Abstract
A hydrolyzable crosslinker (N,O-dimethacryloylhydroxylamine (MANHOMA)) was synthesized by a modified method and was characterized using 1H-NMR, FTIR, and melting point determination. Naltrexone-loaded nanoparticles were prepared by copolymerization of poly(ethylene glycol)1000 monomethyl ether mono methacrylate (PEO-MA), methyl methacrylate (MMA) and N,O-dimethacryloylhydroxylamine (MANHOMA) in 0.4% poly(vinyl alcohol) aqueous solution. The nanoparticles were characterized by FTIR, particle size determination and transmission electron microscope (TEM). The TEM photomicrographs of the nanoparticles show a crosslinked core surrounded by a ring formed by the polyethylene glycol tail of PEO-MA. The loading efficiency of the nanoparticles and in vitro drug availability from the nanoparticles were investigated. The naltrexone-loaded hydrolyzable crosslinked nanoparticles were able to sustain the release of naltrexone for different periods of time, depending on the monomer feed composition.
Keywords: Hydrolyzable crosslinked copolymer, Nanoparticles, Drug delivery system, Naltrexone
1. Introduction
Alcohol-related deaths account for about 5% of all deaths in USA. Aside from human suffering, which is difficult to quantify, it is estimated that alcohol dependence costs the society about 116 billion dollars per year (Weinreb and O'Brien, 1997). A glance at literature on alcohol dependence will reveal a catalog of medical complications: cardiovascular, neurological, gastrointestinal, immunologic, psychiatric, and obstetric complications. The main approaches to the treatment of alcoholism include detoxification to manage alcohol withdrawal, non-pharmacological (psychosocial) treatment methods, brief interventions that are designed to be delivered by the primary physicians rather than alcoholism treatment specialists, and pharmacotherapy (Fuller and Hiller-Sturmhofel, 1999).
Given the modest results attained with psychosocial treatment, efforts are geared towards adjunctive medications to help prevent early return to excessive alcohol drinking. The medications used or already investigated for the pharmacotherapy of alcoholism can be divided into two major groups: (a) aversive or deterrent medications; (b) medications that reduce alcohol consumption or anticraving medications (Fuller and Hiller-Sturmhofel, 1999; Kranzler, 2000). Only three medications are officially available for the treatment of alcoholism: disulfiram (Antabuse), naltrexone (REVIA®) and calcium acetylhomotaurinate (Acamprosate). Disulfiram is a deterrent medication that alters body's response to alcohol, making its ingestion unpleasant. Consequently, its effectiveness in treating alcoholism is limited, because of the reluctance by the patients to take it. Medications that reduce alcohol consumption or anticraving medications are by-products of advances in neuroscience research in recent past and point to the possibility for the development of medications to improve the effectiveness of concurrent psychosocial or behavioral treatment of alcoholism (Litten et al., 1996; Johnson and Ait-Daoud, 1999). Glutamate antagonist, acamprosate, has been investigated and found to be very promising. The effectiveness of acamprosate in the treatment of alcoholics has been reported by many workers (Paille, et al., 1995; Swift, 1999). Acamprosate has been approved in Europe for the treatment of alcoholism.
Naltrexone is a safe and potent narcotic antagonist used for the treatment of narcotic addiction. Following the encouraging preclinical data indicating that alcohol drinking is modulated by opioid receptor activity and that excessive alcohol drinking could be reduced with opioid antagonists, clinical utility of naltrexone in alcohol dependence has been investigated in clinical samples of alcohol-dependent subjects. O'Brien and his research associates have recently reviewed the pertinent preclinical and clinical research that led to the FDA's review and approval of naltrexone for the adjunctive treatment of alcoholism (Weinreb and O'Brien, 1997). However, oral naltrexone hydrochloride, as REVIA™ tablet, has been associated with a high early drop out rate. Thus the effectiveness of naltrexone is limited by problem with compliance. This problem is important in that alcoholics show particularly low rates of medication compliance. Consequently, there is a need for a new drug delivery system for naltrexone in the treatment of alcohol dependence in the light of variable compliance with oral medication in this patient population: a system that can deliver a lower constant dose for a long period of time. Aside from the issue of compliance, orally administered naltrexone is highly extracted. Thus hepatic metabolism (>98% metabolized) will result in having a very low concentration of the parent drug in the brain (Johnson, 1984). In fact, the bioavailability of an oral naltrexone has been reported to be very low, which is due to the extensive first-pass metabolism (Wall et al., 1981). An injectable long acting delivery system will make naltrexone bypass the liver.
