Abstract
Nonalcoholic Fatty Liver Disease (NAFLD) is the hepatic manifestation of metabolic syndrome and is a marker of Insulin Resistance (IR). Euglycemic-hyperinsulinemic clamp is the gold standard for measuring whole body IR (hepatic + peripheral IR). However, it is an invasive and expensive procedure. Homeostasis Model Assessment Index for Insulin Sensitivity (HOMA-IS), Quantitative Insulin Sensitivity Check Index (QUICKI) for hepatic IR and Insulin Sensitivity Index (ISI0,120), and Whole Body Insulin Sensitivity Index (WBISI) for whole body IR are the indices calculated after Oral Glucose Tolerance Test (OGTT). We used these indices as noninvasive methods of IR (inverse of insulin sensitivity) estimation and compared hepatic/peripheral components of whole body IR in NAFLD. Methods. 113 morbidly obese, nondiabetic subjects who underwent gastric bypass surgery and intraoperative liver biopsy were included in the study. OGTT was performed preoperatively and the indices were calculated. Subjects were divided into closely matched groups as normal, fatty liver (FL) and Non-Alcoholic Steatohepatitis (NASH) based on histology. Results. Whole body IR was significantly higher in both FL and NASH groups (NAFLD) as compared to Normal, while hepatic IR was higher only in NASH from Normal. Conclusions. FL is a manifestation of peripheral IR but not hepatic IR.
1. Introduction
NAFLD is the term used for the spectrum of hepatic pathology ranging from simple steatosis to steatohepatitis and cirrhosis in individuals who do not consume alcohol in amount which is generally considered harmful to the liver. In simple steatosis, fat accumulates in the hepatocytes called FL, which is seen in up to 30% of general and 75% of obese individuals. Development of FL has been reported to be associated with BMI, abnormal glucose tolerance, IR, and other components of MetS [1]. Hepatocytes with excessive intracellular fat “first hit” create a state of oxidative stress and possibly with accumulation of oxidative damage “second hit” FL may progress to NASH which is present in 5% of the general population and 20% of obese subjects [2, 3]. NASH may further progress to advanced fibrosis, cirrhosis, and hepatocellular carcinoma [4].
NAFLD is considered a hepatic complication of MetS. Studies have documented close relationship between MetS, NAFLD, and IR but the underlying mechanisms have not been clarified [1, 5]. Postabsorptive phase hyperglycemia and subsequent hyperinsulinemia are believed to be important physiologic risk factors for hepatocyte injury and progressive hepatic fibrosis [6, 7]. These metabolic derangements are amplified in setting of IR. IR can be defined as altered metabolic condition in which higher than normal insulin levels are needed to achieve normal metabolic responses. IR—the reciprocal of insulin sensitivity, is present long before the DM become clinically manifest. IR can be central (hepatic) or peripheral (muscle, fat) depending upon the primary site of involvement. Peripheral IR impairs glucose uptake from blood into muscles (Postabsorptive and fasting states) while hepatic IR manifests as unrestrained glucose production by liver under fasting conditions [8]. Whole body IR is a composite of hepatic and peripheral IR and is best measured by euglycemic-hyperinsulinemic clamp technique [9]. The method is invasive and cannot be done on larger populations on routine basis. Several authors have developed simpler methods and indices based on various weighted combinations of glucose and insulin levels at fasting or during OGTT. Indices relying upon single fasting blood samples, such as homeostatic model assessment (HOMA) [10] and quantitative insulin sensitivity check index (QUICKI) [11] are widely used in general population for their simplicity for accessing hepatic IR. Several other more comprehensive approaches of non-invasive estimation of IR have been tested in several populations, which use Postabsorptive insulin and glucose levels in addition to fasting ones and provide more elaborative and physiologic assessment of IR. Two of such methods are Insulin Sensitivity Index (ISI0,120) [12, 13] and Whole Body Insulin Sensitivity Index (WBISI) [14] which have also been validated as reliable indices of whole body insulin sensitivity particularly in obese population [15–17].
This study was conducted to explore the association between NAFLD and glucose metabolism with components of IR which was estimated by indices for both hepatic and whole body IR.
