Abstract
Herein, we demonstrate that the surface charge of gold nanoparticles (AuNPs) plays a critical role in modulating membrane potential of different malignant and non-malignant cell types and subsequent downstream intracellular events. The findings presented here describe a novel mechanism for cell-nanoparticle interactions and AuNP uptake: modulation of membrane potential and its effect on intracellular events. These studies will help understand the biology of cell-nanoparticle interactions and facilitate the engineering of nanoparticles for specific intracellular targets.
Keywords: Gold nanoparticles, surface properties, cancer, lung, ovary, plasma membrane, membrane potential, calcium, apoptosis, proliferation
In recent years, significant effort has been devoted to develop nanotechnology for the delivery of small molecular weight drugs, as well as macromolecules such as proteins, peptides, or genes into cells and tissue1–7. Targeted nanoparticle-mediated drug delivery may be used to direct the particles to specific tissues (minimizing toxicity), improve oral bio-availability, sustain drug/gene effect in the target tissue, solubilize drugs for intravascular delivery, and/or improve the stability of therapeutic agents against enzymatic degradation8. In spite of the fantastic potential for nanoparticle use in medicine, fundamental studies to understand the molecular interactions of nanoparticles with their target cells (normal as well as malignant) remain largely unexplored. One such mechanism of action may be ionic interactions: the negative membrane potential of most cells likely interacts differently with nanoparticles of a positive vs. negative charge density. These interactions could, in turn, determine intracellular uptake and localization of the nanoparticles and their biological functions. Understanding such interactions between cells and nanoparticles with different surface properties is important not only for engineering of nanoparticles that exhibit selective intracellular uptake (to subsequently modulate cellular processes of interest) but also for determining the relative cytotoxicity of nanoparticles.
All living cells have an inherent membrane potential that is determined by ionic permeability and modulated by processes including electrical or agonist stimulation, ion channels and changes in intracellular vs. extracellular ionic concentrations. Furthermore, the membrane potential itself can modulate a number of intracellular pathways, including intracellular Ca2+ concentration ([Ca2+]i), the cell cycle, and cellular proliferation vs. apoptosis; each important not only for normal cell structure and function but also in the progression of diseases, especially cancer9, 10. Additionally, changes in [Ca2+]i induced by altered membrane potential or by other mechanisms serves to regulate cell growth. Accordingly, if nanoparticles are to realize their potential in biomedical applications it is important to determine the nature of their interactions with cells (particularly the plasma membrane), and their concomitant modulation of subsequent signaling pathways (especially [Ca2+]i regulation). We address here these important issues in nanoparticle biology by testing the hypothesis that membrane potential is a key player in determining intracellular uptake of nanoparticles. Using both malignant cells (ovarian cancer CP70 and A2780 cells) and non-malignant, excitable cells (human bronchial epithelial cells (BECs) and human airway smooth muscle (ASM) cells), we investigated whether cellular membrane potential plays a role in uptake of gold nanoparticles (AuNPs) of different charges (positive, +AuNP; negative, −AuNP; neutral; 0AuNP; and zwitterionic. ±AuNP; Figure 1A), and likewise quantified the subsequent effects on [Ca2+]I and cellular proliferation vs. apoptosis.
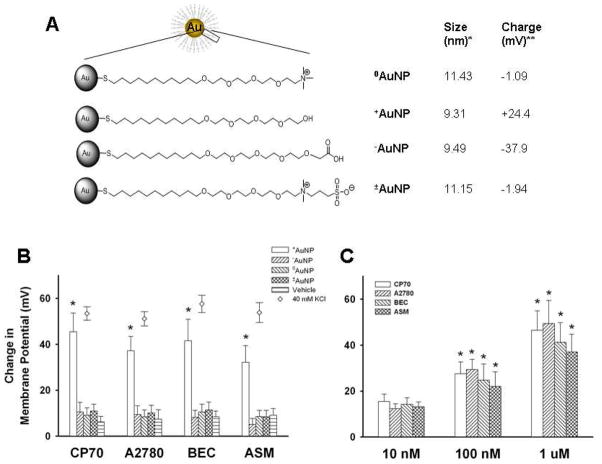
Figure 1.
