Abstract
Malaria is one of today’s most serious diseases with an enormous socioeconomic impact. While anti-malarial drugs have existed for some time and vaccines development may be underway, the most successful malaria eradication programs have thus far relied on attacking the mosquito vector that spreads the disease causing agent Plasmodium. Here we will review past, current and future perspectives of malaria vector control strategies and how these approaches have taken a promising turn thanks recent advances in functional genomics and molecular biology.
Keywords: Malaria, Plasmodium, Anopheles, mosquito vector
1. INTRODUCTION
Malaria affects 300-500 million people and causes1-2 million deaths annually [1]. Drug resistance, limitations in the ability to store and distribute drugs or vaccines, and the poverty of the target population are just some of the major barriers to providing effective treatment in endemic areas. In areas in which malaria has been eradicated, disease elimination has largely been achieved because of local control of the mosquito vector population. In the past, mosquitoes (and, consequently, malaria) have been controlled by environmental and chemical means that reduce mosquito survival and/or population expansion (Section 3). These methods have been successful in many areas of the world, yet there are roadblocks that prevent these classical approaches from eradicating malaria (Section 3); some limitations come from the infrastructure of endemic areas, while others are purely biological (such as insecticide resistance). The simultaneous uncovering of the genomes of the parasite, human host and mosquito vector has created an unprecedented opportunity to study the mosquito biology and how this vector interacts with the malaria parasite, humans, and the environment. The data gleaned from molecular methods are the result of a multifaceted approach that strives to understand not only the molecular mechanisms governing mosquito biology and mosquito-malaria interactions (Section 4) but also how the insects can be manipulated to reduce malaria transmission (Section 5 and 6) and how these mechanisms and manipulations play out across populations. It is the synergy of these areas of molecular entomology that has generated the possibility of applied, vector-based malaria control strategies and has given new hope to efforts at malaria eradication. These new strategies, however, also face challenges that will need to be addressed before they can be implemented in the field (Section 7). A welcome but unexpected outcome of studying a medically relevant insect is the discoveryof new pharmacologic agents, including antibacterials, anti-coagulants, and vasodilators, that may also be of great use outside the field of malaria intervention (Section 8). In this review, we outline the recent advances in each of these areas and discuss how they can be of importance to the fight against malaria.
2. THE MOSQUITO LIFECYCLE
Malaria is caused by blood-borne protozoan parasites of the genus Plasmodium; Plasmodium falciparum is by far the most costly species in terms of its effects on both human life and economic progress. Mosquitoes of the genus Anopheles function as vectors for the Plasmodium parasite and are thus essential for disease transmission. Vector-based malaria control is promising because the course of malaria infection is inextricably dependent on the lifecycle of these mosquitoes. There are over 400 species of mosquito in this genus; only 10% of these are suitable malaria vectors, and one, A. gambiae, is the major vector in sub-Saharan Africa (although others can be important vectors in particular regions). It is particularly significant that the comprehensive characterization of mosquito populations conducted thus far has revealed a high degree of molecular diversity and complexity within the Anopheles genus that must be addressed if a control measure is to be effective. Although Anopheles is the major genus responsible for malaria transmission, there is a spectrum of species, sub-species, and molecular forms within this category. Identification of the areas in which these sub-populations exist and an understanding of how they differ biologically are essential for the creation of a widespread control strategy based on vector biology [reviewed in 2, 3]. Despite the many differences that exist within the genus, the lifecycle of all Anopheles mosquitoes is generally the same: Eggs hatch in water, where they undergo the transition to larvae; there are four aquatic larval stages, followed by an aquatic pupal stage, before the adult emerges. Adults feed on nectar and other sugar sources and, within days of emergence, adult males form mating swarms into which females fly to mate. The female must then take a blood meal before she is able to lay 50-200 eggs. Most adults can live up to 2 weeks in the field, and longer under laboratory conditions, but within this time period a female can take multiple blood meals and transmit malarial parasites. This biological framework has provided the basis for a variety of vector control approaches.
3. CURRENT AND PAST APPROACHES TO MALARIA VECTOR CONTROL
Since the discovery by Ronald Ross (1897) that mosquitoes are the vectors of the malaria parasite, vector control has become an important part of malaria control programs. Several strategies have been designed and put into place to reduce the mosquito population, and several others are currently being investigated as possible solutions for rendering the mosquito vector less competent to transmit malaria. Among these strategies are environmental management, insecticide treatments, and molecular entomology approaches.
3.1. Environmental Management
Environmental management to control malaria consists of environmental modification, manipulation, and changes in human habitations and behavior [4, 5]. Initial vector control strategies in this area involved limiting the mosquito population through the draining of wetlands, removal of potential breeding habitats, installation of house screens, and use of larvivorous fish [5,6]. This approach has had some success in controlling the mosquito population in Rome, Israel, India, Brazil, Egypt, and Zambia [7, 8], and it is still used and recommended as an alternative approach in a few areas [9]. However, the wide array of vectors and their diverse habitat requirements make this strategy impractical in some situations. For example, the draining of wetlands is not suitable in areas where it might adversely affect biodiversity and conservation efforts [10, 11], and the formation of large numbers of temporary pools during the rainy season in some areas can make this strategy impractical [6]. Although environmental management strategies present their own challenges, and their implementation has diminished with the introduction of synthetic pesticides, there is now renewed interest in integrating this technique into malaria control programs. The appearance of pesticide-resistant mosquitoes and the availability of less toxic and more eco-friendly larvicidal agents, among other compounds, point to the potential usefulness of such agents as an alternative cost-effective strategy in certain urban and peri-urban areas [12]. The efficacy of environmental control strategies has been found to depend on adapting such approaches to match the habitat requirements of the local vector species and local environmental conditions, conducting entomological monitoring, integrating these strategies to agricultural practices, and coordinating activities at the local and regional level [5, 13].
3.2. Biological Control
Effective biological control of larval stages through the use of larvivorous fish and the bacterial pathogens Bacillus thuringiensis israelensis and Bacillus sphaericus has been reported [14-16]. Use of the larvivorous fish Gambusia affinis was initially implemented as a biological control throughout the world, but its negative impact on the native fauna has discouraged its further use [5]. Currently, such efforts have been replaced by the use of native fish to control vector populations, with relative success being achieved [17, 18]. The microbial agents B. thuringiensis israelensis and B. sphaericus are other environmentally friendly alternatives, given that the toxins they produce are non-toxic to other species and do not persist for a long period of time in the environment [5]. B. thuringiensis israelensis is widely used in the USA to control nuisance mosquitoes [13], and field trials in malaria-endemic zones have indicated success in controlling malarial vectors. The duration of their effectiveness, however, tends to vary across regions [14]. Another type of microbial approach in use against agricultural pests, and currently being tested against mosquito adults, is the use of entomopathogenic fungi. This strategy involves spraying mosquitoes’ resting places with a suspension of fungal spores. Upon exposure, the fungus readily invades and multiplies inside the mosquito, killing it within 15 days, and thus reducing the parasite’s transmission intensity. Laboratory and field studies have identified two fungal species, Beauveria bassiana and Metarhizium anisopliae, that are effective against A. gambiae [19, 20]. Although current data show that this approach has potential for reducing parasite transmission, there are several barriers that need to be addressed before its widespread application. These hurdles includes fungal spore viability, fungal specificity, and the development of resistance in the mosquitoes [21, 22].
A recent strategy that is considered an environmentally friendly alternative for insect control, but is not currently being applied to mosquitoes, is the sterile insect technique (SIT) [23, 24]. SIT involves the mass rearing and release of sterile males which, upon mating with the native population, are unable to produce viable offspring and thereby drive the native population into decline or eradication [25, 26]. Sterilization of insects has been accomplished by irradiation or chemosterilization [27], with transgenic techniques recently being recognized as potential new methods for sterilization [23, 25, 28]. The successful employment of this technique to control important disease vectors and agricultural pests such as the screworm and the Mediterranean fruit fly has created optimism about its usefulness as a potential malaria control strategy [29, 30]. The most successful SIT project against a malaria vector has involved using A. albimanus in El Salvador, where a degree of population suppression was observed [31-33]. Although SIT appears to be a suitable alternative, significant hurdles have prevented it from attaining the same level of success that has been observed for otherinsect pests. Among these hurdles are the loss of male fitness after sterilization, the need to produce sufficient numbers of sterile individuals, and the challenges posed by the biology of the mosquito population [24].
3.3. Chemical Control
Early mosquito management relied on the use of Paris green (copper acetoarsenite) and petroleum byproducts, but the use of these chemicals has been discontinued because of their high toxicity and pollution of water sources [5, 34]. With the discovery of dichlorodiphenyltrichloroethane (DDT), the focus of malaria control strategies shifted to managing the adult mosquito population and resulted in the abandonment of early vector control approaches. The outstanding results obtained in controlling other vector-borne diseases and, in particular, the reduction of mosquito vectors in the USA, Southern Europe, the former Soviet Union, and parts of South Africa created the false impression that malaria could be eradicated from the planet [9, 35-37]. The reduction in malaria incidence obtained through the widespread application of DDT and other synthetic insecticides only added to this optimism. Soon after, the appearance of insecticide-resistant mosquitoes, an increased public rejection of the application of DDT because of its ecological impact, and changes in the feeding behavior of certain vectors, among other factors, eroded this optimism [38]. Currently, insecticide use still plays a significant role in malaria control programs involving the use of insecticide-treated bednets and indoor residual spraying [39]. Both strategies are based on the feeding behavior of A. gambiae, which is anthropophilic and endophagic and in which the reduction in transmission is attained by reducing the lifespan of the mosquito vector [6]. Insecticide-treated bednets alone have been regarded as an excellent tool to reduce malaria transmission in highly endemic countries, especially by reducing child mortality and morbidity [1]. The efficacy of pyrethorid-treated bednets is well known from its implementation in Asia, where it has successfully helped control malaria transmission [40]. It has been noted that in order to achieve a greater efficacy in Africa, insecticide-treated bednets need to be widely distributed among the population, and insecticide re-impregnation services have to be provided at a relatively low cost if continuous protection is to be maintained [41]. The same is true for indoor residual spraying, which requires frequent supervision and inspection as well as appropriate application to be effective [1, 6].
Although pesticide application is primarily aimed at controlling the adult mosquito, larval control strategies are still in practice, but less common than in earlier years. Recently, larval control through the use of insect growth regulators (IGRs) has been recommended because of its low non-target impact and persistence [12]. Furthermore, the cost-effectiveness of this approach has been demonstrated making it an efficient alternative to larvicidal organophosphates, to which larval resistance has already been observed [5, 12, 42].