2. Methods and Materials
2.1. Population Selection
Patients were selected from the database of 445 individuals with clinically severe obesity that consecutively underwent laparoscopic gastric bypass surgery and intra-operative liver biopsy at University of Alabama-Birmingham between January 2002 and March 2007. NIH guidelines (BMI > 40 kg/m2 or BMI > 35 kg/m2 associated with medical comorbidities) were used to determine surgical eligibility. Most subjects underwent a 6-month physician-monitored weight reduction program before being considered for surgery. Only those subjects were included in the database who had clinically diagnosis of NAFLD based on history of alcohol consumption in amounts less than what is generally considered to be hepatotoxic and absence of other liver diseases [18]. Alcohol intake (<20 gm/day in females and <40 gm/day in males) was assessed separately by both physician and surgeon who were involved in the patient care. Preoperatively, all patients had several laboratory tests done, including hepatitis B and C serology, ceruloplasmin, iron studies, alpha1-antitrypsin level, and phenotype and autoimmune markers to exclude various causes of liver disease. None of the subjects was taking medications such as methotrexate, amiodarone, tetracycline, high dose estrogen, tamoxifen, steroids, or calcium channel blockers. For the present study only those patients were selected from the database who did not have a past history of DM and were not taking any medicines for glucose control. OGTT was done as a part of pre-operative evaluation of all such patients. The patients did not undergo any specialized diet plan prior to OGTT which was done 113 ± 71 days prior to the procedure. From the selected 232 patients who did not have known DM and who underwent OGTT, 169 patients were selected in whom fasting and 2 hours postload levels of both glucose and insulin were documented. 56 patients were further excluded who were diagnosed as diabetic according to ADA guidelines (fasting glucose>126 mg/dl or 2 hours glucose>200 mg/dl) after OGTT. Finally, 113 non-diabetic morbidly obese subjects were included in this study for comparison of surrogate markers of hepatic and whole body IR. All subjects had previously provided informed consent and protocol of the study was approved by the Human Investigative Committee of the University of Alabama at Birmingham.
2.2. Pre-Operative Evaluation
Laboratory evaluation included routine liver tests panel (AST, ALT, total bilirubin, alkaline phosphatase, total protein, and albumin), lipid panel (cholesterol, TAG, HDL, and LDL), and complete blood count. These tests were done on the same day. OGTT was done according to the standard ADA guidelines. In overnight fasting state, all patients were given 75 gm glucose dissolved in water and blood was drawn at 0 (fating state), 0.5, 1 and 2 hours interval. Glucose and insulin levels were estimated by standard laboratory methods. Surrogate markers of IR were calculated from the fasting (0 hour) and 2 hours insulin and glucose levels.
The formulae of IR are given below.
HOMA-IR = [Glucose at 0 hours (mg/dl)]×[Insulin at 0 hours (mIU/L)]/405.
1/QUICKI = log [Glucose at 0 hours (mg/dl)] × log [Insulin at 0 hours (mIU/L)].
1/ISI0,120 = log mean insulin/MCR; where, log mean insulin = log [(Insulin at 0 hours (mIU/L) + Insulin at 2 hours (mIU/L))/2] and MCR = m/mean glucose, m = [75000 + (Glucose at 0 hours (mg/L)—Glucose at 2 hours (mg/L)) × 0.19 × weight (kg)]/120 and mean glucose = [Glucose at 0 hours (mmol/L) + Glucose at 2 hours (mmol/L)]/2.
1/WBISI = square root of [Insulin at 0 hours (mIU/L) × Glucose at 0 hours (mmol/L) × mean glucose (mmol/L) × mean insulin (mIU/L)]/10,000; where mean glucose = [Glucose at 0 hours (mmol/L) + Glucose at 2 hours (mmol/L)]/2 and mean insulin = [Insulin at 0 hours (mIU/L) + Insulin at 2 hours (mIU/L)]/2.
