Abstract
Numerous small RNAs regulators of gene expression exist in bacteria. A large class of them binds to the RNA chaperone Hfq and act by base-pairing interactions with their target-mRNA, thereby affecting their translation and/or stability. They often have multiple direct targets, some of which may be regulators themselves, and production of a single sRNA can therefore affect the expression of dozens of genes.
We show in this study that the synthesis of the E. coli pleiotropic PhoPQ two-component system is repressed by MicA, a σE-dependent sRNA regulator of porin biogenesis. MicA directly pairs with phoPQ mRNA in the translation initiation region of phoP and presumably inhibits translation by competiting with ribosome binding. Consequently, MicA down-regulates several members of the PhoPQ regulon. By linking PhoPQ to σE, our findings suggest that major cellular processes such as Mg2+ transport, virulence, LPS modification or resistance to antimicrobial peptides are modulated in response to envelope stress.
In addition, we found that Hfq strongly affects the expression of phoP independently of MicA, raising the possibility that even more sRNAs, that remain to be identified, could regulate PhoPQ synthesis.
Keywords: ompT, OmrA, OmrB
Introduction
A major challenge faced by bacteria is the necessity to adapt rapidly to ever-changing environments, which partly relies on their ability to regulate gene expression as a function of the environmental conditions. Two-component systems (TCS) are key components of this process. The model organism Escherichia coli has more than 30 TCS, which directly regulate transcription of hundreds of genes. Typically, TCS consist of a sensor kinase that autophosphorylates in response to a given external stimulus and then transfers its phosphate group to its cognate response regulator. The activated response regulator then binds to promoter regions and regulates transcription of its targets. In most cases, the genes encoding the regulator and the kinase are organized within an operon, which allows their synthesis to be coordinated. It is also common that expression of TCS operons is subject to feedback control. In the case of the E. coli EnvZ-OmpR TCS, this feedback is not direct, but is mediated by two homologous small RNAs (sRNAs), OmrA and OmrB, whose transcription is activated by EnvZ-OmpR and which in turn repress the expression of multiple genes, including the ompR-envZ operon itself (Guillier and Gottesman, 2008).
sRNAs are another class of widespread regulators in bacteria (Waters and Storz, 2009 for a recent review). Different experimental searches led to the identification of almost a hundred of them in E. coli (Sharma and Vogel, 2009). In general they are synthesized as discrete transcripts whose size varies between 50 and 400 nts in length. Most of them are post-transcriptional regulators that can act according to two major mechanisms: sRNAs that titrate a protein and therefore modulate its activity or sRNAs that directly base-pair with target-mRNAs. In this later class, one can distinguish sRNAs that are encoded on the opposite DNA strand to their target, and therefore share perfect complementarity with their target-mRNA, and sRNAs referred to as “trans-encoded” that often regulate multiple targets via limited complementarity. All trans-encoded sRNAs studied so far in E. coli bind the RNA chaperone Hfq, which has been shown to stabilize several sRNAs as well as facilitating sRNA-mRNA duplex formation by a mechanism that is still unclear (Brennan and Link, 2007; Valentin-Hansen et al., 2004). Most, but not all, trans-encoded sRNAs are transcribed under specific conditions as part of major regulons. For instance, MgrR, RyhB and CyaR sRNAs belong respectively to the regulons of PhoPQ TCS (Moon and Gottesman, 2009), Fur (Massé and Gottesman, 2002) and CRP (De Lay and Gottesman, 2009; Johansen et al., 2008; Papenfort et al., 2008). Pairing of sRNAs to their targets leads to regulation of gene expression by affecting translation and/or stability of the mRNA, either positively or negatively. Genes subject to post-transcriptional control by sRNAs are involved in numerous cellular processes, such as iron homeostasis, envelope homeostasis and quorum sensing, to name just a few. The expression of several regulators has also been shown to be controlled by sRNAs: for example, at least three of them directly activate translation of rpoS, the gene for the stationary phase sigma factor (Lease et al., 1998; Majdalani et al., 1998; Majdalani et al., 2001; Mandin and Gottesman, manuscript in preparation; Majdalani et al., 2002) and, as mentioned above, OmrA/B repress expression of the EnvZ-OmpR TCS.
Interestingly, regulation of TCS by sRNAs is not restricted to EnvZ-OmpR. Other examples include regulation of E. coli dpiBA, encoding a TCS induced by β-lactams and involved in the SOS response, by RybC (Mandin and Gottesman, 2009), as well as regulation of luxOU, encoding the response regulator LuxO and the phosphotransfer protein LuxU, by Qrr1-4 sRNAs in Vibrio cholerae (Svenningsen et al., 2009).
In this study, we show that yet another TCS, PhoPQ (where PhoQ is the sensor kinase and PhoP the cognate response regulator), is subject to direct post-transcriptional control by MicA sRNA. PhoPQ has been extensively studied in bacteria and especially in Salmonella. It is induced in response to low Mg2+ and Ca2+, as well as in the presence of antimicrobial peptides. Genes that are regulated by PhoPQ are involved in Mg2+ transport, resistance to antimicrobial peptides, acid resistance, LPS modification and virulence (Groisman, 2001). MicA is an Hfq-binding sRNA that was previously shown to directly repress synthesis of at least two bacterial outer membrane proteins (OMPs), OmpA and LamB (Bossi and Figueroa-Bossi, 2007; Rasmussen et al., 2005; Udekwu et al., 2005). MicA transcription is dependent on the alternative sigma factor σE which, in turn, responds to envelope stress (Johansen et al., 2006; Papenfort et al., 2006; Udekwu and Wagner, 2007). Our results show that expression of phoPQ is repressed in a MicA-dependent manner upon activation of σE, and that several PhoP-regulated genes are also controlled by MicA in a PhoP-dependent fashion. Therefore, expression of the PhoP-PhoQ regulon, or at least part of it, is likely to be modulated in response to cell envelope stress. Finally, preliminary findings indicate that, in addition to MicA, other sRNAs might also be regulators of PhoPQ synthesis.
Results
Hfq and MicA affect the expression of ompT in the absence of OmrA and OmrB sRNAs
Expression of the outer membrane protease OmpT was previously shown to be regulated by two redundant Hfq-binding sRNAs, OmrA and OmrB, via a direct interaction between the conserved 5’ end of OmrA/B and the early coding region of the ompT mRNA (Fig. 1, Guillier and Gottesman, 2006; Guillier and Gottesman, 2008). However, in a strain lacking OmrA/B, the expression of an ompT-lacZ translational fusion is still 1.3-fold higher in an hfq mutant compared to a wild-type strain (Fig. 2A, Table S1). One possible explanation for this observation is that, in addition to and independently of OmrA/B, other sRNAs could control OmpT synthesis.