Summary of gold nanoparticles (AuNPs) effects on cellular membrane potential. AuNPs of different surface charges were generated by chemical modification of the terminal portion of the ligand bonded to the nanoparticle core. Four types of AuNPs were examined (A): neutral (0AuNP), positive (+AuNP), negative (−AuNP) and zwitterionic (±AuNP). The diameter (*) and surface charge (**) were measured by dynamic light scattering and zeta potential, respectively. Using the cell-permeant fluorescent membrane potential indicator RH414 and real-time fluorescence microscopy, membrane potential changes following exposure to AuNPs of different surface charges were measured for two ovarian cancer cell lines (CP70, A2780), human bronchial epithelial cells (BEC) and human airway smooth muscle (ASM) cells (also see supplemental Figure S1). +AuNPs (1.2 μM) produced rapid and significant membrane depolarization (panel B; bars; comparable to that induced by 40 mM KCl, diamonds). The extent of membrane potential change was dependent on +AuNPs concentration (C), with minimal changes at 10 nM. In comparison to +AuNPs, those with other charges had negligible effects on membrane potential in any cell type (B). Values are means ± SE. * indicates significant AuNP effect (p<0.05).
To assess the role of surface charge of nanoparticles on membrane potential, we synthesized AuNPs (~2 nm core size) using the Brust-Schiffrin two-phase synthesis method11, 12. Surface functionalization was achieved via the Murray place-exchange method 13. These particles were applied to CP70 14, A2780, BEC15 and ASM16 cells loaded with the fluorescent, fast-response membrane potential-sensitive dye RH414. The baseline plasma membrane potential ranged between −75 mV and −55 mV depending on cell type. With images taken at 1–2 frames/s, fluorescence levels remained stable for at least 5 min in vehicle controls16. Among the four species of AuNPs, only +AuNPs induced membrane depolarization across different cell types (Figure 1B). In comparison, membrane depolarization induced by −AuNP, 0AuNP or ±AuNP was negligible (Figure 1B, also see supplemental Figure S1). The extent of membrane depolarization was found to be dependent on +AuNP concentration (Figure 1C; p<0.05 compared to vehicle control) with minimal depolarization at 10 nM, and substantial depolarization at 1.2 μM +AuNP in less than 10 s, with maximum depolarization reached in ~5 min across cell types (supplemental Figure S1). Among cell types, the extent of depolarization was greatest in ovarian cancer cells (CP70, A2780), and comparable to that achieved with 40 mM KCl (which produces a depolarization to ~−25 mV) (Figure 1B, and Figure S1). We verified lack of fluorescence quenching by examining the effect of AuNPs on RH414 fluorescence in an in vitro acellular preparation (not shown).
Next, we wanted to investigate the factors that determined intracellular uptake of AuNPs, focusing on membrane potential. In CP70, A2780, BEC and ASM cells, uptake of +AuNPs (as determined by INAA17) was significantly higher than AuNPs of other charges (Figure 2A; p<0.05). However, prior depolarization of the plasma membrane using 40 mM or 80 mM KCl (which changed membrane potential to ~−25 mV and ~−8 mV, respectively) resulted in significant reduction in the extent of +AuNP uptake in all cell types (Figure 2B; p<0.05). Furthermore, in cells pre-exposed to KCl, the extent of membrane depolarization induced by 1.2 μM +AuNPs was significantly smaller, confirming the inability of these particles to depolarize the membrane under these conditions (Figure 2C; p<0.05). In summary, these data clearly demonstrate a key role for membrane potential in intracellular uptake of AuNPs. Furthermore, by altering membrane potential, AuNPs may modulate their own uptake.
Figure 2.