2.3. Histological Evaluation
Liver biopsies were performed on the left lobe of liver at the beginning of the operation using a core biopsy needle (Bard Max-Core, Covington, GA). The liver biopsy was interpreted by a single pathologist blinded to all clinical data. NAFLD is typically associated with macrovesicular fat accumulation in hepatocytes with nucleus pushed to periphery, giving the classic “signet ring” appearance. Hepatic inflammation in NASH is characterized by lymphocytic infiltration into the hepatic lobules. Steatosis was graded according to amount of fat present in the hepatocytes in biopsy specimen as 0: normal (1%–5%), 1: mild (6%–33%), 2: moderate (34%–66%), and 3: severe (67%–100%). The cellular infiltration was identified as panlobular, portal, or both and subjectively assessed as mild, moderate, or severe. The pathological diagnosis NASH was made in accordance with Kleiner et al. criteria [19] (i.e., steatosis and ballooning degeneration and/or fibrosis in zone 3) and staging of fibrosis was done in accordance with the National Institutes of Health sponsored NASH Clinical Research Network guidelines: stage (1) (1a/b—mild/moderate zone 3 fibrosis; 1c—zone 1 fibrosis only); stage (2) both zone 3 and zone 1 fibrosis; stage (3) bridging; and stage (4) cirrhosis. Steatosis alone (no zone 3 injury or fibrosis) ± zone 1 (portal/periportal) fibrosis was identified as FL. Biopsies showing no or minimal (<5%) steatosis and absent injury or fibrosis were considered as Normal. Study population was divided into 3 groups; Normal, FL and NASH on the basis of liver biopsy diagnosis criteria.
2.4. Statistical Analysis
All the indices used in the study were the measures of Insulin Sensitivity. The values were inversed to use them as indices of IR. This also helped in reciprocal transformation of skewed data to symmetrical distribution and to reliably create confidence intervals. Data was analyzed as both continuous and categorical variables. All continuous variables were expressed as Mean ± (SD) and categorical as Numbers or percentages. Independent t test and ANOVA were used for normally distributed continuous variables. Nonparametric tests (Mann-Whitney and Kruskal-Wallis with post hoc analysis) were applied for ordinal or continuous variables that showed significance on Kolmogorov-Smirnov test. Categorical variables were analyzed using Chi-square or Fisher exact tests with correction, when appropriate. A P value cut-off was selected to be <.01 to get stronger evidence against null hypothesis. Statistical analysis was performed by using SPSS (SPSS Inc., Chicago, IL; V13.0 for windows).
3. Results
Table 1 presents the clinical and biographical data of population which consists of 113 non-diabetic, obese patients who underwent pre-operative testing and intra-operative liver biopsy. Table 2 shows histological classification study populations. Biopsy sample was adequate for histological assessment of NAFLD and comparable among groups. Population mean age was 39.4 years and BMI was 49 kg/m2. The study population was divided into three groups on histological basis as already described. FL (51%), NASH (27%) and Normal (22%) were statistically similar in age, BMI, gender, and race distribution. Overall there was female and Caucasian predominance in our population (89% and 79%, respectively). The means of other biochemical parameters including LDL, HDL, TAG, and Albumin were not statistically different among the groups. However liver transaminases (both AST and ALT) were significantly elevated in NASH group as compared to both FL and Normal groups.
Table 1.
Population Description. Breakup of study population according to the NAFLD spectrum is shown. Data represents Mean ± (SD).
| Total | Normal | FL | NASH | |||||
|---|---|---|---|---|---|---|---|---|
| N | 113 | 26 | 58 | 29 | ||||
| Age (years) | 39.4 ± (8.3) | 38.4 ± (11.0) | 38.9 ± (7.2) | 41.4 ± (7.6) | ||||
| Gender (males/females) | 12/101 | 1/25 | 5/53 | 6/23 | ||||