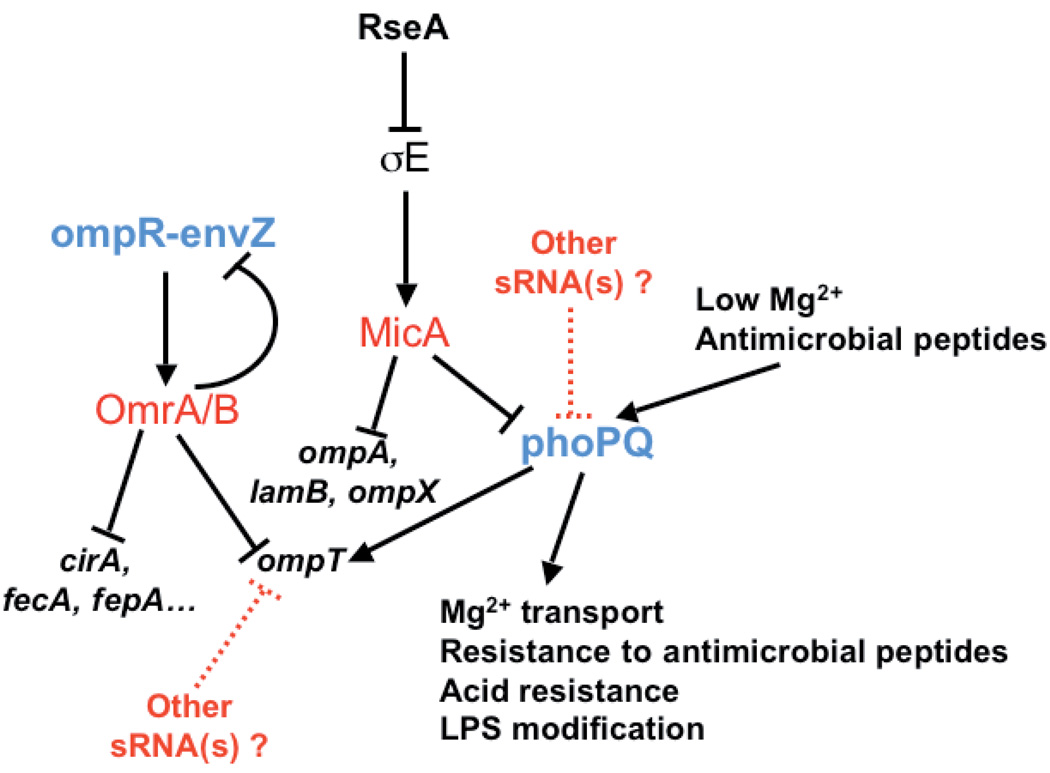
Fig. 1. Model of regulation of ompT and phoPQ expression.
sRNAs are shown in red and two component-systems are in blue. Positive and negative regulation events are indicated by arrows and horizontal bars respectively. Dashed lines correspond to putative regulation events. Note that overproduction of MicA should down-regulate the σE response by down-regulating the level of several OMPs.
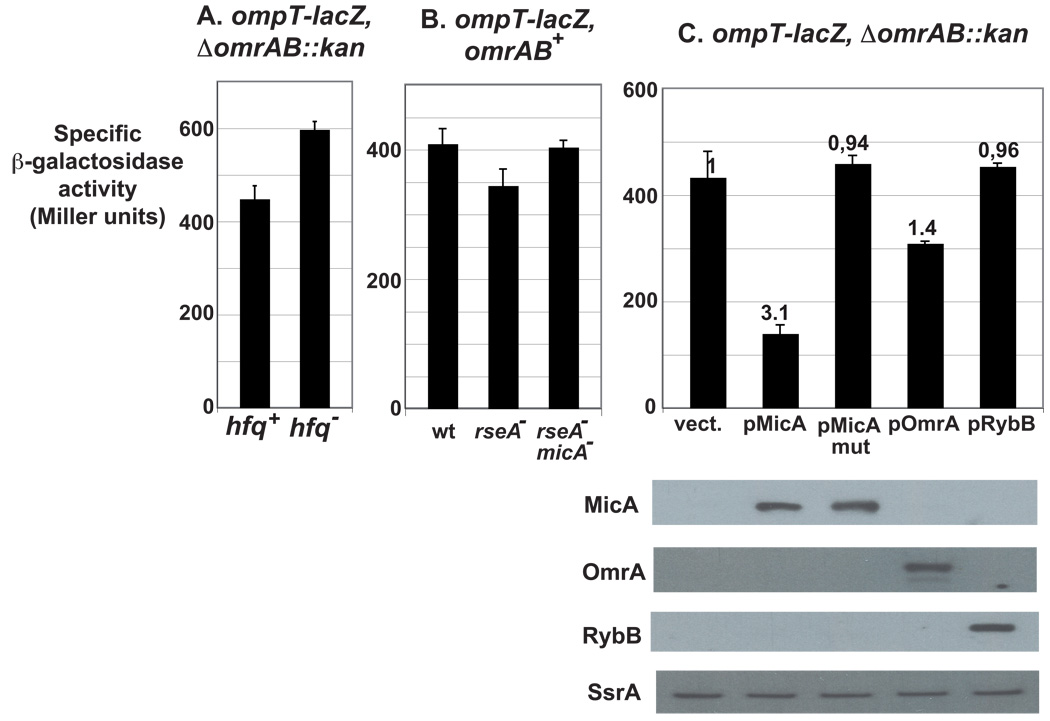
Fig. 2. Both Hfq and MicA affect ompT expression in the absence of OmrA and OmrB.
(A) The β-galactosidase activity of an ompT-lacZ translational fusion is increased in an hfq mutant (strain MG1194) compared to the hfq+ isogenic strain (strain MG1188) in the absence of OmrA and OmrB sRNAs. (B) Activity of the same fusion is slightly decreased in an rseA− strain, but not in an rseA− micA− double mutant. Strains used here are MG1173 (wt), MG1447 (rseA−) and MG1461 (rseA− micA−). (C) The activity of the fusion in a strain lacking omrA and omrB chromosomal copies was measured after transformation of the MG1188 strain with the pBRplac empty vector (vect.) or its derivatives overexpressing different sRNAs. Numbers on the top of the bars correspond to the repression factors. Levels of the different sRNAs in this experiment were analyzed by Northern-Blot with SsrA used as a loading control.
σE is an alternative sigma factor whose activity is induced under conditions of envelope stress leading to the accumulation of unfolded OMP in the periplasm (Ruiz and Silhavy, 2005). σE activates the transcription of numerous genes, including genes for periplasmic chaperones and proteases (Mutalik et al., 2009; Rhodius et al., 2006). It also activates transcription of two Hfq-binding sRNAs, RybB and MicA, that down-regulate the synthesis of major OMPs, such as OmpC and OmpA in E. coli, thereby reducing the accumulation of unassembled periplasmic OMPs (Johansen et al., 2006; Papenfort et al., 2006; Rasmussen et al., 2005; Thompson et al., 2007; Udekwu et al., 2005; Udekwu and Wagner, 2007). We reasoned that, since OmpT is a rather abundant OMP (Molloy et al., 2000), its expression could be repressed by σE-dependent sRNAs.
The activation of σE relies on a proteolytic cascade that leads to the degradation of RseA, an anti-sigma factor that sequesters σE at the inner membrane under non-inducing conditions (Fig.1, Ades, 2008). To test whether the expression of ompT was affected by σE, we compared the activity of the ompT-lacZ fusion in rseA+ and rseA− strains. There is a decrease of about 1.2 fold in the β-galactosidase activity in the rseA− strain (Fig. 2B and Table S1). Even though this effect is very modest, it was enough to convince us to look for σE-dependent sRNAs involved in ompT regulation.
To date, two such sRNAs have been identified in E. coli and related enterobacteria, MicA and RybB (see above). The effect of these two sRNAs on the ompT-lacZ fusion was therefore analyzed by measuring the β-galactosidase activity of strains transformed with a plasmid overexpressing either MicA or RybB. In these experiments, overexpression of MicA resulted in a 3-fold decrease in the β-galactosidase activity compared to the vector control, whereas overexpression of RybB had no noticeable effect on the expression of the fusion (Fig. 2C). Consistent with what was found in previous studies, overexpression of OmrA, a direct regulator of ompT, down-regulated the expression of the ompT-lacZ by only 1.4-fold. The experiments shown in Fig. 2C were carried out in a strain deleted for the chromosomal copies of OmrA and OmrB, and the effect of MicA is therefore independent of these two sRNAs. In addition, MicA is responsible for the decrease in ompT-lacZ activity observed in an rseA− strain (see above), as this effect is abolished in a micA− rseA− double mutant (Fig. 2B, Table S1).
Repression of ompT by MicA is dependent on the PhoP transcriptional regulator
MicA (previously SraD) is an Hfq-binding sRNA that was identified in several searches for bacterial sRNAs (Argaman et al., 2001; Zhang et al., 2003). It was shown to inhibit synthesis of two OMPs, OmpA and LamB, by pairing in the translation initiation region of their mRNAs (Bossi and Figueroa-Bossi, 2007; Rasmussen et al., 2005; Udekwu et al., 2005). However, no obvious complementarity was found between MicA and the ompT mRNA region present in our fusion, suggesting that control of ompT by MicA might be indirect. The translational ompT-lacZ fusion used in this study carries 220 nts of ompT upstream of the start codon and its transcription is driven by the ompT promoter. Therefore, this reporter fusion should be sensitive to regulation exerted either at the level of transcription and/or translation initiation.
