Abstract
Gamma-Interferon-inducible Lysosomal Thiolreductase (GILT) promotes Major Histocompatibility Complex (MHC) class II-restricted presentation of exogenous antigens containing disulfide bonds. Here we show that GILT also facilitates MHC class I-restricted recognition of such antigens by CD8+ T cells, or cross-presentation. GILT is essential for cross-presentation of a CD8+ T cell epitope of glycoprotein B (gB) from Herpes Simplex Virus (HSV)-1 but not for its presentation by infected cells. Initiation of the gB-specific CD8+ T cell response during HSV-1 infection, or cross-priming, is highly GILT-dependent, as is initiation of the response to the envelope glycoproteins of influenza A virus. Efficient cross-presentation of disulfide-rich antigens requires a complex pathway involving GILT-mediated reduction, unfolding and partial proteolysis, followed by translocation into the cytosol for proteasomal processing.
Cross-priming (1) is important for the development of specific CD8+ T cell responses to viruses that do not directly infect antigen presenting cells (APCs) (2). The critical APCs for cross-presentation are dendritic cells (DCs), which acquire antigens by phagocytosis of apoptotic and necrotic infected cells and migrate to secondary lymphoid organs to activate resident naïve CD8+ T cells (3). Transfer of antigen from migratory DCs to resident CD8α+ DCs may be required (4, 5). The pathways that generate complexes of MHC class I molecules with peptides derived from internalized antigens are not well understood. Occasionally the peptides are generated in the endocytic pathway and bind to recycling MHC class I molecules (6). However, the dominant mechanism involves translocation of the antigens into the cytosol, where proteasomal degradation generates peptides which are transported via the Transporter associated with Antigen Processing (TAP) and bind to newly synthesized MHC class I molecules (7). The translocation mechanism may involve components of the endoplasmic reticulum-associated degradation (ERAD) machinery (8, 9).
Intact functional proteins can enter the DC cytosol after internalization (10–12), and recently we showed that luciferases can be unfolded in the endocytic pathway, translocated, and cytosolically refolded by the chaperone Hsp90 (12). The suggestion that translocation may require unfolding led us to investigate the role of GILT, a soluble enzyme expressed constitutively in APCs, in cross-presentation. GILT is the only known thiol reductase localized in lysosomes and phagosomes (13, 14), and we hypothesized that acidification combined with GILT-mediated reduction could mediate the unfolding of internalized disulfide-containing antigens and facilitate their translocation into the cytosol.
Viral glycoproteins are often recognized by CD8+ T cells and are rich in disulfide bonds. We selected gB from HSV-1, which has a well-characterized MHC class I-restricted epitope (15), as a model antigen. In vitro cross-presentation assays were established using bone marrow-derived DCs from wild type and mice lacking Ifi30, the gene encoding GILT, and HSV-infected HeLa cells to provide apoptotic or necrotic bodies for antigen uptake (16). Using an H2-Kb-restricted CD8+ T cell hybridoma specific for gB498–505, we found that cross-presentation of gB is indeed dependent upon GILT expression (Fig. 1A). Cross-presentation of a second HSV-1 epitope, ICP6822–829 from a viral ribonucleotide reductase, was GILT-independent. gB contains five disulfide bonds (17), while ICP6 is a cytosolic protein and likely has none. Wild type and GILT-negative DCs presented both the ICP6 and gB epitopes when directly infected by HSV-1 (Fig. 1B). To determine whether the enzymatic activity of GILT is required for cross-presentation, wild type GILT or single or double cysteine active site mutants were introduced into Ifi30−/− DCs. Only wild type GILT restored cross-presentation (Fig. 1C). Cross-presentation of purified recombinant gB by DCs was also mediated by wild type DCs but not those lacking GILT (Fig. 1D). If the disulfide bonds in gB were first reduced, however (Fig. S1), GILT-negative cells were able to cross-present the gB epitope (Fig. 1D). Both wild type and GILT-negative DCs were capable of cross-presenting an ovalbumin epitope regardless of reduction (Fig. 1D).
Fig. 1.
