Abstract
Background
Observational studies have shown reduced risk of Alzheimer dementia in users of nonsteroidal anti-inflammatory drugs.
Objective
To evaluate the effects of naproxen sodium and celecoxib on cognitive function in older adults.
Design
Randomized, double-masked chemoprevention trial.
Setting
Six US memory clinics.
Participants
Men and women aged 70 years and older with a family history of Alzheimer disease; 2117 of 2528 enrolled had follow-up cognitive assessment.
Interventions
Celecoxib (200 mg twice daily), naproxen sodium (220 mg twice daily), or placebo, randomly allocated in a ratio of 1:1:1.5, respectively.
Main Outcome Measures
Seven tests of cognitive function and a global summary score measured annually.
Results
Longitudinal analyses showed lower global summary scores over time for naproxen compared with placebo (−0.05 SDs; P=.02) and lower scores on the Modified Mini-Mental State Examination over time for both treatment groups compared with placebo (−0.33 points for celecoxib [P=.04] and −0.36 points for naproxen [P=.02]). Restriction of analyses to measures collected from persons without dementia attenuated the treatment group differences. Analyses limited to measures obtained while participants were being issued study drugs produced results similar to the intention-to-treat analyses.
Conclusions
Use of naproxen or celecoxib did not improve cognitive function. There was weak evidence for a detrimental effect of naproxen.
Trial Registration
clinicaltrials.gov identifier: NCT00007189
Cytokine-mediated in-flammatory processes may play a role in neurodegenerative disorders and the development of cognitive impairment in the elderly. Consistent with this hypothesis, observational studies have shown an association between the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and a lower risk of Alzheimer disease (AD).1
In addition to possibly modifying risk of dementia, extended NSAID use might protect against age-related cognitive decline (a possible forerunner of AD diagnosis). In the Established Populations for Epidemiological Studies of the Elderly cohort, long-time NSAID users had higher scores after 3 years on the Short Portable Mental Status Questionnaire than did non-users.2 The magnitude of this effect was comparable with that of a 3.5-year difference in participant age. A modest association between NSAID use and reduced decline during 54 months in paired-associate learning was found in the United Kingdom hypertension treatment trial.3
Not all studies have shown a beneficial effect of NSAIDs on cognition, however, and some have found them to be deleterious.4,5 Whether better or poorer cognitive performance is caused by NSAID use is unknown, and controlled clinical trials are required. We report the early results of such a primary prevention trial. More than 2500 cognitively normal elderly participants in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) were randomly assigned to take naproxen sodium, celecoxib, or placebo, and had annual cognitive assessments. This article describes changes in neuropsychological test performance in this sample during 1 to 4 years following randomization.
METHODS
STUDY DESIGN, FUNDING, AND APPROVAL
The Alzheimer's Disease Anti-inflammatory Prevention Trial is a randomized, placebo-controlled, multicenter, primary prevention trial sponsored by the National Institute on Aging. All pertinent institutional review boards approved the study protocol.
PARTICIPANTS AND ENROLLMENT
Participants were recruited primarily through mailings to Medicare beneficiaries targeted by age and zip code in areas surrounding the trial's 6 field sites (Baltimore, Maryland; Boston, Massachusetts; Rochester, New York; Seattle, Washington; Sun City, Arizona; and Tampa, Florida). Participants were required to be aged 70 years or older, to have a history of at least 1 first-degree relative with AD-like dementia, and to score satisfactorily on an eligibility test battery for excluding cognitive impairment. Persons who regularly used NSAIDs were excluded, but aspirin use of 81 mg or less per day was allowed. More specific information on eligibility criteria is available in the trial's protocol (http://www.jhucct.com/adapt/manall43.pdf). Written consent was obtained from each participant and a collateral respondent. Enrollment commenced in March 2001.
INTERVENTIONS AND RANDOMIZATION
Participants were randomly assigned to receive celecoxib (200 mg twice daily), naproxen sodium (220 mg twice daily), or placebo. The randomization scheme was generated by the trial's coordinating center in permuted blocks stratified by 3 age groups (ages 70-74, 75-79, and ≥80 years) and by the 6 field sites, with an assignment ratio of 1:1:1.5, respectively. Treatment assignments were released only after baseline data were keyed and eligibility was confirmed. Masking was achieved using a double-placebo design.6
SAMPLE SIZE
Sample size calculations were performed using a SAS macro for time-to-event outcomes (SAS Institute Inc, Cary, North Carolina).7 Based on parameter assumptions outlined in the trial's protocol, the sample size provided 80% power to detect a 30% reduction in the incidence of Alzheimer dementia across up to 7 years of follow-up.
DATA COLLECTION
The eligibility test battery was designed to provide acceptable sensitivity for exclusion of those with dementia or other cognitive impairment. It included the Modified Mini-Mental State Examination (3MS-E), the Hopkins Verbal Learning Test–Revised, and the informant-rated Dementia Severity Rating Scale. Those meeting eligibility criteria returned for a more extensive baseline Cognitive Assessment Battery before randomization. In addition to these 3 tests, this battery also included the Digit Span Test, a generative verbal fluency of naming as many supermarket items as possible in 1 minute, narratives from the Rivermead Behavioral Memory Test, the Brief Visuo-spatial Memory Test–Revised, self-rating of memory functions, and the Geriatric Depression Scale. The Cognitive Assessment Battery was then administered annually thereafter. Participants who scored below predetermined cutoffs received a dementia evaluation that included more extensive psychometric testing and physical and neurological examinations. These tests and procedures are documented in the study protocol.