3.4. Roadblocks Preventing Successful Malaria Control
The challenges inherent in developing successful malaria control strategies encompass biological, socioeconomic, and political issues [1]. One of the major challenges in biological terms is the fact that at least 150 different mosquitoes have been identified as malaria vectors, with A. gambiae, the most important malaria vector [43]. Each of these subspecies can serve as a vector of malaria but has different habitat requirements and feeding behaviors that require adjustments in the control program to reflect the local vector species [6]. Furthermore, molecular approaches to render the vector less competent are not immune to the development of Plasmodium resistance, and it has been noted that multiple anti-Plasmodium effector genes may be required to prevent this phenomenon from occurring [44]. Insecticide resistance in mosquitoes is another roadblock to proper malaria control that was documented soon after the implementation of early insecticides such as DDT. Such resistance has hampered eradication efforts and has been considered a serious threat to current malaria control strategies [45]. According to the WHO, mosquitoes in certain areas have become resistant to all the major groups of insecticides [46]. Insecticides such as DDT and pyrethorids primarily target the nervous system of the insect, acting on the insect’s neuronal voltage-gated sodium ion channels. Insecticide resistance can be based on increased glutathione-S-transferase detoxification (GTS), knockdown resistance, which confers target site insensitivity on the mosquitoes and decreased penetration, and active insecticide avoidance by the mosquito [47-49]. The molecular mechanisms of resistance are not yet well understood, but research suggest that it is based on mutations in synapse acetylcholinesterase (targets of organophosphates and carbamate) and sodium channels (the targets of DDT and pyrethorids) [47]. The availability of the A. gambiae genome sequence has made possible the use of functional genomics tools to characterize the molecular mechanisms that regulate insecticide resistance.
4. MOSQUITO-BASED MALARIA CONTROL IN THE POST-GENOMIC ERA: IDENTIFICATION OF MOLECULAR TARGETS FOR DEVELOPMENT OF NEW APPROACHES
Understanding vector biology and its relation to malaria biology has opened the door to new control strategies. Most new ideas in this field make use of molecular data and methods to add to the insights of entomologists from the pre-genomic era and explain the molecular mechanisms behind vector-parasite interactions and mosquito biology and behavior. The genomic analyses made available by the sequencing of the A. gambiae genome have provided significant insight into the vector’s biological processes that can potentially be disrupted or exploited in an effort to control malaria. Understanding vector biology and the biology of malaria transmission opens doors for new eradication strategies and allows for multi-faceted approaches to malaria control. Here, we outline some of the recent advances made in this field and how these data can be used in the context of control applications.
4.1. Blocking the Parasite
4.1.1. Targeting Gametocytogenesis and Gametocytes
Plasmodium gametocytes that are coursing through the human host’s circulation are taken up into the blood meal as the female mosquito feeds (the Plasmodium lifecycle in the mosquito is illustrated in Fig. (1). The blood bolus moves to the insect’s midgut, where the environmental change triggers gametocytes to immediately begin to mature into male and female gametes and begin their sexual cycle. As the first event that occurs in the mosquito vector and one that involves a number of molecular and environmental factors, gametocytogenesis is an attractive target for molecular intervention. Gene expression and proteomic analyses have revealed a range of transcripts and proteins that fluctuate with gametocytogenesis [50, 51]. Many of these molecules are involved in the signaling or mechanics that govern stage shifts, gametocytogenesis, and/or fertilization, all of which take place in the mosquito host [52]. Several Plasmodium key proteins such as Pfs230, Pfs48/45, and Pfs25 are major players in sexual stage progression and, as such, are considered prime targets for transmission-blocking vaccines [53]. One mechanism of gene regulation that is beginning to be better understood is translational repression, in which transcripts are made and then stored in P-bodies until release; only then are these transcripts expressed. In the case of the rodent Plasmodium, this appears to be an influential mechanism that may be responsible for coordinating gametocytogenesis, and understanding this process and the key players involved could reveal a vulnerable mechanism that is unique to the parasite [54]. Thus, stage-specific proteins or proteins involved in translational repression or other mechanisms of gametocytogenesis appear to be excellent targets for disrupting or preventing stage progression.
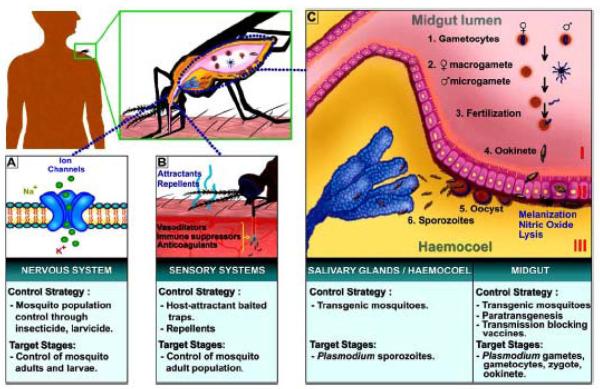
Fig. (1). Targets for malaria control strategies.
Panel A, the mosquito nervous system serves as a target for the control of mosquito adults and larvae, through the action of insecticides and larvicides. Panel B, a set of strategies targets the sensory system of mosquitoes through the use of host attractant-baited traps and behavior modification through the use of repellents. Lower Panel B, mosquito-human interactions: anticoagulants, vasodilators, and immune suppressor compounds are synthesized and used by mosquitoes to successfully feed on blood. Panel C, Plasmodium lifecycle in the mosquito and targets for control strategies: 1) Plasmodium gametocytes enter the mosquito midgut with the ingestion of an infectious blood meal; 2) gametocytes mature into female (macrogamete) and male (microgamete) gametes; 3) fertilization of both gametes occurs in the mosquito midgut, giving rise to a zygote; 4) the zygote further matures into an ookinete that is able to penetrate the midgut epithelium; 5) once the Plasmodium ookinete reaches the area between the epithelium and the basement membrane, it matures into an oocyst; 6) aproximately10 days after blood feeding, the mature oocyst bursts, releasing thousands of sporozoites that migrate and invade the salivary glands. The sporozoites are then inoculated during feeding, along with the mosquito saliva, into the skin of the next human host. Numbers in red represent three major mosquito compartments in which mosquito-Plasmodium interactions occur: I = midgut lumen, II = midgut epithelium, III = hemocoele and salivary glands.
4.1.2. Targeting Ookinetes
Fertilization produces zygotes, which then mature to the next stage, forming elongated, motile ookinetes. These ookinetes are then able to penetrate the epithelial layer of the midgut, where they lodge between the epithelium and the basement membrane and continue their maturation into oocysts. Traversal of the epithelium occurs within 24-48 hr after blood ingestion, and oocyst formation is evident 5 to 7 days later. Most immune- related analyses, as well as analyses that incorporate midgut receptor blockers or genes with promoters induced by feeding, make use of a reduction in oocysts as a quantitative indicator of ookinete killing.
4.1.2.1. Physical Barriers: Targeting Ookinete Penetration into the Epithelium
Research concerning midgut biology has uncovered ways to exploit the parasite’s entry mechanism, attempting to block the pathogen from leaving the midgut. Although its receptor ligand is unknown, the SM1 peptide binds to the salivary glands and the luminal side of the midgut epithelium. This binding interferes with infection progression, suggesting that SM1 may physically block or otherwise prevent midgut invasion [55]. Similarly, jacalin, a lectin, is known to inhibit ookinete attachment in mosquitoes and has recently been found to target an aminopeptidase called AgAPN1 [56, 57]. Antibodies raised against AgAPN1 are able to block infection in a manner similar to that of jacalin, suggesting that this aminopeptidase is a viable option for transmission-blocking strategies [57]. A third candidate target, cpbAg1 (a carboxypeptidase B), has been identified as a mosquito gene that is induced upon infection, and its activity has been found to correlate with the establishment of infection [58]. When antibodies against this molecule are included in a P. falciparum-laden blood meal, infection is significantly limited [59]. While these strategies utilize mosquito molecules, others seek to use transmission-blocking antibodies to neutralize Plasmodium proteins that facilitate penetration. Antisera against proteins that are stage specifically expressed, such as circumsporozoite and TRAP-related protein (CTRP) and von Willebrand factor A domain-related protein (WARP), and against proteins that can act against the midgut barrier, such as chitinase (PgCHT1), are effective in reducing Plasmodium infectivity [60]. Even in the absence of synthetic compounds, a sizeable bottleneck in the parasite population occurs between gametocyte ingestion and oocyst formation, in no small part due to an aggressive immune response mounted by the mosquito. Insect immunologists are exploiting this drastic reduction in population by identifying immune proteins and mechanisms that could eliminate those few parasites that would otherwise survive and propagate. The variety of parasite stages to which the mosquito is exposed presents a challenge for the mosquito to combat but also provides the opportunity to devise an integrated approach that uses mosquito immunity to target multiple parasite stages, thereby increasing the efficacy of the treatment.
4.1.2.2. Exploiting the Mosquito’s Immune Response to Ookinetes
Mosquitoes mount an immune response to parasites, bacteria, viruses, and fungi. These responses are generally triggered when characteristic molecular patterns such as LPS or peptidoglycan are detected by circulating pattern recognition receptors (PRRs) in the mosquito. PRRs then initiate a signal amplification cascade that triggers the major immune signaling pathways and mechanisms. These pathways share commonality with mammalian pathways that are mediated by Toll-like receptors, tumor necrosis factor, and Stat proteins and cause the nuclear translocation of transcription factors and thereby induce transcription of immune effector genes such as antimicrobial peptides. Effector mechanisms can be insect-specific, such as melanization, or conserved, such as phagocytosis, and act directly on the pathogen. Anti-malarial proteins and processes have been ascribed to each of these immune system constituents. Although a global picture of how these constituents are integrated to limit malaria is as yet incomplete, the contributions of individual effectors illustrate how powerful this defense really is.
4.1.2.3. Melanization
The melanization process is initiated by humoral factors that cause the production of melanin and protein crosslinking around a foreign body, walling it off in an inert capsule. This reaction is active against Plasmodium parasites and is mediated by a serine protease cascade that culminates in the conversion of prophenoloxidase (PPO) to phenoloxidase (PO), which then initiates melanin synthesis. Clip domain serine proteases (CLIPs) and serine protease inhibitors (serpins or SRPNs) make up a complex regulatory network, with SRPNs being antagonistic and CLIPs playing either agonistic or antagonistic roles, depending on the specific protease involved [61]. CLIPs are transcriptionally regulated in response to malaria infection, and a comprehensive RNAi analysis of almost every CLIP in the Anopheles genome has shed light on the roles of individual CLIPs in the immune response to ookinetes [62-65]. Gene silencing of CLIP family members affects the lytic killing of Plasmodium ookinetes, the melanization of ookinetes, or both processes, indicating that killing and melanization are separate but coordinated events that are highly dependent on serine proteases [65].Unchecked melanization can be toxic to the mosquito, so SRPNs act as regulators and consequently have an effect on the anti-Plasmodium response. Knockdown of SRPN2 or SRPN6 increases the mosquitoes’ ability to melanize parasites, suggesting suppressive roles for these proteins [66, 67]. The utility of major factors of the CLIP-SRPN-PPO cascade is highly dependent on the mosquito species and strain as well as the Plasmodium strain. Silencing of some components causes marked phenotypic changes in one mosquito strain but has negligible effects in another [65]. Other components show clear roles in defense against a rodent malaria parasite but are ineffective against the human pathogen [68]. The fact that the genetic background of both the vector and the parasite plays such an intimate role in the anti-Plasmodium efficacy of this pathway means that population genetics must be considered a crucial part of any control strategy that involves melanization.