Reduction by GILT is necessary for cross-presentation of gB498–505. IL-2 production, measured by ELISA, by Kb-restricted gB498–505- and ICP6822–829-specific CD8+ T cell hybridomas in response to (A) wild type and GILT-negative DCs co-cultured with HSV-1-infected HeLa cell debris, (B) wild type and GILT-negative DCs directly infected by HSV-1 or (C) GILT-negative DCs retrovirally transduced with wild type GILT or inactive single or double cysteine mutants following co-culture with infected cell debris. (D) IL-2 production by gB498–505- and ovalbumin-specific CD8+ T cell hybridomas in response to wild type and GILT-negative DCs co-cultured with indicated concentrations of recombinant soluble gB or ovalbumin, untreated or treated with dithiothreitol (DTT). Each of the experiments shown is representative of three. *P < 0.05; **P < 0.01, calculated by t-tests. Graphs show mean ± SEM.
A central question is whether cross-presentation depends on reduction of intact gB by GILT. Immunofluorescence analysis clearly showed that GILT and gB were both present in the same intracellular compartment as LAMP-1, a lysosomal/phagosomal marker, in DCs incubated with necrotic infected HeLa cells (Fig. 2A). To demonstrate that GILT mediates gB reduction we used a GILT trapping mutant with a mutation in the second cysteine of the CXXC active site, which leads to accumulation of disulfide-linked enzyme-substrate complexes because substrate release is blocked (14, 18). When necrotic infected HeLa cells were incubated with DCs expressing the trapping mutant, a gB-GILT mixed disulfide was clearly detectable (Fig. 2B). Under reducing conditions the GILT-associated gB had the same mobility in SDS-PAGE as in the HeLa cells (Fig. 2C). The doublet likely results from differential glycosylation. These data argue that GILT directly reduces disulfide bonds in the intact glycoprotein.
Fig. 2.
GILT interacts with gB in DCs. (A) Visualization of intracellular location of GILT and gB wild type and GILT-negative DCs that have taken up infected HeLa cell debris. LAMP-1 is a lysosomal/phagosomal marker. Wild type and GILT-negative DCs were incubated with either uninfected or HSV-1-infected HeLa cells debris for 3 hours. Cells were then harvested, permeabilized, and stained for immunofluorescence. (B) Wild type DCs, GILT-negative DCs, or GILT-negative DCs reconstituted with the GILT C71S trapping mutant were incubated with infected HeLa cell debris for 3 hours prior to detergent solubilization and immunoprecipitation with an H2-Kb control antibody (Y3) or a GILT mAb (MaP.GILT6), non-reducing SDS-PAGE and western blotting. Top panel: gel probed with a gB-specific rabbit antiserum. Middle panel: the DC or HeLa cell lysates were probed with mouse or human calreticulin antibodies as a loading control. Bottom panel: lysates were probed with a GILT antibody. Note GILT is only present in the wild type DC and the GILT-negative DC samples reconstituted with the mutant. The first 9 lanes are DC lysates. The final two lanes in the top panel are uninfected (UI) or infected (I) HeLa cell lysates. (C) Identical to panel B except SDS-PAGE was performed under reducing conditions. Each experiment was done at least three times and a representative experiment is shown.
Vesicular acidification is usually required for MHC class II presentation and may be required for cross-presentation (19–21). Blocking acidification using bafilomycin or concanamycin B abrogated cross-presentation of the gB498–505 epitope (Fig. 3A). GILT has an acidic pH optimum, but neutralization could also inhibit essential lysosomal proteolysis. We examined the effects of pepstatin A, which mainly inhibits cathepsin D, an aspartyl protease, and leupeptin, an inhibitor of cysteine proteases, including cathepsin B. Both blocked gB cross-presentation, suggesting that multiple proteases are required (Fig. 3B and C). Furthermore, when we examined by immunofluorescence microscopy the turnover of gB within wild type and GILT-negative DCs incubated with necrotic infected cells, gB expression decreased much more rapidly in the wild type DCs (Fig 3D). The data suggest that an interplay between GILT-mediated reduction and degradation by several proteases generates gB fragments that are then cross-presented.
Fig. 3.
