Abstract
A new MPA assay based on the enzymatic activity of recombinant IMPDH II (the pharmacological target of MPA) with excellent correlation with HPLC has recently been released for the measurement of MPA plasma levels. This study aimed to: (i) compare this new assay with LC-MS/MS for MPA pharmacokinetic studies in different populations of allograft recipients given mycophenolate mofetil (MMF); (ii) develop specific Bayesian estimators for this inhibition assay and test their accuracy; and (iii) compare the resulting MPA area under the curve (AUC0–12h) estimates with those of Bayesian estimators developed based on the LC-MS/MS results.
Sixty-four adult or pediatric, renal or lung transplant patients who were administered MMF in association with cyclosporine, tacrolimus or sirolimus at different post-transplant periods were enrolled as part of different PK studies. 894 patients’ samples were analyzed in parallel with the enzymatic MPA assay and a reference LC-MS/MS method. Repeated analysis of quality control samples showed a mean difference of 6% between the two assays, while the results obtained in different populations of transplanted patients showed excellent correlation (r2 > 0.96) and small mean relative differences (2.0 to 16.9%). The full profiles obtained with both assays were adequately fitted using either a two-compartment model with one “gamma” absorption phase, or a one-compartment model with two gamma inputs. Several PK parameters were significantly affected by the analytical method used. Accurate Bayesian estimators could be specifically developed for the enzymatic MPA assay, using the same three concentration-time points (20 min, 1h, 3h post-dose) as with LC-MS/MS, with a median bias versus reference (trapezoidal) AUC0–12h values of −1.3% (range −45.2 to 40.4%) and 83% of the patients within ± 20% of the reference. These Bayesian estimates were significantly higher than those obtained with LC-MS/MS in patients on cyclosporine or sirolimus, but not in patients on tacrolimus.
Keywords: Adult; Anti-Bacterial Agents; pharmacokinetics; Child; Cyclosporine; pharmacokinetics; Dose-Response Relationship, Drug; Drug Interactions; Female; Graft Survival; immunology; Humans; Immunosuppressive Agents; pharmacokinetics; Kidney Transplantation; Male; Mycophenolic Acid; analogs & derivatives; pharmacokinetics; Sirolimus; pharmacokinetics; Tacrolimus; pharmacokinetics; Transplantation, Homologous
Keywords: mycophenolic acid, monitoring, enzymatic assay, LC-MS/MS, Bayesian estimators.
Introduction
Mycophenolate (MPA) is an antiproliferative agent that acts by uncompetitive, reversible and selective inhibition of type II inosine monophosphate dehydrogenase (IMPDH II), a key enzyme in the de novo biosynthesis of purines (1). MPA is metabolized by glucuronidation primarily to MPA-phenyl-glucuronide (MPAG), which is pharmacologically inactive (2), as well as to the presumably toxic MPA-acyl-glucuronide (AcMPAG) (3–5). A wide interpatient and intrapatient variability was reported for MPA pharmacokinetics (6–8), leading to the recommendation of therapeutic drug monitoring of mycophenolate mofetil, preferably based on MPA inter-dose area under the concentration-time curve (AUC0–12h) (9,10).
However, differences in analytical techniques were found to affect plasma MPA measurement. Previous comparative studies between the enzyme-multiplied immunoassay technique (EMIT, Dade-Behring) and LC-MS/MS (11,12) for the determination of mycophenolate (MPA) in renal transplant patients showed that: when mycophenolate mofetil (MMF) was associated with cyclosporine, EMIT significantly overestimated MPA levels in clinical samples (mean +61.4%, SD 57.94%; median 46.51%, range −27.7 to +422.8%), with large variations depending on patients, time elapsed since transplantation, sampling time and concentration levels (11); when MMF was combined with sirolimus, the EMIT kit gave a significant but lesser overestimation of 18.7 ± 26.8% as compared to LC-MS/MS, also with variations depending on post-transplantation periods (12). Therefore HPLC-UV and LC-MS/MS have so far been the standard technologies used for MMF therapeutic drug monitoring. Roche Diagnostics designed and recently released a new MPA assay based on the enzymatic activity of recombinant IMPDH (the pharmacological target of MPA), the Roche Mycophenolic Acid™ assay (called herein ‘enzymatic MPA assay’), which was recently shown to precisely measure total MPA plasma levels in the range of 0.31 to 15 mg/l with excellent correlation with HPLC and LC-MS/MS methods and only 5% cross-reactivity with AcMPAG, though it tended to overestimate MPA concentrations near the quantification limit (13).
A multi-centre, randomized, prospective trial in 137 de novo renal transplant recipients showed that MMF dose adjustment based on MPA AUC0–12h resulted in significantly less rejections in the first year post-transplantation (14). This clinical trial was conducted using pharmacokinetic models and Bayesian estimators specifically set up for plasma MPA levels as determined using HPLC (15,16), which are now available on the ISBA (ImmunoSuppressants Bayesian Adjustment) website (at https://pharmaco.chu-limoges.fr/abis.htm) (17). The larger, comparative, randomized FDCC trial did not show significant differences between the concentration-controlled and the fixe-dose arms, but the renal transplant patients were much more diverse (adults and pediatrics, on cyclosporine or tacrolimus, with MPA measurements made by HPLC or EMIT) and the tools used for dose adjustment (i.e., multilinear regression equations) were probably not as robust and accurate as Bayesian estimation (18). On top of that, physician’s compliance with dose adjustment proposals was only 52% in FDCC (18), versus 85% on average in the smaller trial (14).
