Abstract
Crosstalk between intracellular signalling systems is recognized as the principal means by which a cell orchestrates coordinate responses to stimulation by neurotransmitters, hormones or growth factors. The functional consequences of crosstalk are evident at multiple levels within a given signalling cascade, including the regulation of receptor–ligand interactions, guanine nucleotide-binding proteins, enzyme activities, ion channel function and gene expression. Here we focus on the pancreatic β-cells of the islets of Langerhans to illustrate the important role crosstalk plays in the regulation of glucose-induced insulin secretion. Recent studies indicating a synergistic interaction in β-cells between the glucose-regulated ATP-dependent signalling system and the hormonally regulated cAMP-dependent signalling system are emphasized. This interaction gives β-cells the ability to match the ambient concentration of glucose to an appropriate insulin secretory response, a process we refer to as the induction of glucose competence. The glucose competence concept may provide new insights into the etiology and treatment of non-insulin-dependent diabetes mellitus (Type II diabetes).
The Sensitivity of a Cell to externally applied transmitters is determined not only by the functional expression of receptors, G proteins, enzymes and protein kinases, but also by the level of metabolic activity that the cell exhibits. Conversely, the level of metabolic activity is regulated not simply by the availability of substrates, enzymes or cofactors, but also by the influence of second messenger-mediated signalling systems that originate at the plasma membrane. An example of this type of bidirectional crosstalk is the hormonal regulation of glucose-induced insulin secretion from pancreatic β-cells of the islets of Langerhans. The β-cells are highly specialized endocrine cells in which a coordinate response (insulin secretion) results from a complex interplay between intermediary metabolism and second messenger-mediated signal transduction1,2.
Pathways that mediate glucose-induced insulin secretion from β-cells
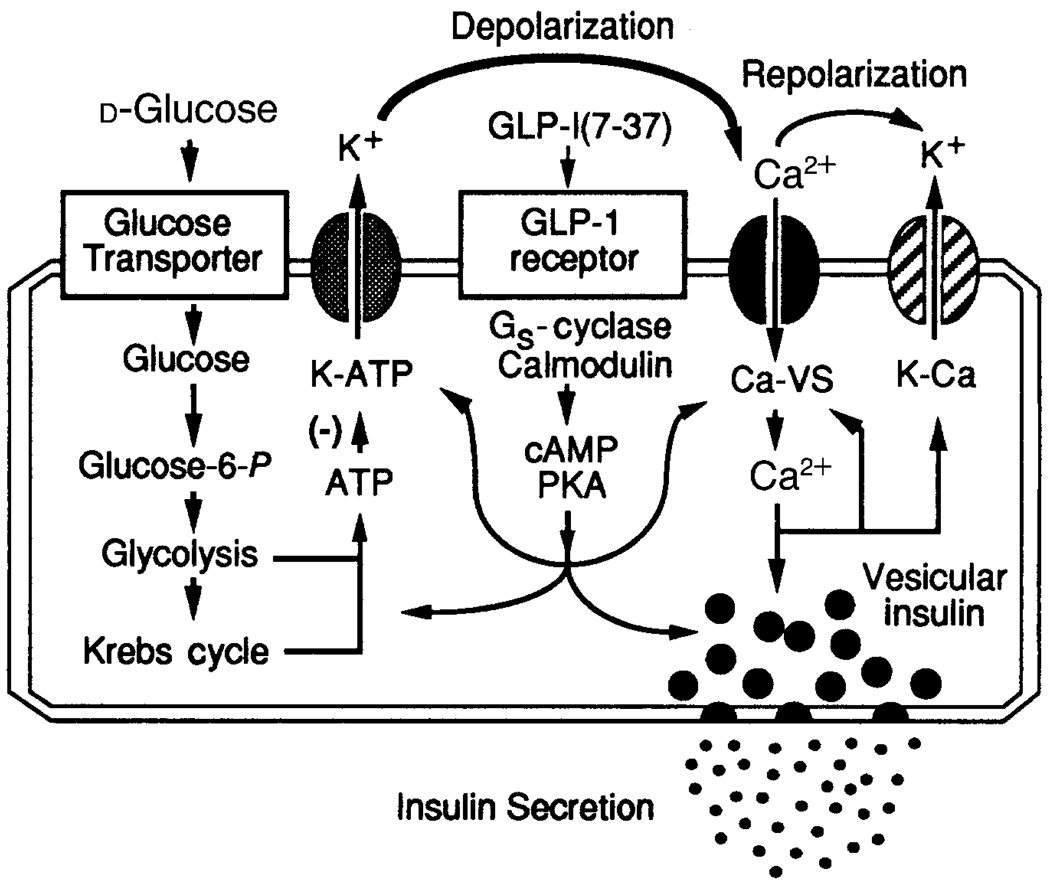
To appreciate fully the role that bidirectional crosstalk plays in the hormonal regulation of glucose-induced insulin secretion, it is necessary to realize the intricate nature of the β-cell glucose-signalling system. As summarized in Fig. 1, stimulatory concentrations of d-glucose (≥ 7.5 mm) trigger insulin secretion, a process that requires the uptake of glucose, its metabolic conversion by aerobic glycolysis, and the generation of signals (yet to be fully characterized) necessary for the mobilization and exocytosis of insulin-containing secretory vesicles. The initial uptake of glucose and its conversion to glucose 6-phosphate constitute what is referred to as the glucose-sensing mechanism3. Glucose uptake is mediated by a facilitative glucose transporter that probably corresponds to the type-2 transporter