Abstract
Much of the work aimed at elucidating the pathogenesis of osteonecrosis (ON) of the femoral head has focused on bone blood supply, with little attention to the surrounding synovial tissue (ST). We hypothesized that patients with ON exhibit synovial inflammation. Using immunohistological techniques, we found that a large population of patients with ON had synovial inflammation. Moreover, a population of ON patients had inflamed ST without having an inflammatory disease co‐morbidity. The inflammatory infiltrate in these patients comprised primarily CD4+ T cells and CD68+ macrophages, the latter of which expressed increased levels of cellular adhesion molecules. Our results suggest the presence of a previously unrecognized population of ON patients without a diagnosed inflammatory co‐morbidity and a highly inflammed synovium consisting primarily of a macrophage and CD4+ T‐cell infi ltrate.
Keywords: osteonecrosis, synovium, inflammation, macrophage, adhesion molecules
Introduction
Osteonecrosis (ON) of the femoral head, also known as avascular necrosis, is a progressively debilitating disease that usually leads to the destruction of the hip joint. It is a fairly common disease that is responsible for 5% to 12% of all total hip replacement surgeries, with an incidence estimated to be between 10,000 and 20,000 cases per year in the United States. 1 Afected individuals are typically between 20 and 50 years of age with only 20% of patients being over 50 years of age at the time of surgery, much younger than the typical patient requiring joint replacement surgery. 1
Although a number of risk factors have been associated with the disease, its etiology is unknown. Associated risk factors include trauma to the joint and nontraumatic factors such as high‐dose glucocorticoid therapy, dyslipidemia, systemic lupus erythematosus (SLE), smoking, and alcohol abuse, among others. 2 In addition, genetic risk factors have been identified, including inherited coagulation disorders, and gene polymorphisms in alcohol‐metabolizing enzymes, the drug transport protein P‐glycoprotein, and the collagen type‐II gene. 3 However, 10% to 20% of patients with ON do not have an identifiable risk factor for the disease, and are thus classified as idiopathic. 2 Regardless of the initiating factor, ischemia in the femoral head is the central effect that eventually leads to ON. 2
It is well understood that synovial inflammation plays a primary role in the pathogenesis of rheumatoid arthritis (RA) and other inflammatory arthritides. 4 This is in contrast to osteoarthritis (OA) where synovial inflammation plays a lesser role in joint destruction. 5 Recently, Pessler et al. 6 examined synovial inflammation in patients with select orthopaedic noninflammatory arthropathies, including patients with femur fracture, plica syndrome, meniscus and/or ligament injury, and ON of the femoral head. They demonstrated that collectively, these patients have increased synovial inflammation compared to normal synovium. 6 However, as their study was designed to examine synovial inflammation in patients with several different orthopaedic noninflammatory arthropathies, only three patients with ON of the fermoral head were examined, and the inflammatory characteristics for this patient population were not presented.
We recently observed that patients with ON undergoing total hip replacement surgery often have a synovium that appears inflamed upon gross examination at the time of surgery. Therefore, we hypothesized that patients with ON of the femoral head have significant synovial inflammation that may play a role in disease progression. Moreover, we sought to determine if the inflammation in these patients is independent of previously diagnosed inflammatory conditions and to characterize the inflammatory infiltrate.
Materials and Methods
Patient samples
A total of 20 ON hip synovial tissue (ST) samples were obtained from patients undergoing total hip replacement surgery. All cases were seen by a single surgeon over the period the study was conducted. ON of the femoral head was diagnosed by radiograph, and all patients had subchondral collapse or greater prior to surgery and were classified according to the University of Pennsylvania ON staging system ( Table 1 ). 7 For a comparison population, 10 OA knee ST samples were obtained from patients undergoing knee replacement surgery or from tissue obtained through the Cooperative Human Tissue Network. All protocols were reviewed and approved by the Institutional Review Board at the University of Michigan. Fresh ST was placed in optimal cutting temperature (OCT) freezing media, frozen in liquid nitrogen, and stored at –80°C until sectioning.
Table 1.