4.1.2.4. Free Radicals and the Time Bomb Model
Free radical reactions and the proteins responsible for such reactions respond to and are active against malarial parasites. Nitric oxide (NO) is produced by the activity of nitric oxide synthase (NOS), which is transcriptionally elevated during blood feeding and Plasmodium infection [69]. After ingestion of P. falciparum, released hemozoin or surface glycosylphosophotidylinositol (GPI) moieties trigger NO production via MAP kinases [70]. NOS expression is highest in parasite-rich regions of the midgut and correlates with increased activity of the enzyme, while biochemical depletion of certain NO metabolites causes an increase in parasite burden [69]. Taken together, these data indicate an induced anti-malarial response that hinges on NO production and metabolism. Other studies have shown that oxygen metabolism can play a major role in limiting infection and may be coupled to cell death in the midgut epithelium [71-74]. The “time bomb model” of midgut traversal by the ookinetes depicts a time-sensitive response of the midgut cells. As the motile ookinetes invade, protein nitration and reactive oxygen species are triggered, creating a toxic and unstable environment inside the target epithelial cell. The toxic products inside the host cell are considered “bombs” that are set off by peroxidases. Once this happens, the damaged cell undergoes apoptosis and is extruded from the epithelial layer. If the parasite has not made it through the cell by this time, it is destroyed by the toxic environment, the apoptotic process, or both [71, 75, 76].
4.1.2.5. Immune Pathways and Anti-Plasmodium Genes
Molecular immune pathways and their downstream effector genes have been implicated in limiting the development of multiple stages of parasite progression. Microarray and RNAi studies have identified many key genes involved in anti-malarial immunity, and silencing many of these genes drastically changes the vector’s susceptibility to infection. Often, targeting of such genes indicates that they have a significant impact on infection, yet the results give little insight into the mechanism of elimination. Many of these genes are considered PRRs because they have PRR orthologs in Drosophila, are known to bind bacteria or other pathogens, have typical binding domains, or can colocalize with the parasite. TEP1 is the best-studied antimalarial PRR. It is a thioester-containing protein with known antibacterial activity as well as sequence and structural similarity to mammalian complement factors [77, 78]. It co-localizes with ookinetes, and knocking down this protein causes a significant increase in oocysts, probably as a result of TEP1’s involvement in killing at the ookinete stage [79]. Likewise, expression of a leucine-rich repeat containing molecule, LRIM1, increases during infection, and depletion of this molecule causes an increase in oocyst numbers [80]. Other leucine-rich repeat domain-containing proteins, Anopheles Plasmodium-responsive leucine-rich repeat 1 and 2 (APL1 and 2), which are also known as leucine rich repeat domain (LRRD) 19 and 7, respectively, have been mapped to a chromosomal region contributing to P. falciparum resistance in certain natural mosquito populations and are up-regulated during Plasmodium infection [63, 81]. Two C-type lectins are implicated as melanization factors and, like the serine proteases, show specificity with regard to mosquito species [80]. Both gene expression data and gene silencing data show that Dscam, a member of the immunoglobulin superfamily, is active against both rodent and human malaria. Dscam is differentially expressed during infection in both cases, and oocyst numbers increase after targeted silencing of the molecule [82]. Furthermore, a combination of microarray and RNAi data have indicated that several members of the ficolinlike fibrinogen-domain immunolectin (FBN) family are able to limit P. falciparum infection [Dong, unpublished data]. The challenge from a control strategy standpoint is to determine what these putative PRRs are recognizing and how best to exploit their ability to recognize parasites and subsequently incite an immune response. PRRs generally trigger serine protease-mediated signal amplification cascades, which then set off immune signaling pathways [83]. Components of these immune pathways have been examined to assess their impact on other infections, and in recent years they have become a focus for responses toward Plasmodium. A major benefit of a control strategy rooted in immune pathway manipulation is that a battery of immune genes can be enhanced, rather than only a select few, and the result may resemble a typical immune response more closely than does the enhancement of a few select genes. The potential drawback that needs to be investigated is the possibility of disrupting other biological processes that depend on that pathway or its products. Since insect immune pathways are used as models for some mammalian immune pathways, an understanding of how the vector combats malaria could provide insights into how the mammalian innate immune system interacts with the parasite. The Toll and Imd pathways, loosely analogous to the TLR- and TNF-alpha-mediated immune pathways in mammals, both rely on NF-kappaB transcription factors for increased immune gene expression. Silencing of Imd pathway components has a direct effect on the ability of parasites to develop [84, Garver, submitted], and effector genes resulting from either pathway are regulated during malarial infection and their depletion or over-expression can influence parasite populations. Over-expression of the antimicrobial peptide CecropinA causes a 60% reduction in oocysts in the South American vector A. albimanus, while expression of two other anti-microbial peptides, defensin and gambicin, is induced during Plasmodium infection in A. gambiae [85-88]. Knock-down of gambicin, but not defensin, causes a marked increase in the midgut Plasmodium population [63, 89]. Expression of PRRs such as TEP1 and LRIM1 also seems to be dependent on the activation of both immune pathways [90], which may indicate that targeting pathways as part of a control strategy would enhance Plasmodium detection as well as killing.
The emerging picture of the mosquito’s anti- Plasmodium immune repertoire is full of possibilities for control targets. The response is multi-faceted, offering a number of diverse mechanisms that could be manipulated to act synergistically, ensuring a more complete elimination and guarding against parasite resistance to the control. We know that these are biological immune strategies that mosquitoes have evolved to limit infection. The immune response is also an interesting avenue, as it could be incorporated into a variety of control methods (summarized in a later section of this review).
As with any control measure, there are caveats that must be considered. Overlaps exist between the mosquito’s immune responses toward malaria, bacteria, viruses, and fungi. How does the immune repertoire shift to address these different pathogens? Would an anti-malarial immune response be weakened or strengthened by the presence of other pathogens? What role do the mosquito’s endogenous flora play? This is an emerging area of interest, since different Anopheles strains harbor quantitatively and qualitatively different microbial flora, and the microbial environment can influence Plasmodium development within the mosquito (Pumpuni 1996, Baton unpublished data).
Understanding the dynamics of endogenous bacteria, malaria parasites and insect immune components will help us to hone vector-based control efforts and may offer some unique control possibilities. It is important to note that there are inconsistencies in the molecules’ immune roles between mosquito and parasite strains— how do we factor this into plans for widespread control?
Does the ecological niche influence immunity and vector competence? These are questions that must be addressed before we rely on immunity-driven control; however, we continue to find evidence that while the parasite has adapted to complete a successful portion of its lifecycle in the vector, the vector has also established powerful means of reducing parasite populations that have great potential for vector-based malaria control efforts. As outlined in this section, a predominant focus of research is on the limitation of the parasite during its sexual stage transition and midgut penetration of the host, since the parasite population at this point is still manageable for the mosquito, and outcomes can be easily ascertained by simple staining and microscopy. Crossing of the midgut by the ookinetes is a population bottleneck which, in natural populations, few individual parasites survive to become oocysts that lodge between the epithelium and the basement membrane of the midgut. However, at about 10 days after blood feeding, each oocyst bursts and releases many thousands of sporozoites that migrate through the mosquito’s open circulatory system to the salivary glands. Here, the sporozoites penetrate the epithelial layer of the gland and, along with the mosquito’s saliva, they can be deposited in the skin of the next human that is bitten, thus completing the transmission process. As a critical and final step in malaria transmission, the sporozoite stage and the insect salivary gland have been subject to study. These analyses are particularly relevant to human studies, since sporozoites are immunogenic in both insects and humans, and it is this stage that survives through the interaction of mosquito saliva with the components of human skin and blood.
4.1.3 Targeting the Oocyst Stage
Molecular entomologists have yet to probe deeply into the possibility of a mosquito response against the oocyst stage. Ookinetes develop into oocysts, which lodge beneath the basal lamina of the midgut epithelium. The oocysts remain stationary, growing as thousands of sporozoites develop within them. The rupture of an oocyst releases these sporozoites, causing the final change in infection stage within the insect. Oocysts are presumed to be minimally antigenic, since they are covered by an inert capsule layer and are thought to incorporate some of the mosquito’s extracellular matrix proteins; however, some refractory strains of Anopheles can melanize oocysts, suggesting a genetic component of those mosquitoes that regulates that immune response [90, 91]. In particular, it is thought that laminin incorporation on the oocyst surface inhibits immune responses such as melanization [92].
Parasite-derived proteins have been implicated in the ookinete-to-oocyst-to sporozoite portion of the developmental cycle, including circumsporozoite- and TRAP-related protein (CTRP), circumsporozoite protein (CSP), secreted ookinete adhesive protein (SOAP), and thrombospondin-related sporozoite protein (TRAP). Some proteins, such as CSP and CTRP, are present and may be essential for both oocyst development and sporozoite entry into the salivary gland and are thus developmental targets that are not limited to a single point of effect.
4.1.4. Targeting the Sporozoite Stage
Once they burst out of the late oocyst, sporozoites course through the mosquito’s hemolymph, ultimately trying to invade the salivary gland (Fig. (1)). Many of the previously mentioned proteins, such as CSP and TRAP and others such as MAEBL and saglin, are essential for salivary gland invasion and are therefore crucial molecular targets [93, 94]. Parasite proteins that aid salivary gland invasion, such as CS, often have an affinity for heparin, as do those that aid liver invasion in humans; these similarities make for interesting correlations between these stages [95, 96]. The sporozoite is far more difficult to target molecularly, simply because there are so many more individual organisms to eliminate, and they occupy both the circulatory system and the salivary gland. This is not to say the sporozoites are inert or should be ignored. Arguably, targeting sporozoites could be part of a multifaceted approach, a back-up plan for any inefficiency in a gametocyte-, ookinete- or oocyst-targeted approach. Some of the same types of approaches that are used against other stages can also be used against sporozoites.