isoform4,5. Glucokinase6,7, a hexokinase that is rate-limiting for β-cell glucose-sensing, converts glucose to glucose 6-phosphate, which must then be processed by glycolysis (Emden–Myerhof pathway) and mitochondrial metabolism (Krebs cycle) in order for the full insulin secretory response to be observed. Although the nature of the signal that mediates the action of glucose remains controversial, evidence suggests that alterations in the intracellular phosphate potential (an increased ratio of ATP relative to ADP), cytosolic redox state [conversion of NAD(P) to NAD(P)H], increased pH, stimulation of malonyl coenzyme-A synthesis, or generation of phospholipid metabolites can induce insulin secretion, and in fact, all of these changes may normally be required8.
Figure 1.
Hormonal regulation of glucose-induced insulin secretion from pancreatic β-cells. On the left are illustrated essential features of the glucose-regulated ATP-dependent signalling system. The initial uptake of glucose is facilitated by the type-2 glucose transporter, whereas the conversion of intracellular glucose to glucose 6-phosphate is catalysed by glucokinase. Stimulation of aerobic glycolysis generates multiple signals, one of which is an increased ratio of intracellular ATP relative to ADP. Binding of ATP to ATP-sensitive potassium channels (K-ATP) induces closure of the channels through a (yet to be determined) mechanism that does not require hydrolysis of the nucleotide. Closure of ATP-sensitive potassium channels results in membrane depolarization which is necessary for the opening of voltage-sensitive Ca2+ channels (Ca-VS). The opening of Ca2+ channels may also be favoured by glucose-derived signalling molecules of undetermined origin. Entry of Ca2+ across the plasma membrane triggers vesicular insulin secretion by Ca2+-dependent exocytosis. Repolarization of the membrane results from the action of intracellular Ca2+ to activate Ca2+-dependent potassium channels (K-Ca) and to inhibit voltage-sensitive Ca2+ channels. Each of these steps in the glucose-regulated ATP-dependent signalling system is viewed as a potential target for modulation by the hormonally regulated cAMP-dependent signalling system. In this example, GLP-1 binds to cell-surface receptors and activates Gs, a heterotrimeric G protein that stimulates adenylate cyclase. Stimulation of adenylate cyclase by GLP-1 is proposed to require the activated form of calmodulin. Since calmodulin is activated by the glucose-induced rise in intracellular Ca2+, the stimulation of adenylate cyclase by GLP-1 is glucose-dependent. The production of cAMP by adenylate cyclase results in the activation of protein kinase A (PKA) which catalyses the phosphorylation of multiple targets within the glucose-signalling cascade. These targets may include elements of the glucose-sensing mechanism, ion channels, gap junctions and components of the secretory apparatus that are responsible for mobilization and exocytosis of insulin-containing vesicles.