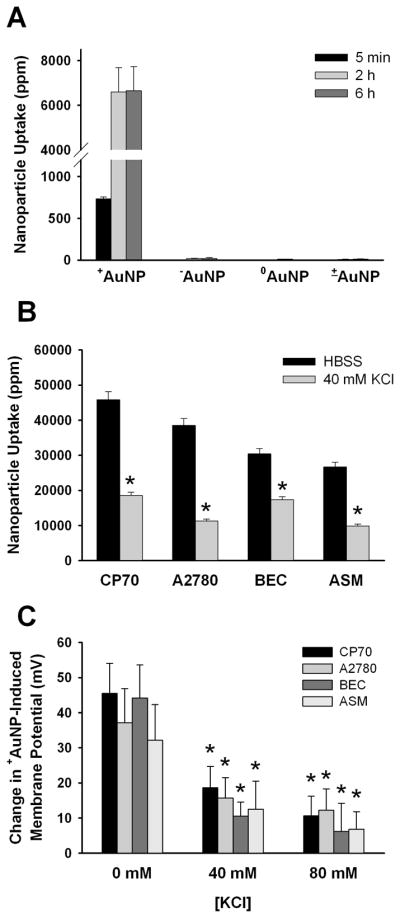
AuNP uptake and membrane potential. (A) In CP70 cells, only +AuNPs (0.4 μM) showed significant uptake, with substantial intracellular levels present at 5 min, followed by a higher level beyond 2h. (B) Prior exposure to KCl (inducing membrane depolarization) significantly (p<0.05) reduced the extent of uptake of +AuNPs (1.2 μM for 30 minutes treatment) in four different types of cells. (C) In all the cells investigated, prior exposure to KCl significantly blunted the extent of membrane depolarization subsequently induced by +AuNPs. Values are means ± SE. * indicates significant AuNP effect (p<0.05).
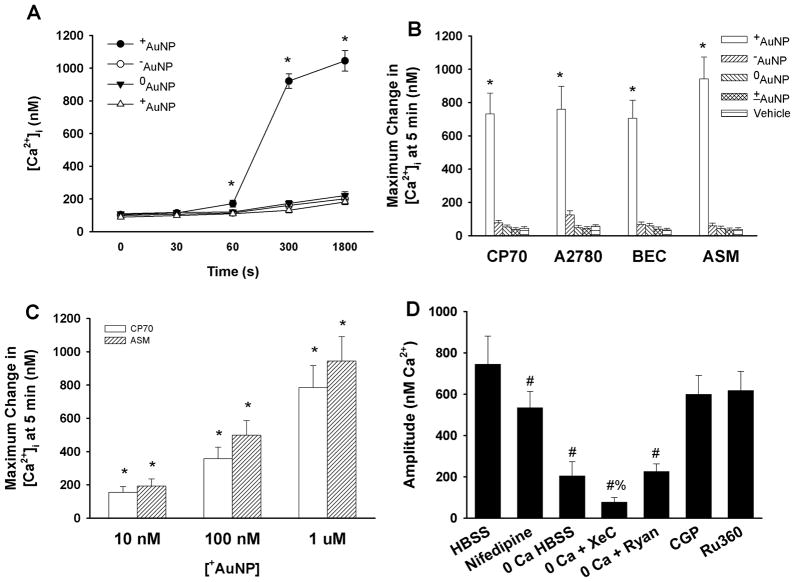
In most cells, membrane depolarization leads to increases in [Ca2+]i that can result in further modulation of cellular events (such as proliferation vs. apoptosis) 9,18. To test whether such membrane depolarization by +AuNPs and their intracellular uptake had any effect on intracellular signaling events, we first determined changes in [Ca2+]i. In CP70, A2780, BEC and ASM cells loaded with the ratiometric fluorescent Ca2+-sensitive dye fura-2/AM, baseline [Ca2+]i ranged between 75 and 120 nM (depending on cell type). In conformity with changes in membrane potential, addition of 1.2 μM +AuNP to CP70 and A2780 cells resulted in immediate and sustained increases in [Ca2+]i while in BEC and ASM cells, the increase in [Ca2+]i was slightly delayed. In all cell types, [Ca2+]i levels increased rapidly to a plateau level (Supplemental Figure S2), with maximum [Ca2+]i reached in ~5 min (Figure 3A). Some cells displayed an initial higher [Ca2+], followed by a decay to a lower level above baseline (supplemental Figure S2). Addition of AuNPs of other surface charges produced negligible changes in [Ca2+]i levels (Figure 3A, 3B and Supplemental Figure S2). In control experiments, each of these cell types were exposed to 40 mM KCl which produced [Ca2+]i elevations across cell types albeit with different time delays and profiles (supplemental Figure S2). The extent of change in [Ca2+]i was concentration-dependent, with significant changes observed even at 10 nM +AuNPs (Figure 3C; p<0.05). As with RH414, lack of fura-2 quenching by AuNPs was verified using the cell-impermeant pentapotassium form of fura-2 (not shown).