We reported analyses and results of 7 of the Cognitive Assessment Battery tests (excluding the self-rated, informant-rated, and depression scales) collected through June 17, 2005 (6 months after cessation of study treatment). Specifically, we used the education-adjusted score for the 3MS-E, the age- and sex-adjusted scores from trial 4 of the Hopkins Verbal Learning Test–Revised, and the delayed recall scores of the Rivermead Behavioral Memory Test and Brief Visuospatial Memory Test–Revised. For all tests, higher scores reflect better cognitive functioning. Additionally, we calculated a global summary score. Each participant's score on each test and at each point were standardized to the mean and SD of the entire sample's baseline test scores. The global summary score was then calculated for each administration of the test battery as an un-weighted average of the standardized scores for the 7 tests.
STATISTICAL ANALYSIS
Analyses included participants who had at least 1 set of follow-up cognitive measures. The mean change from baseline to 1, 2, and 3 years after randomization was calculated by treatment group for each test and for the global summary score. Each active treatment group was compared with the placebo group using t tests of the change from baseline to each follow-up point. Longitudinal analyses of change from baseline to all follow-up points were conducted with generalized estimating equations regression to account for the correlation of within-person measures. This method provided estimates of the difference between the active treatment groups and the placebo group averaged across all follow-up points, with confidence intervals around these estimates.
In secondary analyses, we investigated the change in normal cognitive function by treatment group, after exclusion of scores obtained at the time of or after a diagnosis of dementia in 37 participants (event rates by treatment group are published elsewhere8). Longitudinal analyses were conducted as for all scores. Additionally, we calculated odds ratios for each treatment compared with placebo for the outcome of a decline from baseline of 5, 6, 7, 8, 9, or 10 points on the 3MS-E at any time during follow-up. Similarly, we calculated the odds ratios for a decline from baseline of 0.50, 0.75, 1.00, and 1.25 SDs on the global summary score.
Finally, we performed longitudinal analyses after exclusion of observations obtained after participants were no longer being issued the study drug. Thus, cognitive measures were included if they were obtained on or before the first semiannual visit at which participants were not given a new 6-month supply of the study drug. All analyses were conducted using SAS, version 8.1 (SAS Institute Inc).
The trial's treatment effects monitoring committee was advisory to the steering committee and the National Institute on Aging. The committee met in person twice a year to review efficacy and safety data classified by treatment assignment.
TREATMENT DISCONTINUATION
On December 17, 2004, enrollment and treatment with both celecoxib and naproxen were suspended after increased cardiovascular risk was observed with celecoxib in another prevention trial. The rationale for suspending treatments in ADAPT was presented at the joint meeting of the Food and Drug Administration Arthritis Advisory Committee and Drug Safety and Risk Management Advisory Committee on February 18, 2005.9
RESULTS
STUDY POPULATION
A total of 2528 participants were enrolled in ADAPT from March 2001 to December 2004, and 2117 contributed follow-up cognitive measures. Table 1 provides baseline characteristics and cognitive function scores by treatment group. More men than women were enrolled and race/ethnicity was predominantly white. More than three-quarters of the population had post–high school education. Median 3MS-E scores were well above the eligibility requirement. These baseline characteristics were similar for the 3 treatment groups.
Table 1.
Characteristics of Study Population by Treatment Group
| Characteristic | Randomization |
Follow-up |
||||||
|---|---|---|---|---|---|---|---|---|
| Total | Celecoxib | Naproxen Sodium |
Placebo | Total | Celecoxib | Naproxen | Placebo | |
| No. of participants | 2528 | 726 | 719 | 1083 | 2117 | 617 | 596 | 904 |
| Age, y | ||||||||
| Median | 74 | 74 | 74 | 74 | 74 | 74 | 74 | 74 |
| First/third quartile | 72/77 | 72/77 | 72/77 | 72/77 | 72/77 | 72/77 | 72/77 | 72/77 |
| Range | 70-90 | 70-90 | 70-88 | 70-90 | 70-90 | 70-88 | 70-89 | 70-90 |
| Sex, % | ||||||||
| F | 45.9 | 47.1 | 45.9 | 45.1 | 45.5 | 47.5 | 45.6 | 44.1 |
| M | 54.1 | 52.9 | 54.1 | 54.9 | 54.5 | 52.5 | 54.4 | 55.9 |
| Ethnicity/race, %a | ||||||||
| White | 97.0 | 96.1 | 97.1 | 97.4 | 96.8 | 96.1 | 97.0 | 97.1 |
| African American | 1.5 | 1.8 | 1.8 | 1.0 | 1.6 | 1.9 | 1.9 | 1.2 |
| Hispanic | 0.7 | 1.4 | 0.3 | 0.6 | 0.8 | 1.5 | 0.3 | 0.7 |