The aims of the present study were: (i) to further evaluate the enzymatic MPA assay as compared to a reference LC-MS/MS technique for MPA pharmacokinetic studies in different populations of allograft recipients given MMF; (ii) to develop specific Bayesian estimators for this enzymatic MPA assay and test their accuracy; and (iii) to compare the resulting MPA AUC0–12h estimates with those of Bayesian estimators developed for LC-MS/MS in the same patients’ groups.
Material & methods
Patients and samples
Plasma samples were collected from 64 patients who gave their informed consent to participate in one of various pharmacokinetic studies approved by regional ethic committees and authorized by the Agence Française de Sécurité Sanitaire des Produits de Santé, the French Drug Agency. Sampling times ranged from pre-dose to 6–12 h post-dose, at post-transplant periods varying from day 7 to several years. Twenty profiles came from 20 pediatric renal transplant recipients on cyclosporine (beyond one year post-transplantation), 16 profiles from 10 adult renal transplants on cyclosporine (10 early, i.e. within the first three months post-transplant, and 6 late – or stable - periods), 30 profiles from 10 adult renal transplants on tacrolimus (collected at D7, M1 and M3), 28 profiles from 14 adult renal transplants on sirolimus (stable post-transplant periods) and 14 profiles from 10 adult lung transplants on tacrolimus (early and stable post-transplant periods).
All samples were kept frozen for a period of time ranging from 2 months to 2 years before the comparison study.
Analytical methods
The enzymatic MPA assay, based on a two-reagent system, was run on a COBAS INTEGRA 400 system. After sample addition to the reaction mixture, MPA in the sample inhibits IMPDH II that normally catalyzes the conversion of IMP (inosine monophosphate) and NAD (nicotinamide adenine dinucleotide) to XMP (xanthosine monophosphate) and NADH. The enzymatic reaction is monitored by measuring the rate of NADH formation at 340 nm (13). MPA concentration is inversely proportional to the rate of NADH formation. The measuring range used in this study was 0.4 to 15 mg/L. Using control samples for total MPA at three levels (0.8, 3.5 and 12 mg/L), the intra-assay CV% was 1.0%, 0.6% and 1.1%, respectively (n = 21).
The LC-MS/MS technique employed was previously described in detail (11). Briefly, to 100 μL of plasma were added 50 μL of indomethacin (internal standard) at 50 mg/L and 250 μL of a mixture of acetonitrile and 99% pure formic acid (97/3, v/v). After vortex-mixing and centrifugation, 3 μL of the supernatant were injected into a Nucleosil C18, 5 μm (150 × 1 mm ID.) column. The mobile phase, delivered at a flow-rate of 50 μL.min−1, was a gradient of acetonitrile in 5mmol/L, pH 3.0 ammonium formate. Detection was performed using a 2000 Q-TRAP tandem mass-spectrometer (Applied-Biosystems/Sciex, Foster, CA, USA) in the negative ion, multiple reaction monitoring mode using the following transitions: MPA (m/z −319 → −191 for quantification and m/z −319 → −275 for confirmation) and indomethacin (m/z −356 → −312 for quantification and m/z −356 → −297 for confirmation). The limit of detection was 0.05 mg/L and the limit of quantitation (LLOQ) 0.1 mg/L. Linearity was verified up to 30 mg/L (r = 0.999). The within-day and between-day precision (CV% < 10% and < 15%, respectively) and accuracy (mean bias < 7% for both) were satisfactory over the calibration range. Samples with MPA concentration > 30 mg/L (upper limit of quantitation) were diluted 1/2 with blank human plasma and re-analyzed.
Method comparison
The three QC samples provided by Roche Diagnostics for the enzymatic MPA assay and two commercial QC samples for MPA monitoring (ChromSystems, level I: 1.8 mg/L; and level II: 4.86 mg/L) were analyzed with each batch of patients’ samples using both assays, for cross-checking.
Furthermore, six external quality control samples from the International Mycophenolate Proficiency Testing Scheme (3 pools of blank plasma spiked to a known concentration of MPA and 3 pools of plasma from treated patients) were purchased from Analytical Services International (London, UK) in sufficient volumes and analyzed by both methods with 15 different batches of patients’ samples.
Patients’ plasma samples were thawed in batches and analyzed in parallel, on the same day, with both methods. For the enzymatic MPA assay, samples with MPA concentration above 15 mg/L (upper limit of quantitation) were diluted 1/4 with blank human plasma and re-analyzed.
PK modeling and development of Bayesian estimators
Two previously published PK models able to fit MPA absorption profiles were tested (15): a two-compartment open model with first order elimination and a single gamma input to describe the absorption phase; and a one-compartment open model with first order elimination and a sum of two gamma distributions to describe the absorption phase, theoretically able to describe the double-peak concentration-time curves frequently observed with MPA.
Population parameters were estimated using the iterative 2-stage method (ITS) as implemented in an in-house computer program. Bayesian fitting of the PK model to each individual data set (all available concentrations) employed the simplex algorithm to minimize the individual objective function value:
where Ci denotes an observed concentration, Si its standard deviation, Ei the corresponding theoretical concentration, θ the vector of fitted parameters, μ the mean parameter values in the reference population, and Σ−1 the inverse variance-covariance matrix. The symbol T denotes matrix transposition. The calibration experiments showed that Si could be expressed as a power function: Si = 0.015×Ci1.2, where Ci and Si are in mg/L. The program used the individual parameter estimates together with their variance-covariance matrix to generate a new set of population parameter estimates. These population estimates were then used as Bayesian priors and refined by successive population iterations until convergence.