In recent years, there has been increasing impetus for the use of colloidal drug delivery systems (microspheres, nanospheres, liposomes, and block copolymer micelles) for temporal and spatial delivery of bioactive agents, because of their ability to increase therapeutic index and improve the selectivity of drugs. We have embarked on the investigation of colloidal polymeric controlled drug delivery systems for naltrexone for the treatment of alcoholism. The goal is to develop polymeric injectable nano- and/or microparticulate naltrexone controlled delivery systems capable of sustaining availability of naltrexone for a long period of time. Generally, the fabrication of nanoparticles can proceed in one of two major ways: dispersion of preformed polymers and in situ polymerization of monomers (Kreuter, 1994; Fattal and Couvreur, 2000). Further, at the appropriate concentration and when block copolymers with blocks of different solubility properties are placed in a selective solvent for one block, self-assembly into micelles occurs. These micelles are formed from individual block copolymer molecules, each of which contains a hydrophobic and a hydrophilic block. The amphiphilic nature of the block copolymers enables them to self-assemble to form aggregates of various sizes and morphologies in aqueous solution. The hydrophobic blocks form the core of the micelle, which is surrounded by the hydrophilic blocks, which form the outer shell (Zhang and Eisenberg, 1995). The inner core of the micelle creates a hydrophobic microenvironment for the nonpolar drug; while the hydrophilic shell provides a stabilizing interface between the micelle core and the aqueous medium (Allen et al., 1998). The drug delivery systems based on polymeric micelles are generally not stable and drug release is often very fast. The block copolymer micelles are held together by van der Waals forces and need to be stabilized further. One way to achieve this is to crosslink either the core or the shell of the micelles. The synthesis of core-crosslinked poly(butadiene)-b-poly(ethylene oxide) diblock copolymer in aqueous solution has been reported (Won et al., 1999). They obtained cylindrical structures in water as a selective solvent for poly(ethylene oxide). Thrumond et al. (1997) reported on crosslinking of the shell of amphiphilic diblock copolymer micelles. Nanometer-sized spherical particles composed of a core-shell morphology were prepared by intramicellar polymerization of side chain functionalities along the backbone of the hydrophilic block of amphiphilic polystyrene-b-poly(vininylpyridine) block copolymer organized into micellar assemblies. However, no drug was entrapped in the two types of crosslinked nanoparticles. Birrenbach and Speiser (1976) and Seijo et al. (1990) reported on the preparations of drug-loaded crosslinked polymeric nanoparticles by emulsion polymerization of monomers in a continous organic phase (aqueous micelles) and a continous aqueous phase (lipophilic micelles), respectively. The synthesis of crosslinked nano-sized poly(methacrylic acid-gethylene glycol) gels by micellar polymerization was reported recently (Ichikawa and Peppas, 1999). The pH-dependent swelling properties of the nano-sized gels were discussed.
In this work we have studied the use of hydrolyzable crosslinked poly(ethylene glycol-graft-methyl methacrylate) diblock copolymers as nanoparticulate carriers for naltrexone. Naltrexone-loaded hydrolyzable crosslinked poly(ethylene glycol-graft-methyl methacrylate) nanoparticles were synthesized by crosslinking copolymerization of hydrophilic and hydrophobic monomers in an aqueous medium. Naltrexone base could be effectively encapsulated in the crosslinked polymer networks by controlling the polymerization conditions. We report here the synthesis and characterization of naltrexone-loaded hydrolyzable crosslinked nanoparticles.
2. Experimental
2.1. Materials
Methacrylol chloride (98%) obtained from Bimax Corp. and methyl methacrylate (MMA) purchased from Aldrich were vacuum-distilled prior to use. Hydroxylamine hydrochloride (99%, Aldrich), pyridine (99%+, Aldrich), diethyl ether (99.8%, Aldrich), and hydrochloric acid (37%, Fisher Scientific) were used as received. Poly(ethylene glycol)n monomethyl ether mono methacrylate (PEO-MA), (n=1000, Polyscience, Inc.), poly(vinyl alcohol) (PVA, average molecular weight, 30000–70000, Sigma), chloroform (HPLC Grade, Aldrich), and acetone (99.9+%, Aldrich) were used as received. 2,2′-azobisisobutyronitrile (98%, Aldrich) was recrystallized before use.
2.2. Synthesis of hydrolyzable crosslinker (N,O-dimethacryloylhydroxylamine)
We modified the method for the synthesis of N,O-dimethacryloylhydroxylamine reported in the literature (Ulbrich et al., 1993; Akala et al., 1998). To a solution of hydroxylamine hydrochlo-ride (9.0 g) in 72 ml of pyridine, methacrylol chloride (27 ml) was added dropwise with stirring. The temperature of the mixture was kept below 30 °C. The reaction mixture was left at room temperature for 10 h. It was dissolved in 150 ml chloroform and then neutralized with 48 ml HCl. The product was extracted with distilled water (4×150 ml), dried over magnesium sulfate and then dissolved in ether, followed by filtration, drying and recrystallization. Yield: 28%, m.p.: 55±1 °C, 1H-NMR (300 MHz, CDCl3): (C8H11NO3) δ=2.01 [s, 3H, –CO–C(CH3)=CH2], 2.04 [s, 3H, –O–CO–C(CH3)=CH2], 5.6, 5.85 [s, 2H, –O–CO–C(CH3)=CH2], 6.0, 6.4 [s, 2H, –N–CO–C(CH3)=CH2], 9.55 [s, 1H, –CO–NH–O–]. FTIR (KBr pellet): 1770 cm−1 [–C=O, ester], 1665 cm−1 [–C=O, amide], 1095 cm−1 [C–O].