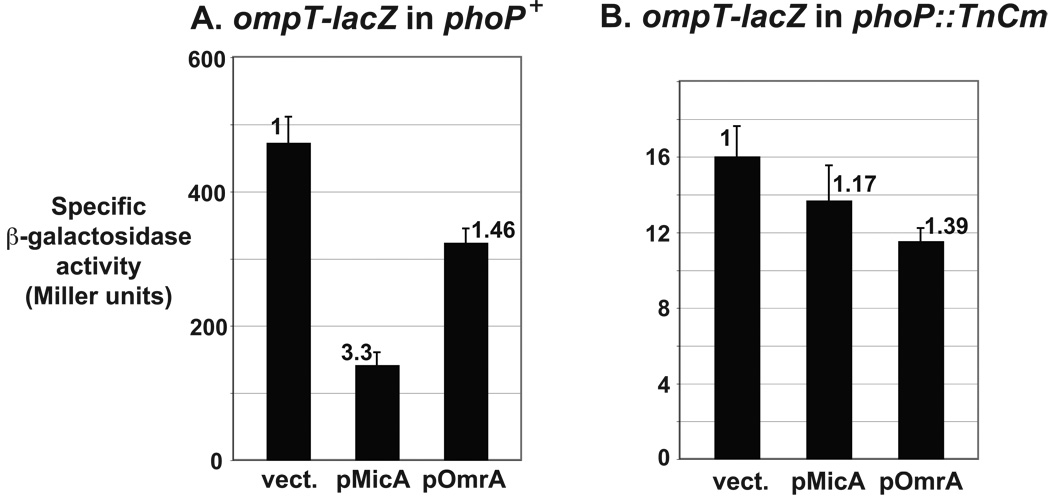
ompT transcription was shown to be activated by PhoP, the response regulator of the PhoP-PhoQ TCS, probably in a direct manner (Eguchi et al., 2004; Zwir et al., 2005; Fig. 1). Therefore, one possible explanation of our results is that MicA controls ompT through PhoPQ. To test this hypothesis, we measured the effect of MicA sRNA on the expression of the ompT-lacZ fusion in a strain where phoP was inactivated by a transposon insertion. As expected, the activity of the fusion was strongly decreased compared to the phoP+ background, but was still sufficient to observe a potential repression. The effect of MicA dropped from 3.3-fold in the wt strain to 1.2-fold in the phoP mutant, whereas repression by OmrA was similar in the wt and mutant strains (roughly 1.4-fold) (Fig. 3 and Table S1). These results clearly show that repression of ompT by MicA requires PhoP.
Fig. 3. Control of ompT expression by MicA requires PhoP.
The effect of ectopic overexpression of MicA and OmrA sRNAs on the ompT-lacZ fusion was determined in both phoP+ (A) and phoP− (B) backgrounds (strains MG1188 and MG1423 respectively).
MicA regulates the expression of phoPQ through direct base-pairing interaction
The next logical question was to ask whether MicA directly controlled PhoPQ synthesis. As is the case for most two component-systems, the genes for the response regulator and the sensor kinase are organized in a bicistronic operon, here phoP-phoQ, and interestingly, a base-pairing interaction can be predicted between MicA and the phoPQ mRNA in the phoP translation initiation region (Fig. 4A). Most of this pairing is conserved in several enterobacteria, including Salmonella, Shigella and Enterobacter. However, in some members of Enterobacteriaceae where both micA and phoP genes are present, complementarity between MicA and the phoP translation initiation region is poorer. This is the case in Yersinia pestis, Klebsiella pneumoniae and Photorhabdus luminescens (data not shown).
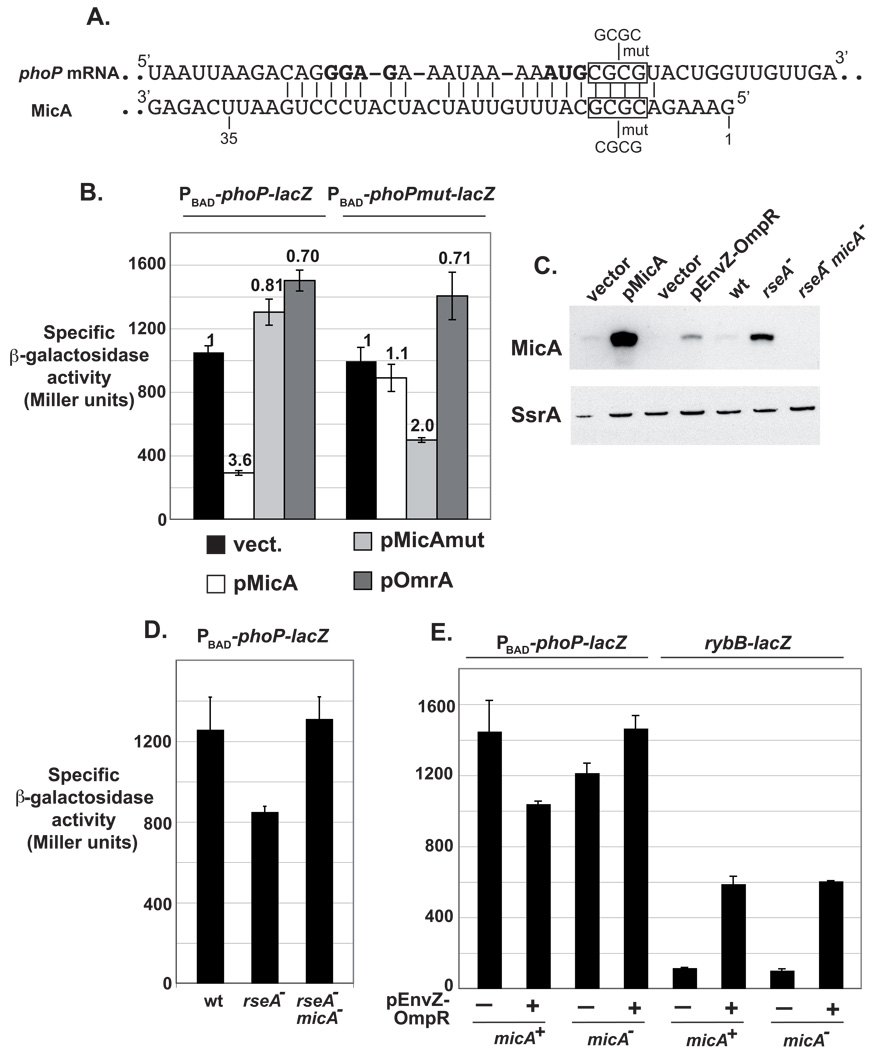
Fig. 4. MicA directly represses phoPQ expression through base-pairing interaction.
(A) Base-pairing prediction between MicA and phoPQ mRNA in Escherichia coli. phoP translation initiation codon and putative Shine-Dalgarno sequence are in bold. Numbering of MicA refers to transcription initiation. The nature of the compensatory changes introduced in the RNAs is indicated by “mut”. (B) Compensatory changes in MicA and phoP-lacZ mRNA restore the control of a PBAD-phoP-lacZ construct. Strains used in this experiment are MG1430 (PBAD-phoP-lacZ fusion) and MG1431 (PBAD-phoPmut-lacZ fusion) and are deleted for the chromosomal micA gene. (C) Comparison of MicA levels by Northern-Blot when overexpressed from a plasmid or induced from the chromosome using EnvZ-OmpR overproduction or an rseA− allele. Blot was also probed for SsrA as a loading control. RNA was extracted from strain MG1425 transformed with pBRplac or pMicA, MG1490 transformed with pHDB3 or pEnvZ-OmpR, or from strain MG1425, MG1459 or MG1460 as described in Experimental Procedures. (D, E) Activity of the PBAD-phoP-lacZ fusion is decreased upon induction of micA chromosomal copy in a MicA-dependent manner. Strains are MG1425 (wt), MG1459 (rseA−) and MG1460 (rseA− micA−) on panel D. On panel E, they are MG1490 and MG1491 (PBAD-phoP-lacZ, micA+ and micA− respectively), or MG1492 and MG1493 (rybB-lacZ, micA+ and micA− respectively), transformed with pHDB3 (−) or pEnvZ-OmpR (+).