For each PK profile, the two PK models (i.e., the two-compartment model with one gamma law and the one-compartment model with two gamma laws) were applied and the best one was selected based on the lowest Akaike criterion (19), calculated as follows:
where Φmin is the value of the objective function at minimum and p the number of parameters in the model.
Using the appropriate pharmacokinetic model and the final set of population parameter estimates (mean, standard deviation and correlation matrix), maximum a posteriori Bayesian estimators (MAP-BE) were developed as previously described (16,20), for each group of patients defined by the associated immunosuppressant and post-transplantation period. Every combination of 3 sampling times within the first 3 hours post-dose was tested for MAP-Bayesian forecasting.
Statistical analyses
Method comparison was performed globally, as well as separately for the different sub-populations. Correlation analysis was conducted using the non-parametric Passing-Bablok method (21). The relative difference was calculated as (enzymatic MPA assay-LC-MS/MS)/LC-MS/MS, and normalized relative difference as (enzymatic MPA assay-LC-MS/MS)/mean (enzymatic MPA assay and LC-MS/MS).
For each individual post-transplantation period, the best limited sampling strategies (LSS) for Bayesian estimation were selected on the basis of: (i) the best squared correlation coefficients (r2); and (ii) the lowest bias between observed and estimated AUC0–12h (AUC being the main index targeted). Then, for practical reasons, a common LSS over the 3 periods was sought and evaluated. The whole procedure was repeated for MPA PK profiles generated using the enzymatic MPA assay and LC-MS/MS.
The influence of age, transplanted organ, associated drug and post-transplantation period on the relative differences between the two techniques was tested using one-way ANOVA, then two-by-two comparisons for the influence of the associated immunosuppressive drug by the Scheffe test.
Finally, the PK parameters obtained with the enzymatic MPA assay and LC-MS/MS at different post-transplantation periods on the one hand, and the respective Bayesian AUC0–12h estimates on the other hand were compared using paired t-tests.
All statistic analyses were performed using Statview 5.0 (SAS Institute Inc., USA).
Results
The cross-checking of internal quality control samples analyzed routinely with the batches of patients’ samples showed that there was no systematic difference between the enzymatic MPA assay and LC-MS/MS and that the inter-assay CVs obtained with the former were always less than those obtained with the latter (table I). Moreover, repeated analysis (15 or 16 replicates) of spiked or patients’ samples from the Mycophenolate International Proficiency Testing Scheme showed a mean difference ≤ 6% between the two assays and lower CV% values with the enzymatic MPA assay than with LC-MS/MS, except for the spiked sample at the highest concentration (table II). The MPA concentrations as measured by LC-MS/MS in 894 patients samples taken over dosing intervals ranged between BLOQ (i.e., below 0.1 mg/L) and 33.4 mg/L. The concentration measured by the enzymatic MPA assay was lower than 0.4 mg/L, limit of quantitation of the assay, in 29 samples. When comparing the results obtained for the remaining 865 patients’ samples, the mean overestimation of the enzymatic MPA assay was 7.8%±12.7% (median 7%, range −19.1 to + 54.5%), translating into mean = 6.8%±11.7% (median 6.8%, range −21.2 to + 42.8%) for the normalized relative difference (enzymatic MPA assay – LC-MS/MS)/mean (enzymatic MPA assay and LC-MS/MS), with r = 0.9839 and more than 95% samples within −20% to +30% (figure 1).
Table I.
Cross-checking of internal quality controls analysed using the enzymatic MPA assay and LC-MS/MS in parallel.
| Method | IQC sample | Target (mg/L) | N = | Median concentration (range) (mg/L) | Coefficient of variation (%) | Median accuracy (range) (%) |
|---|---|---|---|---|---|---|
| Enzymatic MPA assay | TDM Online 1 | 0.8 | 45 | 0.85 (0.79 – 0.93) | 4.2% | 106.3 (98.8 – 116.3) |
| TDM Online 2 | 3.5 | 45 | 3.54 (3.43 – 3.71) | 1.8% | 101.1 (98.0 – 106.0) | |
| TDM Online 3 | 12.0 | 45 | 12.0 (11.38 – 12.55) | 2.5% | 100.0 (94.8 – 104.6) | |
| LC-MS/MS | TDM Online 1 | 0.8 | 44 | 0.81 (0.69 – 0.94) | 8.4% | 101.3 (86.0 – 117.4) |
| TDM Online 2 | 3.5 | 45 | 3.39 (3.00 – 3.91) | 7.1% | 96.9 (85.7 – 111.7) | |
| TDM Online 3 | 12.0 | 45 | 11.60 (10.30 – 14.30) | 8.6% | 96.7 (85.83 – 119.17) | |
| Enzymatic MPA assay | ChromSystem 1 | 1.8 | 68 | 2.08 (1.80 – 2.22) | 4.5% | 115.6 (100.0 – 123.3) |
| ChromSystem 2 | 4.86 | 68 | 6.22 (5.57 – 6.59) | 4.1% | 128.0 (114.6 – 135.6) | |
| LC-MS/MS | ChromSystem 1 | 1.8 | 68 | 1.94 (1.56 – 2.14) | 7.1% | 107.8 (86.7 – 118.9) |
| ChromSystem 2 | 4.86 | 67 | 5.16 (4.27 – 5.77) | 6.5% | 106.2 (87.9– 118.7) | |
Table II.