| Race (Caucasian white/AA) | 89/24 | 18/8 | 46/12 | 25/4 | ||||
| BMI (kg/m2) | 49.0 ± (8.0) | 47.0 ± (6.4) | 49.1 ± (8.2) | 50.5 ± (8.7) | ||||
| Fasting c-peptide (ng/ml)1 | 4.4 ± (1.6) | 3.7 ± (1.6) | 4.3 ± (1.6) | 5.1 ± (1.4) | ||||
| Insulin (mIU/L) at 0 hours in OGTT 1 | 23.5 | (14.2) | 17.3 | (7.6) | 22.9 | (11.2) | 30.3 | (20.3) |
| Fasting c-peptide to insulin ratio | 0.22 | (0.1) | 0.24 | (0.1) | 0.22 | (0.1) | 0.21 | (0.1) |
| Insulin (mIU/L) at 2 hours in OGTT | 95.1 | (73.4) | 63.7 | (53.2) | 106.1 | (80.5) | 101.2 | (68.1) |
| Glucose (mg/dl) at 0 hours in OGTT | 99.4 | (10.4) | 95.5 | (8.6) | 99.9 | (10.6) | 101.8 | (10.9) |
| Glucose (mg/dl) at 2 hours in OGTT2 | 119.3 | (29.9) | 104.5 | (26.3) | 125.4 | (30.7) | 120.3 | (27.7) |
| AST (U/L)3 | 22.8 | (8.6) | 21.4 | (8.4) | 20.7 | (6.1) | 28.4 | (10.9) |
| ALT (U/L)3 | 27.2 | (15.8) | 25.4 | (17.6) | 22.7 | (7.8) | 37.6 | (21) |
| Albumin (g/dl) | 4.5 | (8.8) | 3.6 | (0.2) | 5.3 | (12.1) | 3.8 | (0.3) |
| Total Cholesterol (mg/dl) | 191.6 | (32.2) | 189.0 | (23.9) | 187.3 | (34.4) | 202.6 | (32.7) |
| TAG (mg/dl) | 139.4 | (104) | 104.2 | (51.3) | 130.8 | (71.2) | 189.0 | (164) |
| HDL (mg/dl) | 44.4 | (10.2) | 48.0 | (10.4) | 43.5 | (8.6) | 42.8 | (12.3) |
| LDL (mg/dl) | 122.2 | (29) | 122.7 | (16.9) | 120.4 | (31.3) | 125.3 | (33.4) |
| No. of portal tracts in biopsy | 12.1 | (4.6) | 11.0 | (5.7) | 11.5 | (5.3) | 13.3 | (3.5) |
1 = P < .01 for NASH as compared to Normal only, 2 = P < .01 for FL as compared to Normal only, 3 = P < .01. for NASH as compared to both FL and Normal.
Table 2.
Histological classification of study population for the spectrum of NAFLD.
| N | N | |
|---|---|---|
| Normal | 26 | |
| FL (hepatic steatosis) | 58 | |
| (i) Without Portal Fibrosis | 37 | |
| 21 | ||
| (ii) With Portal Fibrosis (1c) | ||
| NASH (steatosis + ballooning/zone3 fibrosis) | 29 | |
| (iii) With ballooning | 25 | |
| (1) No fibrosis (ballooning only) | 3 | |
| (2) With fibrosis | 22 | |
| (a) Stage 1a & 2 | 15 | |
| (b) IPF (1c) | 3 | |
| (c) Advanced fibrosis | 3 | |
| (iv) With zone 3 fibrosis only (no ballooning) | 5 | |
| Total | 113 | |
| Stage | ||
| 0 | 65 | |
| 1 | 32 | |
| 2 | 13 | |
| 3 | 3 |
Parameters of OGTT were compared among groups as shown in Table 1. Fasting glucose was comparable among all the three groups of this non-diabetic population. However fasting insulin and c-peptide levels were significantly higher in NASH as compared to Normal group only. FL group was seen in transition between Normal and NASH group. Fasting c-peptide to insulin ratio was similar among groups which showed insulin extraction by liver was similar in all the groups. 2 hours after glucose ingestion insulin levels were similar among groups but glucose levels were significantly elevated in FL as compared to Normal group only.
The comparison of metabolic status of our study population revealed comparatively large proportion of NAFLD patients with underlying impaired glucose tolerance as shown in Table 3. Abnormal glucose tolerance has been shown to be present in NAFLD in previous studies but our study further broke NAFLD into biopsy proven FL and NASH. Among FL, 24% had IGT and 12% had IFG. In subjects with NASH, 20% and 14% were diagnosed with IGT and IFG, respectively. Taken together, no significant difference in the prevalence of IGT or IGF was noted in the FL and NASH groups. IGT and IFG patients were significantly less prevalent in the Normal group (only 7% and 4%, respectively).
Table 3.
Metabolic status of population from 2 hours oral glucose load testing.
| Normal | FL | NASH | Total | |
|---|---|---|---|---|
| Normoglycemic | 23 | 37 | 19 | 79 |
| Impaired Fasting Glucose (IFG) | 2 | 7 | 4 | 13 |
| Impaired Glucose Tolerance (IGT) | 1 | 14 | 6 | 21 |
| 26 | 58 | 29 | 113 |
Normoglycemic = Fasting glucose<110 and 2 hours glucose <140, Impaired Fasting Glucose = Fasting glucose 110 to 126, Impaired glucose tolerance = 2 hours glucose 140 to 200, Units = mg/dl.