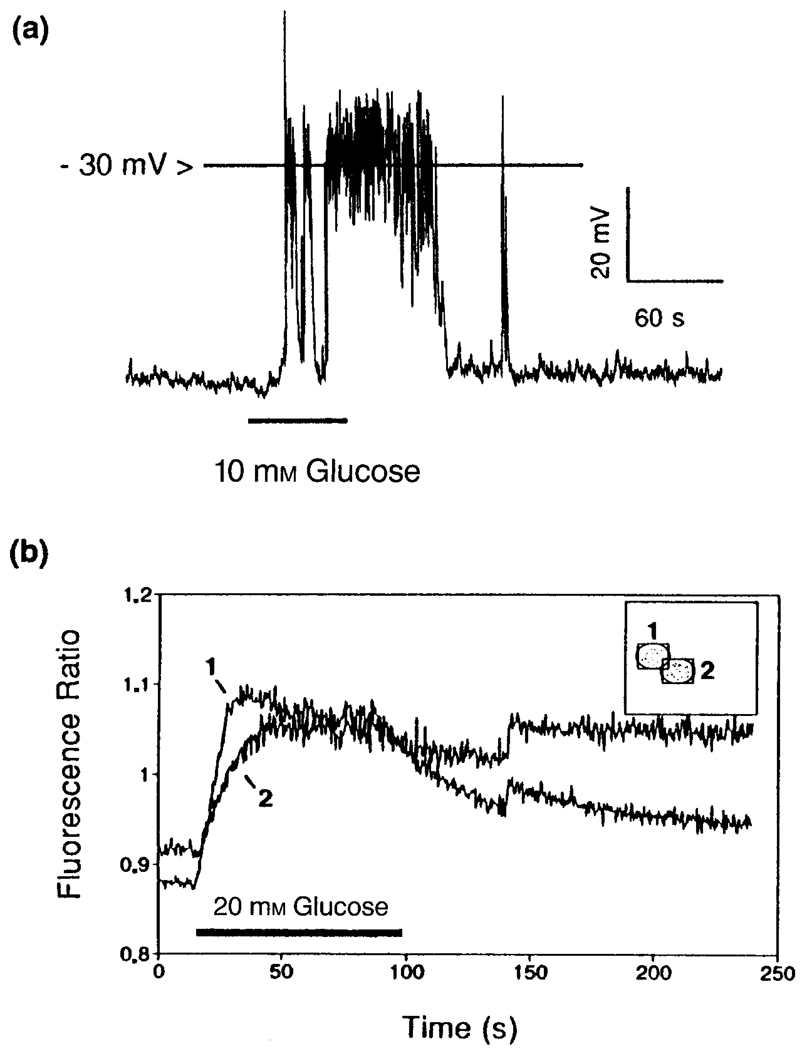
What is certain is that glucose-induced insulin secretion results from vesicular exocytosis, a process that is triggered by the entry of Ca2+ across the plasma membrane1,9. Since Ca2+ is known to enter through voltage-sensitive calcium channels, this requirement implies that glucose generates a signal capable of depolarizing the β-cell membrane potential from an initial resting value of approximately −70 mV to a value (approximately −30 mV) at which the relevant Ca2+ channels begin to open. Electrophysiological studies of β-cells provide the clearest support for the role of ATP as a mediator of the depolarizing action of glucose, and it is now recognized that this adenine nucleotide inhibits the activity of metabolically regulated potassium channels (the ATP-sensitive potassium channels) that play a dominant role in maintaining the β-cell resting membrane potential10. ATP is therefore considered a prototypic glucose-derived signalling molecule that is capable of triggering insulin secretion in an indirect manner. These features of the β-cell glucose-signalling system are summarized in Fig. 2, which illustrates the ability of glucose to depolarize β-cells and to increase the concentration of intracellular free ionized calcium.
Figure 2.
Glucose-induced insulin secretion from β-cells results from depolarization-induced increases in the concentration of intracellular Ca2+. The recordings illustrated were obtained from clusters of pancreatic β-cells using either the perforated patch configuration of the patch–clamp technique30 (a), or fluorescence ratio imaging with the membrane-permeant Ca2+ indicator dye FURA-2 AM9. As illustrated in a, recordings of the resting membrane potential revealed that 10 mm glucose shifted the membrane potential in the depolarizing direction and generated action potentials (spike-like phenomena) that resulted from Ca2+ influx through voltage-sensitive calcium channels. In a separate experiment the depolarizing action of glucose was accompanied by an increased concentration of intracellular Ca2+, as indicated by the increased fluorescence ratio (b). Note that in this example the rise in intracellular Ca2+ was detected simultaneously in two adjacent cells (labelled 1 and 2), thereby indicating that the cells were electrically and metabolically coupled by gap junctions.
A noteworthy feature of glucose-induced insulin secretion is that it occurs in a pulsatile and oscillatory manner. Furthermore, the kinetics of insulin secretion are biphasic such that the response to glucose exhibits a fast transient first phase followed by a slowly developing and sustained second phase11. The oscillatory manner in which glucose induces insulin secretion is thought to reflect slow fluctuations in the resting membrane potential whereby intermittent periods of sustained depolarization trigger Ca2+ influx. These oscillations, which result from underlying fluctuations in the activity of the glucose-signalling system, are generated in a synchronous manner amongst neighbouring β-cells by virtue of the fact that these cells are electrically and metabolically coupled by gap junctions12. In fact, the integrity of gap junctional communication appears to be a critical determinant for the induction of pulsatile insulin secretion by glucose. With respect to the kinetics of insulin secretion, the fast transient first phase may reflect the availability of a readily releasable pool of insulin-containing vesicles, whereas the slowly developing second phase may be a consequence of the mobilization of a reserve source of insulin13.