Analysis of blinded control samples from the Mycophenolate International Proficiency Testing Scheme (www.bioanalytics.co.uk) by the enzymatic MPA assay and LC-MS/MS in parallel.
| M22A Patient pool | M23A Patient pool | M30A Spiked sample | M30 B Spiked sample | M31A Patient Sample | M32 B Spiked sample | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enzymatic MPA assay | LC-MS/MS | Enzymatic MPA assay | LC-MS/MS | Enzymatic MPA assay | LC-MS/MS | Enzymatic MPA assay | LC-MS/MS | Enzymatic MPA assay | LC-MS/MS | Enzymatic MPA assay | LC-MS/MS | |
| Number of replicates | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 15 | 15 | 15 | 15 |
| Median (mg/L) (range) | 1.55 (1.45–1.62) | 1.58 (1.34–1.76) | 1.58 (1.50–1.64) | 1.51 (1.30–1.86) | 3.66 (2.60–3.78) | 3.58 (2.92–3.88) | 1.13 (1.06–1.20) | 1.09 (0.90–1.20) | 1.86 (1.80–1.91) | 1.75 (1.58–1.95) | 10.47 (10.08–14.14) | 10.65 (9.23–11.30) |
| Mean (mg/L) | 1.55 | 1.57 | 1.57 | 1.53 | 3.59 | 3.52 | 1.13 | 1.06 | 1.85 | 1.76 | 10.89 | 10.40 |
| CV% | 2.8 | 8.7 | 2.67 | 10.7 | 7.6 | 9.4 | 3.8 | 8.4 | 1.7 | 6.0 | 12.2 | 6.9 |
Figure 1.
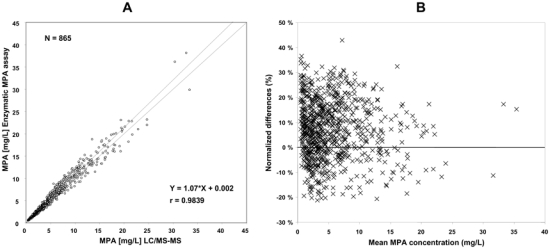
Comparison of the Roche enzymatic MPA assay run on the COBAS INTEGRA system with LC-MS/MS using plasma samples with MPA concentration > 0.4 mg/L, as determined by the enzymatic MPA assay. A) Passing-Bablok correlation analysis. B) Normalized relative differences (i.e., (enzymatic MPA assay – LC-MS/MS)/mean of (enzymatic MPA assay and LC-MS/MS) plotted against increasing mean concentration, as represented by sequence number.
Though samples above 15 mg/L (n = 47) were determined with the same accuracy and precision after dilution with blank plasma as samples in the linearity range (figure 1), method comparison within the different sub-groups defined by age, transplanted organ, co-administered immunosuppressant and post-transplantation period (table III) were limited to the 818 samples with concentration values within the linearity range of the enzymatic MPA assay (0.4 to 15 mg/L), in order to avoid sample dilution as a confusing factor. They showed that the agreement between the two methods was excellent in all cases, with slightly though significantly higher overestimation of the enzymatic MPA assay in adults than in children, in patients on cyclosporine than in those on tacrolimus or sirolimus, and in the stable than in the early (i.e., within the first three months) post-transplantation periods (table III). In contrast, there was no significant difference between lung- and kidney transplant recipients.
Table III.
Summary of correlation and bias analysis
| Population (number of patients) | N° samples | Regression equation | r | Median normalized relative difference (range) (%) |
|---|---|---|---|---|
| All (n = 64) | ||||
| MPA > 0.4 mg/L# | 865 | Y = 1.070 * X + 0.002 | 0.9839 | 6.8 (−21.2; 42.8) |
| 0.4 mg/L<MPA<15 mg/L# | 818 | Y = 1.076 * X − 0.013 | 0.9806 | 7.0 (−21.2; 42.8) p = 0.1134 (NS) |
| Age | ||||
| paediatric*(N = 20) | 96 | Y = 1.016 * X − 0.001 | 0.9678 | 1.5 (−21.2; 26.9) |
| adults (N = 44) | 722 | Y = 1.083 * X + 0.011 | 0.9831 | 7.7 (−20.2; 42.8) p<0.0001 |
| Transplanted organ | ||||
| kidney (N = 34) | 694 | Y = 1.079 * X − 0.031 | 0.9783 | 6.7 (−21.2; 42.8) |
| lung (N = 10)§ | 109 | Y = 1.045 * X + 0.048 | 0.9898 | 8.1 (−14.0; 33.7) p = 0.2251 (NS) |
| Associated IS (N = 34 adult renal transplant recipients) | ||||
| tacrolimus (N = 10) | 356 | Y = 1.038 * X + 0.04 | 0.9848 | 5.4 (−20.2; 36.6) |
| cyclosporine (N = 10) | 233 | Y = 1.123 * X − 0.012 | 0.9704 | 10.7 (−21.2; 42.8) |
| sirolimus (N = 14) | 229 | Y = 1.117 * X − 0.153 | 0.9838 | 6.9 (−18.8; 35.3) p<0.0001 |
| Post-transplantation period (N = 44 adults) | ||||
| early (< 3 months) | 344 | Y = 1.108 * X − 0.022 | 0.9813 | 9.3 (−20.2; 42.8) |
| stable (≥ 3 months) | 378 | Y = 1.064 * X − 0.001 | 0.9848 | 6.1 (−20.2; 36.6) p=0.0040 |
As determined using the enzymatic MPA assay
All paediatric patients were renal transplant recipients
All lung transplant recipients were on tacrolimus
In the study database, 68 PK profiles containing at least 10 concentration-time points could be used to develop PK models and Bayesian estimators. These PK profiles analyzed with both the enzymatic MPA assay and the LC-MS/MS technique were adequately fitted using the same types of PK models as those previously reported for MPA (16), though with a different equation for the analytical error, specifically calculated from the assay validation data. In the vast majority of cases, the one-compartment open model with two gamma laws (i.e., two peaks) best fitted the profiles in the early post-transplantation period and the two-compartment open model with one gamma law (i.e., one peak) those in the stable post-transplantation period. Considering this database (n = 668 and 743 concentration values for LC-MS/MS and for the enzymatic MPA assay, respectively), excellent correlation was found between observed and modelled MPA plasma concentrations (r2= 0.93 for both methods). However, significant differences in the PK parameter values were found between the models developed for the enzymatic MPA assay and those for LC-MS/MS in patients on cyclosporine: 1 out of 7 parameters in the stable post-transplantation period (namely C0; model with one peak) and 4 out of 8 parameters in the early post-transplantation phase (namely C0, a1, a2 and b2; model with two peaks) were significantly different between the two techniques (p<0.01). No significant difference was found in patients on sirolimus or tacrolimus.