ON patient characteristics.
| Patient number | Sex | Age | Pathological changes | Steinberg classification | inflammatory co‐morbidities | Identifiable risk factors for ON |
|---|---|---|---|---|---|---|
| 1 | Female | 80 | Acetabulum, femoral head collapse | V | Temporal arthritis | Steroid use |
| 2 | Female | 44 | Acetabulum, crescent sign | III | SLE, RA, leukocyto‐clastic vasculitis | SLE |
| 3 | Female | 66 | Acetabulum, femoral head collapse, sclerosis | V | Hyperlipidemia | |
| 4 | Female | 61 | Acetabulum, femoral head collapse | V | Hyperlipidemia | |
| 5 | Male | 61 | Crescent sign | III | ||
| 6 | Female | 23 | Femoral head collapse | IV | SLE | SLE |
| 7 | Female | 69 | Femoral head collapse | IV | Psoriatic arthritis | Hyperlipidemia, Smoking |
| 8 | Female | 53 | Crescent sign | III | Smoking | |
| 9 | Female | 50 | Acetabulum, femoral head collapse | V | ||
| 10 | Female | 73 | Acetabulum, femoral head collapse, dysplastic hip | V | Hyperlipidemia | |
| 11 | Male | 32 | Crescent sign | III | SLE | Hyperlipidemia |
| 12 | Female | 49 | Acetabulum, femoral head collapse | V | ||
| 13 | Female | 39 | Femoral head collapse, sclerosis | IV | Smoking | |
| 14 | Male | 56 | Femoral head collapse, sclerosis | IV | Steroid use | |
| 15 | Female | 54 | Acetabulum, femoral head collapse | V | ||
| 16 | Female | 74 | Acetabulum, femoral head collapse | V | ||
| 17 | Female | 78 | Acetabulum, femoral head collapse | V | Hyperlipidemia, Alcohol abuse | |
| 18 | Female | 55 | Femoral head collapse | IV | Crohns disease, OA | Hyperlipidemia |
| 19 | Male | 43 | Acetabulum, femoral head collapse | V | RA | |
| 20 | Female | 64 | Acetabulum, femoral head collapse | V | Microscopic polyangiitis | Microscopic polyangiitis |
SLE = systemic lupus erythematosus; RA = rheumatoid arthritis; OA = osteoarthritis.
Antibodies and reagents
Mouse anti‐human CD3 (pan T‐cell marker), mouse anti‐human CD4 (T helper cell marker), mouse anti‐human CD8 (cytotoxic T‐cell marker), mouse anti‐human CD68 (macrophage marker), and rabbit anti‐human von Willebrand factor (vWF, endothelial cell marker) antibodies were purchased from Dako (Glostrup, Denmark). Mouse anti‐human CD19 antibody was purchased from BD Pharmingen (San Diego, CA, USA). Rabbit anti‐human integrin αv and rabbit anti‐human ICAM‐1 antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Species‐specific biotinylated secondary antibodies were purchased from Vector Laboratories (Burlingame, CA, USA). Mouse and rabbit purified IgG were purchased from Pierce (Rockford, IL, USA) and used as negative controls throughout.
Immunohistology
Frozen ST samples obtained from patients with ON or OA were cut (approximately 5 μm) and stained using an immunoperoxidase method. Slides were fixed in cold acetone for 20 minutes. Following incubation with 3% H2O2 for 5 minutes to block endogenous peroxidase, STs were blocked with blocking buffer (20% fetal bovine serum (FBS) and 5% goat, rabbit, or horse serum in phosphate buffered saline [PBS]) at 37°C for 1 hour, and then incubated with specific primary antibody (10 μg/mL) or purified nonspecific goat IgG for 1 hour at 37°C in blocking buffer. The STs were washed with PBS, and a 1:200 dilution (in blocking buffer) of biotinylated secondary antibody was added and incubated for an additional 1 hour at 37°C. After washing with PBS, antibody binding was detected using Vectastain ABC Elite kit (Vector Labs, Burlingame, CA, USA) and the chromogen 3,3’‐diaminobenzidine (DAB) (Vector Labs). STs were rinsed in tap water, counterstained with Gill's hematoxylin, and dipped in saturated lithium carbonate solution for bluing. Staining was evaluated under blinded conditions and graded by a pathologist. Slides were examined for cellular immunoreactivity and cell types were distinguished based on their characteristic morphology. ST inflammation was determined on a 0 to 4 scale depending on the inflammatory cell invasion, as we have established previously. 8 The inflammatory score was obtained using the following scoring system: 0 = normal; 1 = few scattered inflammatory cells; 2 = mild but distinct increase in scattered inflammatory cells; 3 = moderate inflammation, including discrete aggregates; and 4 = marked diffuse inflammatory cell infl ltrate. To determine the relative number of each specific cell type, the percent staining of each specific cell surface marker for each cell type was multiplied by the inflammatory score for each patient. A macrophage positivity score was assigned by a blinded pathologist to determine how many macrophages were expressing a given adhesion molecule in the section based on the following scoring system: 0 = normal; 1 = few scattered cells; 2 = more abundant individual cells and small clusters; 3 = large clusters of cells; 4 = diffuse sheets of cells.
