Abstract
Human immunodeficiency virus type 1 (HIV-1) tropism plays an important role in HIV-associated dementia. In this study, aimed at determining if the tropism and coreceptor usage of circulating viruses correlates with cognitive function, the authors isolated and characterized HIV from the peripheral blood of 21 Hispanic women using antiretroviral therapy. Macrophage tropism was determined by inoculation of HIV isolates onto monocyte-derived macrophages and lymphocyte cultures. To define coreceptor usage, the HIV isolates were inoculated onto the U87.CD4 glioma cell lines with specific CCR5 and CXCR4 coreceptors. HIV isolates from cognitively impaired patients showed higher levels of replication in mitogen-stimulated peripheral blood mononuclear cells than did isolates from patients with normal cognition (P < .05). The viral growth of HIV primary isolates in macrophages and lymphocytes did not differ between patients with and those without cognitive impairment. However, isolates from the cognitively impaired women preferentially used the X4 coreceptor (P < .05). These phenotypic studies suggest that cognitively impaired HIV-infected women receiving treatment may have a more highly replicating and more pathogenic X4 virus in the circulation that could contribute to their neuropathogenesis.
Keywords: cognitive impairment, coreceptor usage, HIV dementia, HIV tropism
Introduction
Human immunodeficiency virus type 1 (HIV-1)–associated dementia (HAD) is a metabolic encephalopathy induced by increased viral infection and fueled by immune activation of brain mononuclear phagocytes (perivascular and parenchymal macrophages and microglia) (Diesing et al, 2002; Dou et al, 2004; Marder et al, 1996). HAD occurs in advanced stages of HIV-1 disease and after acquired immunodeficiency disease (AIDS) progression because of the loss of immune control, increased trafficking of activated and/or infected monocytes into the central nervous system (CNS), and the late emergence of viral variants that affect CNS disease progression (Ranki et al, 1995; Williams and Hickey, 2002). HIV-1 enters the CNS soon after the systemic infection and affects the white matter, basal ganglia, and cerebral cortex (Jones and Power, 2005; McArthur et al, 1999).
HIV-1 entry occurs by direct fusion of the viral envelope protein gp120 with the target cell membranes. The viral envelope glycoprotein (Env), which, binds with high affinity to the cellular CD4 receptor, mediates the fusion with the primary receptor on the targeted cell surface (Berger et al, 1999; Chang et al, 2005; Huang et al, 2005; Sharma et al, 2005). The seven-transmembrane G protein–coupled chemokine receptors (CXCR4 and CCR5) function as coreceptors for HIV entry. These coreceptors are expressed by primary lymphocytes and macrophages, the main cellular targets for HIV infection, and may influence cell tropism (Goodenow and Collman, 2006).
HIV isolates may exhibit a macrophage, lymphocyte, or dual (i.e., growth in macrophages and lymphocytes) tropism. Macrophage (M)-tropic isolates primarily infect macrophages and lymphocytes, but not CD4-positive transformed cell lines; they typically are not syncytia-inducing (NSI) in infected lymphoid targets. These M-tropic isolates use predominantly the CCR5 coreceptor and have been termed R5 viruses (Berger et al, 1998). T-cell line (T)-tropic isolates infect lymphocytes and CD4-transformed cells, causing the formation of multinucleated giant cells or syncytia; they often are syncytia-inducing (SI)(Bannert et al, 2000; Mack et al, 2005). T-tropic isolates use predominantly CXCR4 and have been termed X4 viruses (Berger et al, 1998). Some viruses, such as the X4 primary isolate HIV-Tybe, do have the capacity to infect macrophages through the CXCR4, depending on the coreceptor’s density level or conformation (Yi et al, 2003, 2005). The determinants of coreceptor usage and cell tropism lie mainly within the V3 loop in gp120, although other envelope regions, particularly V1/V2, may also contribute (Hibbitts et al, 1999; Sirois et al, 2005; Watabe et al, 2005). Viral tropism and coreceptor utilization are determined by virus-cell specific interactions and thus may influence the development of clinical therapies. For example, CCR5 and CXCR4 antagonists could be used to inhibit selectively the entrance of HIV-1 strains that use specific coreceptors for cell entry (Yi et al, 2005). The interplay between viral tropism and coreceptor utilization is still a subject of intense debate (Goodenow and Collman, 2006; Poveda et al, 2006).
Coreceptor utilization has been associated to the pathogenesis of CNS (Spudich et al, 2005). Most of the brain-derived viruses (i.e., SF-162, JR-CSF, JR-FL, Yu2) are M-tropic and principally use CCR5 for cell entry (D’Aversa et al, 2005; Gorry et al, 2001, 2002). M-tropic variants are found throughout all stages of disease and have been associated with increased secretion of inflammatory mediators by macrophages and development of HAD (Wojna et al, 2004a). T-tropic and dual-tropic variants develop with disease progression (Neil et al, 2005; Simmons et al, 1996). These dual-tropic strains (R5X4) could use CCR5 and CXCR4 coreceptors to enter the macrophages and T-cell lines. CXCR4 is expressed in T cells, in macrophages, and in various tissues including brain (astrocytes, cortical neurons, cerebellar granule cells, and microglia) and is the major receptor preferred by strains of HIV-1 that arise during progression to immunodeficiency and development of HAD (Gendelman, 2006; Yi et al, 2003, 2004). T-tropic viruses generally do not replicate well in microglia, despite the presence of the CXCR4 coreceptor, but they can traffic in and out of the brain during progressive HIV-1 disease. During disease development, the blood-brain barrier is compromised, and X4 viruses may play an important role in the neuropathogenesis of HAD by activating macrophages, microglia, and astrocytes expressing the CXCR4 coreceptor by producing neurotoxic factors and inducing apoptosis in neurons (Fischer-Smith and Rappaport, 2005; Gendelman et al, 2006; Nath, 2002; Yi et al, 2003, 2004).
Studies comparing HIV tropism in the peripheral blood and in the CNS are needed to define the phenotypic determinants of HAD. Spudich and collaborators have reported a mixture of R5 and X4 viruses that show promiscuity in their coreceptor preference and can traffic between the peripheral blood and the CSF (Spudich et al, 2005). Despite various studies showing that genetic factors and adaptation processes are responsible for the differences between cerebrospinal fluid (CSF)–derived and plasma-derived viral populations (Di Stefano et al, 1998; Pillai et al, 2006; Strain et al, 2005), Burkala and collaborators reported that viral isolates with characteristics of peripheral origin were present in the choroid plexus (Burkala et al, 2005). Consonant with these findings, Korber and collaborators found that isolates from blood and brain shared genetic viral sequences, suggesting that there is a trafficking of virus between the CNS and the periphery (Korber et al, 1994). In recent studies using heteroduplex tracking assays, Harrington and collaborators found that besides CSF autogenous viral populations, there were strains that were shared between the CSF and the peripheral blood (Harrington et al, 2005). Recently, important associations have been found between the proviral DNA in circulating monocytes and the pathogenesis of HAD (Shiramizu et al, 2005).
In the era of highly active antiretroviral therapy (HAART), the CNS has become increasingly important as a sanctuary for HIV-1. In addition to reducing viral load and restoring T-cell function, HAART may affect the HIV tropism, thereby causing a selection of X4 viruses rather than R5 variants (Poveda et al, 2006). However, others have shown that HAART can cause a delay in the emergence of X4 variants (Poveda et al, 2006).
In this study, we examined the hypothesis that HIV tropism and coreceptor usage of the viruses present in the peripheral blood correlate with cognitive function. Experiments were conducted to isolate and determine the tropism of HIV variants from a cohort of HIV-infected Hispanic women characterized for cognitive function (Wojna et al, 2006). The prevalence of HAD in this cohort (28.6 %) has been reported to be higher than that in other longitudinally studied cohorts in the United States and the use of this cohort was particularly valuable in testing our hypothesis.