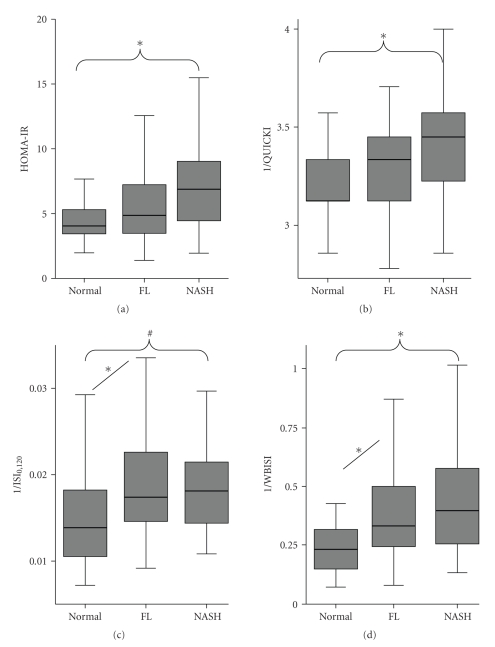
Four indices of IR were compared in our study (Table 4). When compared as NAFLD versus. Normal, all indices showed statistically significant difference indicating that IR is present in NAFLD irrespective of the spectrum of pathology. Study population consisted of severely obese individuals with BMI of 49.0 ± (8.0) kg/m2 so the IR values were already high as shown in other studies [20]. Figure 1 shows further comparison of these indices along the spectrum of NAFLD. Fasting-based indices HOMA and QUICKI were statistically different among NASH and Normal group. While OGTT based whole body IR as measured by ISI0,120 and WBISI were significantly different in both NASH and FL group when compared to Normal.
Table 4.
Indices of Insulin resistance calculated from insulin and glucose levels during OGTT. Data represented as Mean ± (SD).*
| Total | Normal | NAFLD | ||||
|---|---|---|---|---|---|---|
| N | 113 | 26 | 87 | |||
| HOMA-IR | 5.882 | (3.818) | 4.105 | (1.924) | 6.413 | (4.083) |
| 1/QUICKI | 3.303 | (0.252) | 3.177 | (0.217) | 3.341 | (0.251) |
| 1/ISI0,120 | 0.018 | (0.007) | 0.015 | (0.005) | 0.019 | (0.007) |
| 1/WBISI | 0.385 | (0.24) | 0.252 | (0.139) | 0.425 | (0.25) |
∗ = P < .01 for all four indices of insulin resistance for NAFLD as compared to Normal by non-parametric test (Mann-Whitney test).
Figure 1.
Box plot analysis of surrogate markers of hepatic and whole body insulin resistance along the spectrum of NAFLD. (a) HOMA-IR and (b) 1/QUICKI calculated from fasting insulin and glucose levels represent hepatic (central) insulin resistance. (c) 1/ISI0,120 and (d) 1/WBISI calculated from both fasting and 2 hours postglucose challenge insulin and glucose levels represent whole body insulin resistance, which is composite of hepatic and peripheral insulin resistance. Groups were compared by nonparametric test (Kruskal-Wallis H test) and post hoc corrections. ∗ = P < .01 and # = P < .05.
4. Discussion
IR is an important clinical problem that occurs nearly in all patients with NAFLD and MetS. The presence of IR in non-diabetic individuals portends higher risk of developing NAFLD, DM or cardiovascular disease [21]. In vivo, IR can be measured directly by sophisticated tests like glucose clamp techniques and Insulin suppression tests. However, direct measures of IR are frequently expensive, time demanding, and labor intensive. For large clinical studies involving measurement of IR, use of the surrogate markers is a more practical option. HOMA and QUICKI estimate IR in fasting state and measure central (hepatic) IR. Central IR manifests clinically as fasting hyperglycemia due to unrestrained hepatic gluconeogenesis. Insulin mediates glucose disposal in the peripheral tissues. This mechanism is mostly determined by peripheral IR and it has been shown that the pathways involved in insulin stimulated peripheral glucose uptake are deranged in patients with MetS [22]. Peripheral IR can be estimated indirectly with the whole body IR using ISI0,120 and WBISI as surrogate measures. Although changes in hepatic IR parallel with changes in peripheral IR frequently, it has been shown that significant number of individuals with normal or near normal hepatic IR have impaired peripheral IR [23].