For example, there is already proof of principle that the same antibody-based transmission blocking strategy works at the salivary gland level as well as the midgut. Antibodies against a female-specific A. gambiae salivary gland protein can reduce sporozoite invasion, but the biology of the target molecule is unclear; similarly, antibodies against several unknown and one known parasite surface protein administered to Aedes aegypti can prevent P. gallinacium invasion [97-101]. On the other hand, several potential sporozoite and salivary gland targets have been described, but their role in application has yet to be determined. They have stage-specific expression profiles, represent an immune response to the sporozoite, or are necessary for sporozoite penetration into the salivary gland. A mechanism for sporozoite destruction in the mosquito’s hemolymph has been identified although the details are unclear; and there are infection-responsive increases in transcripts and proteins in the salivary gland, such as defensin and cecropin, that have known immune function and could be protective [102-106]. The fact that the mosquito can actively limit sporozoites populations in both the hemocoele and the gland offers options for molecular control at this stage. Serpin 6 is one the best-studied immune response-related mosquito proteins that has the ability to limit invading sporozoite numbers [107]. The chief goal of the pharmacological development of any transmission-blocking peptide and/or antibody or an alteration of mosquito immunity or other biological process is that such an intervention would not prevent the mosquitoes from feeding on humans but would render them unable to support the malarial infection. The ultimate goal is to incorporate these molecular data into novel applied strategies for vector-based malaria control. Several of these strategies are designed specifically for targets that are only identifiable through molecular means. Here, we describe five future malaria control strategies that exploit either anti-malarial effector systems within the mosquito or mosquito behavior modification, and we comment on the development of pharmacological agents using mosquito-derived data.
Although several methods have been described in detail and are being proven effective in an experimental laboratory situation, no methods have yet been implemented or developed on a large scale.
4.2. Effector Delivery Systems
Once anti-Plasmodium factors have been identified (as described above), the next challenge is to generate a way to introduce or employ them into the natural population.
There are several ways to accomplish delivery and, though none have yet been utilized in the field, great strides are being made to streamline each method and make them as effective, safe, easy and successful as possible before a wide-scale effort is attempted.
4.2.1 Transgenic Mosquitoes: A Requirement for Genetically Inherited Resistance
The genetic transformation of mosquitoes to make them refractory to Plasmodium infection is regarded as a potential strategy to control malaria transmission. Three key components are needed for a successful mosquito transformation [108]. First, an efficient germline transformation system has to exist. Advances in molecular biology have allowed the development of stable transformation of mosquitoes and a reduction in Plasmodium transmission by transformed-anopheline mosquitoes has been observed [109, 110].
Second, suitable promoters that will drive stage-, tissue-, and sex-specific expression of anti-Plasmodium genes need to be selected [111, 112]. Given that malaria transmission requires that the parasite move through three mosquito compartments (midgut, hemocoele and salivary glands), suitable promoters will be those induced in these tissues at the right time after feeding [111]. Among the promoters currently being tested are a carboxypeptidase promoter driving expression in the mosquito gut epithelium, an anopheline antiplatelet protein promoter (AAPP), which has been found to be highly induced after a blood meal in the mosquito salivary glands, and a vitellogenin promoter obtained from Anopheles stephensi that shows promise as an excellent promoter [113-116]. Other potentially suitable promoters are those involved in mosquito immunity and those expressed in the hemolymph at the time of sporozoite release [62, 111]. The third component needed for a successful mosquito transformation is the selection of effector genes that either impair parasite development or serve as parasiticidal agents with 100% efficiency and a low fitness cost [108]. Five different categories of effector genes have been recognized on the basis of their targets of action [117]. These five categories target parasite ligands and tissue recognition receptors, block parasite gene expression, act as immune response effectors, and produce toxins that kill the parasite. Even though current effector genes are not 100% effective, recent results in these areas serve as proof of concept of what can be achieved with this approach. For example, by using single-chained antibodies against Plasmodium surface proteins (anti-Pbs21), Yoshida et al. [118] observed a 95% interference with oocyst formation. A series of other effector genes under the control of the carboxypeptidase promoter, such as the SM1 tetramer peptide, which binds to the midgut and salivary glands, and phospolipase A2 (PLA2) which binds to the midgut, have been shown to be effective at interfering with P. berghei oocyst formation in A. stephensi mosquitoes [110, 119]. Furthermore, functional genomics have provided an insightful view of Plasmodium-mosquito interactions from which new effector genes involved in immunity could be selected [62] and which are discussed earlier in this review. Finally, since this control strategy relies on the introduction of refractory transgenes into the native population, it is critical that the transgenic mosquito’s fitness is as good or better than that of its wild-type counterpart. Although fitness assessment of transgenic mosquitoes has indicated a loss of fitness, at least one study involving A. stephensi expressing the peptide SM1 has shown a fitness advantage over the wild-type strain when fed on Plasmodium-infected mice [109, 111, 120]. Another constraint related to the eventual release of transgenic mosquitoes is the lack of an effective drive mechanism to introduce the transgene into a population. Several genetic drive mechanisms such as transposable elements, homing endonucleases, and the use of the symbiotic bacteria Wolbachia have been proposed and are currently being assessed [121-123]. Future studies on transgenesis will likely focus on P. falciparum models. Most of the work until now has made use of the model parasite P. berghei, which infects rodents. It is a useful model organism but does not affect the mosquito in exactly the same way that P. falciparum does [63, 123].
4.4.2. Paratransgenesis: Acquired (Transient) Resistance
Another method that has been proposed as an anti-Plasmodium effector delivery strategy is paratransgenesis. Paratransgenesis is the genetic modification of symbiotic bacteria to deliver anti-pathogenic gene products and thus reduce vector competence [124]. A successful paratransgenic approach must meet five general requirements: 1) the selection of a symbiont closely associated with the vector and in contact with the targeted pathogen, 2) a bacterial species that is cultivable and amenable to genetic transformation, 3) uncompromised fitness of the transformed symbiont, 4) a suitable method for reintroduction, and 5) the spread of the transformed symbiont in the vector population [124, 125]. The best example of paratransgenesis is the pioneering work conducted by Duvarsula et al. to break the transmission of American trypanosomiasis (Trypanosoma cruzi) by Rhodnius prolixus [125]. The ability to engineer bacteria that are capable of expressing multiple effector molecules, compatibility with current control programs that use insecticide treatment, and relative simplicity of the logistics needed for reintroduction are some of the identified advantages of a paratransgenetic approach over the genetic transformation of mosquitoes [126, 127]. Thus far, several bacteria have been isolated from anophelines, from both laboratory colonies and wild populations and at least one bacterium, Enterobacter agglomerans, has been identified as an excellent candidate for paratransgenesis [127-129]. Furthermore, in a recent study, the expression of two anti-Plasmodium effector molecules (SM1 and PLA2) on the surface of Escherichia coli partially inhibited P. berghei development, showing thepotential of this technique as part of a malaria control strategy [127].
5. TRANSMISSION-BLOCKING VACCINES
There are three areas in which vaccine development against malaria is carried out: vaccines against 1) the pre-erythrocytic or liver stage of the parasite, 2) the blood stage, and 3) the sexual stages in the mosquito vector. It is this third area in which transmission blocking vaccines (TBVs) are being assessed as a way to control the spread of malaria. TBVs work by inducing the immune response of the human host against antigens expressed by the sexual stages (gametocyte, gamete, zygote, ookinete) of Plasmodium or against the mosquito midgut receptor molecules, which are important for subsequent Plasmodium development [130]. It has been noted that the molecules targeted for the development of TBVs should have limited antigenic diversity, they should be highly immunogenic to block Plasmodium development in the mosquito, and they should provide long-lasting immunity in the vaccinated population [59]. Several candidates for TBV development have been found and the mechanisms of immune killing are thought to include complement-dependent lysis, neutralization, gamete agglutination, opzonization, and steric interference [59, 130]. Promising results have been obtained from the research conducted in this area. Hisaeda et al., for instance, have reported a significant blockage in transmission with TBVs that targeted P. vivax ookinete surface proteins (P25 or P28) [131]. Recently, two other reports have shed light on other potential TBV candidates that were described earlier in this review [57, 59]. Another novel strategy, recently suggested and quite different from the TBV approach, is the use of mosquito salivary components in antimalarial vaccines that can induce a Th-1 response capable of controlling malaria infection [132]. Using such a strategy in a murine malaria model, Donovan et al. showed that mice exposed to bites from uninfected mosquitoes developed a biased Th1 response that limited the development of P. yoelii [132]. Among the perceived benefits of a TBV are the prevention of the spread of drug- or vaccine-resistant Plasmodium strains, a decrease in the incidence in low-endemic areas, and a reduction in morbidity and mortality in highly endemic areas [1, 133]. As with other mentioned malaria control programs, concerns have been raised regarding the TBV approach, such as the potential loss of natural immunity in the population and the lack of any direct benefit to the vaccine [134]. These problems, however, have also been seen to affect current control strategies [130]. The other limitation of a TBV approach is that the antibodies generated do not directly benefit the vaccinated person, but they instead prevent the development of the parasite in the mosquito, thereby benefitting the community. This situation is limiting because the efficacy depends on almost complete compliance. Unvaccinated individuals become a risk to the whole population, not just themselves. One proposal suggests the development of spray-able transmission blocking agents that can be applied in dwellings; this strategy transfers the responsibility from inhabitants to the control program administration.