| Other | 0.8 | 0.6 | 0.7 | 0.9 | 0.7 | 0.5 | 0.7 | 0.9 |
| Did not answer | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.2 | 0.1 |
| Marital status, % | ||||||||
| Married | 71.9 | 70.2 | 75.0 | 71.0 | 71.9 | 70.2 | 74.7 | 71.2 |
| Widowed | 18.2 | 19.7 | 16.1 | 18.7 | 18.7 | 20.3 | 16.6 | 19.1 |
| Divorced/separated | 7.3 | 7.3 | 6.1 | 8.0 | 6.7 | 6.7 | 5.5 | 7.4 |
| Single | 2.6 | 2.8 | 2.8 | 2.3 | 2.7 | 2.9 | 3.2 | 2.3 |
| Education, % | ||||||||
| <High school | 4.0 | 3.9 | 4.9 | 3.6 | 4.1 | 3.6 | 5.2 | 3.7 |
| High school degree | 19.9 | 20.8 | 17.5 | 20.9 | 19.5 | 21.1 | 17.1 | 20.0 |
| College, no degree | 27.5 | 27.7 | 28.4 | 26.8 | 27.0 | 27.6 | 27.7 | 26.2 |
| College degree | 19.2 | 19.2 | 17.0 | 20.6 | 19.5 | 19.3 | 17.5 | 21.0 |
| Postgraduate | 29.4 | 28.5 | 32.3 | 28.2 | 29.9 | 28.5 | 32.6 | 29.1 |
| Karnofsky scoring, % | ||||||||
| 100 | 82.3 | 84.3 | 80.1 | 82.5 | 84.0 | 85.3 | 83.2 | 83.6 |
| 90 | 15.3 | 13.5 | 18.2 | 14.6 | 14.2 | 12.8 | 15.9 | 13.9 |
| 80 | 2.2 | 2.1 | 1.4 | 2.8 | 1.7 | 1.8 | 0.8 | 2.2 |
| 60-70 | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.2 | 0.0 | 0.2 |
| Aspirin use, % | 56.1 | 57.0 | 56.2 | 55.4 | 49.7 | 51.4 | 48.8 | 49.1 |
| Cognitive measure, median score | ||||||||
| Global summary | 0.01 | 0.00 | 0.02 | 0.00 | 0.02 | −0.01 | 0.01 | 0.03 |
| Adjusted 3MS-E | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 |
| GVF | 25.0 | 24.0 | 24.0 | 25.0 | 25.0 | 24.0 | 24.0 | 25.0 |
| RBMT delayed recall | 6.0 | 6.5 | 6.0 | 6.0 | 6.5 | 6.5 | 6.0 | 6.5 |
| BVMT-R delayed recall | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| Adjusted HVLT-R trial 4 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 |
| Digit Span, forward | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| Digit Span, backward | 7.0 | 7.0 | 6.0 | 7.0 | 7.0 | 7.0 | 6.0 | 7.0 |
Abbreviations: BVMT-R, Brief Visuospatial Memory Test–Revised; GVF, generative verbal fluency; HVLT-R, Hopkins Verbal Learning Test–Revised; RBMT, Rivermead Behavioral Memory Test; 3MS-E, Modified Mini-Mental State Examination.
This variable was collected for the tracking of minority participation in this trial. Participants were asked to designate their race or ethnicity as 1 of the listed categories including “other.”
FOLLOW-UP AND TREATMENT ADHERENCE
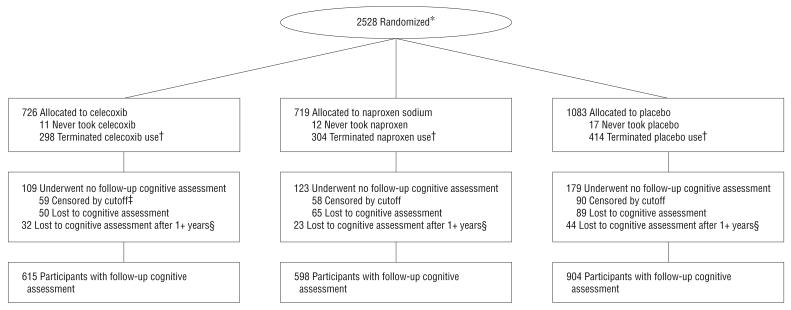
Figure 1 shows the flow of participants from randomization to analysis. A total of 411 participants did not contribute to the analyses either because the data were censored before their first annual follow-up (n=207) or because they did not return for cognitive follow-up (n=204). These losses were distributed evenly across treatment groups (P=.53). There were 51 deaths: 16 participants who were taking naproxen (2.2% of all participants assigned to naproxen), 17 who were taking celecoxib (2.3%), and 18 who were taking placebo (1.7%). Death rates were comparable between placebo and naproxen (P=.38) and placebo and celecoxib (P=.30). For those with follow-up cognitive assessments, the median time to last cognitive assessment was 736 days for the celecoxib, 737 days for the naproxen, and 736 days for the placebo groups. Median time to discontinuation of treatment (for early terminators, the first semiannual visit at which the study drug was not issued or, for the remainder of participants, December 17, 2004) was 546 days for the celecoxib, 520 days for the naproxen, and 544 for the placebo groups (P=.46).
Figure 1.
Flowchart of the Alzheimer's Disease Anti-inflammatory Prevention Trial. * Indicates numbers available only for those randomized, not those screened for eligibility; †, participants considered to have terminated study drug if study drugs had been started but were no longer being issued at scheduled visits before December 17, 2004 (does not include temporary interruptions); ‡, participants considered administratively censored if their 1-year visit window had not closed by June 17, 2005; §, participants considered lost to cognitive assessment after 1 or more years if they did not have cognitive assessment data in the 1.5 years before June 17, 2005 (losses include death).
COGNITIVE FUNCTION SCORES BY TREATMENT GROUP
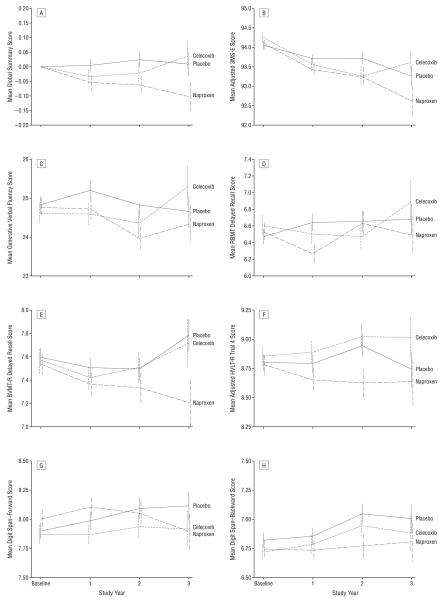
Figure 2 shows the raw scores for each of the 7 tests and the global summary over time by treatment group. Changes from baseline to 1, 2, and 3 years after randomization and t tests of the differences by treatment group at each point in time are presented in Table 2. Mean scores mostly decline with time, though the declines are small relative to the SDs of the changes from baseline and most were not statistically significant at individual points. Decline over time is most apparent in 3MS-E scores; declines in 3MS-E scores are greater in both treatment groups than in the placebo group at all follow-up points.
Figure 2.