In order to determine whether the predicted interaction in E. coli takes place in vivo, we constructed a translational phoP-lacZ reporter fusion. This fusion encompasses nts −36 (i.e. transcription start site of the phoPp1 promoter) to +30 of the phoP mRNA placed at the lacZ locus in frame with the 9th codon of lacZ. Transcription of this fusion is driven by a PBAD promoter; only control at the post-transcriptional level is therefore expected to affect its expression.
Overproduction of MicA down-regulated this fusion by 3.6-fold, whereas OmrA had no inhibitory effect, but rather increased expression of the fusion by 1.4-fold (Fig. 4B). A slight increase in expression was also observed upon overproduction of MicAmut, a mutant derivative of MicA where 4 nts involved in the predicted interaction with phoP mRNA were mutated (Fig. 4A). This mutant was shown to be present at a level similar to that of wt MicA in a previous experiment and, in agreement with MicA modulating ompT expression through PhoP, we found that MicAmut overproduction does not affect the activity of an ompT-lacZ fusion (Fig. 2C). A phoP-lacZ fusion carrying the compensatory changes was no longer controlled by wt MicA, whereas control was partially restored by MicAmut (repression of 1.1- and 2-fold respectively) (Fig. 4B and Table S1). As for the wt phoP-lacZ fusion, overproduction of OmrA up-regulated expression of the mutant fusion by 1.4-fold. The reason for the positive action of OmrA on these fusions, as well as of MicAmut on the wt fusion, is not clear and we suspect that these effects are most likely indirect, possibly through Hfq titration. Together, these results unambiguously show that MicA base-pairs with phoPQ mRNA around the phoP start codon. This leads to down-regulation of phoP expression. phoQ, in an operon with phoP, is also down-regulated, as measured with a phoPQ-lacZ fusion (data not shown).
MicA sRNA links PhoP synthesis to σE activity
In the previous experiment, MicA was expressed from a multicopy plasmid and its transcription was driven by an IPTG-inducible promoter. Because MicA is strongly overexpressed in these experiments (see lane pMicA of Fig. 4C), we decided to look at the effect of MicA induction on phoP expression, when MicA was transcribed from its own promoter under more physiological conditions. In a first experiment, σE was activated using an rseA− strain. Consistent with a control of phoPQ by MicA, this resulted in a 1.5-fold decrease in the expression of the PBAD-phoP-lacZ fusion. This effect was due to MicA, since activity was no longer decreased in an rseA− micA− double mutant (Fig. 4D and Table S1). We then used different envelope stresses to induce σE. Surprisingly, we found that several of them had no effect on phoP expression and so far, we do not have a clear explanation for this. However, other stresses, such as addition of procaine for instance, slightly decreased phoP expression in a reproducible manner (data not shown). Procaine most likely induces σE through EnvZ-OmpR activation, which is expected to result in an elevated synthesis of OMPs. In agreement with this, Thompson and Gottesman isolated a plasmid carrying envZ-ompR as a multicopy activator of the σE-dependent rybB-lacZ fusion in a genetic screen (Thompson et al., 2007). This plasmid is referred here as pEnvZ-OmpR.
In the presence of pEnvZ-OmpR, MicA was produced to a much lower level than in the rseA mutant (Fig. 4C, compare lanes pEnvZ-OmpR and rseA−). Nevertheless, this was sufficient to observe a slight decrease in the expression of phoP: 1.4-fold at the best (Fig. 4E and Table S1). Since this effect is modest, we repeated this experiment 7 times, and results were similar. Shown in Fig. 4E is a representative experiment where β-galactosidase activity was measured on two duplicate samples. As expected, this decrease is reproducibly dependent on MicA as it is not observed in the micA mutant strain. In contrast, pEnvZ-OmpR activates the expression of the σE-dependent rybB-lacZ fusion in a MicA-independent manner (induction of 5.4- and 5.8-fold respectively in micA− and micA− cells) (Fig. 4E and Table S1). Note that these experiments were performed in the absence of ompA, a major target of MicA. However, in several experiments, a similar effect of pEnvZ-OmpR on phoP was observed in ompA+ cells.
Regulation of different members of the PhoP regulon by MicA
Expression of the porin-encoding genes ompC and ompF is regulated by the EnvZ-OmpR TCS. Surprisingly however, their expression was shown to be rather constant over a large range of OmpR and EnvZ levels (Batchelor and Goulian, 2003). One can therefore wonder what the effect of regulating a TCS will be on the expression of its target-genes. To gain insight into this question, we looked at how MicA overproduction affects the expression of different genes whose transcription is controlled by PhoQ-PhoP, in addition to ompT. We focused on two targets of PhoPQ: MgrR, a sRNA regulator of LPS modification (Moon and Gottesman, 2009), and yneM, that encodes a small membrane protein (Hemm et al., 2008). Interestingly, MgrR and yneM are differentially regulated by PhoPQ in response to Mg2+ levels, with MgrR being expressed even at rather high Mg2+ (half-maximal expression occurs at [Mg2+]50% ~ 5 mM) whereas yneM is expressed only at fairly low Mg2+ ([Mg2+]50% ~ 0.005 mM), which is closer to what is observed for other PhoP targets (Moon and Gottesman, 2009, and Moon and Gottesman, personal communication).