GILT-dependent cross-presentation of gB requires lysosomal and proteasomal processing and is TAP-dependent. (A) IL-2 production, measured by ELISA, by Kb-restricted gB498–505-specific CD8+ T cell hybridoma in response to wild type DCs treated with bafilomycin or concanamycin B prior to HeLa cell uptake. IL-2 production by gB498–505-specific CD8+ T cell hybridomas in response to wild type DCs treated with (B) pepstatin A or (C) leupeptin. (D) Kinetics of gB degradation, determined by immunofluorescence, in wild type or GILT-negative DCs incubated with infected HeLa cell debris. A total of 1000 DCs were counted per time point and analyzed by gB antibody staining. (E) IL-2 production by gB498–505-and ICP6822–829- specific CD8+ T cell hybridomas to wild type or GILT-negative DCs treated with lactacystin. (F) IL-2 production by gB498–505-specific CD8+ T cellhybridomas to wild type or Tap1−/− DCs. A representative example of three individual experiments is shown for each panel. *P < 0.05; **P < 0.01, calculated by t-tests. Graphs show mean ± SEM.
Proteolysis in the phagosome could give rise to gB498–505 that binds directly to Kb molecules or result in gB fragments that are translocated into the cytoplasm. To determine whether cytosolic access is required we examined the roles of TAP and proteasomes in gB cross-presentation. When DCs from Tap−/− mice were incubated with necrotic infected cells gB cross-presentation was completely eliminated (Fig. 3F). In addition, cross-presentation of gB, as well as ICP6, was inhibited by lactacystin, indicating dependence on proteasomal processing (Fig. 3E). Cross-presentation thus depends on cytosolic processing of gB fragments generated in the phagosome by GILT-mediated reduction and cathepsin-mediated proteolysis.
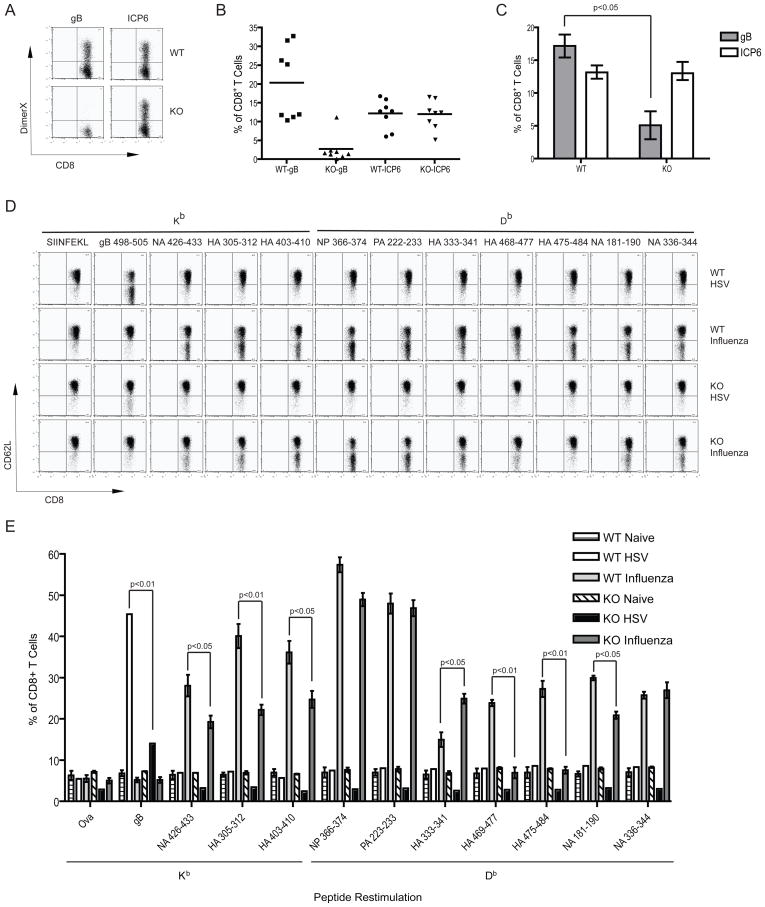
A requirement for GILT in the induction of the CD8+ T cell response to gB498–505 during an infection would argue that cross-priming is important for the in vivo anti-HSV-1 immune response. Wild type and Ifi30−/− mice were infected with HSV-1 and the draining lymph nodes (LN) were examined for the induction of Kb-gB498–505-specific and Kb-ICP6822–829 specific CD8+ T cells. While mice lacking GILT generated the same average percentage of ICP6822–829-specific CD8+ T cells when infected with HSV-1 as wild type mice, the number of gB498–505-specific CD8+ T cells was significantly reduced (Fig. 4A–C). There was no difference in the survival of the infected mice. Responses to GILT-independent epitopes such as ICP6822–829 may make up for any deficiency.