6. BEHAVIORAL MODIFICATION: TARGETING THE BEHAVIOR OF THE MOSQUITO
6.1. Chemosensory: Mosquito Olfaction, the Use of Odorant Receptors to Control Mosquito Populations
Increased interest has been directed towards the understanding of the molecular basis of olfaction in insects of agricultural and medical importance, and mosquitoes in particular [135]. Mosquitoes rely extensively on chemoreception for mating, host location, and host preference. This trait makes olfaction amenable as a target for control, providing the basis for strategies that interfere with mosquito olfaction and disrupt vector-host interactions and various functions of the mosquito (i.e., location of oviposition sites, mating), and ultimately reducing pathogen transmission [136]. Transmission is only completed if the infected female finds a new vertebrate host from which to feed, an accomplishment that is mediated by the ability to sense heat, shapes, and odors. For years, entomologists have known that most anthropophilic mosquitoes are attracted to compounds given off in human perspiration and exhalation [137]. This attraction is mediated by a complex network of odorant receptors (ORs) and odorant binding proteins (OBPs) that subsequently affect host-seeking behavior via the firing of olfactory receptor neurons (ORNs) in the head. How these ORs, OBPs, and ORNs respond positively or negatively to certain odors and how these olfactory proteins interact with each other are major questions in the field that are being answered via genomic methods. Carbon dioxide, ammonia, and other individual compounds have been shown to affect mosquito behavior, but a synergism of the responses to multiple odorants seems to regulate the odor-driven host-seeking of females [138, 139]. An effective control strategy based on odorants would probably require elucidation of the molecular signaling for several key attractants such as carbon dioxide, L-lactic acid, and ammonia. Lu et al. have identified specific proteins in the A. gambiae odorant reception network that respond to carbon dioxide while Qiu et al. have translated the mosquitoes’ physical responses to a panel of stimuli into molecular identification of the neurons that fire [140, 141]. A slightly different strategy would focus not on the responses to specific odorants, but rather on the panel of ORs and OBPs that are uniquely up-regulated in host-seeking females (as compared to males or sugar-feeding females) [142]. From the implementation point of view, the Ors discovered thus far (Fox et al. 2002, Hallem et al. 2004a and 2004b, Li et al. 2005) are currently being tested for their specificity to a wide variety of chemicals and human-derived odorant ligands and may provide the basis for the development of more effective repellants and insect traps (Fox et al. 2002, Hallem et al. 2006, Kwon et al. 2006) [136, 143-147]. Research in this area involves using a combination of transgenic, biochemical, and behavioral studies to finely pinpoint the best odorant candidates. In particular, an A. gambiae female-derived odorant receptor (AgOr1) has recently been found to respond strongly to a component of human sweat (4-methylphenol) [143]. The repellents and attractants that have been discovered by the use of this method are thought to be safer than those in current use, to work more efficiently, and to be economically cost-effective. Current repellents such as DEET are sub-optimal under some conditions because they require continuous application to all exposed body parts and are less effective against the Central American malaria vector A. albimanus [148]. In order to prevent the development of resistance, researchers in this area are contemplating the use odorant ligands that target several ORs [148]. Currently, mosquito traps are being used for the surveillance of mosquito populations [149]. Such traps make use of carbon dioxide as a mosquito attractant that takes advantage of olfaction to attract mosquitoes, in the hope of reducing the vector population. Also, a wide array of mosquito traps such as the Mosquito Magnet Liberty, Mega-Catch Premier, and the SkeeterVac Mosquito Trap, are commercially available in the USA and employ a combination of attractants, heat, vacuum, and light to catch mosquitoes. An intriguing observation that has been made is that the host-seeking behaviors of malaria-infected mosquitoes can apparently be manipulated by the parasite. In model systems, infected mosquitoes are more likely to take a second blood meal, and mosquitoes exhibit a slight biting preference for infected vertebrate hosts while in a natural setting, infected mosquitoes seem to be more aggressive biters [150-152]. Elucidating the vector-parasite interactions that contribute to such manipulation is crucial for control strategies that address host-seeking behavior.
6.2. Photosensory: Trapping Through Light Attraction and Circadian Manipulation
Mosquitoes rely heavily on photosensory mechanisms for the maintenance of their circadian rhythms and for their proper biological functioning. Research devoted to better understanding these biological mechanisms is providing insights that can improve current control and mosquito surveillance strategies. For example, recent research has found that arrestins, which are important in regulating signal transduction, are involved in insect neurotransmission, visual sensory reception, and olfaction, making them important for the mosquito’s vectorial capacity. Light deprivation has been found to reduce the levels of arrestin in the pupal stage, leading to a reduction in the number of emerging adult mosquitoes [153]. Mosquito traps, such as the CDC miniature light traps, make use of the photophilic characteristic of insects to trap host-seeking mosquitoes; these traps are frequently used for mosquito surveillance, providing reliable information regarding the best time and place to control mosquito populations effectively [149, 154]. In many cases, mosquito control strategies combine light traps with attractants such as carbon dioxide and octenol to enhance the efficacy of the approach [149, 155-157]. On a behavioral and molecular level, insight into the mechanisms controlling mosquitoes’ light-cued feeding behavior could lead to interference with feeding and hence transmission. This field is still in its early stages but shows promise as a novel way to control transmission dynamics at the level of mosquito behavior.
7. FROM THE LAB TO THE FIELD: CHALLENGES TO THE FIELD IMPLEMENTATION OF MOLECULAR-BASED VECTOR CONTROL STRATEGIES
Although, great advances have been made in developing transgenic mosquitoes and identifying potent anti-parasite effector genes, an effective gene drive mechanism that will allow the introduction and fixation of the transgene in the wild population is still needed. Transposable elements, homing endonucleases and Wolbachia are potential gene drive mechanisms that are currently being studied, each posing their own challenges [122, 123]. Transposable elements have the potential to spread through the population but there are concerns over the random integration that can lead to a compromise in fitness [111] and their efficiency as gene drive still needs to be fully evaluated. Homing endonucleases can be used for the development of potentially useful gene drive mechanisms while their effectiveness is still under investigation [158]. Another potential gene drive mechanism is the intracellular symbiont Wolbachia. Wolbachia-induced cytoplasmic incompatibility results in non-viable offspring when an infected male mates with an uninfected female but viable offspring when at least the female is infected [159]. The increased reproductive success of infected females allows for the rapid dissemination of Wolbachia through the population. Wolbachia could therefore serve as a facilitator to drive a transgene through the target mosquito population. Although Wolbachia is present in culicine mosquitoes and has been successfully transferred from Aedes albopictus to Ae. aegypti [160] there is no anopheline mosquito known to carry this endosymbiont. Successful use of this approach for gene drive will hence depend on successfully infecting Anopheline mosquitoes. Furthermore, there are concerns that the drive mechanism itself could pose additional fitness costs [161]. This requires that the refractoriness effector gene be linked to the gene drive mechanism to attain the desired impact on pathogen transmission [161, 162]. The existence of several mosquito vectors, cryptic species, molecular forms and subspecies possesses another challenge, since they would have to be manipulated and introduced to the field independently [43, 6]. It is also of critical importance that we understand the biology and the geographic and seasonal distributions of vector species and the gene flow dynamics between them [111]. Another obstacle to the field application of genetically modified (GM) mosquitoes relates to awareness and ethical attributes of this approach. The eventual release of GM mosquitoes is likely to raise considerable attention and opposition from politicians, non-governmental institutions and environmental groups that may see potential risks with this approach. Thus any strategy that involves the release of transgenic mosquitoes will have to go through the regulatory process and overcome ethical and social oppositions to obtain national and international acceptance prior to its release [162]. The need for a better regulation of GM organisms has encouraged scientist and government institutions to develop recommendations and guidelines for conducting contained field trials and open field release of GM organisms (Benedict et al. 2008). Despite these challenges it is now widely accepted that a successful malaria control will require an integrated approach, merging current vector control strategies with other potential strategies such as the use of GM mosquitoes or paratransgenesis [163]. The trade-offs of any disease control strategy need to be considered carefully; where the cost of the disease is weighted against the cost of the control strategy.
8. MOSQUITO RESEARCH SPIN-OFFS: DISCOVERY OF PHARMACOLOGICAL AGENTS
Molecular and functional genomic studies are not only providing a better understanding of mosquito biology that can contribute to better vector control but also offer a new resource for the discovery of novel pharmacological agents. The range of antimicrobial factors used by insects to combat bacteria, fungi, and parasites and the anticoagulant and vasodilator compounds used during blood-feeding are providing the molecular basis for new antimicrobials, anti-inflamatory/analgesic, anti-hemostatic, and anticoagulant drugs as well as serving as potential targets for new vaccines [103, 164-170]. Biotechnology companies have synthesized invertebrate antimicrobial peptides, such as cecropins, defensins, gambicin, and hemocyanins, with the hope that such agents will be part of a new wave of therapy [refs 171, 172 and http://www.inimexpharma.com; http://biotech.deep13.com/Alpha/alpha.html; http://www.geniconsciences.com/]. These “natural antibiotics” may help alleviate drug resistance and work synergistically with the host’s immune response rather than in parallel (reviewed by [171]). To date, no insect antimicrobial peptide has received FDA approval for antibacterial therapy; nonetheless, many of the same compounds are being investigated as agents for surface sterilization [171]. Other candidates for therapeutic pharmacological agents come from the mosquito’s saliva. Mosquitoes have been referred to as “invertebrate pharmacologists” because their hematophagous nature requires them to synthesize compounds that allow them to easily feed on blood [173]. For instance, an anticoagulant salivary compound isolated from A. albimanus, anophelin, has been found to act as an α-thrombin inhibitor, and because its unique primary sequence, it has been hypothesized to be useful for studying α-thrombin structure and function [174, 175]. Another example is a myeloperoxidase from A. albimanus that supports vasodilation by destroying vasoconstrictors [176, 177]. Apyrases, ADP-depleting molecules that mosquitoes deposit into blood vessels to prevent platelet activation and wound repair, have been characterized in Anopheles as well as in the yellow fever mosquito Aedes aegypi and other arthropods [178, 179].
9. CONCLUSION
It is becoming obvious that a comprehensive strategy is needed if we are to make significant progress toward malaria eradication. Drug and vaccine development may not be sufficient to halt transmission, and vector-based initiatives in other areas have proved effective. Not only do vector-based control strategies target an essential step in the transmission process, but they also address a step that precedes infection (thus preventing rather than treating the illness), and they do not rely on human compliance. Some control strategies are already in place and found to be effective to varying degrees in controlling the mosquito as a disease vector and as a pest, yet roadblocks still exist. The genomic era has offered the opportunity to rethink old strategies and to develop novel approaches with exciting promise.
Innovation and development require an intimate knowledge of three elements: 1) the molecular, physiological, and population biology of the mosquito; 2) the interactions between the parasite, vector, and host; and 3) potential delivery mechanisms. The synergy of these elements may be the key to eliminating a pest and eradicating a deadly disease.