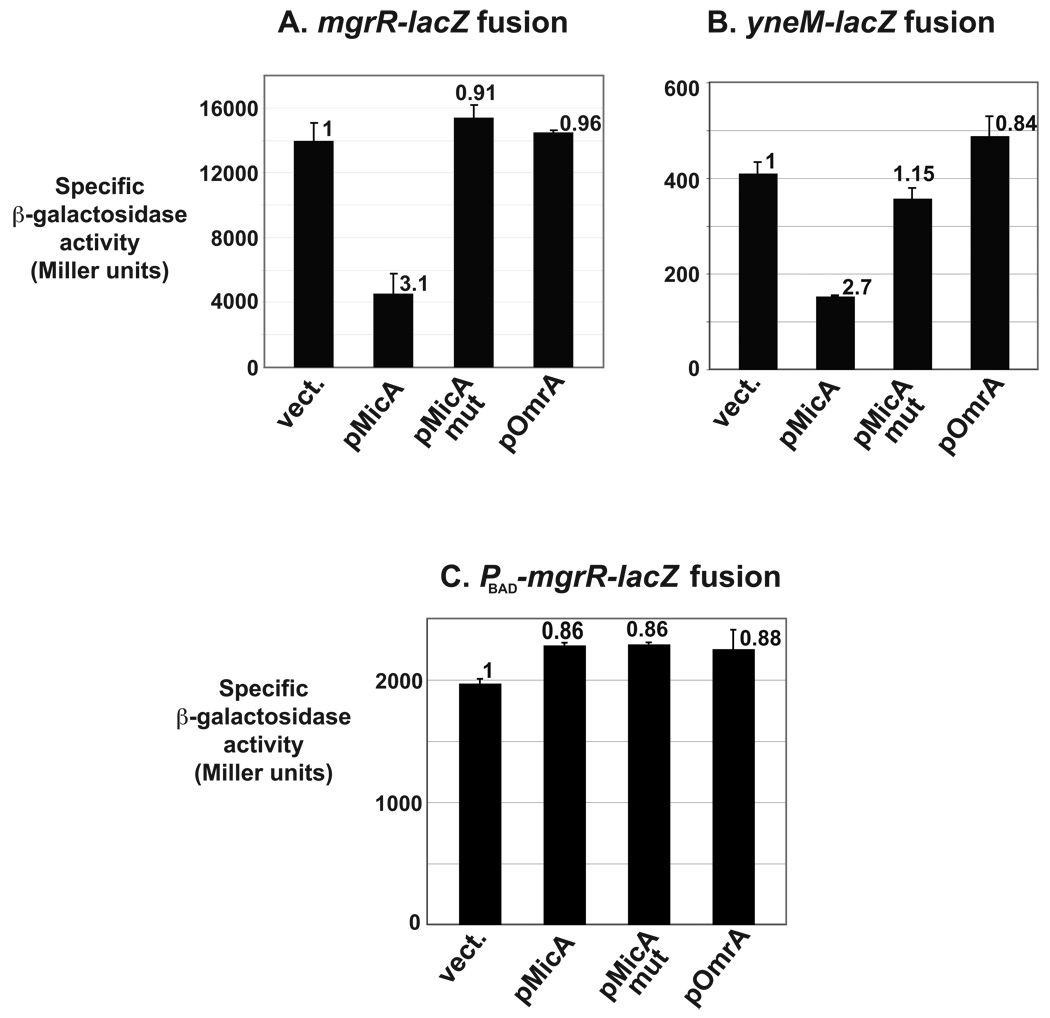
Despite their differential responses to Mg2+, repression of lacZ fusions to these two genes by MicA was similar (repression factors of 3.1 and 2.7 for mgrR-lacZ and yneM-lacZ respectively), whereas, as expected, activity of these fusions was either not affected or slightly increased by ectopic expression of either MicAmut or OmrA (Fig. 5A, 5B, and Table S1). It is interesting that MicA also repressed the activity of ompT-lacZ and phoP-lacZ fusions to a similar extent (repression of 3.1 and 3.6-fold respectively, see above). Therefore, as for ompT, MicA represses mgrR and yneM expression, presumably through phoPQ. In agreement with this, we found that MicA no longer controls the expression of an mgrR-lacZ fusion expressed from the PhoP-independent promoter PBAD (Fig. 5C and Table S1). It will be interesting to determine whether MicA also controls other targets of this TCS that can be differentially regulated as a function of PhoPQ stimulation (Miyashiro and Goulian, 2007).
Fig. 5. Regulation of different members of the PhoP regulon by MicA.
The β-galactosidase activity of two PhoP-regulated fusions (Moon and Gottesman, 2009) was measured in presence of plasmids overexpressing MicA, MicAmut or OmrA sRNAs. Strains used in this experiment are KM112 (panel A) and KM194 (panel B). (C) A similar experiment was performed with an mgrR-lacZ fusion under the control of a PhoP-independent promoter, PBAD (strain MG1484).
Additional sRNAs regulating ompT and/or phoP expression ?
Starting from the observation that ompT expression increases in an hfq mutant strain in the absence of OmrA/B, we showed in this study that MicA, an Hfq-dependent sRNA, directly represses the expression of phoPQ, a TCS regulator of ompT transcription. However, this does not necessarily mean that the effect of Hfq on ompT is due to MicA. To determine this, we compared the activities of ompT-lacZ in hfq+ and hfq− strains in the presence and absence of MicA and/or the OmrA/B sRNAs. In all cases, expression of the fusion was increased in the hfq mutant (between 1.2 and 1.4-fold compared to the hfq+ strain, Fig. 6A and Table S1), clearly showing that, as OmrA/B, MicA does not account for the full effect of Hfq on ompT-lacZ. Even though Hfq can affect gene expression independently of sRNAs (Folichon et al., 2003; Hajnsdorf and Regnier, 2000), a possible explanation for this observation is that additional sRNAs act on ompT-lacZ. If this is true, they could either affect transcription of the fusion, by regulating phoPQ for instance, or act at the post-transcriptional level.
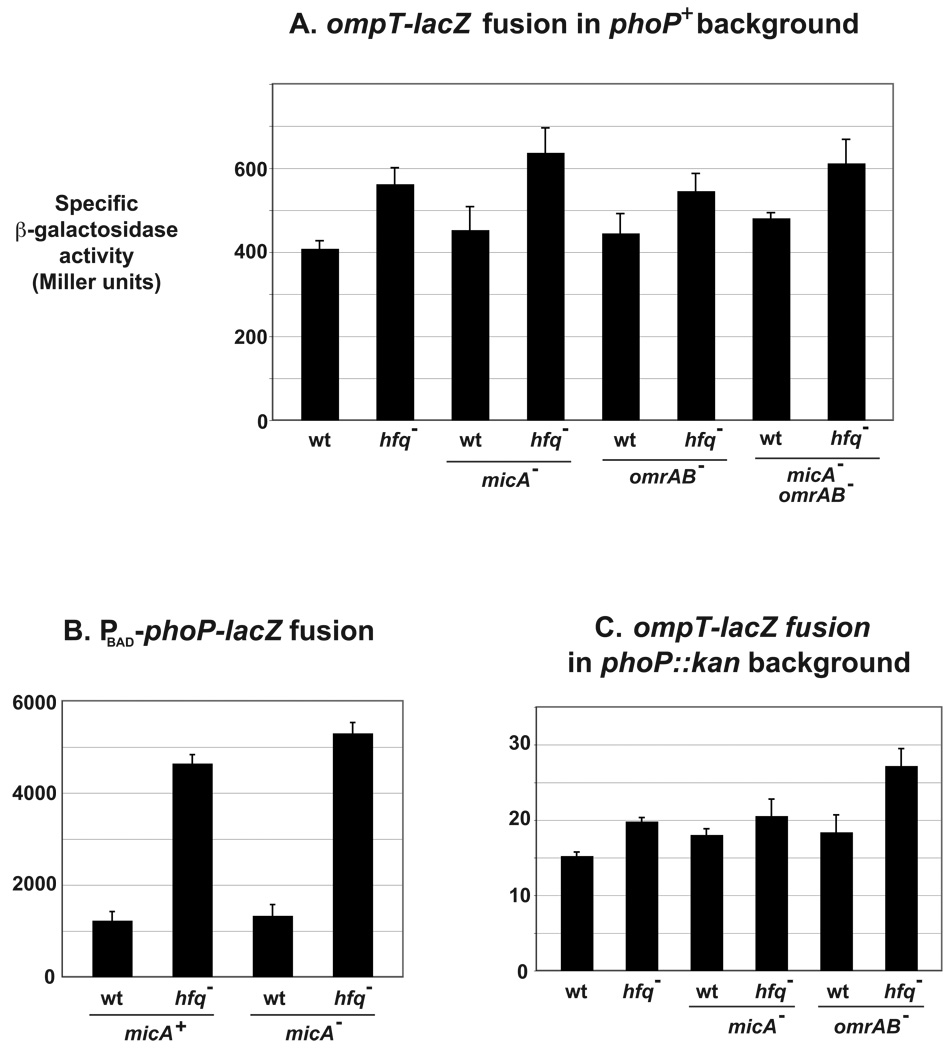
Fig. 6. Additional sRNAs regulating ompT-lacZ and phoP-lacZ expression ?
(A) β-galactosidase activity of ompT-lacZ was compared in hfq+ and hfq− isogenic strains, in the presence or absence of MicA and/or OmrA/B. Strains are MG1173 (wt), MG1196 (hfq−), MG1451(micA−), MG1455 (micA− hfq−), MG1188 (omrAB−), MG1194 (omrAB− hfq−), MG1449 (micA− omrAB−) and MG1450 (micA− omrAB− hfq−). The same experiment was done using the PBAD-phoP-lacZ fusion in micA+ and micA− strains (B), or the ompT-lacZ fusion in a phoP::kan context in the absence of MicA or OmrA/B (C). Strains used in panel B are MG1425, MG1453, MG1452 and MG1458 (wt, hfq−, micA− and hfq− micA− respectively); and in panel C MG1446, MG1454, MG1456, MG1457, MG1465 and MG1466 (wt, hfq−, micA−, hfq− micA−, omrAB− and hfq− omrAB− respectively).