Hormonal regulation of insulin secretion requires bidirectional crosstalk
Glucose-induced insulin secretion from β-cells is a tightly regulated process that is subject to fast (occurring within seconds) stimulatory and inhibitory modulation by a remarkably large number of hormones and neurotransmitters14. These include glucagon and the glucagon-related peptides, gastric inhibitory polypeptide, vasoactive intestinal polypeptide, cholecystokinin, arginine vasopressin, acetylcholine, epinephrine, somatostatin, calcitonin gene-related peptide and galanin. This diversity of ligands reflects the fact that insulin secretion is controlled not simply by the ambient concentration of glucose, but also by hormonal, neural and intra-islet paracrine influences that modulate the responsiveness of β-cells to glucose. Current concepts of β-cell signal transduction envision that modulation results from the coordinate up- or down-regulation of those critical steps (glucose uptake and metabolism, generation of signalling molecules, depolarization and Ca2+ influx, vesicular mobilization and exocytosis) that are necessary for the induction of insulin secretion by glucose. It is evident that the metabolic, ionic and secretory processes that constitute the glucose-signalling system exhibit significant plasticity at multiple levels within this cascade.
A remarkable example of such modulatory influences is to be found in reports indicating that glucagon15 and glucagon-like peptide-1(7–37) (GLP-1)16 potentiate glucose-induced insulin secretion from β-cells. Glucagon and GLP-1 are structurally related peptides which are synthesized by tissue-specific alternative post-translational processing of proglucagon. Glucagon is a secretory product of the α-cells of the islets of Langerhans, whereas GLP-1 is an intestinally derived hormone secreted by specialized neuroendocrine L-cells of the gut. Glucagon and GLP-1 are insulinotropic by virtue of their ability to stimulate β-cell insulin secretion, insulin biosynthesis, and proinsulin gene expression17. These actions are believed to be mediated by distinct G protein-coupled cell-surface receptors18,19, and are likely to be secondary to the known ability of these hormones to stimulate cAMP production20,21. Notably, all insulinotropic actions of glucagon and GLP-1 are glucose-dependent, and in fact both hormones fail to stimulate cAMP production in the absence of glucose. This requirement for glucose may reflect the fact that the β-cell adenylate cyclase is also a calmodulin-regulated enzyme, the activity of which is stimulated by the rise in intracellular Ca2+ that accompanies glucose-induced depolarization. The metabolism of glucose is therefore a near absolute requirement for the functional integrity of the cAMP-dependent signalling system that mediates the modulatory actions of glucagon and GLP-1 on the glucose-signalling system. It is the requirement of the glucagon and GLP-1 signalling systems for glucose on one hand, and the ability of glucagon and GLP-1 to potentiate glucose-induced insulin secretion on the other, that establishes the bidirectional nature of crosstalk in the β-cell system.
The glucose competence concept
The functional consequences of bidirectional crosstalk in the β-cell system are easiest to understand from the standpoint of the glucose competence concept. By definition, β-cells will secrete insulin in response to glucose only when they are glucose-competent. By contrast, diminished glucose-induced insulin secretion is typical of single β-cells maintained in primary cell culture, and is characteristic of fetal β-cells in which the glucose-signalling system is incompletely developed. It is also symptomatic of the metabolic disorder of glucose homeostasis known as Type II diabetes mellitus in which the β-cell glucose-signalling system becomes impaired (see below). Under these less than optimal conditions β-cells can be viewed as relatively glucose-incompetent. The induction of glucose competence is proposed to result from the conditioning influences of circulating insulinotropic hormones which render the β-cell glucose-signalling system capable of responding to glucose. Glucose competence may therefore be envisioned as a metabolic state in which the glucose signalling system is fully ‘primed and ready to go’.
Evidence in support of the glucose competence concept was originally provided by Pipeleers and co-workers in studies examining the modulation of glucose-induced insulin secretion by glucagon22. It was reported that nearly pure preparations of β-cells, derived from dispersed islets of Langerhans, secreted abnormally low amounts of insulin when exposed to glucose. Glucose-induced insulin secretion from these isolated β-cells was potentiated by physiologically relevant concentrations of glucagon, and in fact glucagon appeared to restore the glucose sensitivity of the isolated cells. This interaction was synergistic in nature because a potentiation of insulin secretion was observed using concentrations of glucose and glucagon that had little or no effect on their own. As might be expected for a process of bidirectional crosstalk, the action of glucagon was positively correlated with its effectiveness in stimulating β-cell cAMP production. On the basis of these findings, Pipeleers proposed that glucagon in the pancreas does not simply potentiate insulin secretion, but also enables β-cells to respond to glucose. Inasmuch as the circulating concentrations of glucagon are elevated between meals when blood glucose levels and insulin secretion are reduced, we now interpret these findings to indicate that in the intact pancreas, glucagon maintains the β-cell glucose-signalling system in a functionally competent state during periods of fasting.