2.3. Synthesis of naltrexone-loaded hydrolyzable crosslinked poly(ethylene glycol-co-methyl methacrylate) (PEO–MMA) nanoparticles
Naltrexone base (0.2 g), methyl methacrylate (MMA) and N,O-dimethacryl oylhydroxylamine (MANHOMA) were dissolved in 2.0 ml CHCl3. The solution was dispersed in 25 ml 0.4% PVA aqueous solution containing PEO-MA in a three-neck flask with rigorous stirring to form an emulsion. PEO-MA and MMA were used at different ratios (1:1 and 1:4). The total weight of the monomers and the crosslinker was 2 g. Nitrogen was bubbled into the three-neck flask to purge it free of oxygen for more than half hour before the mixture was heated to 65 °C. Then 2.0 ml of CHCl3 containing 0.05 g AIBN was added drop wise into the three-neck flask. After polymerization had proceeded for about 1 h, nitrogen was bubbled into it again and the polymerization was allowed to proceed for 19 h. On the completion of the polymerization, the product was opaque. It was filtered with 0.1 μm nylon filter paper (Osmonics Inc.) and washed with distilled water several times to remove residual PVA and free naltrexone. The nanoparticles were dried under vacuum..
2.4. Determination of particle size
The nanoparticles were dispersed in 5 ml of deionized water using a sonicator. Particle size determination was carried out using asymmetric field-flow fractionation (AFFF) coupled with a multi-angle light scattering (MALS) technique (Wyatt technology Corporation, Santa Barbara, California). It is based on light scattering measurement following fractionation. AFFF is a novel chromatographic separation technique used to separate complex fluids or colloidal materials based on differences in size. The particles were separated into aliquots by AFFF, each of which consisted essentially of a single size. Connecting the fractionator output to MALS unit permitted the measurement of the scattered light from each eluting fraction and the determination of the particle size.
2.5. Transmission electron microscopy studies
Transmission electron microscope (TEM) was used to study the structure of the nanoparticles. Nanoparticles were dispersed in distilled water and then resuspended in 0.2% sodium phosphotungstate (PTA) solution for 30 min at room temperature. A droplet of the mixture was then placed on a 200 mesh copper grid coated with 2% collodion. Excess PTA solution was syphoned off with filter paper and the PTA solution allowed to dry on the grid by evaporation. The samples were observed in a Zeiss EM- 1OA electron microscope at magnifications ranging from 10000 to 200000×. The developed negatives were examined and scanned with a DuoScan T1200 (Agfa) in a I-Mac computer equipped with adobe photoshop 6 program.
2.6. FTIR characterization of the nanoparticles
The structures of naltrexone, naltrexone-loaded nanoparticles and nanoparticles without nalrexone were characterized by FTIR. A solution of naltrexone base was cast on potassium bromide plate; while a suspension of the nanoparticles prepared by sonication was also cast on potassium bromide plate. The spectra were recorded on Nicolet 560 FTIR.
2.7. Determination of the loading efficiency of the nanoparticles
The nanoparticles were hydrolyzed for more than 1 month till all the natrexone was released (each sample in triplicate). Then the naltrexone content was analyzed on a C18 reverse-phase Zorbax HPLC column using HP series 1100 system. UV–visible detection was at 280 nm. Mobile phase was monobasic potassium phosphate buffer (pH 7.4)/methanol (85:15). Flow rate was 1.5 ml min−1. Quantitation was carried out according to the standard curve prepared from HPLC analysis of naltrexone base dissolved in buffer (pH 7.4). Encapsulation property of nanoparticles can be expressed as loading capacity (amount of drug encapsulated expressed as a percentage of the nanoaparticles weight) or entrapment efficiency, which is the percentage of the initial drug in the encapsulation solution that is entrapped (Gref et al., 1994). While drug loading can be expressed as loading capacity or encapsulation efficiency for nanoparticles fabricated from prepolymers, loading capacity is appropriate for nanoparticle network made by in situ polymerization.
| (1) |
where Md is the weight of drug (naltrexone) in a known weight (Mn) of the nanoparticle network.
2.8. In vitro availability of naltrexone from the nanoparticles
Naltrexone base-loaded nanoparticles (5 mg) were put in a dialysis bag containing 2 ml buffer solution (pH 7.4) and placed in a 15 ml tube which contained 12 ml buffer solution (pH 7.4) in triplicate. Then each tube was clamped onto a Labquake Tube Shaker (Fisher Scientific) capable of 360° rotation. The temperature was maintained at 37 °C. At predetermined time intervals, 2 ml solution was taken out and replaced with 2 ml fresh buffer solution maintained at 37 °C. The solution was filtered with 0.2 μm filter. The filtrate was analyzed using HPLC as described earlier under the determination of the loading efficiency.
3. Results and discussion
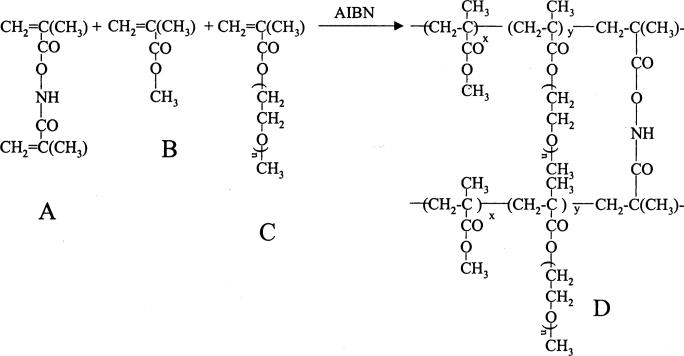
Fig. 1 shows the synthesis of naltrexone-loaded hydrolyzable crosslinked PEO–MMA nanoparticles. The polymerization proceeded by a micellar polymerization mechanism. In 0.4% PVA aqueous solution, AIBN, MMA, naltrexone base, and MANHOMA dissolved in chloroform constituted the organic phase of the micelles formed and stabilized by PEO-MA and PVA. The amphiphilic nature of PEO-MA would make its polymerizable methacrylate moiety extend into the organic phase and its PEO segment would be left in the aqueous phase. Then AIBN would attack the reactive monomers in the micelles to initiate the crosslinking polymerization because of its lipophilic nature. The crosslinking copolymerization of MMA, MANHOMA, and methacrylate moiety of PEO-MA occurred in the micelles. Thus the micelles served as the site of nucleation and polymerization (Jaeghere et al., 1999). Outside the crosslinked-cores would be a hydrophilic shell comprising long chain PEO segments. Consequently, the nanoparticles were found to be easily dispersible in water.