If the effect of Hfq on ompT-lacZ is through sRNA regulation of phoPQ, then the expression of the PBAD-phoP-lacZ fusion should also be increased in an hfq mutant. To verify this, we measured the activity of this fusion in hfq+ and hfq− strains: hfq inactivation resulted in a 3.7-fold increase in β-galactosidase activity (Fig. 6B, Table S1). Somewhat surprisingly, this effect was completely independent of MicA, since a similar increase (3.85 fold) was observed in a micA− strain. Again, it is conceivable that yet another Hfq-dependent sRNA regulator of phoPQ is responsible for this effect (Fig. 1), but it is not the only potential explanation.
This large MicA-independent effect of hfq on phoPQ translation would be sufficient to explain the effects of an hfq mutant on ompT-lacZ. However, to directly test this, the activity of ompT-lacZ in both hfq+ and hfq− cells was determined in the absence of a functional PhoP regulator. Despite a lower level of expression, the results were similar to those obtained in a phoP+ strain : the activity was increased by 1.3-fold in the hfq mutant. This is independent of the sRNAs known to directly regulate ompT at the post-transcriptional level, OmrA/B, since the increase was almost 1.5-fold in an omrAB− background (Fig. 6C and Table S1). Therefore, ompT expression is regulated by Hfq in a way that is, at least partially, independent of PhoP. This could be due to one or more unknown sRNAs acting directly on ompT (Fig. 1).
Discussion
We show here that PhoPQ synthesis is negatively regulated at the post-transcriptional level by MicA, a σE-dependent sRNA. Consistent with this observation, MicA overproduction represses expression of several PhoP-regulated genes, such as ompT, mgrR and yneM. Regulation of ompT by MicA is abolished in a strain where PhoP is inactivated (Fig. 3). Similarly, the involvement of PhoPQ in the regulation of mgrR by MicA is strongly suggested by our result showing that MicA does not affect expression of an mgrR-lacZ fusion under the control of the araBAD promoter (Fig.5). This is presumably true for yneM as well.
Compensatory changes clearly showed that MicA pairs with phoPQ mRNA around the phoP start codon (Fig. 4B). Therefore, MicA most likely represses expression of phoPQ operon by competition with ribosome binding, as was shown to be the case forseveral negatively acting sRNAs (Chen et al., 2004; Udekwu et al., 2005 for instance). This is likely to be accompanied by degradation of the mRNA, since a decrease in phoP mRNA level was observed by transcriptome analysis after MicA induction (Gogol and Gross, manuscript in preparation). Again, this is true for many sRNAs acting by pairing (Aiba, 2007).
For Hfq-dependent sRNAs that directly regulate multiple targets, it is also common to find that the same region of the sRNA is involved in the pairing with different mRNAs (Guillier and Gottesman, 2008; Sharma et al., 2007). This seems to be the case for MicA since nts that are paired with phoPQ mRNA are also predicted to pair with ompA and lamB messages (Bossi and Figueroa-Bossi, 2007; Rasmussen et al., 2005; Udekwu et al., 2005). However, it is worth noting that, whereas MicAmut no longer controls phoPQ expression (Fig. 4B), it still causes a decrease in ompA mRNA levels when overproduced (data not shown). In this case, the stretch of 12 consecutive bp between nts 13 to 24 of MicA and nts −11 to −22 of ompA mRNA, some of which were shown to be involved in the repression in vivo by compensatory changes (Udekwu et al., 2005), is presumably sufficient for pairing. The effect of MicAmut on lamB expression has not been tested so far.
In addition to ompA and lamB mRNAs, MicA was also shown to affect the level of the message for another OMP, ompX, which is the direct target of yet another sRNA, CyaR (De Lay and Gottesman, 2009; Johansen et al., 2008; Papenfort et al., 2008). We therefore wondered whether CyaR could also be a regulator of phoP expression. This does not seem to be the case however, since despite a complementarity of 7 bp between CyaR and the TIR region of phoP, we found that overproduction of CyaR did not affect the activity of our phoP-lacZ fusion (our unpublished results).
These results uncover a novel link between sigma factors and TCS, two major classes of transcriptional regulators. To our knowledge, this is the first example of such a connection, which highlights the complexity of regulation of TCS synthesis. Multiple input signals are therefore integrated to modulate TCS expression and/or activity: signals that are directly sensed by the sensor kinase, leading to phosphorylation of the regulator, but also signals that modulate the synthesis of the TCS through production of a regulator, such as a sRNA for instance. It is not clear how this latter control affects the level of the active, ie phosphorylated, form of the regulator. Nonetheless, modulating the levels of TCS regulators, such as PhoP and OmpR, without affecting the inducing signals, clearly leads to regulation of target-genes (this study and Guillier and Gottesman, 2006 for instance). The connection between regulation of expression and phosphorylation remains to be investigated in detail.
More specifically, our results indicate that some conditions of cell envelope stress known to induce the σE response will lead to repression of the PhoPQ regulon, which includes genes involved in magnesium transport, resistance to antimicrobial peptides, acid resistance and LPS modification. From a physiological standpoint, it is not surprising that resistance to antimicrobial peptides, acid resistance and LPS modification could be affected by cell envelope stress. Other members of the PhoPQ regulon are involved in magnesium transport and their expression could therefore be regulated by MicA as well. The physiological interest of such a link, if it exists, between envelope stress and magnesium transport remains unclear.
Interestingly, Moon and Gottesman recently identified eptB as a major target of the PhoP-regulated sRNA MgrR. eptB is involved in LPS modification and is regulated by σE, most likely in an indirect manner (Moon and Gottesman, 2009). While results from Moon and Gottesman demonstrate that there clearly is an effect of σE on the transcription initiation of eptB (K. Moon and S. Gottesman, unpublished results), it is possible that down-regulation of MgrR by MicA (via PhoP) also contributes to the observed σE up-regulation of eptB. Irrespective of the mechanism of this regulation, this provides another example of intertwined regulation of the σE and PhoPQ regulons.
It is also worth noting that the extent of these controls is limited, as is most often the case for regulation exerted by sRNAs. For instance, whereas the activity of the ompT-lacZ fusion used in this study was decreased by almost 30-fold when PhoP was inactivated (Fig. 3), down-regulation of phoPQ by MicA resulted only in a 3.1-fold or 1.2-fold decrease in ompT-lacZ when MicA was overproduced from a plasmid or induced from the chromosome using an rseA− mutant allele respectively (Fig. 2). Therefore, MicA likely participates in fine-tuning the regulation of phoPQ under conditions where the σE regulon is induced, such as envelope stress and to a lesser extent stationary phase. The limited extent of this regulation is highlighted by the fact that induction of MicA from the chromosome has only a modest effect on phoP expression (Fig. 4E). It is possible however that other envelope stresses will have a stronger effect on MicA accumulation and phoP control.
Our observation of a strong effect of an hfq mutant on the phoP-lacZ fusion, even in the absence of MicA, raises the exciting possibility that sRNAs other than MicA will regulate phoPQ. Two-component systems are widely used in bacteria as a way to sense and adapt to the environment. More than 30 of them have been described in the E. coli genome and it is tempting to speculate that, in addition to PhoPQ, DpiBA and OmpR-EnvZ, many more will be subject to sRNA-mediated regulation. It will also be interesting to elucidate how these controls affect the expression of the downstream genes and the overall physiology of the cell.
Experimental procedures
Bacterial strains
With the exception of DH5α that was used as the recipient strain for cloning procedures, all strains used in this study are derivatives of MG1655. They were grown aerobically in LB medium. When needed, antibiotics were added at the following concentrations : kanamycin 25 µg/ml, chloramphenicol 10 µg/ml (for transduction of the hfq::cm allele) or 25 µg/ml, tetracycline 10 µg/ml, ampicillin 150 µg/ml.