Results
Patient cohort
Viral and immune parameters, along with cognitive status, of the patient cohort are presented in Table 1. The age range of the patients was 22 to 47 years, with a mean ± SD of 34 ± 6 years. The mean CD4 cell count was 234 ± 140 cells/mm3. The mean HIV viral load was 4.1 ± 1.1 log10 copies/ml in plasma and 2.4 ± 0.7 log10 copies/ml in CSF. Mean age, CD4 counts, and viral load did not significantly differ between those with and those without cognitive impairment (P >.05), as has been reported elsewhere (Wojna et al, 2006). The predominant risk factor for HIV infection in this cohort was heterosexual transmission (Wojna et al, 2006).
Table 1.
Viral and immune parameters of a Hispanic Puerto Rican women cohort, by cognitive status
| Viral load (log10 copies/ml) |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Viral isolate | m-AANa | CD4 nadir (cells/mm3) | CD4 count (cells/mm3) | Plasma | CSF | Treatmentc |
| Normal cognition | 1 | NC | 39 | 190 | 4.67 | 2.53 | None |
| 2 | NC | 78 | 42 | 5.47 | 3.06 | HAART | |
| 3 | NC | 185 | 100 | 4.99 | NDb | HAART | |
| 4 | NC | 192 | 223 | 4.48 | 2.51 | HAART | |
| 5 | NC | 309 | 343 | 4.33 | 2.40 | HAART | |
| 6 | NC | 127 | 127 | 4.34 | 2.58 | HAART | |
| 7 | NC | 426 | 466 | <1.7 | <1.7 | HAART | |
| 8 | NC | 171 | 272 | 4.53 | 4.14 | HAART | |
| 9 | NC | 320 | 410 | <1.7 | <1.7 | HAART | |
| Cognitive impairment | 10 | A | 5 | 15 | 3.59 | <1.7 | HAART |
| 11 | A | 22 | 22 | 5.68 | 2.27 | HAART | |
| 12 | A | 404 | 278 | 4.07 | ND | HAART | |
| 13 | A | 324 | 309 | 4.28 | 3.34 | None | |
| 14 | A | 168 | 168 | 5.16 | 3.45 | Monotherapy | |
| 15 | MCMD | 296 | 296 | 3.14 | 1.83 | HAART | |
| 16 | MCMD | 225 | 210 | 4.17 | ND | HAART | |
| 17 | MCMD | 48 | 413 | 3.48 | ND | Monotherapy | |
| 18 | HAD | 42 | 388 | 4.50 | 1.85 | HAART | |
| 19 | HAD | 304 | 385 | 2.97 | <1.7 | HAART | |
| 20 | HAD | 337 | 204 | 4.32 | ND | None | |
| 21 | HAD | 35 | 46 | 4.83 | 1.97 | HAART | |
Cognitive function determined using the American Academy of Neurology HIV-associated dementia criteria modified to include an asymptomatic cognitively impaired group (m-AAN): normal cognition (NC), asymptomatic (A), minor cognitive motor disturbance (MCMD), HIV-associated dementia (HAD).
Not done.
Antiretroviral treatment.
Viral isolation
HIV was recovered from only 21 women (Table 1) following coculture of peripheral blood mononuclear cells (PBMCs) from 62 HIV-seropositive women using combined antiretroviral therapy. These co-cultures were amplified with fresh phytohemagglutinin (PHA)-activated PBMCs in three separate experiments, each using blood from different HIV-seronegative donors. Viruses isolated from patients with cognitive impairment had significantly higher HIV p24 antigen levels (median 382.80) than did viruses isolated from patients with normal cognition (median 311.90) at day 14 post infection (P < .05) (Figure 1).
Figure 1.

Isolation of HIV-1 variants from peripheral blood of HIV-positive women characterized for cognitive function. Peripheral blood mononuclear cells (PBMCs) were isolated from women with normal cognition and women with cognitive impairment and cocultured with HPV-1-negative PBMCs. Culture supernatants were tested for HIV p24 antigen concentration at day 14 post infection to detect virus replication. Isolates from HIV patients with cognitive impairment showed significantly higher HIV p24 titers (* P = .049) than those from HIV patients with normal cognition.
Macrophage and lymphocyte tropism of HIV-1 primary isolates
To determine the tropism of viruses present in the blood of women with cognitive impairment as compared with that in women with normal cognition, macrophages from an individual HIV-seronegative donor were inoculated with HIV isolates from patients with normal cognition or with cognitive impairment.
For the characterization of M-tropism, 14-day supernatants from HIV-infected monocyte-derived macrophages (MDMs) were tested for replication levels by HIV p24 antigen, and only 8 of 21 (38%) HIV isolates replicated in macrophages (Table 2). Five isolates from patients with normal cognition replicated in MDMs: four showed high HIV p24 antigen levels that ranged from 5 to 446 ng/ml; one (viral isolate 7) showed low levels of HIV p24 antigen in MDMs and was not syncytia-inducing (NSI) on MT-2 cells. Three isolates from women with cognitive impairment showed higher replication in MDMs (levels that ranged from 26 to 4912 ng/ml) (Table 2). There was no significant difference between the M-tropism of viral isolates from patients with and those without cognitive impairment (P = .538).
Table 2.
Tropism and coreceptor usage of HIV-1 primary isolates from peripheral blood of women
| U87.CD4c |
|||||||
|---|---|---|---|---|---|---|---|
| Viral isolate | Cognitive functiona | MT-2 cells | MDMb | Cell tropism | CCR5 | CXCR4 | Coreceptor tropism |
| 1 | NC | NSI | (+++) | M-tropic | (+++) | (−) | R5 |
| 2 | NC | SI | (+++) | Dual | (−) | (+++) | X4 |
| 3 | NC | SI | (+++) | Dual | (+++) | (+++) | Dual |
| 4 | NC | NSI | (−) | N/Dd | (+++) | (−) | R5 |
| 5 | NC | SI | (−) | T-tropic | (−) | (+++) | X4 |
| 6 | NC | NSI | (−) | N/D | (+/−) | (+) | Dual |
| 7 | NC | NSI | (+/−) | M-tropic | (+++) | (−) | R5 |
| 8 | NC | NSI | (+++) | M-tropic | (+++) | (+/−) | Dual |
| 9 | NC | NSI | (−) | N/D | (+++) | (−) | R5 |
| 10 | CI | SI | (−) | T-tropic | (+++) | (+++) | Dual |
| 11 | CI | NSI | (+++) | M-tropic | (+++) | (+++) | Dual |
| 12 | CI | SI | (−) | T-tropic | (−) | (+++) | X4 |
| 13 | CI | SI | (−) | T-tropic | (+++) | (+++) | Dual |
| 14 | CI | SI | (−) | T-tropic | (−) | (+++) | X4 |
| 15 | CI | SI | (−) | T-tropic | (−) | (+++) | X4 |
| 16 | CI | NSI | (−) | N/D | (+/−) | (−) | R5 |
| 17 | CI | SI | (−) | T-tropic | (+++) | (+++) | Dual |
| 18 | CI | SI | (−) | T-tropic | (−) | (++) | X4 |
| 19 | CI | SI | (−) | T-tropic | (+++) | (+++) | Dual |
| 20 | CI | SI | (+++) | Dual | (+++) | (+++) | Dual |
| 21 | CI | NSI | (+++) | M-tropic | (−) | (+++) | X4 |
| LAIe | N/A | SI | (−) | T-tropic | (−) | (+++) | X4 |
| BALf | N/Ag | NSI | (+++) | M-tropic | (+++) | (−) | R5 |
Cognitive function includes two groups: NC (normal cognition) and CI (cognitive impairment). Cognitive impairment was determined using the American Academy of Neurology HIV-associated dementia criteria. The CI group includes asymptomatic (A), minor cognitive motor disturbance (MCMD), and HIV-associated dementia (HAD).