Figure 3.
Summary of +AuNPs effects on intracellular Ca2+ ([Ca2+]i) levels. (A, B) In ovarian cancer cells (CP70, A2780) and airway cells (BEC, ASM) loaded with the ratiometric fluorescent Ca2+ indicator fura-2, +AuNPs (1.2 μM) produced substantial increases in [Ca2+]i levels that reached a maximum value in ~5 minutes and in some cell types decayed to a lower level above baseline (see supplemental Figure S2). In comparison, AuNPs with other charges had negligible effects on [Ca2+]i levels. The effect on [Ca2+]i levels was dependent on the concentration of +AuNPs (C), with even 10 nM AuNPs producing a substantial increase in [Ca2+]I (compared to small effects on membrane potential). In ovarian cancer CP70 cells, the role of specific [Ca2+]i regulatory mechanisms were examined by first inhibiting specific Ca2+ regulatory mechanisms, and then exposing cells to +AuNPs (1.2 μM) (D). Inhibition of plasma membrane Ca2+ influx via L-type Ca2+ channels resulted in significant reduction in positively charged AuNP effects on [Ca2+]i levels. AuNP effects were even more reduced in the absence of extracellular Ca2+ (0 Ca HBSS) suggesting that Ca2+ influx (partly via L-type channels) contributes to the observed change in [Ca2+]i levels with AuNPs. The remainder of the [Ca2+]i response appears to be derived from endoplasmic reticulum Ca2+ release (since the response persists in the absence of extracellular Ca2+) especially via IP3 receptor channels (evidenced by lack of AuNP effects when the channels are inhibited by Xestospongin C). Lack of effect of mitochondrial Ca2+ pathways (CGP 37,157 for mitochondrial Na+/Ca2+ exchange and Ru360 for mitochondrial Ca2+ uniporter) suggests that AuNPs may not be affecting mitochondria. Values are means ± SE. * indicates significant AuNP effect, and # indicates significant effect of 0 Ca HBSS, and % indicates significant effect of inhibitor (p<0.05).
To determine the temporal relationship between membrane depolarization and elevated [Ca2+]i we simultaneously visualized both parameters by loading cells with RH414 and the non-ratiometric Ca2+ indicator fluo-3/AM. Immediately following exposure to +AuNPs, distinct membrane depolarization occurred, prior to any changes in [Ca2+]I (not shown). As membrane potential reached ~−30 mV, increases in [Ca2+]i were observed. Clearly, the membrane potential had reached a maximum state of depolarization prior to maximum changes in [Ca2+]I. The temporal relationship between depolarization and [Ca2+]i was further verified using 40 or 80 mM KCl, which induced RH414 changes prior to increasing [Ca2+]i detected using fluo-3. In this regard, the change in +AuNP-induced changes in membrane potential and [Ca2+]i were comparable to that by 40 mM KCl. Taken together, these data links membrane depolarization induced by +AuNPs to increased [Ca2+]i.