Construction of ΔmicA::cm, ΔmicA::tet, ΔomrAB::tet and ΔompA::kan was as follows : antibiotic resistance cassettes were amplified by PCR using forward (for) and reverse (rev) oligonucleotides (sequence in Table S2) and the Phusion polymerase (Finnzymes) following manufacturer’s instructions. These PCR products were then recombined in strain NM300 for ΔmicA::cm and ΔompA::kan or NM1200 for ΔmicA::tet and ΔomrAB::tet, that carry a mini-lambda (Court et al., 2003), as described previously (Yu et al., 2000).
These alleles, as well as hfq::cm (Tsui et al., 1997), ΔomrAB::kan (Guillier and Gottesman, 2006), phoP::TnCm, phoP::kan (Bougdour et al., 2008) and ΔrseA::kan (Thompson et al., 2007) were then moved by P1vir transduction as requested. Note that in ΔmicA::cm and ΔmicA::tet constructs, the promoter region for the adjacent and divergently transcribed luxS gene is removed; therefore, luxS should not be expressed in these micA mutants.
Construction of the ompT-lacZ translational fusion was described previously (Guillier and Gottesman, 2008). For the PBAD-phoP-lacZ fusion, a PCR product carrying nts −36 to +30 of phoP relative to the start codon was amplified from genomic DNA from strain MG1655 using the Phusion polymerase and primers phoP-lac for and phoP-lac rev, that carry homology regions to PBAD promoter and lacZ ORF respectively (Table S2). It was then recombined in the strain PM1205 (Mandin and Gottesman, 2009) to replace the cat-sacB cassette of the PBAD-cat-sacB-lacZ construct as described (Yu et al., 2000). Recombinants were selected on LB plates without NaCl supplemented with 6% sucrose. Chloramphenicol sensitive colonies were purified three times on the same medium and the PBAD-phoP-lacZ construct was confirmed by sequencing. For the construction of the PBAD-phoPmut-lacZ fusion, the PCR product was obtained as follows. First, a PCR fragment was amplified from genomic DNA of strain MG1655 using primers phoP-lac for and phoPmut (Table S2) and the Phusion polymerase. This reaction was then treated with DpnI enzyme, purified and the resulting PCR product was used as a template in a second PCR reaction, with primers phoP-lacZ for and phoP-lacZ rev and Phusion DNA polymerase. Recombination in strain PM1205 and selection of recombinants was then carried out as above.
To construct the PBAD-mgrR-lacZ fusion (strain MG1484), a PCR product synthesized with the partially complementary primers 5’PBAD-mgrR and 3’mgrR-lacZ was recombined in strain PM1205 as previously. Recombinants were selected on M plates containing 6% sucrose, 0.2% arabinose and 20µg/ml Xgal. Verification of recombinants was as above.
Plasmids
pBRplac and pOmrA (also referred to as pBRplacOmrA) have been previously described (Guillier and Gottesman, 2006).
pMicA and pRybB : MicA and RybB were PCR-amplified from genomic DNA of strain MG1655 using primers AatII-MicA and Hind-MicA or AatII-RybB and Eco-RybB respectively, and the Expand High-Fidelity System (Roche). PCR products were then digested with EcoRI and AatII (RybB) or AatII and HindIII (MicA) and ligated in the pBRplac vector cut with the same enzymes. Plasmids were checked by sequencing.
pMicAmut : mutation was introduced in pMicA using the Quickchange Site-directed mutagenesis kit (Stratagene) with MicAmut for and MicAmut rev primers. After sequencing, the AatII-HindIII fragment was cloned in pBRplac to avoid any unwanted mutations.
The plasmid pEnvZ-OmpR (a.k.a pK4-55) was isolated from a genomic library constructed in pHDB3 (Ulbrandt et al., 1997) as an activator of a rybB-lacZ fusion (Thompson et al., 2007).
β-galactosidase assays
Cells were grown overnight in LB medium – supplemented with ampicillin, arabinose or IPTG when necessary, see below - and diluted 500-fold in fresh medium. When the OD600 reached 0.4, the β-galactosidase activity was assayed as described in (Miller, 1992) using 0.5 ml of culture, or 0.2 ml for experiment shown in Fig. 5A.
Media used in the different experiments are LB (Fig. 2A, 2B, 6A and 6C), LB + ampicillin (Fig. 4E, rybB-lacZ fusion), LB + 0.02% arabinose (Fig. 4D and 6B), LB + ampicillin + IPTG 100 µM (Fig. 2C, 3, 5A and 5B), LB + ampicillin + IPTG 100 µM + 0.02% arabinose (Fig. 4B), LB + ampicillin + 0.02% arabinose (Fig. 4E, PBAD-phoP-lacZ fusion) or LB + ampicillin + IPTG 100 µM + 0.004% arabinose (Fig. 5C).
Results are the average value of at least two (Fig. 2, 5A, 5B, 6B and 6C) or three (Fig. 3, 4B, 4D and 6A) independent experiments, except for Fig. 4E and 5C where a single representative experiment carried out on two duplicate samples is represented.
RNA extraction and Northern-Blot analysis
RNA was extracted from cells diluted 500-fold from an overnight culture and grown to exponential phase (OD600 ~ 0.4) in LB medium, supplemented if necessary. For Fig. 2C, RNA was extracted at the same time as samples taken to assess the β-galactosidase activity. Extraction was done as previously described using 650 µl of culture (Guillier and Gottesman, 2006). A constant amount of total RNA (3 µg for Fig. 2C and 2.5 µg for Fig. 4C) was then separated on an 8% acrylamide TBE-urea gel, and transferred in TAE 1X to an Hybond-N+ membrane (Amersham). RNA was finally detected using specific biotinylated probes (sequence in Table S2) and the Brighstar Biodetect kit (Ambion). After boiling in SDS 0.5% for 15 minutes, the same membrane could be hybridized with a different probe.
Supplementary Material
Table 1.