Using these PK models, specific Bayesian estimators were then developed for accurate estimation of MPA AUC0–12h as measured with the enzymatic MPA assay, as well as with LC-MS/MS in the same groups of patients. The best sampling strategy over the different post-transplantation periods and across transplant groups comprised three time points at 20 min, 1h and 3h post-dose, for both analytical techniques.
For the enzymatic MPA assay, depending on the post-transplantation period and concomitant immunosuppressive drug, the median bias of the Bayesian versus reference (trapezoidal) AUCs was −1.3 % (range −45.2 to 40.4%), with 83% of the patients with AUC bias < 20% (figure 2). Examples of concentration-time profiles of MPA measured using the enzymatic MPA assay and estimated using the Bayesian method and the 20 min-1h–3h limited sampling schedule are shown in figure 3. The relative difference in AUC estimates obtained using the enzymatic MPA assay or LC-MS/MS was 12.3 ± 15.3 % on average. The individual relative differences were highest and significant in patients given cyclosporine (13.3 – 20.4%; p<0.01), moderate and still significant in those on sirolimus (10.1 – 10.8%; p<0.01), lower and not significant in patients given tacrolimus (4.1 – 9.1%; n.s). Figure 4 shows the Bland-Altman (22) representation of these relative differences.
Figure 2.
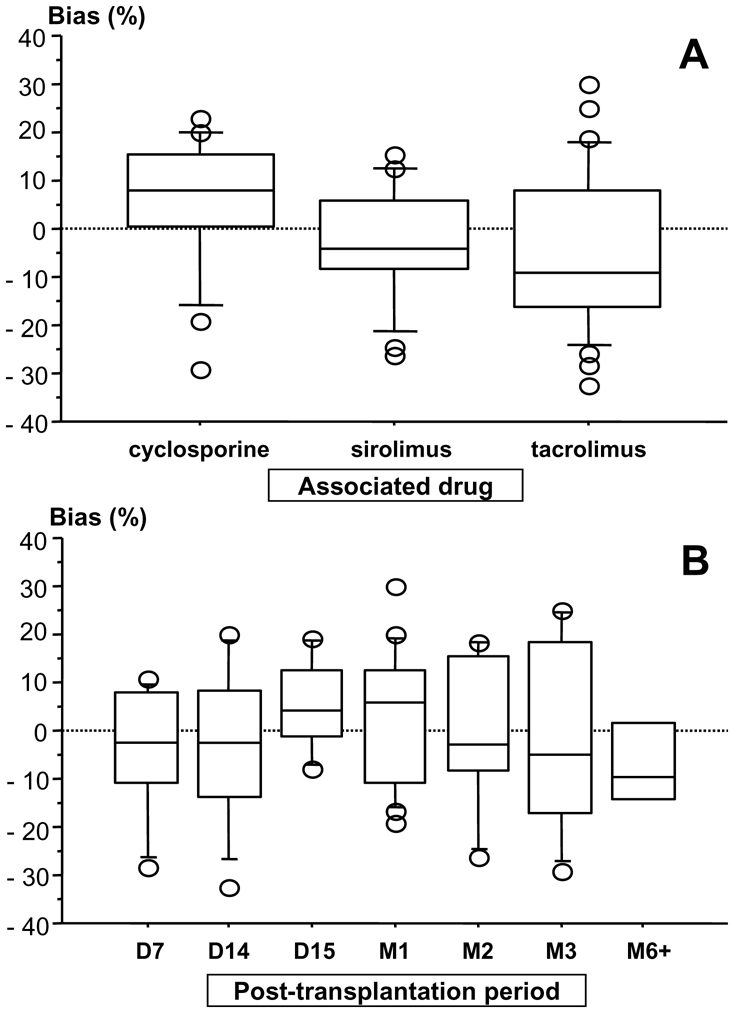
Bias of the Bayesian estimates of MPA AUC0–12h obtained using the 20 min-1h–3h sampling schedule with respect to the “true”, trapezoidal AUC0–12h sorted out by: A) associated immunosuppressant; and B) post-transplantation periods.
Figure 3.