Fig. 1.
Synthesis of naltrexone-loaded hydrolyzable crosslinked PEO–MMA nanoparticles (A=N,O-dimethacryloylhydroxylamine; B=Methylmethacrylate; C=Plolyethyleneglycol monomethylether monomethyacrylate; D=Crosslinked copolymer).
The yields of naltrexone-loaded nanoparticles (the weight of nanoparticles expressed as a percentage of the weight of initial feed composition) at different monomer feed ratios of PEO-MA–MMA are as follows: 4:1 (0%); 1:1 (5%); 1:4(10%) and 0:1(0%). The low efficiency of AIBN as an initiator in an aqueous environment probably precluded the formation of nanoparticles when a high amount of PEO-MA (i.e. PEO-MA–MMA, 4:1) was used. This reasoning is supported by the increase in the yield of the nanoparticles when more of MMA was present in the feed ratio (PEO-MA–MMA 1:4). The absence of stabilization by PEO-MA in PEO-MA–MMA (0:1) monomer feed ratio might have prevented the formation of nanoparticles. The low yields of nanoparticles in the two types of monomer feed composition might be due to low conversion of the monomers and possible formation of a large amount of nanoparticles less than 100 nm (0.1 μm nylon filter paper (Osmonics Inc.)) was used for the recovery of the nanoparticles.
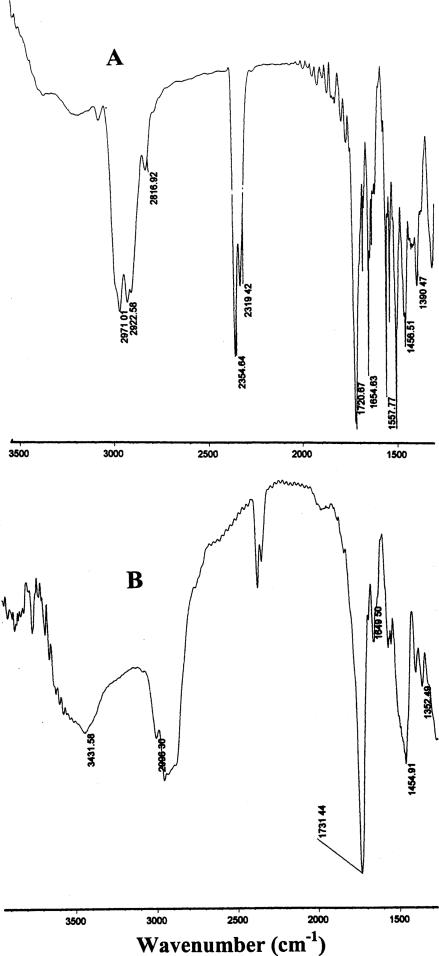
The data obtained on the FTIR experiment are shown in Figs. 2 and 3. Comparing FTIR spectrum of naltrexone base with that of the naltrexone-loaded nanoparticles (Fig. 2), it was found that:
Absorption bands (1655, 1558, 1457 cm−1 assigned to the benzene rings in naltrexone base, (A) were observed in naltrexone-loaded hydrolyzable PEO–MMA nanoparticles; (B) (Fig. 2), but their intensities greatly decreased.
A broad absorption band from 3600 to 3300 cm−1 with a maximum peak at 3431 cm−1 was assigned to a combination of signals from –N–H group in N,O-dimethacryloylhydroxylamine moieties and –O–H group in naltrexone base (Fig. 2B).