In the present study, we assessed the association of NAFLD and the components of IR among morbidly obese individuals. The study suggests that NAFLD is related to an increase in IR and there are comparative differences in hepatic and whole body IR along the spectrum of NAFLD. Importantly, these differences were identified by using simple non-invasive methods. The presence of IR in NAFLD and in obesity has already been widely reported but the nature and direction of this connection still remains a matter of debate. Our study confirms the presence of IR among morbidly obese NAFLD patients. A significant number of these obese subjects with IR had normal liver biopsies. Although sampling error is a possibility, this observation points out the importance of other factors which might be involved in pathogenesis of NAFLD. DM is the advanced form of IR and has a strong association with NAFLD. To eliminate its confounding effect, we selected to investigate only the non-diabetic individuals based on ADA guidelines. Thus, all included subjects had fasting glucose levels in non-diabetic range. The study population was divided into three groups of Normal, FL, and NASH according to the well accepted histological definition of NAFLD. The NASH group demonstrated elevated fasting Insulin levels due to underlying hepatic IR and this was not a consequence of decreased insulin extraction, which occurs in advanced liver fibrosis. In FL, there was a sustained postload hyperglycemia, secondary to peripheral IR and inability to efficiently move glucose into peripheral tissues.
The important finding in this study was the gradual increase in the whole body IR and its component—hepatic IR along the spectrum on NAFLD. Whole body IR was significantly different in both FL and NASH groups when compared to Normal. However, the difference in hepatic IR became significant only in NASH as compared to Normal. Thus, indirectly—peripheral IR significantly increased in FL as compared to Normal. This observation leads to the assumption that FL is a manifestation of peripheral IR but not hepatic IR. NASH is associated with increase in hepatic IR and further worsening of whole body IR. Similar findings were also observed in hypertensive individuals where up to a 40% increase in peripheral IR was noted in the studies [24].
Limitations in this study included intraindividual variation of WBISI and ISI0,120 secondary to significant variations in 2 hours glucose and insulin levels in individuals subjected to OGTT at different times [25]. Various medications such as beta blockers and diuretics have been shown to influence IR in hypertensive populations [26]. There is a selection bias in this study as the cohort investigated included only morbidly obese patients who were undergoing gastric bypass surgery and this may not be extrapolated to the general obese population. The subjects investigated comprised predominantly females and Caucasians. Liver biopsy sampling error and observer variability were also important factors pertaining to our study [27].
In summary, by using simple non-invasive measures we showed that NAFLD is associated with worsening of whole body IR. This increase in IR is significant only in peripheral tissues in FL population. Possibly, this leads to further channeling of lipids and glucose to the liver and subsequent development of hepatic IR. Hepatic IR results in a sustained postprandial hyperglycemia and hyperinsulinemia—which are important factors involved in cellular injury and fibrosis characteristic of NASH and progression of NAFLD. Further studies are needed for more elaborative evaluation of these components of IR in NAFLD in an effort to identify the pathogenesis of progression of this disease.
Abbreviations
- ADA:
American Diabetes Association
- ALT:
Alanine Aminotransferase
- ANOVA:
Analysis of Variance
- AST:
Aspartate Aminotransferase
- BMI:
Body Mass Index
- DM:
Diabetes Mellitus
- FL:
Fatty Liver
- HDL:
High Density Lipoprotein
- HOMA-IS:
Homeostasis Model Assessment Index for Insulin Sensitivity
- IFG:
Impaired Fasting Glucose
- IGT:
Impaired Glucose Tolerance
- IPF:
Isolated Portal Fibrosis
- IR:
Insulin Resistance
- ISI0,120:
Insulin Sensitivity Index
- LDL:
Low Density Lipoprotein
- MetS:
Metabolic Syndrome
- NAFLD:
Non Alcoholic Fatty Liver Disease
- NASH:
Non Alcoholic Steatohepatitis
- OGTT:
Oral Glucose Tolerance Test
- QUICKI:
Quantitative Insulin Sensitivity Check Index
- SD:
Standard Deviation
- TAG:
Triacylglycerol; Triglycerides
- WBISI:
Whole Body Insulin Sensitivity Index.