Statistical analysis
Data was analyzed using a Student's t‐test assuming equal variances. P‐values < 0.05 were considered statistically significant. Data are represented as the mean ± standard error of the mean (SEM).
Results
Patients with ON have synovial inflammation
To test our hypothesis, we determined the extent of inflammation in ON and OA ST. We found that of the 20 ON STs examined, 13 (65%) had significant synovial inflammation ( Figure 1 ). When grouped together, these inflammatory ON STs had a significantly higher inflammatory score than OA ST ( Figure 1 ). It was also noted that the inflammatory infiltrate in ON ST looked different than that observed in OA ST, and that which has been previously observed in RA ST. The inf ltrate in ON ST contained lymphoid aggregates largely centered around vessels, with little inflammation in the interstitium ( Figure 1 , arrows). In addition, the ON ST lining layer was much thicker than the synovial lining from OA ST (data not shown).
Figure 1.
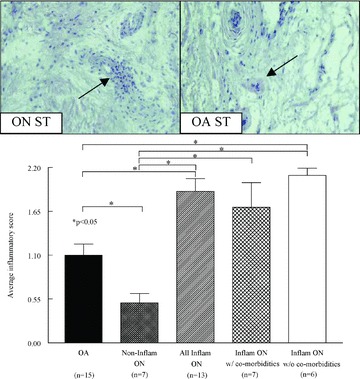
A subset of patients with ON have an inflamed synovium without having an inflammatory disease co‐morbidity. n= number of patients. Values represent the mean ± SEM.
After observing that a high percentage of patients with ON had significant ST inflammation, we examined the prior medical histories of these patients. We found that eight of the 20 (40%) study patients had an inflammatory co‐morbidity ( Table 1 ); however, two of these patients did not have any synovial inflammation. Therefore, for future experiments, ON patients having synovial inflammation were segregated based on a previous diagnosis of an inflammatory disease. We observed that a population of ON patients (35%) exists that does not have any inflammatory disease co‐morbidity and that has significantly greater synovial inflammation than ST from patients with OA ( Figure 1 ). Despite the increase in inflammation, no differences in vascularity were observed between any of the groups (data not shown). These results suggest the existence of a population of ON patients without a diagnosed inflammatory disease co‐morbidity that has significantly more synovial inflammation than OA.
Inflammatory infiltrates in patients with ON comprised CD4+ T cells and CD68+ macrophages
After identifying the presence of Inflammatory cells in ON ST, we sought to determine which cells we predominantly present. CD3+ T cells were present to a large degree in all patient populations except for the noninflammatory ON STs, which had low numbers of all inflammatory cells (p < 0.05) ( Table 2 and Figure 2 ). The majority of T cells present in ON STs from patients without inf ammatory disease co‐morbidities were CD4+, with smaller amounts of CD8+ T cells also present ( Table 2 ). However, the relative amount of each T‐cell type was similar to that observed in OA ST. Low levels of CD19+ B cells were also present in OA ST and ON ST (data not shown). In contrast to these cell types, we found a significant increase in the relative amount of CD68+ macrophages in the ST of patients with ON and without other inflammatory conditions compared to OA ST ( Table 2 and Figure 2 ).
Table 2.
Inflammatory infi ltrate cellular composition in OA and ON ST.
| Cell* type | OA | Noninflammatory ON | All inflammatory ON | Inflammatory ON w/co‐morbidities | Inflammatory ON w/o co‐morbidities |
|---|---|---|---|---|---|
| (n ≥ 9) | (n ≥ 6) | (n ≥ 11) | (n ≥ 4) | (n ≥ 6) | |
| CD3+ | 108.9 (22.6) | 21.4 (13.9)†,‡,§,,∥ | 105 (9.5) | 91.3 (21.3) | 105 (7.2) |
| CD4+ | 70.0 (11.1) | 12.5 (11.5)†,‡,§,,∥ | 96.3 (15.6) | 80.8 (23.7) | 111.7 (20.2) |
| CD8+ | 6.9 (3.0) | 1.7 (1.4)‡,§,,∥ | 12.9 (2.4) | 10.5 (3.6) | 14.0 (3.7) |
| CD68+ | 117.5 (22.4)∥ | 45.8 (31.0)‡,§,∥ | 179.2 (21.4) | 149.2 (43.1) | 207.5 (18.9) |
*Values represent the mean relative number of each specific cell type present in each group along with the SEM in parenthesis. Values were derived by multiplying the percent staining of each antibody on each cell type by the inflammatory score. n= the number patients.