Raw scores for each of the 7 tests of cognitive function and the global summary over time by treatment group (baseline, N=2528; year 1, n=2088; year 2, n=1485; year 3, n=700). BVMT-R indicates Brief Visuospatial Memory Test–Revised; HVLT-R, Hopkins Verbal Learning Test–Revised; RBMT, Rivermead Behavioral Memory Test; and 3MS-E, Modified Mini-Mental State Examination.
Table 2.
Mean Changes From Baseline in Cognitive Function by Treatment Group
| Measure | Treatment Group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Celecoxib |
Naproxen Sodium |
Placebo |
P Value |
|||||
| No. of Participants |
Mean Change in Score (SD) |
No. of Participants |
Mean Change in Score (SD) |
No. of Participants |
Mean Change in Score (SD) |
Celecoxib vs Placebo |
Naproxen vs Placebo |
|
| Global summary, y | ||||||||
| 1 | 608 | −0.04 (0.40) | 589 | −0.06 (0.39) | 889 | −0.03 (0.39) | .61 | .15 |
| 2 | 432 | −0.05 (0.45) | 424 | −0.07 (0.45) | 629 | −0.02 (0.40) | .22 | .07 |
| 3 | 195 | −0.01 (0.44) | 202 | −0.10 (0.51) | 303 | −0.04 (0.42) | .47 | .12 |
| Adjusted 3MS-E, y | ||||||||
| 1 | 608 | −0.79 (3.22) | 589 | −0.67 (3.47) | 889 | −0.46 (3.22) | .06 | .21 |
| 2 | 432 | −1.19 (4.28) | 424 | −0.95 (4.03) | 629 | −0.50 (3.41) | .004 | .06 |
| 3 | 195 | −1.03 (3.53) | 202 | −1.39 (4.84) | 303 | −0.99 (3.59) | .92 | .28 |
| GVF, y | ||||||||
| 1 | 608 | −0.01 (5.38) | 589 | −0.06 (5.76) | 889 | 0.32 (5.59) | .25 | .19 |
| 2 | 432 | −0.38 (5.66) | 424 | −0.81 (5.79) | 627 | −0.01 (5.45) | .29 | .02 |
| 3 | 195 | −0.18 (5.10) | 202 | −0.60 (6.02) | 303 | −0.33 (6.02) | .77 | .61 |
| RBMT delayed recall, y | ||||||||
| 1 | 608 | −0.05 (3.39) | 589 | −0.27 (3.19) | 889 | 0.04 (3.26) | .59 | .08 |
| 2 | 432 | −0.15 (3.28) | 424 | 0.17 (3.39) | 628 | 0.02 (3.29) | .41 | .47 |
| 3 | 195 | 0.35 (3.12) | 201 | 0.16 (2.73) | 303 | 0.26 (2.79) | .73 | .70 |
| BVMT-R delayed recall, y | ||||||||
| 1 | 608 | −0.15 (2.25) | 589 | −0.22 (2.35) | 889 | −0.20 (2.14) | .69 | .87 |
| 2 | 431 | −0.17 (2.50) | 424 | −0.23 (2.44) | 628 | −0.28 (2.39) | .46 | .74 |
| 3 | 195 | 0.14 (2.59) | 202 | −0.24 (2.57) | 302 | 0.04 (2.36) | .64 | .22 |
| Adjusted HVLT-R trial 4, y | ||||||||
| 1 | 608 | 0.02 (2.32) | 589 | −0.07 (2.46) | 888 | −0.11 (2.29) | .30 | .78 |
| 2 | 432 | 0.00 (2.36) | 424 | −0.03 (2.66) | 628 | 0.07 (2.41) | .62 | .51 |
| 3 | 195 | −0.12 (2.28) | 202 | −0.22 (2.65) | 303 | −0.18 (2.26) | .80 | .85 |
| Digit Span, forward, y | ||||||||
| 1 | 607 | 0.00 (1.74) | 589 | 0.07 (1.71) | 889 | 0.02 (1.73) | .88 | .56 |
| 2 | 430 | 0.09 (1.70) | 422 | −0.03 (1.82) | 627 | 0.11 (1.86) | .83 | .22 |
| 3 | 195 | 0.07 (1.71) | 202 | −0.19 (1.74) | 303 | 0.06 (1.80) | .92 | .13 |
| Digit Span, backward, y | ||||||||
| 1 | 606 | 0.05 (1.68) | 589 | −0.04 (1.72) | 889 | 0.00 (1.76) | .53 | .69 |
| 2 | 430 | 0.20 (1.75) | 423 | −0.05 (1.70) | 625 | 0.09 (1.84) | .33 | .19 |
| 3 | 195 | 0.17 (1.82) | 202 | −0.04 (2.01) | 302 | −0.03 (1.65) | .23 | .95 |
Abbreviations: BVMT-R, Brief Visuospatial Memory Test–Revised; GVF, generative verbal fluency; HVLT-R, Hopkins Verbal Learning Test–Revised; RBMT, Rivermead Behavioral Memory Test; 3MS-E, Modified Mini-Mental State Examination.
Results of longitudinal analyses are presented in Table 3. Coefficients represent the difference between the active treatment groups and the placebo group averaged across all follow-up points. For naproxen, coefficients for all 7 tests were negative and global summary scores were significantly lower over time (−0.05 SDs; P=.02). For celecoxib, coefficients were negative for 4 of the 7 tests and global summary scores were not different from placebo (−0.01 SDs; P=.47). Scores over time on the 3MS-E were lower for both treatment groups compared with the placebo group (−0.32 points for celecoxib [P=.04] and −0.36 points for naproxen [P=.03]).
Table 3.