Important new insights into the nature of glucose competence were recently elucidated in studies examining the insulinotropic actions of GLP-1 on β-cells. GLP-1 is one member of a class of circulating hormones known as incretin hormones that subserve humoral communication between the gastrointestinal tract and the pancreas (the enteroinsular axis)17. The secretion of GLP-1 into the bloodstream from intestinal L-cells is triggered by the autonomic nervous system and by nutrient stimuli acting at the luminal surface of the gut following a meal. The timing of the secretion of GLP-1 is such that circulating levels of the hormone rise coincident with the postprandial increase in the concentration of blood glucose. By binding to specific β-cell receptors and stimulating cAMP production, GLP-1 synergizes with glucose to induce insulin secretion, thereby increasing the concentration of circulating insulin to a level above and beyond that attributable to glucose alone.
Of special significance to the glucose competence concept is the existence of a reciprocal relationship between the concentrations of glucagon and GLP-1 in the bloodstream. This is a consequence of the inhibition of glucagon secretion from the α-cells of the islets of Langerhans when blood concentrations of glucose and insulin rise. Such a fall in circulating glucagon levels would ultimately lead to diminished β-cell glucose competence if it were not for the compensatory ability of GLP-1 to maintain the glucose-signalling system in a fully functional state. The action of GLP-1 therefore contrasts with that of glucagon in that GLP-1 confers glucose competence to β-cells during the feeding state.
Signalling pathways that mediate glucose competence
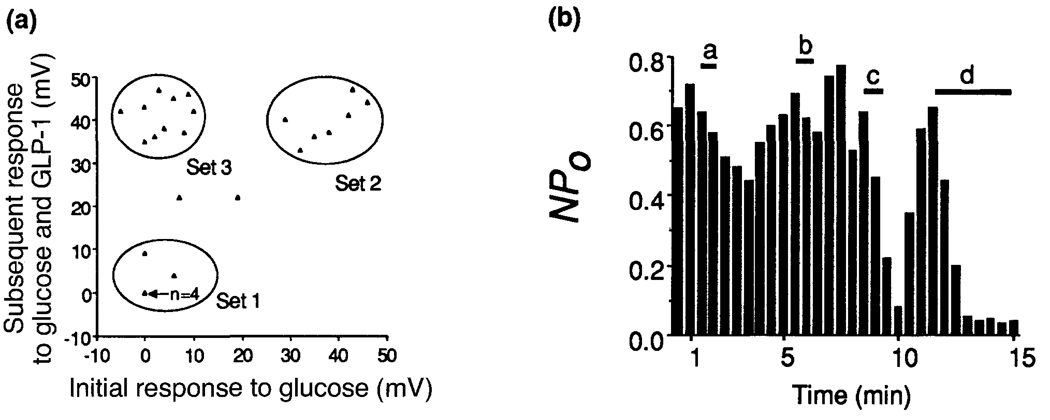
An important clue as to how glucose competence is achieved is provided by recent electrophysiological studies of β-cells using the patch–clamp recording technique23. This approach allows an analysis of the interaction between glucose and the insulinotropic hormones at the single cell level, and provides a direct test of the validity of the glucose competence concept. Measurements of the resting membrane potential obtained from single β-cells isolated from dispersed islets of Langerhans demonstrated that the majority of these cells exhibited little or no depolarizing response when initially exposed to a stimulatory concentration (10 mm) of glucose (Fig. 3). This was expected since, under these in vitro conditions, the β-cells are no longer subject to the conditioning influences of circulating hormones that maintain the glucose-competent state. These initially glucose-insensitive β-cells were rendered fully responsive to glucose by simultaneous exposure to GLP-1, and only then was a substantial depolarizing response observed. It is therefore evident that this induction of glucose competence by GLP-1 resulted from its ability to modulate the glucose-signalling system at a step proximal to the depolarization-induced entry of Ca2+, an event that triggers insulin secretion.
Figure 3.