Monocyte-derived macrophages.
p24 antigen in ng/ml: (−): <0.2 ng/ml; (+/−): 0.2–0.5 ng/ml; (+): 0.5–1 ng/ml; (++): 1–2 ng/ml; (+++): >2 ng/ml.
N/D: Not determined because of lack of viral replication in phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs).
HIV-1 laboratory strain, prototype T-tropic.
HIV-1 laboratory strain, prototype M-tropic.
N/A: Not applicable.
Characterization of T-tropism was determined by the formation of syncytia on MT-2 cells. Among the isolates from women with normal cognition, 3 of 9 (33%) formed syncytia; among those from women with cognitive impairment, 9 of 12 (75%) did so. The difference between the T-tropism of isolates from women with normal cognition and that from women with cognitive impairment approached statistical significance (Fisher’s exact test, P = .087) (Table 2).
When the results from MT-2 and MDM assays were combined to determine the predominant cell tropism, we found that among the viruses isolated from patients with normal cognition, three of the six tested (50%) demonstrated preferential macrophage tropism, one of six (17%) demonstrated preferential lymphocyte tropism, and two of six (33%) demonstrated dual tropism. Three viruses (isolates 4, 6, and 9) were not tested for tropism on lymphocyte and macrophage cultures because of insufficient viral replication levels in both target cells. Among those viruses isolated from women with cognitive impairment, 2 of 11 (18%) demonstrated preferential macrophage tropism, 8 of 11 (73%) demonstrated preferential lymphocyte tropism, and 1 of 11 (9%) demonstrated dual tropism. There was one viral isolate (number 16) that did not grow in any of the target cells (Table 2).
HIV-1 coreceptor usage
We determined the coreceptor usage of the HIV isolates by using the transfected glioma cells U87.CD4.CCR5 and U87.CD4.CXCR4, which express the R5 and X4 chemokine coreceptors, respectively. We then compared the coreceptor usage of these primary isolates with the cognitive status of the patients. In each cell line, we first confirmed by flow cytometry that R5 and X4 coreceptor expression was optimal. Both cell lines (U87.CD4.CCR5 and U87.CD4.CXCR4) showed R5 and X4 coreceptor expression of more than 60% (data not shown).
The X4 and R5 coreceptor usage of the 21 HIV isolates was determined by monitoring the levels of HIV p24 antigen on the U87.CD4.CXCR4 and U87.CD4.CCR5 cell lines inoculated with HIV isolates containing 25 ng/ml of HIV p24 antigen. Five of nine (55%) viral isolates from women with normal cognition released more than 0.2 ng/mL of HIV p24 antigen from CXCR4 cell supernatants, thus demonstrating preferential use of the X4 coreceptor (Figure 2A). Of these five isolates, two (isolates 2 and 5) showed preference for the utilization of the X4 coreceptor, whereas three (isolates 3, 6, and 8) were characterized as dual-tropic for coreceptor usage because they also demonstrated replication in the CCR5 cell line (Table 2). Isolates 4 and 8 showed a rapid replication in X4 cells that was followed by a reduction in the HIV p24 antigen levels at day 13 post infection (Figure 2A). Even the CXCR4-positive control, HIVLAI, demonstrated a similar reduction. Seven of nine (77%) viral isolates from patients with normal cognition replicated well (HIV p24 > 0.2 ng/ml) in the R5 cell line (Figure 2B). Four of nine isolates from patients with normal cognition showed predominant CCR5 coreceptor usage, three were considered dual because of an efficient replication in the CCR5 and CXCR4 cells, and two showed predominance for the X4 coreceptor (Table 2). Similar to the X4 line, some viruses (isolates 2, 5, and the CCR5-positive control HIVBAL) showed a rapid replication in the CCR5 cells that was followed by decreased HIV p24 antigen titer 8 days post infection (Figure 2B). The predominant coreceptor usage of these isolates was as follows: four of nine (44%) isolates used CCR5, two of nine (22%) used CXCR4, and three of nine (33%) used both coreceptors. Among the three HIV isolates whose predominant tropism could not be determined because of insufficient growth on lymphocytes, MT-2 cells, and macrophages, two showed R5 coreceptor usage and one was dual-tropic, thus demonstrating the increased sensitivity of coreceptor usage over replication capacity for tropism determination.
Figure 2.
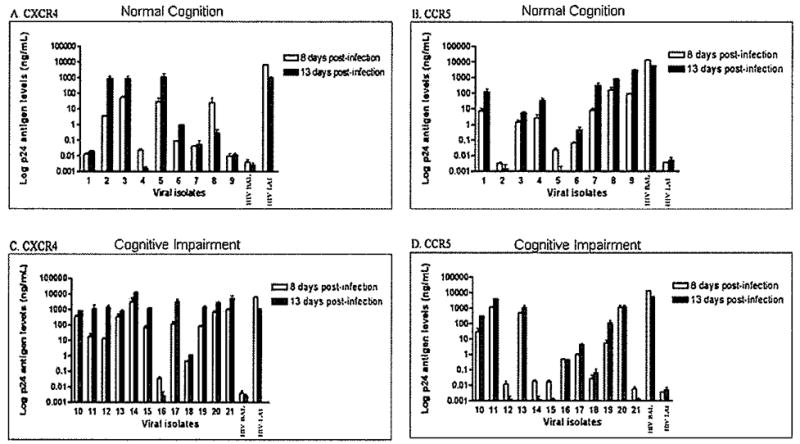
Coreceptor usage of peripheral blood primary isolates. Coreceptor specificity was defined through the transfected glioma cell lines U87.CD4.GCR5 and U87.CD4.CXCR4, which express CCR5 and CXCR4 coreceptors, respectively. Cells were inoculated with 25 ng of HIV p24 antigen of primary HIV isolates from women with normal cognition (isolates 1 to 9) and with cognitive impairment (isolates 10 to 21). Infection was monitored by HIV p24 antigen ELISA at 8 and 13 days post infection. The laboratory-adapted isolates, HIVBAL, and HIVLAI, were used as the R5 and X4 positive controls, respectively. CXCR4 and CCR5 coreceptor utilization by HIV-1 primary isolates from patients with normal cognition is shown in A and B, respectively. CXCR4 and CCR5 coreceptor usage of HIV-1 primary isolates from patients with cognitive impairment is shown in C and D, respectively. Results are representative of one experiment in duplicate. There was no significant difference between HIV p24 titers in the R5 line between isolates from patients with (D) and those without (B) cognitive impairment at 8 (P = .538) or 13 (P = .537) days post infection. Isolates from patients with cognitive impairment (C) showed higher HIV p24 levels in the X4 line than the isolates from women with normal cognition (A) at 8 (P = .048) and 13 (P = .047) days post infection.
Among those from women with cognitive impairment, 11 (92%) viral isolates demonstrated HIV p24 antigen levels of more than 0.2 ng/ml in CXCR4 cells (Figure 2G). Most of these isolates showed higher infection levels in the X4 cells than did the isolates from patients with normal cognition (Figure 2C; Table 2). Among the 11 isolates that used the CXCR4, 6 also used the CCR5, showing a dual coreceptor usage. There was more homogeneity between the viruses from both groups of patients in terms of infection levels in CCR5 cells (Figure 2D; Table 2), Only one virus (isolate 16) showed preference for R5 usage alone, and its replication levels were relatively low compared with the other isolates that used the R5 coreceptor. There was no difference in HIV p24 antigen levels on the CCR5 line between those with cognitive impairment and those with normal cognition at 8 (P = .538) or 13 (P = .537) days. However, patients with cognitive impairment had significantly higher HIV p24 antigen levels on the CXCR4 line than did those with normal cognition at 8 (P = .048) and 13 (P = .047) days.