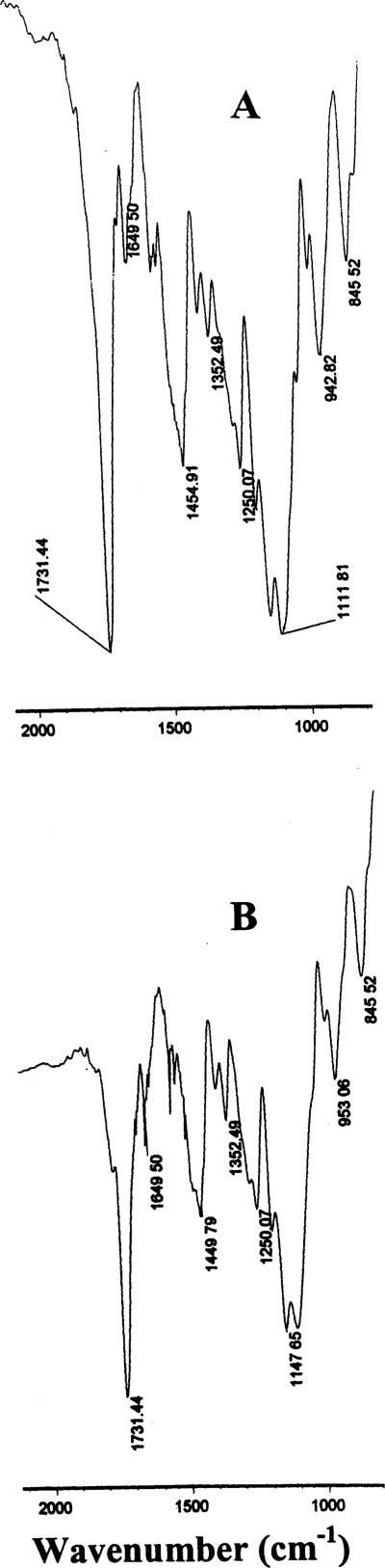
The intensities of absorption bands at 1148 and 1112 cm−1 (Fig. 3B; nanoparticles without naltrexone) which were assigned to O=C–O and C–O were obviously increased (Fig. 3A; nanoparticles containing naltrexone), with a small shoulder occurring at approximately 1080 cm−1. These results indicate that the nanoparticles composed of the crosslinked copolymers (MMA, PEO-MA and N,O-dimethacryloylhydroxylamine moieties) and naltrexone were obtained, as expected; there exist some forms of association between crosslinked copolymer networks and naltrexone base in the nanoparticles.
Fig. 2.
FTIR spectra of naltrexone base (A) and naltrexone-loaded hydrolyzable crosslinked PEO–MMA nanoparticles (B).
Fig. 3.
FTIR spectra of naltrexone-loaded hydrolyzable crosslinked PEO–MMA nanoparticles (A) and hydrolyzable crosslinked PEO–MMA nanoparticles containing no drug (B).
The loading capacities of the nanoparticles were 21.4 and 34%, respectively, for nanoparticles made from PEO-MA–MMA (1:1) and PEO-MA–MMA (1:4). The loading capacity is strongly dependent on the composition of the crosslinked copolymer networks. When the 1:1 weight ratio of PEO-MA and MMA was employed in crosslinking copolymerization, the loading capacity was 34% for naltrexone base was 21.4%. When the weight ratio of PEO-MA and MMA was 1:4, the loading capacity was 21.4%. This might be due to the fact that at the PEO-MA–MMA ratio of 1:1, naltrexone was solubilized due to the presence of a large amount of PEO-MA and consequently, had higher solubility in water; while at the PEO-MA–MMA ratio of 1:4, naltrexone was mainly dissolved in MMA with the formation of organic phase of the micelles, which was the site of nucleation and polymerization.
Multiple light scattering (MALS) is a well-established method for absolute determination of molar masses and root mean square (rms) radii of polymeric materials (Wyatt, 1993). For each eluting fraction or slice across the distribution, the usual light scattering equation (Eq. (2)) can be used to calculate the molar mass (Mw) and the square root of the mean square radius or the rms radius (RG).
| (2) |
where K is the light-scattering constant, c is the sample concentration, and Rθ is the excess Rayleigh ratio (which is proportional to the amount of light scattered in excess of that due to the solvent alone). Pθ is the scattering form factor and it describes the angular dependence of the intensity of scattered light.
For very low concentrations, the second and higher orders of Eq. (2) can be ignored (this condition is valid for fractionated samples or those obtained from size exclusion chromatography (SEC)) and Eq. (2) reduces to Eq. (3).
| (3) |
The form factor or particle scattering function (Pθ), which is a mathematical relationship describing the angular variation of the scattered light intensity as a function of particle size, is often represented as an alternating power series in sin2 θ/2 as shown in Eq. (4) below (Wyatt, 1993).
| (4) |
where kn is a constant whose value depends on which of the higher order terms in Eq. (4) is considered. From this angular dependence of the intensity of the scattered light, the rms radius (RG) can be derived. It is believed that the detailed form of Pθ in Eq. (4) depends on the size of the scatters and their conformation: spheres, rods, and random coils (Kratochvil, 1972; Wyatt, 1993). For a sphere of a characteristic diameter d and which scatters light of wave length λ, Pθ can be expressed as follows:
| (5) |
where,
| (6) |
and d is the diameter of the sphere or particle.
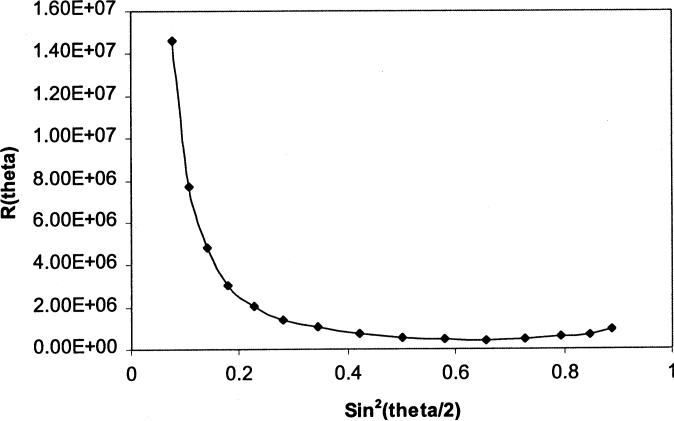
From Eq. (3), for a particular fraction of the eluting sample, Rθ is proportional to Pθ and the quantity KcMw is considered a proportional factor. Consequently, measurement of Rθ at various angles can then be fitted into the appropriate model (i.e. sphere model as shown in Eq. (5)). The astra chromatography software (Wyatt Technology) fits the light scattering data acquired to the appropriate model selected (sphere model in this work) and the rms radius is calculated for the eluting fraction from the initial slope of the angle dependence of the intensity of the scattered light (Kratochvil, 1972). A typical example from our experiment (generated by astra chromatography software) is shown in Fig. 4. Performing a similar fit for all other fractions will yield the average rms radius, which can be used to calculate the diameter of the spheres (dls) based on light scattering.