The induction of glucose competence by GLP-1 is observed at the single cell level, is specific for a distinct subpopulation of β-cells, and is attributable to the inhibition of ATP-sensitive potassium channels. In (a) the actions of glucose and GLP-1 were tested on solitary β-cells isolated from dispersed islets of Langerhans and maintained in short-term primary cell culture23, conditions under which the cells are known to exhibit diminished glucose-induced insulin secretion22. Scatter plot analysis (where each triangle represents observations obtained from a single cell) revealed that the majority of the cells (those comprising Sets 1 and 3) also showed relatively little change in resting membrane potential when exposed to 10 mm glucose (x-axis). In contrast, a subpopulation of these cells (comprising Set 3) exhibited a large depolarizing response when challenged with a combined application of 10 mm glucose and 10 nm GLP-1 (y-axis). This induction of glucose competence by GLP-1 was observed in 40% of all cells tested. (b) A graphical presentation of how the synergistic interaction of 10 mm glucose and 10 nm GLP-1 depolarizes β-cells by inhibiting ATP-sensitive potassium channels, as recorded in the perforated vesicle configuration31. Channel activity is expressed as a function of ‘openness’ vs time where openness is defined as the product of N and Po (N is the number of channels in the patch and Po is the probability that an individual channel is open within a given time frame). In this cell neither glucose (a) nor GLP-1 (b) were effective inhibitors of channel activity when applied alone, whereas a nearly complete inhibition of channel activity was observed when both substances were applied together (c). Channel activity recovered after removal of glucose/GLP-1, and was then blocked by application of 10 nm glyburide (d), indicating that these channels do in fact correspond to the ATP-sensitive potassium channels.
The complexity of the β-cell glucose-signalling system allows for a wide range of possibilities when considering at which step(s) in the signalling cascade GLP-1 exerts its modulatory action. Although GLP-1 might confer glucose competence by modulating the activity of the β-cell glucose transporter and/or glucokinase, it remains to be demonstrated that these two key components of the glucose-sensing mechanism are susceptible to rapid hormonal regulation. Perhaps a more likely target is the ATP-sensitive potassium channel which regulates the resting membrane potential of the β-cell. In support of this hypothesis, we have observed a synergistic interaction between glucose and GLP-1 which inhibits the activity of the ATP-sensitive potassium channel. Patch–clamp recordings from single β-cells isolated from dispersed islets of Langerhans show that the activity of the ATP-sensitive channel is relatively unaffected by concentrations of glucose that normally suppress channel activity in the intact islet (Fig. 3b). By contrast, the activity of these channels is nearly completely blocked by the combined application of glucose and GLP-1. Therefore, the induction of glucose competence by GLP-1 is observed not only at the level of membrane depolarization, but also at the level of the modulation of single ATP-sensitive potassium channels.
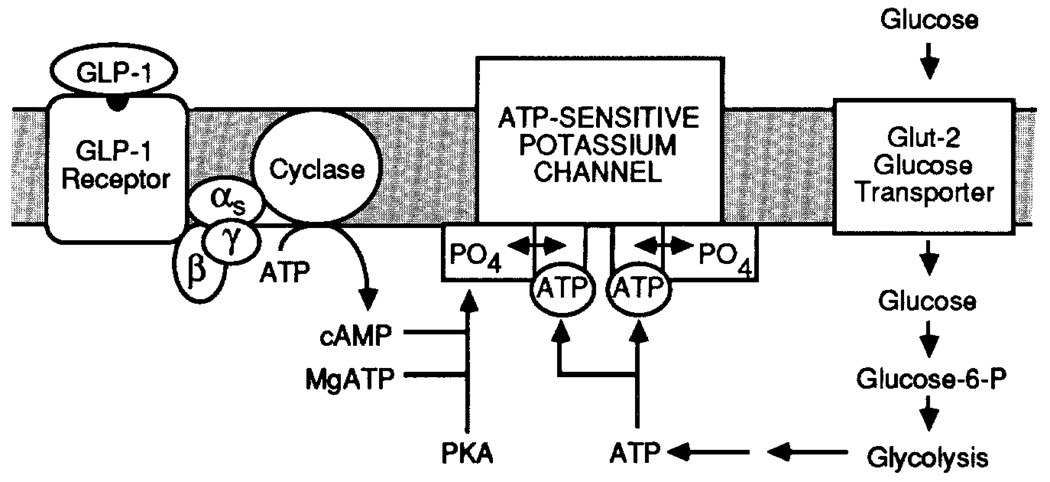
Since ATP-sensitive potassium channels are inhibited by a signalling molecule (ATP) generated by glucose metabolism, it is reasonable to imagine that these channels might also be targeted for modulation by a second messenger, such as cAMP, generated in response to glucagon or GLP-1. Such a scenario provides a logical explanation for the requirement of crosstalk among signalling systems for the induction of β-cell glucose competence (Fig. 4). In this hypothetical scheme the activity of the ATP-sensitive potassium channel is inhibited by the synergistic interaction of intracellular ATP and cAMP. Neither the glucose (ATP) nor the GLP-1 (cAMP) signalling systems are viewed as sufficient, alone, to cause closure of the channels. Thus, the inhibition of channel function requires bidirectional crosstalk. Glucose competence is thought to result from cAMP-dependent, protein kinase A (PKA)-mediated phosphorylation of the channel. This facilitates the inhibitory action of ATP on the channel, possibly by increasing the affinity of the channel for ATP. An intriguing possibility suggested by this model is that a molecular defect in the ATP-sensitive channel would prevent its phosphorylation by PKA, and that under these conditions the channel would no longer be inhibited by ATP. We envision that such a defect in turn would render β-cells glucose-incompetent, as may be the case in certain forms of diabetes mellitus (see below). This model is reminiscent of that which has been proposed to explain the defective interaction between cAMP and ATP in the regulation of the cystic fibrosis transmembrane regulator (CFTR) chloride channels24.
