reactive antibodies that otherwise might contribute to inflammation. However, no significant effect of the herbal mixture was observed on serum NO, one of the biochemical mediators of inflammation.
Immunization of Lewis rats with Mtb not only induces inflammation, but also primes and expands T cells directed against mycobacterial antigens (Durai et al., 2004). One dominant antigen in this regard is Bhsp65 (Durai et al., 2004). Activation of T cells following Mtb injection involves the processing and presentation of mycobacterial antigens to specific T cells and subsequent clonal proliferation of the activated T cells.
Our results showing that HLXL induces a significant reduction of the T cell proliferative response to Bhsp65 demonstrate that this herbal mixture influences cell-mediated immunity effected via the T cells. However, the mechanism by which HLXL might suppress T cell proliferation remains to be examined.
The influence of HLXL on the T cell response is further validated by the results showing that HLXL modulates the cytokine response to Bhsp65 in Mtb-immunized rats. The cytokines tested in our study belong to two main categories - pro-inflammatory cytokines (IFN-γ and IL-17) and anti-inflammatory cytokine (IL-10) (Romagnani, 2006; Chen and O’Shea, 2008). The type of cytokine response induced is dependent on the nature of the antigen besides other critical factors (Tao et al., 1997; Rogers and Croft, 1999; Satpute et al., 2008). We observed that the control rats raised a predominantly pro-inflammatory cytokine response following Mtb injection, whereas HLXL-treated rats showed significantly reduced IL-17 response. In addition, the enhanced IL-10 response in HLXL-fed rats resulted in shifting of the overall cytokine milieu of the antigen-draining lymphoid tissues toward an anti-inflammatory type. Such a shift or deviation of the cytokine response (immune deviation) is a well-documented phenomenon that provides the rationale for the beneficial effects of immunotherapeutic approaches against RA, diabetes mellitus, multiple sclerosis and some other autoimmune disorders (Tao et al., 1997; Harber et al., 2000; Romagnani, 2006; Satpute et al., 2008). One of the most studied therapeutic approaches in this regard is the induction of immune tolerance by the administration (i.v., i.p., or mucosally) of soluble antigen without an adjuvant (Tao et al., 1997; Harber et al., 2000; Romagnani, 2006; Satpute et al., 2008), which leads to the deviation of Th1 type (IFN-γ) to Th2 type (IL-4, IL-10) of response (Tao et al., 1997; Rogers and Croft, 1999; Harber et al., 2000; Romagnani, 2006; Satpute et al., 2008). This deviation can result from a decrease of IFN-γ, an increase of IL-10, or both. Recently, IL-17-producing Th17 cells have been invoked in the pathogenesis of autoimmunity (Romagnani, 2006; Bettelli et al., 2007; Chen and O’Shea, 2008; Kim et al., 2008; Satpute et al., 2008). The Th17 subset of cells is distinct from the Th1 cells. In our study, we observed suppression of IL-17 coupled with an altered ratio of IFN-γ/IL-10. Thus, HLXL not only suppressed the proliferation of Bhsp65-reactive T cells, but it also led to an alteration of the cytokine balance towards a predominantly anti-inflammatory type. These results are further supported by those of our previous study (Fan et al., 2005) showing that an acetone extract of Boswellia carterii Birdw. gum resin, which is the major ingredient of HLXL, has anti-inflammatory activity that involves a significant reduction of the local tissue concentrations of the pro-inflammatory cytokines TNF-α and IL-1β. In our other study, a mixture of boswellic acids from B. carterii was shown to alter cytokine production in vitro by splenic cells such that Th1 cytokine production was reduced, but Th2 cytokine response was enhanced leading to immune deviation (Chevrier et al., 2005).
Generally, both the cell-mediated and the humoral arm of the immune system contribute to an effective immune response to an antigen. In this regard, our results showed that the control Lewis rats immunized with Mtb raised not only a potent T cell response to Bhsp65, but also developed an antibody response to that antigen. The IgG2a type of antibody subset represented the dominant antibody isotype as IgG1 subset was barely detectable. Antibodies can contribute to inflammation by forming antigen-antibody complexes and subsequent activation of the complement pathway, whose components can mediate an inflammatory response (Gao et al., 2006; McMahon and Hahn, 2007; Lutz and Fumia, 2008). The inhibition of antibody production in turn may circumvent the propagation of an inflammatory response in Mtb-injected rats. Thus, HLXL was capable of modulating both the T cell response to Bhsp65 and the antibody response to that antigen.