A number of mechanisms regulate [Ca2+]i, with the relative contribution of plasma membrane vs. intracellular mechanisms differing between cell types19, 20. Indeed, it is now recognized that a number of disease states involve dysregulation of this universal intracellular messenger, modulating cellular proliferation vs. apoptosis (as in cancers and other proliferative diseases), cellular contraction (as in asthma and other reactive airway diseases) and fibrosis9, 10, 16, 20, 21. Accordingly, we determined the mechanisms by which AuNPs modulate [Ca2+]i. This was performed in CP70 cells by first inhibiting specific Ca2+ regulatory mechanisms, and then exposing cells to 1.2 μM +AuNPs (Figure 3D). Inhibition of plasma membrane voltage-gated Ca2+ influx via L-type Ca2+ channels (using nifedipine) resulted in a reduction in +AuNP effects on [Ca2+]i levels (Figure 3D), indicating a significant contribution via this mechanism, especially given that +AuNP induces membrane depolarization. +AuNP effects on [Ca2+]i were even more blunted in the absence of extracellular Ca2+ (0 Ca HBSS) suggesting that Ca2+ influx via mechanisms other than L-type channels contributes to the observed change in [Ca2+]i with +AuNPs. The remainder of the [Ca2+]i response may be derived either from endoplasmic reticulum (ER) or mitochondria (since some of the response persists in the absence of extracellular Ca2+). To distinguish between these effects, inhibitors of the two well-known ER Ca2+ release channels (inositol trisphosphate (IP3) receptor channels inhibited by Xestospongin C, and ryanodine receptor (RyR) channels inhibited by high ryanodine concentrations)19, and of mitochondrial Ca2+ pathways (Ca2+ uniporter inhibited by Ru360, and mitochondrial Na+/Ca2+ exchange inhibited by CGP 37,157) 22–24 were used. In the presence of Xestospongin C (but in the absence of extracellular Ca2+), +AuNP-induced increase in [Ca2+]i was small, confirming a role for Ca2+ release from ER stores via these channels. In comparison, blocking RyR channels had no effect. The lack of effect of mitochondrial Ca2+ pathway inhibitors (Ru360 and CGP 37,157) suggests that AuNPs may not be affecting mitochondrial Ca2+ regulation. Overall, these data suggest that +AuNPs elevate [Ca2+]i by stimulating plasma membrane Ca2+ influx and ER Ca2+ release.
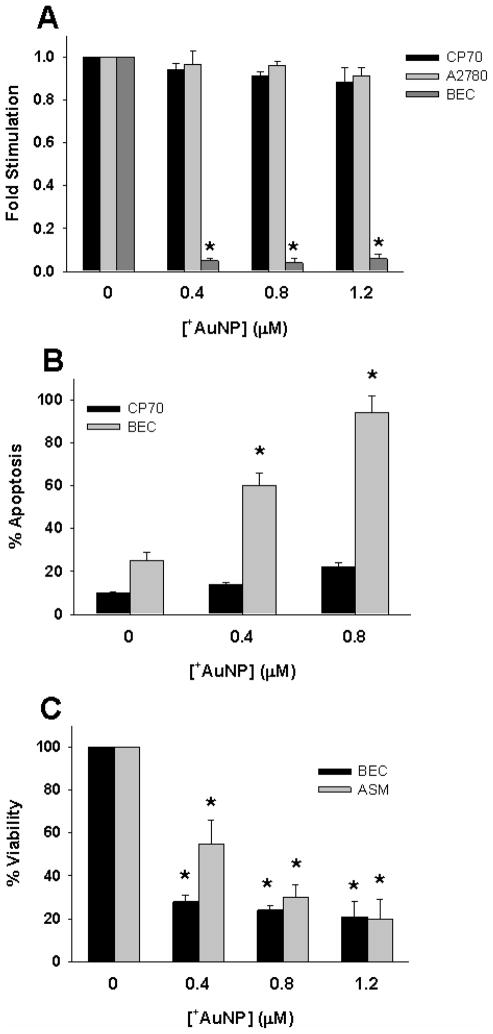
Finally we wanted to investigate whether +AuNP modulation of membrane potential, and +AuNP uptake affects cellular proliferation or viability. +AuNPs completely inhibited proliferation (determined by 3H-thymidine incorporation4) of BECs, whereas proliferation of CP70 and A2780 cells remained largely unaffected (Figure 4A; p>0.05). Furthermore, apoptosis (determined using annexin-propidium iodide assay) was only slightly increased in CP70 cells following +AuNP exposure (Figure 4B). In