Figure 4.
A model in which the induction of glucose competence by GLP-1 is proposed to result from the synergistic interaction of ATP and cAMP to inhibit ATP-sensitive potassium channels. The binding of GLP-1 to its receptor leads to activation of a heterotrimeric G protein composed of α-, β- and γ-subunits. G protein activation stimulates the activity of the enzyme adenylate cyclase which catalyses the formation of intracellular cAMP. The rise in cAMP levels results in the activation of protein kinase A which catalyses the phosphorylation of regulatory sites (indicated by PO4) on the channel. Conformational switches (indicated by the double-ended arrows) in response to phosphorylation increase the affinity of the channel for ATP, thereby favouring channel closure. In this manner, GLP-1 favours the inhibition of the channel by ATP. Neither cAMP or ATP are viewed as sufficient, alone, to inhibit channel function, and for this reason bidirectional crosstalk between the GLP-1 and glucose signalling system is required for complete inhibition of channel activity.
Is the glucose competence concept relevant to our understanding of diabetes mellitus?
Type II diabetes mellitus is a disorder of glucose homeostasis characterized by the development of insulin resistance in peripheral tissues such as fat and muscle where the uptake and metabolism of glucose is an insulin-stimulated process25. Type II diabetes is also characterized by excessive hepatic glucose production, impaired secretion of insulin from pancreatic β-cells, and chronic hyperglycaemia. Recent attempts at unraveling the etiology of this disorder have established that it is multifactorial (polygenic) in origin; it is thought to arise as a consequence of any one of a potentially large number of molecular defects that disrupt glucose production, uptake or utilization26. Because the β-ce11 acts as a glucose sensor, it is understandable that Type II diabetes may arise, at least in part, from a defect in the β-cell glucose-signalling system.
One characteristic feature of Type II diabetes is that the circulating concentrations of insulin are insufficient to compensate for peripheral insulin resistance. Furthermore, the secretion of insulin from the pancreas is less than expected, given that the concentration of blood glucose is chronically elevated. It is evident, therefore, that in Type II diabetes the β-cells fail to match ambient concentrations of glucose to an appropriate insulin secretory response. Insufficient secretion of insulin does not result from decreased numbers of β-cells, as is the case for Type I diabetes (insulin-dependent diabetes mellitus), nor is there a decreased capacity of the β-cells to synthesize and package insulin in vesicular stores. In addition, the processes that mediate exocytosis of insulin are not disrupted since large amounts of insulin are secreted in response to secretagogues other than glucose. Instead, it now appears that stimulus–secretion coupling is disrupted at earlier steps in the glucose-signalling cascade. For example, a molecular defect that renders the β-cell glucose transporter and/or glucokinase dysfunctional may contribute to the onset or progression of Type II diabetes. Alternatively, Type II diabetes may result from a functional uncoupling of the signal transduction pathways that mediate glucose competence. Since the circulating concentrations of glucagon and GLP-1 are elevated in Type II diabetes, such an uncoupling might easily result from desensitization and down-regulation of the β-cell glucagon/GLP-1 receptors that serve to maintain the glucose-competent state27. The restoration of glucose competence would then require pharmacological concentrations of the hormones, as recently demonstrated in clinical studies examining the actions of GLP-1 in Type II diabetic patients28,29.
Concluding remarks
The glucose competence concept provides a simplified, but useful model of a complex process, namely the role of crosstalk in the regulation of insulin secretion. From this perspective it is possible to appreciate fully that there is a synergistic, bidirectional and mutually interdependent interaction between the β-cell signalling pathways that subserve intermediary metabolism and second messenger-mediated signal transduction. The failure of these two systems to interact properly is hypothesized to be an important determinant for the onset and/or progression of a common metabolic disorder, Type II diabetes. These observations reinforce our view that cellular signal transduction is achieved through the coordinate and fully integrated interaction of what at first might seem to be relatively unrelated signalling systems.