REFERENCES
- [1].WHO . Insecticide-treated mosquito nets: a WHO position statement. WHO; Geneva: 2007. [Google Scholar]
- [2].Fontenille D, Lochouarn R. Parassitologia. 1999;41:267–71. [PubMed] [Google Scholar]
- [3].Fontenille D, Cohuet A, Awono-Ambene PH, Antonio-Nkondjio C, Wondji C, Kengne P, Dia I, Boccolini D, Duchemin JB, Rajaonarivelo V, Dabire R, Adja-Akre M, Ceainu C, Goff G. le, Simard F. Med. Trop.(Mars) 2003;63:247–53. [PubMed] [Google Scholar]
- [4].Ault SK. Am. J. Trop. Med. Hyg. 1994;50:35–49. doi: 10.4269/ajtmh.1994.50.35. [DOI] [PubMed] [Google Scholar]
- [5].Walker K, Lynch M. Med.Vet. Entomol. 2007;21:2–21. doi: 10.1111/j.1365-2915.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- [6].Shiff C. Clin. Microbiol. Rev. 2002;15:278–293. doi: 10.1128/CMR.15.2.278-293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Utzinger J, Tozan Y, Singer BH. Trop. Med.Int. Health. 2001;6:677–687. doi: 10.1046/j.1365-3156.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- [8].Killeen GF, Fillinger U, Kiche I, Gouagna LC, Knols BGJ. Lancet Infect. Dis. 2002;2:618. doi: 10.1016/s1473-3099(02)00397-3. [DOI] [PubMed] [Google Scholar]
- [9].Killeen G, Fillinger U, Knols BGJ. Malar. J. 2002;1:8. doi: 10.1186/1475-2875-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grillet ME. J. Med. Entomol. 2000;37:231–238. doi: 10.1603/0022-2585-37.2.231. [DOI] [PubMed] [Google Scholar]
- [11].Utzinger J, Tozan Y, Doumani F, Singer BH. Trop. Med. Int. Health. 2002;7:657–77. doi: 10.1046/j.1365-3156.2002.00916.x. [DOI] [PubMed] [Google Scholar]
- [12].De Castro MC, Yamagata Y, Mtasiwa D, Tanner M, Utzinger J, Keiser J, Singer BH. Am. J. Trop. Med. Hyg. 2004;71:103–117. [PubMed] [Google Scholar]
- [13].Lacey LA, Lacey CM. J. Am. Mosq. Control. Assoc. 1990;6:1–93. [PubMed] [Google Scholar]
- [14].Fillinger U, Knols BGJ, Becker N. Trop. Med. Int. Health. 2003;8:37–47. doi: 10.1046/j.1365-3156.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- [15].Russell TL, Brown MD, Purdie DM, Ryan PA, Kay BH. J. Econ. Entomol. 2003;96:1786–1791. doi: 10.1093/jee/96.6.1786. [DOI] [PubMed] [Google Scholar]
- [16].Das PK, Amalraj DD. Indian J. Med. Res. 1997;106:174–197. [PubMed] [Google Scholar]
- [17].Fletcher M, Teklehaimanot A, Yemane G. Acta Trop. 1992;52:155–166. doi: 10.1016/0001-706x(92)90032-s. [DOI] [PubMed] [Google Scholar]
- [18].Chapman R. MSc Thesis. London School of Hygiene and Tropical Medicine; 2000. [Google Scholar]
- [19].Blanford S, Chan BH, Jenkins N, Sim D, Turner RJ, Read AF, Thomas MB. Science. 2005;308:1638–41. doi: 10.1126/science.1108423. [DOI] [PubMed] [Google Scholar]
- [20].Scholte EJ, Knols BG, Takken W. J. Invertebr. Pathol. 2005;91:43–9. doi: 10.1016/j.jip.2005.10.006. [DOI] [PubMed] [Google Scholar]
- [21].Scholte EJ, Knols BG, Takken W. Malar J. 2004;3:45. doi: 10.1186/1475-2875-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kanzok SM, Jacobs-Lorena M. Trends Parasitol. 2006;22:49–51. doi: 10.1016/j.pt.2005.12.008. [DOI] [PubMed] [Google Scholar]
- [23].Alphey L, Beard CB, Billingsley P, Coetzee M, Crisanti A, Curtis C, Eggleston P, Godfray C, Hemingway J, Jacobs-Lorena M, James AA, Kafatos FC, Mukwaya LG, Paton M, Powell JR, Schneider W, Scott TW, Sina B, Sinden R, Sinkins S, Spielman A, Touré Y, Collins FH. Science. 2002;298:119–121. doi: 10.1126/science.1078278. [DOI] [PubMed] [Google Scholar]
- [24].Phuc HK, andreasen MH, Burton RS, Vass C, Epton MJ, Pape G, Fu G, Condon KC, Scaife S, Donnelly CA, Coleman PG, White-Cooper H, Alphey L. BMC Biol. 2007;5:11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Science. 2000;287:2474–2476. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- [26].Dyck A, Hendrichs J, Robinson AS. The Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Springer; Heidelberg, Germany: 2005. [Google Scholar]
- [27].Sharma VP, Razdan RK, Ansari MA. J. Econ. Entomol. 1978;71:449–452. doi: 10.1093/jee/71.3.449. [DOI] [PubMed] [Google Scholar]
- [28].Andreasen MH, Curtis CF. Med. Vet. Entomol. 2005;19:238–44. doi: 10.1111/j.1365-2915.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- [29].Lindquist DA, Abusowa M, Hall MJ. Med. Vet. Entomol. 1992;6:2–8. doi: 10.1111/j.1365-2915.1992.tb00027.x. [DOI] [PubMed] [Google Scholar]
- [30].Hendrichs J, Franz G, Rendón P. J. Appl. Entomol. 1995;119:371–377. [Google Scholar]
- [31].Breeland SG, Jeffery GM, Lofgren CS, Weidhaas DE. Am. J. Trop. Med. Hyg. 1974;23:274–281. doi: 10.4269/ajtmh.1974.23.274. [DOI] [PubMed] [Google Scholar]
- [32].Weidhaas DE, Breeland SG, Lofgren CS, Dame DA, Kaiser R. Am. J. Trop. Med. Hyg. 1974;23:298–308. doi: 10.4269/ajtmh.1974.23.298. [DOI] [PubMed] [Google Scholar]
- [33].Benedict MQ, Robinson AS. Trends Parasitol. 2003;19:349–355. doi: 10.1016/s1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- [34].Rozendaal JA. Vector Control: Methods for Use by Individuals and Communities. World Health Organization; Geneva: 1997. [Google Scholar]
- [35].Killeen GF. Lancet Infect. Dis. 2003;3:663. doi: 10.1016/s1473-3099(03)00776-x. [DOI] [PubMed] [Google Scholar]
- [36].Curtis CF. Science. 2000;290:1508. doi: 10.1126/science.290.5496.1508. [DOI] [PubMed] [Google Scholar]
- [37].Pardo G, Descalzo MA, Molina L, Custodio E, Lwanga M, Mangue C, Obono J, Nchama A, Roche J, Benito A, Cano J. Malar. J. 2006;5:1–10. doi: 10.1186/1475-2875-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Potikasikorn J, Chareonviriyaphap T, Bangs MJ, Prabaripai A. Am. J. Trop. Med. Hyg. 2005;73:343–349. [PubMed] [Google Scholar]
- [39].WHO 2006. (WHO technical report series 936).
- [40].Hung LQ, de Vries PJ, Giao PT, Nam NV, Binh TQ, Chong MT, Quoc NTTA, Thanh TN, Hung LN, Kager PA. Bull. WHO. 2002;80:660–666. [PMC free article] [PubMed] [Google Scholar]
- [41].Curtis CF, Mnzava AE. Bull. WHO. 2000;78:1389–1400. [PMC free article] [PubMed] [Google Scholar]
- [42].Yapabandara AMGM, Curtis CF, Wickramasinghe MB, Fernando WP. Acta Trop. 2001;80:265–276. doi: 10.1016/s0001-706x(01)00178-4. [DOI] [PubMed] [Google Scholar]
- [43].Collins FH, Kamau L, Ranson HA, Vulule JM. Bull. WHO. 2000;78:1412–1423. [PMC free article] [PubMed] [Google Scholar]
- [44].James AA. J. Exp. Biol. 2003:3817–3821. doi: 10.1242/jeb.00616. [DOI] [PubMed] [Google Scholar]
- [45].Hemingway J, Field L, Vontas J. Science. 2002;298:96–97. doi: 10.1126/science.1078052. [DOI] [PubMed] [Google Scholar]
- [46].WHO WHO; Geneva: Pesticides and Their Application for the Control of Vectors and Pests of Public Health Importance. 2006
- [47].Pasteur N, Raymond M. J. Hered. 1996;87:444–449. doi: 10.1093/oxfordjournals.jhered.a023035. [DOI] [PubMed] [Google Scholar]
- [48].Ranson H. Science. 2002;298:179–181. doi: 10.1126/science.1076781. [DOI] [PubMed] [Google Scholar]
- [49].Matambo TS, Abdalla H, Brooke BD, Koekemoer LL, Mnzava A, Hunt RH, Coetzee M. Med. Vet. Entomol. 2007;21:97–102. doi: 10.1111/j.1365-2915.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- [50].Silvestrini F, Bozdech Z, Lanfrancotti A, Di Giulio E, Bultrini E, Picci L, Derisi JL, Pizzi E, Alano P. Mol. Biochem. Parasitol. 2005;143:100–10. doi: 10.1016/j.molbiopara.2005.04.015. [DOI] [PubMed] [Google Scholar]
- [51].Khan SM, Franke-Fayard B, Mair GR, Lasonder E, Janse CJ, Mann M, Waters AP. Cell. 2005;121:675–87. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- [52].Alano P. Mol. Microbiol. 2007;66:291–302. doi: 10.1111/j.1365-2958.2007.05904.x. [DOI] [PubMed] [Google Scholar]
- [53].Pradel G. Parasitology. 2007:1–19. doi: 10.1017/S0031182007003381. [DOI] [PubMed] [Google Scholar]
- [54].Mair GR, Braks JA, Garver LS, Dimopoulos G, Hall N, Wiegant JCAG, Dirks RW, Khan SM, Janse CJ, Waters AP. Science. 2006;313:667–9. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ghosh AK, Ribolla PE, Jacbos-Lorena M. Proc. Natl. Acad. Sci. USA. 2001;98:13278–81. doi: 10.1073/pnas.241491198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zieler H, Garon CF, Fischer ER, Shahabuddin M. J. Exp. Biol. 2000;203:1599–1611. doi: 10.1242/jeb.203.10.1599. [DOI] [PubMed] [Google Scholar]
- [57].Dinglasan RR, Kalume DE, Kanzok SE, Ghosh AK, Muratova O, Pandey A, Jacobs-Lorena M. Proc. Natl. Acad. Sci. USA. 2007;104:13461–6. doi: 10.1073/pnas.0702239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lavazec C, Bonnet S, Thiery I, Boisson B, Bourgouin C. Insect Mol. Biol. 2005;14:163–174. doi: 10.1111/j.1365-2583.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- [59].Lavazec C, Boudin C, Lacroix R, Bonnet S, Diop A, Boisson B, Tahar R, Bourgouin C. Infect. Immun. 2007;75:1635–42. doi: 10.1128/IAI.00864-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li F, Templeton TJ, Popov V, Comer JE, Tsuboi T, Torii M, Vinetz JM. J. Biol. Chem. 2004;279:26635–44. doi: 10.1074/jbc.M401385200. [DOI] [PubMed] [Google Scholar]