The predominant coreceptor usage of the HIV isolates was as follows: 5 of 12 (42%) of those with cognitive impairment showed preference for the CXCR4, 1 of 12 (8%) showed preference for the CCR5, and 6 of 12 (50%) were dual-tropic (Table 2). These isolates from cognitively impaired patients were predominantly dual-tropic and X4 tropic.
Cytopathic effects induced by primary isolates
The cytopathic effect was evaluated to determine the ability of the isolates to infect cell lines expressing each coreceptor. The formation of multinucleated giant cells or syncytia occurs as a consequence of the cell-to-cell fusion or interactions between the viral envelope glycoprotein of infected cells and the CD4 surface molecules present in uninfected cells. We found that syncytia formation correlated with, the replication of 17 of the 21 (81%) isolates in the cell lines expressing coreceptors. Figure 3F–I shows a representation of the multinucleated giant cells formed as a result of an effective replication in the U87.CD4 lines (R5 and X4). Five of the seven viruses isolated from patients with normal cognition that showed replication in the R5 line also induced syncytia formation. However, two viral isolates (3 and 6) showed a productive infection over time (HIV p24 antigen >0.2 ng/ml), but did not cause any cytopathic effect in these cells (Figure 2). In particular, isolate 3 showed productive infections in all cell types and was characterized as dual-tropic, but it did not induce any cytopathology in the CCR5 cell line. Isolate 6 grew efficiently in mitogen-activated PBMCs, as well as in the CCR5 and CXCR4 cell lines (Table 2), but it did not infect macrophages and did not induce syncytia formation in MT-2, R5, or X4 cell lines.
Figure 3.
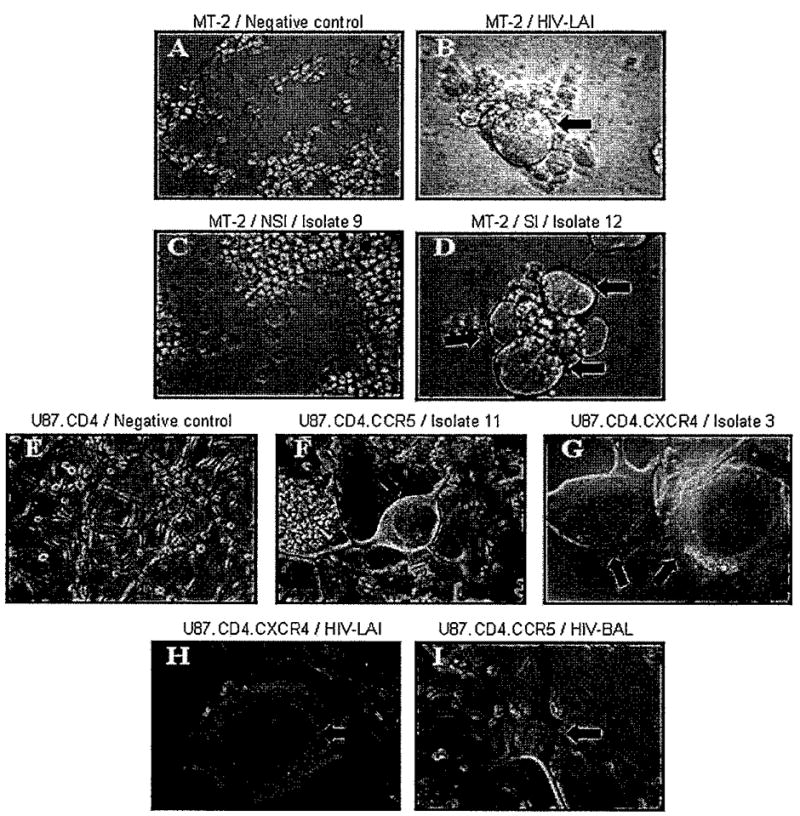
Cytopathic effects of HIV-1 primary isolates in MT-2 and U87.CD4 cell lines. Cytopathic effects for lymphocyte tropism was determined in the MT-2 cell line with the uninfected negative control (A) and the T-tropic isolate as positive control, HIV-1LAI (B). Isolates were characterized as not syncytia-inducing (NSI) (isolate 9) (C) or syncytia-inducing (SI) (isolate 12) (D) variants. Syncytia formation was also used as a parameter to determine coreceptor usage in the U87.CD4 glioma cells with laboratory-adapted isolates HIV-1LAI (H) and HIV-1BAL (I) used as positive controls for the U87.CD4.CXCR4 and U87.CD4.CCR5 lines, respectively. The negative control of the assay is shown in (E). Cells were screened every 3 days for cytopathic effects on U87.CD4.CCR5 (isolate 11) (F) and U87.CD4.CXCR4 (isolate 3) (G) lines. Data are representative from three different experiments performed in duplicate.
Viral isolates from patients with cognitive impairment showed several discrepancies between syncytia formation and coreceptor usage. Of the seven isolates that infected the CCR5 line as determined by high HIV p24 antigen, two (isolates 16 and 17) did not induce syncytia formation in these cells. The cell tropism could not be determined for isolate 16, which (like isolate 6) did not grow in either macrophages or the MT-2 cell line. This isolate grew well on PHA-activated PBMCs, but it did not show any replication in the CXCR4 cells. Isolate 17 showed high replication levels in PHA-activated PBMCs and in MT-2, with no replication in MDMs (Table 2). When characterized for coreceptor usage in the U87.CD4 R5 and X4 cells, it showed high replication levels (HIV p24 > 2 ng/ml) in both cell lines (Table 2), with no syncytia formation in the U87.CD4.CCR5 cell line. A lack of cytopathology was also observed in CXCR4 cells with isolates 11 and 18. Viral isolate 11 was characterized as M-tropic and NSI in the MT-2 cells, but was still considered as dual-tropic because of its high replication levels in both R5 and X4 U87.CD4 lines. However, this virus did not form syncytia in the U87 cultures (Table 2). Isolate 18 was characterized as T-tropic because it showed an effective replication in PHA-activated PBMCs, induced syncytia formation in the MT-2 cells, and did not grow in MDMs. It was also characterized as X4 because it replicated well in the U87.CD4.CXCR4 cells, but did not induce any cytopathic changes in these cells.
Discussion
In this study we tested the hypothesis that HIV tropism and coreceptor usage of the viruses present in the peripheral blood correlate with cognitive function. We found that HIV isolates from women with cognitive impairment did not differ in macrophage and lymphocyte tropism, but the isolates did show significant differences in both their replication capacity and their coreceptor usage, which was predominantly X4 viruses. Our results are in accord with studies demonstrating that HIV tropism and coreceptor usage can be different (Goodenow and Collman, 2006). Although tropism and coreceptor usage are determinants of pathogenesis, our finding of predominant X4 viruses in patients with cognitive impairment has important implications for the use and development of CXCR4 coreceptor-blocking agents to prevent HIV-induced neuropathogenesis (Yi et al, 2005).
We initially performed viral isolation in peripheral blood mononuclear cells (PBMCs) from normal donors by examining replication. We recovered virus from PBMCs of 21 out of 62 (34%) HIV-positive women. The susceptibility of the donor’s PBMCs to infection and the patient’s therapy were factors that possibly contributed to the low viral recovery in this cohort. Studies demonstrate that cell susceptibility to viral infection depends on the genetic variability that affects coreceptor expression, viral entry, and replication (Huang et al, 1996; Samson et al, 1996). In addition, a variety of viral strains develop different levels of replication in each patient, and only the pre-dominant strains are able to infect new and different cells (Steain et al, 2004).