Fig. 4.
GILT-dependent cross-priming to gB and influenza A virus glycoproteins in vivo. (A) Flow cytometric analysis of CD8+ T cells specific for gB498–505 from wild type or Ifi30−/− mice infected with HSV-1, detected using gB498–505- or ICP6822–829 -loaded DimerX:Kb-Ig fusion protein. Draining LNs were examined 6 days after infection. A representative dot plot is shown. (B) The percentage of gB498–505 and ICP6822–829 -specific CD8+ T cells from HSV-1-infected wild type and Ifi30−/− mice. A representative experiment of three individual experiments is shown. (C) As in panel B, showing the average of 3 independent experiments. Graph shows mean ± SEM. (D) Flow cytometric analysis of recall CD8+ T cell responses isolated from HSV-1- or influenza A-infected mice and restimulated with wild type DCs pulsed with the indicated peptides. Cells were cultured for 2 days with APCs loaded with each peptide prior to FACs analysis for activation assessed by the downregulation of CD62L. A representative dot plot of a wild type mouse and a Ifi30−/− mouse infected with HSV or influenza A is shown. (E) As in panel D, but showing the average of 3 independent experiments. Graphs show mean ± SEM. P-values calculated by t-tests.
To determine whether GILT-dependent cross-presentation is a more general phenomenon we examined the CD8+ T cell response of mice infected with the PR8 strain of influenza A virus. LN cells from naïve and infected mice were re-stimulated with wild type DCs pulsed with peptides corresponding to a variety of H2-Kb- and H2-Db-restricted epitopes from hemagglutinin (HA), neuraminidase (HA), polymerase (PA), and nucleoprotein (NP) (http://www.immuneepitope.com/home.do) (22). HA and NA contain 6 and 8 disulfide bonds, respectively, whereas PA and NP have none (23–25). A similar percentage of wild type and GILT-negative CD8+ T cells responded to Db-restricted PA and NP epitopes upon restimulation (Fig. 4D, E). In contrast, the responses of CD8+ T cells from mice lacking GILT were significantly reduced for 4 out of 5 of the HA epitopes and for 2 out of 3 of the NA epitopes. The two HA epitopes to which almost no CD8+ T cells develop in the Ifi30−/− mice contain or are immediately adjacent to a cysteine (C480) involved in a disulfide bond (C21–C480). For both HA and NA one epitope is GILT-independent, strongly arguing against the possibility that any GILT requirement reflects GILT-dependent MHC class II-restricted responses that mediate CD4+ T cell help (26). Although the epitope-specificity of the CD4+ T cells in the Ifi30−/− mice may be different from wild type mice, the total numbers of CD4+ T cells they do generate during a viral immune response is similar (Fig. S2), as are the numbers of CD4+ T cells in the spleens of uninfected wild type and Ifi30−/− mice (data not shown). The data show that GILT-dependent cross-presentation is not restricted to gB, and that cross-priming is important in the CD8+ T cell response to influenza virus. The residual CD8+ T cell responses observed to gB and the HA and NA epitopes by Ifi30−/− animals may reflect priming by directly infected APCs.
The only known function of GILT is to reduce disulfide bonds, and we have shown that GILT is essential for cross-presentation of many peptides from disulfide-containing proteins. We suggest that reduction in the acidic environment of the phagosome facilitates partial proteolysis into fragments that are translocated into the cytosol where they are further degraded by the proteasome to generate peptides. These are transported by TAP and bind in a conventional manner, possibly after amino-terminal trimming (27), to MHC class I molecules. This latter step is likely to occur in the ER, but could occur in phagosomes that have recruited ER membrane components, although this issue remains contentious (28, 29).