Fig. 4.
Debye plot for PEO:MMA (1:4) nanoparticles: representation of the angle dependence of the intensity of the scattered light.
It is frequently desirable to compare the diameter of the spherical particle determined by other methods (e.g. TEM) with the diameter of the particle calculated from the light scattering. Consequently, the average rms radius is related to the diameter of the spherical particle by Eq. (7) (Kratochvil, 1972; Roessner and Kulickle, 1994), as shown below:
| (7) |
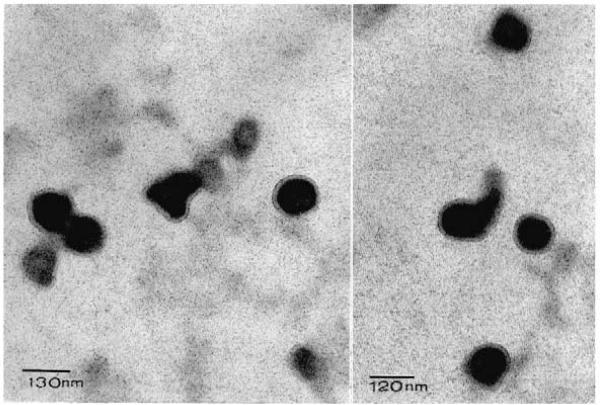
The rms radii of naltrexone-loaded nanoparticles were 141±2.4 and 109±2.87 nm, respectively, for monomer feed ratios (PEO-MA–MMA) of 1:1 and 1:4. The diameter of the nanoparticles calculated from Eq. (6) are as follows: dls=364.06 and 281.44 nm, respectively, for monomer (PEO-MA–MMA) feed ratios of 1:1 and 1:4. Fig. 5 shows the TEM photomicrograph of the nanoparticles obtained with monomer (PEO-MA–MMA) feed ratio of 1:4. It shows a crosslinked core with a ring around it formed from the PEO (Poly(ethylene glycol)1000 monomethyl ether) moiety of PEO-MA. Different particles of same specimen were observed at the same magnification (100000×). Two fields were shown with two scale bars: 120 and 130 nm (Fig. 5). This structure is consistent in all different particles examined under TEM. The data show that the diameters of the nanoparticles as observed under TEM are smaller than the diameter obtained for the same formulation [PEO:MMA(1:4)] from the light scattering experiment. This observation can be accounted for as discussed below. First, the purpose of the TEM experiment was to examine the structure of the most promising batch of the naltrexone-loaded nanoparticles [PEO:MMA(1:4)]. Thus many particles were not examined and characterized in terms of TEM images. Consequently, the determination of the average particle size from TEM experiment is beyond the scope of this work. Second, the smaller size obtained with the TEM might be due to the drying of the particles after staining before examination. It is known that particle shrinkage and distortion often accompany severe preparative processes involved in TEM (Wyatt, 1998). Third, the hydrophilic shell of the nanoparticles formed from polyethylene glycol moiety would swell in water after dispersion before the determination of the rms radius by light scattering (Ichikawa and Peppas, 1999).
Fig. 5.
TEM photomicrographs of naltrexone-loaded hydrolyzable crosslinked PEO–MMA nanoparticles (PEO–MMA, 1:4). Different particles of the same specimen observed at a magnification of 100000×. Two fields are shown with scale bars of 130 and 120 nm.