Acknowledgement
The authors acknowledge the valuable contribution of Dr Wiel M. Kuhtreiber to the acquisition and analysis of electrophysiological data presented in this review.
References
- 1.Wollheim CB, Sharp GWG. Physiol. Rev. 1981;61:914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- 2.Zawalich WS, Rasmussen H. Mol. Cell. Endocrinol. 1990;70:119–137. doi: 10.1016/0303-7207(90)90152-x. [DOI] [PubMed] [Google Scholar]
- 3.Matschinsky FM. Diabetes. 1990;39:647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- 4.Bell GI, et al. Diabetes Care. 1990;13:198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- 5.Thorens B, Charron MJ, Lodish HF. Diabetes Care. 1990;13:209–218. doi: 10.2337/diacare.13.3.209. [DOI] [PubMed] [Google Scholar]
- 6.Magnuson MA, Shelton KD. J. Biol. Chem. 1989;264:15936–15942. [PubMed] [Google Scholar]
- 7.lynedjian PB, et al. Proc. Natl Acad. Sci. USA. 1989;86:7838–7842. doi: 10.1073/pnas.86.20.7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald MJ. Diabetes. 1990;39:1461–1466. doi: 10.2337/diab.39.12.1461. [DOI] [PubMed] [Google Scholar]
- 9.Rajan AS, et al. Diabetes Care. 1990;13:340–363. doi: 10.2337/diacare.13.3.340. [DOI] [PubMed] [Google Scholar]
- 10.Ashcroft FM, Rorsman P. Prog. Biophys. MoL Biol. 1991;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 11.Matschinsky FM, Bedoya FJ. In: Endocrinology. DeGroot LJ, editor. W. B. Saunders; 1989. pp. 1290–1303. [Google Scholar]
- 12.Meda P. In: Cell Interactions and Gap Junctions. Sperelakis N, Cole WC, editors. CRC Press; 1989. pp. 59–79. [Google Scholar]
- 13.Grodsky GM, Curry D, Landahl H, Bennett LL. Acta Diabetol. Lat. 1969;6 suppl. 1:554–579. [PubMed] [Google Scholar]
- 14.Dupre J. In: The Endocrine Pancreas. Samols E, editor. Raven Press; 1991. pp. 253–281. [Google Scholar]
- 15.Pipeleers DG, et al. Endocrinology. 1985;117:824–833. doi: 10.1210/endo-117-3-824. [DOI] [PubMed] [Google Scholar]
- 16.Mojsov S, Weir GC, Habener JF. J. Clin. Invest. 1989;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehman HC, Habener JF. Trends Endocrinol. Metabol. 1992;3:158–163. doi: 10.1016/1043-2760(92)90165-w. [DOI] [PubMed] [Google Scholar]
- 18.Van Schravendijk CFH, et al. Endocrinology. 1985;117:841–848. doi: 10.1210/endo-117-3-841. [DOI] [PubMed] [Google Scholar]
- 19.Thorens B. Diabetes. 1992;41 suppl. 1:12. [Google Scholar]
- 20.Schuit FC, Pipeleers DG. Endocrinology. 1985;117:834–840. doi: 10.1210/endo-117-3-834. [DOI] [PubMed] [Google Scholar]
- 21.Drucker DJ, Philipe J, Mojsov S, Chick WL, Habener JF. Proc. Natl Acad. Sci. USA. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pipeleers DG. Diabetologia. 1987;30:277–291. doi: 10.1007/BF00299019. [DOI] [PubMed] [Google Scholar]
- 23.Holz GG, Kuhtrieber WM, Habener JF. The Endocrine Society 74th Annual Meeting; San Antonio, TX. 1992. [Google Scholar]
- 24.Anderson MP, et al. Cell. 1991;67:775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 25.Unger RH, Foster DW. In: Williams Textbook of Endocrinology. Wilson JD, Foster DW, editors. W. B. Saunders; 1992. pp. 1255–1333. [Google Scholar]
- 26.Bell GI. Diabetes. 1991;40:413–422. doi: 10.2337/diab.40.4.413. [DOI] [PubMed] [Google Scholar]
- 27.Fehman HC, Habener JF. Endocrinology. 1991;128:2880–2888. doi: 10.1210/endo-128-6-2880. [DOI] [PubMed] [Google Scholar]
- 28.Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Diabetes Care. 1992;15:270–276. doi: 10.2337/diacare.15.2.270. [DOI] [PubMed] [Google Scholar]
- 29.Gutniak M, Orskov C, Hoist JJ, Ahren B, Efendic S. N. Engl. J. Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 30.Horn R, Marty A. J. Gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levitan ES, Kramer RH. Nature. 1990;348:546–547. doi: 10.1038/348545a0. [DOI] [PubMed] [Google Scholar]