Longitudinal Effect of Treatment on Cognitive Function
| Measure | Celecoxib vs Placebo |
Naproxen Sodium vs Placebo |
||
|---|---|---|---|---|
| β (95% Confidence Interval) | P Value | β (95% Confidence Interval) | P Value | |
| Global summary score | −0.01 (−0.05 to 0.02) | .47 | − 0.05 (−0.09 to -0.01) | .02 |
| Adjusted 3MS-E score | −0.32 (−0.62 to -0.02) | .04 | − 0.36 (−0.68 to -0.04) | .03 |
| GVF score | −0.27 (−0.73 to 0.19) | .24 | − 0.54 (−1.01 to -0.07) | .02 |
| RBMT delayed recall score | −0.09 (−0.32 to 0.15) | .47 | − 0.18 (−0.42 to 0.06) | .14 |
| BVMT-R delayed recall score | 0.03 (−0.15 to 0.21) | .75 | − 0.12 (−0.31 to 0.07) | .22 |
| Adjusted HVLT-R trial 4 score | 0.08 (−0.11 to 0.27) | .4 | − 0.10 (−0.30 to 0.10) | .34 |
| Digit Span score, forward | −0.06 (−0.20 to 0.08) | .42 | − 0.04 (−0.19 to 0.10) | .55 |
| Digit Span score, backward | 0.03 (−0.11 to 0.17) | .67 | − 0.11 (−0.26 to 0.03) | .13 |
Abbreviations: BVMT-R, Brief Visuospatial Memory Test–Revised; GVF, generative verbal fluency; HVLT-R, Hopkins Verbal Learning Test–Revised; RBMT, Rivermead Behavioral Memory Test; 3MS-E, Modified Mini-Mental State Examination.
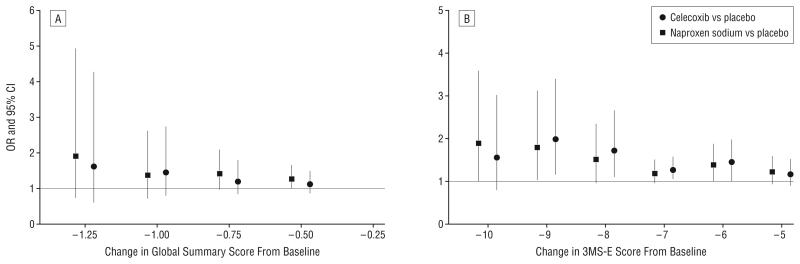
As is shown in Figure 3A and B, odds ratios comparing each treatment to placebo tended to show increasing risk for increasingly larger declines in 3MS-E and global summary scores. In other secondary analyses, after exclusion of measures obtained just before (at the triggering assessment) or after a dementia diagnosis, estimates of longitudinal differences by treatment group were generally closer to 0 without changing negativity or positivity, but the estimates for 3MS-E and global summary scores were no longer statistically significant (Table 4).
Figure 3.
Odds ratios (ORs) comparing treatment groups with placebo for magnitudes of decline from baseline in global summary (A) and Modified Mini-Mental State Examination (3MS-E) (B) scores. CI indicates confidence interval.
Table 4.
Longitudinal Effect of Treatment on Cognitive Function, Excluding Measurements Associated With Incident Dementia
| Measure | Celecoxib vs Placebo |
Naproxen Sodium vs Placebo |
||
|---|---|---|---|---|
| β (95% Confidence Interval) | P Value | β (95% Confidence Interval) | P Value | |
| Global summary score | −0.004 (−0.04 to 0.03) | .84 | −0.03 (−0.07 to 0.01) | .09 |
| Adjusted 3MS-E score | −0.20 (−0.47 to 0.07) | .14 | −0.19 (−0.47 to 0.09) | .19 |
| GVF score | −0.23 (−0.69 to 0.23) | .32 | −0.43 (−0.90 to 0.03) | .07 |
| RBMT delayed recall score | −0.06 (−0.29 to 0.18) | .64 | −0.13 (−0.37 to 0.11) | .28 |
| BVMT-R delayed recall score | 0.05 (−0.14 to 0.23) | .62 | −0.07 (−0.26 to 0.12) | .45 |
| Adjusted HVLT-R trial 4 score | 0.12 (−0.06 to 0.30) | .2 | −0.04 (−0.23 to 0.16) | .7 |
| Digit Span score, forward | −0.05 (−0.19 to 0.09) | .48 | −0.03 (−0.17 to 0.11) | .69 |
| Digit Span score, backward | 0.03 (−0.11 to 0.18) | .64 | −0.09 (−0.23 to 0.05) | .22 |
Abbreviations: BVMT-R, Brief Visuospatial Memory Test–Revised; GVF, generative verbal fluency; HVLT-R, Hopkins Verbal Learning Test–Revised; RBMT, Rivermead Behavioral Memory Test; 3MS-E, Modified Mini-Mental State Examination.
Finally, analyses in which cognitive measures were censored after participants were no longer being issued study drugs showed similar results to the intention-to-treat analyses. Scores over time on the 3MS-E again were statistically significantly lower for both treatment groups compared with the placebo group (−0.41 points for celecoxib [P=.02] and −0.47 points for naproxen [P=.01]). For naproxen compared with placebo, the global summary scores also were significantly lower over time (−0.05 SDs; P=.03).
COMMENT
The ADAPT cognitive function results through 6 months after study treatment cessation do not show a protective effect with the use of NSAIDs and may suggest that cognitive scores are lower. The global summary scores, which combine the results from 7 individual tests in the cognitive assessment battery, were significantly lower over time for naproxen, but not for celecoxib, compared with placebo. Scores over time on the 3MS-E, a single measure of global cognitive function, were lower for both treatment groups compared with placebo. The lack of a positive effect is congruent with ADAPT results over the same period regarding diagnoses of AD, any dementia, or mild cognitive impairment/prodromal AD.8 The data for both the cognitive test scores and cognitive diagnoses are limited by the early termination of treatments in ADAPT, associated with emerging concerns regarding the cardiovascular safety of NSAIDs.10
The Alzheimer Disease Anti-inflammatory Prevention Trial is the first primary prevention trial to test the association of NSAIDs with the incidence of AD and the change over time in cognitive function in people without existing cognitive impairment. Other trials have been conducted to test NSAIDs for treating AD11-17 or as secondary prevention agents in people who have mild cognitive impairment.18,19 These trials did not show a benefit with the use of NSAIDs and, if anything, showed an increased risk associated with NSAIDs. Nevertheless, because of the strong epidemiological evidence of a lower incidence of AD in people taking NSAIDs,1 it was thought that these drugs should be tested as primary prevention agents.