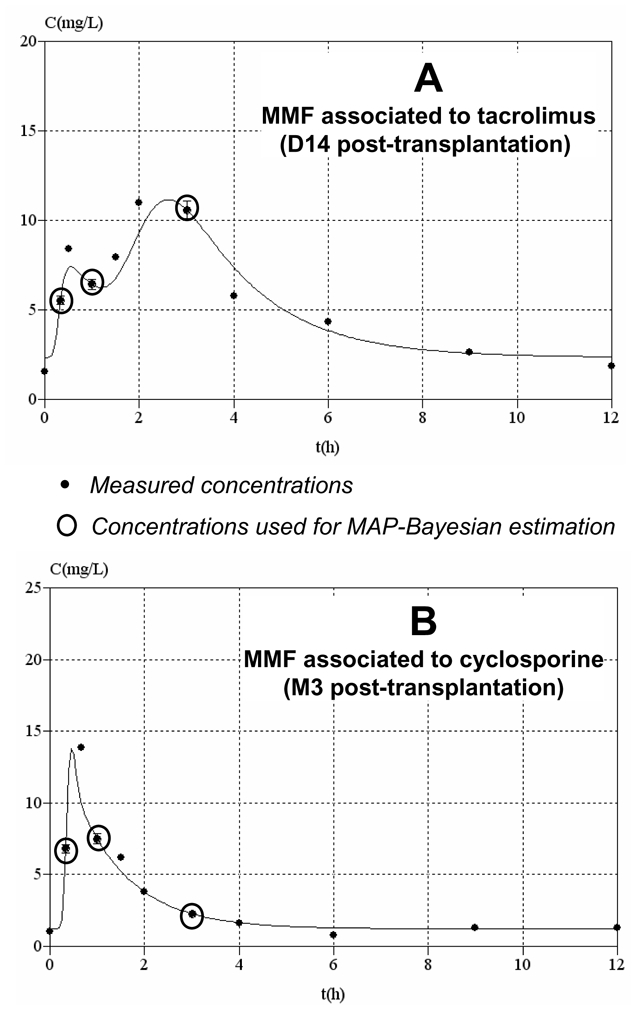
Examples of concentration-time profiles of MPA measured using the enzymatic MPA assay and estimated using the Bayesian method and the 20 min-1h–3h sampling schedule. A) Profile obtained early after transplantation in a patient given tacrolimus, showing a double plasma concentration peak. B) Profile obtained in a stable patient given cyclosporine, with no second concentration peak.
Figure 4.
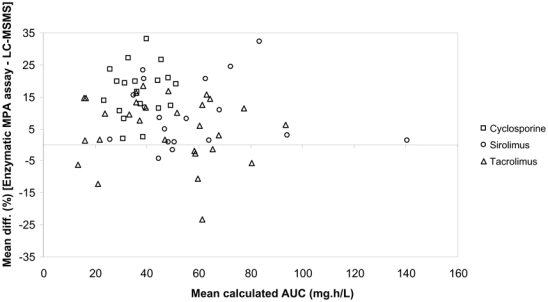
Bland-Altman representation of the relative bias of MPA Bayesian estimates obtained using concentrations measured at approx. 20 min, 1h and 3h post-dose with the enzymatic MPA assay as compared to LC-MS/MS.
Discussion
The new enzymatic MPA assay tested showed excellent correlation with LC-MS/MS for the determination of total MPA in plasma of adult or pediatric, renal or lung transplant patients under cyclosporine, tacrolimus or sirolimus in the early or stable post-transplantation periods. A negligible systematic bias of about +6% was found between the two techniques when analysing spiked plasma samples routinely, with the enzymatic MPA assay showing better precision.
Full PK profiles may result in very low (down to 0.017 mg/L) to quite high (up to 33.4 mg/L) plasma levels, some of which were out of the analytical range of the enzymatic MPA assay. However, very low concentrations were mainly found for trough levels obtained very early after transplantation, when it is now widely accepted that trough levels have limited significance, at least for MMF dose adjustment (10). In the present as well as in previous studies (14,16,17), we proposed Bayesian estimators of MPA AUC0–12h based on plasma samples collected at approx. 20 min, 1h and 3h post-dose, times at which MPA plasma levels were almost always above the LLOQ of the enzymatic MPA assay in our hands (0.4 mg/L). Above this LLOQ, the enzymatic MPA assay gave results very close to those of LC-MS/MS in all patients groups as determined by age, transplanted organ, co-administered immunosuppressant and post-transplantation period, though with slight overestimation of MPA plasma levels in patients on cyclosporine (mean 10.7%). This last finding is in agreement with those of previous reports comparing EMIT to LC-MS/MS, which showed that EMIT overestimated MPA plasma levels more in patients co-administered cyclosporine (11) than sirolimus (12) or tacrolimus (23,24), although overestimation was then globally much higher with EMIT than with the enzymatic MPA assay herein. This might be due to the inhibition of the biliary excretion of MPA phase II metabolites, by cyclosporine but not tacrolimus or sirolimus (25–27), resulting in an increase in plasma concentrations of these metabolites, mainly AcMPAG. However, it was shown that MPAG did not inhibit IMPDH, while MPA-acyl-glucuronide (AcMPAG) exhibited only 5% of MPA inhibition activity on the recombinant enzyme when incubated alone (13), and even less when co-incubated with MPA (28). However, the overestimation of actual MPA levels by the enzymatic MPA assay found in patients on cyclosporine should be of little clinical significance, so much so that this overestimation is proportional across the linearity range and with time post-dose, contrary to that of EMIT (11). It should also be noted that a previously published inter-laboratory validation of the enzymatic MPA assay, which was conducted after the present study and in which we also participated, found no significant overestimation, whether in patients on cyclosporine or on tacrolimus (13). This could be due to the nature of the kit itself, as the beta-version used in the present study was slightly different from the commercial version employed in the interlaboratory validation study.