References
- 1.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 2.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. American Journal of Gastroenterology. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 3.James OFW, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. Journal of Hepatology. 1998;29(3):495–501. doi: 10.1016/s0168-8278(98)80073-1. [DOI] [PubMed] [Google Scholar]
- 4.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36(6):1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 5.Chitturi S, Abeygunasekera S, Farrell GC, et al. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35(2):373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 6.Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54(7):1003–1008. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svegliati-Baroni G, Bugianesi E, Bouserhal T, et al. Post-load insulin resistance is an independent predictor of hepatic fibrosis in virus C chronic hepatitis and in non-alcoholic fatty liver disease. Gut. 2007;56(9):1296–1301. doi: 10.1136/gut.2006.107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World Journal of Gastroenterology. 2007;13(26):3540–3553. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. American Journal of Physiology. 1979;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. Journal of Clinical Endocrinology and Metabolism. 2000;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 12.Cederholm J, Wibell L. Evaluation of insulin release and relative peripheral resistance with use of the oral glucose tolerance test: a study in subjects with normoglycaemia, glucose intolerance and non-insulin-dependent diabetes mellitus. Scandinavian Journal of Clinical and Laboratory Investigation. 1985;45(8):741–751. doi: 10.3109/00365518509155289. [DOI] [PubMed] [Google Scholar]
- 13.Gutt M, Davis CL, Spitzer SB, et al. Validation of the insulin sensitivity index (ISI0,120): comparison with other measures. Diabetes Research and Clinical Practice. 2000;47(3):177–184. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M, Liu Y, Mahankali S, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes. 1999;48(9):1801–1806. doi: 10.2337/diabetes.48.9.1801. [DOI] [PubMed] [Google Scholar]
- 15.Leonetti F, Iacobellis G, Zappaterreno A, et al. Insulin sensitivity assessment in uncomplicated obese women: comparison of indices from fasting and oral glucose load with euglycemic hyperinsulinemic clamp. Nutrition, Metabolism and Cardiovascular Diseases. 2004;14(6):366–372. doi: 10.1016/s0939-4753(04)80027-9. [DOI] [PubMed] [Google Scholar]
- 16.Haeckel R, Raber R, Wosniok W. Comparability of indices for insulin resistance and insulin secretion determined during oral glucose tolerance tests. Clinical Chemistry and Laboratory Medicine. 2006;44(7):817–823. doi: 10.1515/CCLM.2006.153. [DOI] [PubMed] [Google Scholar]
- 17.Perseghin G, Bonfanti R, Magni S, et al. Insulin resistance and whole body energy homeostasis in obese adolescents with fatty liver disease. American Journal of Physiology. 2006;291(4):E697–E703. doi: 10.1152/ajpendo.00017.2006. [DOI] [PubMed] [Google Scholar]
- 18.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. American Journal of Gastroenterology. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 20.Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. American Journal of Gastroenterology. 2007;102(2):399–408. doi: 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 21.Reaven GM. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. Journal of Clinical Endocrinology and Metabolism. 2003;88(6):2399–2403. doi: 10.1210/jc.2003-030087. [DOI] [PubMed] [Google Scholar]
- 22.Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. The New England Journal of Medicine. 1987;317(6):350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- 23.Berrish TS, Hetherington CS, Alberti KGMM, Walker M. Peripheral and hepatic insulin sensitivity in subjects with impaired glucose tolerance. Diabetologia. 1995;38(6):699–704. doi: 10.1007/BF00401842. [DOI] [PubMed] [Google Scholar]
- 24.Shen D-C, Shieh S-M, Fuh MMT, Wu D-A, Chen YDI, Reaven GM. Resistance to insulin-stimulated-glucose uptake in patients with hypertension. Journal of Clinical Endocrinology and Metabolism. 1988;66(3):580–583. doi: 10.1210/jcem-66-3-580. [DOI] [PubMed] [Google Scholar]
- 25.Mooy JM, Grootenhuis PA, de Vries H, et al. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39(3):298–305. doi: 10.1007/BF00418345. [DOI] [PubMed] [Google Scholar]
- 26.Wu K-D, Hsiao C-F, Ho L-T, et al. Clustering and heritability of insulin resistance in Chinese and Japanese hypertensive families: a Stanford-Asian Pacific Program in hypertension and insulin resistance sibling study. Hypertension Research. 2002;25(4):529–536. doi: 10.1291/hypres.25.529. [DOI] [PubMed] [Google Scholar]
- 27.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]