We next determined the predominant viral tropism primarily by examining the replication properties of the primary isolates from women characterized for cognitive function in the lymphocyte cell line, MT-2, and in macrophages (MDMs). Lymphocyte-tropic viruses induced HIV replication and cell fusion in the MT-2 lymphocyte cell line, causing the formation of syncytia. In general, SI variants induce more cytopathic changes than do the NSI (Kwa et al, 2001). Although not significant, we found a higher proportion of SI variants in patients with cognitive impairment. The proportion of macrophage-tropic viruses between patients with normal cognition and cognitive impairment was not significant. The small sample size combined with the low sensitivity of these assays for tropism could contribute to the results reported here.
Macrophages express CD4, CCR5, and low levels of CXCR4, but the ability of a virus to use any of these coreceptors will vary even between macrophage-tropic strains (Bakri et al, 2001; Goodenow and Collman, 2006; Lathey et al, 2000). CCR5 is the major coreceptor used by primary HIV-1 isolates that infect macrophages (M-tropic viruses) and is the major coreceptor used in the primary infection of an individual (Kramer-Hammerle et al, 2005). In late stages of HAD there is a development of strains that preferentially use the CXCR4 (T-tropic viruses) and dual-tropic variants(Bajetto et al, 1999). When we used U87.CD4.R5 and U87.CD4.X4 cell lines to examine the coreceptor usage of the primary isolates, we found that the levels of replication differed between the isolates from the normal cognition and the cognitive impairment groups. The isolates from patients with cognitive impairment showed preference for CXCR4 coreceptor utilization. A switch to CXCR4 and dual-tropic variants could have occurred in patients with cognitive impairment, and this switch may contribute to the HIV neuropathogenesis.
In our study, some HIV isolates showed discrepancies between the predominant tropism observed in the lymphocyte cell lines and MDMs and their coreceptor usage observed in the U87.CD4 cell lines (in either R4 or R5, or in both). For example, viral isolate 3 was characterized as dual-tropic on the basis of its replication capacity in MDMs and its induction of syncytia in MT-2; but when isolate 3 was tested for coreceptor usage, it replicated well only in the U87.CD4.CXCR4 cell line, indicating a preferential X4 coreceptor usage. Thus, this virus could have infected macrophages through the X4 receptor, as has been demonstrated in previous studies (Yi et al, 2003). Two factors might account for a virus’s ability to use the different coreceptors present in a cell. First, each isolate can selectively use the CD4 receptor and coreceptor conformation expressed at different levels in the target cell types (Gorry et al, 2004; Gray et al, 2005; Lee et al, 1999). This can be a limiting factor for viral entry and replication in cell lines used to characterize their coreceptor usage. Second, viral isolates have different molecular abilities to grow in primary target cells and in transfected cell lines. Therefore an isolate that can be characterized as dual-tropic in indicator cell lines will not necessarily be dual-tropic for coreceptor usage in primary cells.
Recent studies indicate that coreceptor usage determination with the U87.CD4 cell lines is the most sensitive method to determine HIV tropism (Poveda et al, 2006). These genetically transformed cells express higher levels of coreceptors than natural target cells such as macrophages or lymphocytes. Thus, Poveda et al (2006) suggested complementing this method with the lymphocyte cell line MT-2 for determination of predominant tropism. We have performed the characterization of HIV isolates with all of these approaches, and indeed the U87.CD4 cell assay, which is linked to coreceptor usage, was more sensitive to the detection of differences related to cognitive function, despite our having a small sample size (Table 2).
When we tested isolates for syncytia formation in MT-2 and in U87.CD4.CCR5 or U87.CD4.CXCR4 cell lines, we found that some isolates that were NSI in the MT-2 cell line induced syncytia in the U87.CD4.CXCR4 line. In addition, 8 of 14 isolates that replicated well in the U87.CD4.CCR5 line did not replicate in MDM, and 2 of 8 isolates that grew well in MDM did not use the R5 receptor. Both of the isolates that grew well in MDM used the X4 coreceptor, suggesting that they infected macrophages through this coreceptor. In addition, we observed that some isolates that produced high HIV p24 antigen levels in the R5 U87.CD4 and/or X4 U87.CD4 lines, did not induce syncytia formation. For example, six viruses (isolates 3, 6, 11, 16, 17, and 18) replicated well in both U87.CD4.CCR5 and U87.CD4.CXCR4, but did not induce syncytia in these cell lines. Therefore, HIV p24 antigen production in these cell lines is a more sensitive method than syncytia formation to determine HIV replication.
Although two different approaches were performed to isolate viruses from the CSF of women with detectable CSF viral load (more than 1000 copies/ml), we were not successful, most likely because of a lower viral load in the CSF than in plasma. Another possibility is that the viruses in the CNS are compartmentalized and sequestered in the brain, or defective owing to the effect of antiretroviral therapy.
One limitation of this study is the small sample size for each cognitive status category reflecting the effects of HAART in decreasing viral load of the 62 patients studied. Despite this limitation, the characterization of these primary isolates showed that HIV patients with cognitive impairment have viral populations in the peripheral blood that exhibit high levels of replication in PHA-activated PBMCs and in CXCR4 cell lines, reflecting a transition to the X4 phenotype. Studies on the effects of antiretroviral therapy on the course of HIV infection show that HAART might cause a switch from R5 to X4 viral variants, a change that can be extremely deleterious to individuals on therapy (Delobel et al, 2005; Johnston et al, 2003). Other studies state that HAART might suppress the emergence of X4 variants and this may give the R5 variants the advantage of being highly replicative (Galan et al, 2004; Skrabal et al, 2003). Our results suggest that antiviral therapy may not be promoting the transition of R5 to X4 because, although both groups of patients were under therapy, the X4 predominance was observed only in the HIV isolates from the patients with cognitive impairment. We observed a tendency toward the predominance of T-tropic strains in patients with cognitive impairment. This finding demonstrates that coreceptor usage cannot always be equivalent to viral tropism despite their relation and importance in HIV pathogenesis. The characterization of HIV variants from our Hispanic women cohort indicates that changes in viral determinants occur in the peripheral blood and could be associated with HIV neuropathology. Further studies are in progress to confirm the neurotoxicity of these HIV isolates and their association with the secretion of toxic factors in the CNS.
Materials and methods
This study was approved by the Institutional Review Board of the University of Puerto Rico, School of Medicine, and was carried out with the informed consent of the participating women.
Patient cohort
This study was conducted as part of the NeuroAIDS Specialized Neuroscience Research Program (SNRP) at the University of Puerto Rico Medical Sciences Campus. HIV-1–seropositive women were recruited from primary HIV clinics at the Puerto Rico Medical Center and the University of Puerto Rico Medical Sciences Campus. The study had the approval of the Institutional Review Board (number 0720102) and was conducted with the informed consent of all participants. HIV was isolated from 21 of 62 HIV-seropositive women who fulfilled the inclusion criteria. The inclusion criteria, exclusion criteria, recruitment, and evaluation have been described previously (Wojna et al, 2004a, 2006). Plasma and CSF viral loads were determined with use of an Ultra-sensitive RNA Roche Amplicor at an ACTG Certified Laboratory with a detection range of 50 to 75000 copies of RNA/ml.
Cognitive impairment was determined in accordance with the American Academy of Neurology HIV associated dementia criteria (1991 (1996) (AAN criteria) (American Academy of Neurology AIDS Task Force), modified (m-AAN criteria) to include an asymptomatic cognitively impaired group (Wojna et al, 2006). The asymptomatic cognitively impaired group is defined as patients with abnormal neuropsy-chological tests (1 SD in two or more tests or 2 SD in one or more tests below the normal control group) but who presented neither functional/emotional nor neurological findings. According to the m-AAN criteria, patients were classified as having normal cognition, asymptomatic cognitive impairment, minor cognitive motor disturbance (MCMD), or HAD (Table 1). For our study, patients with asymptomatic cognitive impairment, MCMD or HAD were grouped together under the term cognitively impaired (CI) and were compared with patients having normal cognition (NC). Of the 21 women whose viruses were isolated, 9 had normal cognition and 12 had cognitive impairment (Table 1). All patients included in this study had negative toxicology results.