For gB, the inability to cross-present is reflected in a reduction in Kb-gB498–505-specific CD8+ T cells in vivo, indicating the importance of cross-priming in CD8+ T cell responses to HSV-1 infection. The similar reduction in HA- and NA-specific CD8+ T cells suggests that cross-priming is also important during influenza A infection. The role played by GILT in cross-priming, combined with its established involvement in MHC class II-restricted CD4+ T cell responses (30) indicates the importance of the enzyme in the immune system. This may have implications for vaccine design and approaches to tumor immunotherapy that involve peptide-based vaccines, in that linear peptides may not represent the optimal vehicles for the expression of GILT-dependent epitopes, and for autoimmunity to self-antigens that contain multiple disulfide bonds.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and NIH grant R37AI23081 (PC).
References and Notes
- 1.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 3.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 4.Allan RS, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 6.Belizaire R, Unanue ER. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proc Natl Acad Sci U S A. 2009;106:17463–17468. doi: 10.1073/pnas.0908583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 8.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Schafer A, Wolf DH. Sec61p is part of the endoplasmic reticulum-associated degradation machinery. Embo J. 2009;28:2874–2884. doi: 10.1038/emboj.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin ML, et al. Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proc Natl Acad Sci U S A. 2008;105:3029–3034. doi: 10.1073/pnas.0712394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–288. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 12.Giodini A, Cresswell P. Hsp90-mediated cytosolic refolding of exogenous proteins internalized by dendritic cells. Embo J. 2008;27:201–211. doi: 10.1038/sj.emboj.7601941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arunachalam B, Phan UT, Geuze HJ, Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT) Proc Natl Acad Sci U S A. 2000;97:745–750. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan UT, Arunachalam B, Cresswell P. Gamma-interferon-inducible lysosomal thiol reductase (GILT). Maturation, activity, and mechanism of action. J Biol Chem. 2000;275:25907–25914. doi: 10.1074/jbc.M003459200. [DOI] [PubMed] [Google Scholar]
- 15.Hanke T, Graham FL, Rosenthal KL, Johnson DC. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol. 1991;65:1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See supporting material on Science Online.
- 17.Heldwein EE, et al. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Jamieson A, Cresswell P. GILT is a critical host factor for Listeria monocytogenes infection. Nature. 2008;455:1244–1247. doi: 10.1038/nature07344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins DS, Unanue ER, Harding CV. Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol. 1991;147:4054–4059. [PubMed] [Google Scholar]
- 20.McCoy KL, et al. Diminished antigen processing by endosomal acidification mutant antigen-presenting cells. J Immunol. 1989;143:29–38. [PubMed] [Google Scholar]
- 21.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Zhong W, Reche PA, Lai CC, Reinhold B, Reinherz EL. Genome-wide characterization of a viral cytotoxic T lymphocyte epitope repertoire. J Biol Chem. 2003;278:45135–45144. doi: 10.1074/jbc.M307417200. [DOI] [PubMed] [Google Scholar]
- 23.Varghese JN, Laver WG, Colman PM. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983;303:35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- 24.Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 25.Ye Q, Krug RM, Tao YJ. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature. 2006;444:1078–1082. doi: 10.1038/nature05379. [DOI] [PubMed] [Google Scholar]
- 26.Rajasagi NK, et al. CD4+ T cells are required for the priming of CD8+ T cells following infection with herpes simplex virus type 1. J Virol. 2009;83:5256–5268. doi: 10.1128/JVI.01997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serwold T, Gonzalez F, Kim J, Jacob R, Shastri N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419:480–483. doi: 10.1038/nature01074. [DOI] [PubMed] [Google Scholar]
- 28.Jutras I, Desjardins M. Phagocytosis: at the crossroads of innate and adaptive immunity. Annu Rev Cell Dev Biol. 2005;21:511–527. doi: 10.1146/annurev.cellbio.20.010403.102755. [DOI] [PubMed] [Google Scholar]
- 29.Touret N, et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell. 2005;123:157–170. doi: 10.1016/j.cell.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Maric M, et al. Defective antigen processing in GILT-free mice. Science. 2001;294:1361–1365. doi: 10.1126/science.1065500. [DOI] [PubMed] [Google Scholar]