- [61].Barillas-Mury C. Trends Parasitol. 2007;23:297–9. doi: 10.1016/j.pt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- [62].Dimopoulos G, Christophides GK, Meister S, Schultz J, White KP, Barillas-Mury C, Kafatos FC. Proc. Natl. Acad. Sci. USA. 2002;99:8814–8819. doi: 10.1073/pnas.092274999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Volz J, Osta MA, Kafatos FC, Muller HM. J. Biol. Chem. 2005;280:40161–8. doi: 10.1074/jbc.M506191200. [DOI] [PubMed] [Google Scholar]
- [65].Volz J, Muller HM, Zdanowicz A, Kafatos FC, Osta MA. Cell Microbiol. 2006;8:1392–405. doi: 10.1111/j.1462-5822.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- [66].Abraham EG, Pinto SB, Ghosh A, Vanlandingham DL, Budd A, Higgs S, Kafatos FC, Jacobs-Lorena M, Michel K. Proc. Natl. Acad. Sci. USA. 2005;102:16327–32. doi: 10.1073/pnas.0508335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Michel K, Budd A, Pinto SB, Gibson TJ, Kafatos FC. EMBO Rep. 2005;6:891–7. doi: 10.1038/sj.embor.7400478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Michel K, Suwanchaichinda C, Morlais I, Lambrechts L, Cohuet A, Awono-Ambene PH, Simard F, Fontenille D, Kanost MR, Kafatos FC. Proc. Natl. Acad. Sci. USA. 2006;103:16858–63. doi: 10.1073/pnas.0608033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Luckhart S, Vodovotz Y, Cui L, Rosenberg R. Proc. Natl. Acad. Sci. USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lim J, Gowda DC, Krishnegowda G, Luckhart S. Infect. Immun. 2005;73:2778–2789. doi: 10.1128/IAI.73.5.2778-2789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Han YS, Barillas-Mury C. Insect Biochem. Mol. Biol. 2002;32:1311–1316. doi: 10.1016/s0965-1748(02)00093-0. [DOI] [PubMed] [Google Scholar]
- [72].Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, Dimopoulos G, Kafatos FC, Barillas-Mury C. Proc. Natl. Acad. Sci. USA. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kumar S, Gupta L, Han YS, Barillas-Mury C. J. Biol. Chem. 2004;270:53475–53482. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- [74].Peterson TM, Gow AJ, Luckhart S. Free Radic. Biol. Med. 2007;42:132–142. doi: 10.1016/j.freeradbiomed.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Han YS, Thompson J, Kafatos FC, Barillas-Mury C. EMBO J. 2000;19:6030–40. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kumar S, Barillas-Mury C. Insect Biochem. Mol. Biol. 2005;35:721–737. doi: 10.1016/j.ibmb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- [77].Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, Kafatos FC. Cell. 2001;104:709–718. doi: 10.1016/s0092-8674(01)00267-7. [DOI] [PubMed] [Google Scholar]
- [78].Baxter RH, Chang CI, Chelliah Y, Blandin S, Levashina EA, Deisenhofer J. Proc. Nat. Acad. Sci. USA. 2007;104:11615–20. doi: 10.1073/pnas.0704967104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- [80].Osta MA, Christophides GK, Kafatos FC. Science. 2004;303:2030–2032. doi: 10.1126/science.1091789. [DOI] [PubMed] [Google Scholar]
- [81].Riehle MM, Markianos K, Niare O, Xu J, Li J, Toure AM, Podiougou B, Oduol F, Diawara S, Diallo M, Coulibaly B, Ouatara A, Kruglyak L, Traore SF, Vernick KD. Science. 2006;312:577–579. [Google Scholar]
- [82].Dong Y, Taylor HE, Dimopoulos G. PLoS Biol. 2006;4:e229. doi: 10.1371/journal.pbio.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu J, Zheng L, Bork P, Kafatos FC. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- [84].Meister S, Kanzok SM, Zheng XL, Luna C, Li TR, Hoa NT, Clayton JR, White KP, Kafatos FC, Christophides GK, Zheng L. Proc. Natl. Acad. Sci. USA. 2005;102:11420–11425. doi: 10.1073/pnas.0504950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rodriguez MC, Zamudio F, Torres JA, Gonzalez-Ceron L, Possani LD, Rodriguez MH. Exp. Parasitol. 1995;80:596–604. doi: 10.1006/expr.1995.1075. [DOI] [PubMed] [Google Scholar]
- [86].Tahar R, Boudin C, Thiery I, Bourgouin C. EMBO J. 2002;21:6673–6680. doi: 10.1093/emboj/cdf664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Vizioli J, Bulet P, Hoffmann JA, Kafatos FC, Muller HM, Dimopoulos GD. Proc. Natl. Acad. Sci. USA. 2001;98:12630–5. doi: 10.1073/pnas.221466798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Vizioli J, Richman AM, Uttenweiler-Joseph S, Blass C, Bulet P. Insect Biochem. Mol. Bio. 2001;31:241–8. doi: 10.1016/s0965-1748(00)00143-0. [DOI] [PubMed] [Google Scholar]
- [89].Blandin S, Moita LF, Kocher T, Wilm M, Kafatos FC, Levashina EA. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. Immunity. 2006;25:677–685. doi: 10.1016/j.immuni.2006.08.019. [DOI] [PubMed] [Google Scholar]
- [91].Adini A, Warburg A. Parasitology. 1999;119:331–336. doi: 10.1017/s0031182099004874. [DOI] [PubMed] [Google Scholar]
- [92].Warburg A, Shtern A, Cohen N, Dahan N. Microbes Infect. 2007;9:192–199. doi: 10.1016/j.micinf.2006.11.006. [DOI] [PubMed] [Google Scholar]
- [93].Kariu T, Yuda M, Yano K, Chinzei Y. J. Exp. Med. 2002;195:1317–23. doi: 10.1084/jem.20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Okulate MA, Kalume DE, Reddy R, Kristiansen T, Bhattacharyya M, Chaerkady R, Pandey A, Kumar N. Insect Mol. Biol. 2007;16:711–22. doi: 10.1111/j.1365-2583.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- [95].Korochkina S, Barreau C, Pradel G, Jeffery E, Li J, Natarajan R, Shabanowitz J, Hunt D, Frevert U, Vernick KD. Cell. Microbiol. 2006;8:163–75. doi: 10.1111/j.1462-5822.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- [96].Myung JM, Marshall P, Sinnis P. Mol. Biochem. Parasitol. 2004;133:53–9. doi: 10.1016/j.molbiopara.2003.09.002. [DOI] [PubMed] [Google Scholar]
- [97].Brennen JD, Kent M, Dhar R, Fugioka H, Kumar N. Proc. Natl. Acad. Sci. USA. 2000;97:13859–64. doi: 10.1073/pnas.250472597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Warburg A, Touray M, Krettli AU, Miller LH. Exp. Parasitol. 1992;75:303–7. doi: 10.1016/0014-4894(92)90215-v. [DOI] [PubMed] [Google Scholar]
- [99].Touray MG, Warburg A, Laughinghouse A, Krettli AU, Miller LH. J. Exp. Med. 1992;175:1607–12. doi: 10.1084/jem.175.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Barreau C, Touray M, Pimenta PF, Miller LH, Vernick KD. J. Exp. Parasitol. 1995;81:332–43. doi: 10.1006/expr.1995.1124. [DOI] [PubMed] [Google Scholar]
- [101].Barreau C, Conrad J, Fischer E, Lujan HD, Vernick KD. Insect Biochem. Mol. Biol. 1999;29:515–26. doi: 10.1016/s0965-1748(99)00025-9. [DOI] [PubMed] [Google Scholar]
- [102].Hillyer JF, Barreau C, Vernick KD. Int. J. Parasitol. 2007;37:673–81. doi: 10.1016/j.ijpara.2006.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Dimopoulos G, Seeley D, Wolf A, Kafatos FC. EMBO J. 1998;17:6115–23. doi: 10.1093/emboj/17.21.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Arca B, Lombardo F, Capurro M, della Torre M, Spanos L, Dimopoulos G, Louis C, James AA, Coluzzi M. Parasitologia. 1999;41:483–7. [PubMed] [Google Scholar]
- [105].Rosinski-Chupin I, Briolay J, Brouilly J, Perrot S, Gomez SM, Chertemps T, Roth CW, Keime C, Gandrillon O, Couble P, Brey PT. Cell. Microbiol. 2007;9:708–24. doi: 10.1111/j.1462-5822.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- [106].Choumet V, Carmi-Leroy A, Laurent C, Lenormand P, Rousselle JC, Namane A, Roth C, Brey PT. Proteomics. 2007;7:3384–94. doi: 10.1002/pmic.200700334. [DOI] [PubMed] [Google Scholar]
- [107].Pinto SB, Kafatos FC, Michel K. Cell Microbiol. 2008;10:891–8. doi: 10.1111/j.1462-5822.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- [108].Jacobs-Lorena M. J. Vect. Borne Dis. 2003;40:73–77. [PubMed] [Google Scholar]
- [109].Catteruccia F, Godfray HC, Crisanti A. Science. 2003;299:1225–1227. doi: 10.1126/science.1081453. [DOI] [PubMed] [Google Scholar]
- [110].Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Nature. 2002;417:452. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- [111].Riehle MA, Srinivasan P, Moreira CK, Jacobs-Lorena M. J. Exp. Biol. 2003;206:3809–16. doi: 10.1242/jeb.00609. [DOI] [PubMed] [Google Scholar]
- [112].Sperança MA, Capurro ML. Mem. Inst. Oswaldo Cruz. 2007;102:425–433. doi: 10.1590/s0074-02762007005000054. [DOI] [PubMed] [Google Scholar]
- [113].Edwards MJ, Lemos FJ, Donnelly-Doman M, Jacobs-Lorena M. Insect. Biochem. Mol. Biol. 1997;27:1063–1072. doi: 10.1016/s0965-1748(97)00093-3. [DOI] [PubMed] [Google Scholar]
- [114].Moreira LA, Edwards MJ, Adhami F, Jasinskiene N, James AA, Jacobs-Lorena M. Proc. Natl. Acad. Sci. USA. 2000;97:10895–10898. doi: 10.1073/pnas.97.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yoshida S, Watanabe H. Insect Mol. Biol. 2006;15:403–410. doi: 10.1111/j.1365-2583.2006.00645.x. [DOI] [PubMed] [Google Scholar]
- [116].Nirmala X, Marinotti O, Sandoval JM, Phin S, Gakhar S, Jasinskiene N, James AA. Insect. Biochem. Mol. Biol. 2006;36:694–700. doi: 10.1016/j.ibmb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- [117].Nirmala X, James AA. Trends Parasitol. 2003;19:384. doi: 10.1016/s1471-4922(03)00188-0. [DOI] [PubMed] [Google Scholar]