†Significantly different compared to OA, p < 0.05.
‡Significantly different compared to all inflammatory ON, p < 0.05.
§Significantly different compared to inflammatory ON w/co‐morbidities, p < 0.05.
∥“Significantly different compared to inflammatory ON w/o co‐morbidities, p < 0.05.
Figure 2.
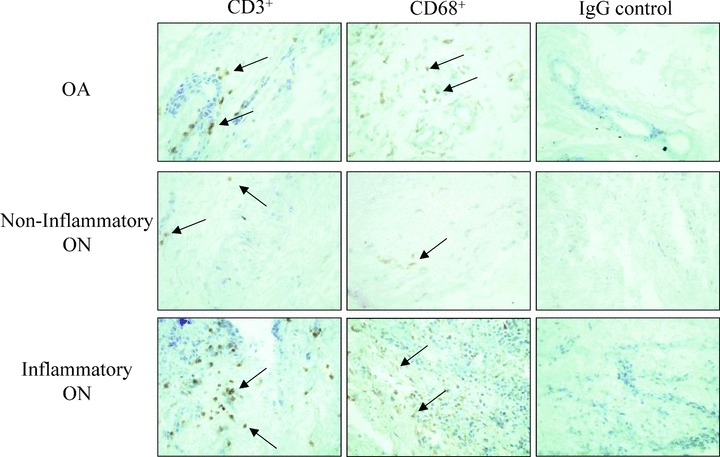
Representative photographs of CD3+ and CD68+ positive cells in OA, noninflammatory ON, and inflammatory ON ST. IgG treated controls are also shown for each group. Photographs were taken at 400×. Arrows indicate positive staining.
ST macrophages from patients with ON and without other inflammatory co‐morbidities have increased cellular adhesion molecule expression
Activated macrophages express higher levels of cellular adhesion molecules than unactivated, resting macrophages. 9 Therefore, we sought to determine if the macrophages that are present in ON ST express an activated phenotype with increased adhesion molecule expression. We found that ON ST macrophages from patients without other inflammatory co‐morbidities had increased expression of integrin αv ( Figure 3a ) and ICAM‐1 ( Figure 3b ) compared to OA ST. These results show that the macrophages in these patients may express increased levels of cellular adhesion molecules.
Figure 3.
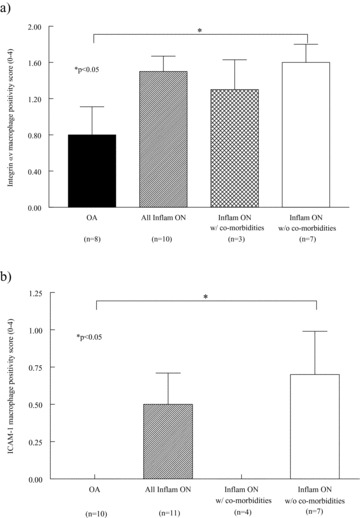
Macrophages from patients with ON and without other inflammatory co‐morbidities have an increased expression of adhesion molecules. (A) integrin αv expression, (B) ICAM‐1 expression. n= number of patients. Values represent the mean ± SEM.
Discussion
After gross examination at the time of surgery suggested the presence of synovial inflammation, we tested the hypothesis that patients with ON of the femoral head have synovial inflammation. Synovial inflammation plays a primary role in the pathogenesis of inflammatory arthritis, such as RA, and a lesser role in the etiology of noninflammatory OA. The synovium in patients with RA is highly inflamed and vascularized. 4 The inflammatory infiltrate in the RA sublining layer works in concert with RA synovial lining cells to produce a large volume of pro‐inf ammatory cytokines, chemokines, and angiogenic mediators that drive the progression of the disease. In contrast to RA, synovial inflammation does not play a primary role in the pathogenesis of OA. Cumulative joint stresses are presumed responsible for the destruction of articular cartilage, ligaments, and bone in the OA joint. 10 However, a level of inflammation greater than in normal synovium, but less than in RA, is present in these patients, with the highest level of inflammation observed early in the disease process. 5
Recently Pessler et al. 6 have observed synovial inflammation in a group of patients with noninflammatory orthopaedic arthropathies. Collectively, ST from patients with femur fracture, plica syndrome, meniscus and/or ligament injury, and ON of the femoral head had a level of ST inflammation between what was observed in normal ST and OA ST. 6 Within this population, three patients with ON of the femoral head were studied. Of these patients, one had a very high level of ST inflammation, while the other two patients had ST inflammation levels similar to what was observed in normal and OA ST. 6
We have now shown that synovial inflammation is present in a population of patients with ON. While a portion of ON patients had little to no inflammation in their STs, others had significant synovial inflammation and confounding inflammatory disease. However, we observed that a distinct population of ON patients exists without an inflammatory disease co‐morbidity and significant synovial inflammation. This report is the f rst to describe that a large portion of patients with ON of the femoral head have synovial inflammation that is independent of any diagnosed inflammatory disease. Moreover, the inflammation observed was greater than that of OA suggesting a role for synovial inflammation in ON of the femoral head. However, in this case we compared inflammation from ON hip synovium to OA knee synovium, and as OA hip and knee synovium may have distinct pathologies, future comparisons of ON and OA hip synovium may be needed to validate this finding.