Viral isolation
Peripheral blood samples were collected from patients and diluted in phosphate-buffered saline (PBS) and processed by Ficoll-Hypaque density gradient for peripheral blood mononuclear cell (PBMC) isolation, as described previously (Melendez-Guerrero et al, 2001). Plasma from HIV-infected patients was collected after centrifugation and stored frozen for viral load determinations. Patients’ PBMCs were co-cultured with the 3-day mitogen phytohemagglutinin (PHA)-activated HIV-1-negative and CD8-depleted PBMCs for virus isolation at a concentration of 1 × 105/ml in coculture medium (RPMI, 5% interleukin-2, 20% fetal bovine serum). One milliliter of plasma from HIV-infected patients was added to the mitogen-activated PBMCs for viral isolation. Culture supernatants were centrifuged and tested for HIV-1 p24 antigen (Ag) secretion with use of an enzyme-linked immunosorbent assay (ELISA) commercial kit (Coulter, Miami, Fl.) at 14 days post infection. Primary cultures with 50 pg/ml of HIV p 24 antigen were considered as positive in accordance with the ATCG Virology Manual (1994). Viral HIV p24 antigen titers were amplified further by an additional macroculture on mitogen-activated cells, as described previously (Arroyo et al, 2002). For viral tropism studies, a titer of 0.2 ng/ml or higher was considered as positive (Arroyo et al, 2002).
Macrophage and lymphocyte tropism by HIV-1 primary isolates
Peripheral blood monocytes were isolated from healthy donors by Ficoll density gradients (Feige et al, 1982) or by leukopheresis, as described (Weiner and Shah, 1980). Monocytes were found to be ~90% pure as determined by fluorescein isothiocyanate (FITC)-conjugated CD14 staining and flow cytometry analysis before cell plating. We resuspended monocytes at a concentration of 1 × 106 cells/ml in monocyte medium (Dulhecco’s modified Eagle’s medium [DMEM], 1000 U macrophage colony-stimulating factor [M-CSF], 10% human serum, 1% L-glutamine, 50 μg/ml gentamicin, and 10 μg/ml ciprofloxacin) and seeded them in a T75 flask for 7 days. Medium was half-exchanged every 2 to 3 days. At day 7, the supernatant was removed and cells were washed with PBS at room temperature to remove serum. Macrophages (2 × 105) were inoculated with HIV isolates containing 25 ng of p24 antigen per well. Cells were incubated at 37 °C overnight with virus and thereafter each flask was washed with DMEM once with serum-free medium at room temperature to remove unbound virus. After three DMEM washes, cells were resuspended with fresh M-CSF–free medium at the same concentration (1 × 106 cells/ml). Supernatants were collected at 14 days post infection and viral titers were determined by ELISA HIV-1 p24 antigen (Coulter), according to the manufacturer’s instructions.
Lymphocyte tropism was determined with the MT-2 lymphocyte cell line by the induction of syncytia. The assay was performed in duplicate in a 96-well flat-bottom culture plate with the use of cell-free supernatants from plasma or PBMC cocultures derived from study subjects. Samples were incubated for 2 weeks and examined every 3rd day for production of syncytia (giant cells or balloons). Syncytia formation was recorded over 14 days in culture. MT-2 cells were obtained from the NIH AIDS Research and Reference Reagent Program and cultured in RPMI 1640 supplemented with 2 mM l-glutamine, 100 units/ml of penicillin, 100 μg/ml of streptomycin, and 10% fetal bovine serum at a concentration of 3.4 × 105 cells/ml. HIV-1LAI, obtained from the NIH AIDS Research and Reference Reagent Program, was used as the positive control. Cells were examined for syncytia formation on days 3, 6, 9, 12, and 14 post infection. Syncytia induction was considered to have occurred if 3 or more syncytia per well were present.
HIV-1 coreceptor usage
U87.CD4 cell lines were used to determine coreceptor usage of the HIV-1 primary isolates. CCR5 and CXCR4 cell lines were cultured in monolayers and maintained in DMEM (GIBCO-BRL) high glucose and l-glutamine, 15% fetal calf serum (FBS), 10,000 IU/ml Pen-Strep, 1 μg/ml puromycin, 300 μg/ml geneticin (G418), 0.1 mM nonessential amino acids, and 1 mM sodium pyruvate. Medium was exchanged twice weekly. For viral characterization experiments, the cells were seeded in 24-well plates at a concentration of 2.5 × 105 cells/ml of medium and incubated at 5% CO2 at 37 °C until the cultures reached half confluence (after 1 to 3 days). HIVBAL and HIVLAI, acquired from the AIDS Reagent Program, were used as CCR5 and CXCR4 positive controls, respectively. Before infection, cells were washed twice with PBS, inoculated with HIV isolates containing 25 ng/well of HIV p24 antigen, and incubated overnight. Then 1 ml of fresh medium was added, and after 48 h supernatants were collected and cells examined for cytopathology (syncytia formation). Supernatants were collected 8 and 13 days post infection for HIV p24 antigen testing, and cells were examined for cytopathology.
Data analysis
The cohort was stratified according to the presence or absence of cognitive impairment, and analysis of variance was used to compare the two groups by age, CD4 cell count, log10 plasma, and log10 CSF HIV viral load. Because the clinical parameters did not differ significantly between the groups, subsequent analyses were not adjusted for these variables. To determine the potential association of cellular tropism with cognitive impairment, the M-tropic, T-tropic, or dual-tropic HIV isolates derived from each patient group were compared using Fisher’s exact test. Fisher’s was also used to test the association between cognitive status and the proportion of viral isolates that used a particular coreceptor that was X4 predominant, R5 predominant, or dual. Statistical analyses were performed using SAS software version 9.1 (SAS Institute, Gary, NC). Significance testing was carried out using a type I error rate of 0.05.
Acknowledgments
The authors thank their patients for supporting this research. Tania Ginebra and Tania de la Torre provided patient outreach, and Dr. Rosa Hechavarría performed neuropsychological testing. The authors thank Drs. Carmen Zorrilla and Hermes García (clinic directors) for referring patients; the RCMI-Clinical Research Center for providing staff and supplies for laboratory sample collections; and Dr. Howard Gendelman for providing elutriated monocytes for studies of macrophage tropism. The authors acknowledge the Swartz Foundation for support of part of this work. Dr. Edmundo Kraiselburd’s continuous support for this project and the Puerto Rico Specialized Neuroscience Program in NeuroAIDS in general are greatly appreciated. The authors are grateful to the NIH AIDS Research and Reference Reagent Program for providing the MT-2 cells, U87.CD4.CCR5/U87.CD4.CXCR4 cell lines, HIV-BAL and HIV-LAI viral isolates. This work was supported by the following grants: NIH-SNRP1 U54NS430, NIH-MBRS-SCORE-SO6GMO822, NIH-RCMI-CRC P20RR11126, and GM61838 from the MBRS-RISE Program.