The ADAPT findings add to the negative or null evidence from treatment trials and secondary prevention trials, and therefore appear to be inconsistent with the epidemiological findings that provided the rationale for the trial. The divergent results may be due to confounding by indication in the observational studies. Also, inflammatory processes are complex and may be hypothesized to have either reparative or detrimental effects on neurons.20 Alternatively, it may be that the ADAPT findings relate specifically to celecoxib or naproxen, perhaps because these drugs are not among those NSAIDs that have been shown in vitro and in vivo in mouse models to lower production of the 42-residue form of amyloid β (Aβ42)21; that is, the epidemiological findings could be due to the effects of other NSAIDs, such as the commonly used ibuprofen, that do lower Aβ42. More speculatively, the ADAPT findings may represent early detrimental effects following initiation of treatment in people who have subclinical neuropathology. That is, NSAIDs might exert protective effects only if given several years before the time when symptoms would otherwise develop. Such a difference in effect has been suggested by results of both the Rotterdam22 and Cache County23 observational studies, which showed apparent protective effects with more distant, but not recent, use of NSAIDs.
In this article, the mean differences by treatment group in the changes from baseline are small; only for the 3MS-E do the differences approach a 10th of an SD, or about one-third of a point on this 100-point scale, over a median follow-up of close to 2 years. The cognitive test measures used have a fair amount of variability both within and among persons, but the large sample size of this trial results in the ability to detect fairly small differences in group means and changes over time as statistically significant. Whether any detected differences are clinically significant must be considered. To aid in clinical interpretation, we may view the difference in 3MS-E scores found between treatment groups in ADAPT as equivalent to the average yearly decline in 3MS-E score in the placebo group.
ADAPT Group Members.
Steering Committee
Resource Center Representatives (Voting). Dr Breitner (study chair), Veteran Affairs Puget Sound Health Care System and University of Washington School of Medicine, Seattle; Neil Buckholtz, PhD (project officer), and Susan Molchan, MD (project officer), National Institute on Aging, Bethesda, Maryland; Dr Evans (steering committee chair), Rush University Medical Center, Chicago, Illinois; Dr Martin (coordinating center deputy director) and Curtis Meinert, PhD (coordinating center director), Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Field Site Directors (Voting). Dr Craft, Veteran Affairs Puget Sound Health Care System and University of Washington School of Medicine; Dr Green, Boston University School of Medicine, Boston, Massachusetts; Constantine Lyketsos, MD, MHS, Johns Hopkins School of Medicine, Baltimore; Dr Mullan, The Roskamp Institute Memory Clinic, Tampa, Florida; Marwan Sabbagh, MD, Sun Health Research Institute, Sun City, Arizona; Pierre N. Tariot, MD (previous); Saleem Ismail, MD, University of Rochester School of Medicine, Rochester, New York.
Other Voting Members. Drs Brandt and Piantadosi, Johns Hopkins School of Medicine.
Staff. Janette Negele (previous) and Melissa Montero, Veteran Affairs Puget Sound Health Care System; and Bonnie Piantadosi, MSW, MPH, Johns Hopkins Bloomberg School of Public Health.
Consultants. Themistocles Dassopoulos, MD, Johns Hopkins School of Medicine; Claudia Kawas, MD, University of California–Irvine, Irvine; Leon Thal, MD, University of California–San Diego, La Jolla; Kathleen Welsh-Bohmer, PhD, Duke University Medical Center, Durham, North Carolina; and Andrew Whelton, MD, Hunt Valley, Maryland.
Research Group
Resource Centers. Chairman's Office, Veteran Affairs Puget Sound Health Care System: Dr Breitner (chairman); Ms Negele (previous coordinator); Ms Montero (coordinator); Elizabeth Aigbe, MS; Jill Dorje; and Brenna Cholerton, PhD.
Coordinating Center, Johns Hopkins Bloomberg School of Public Health: Dr Meinert (director); Dr Martin (deputy director); Ms Piantadosi (coordinator); Robert Casper, MS; Michele Donithan, MHS; Hsu-Tai Liu, MD, MPH; Dr Piantadosi; Anne V. Shanklin, MA, CCRP; and Paul Smith, BA.
Project Office, National Institute on Aging: Dr Buckholtz (project officer) and Susan Molchan, MD.
Field Sites. Johns Hopkins School of Medicine: Constantine G. Lyketsos, MD, MHS (director); Martin Steinberg, MD (associate director); Dr Brandt (neuropsychologist); Julia J. Pedroso, RN, MA (coordinator); Alyssa Bergey; Themos Dassopoulos, MD; Melanie Dieter, MA; Carol Gogel, RN; Chiadi Onyike, MD; Lynn Smith; Veronica Wilson-Sturdivant; and Nadine Yoritomo, RN.
Boston University School of Medicine: Dr Green (director); Sanford Auerbach, MD (associate director); Robert Stern, PhD (neuropsychologist); Patricia Boyle, PhD (previous neuropsychologist); Dawn Cisewski, PhD (previous neuropsychologist); Jane Mwicigi, MD, MPH (coordinator); Mary-Tara Roth, RN, MSN, MPH (previous coordinator); Lorraine Baldwin; Margaret Brickley, MS, RN, NP; Patrick Compton, RN; Debra Hanna, RN, BC, MPH; Sylvia Lambrechts; Janet Nafissi, MSN, APRN, BC; Andreja Packard, MD, PhD; and Mayuri Thakuria, MD, MPH.