A number of different cell types are present in the inflammatory infiltrates of RA ST and OA ST. In RA, CD4+ T cells are largely present throughout the ST and found in aggregates and perivascular sites. 11 CD4+ T cells mediate joint damage both directly, via the production of pro‐inflammatory cytokines such as interleukin‐17 (IL‐17), and by driving non‐T cells to release inflammatory cytokines. CD19+ B cells are also present in RA ST, and can be driven by the CD4+ T cells to produce autoantibodies that may be directly involved in joint damage. In addition, CD19+ B cells are known to be critical in activating CD4+ T cells. CD8+ T cells and polymorphonuclear cells are also present, but to a lesser extent. Tissue macrophages are also observed in RA ST, and are in large part responsible for the production of IL‐1β and tumor necrosis factor‐α (TNF‐α) that drive the progression of the disease. 9 In the early stages of OA, monocytes and CD4+ T cells are also present, but to a much lower extent compared to RA. 5 Moreover, the levels of IL‐1β and TNF‐α produced by infiltrating leukocytes in OA ST are significantly less than that observed in inflammatory arthritis. 12 The pattern of inflammation that we have observed in ON ST more closely resembles that in inflammatory arthritis rather than OA ST. A large number of CD4+ T cells was present in the ON STs, and a significantly greater number of CD68+ macrophages was observed in the ST of ON patients without an inflammatory disease co‐morbidity than in OA ST. T ese results are the first to suggest that significantly higher levels of leukocytes are present in a subset of ON STs compared to noninflammatory OA STs.
In addition to these findings, we also observed that the CD68+ macrophages in the inflamed ON ST express high levels of the adhesion molecules integrin αv and ICAM‐1. The integrin αv subunit has previously been shown to be expressed on macrophages and, when complexed with one of a number of β chains, mediates adhesion to a variety of extracellular matrix macromolecules depending on the β subunit. 9 , 13 Integrins also participate in the firm adhesion of monocytes to endothelial cells and facilitate endothelial cell transmigration into the synovium. 9 ICAM‐1 has also been previously shown to be expressed on a number of cell types, including RA ST macrophages. 14 Moreover, ICAM‐1 is upregulated on RA synovial fluid monocytes compared to peripheral blood monocytes from normal or RA patients. 15 , 16 Collectively these data suggest that the macrophages present in ON ST express a more activated phenotype than OA ST macrophages. The expression pattern of adhesion molecules on these cells in part provides a mechanism for the increased inflammatory infiltrates observed in the STs of these patients.
Conclusions
Here we report the novel finding of a population of ON patients with inflamed synovial membranes. A large portion of patients with ON and without confounding inflammatory arthritis had considerable synovial inflammation consisting primarily of CD4+ T cells and CD68+ macrophages. Since little is known about the pathogenesis of ON, our results suggest that further investigation of the role of synovial inflammation in this disease is needed, including screening for the presence of proinflammatory cytokines and chemokines. In addition, these findings indicate the need for a more comprehensive study of ST inflammation in ON patients, which would enlist more patients with ON of the femoral head, and correlate the extent of ST inflammation with disease progression. Moreover, these results suggest that specific anti‐inflammatory therapies aimed at diminishing inflammatory cell infiltrates, or their products, in the ST of these patients may be a beneficial addendum to therapies aimed at increasing blood flow to the afficted bone.
Clinical Correlations
-
1
A population of patients with ON has synovial inflammation without a diagnosed inflammatory co‐morbidity.