Two different PK models with either one or two gamma laws, previously developed for renal transplant patients (15) were used to fit the present database. As raw data showed that patients could exhibit PK profiles with either one or two peaks, the best model was selected for each profile based on the Akaike criterion (a selection procedure now automatically applied to all three-point profiles sent to the ISBA website).
Significant differences in the PK parameters were found between LC-MS/MS and the enzymatic MPA assay, highlighting that analytical techniques not only impact the actual levels measured and the residual error of the models, but also the distribution of PK parameters in the population. This advocates the need for specific (or possibly adapted) models for pharmacokinetically-guided TDM when different analytical techniques are employed, even when they give close results as in the present study.
Bayesian estimators derived from these models were able to accurately estimate MPA AUCs when measured with the enzymatic MPA assay (i.e., more than 80% of the AUC estimates were within ± 20% of the actual values), with similar efficiency at all post-transplantation periods and for all three associated immunosuppressive drugs. When comparing Bayesian AUC estimates to those obtained with LC-MS/MS, significant although limited mean differences were observed, mainly in patients on cyclosporine or sirolimus. Also, the distribution of the AUC relative differences with respect to LC-MS/MS (figure 4) was very similar to that of the raw concentration data (figure 1). This should not be of clinical significance, in as much as the commercial enzymatic MPA assay apparently presents less overestimation than the beta version used in the present study (see above). This should not affect the accuracy of the Bayesian estimators developed here, which are more sensitive to curve shapes (hence PK parameters) than to absolute concentration values.
These tools were added to the ISBA website (https://pharmaco.chu-limoges.fr/abis.htm) to allow dose adjustment of MMF when measured using the Roche Diagnostics total MPA kit and have been used successfully since.
Acknowledgments
This study was sponsored by Roche Diagnostics.
We express our thanks to: Prof. Yannick Le Meur, Dr Jean-Philippe Rérolle and Dr Jean-Christophe Szelag, who enrolled many patients in all the clinical trials mentioned in this paper; Laetitia Vignaud, Jean-Louis Dupuy, Karine Delaune and Franck Giraudie for excellent technical assistance; Monika Widmann, Roche Diagnostics GmbH Mannheim for technical support; and Karen Poole for English revision.
References
- 1.Ransom JT. Mechanism of action of mycophenolate mofetil. Ther Drug Monit. 1995;17:681–684. doi: 10.1097/00007691-199512000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Bullingham RES, Nicholls AJ, Kamm B. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 1998;34:429–455. doi: 10.2165/00003088-199834060-00002. [DOI] [PubMed] [Google Scholar]
- 3.Shipkova M, Armstrong VW, Wieland E, et al. Identification of glucoside and carboxyl-linked glucuronide conjugates of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mofetil. Br J Pharmacol. 1999;126:1075–1082. doi: 10.1038/sj.bjp.0702399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picard N, Cresteil T, Premaud A, Marquet P. Characterization of a phase 1 metabolite of mycophenolic acid produced by CYP3A4/5. Ther Drug Monit. 2004;26:600–608. doi: 10.1097/00007691-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Picard N, Marquet P. In vitro study of mycophenolic acid glucuronidation. Drug Metab Dispos. 2004;32:1524. doi: 10.1124/dmd.104.001982. [DOI] [PubMed] [Google Scholar]
- 6.van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68:261–266. doi: 10.1097/00007890-199907270-00018. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers DR, Claes K, Evenepoel P, et al. Long-term changes in mycophenolic acid exposure in combination with tacrolimus and corticosteroids are dose dependent and not reflected through plasma concentration: A prospective study in 100 de novo renal allograFt recipients. J Clin Pharmacol. 2003;43:866–880. doi: 10.1177/0091270003256151. [DOI] [PubMed] [Google Scholar]
- 8.Kuypers DR, deJonge H, Naesens M, et al. Current Target Ranges of Mycophenolic Acid Exposure and Drug-Related Adverse Events: A 5-Year, Open-Label, Prospective, Clinical Follow-Up Study in Renal Allograft Recipients. Clin Ther. 2008;30:673–683. doi: 10.1016/j.clinthera.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 9.van Gelder T, Le Meur Y, Shaw LM, Oellerich M, DeNofrio D, Holt C, Holt DW, Kaplan B, Kuypers D, Meiser B, Toenshoff B, Mamelok RD. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit. 2006;28:145–154. doi: 10.1097/01.ftd.0000199358.80013.bd. [DOI] [PubMed] [Google Scholar]
- 10.Knight SR, Morris PJ. Does the Evidence Support the Use of Mycophenolate Mofetil Therapeutic Drug Monitoring in Clinical Practice? A Systematic Review. Transplantation. 2008;85:1675–1685. doi: 10.1097/TP.0b013e3181744199. [DOI] [PubMed] [Google Scholar]