University of Rochester School of Medicine: Saleem Ismail, MD (director); Dr Tariot (previous director); Anton Porsteinsson, MD; J. Michael Ryan, MD (previous associate director); Robin Henderson-Logan, PhD, ABPP-cn (neuropsychologist); Colleen McCallum, MSW (coordinator); Suzanne Decker; Laura Jakimovick, RN, MS; Kara Jones, RN; Arlene Pustalka, RN; Susan Salem-Spencer, RN, MS; and Asa Widman.
Veteran Affairs Puget Sound Health Care System and University of Washington School of Medicine: Dr Craft (director); Mark Fishel, MD (associate director); Laura Baker, PhD (neuropsychologist); Deborah Dahl, RN (coordinator); Kathleen Nelson, RN (previous coordinator); Susan Bigda, RN; Yoshie Biro; Ruth Boucher, RN; Nickolas Dasher; Edward DeVita, MD; Grace Garrett; Austin Hamm; Jeff Lindsey; and Laura Sissons-Ross.
Sun Health Research Institute: Marwan Sabbagh, MD, FAAN (director); Joseph Rogers, PhD (associate director); Donald Connor, PhD, PhD (neuropsychologist); Carolyn Liebsack, RN, BSN, CCRC (coordinator); Nancy Thompson, RN (previous coordinator); Joanne Ciemo, MD; Kathryn Davis; Theresa Hicksenhiser, LPN; Sherry Johnson-Traver; Healther Kolody; Lisa Royer, RN; Nina Silverberg, PhD; and Deborah Tweedy, RN, MSN, CNP.
The Roskamp Institute Memory Clinic: Dr Mullan (director); Cheryl Luis, PhD (associate director, neuropsychologist); Timothy Crowell, PsyD (previous associate director, neuropsychologist); Julia Parrish, LPN (coordinator); Laila Abdullah, (previous coordinator); Theavy Chea; Scott Creamer; Melody Brooks Jayne, MD; Antoinette Oliver, MA; Summer Price, MA; and Joseph Zolton, ERT.
Treatment Effects Monitoring Committee
Voting members: C. Morton Hawkins, PhD (chair), Frontier Science & Technology Research Foundation, Madison, Wisconsin; Bernard Carroll, MBBS, PhD, FRCPsych, Pacific Behavioral Research Foundation, Carmel, California; Dallas M. High, PhD, Sander-Brown Center on Aging, Eustis, Florida; Ronald Petersen, PhD, MD, Mayo Clinic, Rochester; and Thomas Schnitzer, MD, PhD, Northwestern University, Chicago.
Nonvoting members: Dr Buckholtz, National Institute on Aging; Dr Evans, Rush University Medical Center; and Dr Meinert, Johns Hopkins Bloomberg School of Public Health.
An interesting question is whether this treatment difference represents a small decline occurring in a large portion of the study population or might be explained by a larger decline in a small number of people developing dementia. In fact, Figure 3A and B suggest treatment effects of only low magnitude with small changes in 3MS-E or global summary scores, but a higher magnitude of association for large changes. Furthermore, the association of treatment with change from baseline in 3MS-E score was attenuated when measures obtained just before or after a diagnosis of dementia were excluded. Therefore, the treatment group differences could be attributed in part, but not entirely, to the difference by treatment group in the incidence of dementia.
The similarity of the results in secondary analyses of cognitive measures in treatment-adherent participants and the primary intention-to-treat results is reassuring. Thus the trial results do not appear to have been unduly influenced by treatment terminations that occurred before December 17, 2004. Such terminations typically occurred because of perceived adverse effects or health risks of study treatments or because patients required use of NSAIDs for analgesia. Treatment also was terminated after diagnosis of dementia.
The ADAPT experience illustrates the potential difficulty of primary prevention trials in AD, a disease with a long subclinical phase and for which a long duration of exposure to a preventative agent is presumed necessary. It is possible that the effect of treatment may not be consistent over the subclinical phase or the exposure period. If the potential preventive agent has adverse effects and if early results of the trial do not show benefit or even suggest harm, the trial may not be able to continue to its planned completion. Continued follow-up of trial participants, even after cessation of treatment, appears warranted to investigate treatment effects with respect to the timing of exposure. However, for now we suggest that naproxen and celecoxib should not be used for the prevention of AD.
Acknowledgments
Funding/Support: This study was supported by grant U01 AG15477 from the National Institute on Aging.
Role of the Sponsor: National Institute on Aging collaborators participated in the design and conduct of the study and in the interpretation and review of the study results. Neither Pfizer nor Bayer participated in the design, conduct, or analysis of the study but provided comments on the manuscript.