We extended our analysis of HLXL effects beyond immune parameters by including the testing for NO, a biochemical mediator of inflammation (Jang and Murrell, 1998; Bogdan, 2001). Our results showed that HLXL-treatment did not have a measurable effect on serum NO levels compared to control rats. It is likely that HLXL might modulate the local concentration of NO and some other biochemical mediators that were not tested in our study, but apparently this effect was not reflected in the circulating levels of NO. Alternatively, some components of HLXL might increase NO levels, whereas other components might reduce them, rendering the total serum levels of NO unchanged.
The precise mechanisms by which HLXL modulates the cytokine and antibody response to Bhsp65 remain to be defined, as are the immune responses to Bhsp65 when HLXL is administered during established inflammation. The above-mentioned HLXL-modified immune responses to Bhsp65 in Mtb-induced inflammation in Lewis rats might also become significant in the course of subsequent disease process that might ensue if the inflammation is uncontrolled. For example, Lewis rats also are susceptible to the induction of autoimmune arthritis following adjuvant (Mtb) challenge, and immune responses to Bhsp65 are relevant to the pathogenesis of arthritis as well (Durai et al., 2004). However, at present it is not clear as to how much of the observed immune changes to Bhsp65 are applicable to the anti-arthritic activity of HLXL. These aspects of HLXL activity are currently being pursued in our laboratory.
Acknowledgments
This study was supported by NIH/NCCAM grant PO1 AT002605-01A1 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health. Its contents are solely the responsibilities of the authors and do not necessarily represent the official views of NCCAM. We thank Prof. Chen Shi-lin, (IMPLAD, Chinese Academy of Medical Sciences, Beijing, China) for assistance in the acquisition and identities authentication of herbal source materials, and Yinghua Yang, Ruixing Zhang, Elizabeth Pradhan and Margaret Chesney (University of Maryland) for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004:1–19. [PubMed] [Google Scholar]
- Ben-Yedidia T, Arnon R. Epitope-based vaccine against influenza. Expert Rev Vaccines. 2007;6:939–48. doi: 10.1586/14760584.6.6.939. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Chen Z, O’Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008;41:87–102. doi: 10.1007/s12026-007-8014-9. [DOI] [PubMed] [Google Scholar]
- Chevrier MR, Ryan AE, Lee DY, Zhongze M, Wu-Yan Z, Via CS. Boswellia carterii extract inhibits TH1 cytokines and promotes TH2 cytokines in vitro. Clin Diagn Lab Immunol. 2005;12:575–580. doi: 10.1128/CDLI.12.5.575-580.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durai M, Kim HR, Moudgil KD. The regulatory C-terminal determinants within mycobacterial heat shock protein 65 are cryptic and cross-reactive with the dominant self homologs: implications for the pathogenesis of autoimmune arthritis. J Immunol. 2004;173:181–188. doi: 10.4049/jimmunol.173.1.181. [DOI] [PubMed] [Google Scholar]
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. Jama. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- Fan AY, Lao L, Zhang RX, Wang LB, Lee DY, Ma ZZ, Zhang WY, Berman B. Effects of an acetone extract of Boswellia carterii Birdw. (Burseraceae) gum resin on rats with persistent inflammation. J Altern Complement Med. 2005;11:323–331. doi: 10.1089/acm.2005.11.323. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- Gao H, Neff T, Ward PA. Regulation of lung inflammation in the model of IgG immune-complex injury. Annu Rev Pathol. 2006;1:215–242. doi: 10.1146/annurev.pathol.1.110304.100155. [DOI] [PubMed] [Google Scholar]