References
- American Academy of Neurology AIDS Task Force. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a working group of the American Academy of Neurology AIDS task force. Neurol. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- American Academy of Neurology AIDS Task Force. Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurol. 1996;47:1247–1253. doi: 10.1212/wnl.47.5.1247. [DOI] [PubMed] [Google Scholar]
- Arroyo MA, Tien H, Pagan M, Swanstrom R, Hillyer GV, Cadilla CL, Melendez-Guerrero LM. Virologic risk factors for vertical transmission of HIV type 1 in Puerto Rico. AIDS Res Hum Retroviruses. 2002;18:447–460. doi: 10.1089/088922202753614218. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Piccioli P, Costa A, Florio T, Schettini G. Glial and neuronal cells express functional chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1. J Neurochem. 1999;73:2348–2357. doi: 10.1046/j.1471-4159.1999.0732348.x. [DOI] [PubMed] [Google Scholar]
- Bakri Y, Amzazi S, Mannioui A, Benjouad A. The susceptibility of macrophages to human immunodeficiency virus type 1 X4 isolates depends on their activation state. Biomed Pharmacother. 2001;55:32–38. doi: 10.1016/s0753-3322(00)00015-9. [DOI] [PubMed] [Google Scholar]
- Bannert N, Schenten D, Craig S, Sodroski J. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J Virol. 2000;74:10984–10993. doi: 10.1128/jvi.74.23.10984-10993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger EA, Doms RW, Fenyo EM, Korber BT, Littman DR, Moore JP, Sattentau QJ, Schuitemaker H, Sodroski J, Weiss RA. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Burkala EJ, He J, West JT, Wood C, Petito CK. Compartmentalization of HIV-1 in the central nervous system: role of the choroid plexus. AIDS. 2005;19:675–684. doi: 10.1097/01.aids.0000166090.31693.aa. [DOI] [PubMed] [Google Scholar]
- Chang MI, Panorchan P, Dobrowsky TM, Tseng Y, Wirtz D. Single-molecule analysis of human immunodeficiency virus type 1 gp120-receptor interactions in living cells. J Virol. 2005;79:14748–14755. doi: 10.1128/JVI.79.23.14748-14755.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aversa TG, Eugenin EA, Berman JW. NeuroAIDS: contributions of the human immunodeficiency virus-1 proteins Tat and gp120 as well as CD40 to microglial activation. J Neurosci Res. 2005;81:436–446. doi: 10.1002/jnr.20486. [DOI] [PubMed] [Google Scholar]
- Delobel P, Sandres-Saune K, Cazabat M, Pasquier C, Marchou B, Massip P, Izopet J. R5 to X4 switch of the predominant HIV-1 population in cellular reservoirs during effective highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;38:382–392. doi: 10.1097/01.qai.0000152835.17747.47. [DOI] [PubMed] [Google Scholar]
- Di Stefano M, Monno L, Fiore JR, Buccoliero G, Appice A, Perulli LM, Pastore G, Angarano G. Neurological disorders during HIV-1 infection correlate with viral load in cerebrospinal fluid but not with virus phenotype. AIDS. 1998;12:737–743. doi: 10.1097/00002030-199807000-00010. [DOI] [PubMed] [Google Scholar]
- Diesing TS, Swindells S, Gelbard H, Gendelman HE. HIV-1-associated dementia: a basic science and clinical perspective. AIDS Read. 2002;12:358–368. [PubMed] [Google Scholar]
- Don H, Kingsley JD, Mosley RL, Gelbard HA, Gendelman HE. Neuroprotective strategies for HIV-1 associated dementia. Neurotox Res. 2004;6:503–521. doi: 10.1007/BF03033447. [DOI] [PubMed] [Google Scholar]
- Feige U, Overwien B, Sorg C. Purification of human blood monocytes by hypotonic density gradient centrifugation in Percoll. J Immunol Methods. 1982;54:309–315. doi: 10.1016/0022-1759(82)90315-5. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med. 2005;7:1–26. doi: 10.1017/S1462399405010239. [DOI] [PubMed] [Google Scholar]
- Galan I, Jimenez JL, Gonzalez-Rivera M, De Jose MI, Navarro ML, Ramos JT, Mellado MJ, Gurbindo MD, Bellon JM, Resino S, Cabrero E, Munoz-Fernandez MA. Virological phenotype switches under salvage therapy with lopinavir-ritonavir in heavily pretreated HIV-1 vertically infected children. AIDS. 2004;18:247–255. doi: 10.1097/00002030-200401230-00014. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Anderson E, Meléndez L, Zheng J. Chemokines and their receptors in HIV-1 neuropathogenesis: protection versus injury. New York: Springer; 2006. [Google Scholar]
- Goodenow MM, Collman RG. HIV-1 coreceptor preference is distinct from target cell tropism: a dual-parameter nomenclature to define viral phenotypes. J Leukoc Biol. 2006;80:965–972. doi: 10.1189/jlb.0306148. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Bristol G, Zack JA, Ritola K, Swanstrom R, Birch CJ, Bell JE, Bannert N, Crawford K, Wang H, Schols D, De Clercq E, Kunstman K, Wolinsky SM, Gabuzda D. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol. 2001;75:10073–10089. doi: 10.1128/JVI.75.21.10073-10089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry PR, Sterjovski J, Churchill M, Witlox K, Gray L, Cunningham A, Wesselingh S. The role of viral coreceptors and enhanced macrophage tropism in human immunodeficiency virus type 1 disease progression. Sex Health. 2004;1:23–34. doi: 10.1071/sh03006. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Taylor J, Holm GH, Mehle A, Morgan T, Cayabyab M, Farzan M, Wang H, Bell JE, Kunstman K, Moore JP, Wolinsky SM, Gabuzda D. Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. J Virol. 2002;76:6277–6292. doi: 10.1128/JVI.76.12.6277-6292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L, Sterjovski J, Churchill M, Ellery P, Nasr N, Lewin SR, Crowe SM, Wesselingh SL, Cunningham AL, Gorry PR. Uncoupling coreceptor usage of human immunodeficiency virus type 1 (HIV-1) from macrophage tropism reveals biological properties of CCRS-restricted HIV-1 isolates from patients with acquired immunodeficiency syndrome. Virology. 2005;337:384–398. doi: 10.1016/j.virol.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Harrington PR, Haas DW, Ritola K, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 present in cerebrospinal fluid is produced by short-lived cells. J Virol. 2005;79:7959–7966. doi: 10.1128/JVI.79.13.7959-7966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbitts S, Reeves JD, Simmons G, Gray PW, Epstein LG, Schols D, de Clercq E, Wells TN, Proudfoot AE, Clapham PR. Coreceptor ligand inhibition of fetal brain cell infection by HIV type 1. AIDS Res Hum Retroviruses. 1999;15:989–1000. doi: 10.1089/088922299310502. [DOI] [PubMed] [Google Scholar]
- Huang CC, Tang M, Zhang MY, Majeed S, Montabana E, Stanfield RL, Dimitrov DS, Korber B, Sodroski J, Wilson IA, Wyatt R, Kwong PD. Structure ofaV3-containing HIV-1 gp120 core. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Paxton WA, Wolinsky SM, Neumann AU, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau NR, Phair J, Ho DD, Koup RA. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- Johnston ER, Zijenah LS, Mutetwa S, Kantor R, Kittinunvorakoon C, Katzenstein DA. High frequency of syncytium-inducing and CXCR4-tropic viruses among human immunodeficiency virus type 1 subtype C-infected patients receiving antiretroviral treatment. J Virol. 2003;77:7682–7688. doi: 10.1128/JVI.77.13.7682-7688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis. 2005 Jan;21(1):1–17. doi: 10.1016/j.nbd.2005.07.018. 2006. [DOI] [PubMed] [Google Scholar]