-
2
The inflammatory cell infiltrate in these patients comprised CD4+ T cells and CD68+ macrophages.
-
3
Further investigation of the role of synovial inflammation in the pathogenesis of ON is warranted.
-
4
Anti‐inflammatory therapies aimed at diminishing the inflammatory infiltrate in patients with ON may be a beneficial supplementary therapy.
Acknowledgments
This work was supported by NIH grants AI40987 and AR48267. Additional support included the Frederick G.L. Huetwell and William D. Robinson, M.D. Professorship in Rheumatology, and funds from the Office of Research and Development and the Medical Research Service, Department of Veterans Affairs. This work was also supported by a gift from Tom and Nancy Woodworth. The authors declare that they have no competing financial interests.
References
- 1. Mont MA, Hungerford DS. Non‐traumatic avascular necrosis of the femoral head. J Bone Joint Surg. 1995; 77: 459–474. [DOI] [PubMed] [Google Scholar]
- 2. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Ortho Surg. 1999; 7: 250–261. [DOI] [PubMed] [Google Scholar]
- 3. Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg. 2006; 88: 1117–1132. [DOI] [PubMed] [Google Scholar]
- 4. Szekanecz Z, Koch AE. Update on synovitis. Curr Rheumatol Rep. 2001; 3: 53–63. [DOI] [PubMed] [Google Scholar]
- 5. Benito MJ, Veale DJ, FitzGerald O, Van Den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 2005; 64: 1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pessler F, Dai L, Diaz‐Torne C, Gomez‐Vaquero C, Paessler ME, Zheng DH, Einhorn E, Range U, Scanzello C, Schumacher HR. The synovitis of “non‐inflammatory” orthopaedic arthropathies: a quantitative histological and immunohistochemical analysis. Ann Rheum Dis. 2008; 67: 1184–1187. [DOI] [PubMed] [Google Scholar]
- 7. Steinberg ME, Steinberg DR. Classifi cation systems for osteonecrosis: an overview. Orthop Clin N Am. 2004; 35: 273–283. [DOI] [PubMed] [Google Scholar]
- 8. Ruth JH, Volin MV, Haines GK, 3rd , Woodruff DC, Katschke KJ, Jr , Woods JM, Park CC, Morel JC, Koch AE. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant‐induced arthritis. Arthritis Rheum. 2001; 44: 1568–1581. [DOI] [PubMed] [Google Scholar]
- 9. Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007; 19: 289–295. [DOI] [PubMed] [Google Scholar]
- 10. Fernandes J, Martel‐Pelletier J, Pelletier J. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002; 39: 237–246. [PubMed] [Google Scholar]
- 11. Koch AE, Robinson PG, Radosevich JA, Pope RM. Distribution of CD45RA and CD45RO T‐lymphocyte subsets in rheumatoid arthritis synovial tissue. J Clin Immunol. 1990; 10: 192–199. [DOI] [PubMed] [Google Scholar]
- 12. Melchiorri C, Meliconi R, Frizziero L, Silvestri T, Pulsatelli L, Mazzetti I, Borzi RM, Uguccioni M, Facchini A. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis Rheum. 1998; 41: 2165–2174. [DOI] [PubMed] [Google Scholar]
- 13. Szekanecz Z, Koch AE. Adhesion molecules: potent inducers of endothelial cell chemotaxis In: Zilla PP, Greisler H, eds. Tissue engineering of vascular grafts. Austin: R.G. Landes, 1999, 271–277. [Google Scholar]
- 14. Koch AE, Burrows JC, Haines GK, Carlos TM, Harlan JM, Leibovich SJ. Immunolocalization of endothelial and leukocyte adhesion molecules in human rheumatoid and osteoarthritic synovial tissues. Lab Invest. 1991; 64: 313–320. [PubMed] [Google Scholar]
- 15. Ranheim EA, Kipps TJ. Elevated expression of CD80 (B7/BB1) and other accessory molecules on synovial fluid mononuclear cell subsets in rheumatoid arthritis. Arthritis Rheum. 1994; 37: 1637–1646. [DOI] [PubMed] [Google Scholar]
- 16. Takahashi H, Soderstrom K, Nilsson E, Kiessling R, Patarroyo M. Integrins and other adhesion molecules on lymphocytes from synovial fl uid and peripheral blood of rheumatoid arthritis patients. Eur J Immunol. 1992; 22: 2879–2885. [DOI] [PubMed] [Google Scholar]