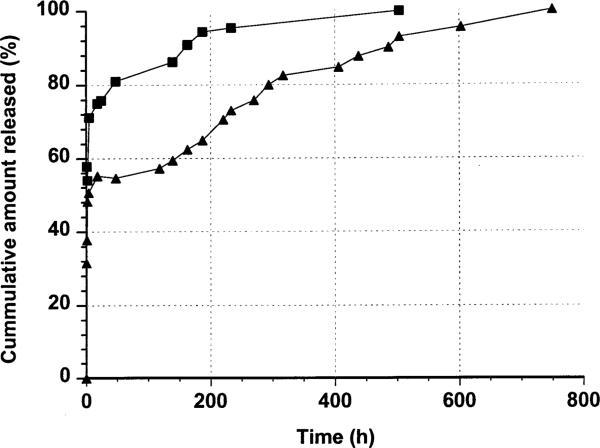
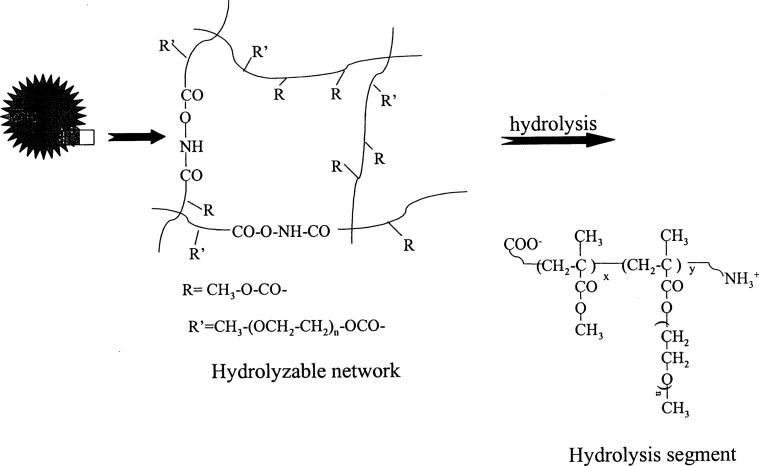
Fig. 6 shows the release isotherms of two naltrexone-loaded hydrolyzable crosslinked PEOMMA nanoparticles. The two nanoparticle systems controlled the release of naltrexone. The release is biphasic. There is an initial rapid release (burst effect) due to the drug embedded on the surface (i.e. embedded on the free PEO tails of hydrolyzable crosslinked PEO–MMA nanoparticles) after the dispersion of the nanoparticles in a buffer solution. The second phase of the release isotherms is due to the hydrolysis of the crosslinked core (Fig. 7) to release the drug encapsulated within the matrix of the nanoparticles: the drug release proceeds more slowly and extends for a long period of time. The hydrolyzable crosslinker in the nanoparticle matrix is susceptible to hydrolysis at pH 7.4 (Ulbrich, et al., 1993; Akala et al., 1998; Akala, 1998) with the production of segments shown in Fig. 7. To make sure the hydrolysis segments will be easily removed from body, 10% w/w of the hydrolyzable crosslinker was introduced into the polymerization system to increase the crosslinking density, (i.e. low Mc (low average molecular weight of the polymer chains between cross-links)). Consequently, a lot of water-soluble groups ( and COO−) would be produced after hydrolysis which associate with water-soluble PEO moieties and then make low molecular weight MMA-containing hydrolysis segments soluble in water. Thus non-hydrolyzable MMA could be easily solubilized in water due to the presence of and COO− groups as well as PEO moieties in the hydrolysis product by controlling the crosslinking density (Fig. 7).
Fig. 6.
In vitro release isotherms of naltrexone-loaded hydrolyzable crosslinked PEO–MMA nanoparticles prepared at different weight ratios of PEO–MMA (■:1/1; ▲: 1/4). Standard deviation ε (0.28; 3.52).
Fig. 7.
Hydrolysis of naltrexone-loaded hydrolyzable crosslinked PEO–MMA nanoparticles to release naltrexone.
The initial burst in PEO–MMA nanoparticles (1:4) was about 30%; while that of PEO–MMA nanoparticles (1:1) was about 50%. This can be explained on the basis of the fact that the core of PEO–MMA nanoparticles (1:4) is more hydrophobic and the surface contains less free PEO tails, thereby permitting less naltrexone to be embedded on the surface of the nanoparticles. Naltrexone-loaded hydrolyzable crosslinked PEO–MMA nanoparticles prepared at weight ratio 1:4 PEO–MMA could sustain the release of the drug for about 750 h, much longer than that of 1:1 weight ratio (about 500 h). Thus the release characteristics were strongly dependent on the ratio of hydrophilic to hydrophobic components. The more hydrophobic crosslinked core of PEO– MMA (1:4) hydrolyzed more slowly and hence could sustain the release of naltrexone for a longer time.
4. Conclusions
Two types of naltrexone-loaded hydrolyzable crosslinked PEO–MMA nanoparticles have been prepared by crosslinking copolymerization of poly(ethylene glycol)1000 monomethyl ether mono methacrylate and methyl methacrylate using N,O-dimethacryloylhydroxylamine as the hydrolyzable crosslinking agent in 0.4% poly(vinyl alcohol) aqueous solution. The properties (e.g. loading capacity, size, and in vitro drug availability) of the naltrexone-loaded hydrolyzable crosslinked PEO–MMA nanoparticles depend on the types of nanoparticles: the monomer feed composition (PEO–MMA 1:1 or 1:4). Nanoparticles with more hydrophobic core (PEO–MMA 1:4) and less hydrophilic shell exhibited a higher loading capacity, less burst effect; and ability to sustain the release of naltrexone for a longer period of time. TEM photomicrograph of the nanoparticles shows a core surrounded by a ring.
Acknowledgements
Supported by NIH Grant #1 R21 AA13407-01 and in part by NIH grant #IU24AAA11898-03. We are indebted to Dr Winston Anderson of the Department of Biology, Howard University, for assistance and discussion on TEM experiment and Dr Andrea Palmer of the Department of Chemistry, Howard University and Dr Michelle Chen of Wyatt Technology Corporation for assistance and discussion on light scattering experiment.