- Harber M, Sundstedt A, Wraith D. The role of cytokines in immunological tolerance: potential for therapy. Expert Rev Mol Med. 2000;2:1–20. doi: 10.1017/S1462399400002143. [DOI] [PubMed] [Google Scholar]
- Imboden JB. The immunopathogenesis of rheumatoid arthritis. Annu Rev Pathol. 2008 Oct 27; doi: 10.1146/annurev.pathol.4.110807.092254. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Jang D, Murrell GA. Nitric oxide in arthritis. Free Radic Biol Med. 1998;24:1511–1519. doi: 10.1016/s0891-5849(97)00459-0. [DOI] [PubMed] [Google Scholar]
- Kim EY, Chi HH, Bouziane M, Gaur A, Moudgil KD. Regulation of autoimmune arthritis by the pro-inflammatory cytokine interferon-gamma. Clin Immunol. 2008;127:98–106. doi: 10.1016/j.clim.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmun Rev. 2008;7:550–7. doi: 10.1016/j.autrev.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Lao L, Fan AY, Zhang RX, Zhou A, Ma ZZ, Lee DY, Ren K, Berman B. Anti-hyperalgesic and Anti-inflammatory Effects of the Modified Chinese Herbal Formula Huo Luo Xiao Ling Dan (HLXL) in Rats. Am J Chin Med. 2006;34:833–844. doi: 10.1142/S0192415X06004326. [DOI] [PubMed] [Google Scholar]
- Lin CL, Kao JH. Hepatitis B viral factors and clinical outcomes of chronic hepatitis B. J Biomed Sci. 2008;15:137–45. doi: 10.1007/s11373-007-9225-8. [DOI] [PubMed] [Google Scholar]
- Lutz HU, Fumia S. Stimulation of complement amplification by F(ab’)(2)-containing immune complexes and naturally occurring anti-hinge antibodies, possible role in systemic inflammation. Autoimmun Rev. 2008;7:508–513. doi: 10.1016/j.autrev.2008.04.017. [DOI] [PubMed] [Google Scholar]
- McMahon M, Hahn BH. Atherosclerosis and systemic lupus erythematosus: mechanistic basis of the association. Curr Opin Immunol. 2007;19:633–639. doi: 10.1016/j.coi.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mia MY, Durai M, Kim HR, Moudgil KD. Heat shock protein 65-reactive T cells are involved in the pathogenesis of non-antigenic dimethyl dioctadecyl ammonium bromide-induced arthritis. J Immunol. 2005;175:219–227. doi: 10.4049/jimmunol.175.1.219. [DOI] [PubMed] [Google Scholar]
- Rogers PR, Croft M. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J Immunol. 1999;163:1205–1213. [PubMed] [Google Scholar]
- Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- Satpute SR, Durai M, Moudgil KD. Antigen-Specific Tolerogenic and Immunomodulatory Strategies for the Treatment of Autoimmune Arthritis. Semin Arthritis Rheum. 2008 Jan 2; doi: 10.1016/j.semarthrit.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon LS, Yocum D. New and future drug therapies for rheumatoid arthritis. Rheumatology (Oxford) 2000;39(Suppl 1):36–42. doi: 10.1093/oxfordjournals.rheumatology.a031493. [DOI] [PubMed] [Google Scholar]
- Stiel D. Exploring the link between gastrointestinal complications and over-the-counter analgesics: current issues and considerations. Am J Ther. 2000;7:91–98. doi: 10.1097/00045391-200007020-00006. [DOI] [PubMed] [Google Scholar]
- Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol. 1997;159:5956–5963. [PubMed] [Google Scholar]
- Weir MR. Renal effects of nonselective NSAIDs and coxibs. Cleve Clin J Med. 2002;69(Suppl 1):SI53–58. doi: 10.3949/ccjm.69.suppl_1.si53. [DOI] [PubMed] [Google Scholar]
- Zhang R-X, Fan AY, Zhou A-N, Moudgil KD, Ma Z-Z, Lee DY-W, Fong HH-S, Berman BM, Lao L. Extract of Chinese herbal formula Huo-Luo-Xiao-Ling Dan inhibited adjuvant arthritis in rats. J Ethnopharmacol. 2008;121:366–371. doi: 10.1016/j.jep.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