contrast, BEC cells displayed substantial apoptosis (Figure 4B; p<0.05). Indeed, cellular viability (determined by an MTS assay) of normal BEC and ASM cells was substantially reduced by +AuNP exposure (Figure 4C; p<0.05). To determine whether these changes in cellular proliferation and apoptosis were a result of +AuNP-induced membrane depolarization and [Ca2+]i elevation, we performed control studies where 40 mM or 80 mM KCl was used to induced membrane potential and [Ca2+]i changes. Cells were exposed to KCl only for 5 or 30 min (to transiently induce [Ca2+]i changes) to mimic +AuNP exposure. Compared to +AuNPs, KCl induced substantially lesser apoptosis, and affected proliferation of BECs to a lesser extent (supplemental Figure S3, compare to Figure 4). Furthermore, unlike +AuNPs, KCl had negligible effects on apoptosis of ASM cells. In all of these experiments, it must be noted that the duration of AuNP (or KCl) exposure was brief (minutes), while apoptosis or proliferation was evaluated after ~24h (overnight). Accordingly, these changes are unlikely to reflect short term cell death resulting from cytotoxicity of AuNPs.
Figure 4.
Effect of AuNPs on cellular proliferation vs. apoptosis. (A) Even brief (30 min) exposure of cells to +AuNPs substantially blunted the proliferation of human BECs, but did not affect the proliferation of ovarian cancer CP70 or A2780 cells. (B). Apoptosis of BEC by +AuNPs was concentration-dependent, and substantial. In contrast, some degree of apoptosis (< 10 %) of CP70 cells occurred only at a higher +AuNP concentration. (C) The viability of both BECs and ASM cells was substantially reduced by +AuNPs, with a concentration-dependence for ASM. * indicates significant effect (p<0.001)
These novel data highlight several characteristics of +AuNPs: 1) uptake of +AuNPs results in substantial inhibition of proliferation and decreased viability of normal cells, but not of cancer cells, even though comparable membrane depolarization and increased [Ca2+]i occurs across cell types. These differential effects of +AuNPs on normal vs. malignant cells, and their potential relevance to nanoparticle design and applications are interesting, and require further investigation; 2) the fact that within a cell type (e.g. BECs), +AuNP effects on proliferation, apoptosis or viability are greater than that of KCl only (in spite of comparable depolarization or [Ca2+]i elevation) indicates that +AuNP effects on cells are mediated not only via altered membrane potential and [Ca2+]I, but additional effects on signaling pathways. Accordingly, an important aspect of understanding AuNP action may be identifying different signaling mechanisms that may be targeted by AuNPs, with normal vs. cancer cells being differently sensitive to alterations in these mechanisms (especially relating to apoptosis and proliferation).