References
- Akala EO. Hydrolysis of linear copolymers containing N,O-dacylhydroxylamine moiety. Pharm. Pharmacol. Lett. 1998;8:129–132. [Google Scholar]
- Akala EO, Kopeckova P, Kopecek J. Novel pH-sensitive hydrogels with adjustable swelling kinetics. Biomaterials. 1998;19:1037–1047. doi: 10.1016/s0142-9612(98)00023-4. [DOI] [PubMed] [Google Scholar]
- Allen C, Yu Y, Maysinger D, Eisenberg A. Polycaprolactone-b-poly(ethylene oxide) block copolymer micelles as a novel drug delivery vehicle for neurotrophic agents FK506 and L-685818. Bioconjugate Chem. 1998;9:564–572. doi: 10.1021/bc9702157. [DOI] [PubMed] [Google Scholar]
- Birrenbach G, Speiser PP. Polymerized micelles and their use as adjuvants in immunology. J. Pharm. Sci. 1976;65:1763–1766. doi: 10.1002/jps.2600651217. [DOI] [PubMed] [Google Scholar]
- Fattal E, Couvreur P. Polymeric nanoparticles and microparticles as carriers for antisense oligonucleotides. In: Couvreur P, Malvy C, editors. Pharmaceutical Aspects of Oligonucleotides. Taylor & Francis; London: 2000. pp. 128–145. [Google Scholar]
- Fuller RK, Hiller-Sturmhofel S. Alcoholism treatment in the United States: an overview. Alcohol Res. Health. 1999;23:69–77. [PMC free article] [PubMed] [Google Scholar]
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Peppas NA. pH-dependent swelling of nano-sized poly(methacrylic acid-g-ehtylene glycol) gels. Polymer Preprints. 1999;40:363–364. [Google Scholar]
- Jaeghere FD, Doelker E, Gurny R. Nanoparticles. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. Vol. 2. Wiley; New York: 1999. pp. 641–664. [Google Scholar]
- Johnson DAW. Observations on the use of long-acting depot neuroleptic injections in the maintenance therapy of schizopherenia. J. Clin. Psychiatry. 1984;5:13–21. [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N. Medications to treat alcoholism. Alcohol Res. Health. 1999;23:99–105. [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR. Pharmacotherapy of alcoholism: gaps in knowledge and opportunities for research. Alcohol Alcohol. 2000;35(6):537–547. doi: 10.1093/alcalc/35.6.537. [DOI] [PubMed] [Google Scholar]
- Kratochvil P. In: Light Scattering from Polymer Solutions. Huglin MB, editor. Academic Press; New York: 1972. pp. 339–340. [Google Scholar]
- Kreuter J. Nanoparticles. In: Swarbrick J, Boylan JC, editors. Encyclopedia of Pharmaceutical Technology. Vol. 10. Marcel Dekker; New York: 1994. pp. 165–190. [Google Scholar]
- Litten RZ, Allen J, Fertig J. Pharmacotherapies for alcohol problems: a review of research with focus on developments since 1991. Alcohol. Clin. Exp. Res. 1996;20:859–876. doi: 10.1111/j.1530-0277.1996.tb05264.x. [DOI] [PubMed] [Google Scholar]
- Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double blind randomized multicenter trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30:239–247. [PubMed] [Google Scholar]
- Roessner D, Kulickle W. On-line coupling of flow field-flow fractionation and multi-angle laser light scattering. J. Chromatogr. A. 1994;687:249–258. [Google Scholar]
- Seijo B, Fattal E, Roblot-Treupel L, Couvreur P. Design of nanoparticles of less than 50 nm diameter: preparation, characterization and drug loading. Int. J. Pharmaceutics. 1990;62:1–7. [Google Scholar]
- Swift RM. Drug therapy for alcohol dependence. New Engl. J. Med. 1999;340:1482–1490. doi: 10.1056/NEJM199905133401907. [DOI] [PubMed] [Google Scholar]
- Thrumond KB, Kowalewski T, Wolley KL. Shell cross-linked knedels: a synthetic study of the factors affecting the dimensions and properties of amphiphilic core-shell nanospheres. J. Am. Chem. Soc. 1997;119:6656–6665. [Google Scholar]
- Ulbrich K, Subr V, Seymour LW, Duncan R. Novel biodegradable hydrogels prepared using divinyl crosslinking agent N,O-dimethacryloylhydroxylamine 1. Synthesis and characterization of rates of gel degradation and rate of release of model drugs in vitro and in vivo. J. Control. Release. 1993;24:181–190. [Google Scholar]
- Wall ME, Brine DR, Perez-Reyes M. Metabolism and disposition of naltrexone in man after oral and intravenous administration. Drug Metab. Dispos. 1981;9:370–375. [PubMed] [Google Scholar]
- Weinreb RM, O'Brien CP. Naltrexone in the treatment of alcoholism. Annu. Rev. Med. 1997;48:477–487. doi: 10.1146/annurev.med.48.1.477. [DOI] [PubMed] [Google Scholar]
- Won Y-Y, Davis HT, Bates FS. Giant wormlike rubber micelles. Science. 1999;283:960–963. doi: 10.1126/science.283.5404.960. [DOI] [PubMed] [Google Scholar]
- Wyatt PJ. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta. 1993;272:1–40. [Google Scholar]
- Wyatt PJ. Submicrometer particle sizing by multipleangle light scattering following fractionation. J. Colloid Interf. Sci. 1998;197:9–20. doi: 10.1006/jcis.1997.5215. [DOI] [PubMed] [Google Scholar]
- Zhang L, Eisenberg A. Multiple morphologies of ‘crew-cut’ aggregates of polystyrene-b-poly(acrylic acid) block copolymers. Science. 1995;268:1728–1731. doi: 10.1126/science.268.5218.1728. [DOI] [PubMed] [Google Scholar]