- 11.Premaud A, Rousseau A, Le Meur Y, Lachâtre G, Marquet P. Comparison of liquid chromatography-tandem mass spectrometry with a commercial enzyme-multiplied immunoassay for the determination of plasma MPA in renal transplant recipients and consequences for therapeutic drug monitoring. Ther Drug Monit. 2004;26:609–619. doi: 10.1097/00007691-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Premaud A, Rousseau A, Picard N, Marquet P. Determination of mycophenolic acid plasma levels in renal transplant recipients co-administered sirolimus: comparison of an enzyme multiplied immunoassay technique (EMIT) and liquid chromatography-tandem mass spectrometry. Ther Drug Monit. 2005;27:354–361. doi: 10.1097/01.ftd.0000197092.84935.ef. [DOI] [PubMed] [Google Scholar]
- 13.Brandhorst G, Marquet P, Shaw LM, Liebisch G, Schmitz G, Coffing MJ, Domke I, Streit F, Luthe H, Oellerich M. Multicenter evaluation of a new IMPDH inhibition assay for the quantification of total mycophenolic acid in plasma. Ther Drug Monit. 2008;30:428–433. doi: 10.1097/FTD.0b013e31817fd590. [DOI] [PubMed] [Google Scholar]
- 14.Le Meur Y, Büchler M, Lavaud S, Etienne I, Westeel PF, Thierry A, Caillard S, Villemain F, Hurault de Ligny B, Rostaing L, Thervet E, Szelag JC, Rérolle JP, Touchard G, Rousseau A, Marquet P. Personalized Mycophenolate Mofetil dosing based on drug exposure significantly improves patient outcome after renal transplantation. Am J Transplant. 2007;7:2496–2503. doi: 10.1111/j.1600-6143.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 15.Prémaud A, Debord J, Rousseau A, Le Meur Y, Toupance O, Lebranchu Y, Hoizey G, Le Guellec C, Marquet P. A double absorption-phase model adequately describes mycophenolic acid plasma profiles in de novo renal transplant recipients given oral mycophenolate mofetil. Clin Pharmacokinet. 2005;44:837–847. doi: 10.2165/00003088-200544080-00005. [DOI] [PubMed] [Google Scholar]
- 16.Prémaud A, Le Meur Y, Debord J, Szelag JC, Rousseau A, Hoizey G, Toupance O, Marquet P. Maximum a posteriori bayesian estimation of mycophenolic acid pharmacokinetics in renal transplant recipients at different postgrafting periods. Ther Drug Monit. 2005;27:354–361. doi: 10.1097/01.ftd.0000162231.90811.38. [DOI] [PubMed] [Google Scholar]
- 17.Marquet P. Clinical application of population pharmacokinetic methods developed for immunosuppressive drugs. Ther Drug Monit. 2005;27:727–732. doi: 10.1097/01.ftd.0000179848.65266.aa. [DOI] [PubMed] [Google Scholar]
- 18.van Gelder T, Silva HT, de Fijter JW, Budde K, Kuypers D, Tyden G, Lohmus A, Sommerer C, Hartmann A, Le Meur Y, Oellerich M, Holt DW, Tönshoff B, Keown P, Campbell S, Mamelok RD. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation. 2008;86:1043–1051. doi: 10.1097/TP.0b013e318186f98a. [DOI] [PubMed] [Google Scholar]
- 19.D’Argenio DZ. Optimal sampling times for pharmacokinetics experiments. J Pharmacokinet Biopharm. 1981;9:739–756. doi: 10.1007/BF01070904. [DOI] [PubMed] [Google Scholar]
- 20.Saint-Marcoux F, Knoop C, Debord J, Thiry P, Rousseau A, Estenne M, Marquet P. Pharmacokinetic study of tacrolimus in cystic fibrosis and non-cystic fibrosis lung transplant patients and design of Bayesian estimators using limited sampling strategies. Clin Pharmacokinet. 2005;44:1317–1328. doi: 10.2165/00003088-200544120-00010. [DOI] [PubMed] [Google Scholar]
- 21.Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21:709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 23.Hosotsubo H, Takahara S, Imamura R, et al. Analytic validation of the enzyme multiplied immunoassay technique for the determination of mycophenolic acid in plasma from renal transplant recipients compared with a high-performance liquid chromatographic assay. Ther Drug Monit. 2001;23:669–674. doi: 10.1097/00007691-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Schütz E, Shipkova M, Armstrong VW, et al. Therapeutic drug monitoring of mycophenolic acid: comparison of HPLC and immunoassay reveals new MPA metabolites. Transplant Proc. 1998;30:1185–1187. doi: 10.1016/s0041-1345(98)00201-2. [DOI] [PubMed] [Google Scholar]
- 25.van Gelder T, Klupp J, Barten MJ, et al. Comparison of the effects of tacrolimus and cyclosporine on the pharmacokinetics of mycophenolic acid. Ther Drug Monit. 2001;23:119–128. doi: 10.1097/00007691-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Sawamoto T, van Gelder T, Christians U, et al. Membrane transport of mycophenolate mofetil and its active metabolite, mycophenolic acid in MDCK and MDR1-MDCK cell monolayers [Abstract] J Heart Lung Transplant. 2001;20:234–235. doi: 10.1016/s1053-2498(00)00525-8. [DOI] [PubMed] [Google Scholar]
- 27.Picard N, Premaud A, Rousseau A, Le Meur Y, Marquet P. A comparison of the effect of ciclosporine and sirolimus on the pharmacokinetics of mycophenolate in renal transplant patients. Br J Clin Pharmacol. 2006;62:477–484. doi: 10.1111/j.1365-2125.2006.02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gensburger O, Picard N, Marquet P. Effect of mycophenolate acyl-glucuronide (AcMPAG) on human recombinant type 2 inosine monophosphate dehydrogenase (IMPDH II) Clin Chem. doi: 10.1373/clinchem.2008.113936. [in Press] [DOI] [PubMed] [Google Scholar]