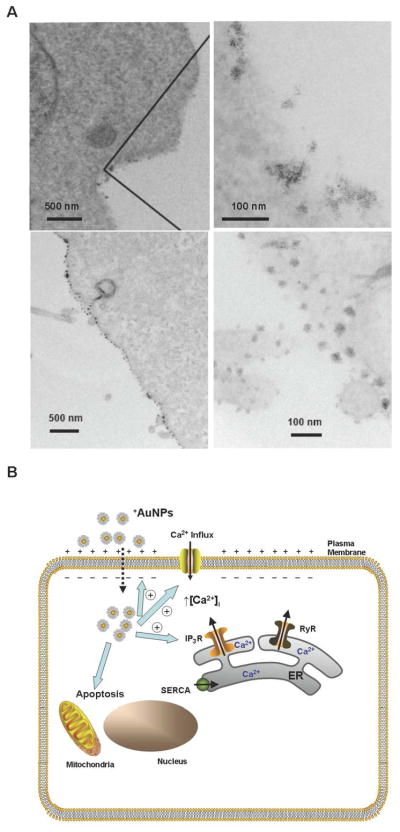
In conclusion, we have demonstrated that cellular membrane potential plays a prominent role in intracellular uptake of AuNPs. Perturbation of the membrane potential is dependent on surface charge of the nanoparticles: positively charged nanoparticles depolarize the membrane to the greatest extent with nanoparticles of other charges having negligible effect. Such membrane potential perturbations result in increased [Ca2+]i, which in turn inhibits the proliferation of normal cells whereas malignant cells remain unaffected. The mechanisms by which positively charged nanoparticles interact with the plasma membrane need to be further investigated. Such interactions may involve AuNPs binding to the plasma membrane. Indeed, this was found using transmission electron microscopy25 (TEM) where nanoparticles were clearly seen to be bound to the cell membrane (Figures 5A, left panels are the low magnification images, right panels being the higher magnification images of the corresponding left panels). Once bound to the plasma membrane, an obvious question is whether AuNPs disrupt the membrane, potentially resulting in depolarization and Ca2+ influx. However, TEM studies did not demonstrate any membrane disruption (Figure 5A). One plausible mechanism for AuNP action is the flipping of membrane areas by these particles. Uptake may also involve lipid rafts, pinocytosis and other plasma membrane mechanisms. Indeed, previous studies have found that modulation of nanoparticle surface properties can influence the mechanism of intracellular uptake (i.e. endosomal, passive diffusion) 26–28. However, the extremely fast membrane depolarization, and rapid uptake of AuNPs that was observed in our study need to be reconciled with the relatively slow rate of such uptake processes. Regardless, the findings of the present study will help to better define the biology of cell-nanoparticle interactions and help engineer nanoparticles to modulate cellular functions of interest. For example, varying surface charge density or combining positive and negative charges on the same nanoparticle may allow for graduated cellular uptake, targeting towards specific intracellular organelles, as well as control of the extent of change in [Ca2+]i and other effects, thus balanced unintended cytotoxicity vs. targeting mechanisms of interest.
Figure 5.
(A) Transmission electron microscopy of +AuNP (concentration = 0.4 μM) interactions with plasma membrane. Top panels demonstrate localization of +AuNPs within the plasma membrane (CP70 cells). Bottom panels demonstrate lack of plasma membrane disruption following +AuNP uptake (BEC cells). (B) Schematic of AuNP effects on cellular function. Based on our findings using +AuNPs and different types of cells, we propose that AuNPs are taken up intracellularly, based on membrane potential. Upon uptake, +AuNPs produce membrane depolarization, and increase [Ca2+]i by enhancing Ca2+ influx and inducing release of intracellular Ca2+ stores (e.g. via IP3 receptor channels of the endoplasmic reticulum; ER). These changes can result in increased apoptosis and decreased cellular proliferation, depending on cell type. Further modulation of apoptosis and proliferation may involve direct nanoparticle effects on intracellular signaling mechanisms.
Supplementary Material
Acknowledgments
Supported by NIH CA135011, CA136494 and UTMD-1 grants (PM), and by NIH grants HL090595 (CMP), GM077173 (VMR) and HL088029 (YSP).