- Korber BT, Kunstman KJ, Patterson BK, Furtado M, McEvilly MM, Levy R, Wolinsky SM. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kwa D, Vingerhoed J, Boeser-Nunnink B, Broersen S, Schuitemaker H. Cytopathic effects of non-syncytium-inducing and syncytium-inducing human immunodeficiency virus type 1 variants on different CD4(+)-T-cell subsets are determined only by coreceptor expression. J Virol. 2001;75:10455–10459. doi: 10.1128/JVI.75.21.10455-10459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathey JL, Brambilla D, Goodenow MM, Nokta M, Rasheed S, Siwak EB, Bremer JW, Huang DD, Yi Y, Reichelderfer PS, Collman RG. Coreceptor usage was more predictive than NSI/SI phenotype for HIV replication in macrophages: is NSI/SI phenotyping sufficient? J Leukoc Biol. 2000;68:324–330. [PubMed] [Google Scholar]
- Lee B, Sharron M, Blanpain C, Doranz BJ, Vakili J, Setoh P, Berg E, Liu G, Guy HR, Durell SR, Parmentier M, Chang CN, Price K, Tsang M, Doms RW. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- Mack M, Pfirstinger J, Haas J, Nelson PJ, Kufer P, Riethmuller G, Schlondorff D. Preferential targeting of CD4-CCR5 complexes with bifunctional inhibitors: a novel approach to block HIV-1 infection. J Immunol. 2005;175:7586–7593. doi: 10.4049/jimmunol.175.11.7586. [DOI] [PubMed] [Google Scholar]
- Marder K, Tang MX, Mejia H, Alfaro B, Cote L, Louis E, Groves J, Mayeux R. Risk of Parkinson’s disease among first-degree relatives: a community-based study. Neurology. 1996;47:155–160. doi: 10.1212/wnl.47.1.155. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Sacktor N, Selnes O. Human immunodeficiency virus-associated dementia. Semin Neurol. 1999;19:129–150. doi: 10.1055/s-2008-1040831. [DOI] [PubMed] [Google Scholar]
- Melendez-Guerrero LM, Arroyo MA, Vega ME, Jimenez E, Hillyer GV, Cadilla CL. Characterization of HIV isolates from Puerto Rican maternal-infant pairs reveal predominance of non-syncytium inducing (NSI) variants with CCR5 genotype. Cell Mol Biol (Noisy-le-grand) 2001;47:OL39–OL47. (published online) [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Neil SJ, Aasa-Chapman MM, Clapham PR, Nibbs RJ, McKnight A, Weiss RA. The promiscuous CC chemokine receptor D6 is a functional coreceptor for primary isolates of human immunodeficiency virus type 1 (HIV-1) and HIV-2 on astrocytes. J Virol. 2005;79:9618–9624. doi: 10.1128/JVI.79.15.9618-9624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai SK, Kosakovsky Pond SL, Liu Y, Good BM, Strain MC, Ellis RJ, Letendre S, Smith DM, Gunthard HF, Grant I, Marcotte TD, Allen McCutchan J, Richman DD, Wong JK. Genetic attributes of cerebrospinal fluid-derived HIV-1 env. Brain. 2006 Jul;129 (Pt):1872–1883. doi: 10.1093/brain/awl136. [DOI] [PubMed] [Google Scholar]
- Poveda E, Briz V, Quinones-Mateu M, Soriano V. HIV tropism: diagnostic tools and implications for disease progression and treatment with entry inhibitors. AIDS. 2006;20:1359–1367. doi: 10.1097/01.aids.0000233569.74769.69. [DOI] [PubMed] [Google Scholar]
- Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth RJ, Collman RG, Doms RW, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Sharma D, Balamurali MM, Chakraborty K, Kumaran S, Jeganathan S, Rashid U, Ingallinella P, Varadarajan R. Protein minimization of the gp120 binding region of human CD4. Biochemistry. 2005;44:16192–16202. doi: 10.1021/bi051120s. [DOI] [PubMed] [Google Scholar]
- Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Walters M, Aguon J, Valcour V. Circulating proviral HIV DNA and HIV-associated dementia. AIDS. 2005;19:45–52. doi: 10.1097/00002030-200501030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Wilkinson D, Reeves JD, Dittmar MT, Beddows S, Weber J, Carnegie G, Desselberger U, Gray PW, Weiss RA, Clapham PR. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois S, Sing T, Chou KG. HIV-1 gp120 V3 loop for structure-based drug design. Curr Protein Pept Sci. 2005;6:413–422. doi: 10.2174/138920305774329359. [DOI] [PubMed] [Google Scholar]
- Skrabal K, Trouplin V, Labrosse B, Obry V, Damond F, Hance AJ, Clavel F, Mammano F. Impact of antiretroviral treatment on the tropism of HIV-1 plasma virus populations. AIDS. 2003;17:809–814. doi: 10.1097/00002030-200304110-00005. [DOI] [PubMed] [Google Scholar]
- Spudich SS, Huang W, Nilsson AC, Petropoulos CJ, Liegler TJ, Whitcomb JM, Price RW. HIV-1 chemokine coreceptor utilization in paired cerebrospinal fluid and plasma samples: a survey of subjects with viremia. J Infect Dis. 2005;191:890–898. doi: 10.1086/428095. [DOI] [PubMed] [Google Scholar]
- Steain MC, Wang B, Dwyer DE, Saksena NK. HIV-1 co-infection, superinfection and recombination. Sex Health. 2004;1:239–250. doi: 10.1071/sh04024. [DOI] [PubMed] [Google Scholar]
- Strain MC, Letendre S, Pillai SK, Russell T, Ignacio CC, Gunthard HF, Good B, Smith DM, Wolinsky SM, Furtado M, Marquie-Beck J, Durelle J, Grant I, Richman DD, Marcotte T, McCutchan JA, Ellis RJ, Wong JK. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol. 2005;79:1772–1788. doi: 10.1128/JVI.79.3.1772-1788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe T, Kishino H, Okuhara Y, Kitazoe Y. Fold recognition of the HIV-1 V3 loop and flexibility of its crown structure during the course of adaptation to a host. Genetics. 2005 Mar;172(3):1385–1396. doi: 10.1534/genetics.105.051508. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner RS, Shah VO. Purification of human monocytes: isolation and collection of large numbers of peripheral blood monocytes. J Immunol Methods. 1980;36:89–97. doi: 10.1016/0022-1759(80)90034-4. [DOI] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Wojna V, Carlson KA, Luo X, Mayo R, Melendez LM, Kraiselburd E, Gendelman HE. Proteomic fingerprinting of human immunodeficiency virus type 1-associated dementia from patient monocyte-derived macrophages: a case study. J Neuro Virol. 2004a;10(Suppl 1):74–81. doi: 10.1080/753312756. [DOI] [PubMed] [Google Scholar]
- Wojna V, Skolasky RL, Hechavarria R, Mayo R, Selnes O, McArthur JC, Melendez LM, Maldonado E, Zorrilla CD, Garcia H, Kraiselburd E, Nath A. Prevalence of hum an immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neuro Virol. 2006;12:356–364. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- Yi Y, Chen W, Frank I, Cutilli J, Singh A, Starr-Spires L, Sulcove J, Kolson DL, Collman RG. An unusual syncytia-inducing human immunodeficiency virus type 1 primary isolate from the central nervous system that is restricted to CXCR4, replicates efficiently in macrophages, and induces neuronal apoptosis. J Neuro Virol. 2003;9:432–441. doi: 10.1080/13550280390218706. [DOI] [PubMed] [Google Scholar]
- Yi Y, Lee C, Liu QH, Freedman BD, Collman RG. Chemokine receptor utilization and macrophage signaling by human immunodeficiency virus type 1 gp120: Implications for neuropathogenesis. J Neuro Virol. 2004;10(Suppl 1):91–96. doi: 10.1080/753312758. [DOI] [PubMed] [Google Scholar]
- Yi Y, Shaheen F, Collman RG. Preferential use of CXCR4 by R5X4 human immunodeficiency virus type 1 isolates for infection of primary lymphocytes. J Virol. 2005;79:1480–1486. doi: 10.1128/JVI.79.3.1480-1486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