- [118].Yoshida S, Ioka D, Matsuoka H, Endo H, Ishii A. Mol. Biochem. Parasitol. 2001;113:89–96. doi: 10.1016/s0166-6851(00)00387-x. [DOI] [PubMed] [Google Scholar]
- [119].Moreira LA, Ito J, Ghosh A, Devenport M, Zieler H, Abraham EG, Crisanti A, Nolan T, Catteruccia F, Jacobs-Lorena M. J. Biol. Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- [120].Marrelli MT, Li C, Rasgon JL, Jacobs-Lorena M. Proc. Natl. Acad. Sci. USA. 2007;104:5580–5583. doi: 10.1073/pnas.0609809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Burt A. Proc. R. Soc. Lond. B. 2003;270:921–928. doi: 10.1098/rspb.2002.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Turelli M, Hoffmann AA. Insect Mol. Biol. 1999;8:243–255. doi: 10.1046/j.1365-2583.1999.820243.x. [DOI] [PubMed] [Google Scholar]
- [123].Cohuet A, Osta MA, Morlais I, Awono-Ambene PH, Michel K, Simard F, Christophides GK, Fontenille D, Kafatos FC. EMBO Rep. 2006;7:1285–1289. doi: 10.1038/sj.embor.7400831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Beard CB, Cordon-Rosales C, Duvarsula RV. Ann. Rev. Entomol. 2002;47:123–141. doi: 10.1146/annurev.ento.47.091201.145144. [DOI] [PubMed] [Google Scholar]
- [125].Durvasula RV, Gumbs A, Panackal A, Kruglov O, Aksoy S, Merrifield RB, Richards FF, Beard CB. Proc. Nat. Acad. Sci. USA. 1997;94:3274–3278. doi: 10.1073/pnas.94.7.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Riehle MA, Jacobs-Lorena M. Insect Biochem. Mol. Biol. 2005;35:699. doi: 10.1016/j.ibmb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- [127].Riehle MA, Moreira CK, Lampe D, Lauzon C, Jacobs-Lorena M. Int. J. Parasitol. 2007;37:595. doi: 10.1016/j.ijpara.2006.12.002. [DOI] [PubMed] [Google Scholar]
- [128].Pumpuni CB, Demaio J, Kent M, Davis JR, Beier JC. Am. J. Trop. Med. Hyg. 1996;54:214–218. doi: 10.4269/ajtmh.1996.54.214. [DOI] [PubMed] [Google Scholar]
- [129].Straif SC, Mbogo CN, Toure AM, Walker ED, Kaufman M, Toure YT, Beier JC. J. Med. Entomol. 1998;35:222–226. doi: 10.1093/jmedent/35.3.222. [DOI] [PubMed] [Google Scholar]
- [130].Sinden RE. Ann. Trop. Med. Parasitol. 1997;91:69. [Google Scholar]
- [131].Hisaeda H, Stowers AW, Tsuboi T, Collins WE, Sattabongkot JS, Suwanabun N, Torii M, Kaslow DC. Infect. Immun. 2000;68:6618–6623. doi: 10.1128/iai.68.12.6618-6623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Donovan MJ, Messmore AS, Scrafford DA, Sacks DL, Kamhawi S, McDowell M. Infect. Immun. 2007;75:2523–2530. doi: 10.1128/IAI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Stowers A, Carter R. Expert Opin. Biol. Ther. 2001;1:619–628. doi: 10.1517/14712598.1.4.619. [DOI] [PubMed] [Google Scholar]
- [134].Carter R. Vaccine. 2001;19:2309–2314. doi: 10.1016/s0264-410x(00)00521-1. [DOI] [PubMed] [Google Scholar]
- [135].van der Goes van Naters W, Carlson JR. Nature. 2006;444:302. doi: 10.1038/nature05403. [DOI] [PubMed] [Google Scholar]
- [136].Fox AN, Pitts RJ, Zwiebel LJ. Chem. Senses. 2002;27:453–459. doi: 10.1093/chemse/27.5.453. [DOI] [PubMed] [Google Scholar]
- [137].Takken W. Insect Sci. Appl. 1991;12:287–95. [Google Scholar]
- [138].Braks MAH, Takken W. J. Chem. Ecol. 1999;25:663–72. [Google Scholar]
- [139].Smallegange RC, Qiu YT, Van Loon JJA, Takken W. Chem. Senses. 2005;30:145–52. doi: 10.1093/chemse/bji010. [DOI] [PubMed] [Google Scholar]
- [140].Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJ, Takken W, Carlson JR, Zwiebel LJ. Curr. Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Oiu YT, van Loon JJ, Takken W, Meijerink J, Smid HM. Chem. Senses. 2006;31:845–63. doi: 10.1093/chemse/bjl027. [DOI] [PubMed] [Google Scholar]
- [142].Biessmann H, Nguyen QK, Le D, Walter MF. Insect Mol. Biol. 2005;14:575–89. doi: 10.1111/j.1365-2583.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- [143].Hallem EA, Fox AN, Zwiebel LJ, Carlson JR. Nature. 2004;427:212–213. doi: 10.1038/427212a. [DOI] [PubMed] [Google Scholar]
- [144].Hallem EA, Ho MG, Carlson JR. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- [145].Li Z, Pickett JA, Field LM, Zhou J. Arch. Insect Biochem. Physiol. 2005;58:175. doi: 10.1002/arch.20047. [DOI] [PubMed] [Google Scholar]
- [146].Hallem EA, Carlson JR. Cell. 2006;125:143–146. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- [147].Kwon H-W, Lu T, Rutzler M, Zwiebel LJ. Proc. Natl. Acad. Sci. USA. 2006;103:13526–13531. doi: 10.1073/pnas.0601107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Schmidt VM, Zucchi R, Barth FG. Apidologie. 2005;36:285–291. [Google Scholar]
- [149].Shone SM, Glass GE, Norris DE. J. Med. Entomol. 2006;43:151–158. doi: 10.1603/0022-2585(2006)043[0151:ttomvi]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Ferguson HM, Read AF. Malar. J. 2004;3:12. doi: 10.1186/1475-2875-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Koella J, Packer MJ. Parasitology. 1996;113:105–9. doi: 10.1017/s0031182000066348. [DOI] [PubMed] [Google Scholar]
- [152].Koella J, Sorenson FL, Anderson RA. Proc. Biol. Sci. 1998;265:763–8. doi: 10.1098/rspb.1998.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Hoffman S, Subramanian GM. J. Med. Entomol. 2005;42:801–804. doi: 10.1093/jmedent/42.5.801. [DOI] [PubMed] [Google Scholar]
- [154].Service MW. Mosquito Ecology, Field Sampling Methods. Halsted Press; 1976. pp. 324–342. [Google Scholar]
- [155].Burkett DA, Lee WJ, Lee KW, Kim HC, Lee HI, Lee JS, Shin EH, Wirtz RA, Cho HW, Claborn DM, Coleman RE, Klein TA. J. Am. Mosq. Control Assoc. 2001;17:196–205. [PubMed] [Google Scholar]
- [156].Cooper RD, Frances SP, Popat S, Waterson DG. J. Am. Mosq. Control Assoc. 2004;20:239–42. [PubMed] [Google Scholar]
- [157].van den Hurk AF, Montgomery BL, Zborowski P, Beebe NW, Cooper RD, Ritchie SA. J. Am. Mosq. Control Assoc. 2006;22:15–21. doi: 10.2987/8756-971X(2006)22[15:DOETCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [158].Windbichler N, Papathanos PA, Catteruccia F, Ranson H, Burt A, Crisanti A. Nucleic Acids Res. 2007;35:5922–5933. doi: 10.1093/nar/gkm632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Hoffman AA, Turelli M, Harshman LG. Genetics. 1990;126:933–948. doi: 10.1093/genetics/126.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Xi Z, Kho CCH, Dobson SL. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- [161].Curtis CF, Coleman PG, Kelly DW, Campbell-Lendrum DH. In: Genetically Modified Mosquitoes for Malaria Control. Boëte C, editor. Eurekah/Landes Bioscience; Georgetown: 2006. pp. 60–78. [Google Scholar]
- [162].Knols BGJ, Bossin HC, Mukabana WR, Robinson AS. Am. J. Trop. Med. Hyg. 2007;77:232–242. [PubMed] [Google Scholar]
- [163].Beaty BJ. Proc. Natl. Acad. Sci. USA. 2000;97:10295–10297. doi: 10.1073/pnas.97.19.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ribeiro JM. J. Exp. Biol. 1992;165:61–71. doi: 10.1242/jeb.165.1.61. [DOI] [PubMed] [Google Scholar]
- [165].Isawa H, Yuda M, Yoneda K, Chinzei Y. J. Biol. Chem. 1999;275:6636–6641. doi: 10.1074/jbc.275.9.6636. [DOI] [PubMed] [Google Scholar]
- [166].Isawa H, Yuda M, Orito Y, Chinzei Y. J. Biol. Chem. 2002;277:27651–27658. doi: 10.1074/jbc.M203505200. [DOI] [PubMed] [Google Scholar]
- [167].Valenzuela JG, Garfield M, Rowton ED, Pham VM. J. Exp. Biol. 2004;207:3717–3729. doi: 10.1242/jeb.01185. [DOI] [PubMed] [Google Scholar]
- [168].Champagne DE, Wasserman HA, Kumar S, Singh S. Physiol. Entomol. 2004;29:269–277. [Google Scholar]
- [169].Valenzuela JG, Belkaid Y, Garfield MK, Mendez SA, Kamhawi S, Rowten ED, Sacks DL, Ribeiro JM. J. Exp. Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Titus RG, Bishop JV, Mejia JS. Parasite Immunol. 2006;28:131–41. doi: 10.1111/j.1365-3024.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- [171].Marshall SH, Arenas G. Electon J. Biotechnol. 2003;6:3. [Google Scholar]
- [172].Hancock REW. Exp. Opin. Invest. Drugs. 2000;9:1723–1729. doi: 10.1517/13543784.9.8.1723. [DOI] [PubMed] [Google Scholar]
- [173].Ribeiro JM. Infect. Agents Dis. 1995;4:143–152. [PubMed] [Google Scholar]
- [174].Valenzuela JG, Francischetti IMB, Ribeiro JMC. Biochemistry. 1999;38:11209–11215. doi: 10.1021/bi990761i. [DOI] [PubMed] [Google Scholar]
- [175].Francischetti IMB, Valenzuela JG, Ribeiro JMC. Biochemistry. 1999;38:16678–16685. doi: 10.1021/bi991231p. [DOI] [PubMed] [Google Scholar]
- [176].Ribeiro JM. Insect Biochem. Mol. Biol. 1996;26:715–720. doi: 10.1016/s0965-1748(96)00040-9. [DOI] [PubMed] [Google Scholar]
- [177].Ribeiro JM, Valenzuela JG. J. Exp. Biol. 1999;202:809–816. doi: 10.1242/jeb.202.7.809. [DOI] [PubMed] [Google Scholar]
- [178].Champagne DE, Smartt CT, Ribeiro JM, James AA. Proc. Natl. Acad. Sci. USA. 1995;92:694–700. doi: 10.1073/pnas.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Arca B, Lombardo F, Capurro M, della Torre A, Dimopoulos G, James AA, Coluzzi M. Proc. Natl. Acad. Sci. USA. 1999;96:1516–1521. doi: 10.1073/pnas.96.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]