Footnotes
Supporting Information Available: Materials and methods; additional data. This material is available free of charge via the internet at http://pubs.acs.org
References
- 1.Ferrari M. Nat Rev Cancer. 2005;5(3):161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 2.Burda C, Chen X, Narayanan R, El-Sayed MA. Chem Rev. 2005;105(4):1025–102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 3.Daniel MC, Astruc D. Chem Rev. 2004;104(1):293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 4.Patra CR, Bhattacharya R, Wang E, Katarya A, Lau JS, Dutta S, Muders M, Wang S, Buhrow SA, Safgren SL, Yaszemski MJ, Reid JM, Ames MM, Mukherjee P, Mukhopadhyay D. Cancer Res. 2008;68(6):1970–8. doi: 10.1158/0008-5472.CAN-07-6102. [DOI] [PubMed] [Google Scholar]
- 5.Mirkin CA, Taton TA. Nature. 2000;405(6787):626–7. doi: 10.1038/35015190. [DOI] [PubMed] [Google Scholar]
- 6.Alivisatos P. Nat Biotechnol. 2004;22(1):47–52. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 7.Whitesides GM. Nat Biotechnol. 2003;21(10):1161–5. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 8.Xu P, Van†Kirk EA, Zhan Y, Murdoch WJ, Radosz M, Shen Y. Angewandte Chemie International Edition. 2007;46(26):4999–5002. doi: 10.1002/anie.200605254. [DOI] [PubMed] [Google Scholar]
- 9.Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Nature Reviews Cancer. 2007;7(7):519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 10.Prevarskaya N, Skryma R, Shuba Y. Trends in Molecular Medicine. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 11.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman RJ. J Chem Soc, Chem Commun. 1994:801–802. [Google Scholar]
- 12.Kanaras AG, Kamounah FS, Schaumburg K, Kiely CJ, Brust M. Chem Commun. 2002:2294–2295. doi: 10.1039/b207838b. [DOI] [PubMed] [Google Scholar]
- 13.Templeton AC, Wuelfing WP, Murray RW. Acc Chem Res. 2000;33:27. doi: 10.1021/ar9602664. [DOI] [PubMed] [Google Scholar]
- 14.Behrens BC, Hamilton TC, Masuda H, Grotzinger KR, Whang-Peng J, Louie KG, Knutsen T, McKoy WM, Young RC, Ozols RF. Cancer Research. 1987;47(2):414–418. [PubMed] [Google Scholar]
- 15.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 16.Prakash YS, Thompson MA, Pabelick CM. Am J Respir Cell Mol Biol. 2009;41(5):603–11. doi: 10.1165/rcmb.2008-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kattumuri V, Katti K, Bhaskaran S, Boote EJ, Casteel SW, Fent GM, Robertson DJ, Chandrasekhar M, Kannan R, Katti KV. Small. 2007;3(2):333–341. doi: 10.1002/smll.200600427. [DOI] [PubMed] [Google Scholar]
- 18.Chakrabarti R. J Cell Biochem. 2006;99(6):1503–16. doi: 10.1002/jcb.21102. [DOI] [PubMed] [Google Scholar]
- 19.Pabelick CM, Sieck GC, Prakash YS. J Appl Physiol. 2001;91(1):488–96. doi: 10.1152/jappl.2001.91.1.488. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Zoghbi JF, Karner C, Ito S, Shepherd M, Alrashdan Y, Sanderson MJ. Pulm Pharmacol Ther. 2009;22(5):388–97. doi: 10.1016/j.pupt.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L26–34. doi: 10.1152/ajplung.00026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balemba OB, Bartoo AC, Nelson MT, Mawe GM. Am J Physiol Gastrointest Liver Physiol. 2008;294(2):G467–476. doi: 10.1152/ajpgi.00415.2007. [DOI] [PubMed] [Google Scholar]
- 23.Michels GMK, Ismail F, PhD, Endres-Becker Jeannette, PhD, Rottlaender Dennis, MD, Herzig Stefan, MD, Ruhparwar Arjang, MD, Wahlers Thorsten, MD, Hoppe Uta C., MD Circulation. 2009;119(18):2435–2443. doi: 10.1161/CIRCULATIONAHA.108.835389. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Brown DA, O’Rourke B. Biophysical Journal. 2009;96(3 Supplement 1):243a–243a. [Google Scholar]
- 25.Bhattacharya R, Patra CR, Earl A, Wang S, Katarya A, Lu L, Kizhakkedathu JN, Yaszemski MJ, Greipp PR, Mukhopadhyay D, Mukherjee P. Nanomed. 2007;3:224–238. [Google Scholar]
- 26.Verma A, Uzun O, Hu Y, Hu Y, Han HS, Watson N, Chen S, Irvine DJ, Stellacci F. Nat Mater. 2008;7(7):588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leroueil PR, Hong S, Mecke A, Baker JR, Orr BG, Banaszak Holl MM. Accounts of Chemical Research. 2007;40(5):335–342. doi: 10.1021/ar600012y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma A, Stellacci F. Small. 2010;6(1):12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.