Footnotes
Financial Disclosure: Pfizer provided celecoxib and matching placebo and Bayer Healthcare provided naproxen sodium and matching placebo for this trial. Competing interests for the ADAPT Writing Committee: Drs Martin, Evans, Mullan, and Szekely have no conflicts of interest. Dr Brandt receives royalties from Psychological Assessment Resources Inc, publisher of the Hopkins Verbal Learning Test–Revised, one of the neuropsychological tests used in this trial; however, Dr Brandt waived royalties from use of the test in ADAPT. Dr Breitner previously held partial royalty interests in 2 United States patents for the use of NSAIDs for the prevention of AD; in January 2005 he assigned these interests irrevocably as a gift to a private charitable foundation in which he has no constructive or beneficial interest. Dr Piantadosi is a consultant for Pfizer. Dr Craft is a consultant for and receives research support from GlaxoSmithKline. Dr Green has received lecture honoraria or educational grants and has served as an advisor or consultant to Forest Laboratories and Pfizer and is an unpaid scientific advisor to Myriad Pharmaceuticals and SmartGenetics. Competing interests for the rest of the ADAPT Steering Committee:Dr Meinert has no conflicts of interest. Dr Lyketsos is a consultant to GlaxoSmithKline and receives lecture honoraria and support for continuing medical education activities from AstraZeneca, Eisai, Forest Laboratories, Janssen, Lilly, Novartis, and Pfizer. Dr Ismail has received research support from Pfizer/Eisai and is a member of their speakers' bureau. Dr Tariot is a consultant to Abbott Laboratories, AstraZeneca, Bristol-Meyers Squibb, Eisai, Forest Laboratories, Janssen, Eli Lilly, Myriad Pharmaceuticals, Pfizer, Sanofi-Aventis, and Schwabe; he has received research support from Abbott Laboratories, AstraZeneca, Elan, Pfizer, Bristol-Myers Squibb, Forest Laboratories, Merck, Neurochem, Ono, and Sanofi-Aventis and lecture honoraria from Pfizer, Forest Laboratories, AstraZeneca, and Eisai. Dr Sabbagh is a speaker for Pfizer, Eisai, Ortho-McNeil, Forest Laboratories, and Abbott Laboratories and receives research support from GlaxoSmithKline, Pfizer, Eisai, Elan, Ono, and Takeda. Dr Welsh-Bohmer, who is a nonvoting member of the ADAPT Steering Committee, holds royalty interests in the 2 US patents for the use of NSAIDs for the prevention of AD; she is also a paid consultant to GlaxoSmithKline and has received speaking honoraria from Pfizer and private gifts to the Bryan Alzheimer's Disease Research Center from GlaxoSmithKline and Novartis.
REFERENCES
- 1.Szekely CA, Thorne JE, Zandi PP, et al. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer's disease: a systematic review. Neuroepidemiology. 2004;23(4):159–169. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- 2.Rozzini R, Ferrucci L, Losonczy K, et al. Protective effect of chronic NSAID use on cognitive decline in older persons. J Am Geriatr Soc. 1996;44(9):1025–1029. doi: 10.1111/j.1532-5415.1996.tb02932.x. [DOI] [PubMed] [Google Scholar]
- 3.Prince M, Rabe-Hesketh S, Brennan P. Do antiarthritic drugs decrease the risk for cognitive decline? an analysis based on data from the MRC treatment trial of hypertension in older adults. Neurology. 1998;50(2):374–379. doi: 10.1212/wnl.50.2.374. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin JS, Regan M. Cognitive dysfunction associated with naproxen and ibuprofen in the elderly. Arthritis Rheum. 1982;25(8):1013–1015. doi: 10.1002/art.1780250817. [DOI] [PubMed] [Google Scholar]
- 5.Karplus TM, Saag KG. Nonsteroidal anti-inflammatory drugs and cognitive function: do they have a beneficial or deleterious effect? Drug Saf. 1998;19(6):427–433. doi: 10.2165/00002018-199819060-00001. [DOI] [PubMed] [Google Scholar]
- 6.Martin BK, Meinert CL. Breitner JCS for the ADAPT Research Group. Double placebo design in a prevention trial for Alzheimer's disease. Control Clin Trials. 2002;23(1):93–99. doi: 10.1016/s0197-2456(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 7.Shih JH. Sample size calculation for complex clinical trials with survival endpoints. Control Clin Trials. 1995;16(6):395–407. doi: 10.1016/s0197-2456(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 8.ADAPT Research Group Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68(21):1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 9.Statement from the Steering Committee of the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) for communication to the FDA Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee. http://www.jhucct.com/adapt/pdf%20documents/FDA%20ADAPT %20STATEMENT_web%20posting.pdf. Published February 18, 2005. Accessed December 22, 2005.
- 10.ADAPT Research Group Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) PLoS Clin Trials. 2006;1(7):e33. doi: 10.1371/journal.pctr.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers J, Kirby LC, Hempelman SR, et al. Clinical trial of indomethacin in Alzheimer's disease. Neurology. 1993;43(8):1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- 12.Scharf S, Mander A, Ugoni A, et al. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer's disease. Neurology. 1999;53(1):197–201. doi: 10.1212/wnl.53.1.197. [DOI] [PubMed] [Google Scholar]
- 13.Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer's disease. Neurology. 2002;58(7):1050–1054. doi: 10.1212/wnl.58.7.1050. [DOI] [PubMed] [Google Scholar]
- 14.Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289(21):2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 15.Reines SA, Block GA, Morris JC, et al. Rofecoxib: no effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004;62(1):66–71. doi: 10.1212/wnl.62.1.66. [DOI] [PubMed] [Google Scholar]
- 16.Sainati S, Ingram D, Talwalker S, Geis G. Results of a double-blind, randomized, placebo-controlled study of celecoxib in the treatment of progression of Alzheimer's disease; Presented at: The Sixth International Stockholm-Springfield Symposium of Advances in Alzheimer's Therapy; Stockholm, Sweden. April 5-8, 2000. [Google Scholar]
- 17.Black SE, Wilcock G, Haworth J, et al. A placebo-controlled, double-blind trial of the selective A 42-lowering agent Flurizan in patients with mild to moderate Alzheimer's disease: efficacy, safety, and follow-on study results [program No. 585.6]; Presented at: the 35th Annual Meeting of the Society for Neuroscience; Washington, DC. November 15, 2005. [Google Scholar]
- 18.Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30(6):1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 19.Trials registration for “Anti-inflammatory treatment for age-associated memory impairment: a double-blind placebo-controlled trial” [ NCT00009230] http://clinicaltrials.gov/ct2/show/NCT00009230?term=NCT00009230&rank=1. Accessed March 22, 2006.
- 20.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease: a double-edged sword. Neuron. 2002;35(3):419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 21.Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 22.in t' Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345(21):1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 23.Zandi PP, Anthony JC, Hayden KM, et al. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59(6):880–886. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]