Strains and plasmids used in this study
| Strain | Description | Construction or source |
|---|---|---|
| MG1655 | Wild-type strain | F. Blattner |
| DJ480 | MG1655 ΔlacX174 | D. Jin |
| DJ624 | DJ480 mal::lacIq | D. Jin |
| NM300 | DJ480 mini-λ tetR | N. Majdalani |
| NM1200 | MG1655 mini-λ cmR | N. Majdalani |
| PM1205 | MG1655 mal::lacIq ΔaraBAD araC+lacI’ :: PBAD-cat- sacB-lacZ, mini-λ tetR |
Mandin and Gottesman, 2009 |
| MG1173 | DJ624 λRSompT-lacZ | This study |
| MG1188 | DJ624 λRSompT-lacZΔomrAB::kan | Guillier and Gottesman, 2008 |
| MG1194 | MG1188 hfq::cm | This study |
| MG1196 | MG1173 hfq::cm | This study |
| MG1423 | MG1188 phoP::TnCm | This study |
| MG1425 | MG1655 mal::lacIq ΔaraBAD araC+ PBAD-phoP-lacZ | This study |
| MG1430 | MG1655 mal::lacIq ΔaraBAD araC+ PBAD-phoP-lacZ ΔmicA::cm |
This study |
| MG1431 | MG1655 mal::lacIq ΔaraBAD araC+PBAD-phoPmut-lacZ ΔmicA::cm |
This study |
| MG1446 | MG1173 phoP::kan | This study |
| MG1447 | MG1173 ΔrseA::kan | This study |
| MG1449 | MG1188 ΔmicA::tet | This study |
| MG1450 | MG1194 ΔmicA::tet | This study |
| MG1451 | MG1173 ΔmicA::tet | This study |
| MG1452 | MG1425 ΔmicA::tet | This study |
| MG1453 | MG1425 hfq::cm | This study |
| MG1454 | MG1446 hfq::cm | This study |
| MG1455 | MG1196 ΔmicA::tet | This study |
| MG1456 | MG1446 ΔmicA::tet | This study |
| MG1457 | MG1454 ΔmicA::tet | This study |
| MG1458 | MG1453 ΔmicA::tet | This study |
| MG1459 | MG1425 ΔrseA::kan | This study |
| MG1460 | MG1459 ΔmicA::tet | This study |
| MG1461 | MG1447 ΔmicA::tet | This study |
| MG1465 | MG1446 ΔomrAB::tet | This study |
| MG1466 | MG1454 ΔomrAB::tet | This study |
| MG1484 | MG1655 mal::lacIq ΔaraBAD araC+ PBAD-mgrR-lacZ | This study |
| MG1490 | MG1425 ΔompA::kan | This study |
| MG1491 | MG1452 ΔompA::kan | This study |
| MG1492 | KMT12000 ΔompA::kan | This study |
| MG1493 | KMT12000 ΔompA::kan ΔmicA::tet | This study |
| KMT12000 | DJ480 rybB-lacZ | Thompson and Gottesman, 2007 |
| KM112 | MG1655 mal::lacIq ΔaraBAD araC+ mgrR-lacZ | Moon and Gottesman, 2009 |
| KM194 | MG1655 mal::lacIq ΔaraBAD araC+ yneM-lacZ | Moon and Gottesman, 2009 |
| Plasmid | Description | Construction or source |
| pBRplac | Modified PLlac0-1 promoter in pBR322, AmpR, TetR | Guillier and Gottesman, 2006 |
| pMicA | micA gene under control of modified PLlacO-1, AmpR | This study |
| pMicAmut | micAmut gene under control of modified PLlacO-1, AmpR | This study |
| pOmrA | omrA gene under control of modified PLlacO-1, AmpR, TetR | Guillier and Gottesman, 2006 |
| pRybB | rybB gene under control of modified PLlacO-1, AmpR, TetR | This study |
| pHDB3 | Empty vector for the genomic library, derivative of pBR322, AmpR |
Ulbrandt et al., 1997 |
| pEnvZ- OmpR |
pHDB3 carrying the fragment ‘pckA-envZ-ompR-greB- yhgF’, AmpR (Plasmid pK4-55 in Thompson and Gottesman, 2007) |
Thompson and Gottesman, 2007 |
Acknowledgements
We thank E. Gogol and C. Gross for sharing unpublished results. We are grateful to K. Moon for providing strains and communicating unpublished results, and to A. Bougdour, K. Thompson and N. Majdalani for sending strains and plasmids. We also thank C. Condon for insightful comments on the manuscript.
This work was supported by the CNRS (UPR 9073) and the University of Paris 7- Denis Diderot and, in part, by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Bibliographic references
- Ades SE. Regulation by destruction: design of the sigmaE envelope stress response. Curr. Opin. Microbiol. 2008;11:535–540. doi: 10.1016/j.mib.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Aiba H. Mechanism of RNA silencing by Hfq-binding small RNAs. Curr. Opin. Microbiol. 2007;10:134–139. doi: 10.1016/j.mib.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, Altuvia S. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr. Biol. 2001;11:941–950. doi: 10.1016/s0960-9822(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Batchelor E, Goulian M. Robustness and the cycle of phosphorylation and dephosphorylation in a two-component regulatory system. Proc. Natl. Acad. Sci. USA. 2003;100:691–696. doi: 10.1073/pnas.0234782100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi L, Figueroa-Bossi N. A small RNA downregulates LamB maltoporin in Salmonella. Mol. Microbiol. 2007;65:799–810. doi: 10.1111/j.1365-2958.2007.05829.x. [DOI] [PubMed] [Google Scholar]
- Bougdour A, Cunning C, Baptiste PJ, Elliott T, Gottesman S. Multiple pathways for regulation of sigmaS (RpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol. Microbiol. 2008;68:298–313. doi: 10.1111/j.1365-2958.2008.06146.x. [DOI] [PubMed] [Google Scholar]
- Brennan RG, Link TM. Hfq structure, function and ligand binding. Curr. Opin. Microbiol. 2007;10:125–133. doi: 10.1016/j.mib.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhang A, Blyn LB, Storz G. MicC, a second small-RNA regulator of Omp protein expression in Escherichia coli. J. Bacteriol. 2004;186:6689–6697. doi: 10.1128/JB.186.20.6689-6697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court DL, Swaminathan S, Yu D, Wilson H, Baker T, Bubunenko M, Sawitzke J, Sharan SK. Mini-lambda: a tractable system for chromosome and BAC engineering. Gene. 2003;315:63–69. doi: 10.1016/s0378-1119(03)00728-5. [DOI] [PubMed] [Google Scholar]
- De Lay N, Gottesman S. The Crp-activated small noncoding regulatory RNA CyaR (RyeE) links nutritional status to group behavior. J. Bacteriol. 2009;191:461–476. doi: 10.1128/JB.01157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y, Okada T, Minagawa S, Oshima T, Mori H, Yamamoto K, Ishihama A, Utsumi R. Signal transduction cascade between EvgA/EvgS and PhoP/PhoQ two-component systems of Escherichia coli. J. Bacteriol. 2004;186:3006–3014. doi: 10.1128/JB.186.10.3006-3014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folichon M, Arluison V, Pellegrini O, Huntzinger E, Regnier P, Hajnsdorf E. The poly(A) binding protein Hfq protects RNA from RNase E and exoribonucleolytic degradation. Nucleic Acids Res. 2003;31:7302–7310. doi: 10.1093/nar/gkg915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillier M, Gottesman S. Remodelling of the Escherichia coli outer membrane by two small regulatory RNAs. Mol. Microbiol. 2006;59:231–247. doi: 10.1111/j.1365-2958.2005.04929.x. [DOI] [PubMed] [Google Scholar]
- Guillier M, Gottesman S. The 5' end of two redundant sRNAs is involved in the regulation of multiple targets, including their own regulator. Nucleic Acids Res. 2008;36:6781–6794. doi: 10.1093/nar/gkn742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnsdorf E, Regnier P. Host factor Hfq of Escherichia coli stimulates elongation of poly(A) tails by poly(A) polymerase I. Proc. Natl. Acad. Sci. USA. 2000;97:1501–1505. doi: 10.1073/pnas.040549897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemm MR, Paul BJ, Schneider TD, Storz G, Rudd KE. Small membrane proteins found by comparative genomics and ribosome binding site models. Mol Microbiol. 2008;70:1487–1501. doi: 10.1111/j.1365-2958.2008.06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Rasmussen AA, Overgaard M, Valentin-Hansen P. Conserved Small Non-coding RNAs that belong to the sigma(E) Regulon: Role in Down-regulation of Outer Membrane Proteins. J. Mol. Biol. 2006;364:1–8. doi: 10.1016/j.jmb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Johansen J, Eriksen M, Kallipolitis B, Valentin-Hansen P. Down-regulation of outer membrane proteins by noncoding RNAs: unraveling the cAMP-CRP- and sigmaE-dependent CyaR-ompX regulatory case. J. Mol. Biol. 2008;383:1–9. doi: 10.1016/j.jmb.2008.06.058. [DOI] [PubMed] [Google Scholar]
- Lease RA, Cusick M, Belfort M. Riboregulation in Escherichia coli: DsrA RNA acts by RNA:RNA interactions at multiple loci. Proc. Natl. Acad. Sci. USA. 1998;95:12456–12461. doi: 10.1073/pnas.95.21.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. USA. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N, Chen S, Murrow J, St. John K, Gottesman S. Regulation of RpoS by a novel small RNA: the characterization of RprA. Mol. Microbiol. 2001;39:1382–1394. doi: 10.1111/j.1365-2958.2001.02329.x. [DOI] [PubMed] [Google Scholar]
- Majdalani N, Hernandez D, Gottesman S. Regulation and mode of action of the second small RNA activator of RpoS translation. RprA. Mol. Microbiol. 2002;46:813–826. doi: 10.1046/j.1365-2958.2002.03203.x. [DOI] [PubMed] [Google Scholar]
- Mandin P, Gottesman S. A genetic approach for finding small RNAs regulators of genes of interest identifies RybC as regulating the DpiA/DpiB two-component system. Mol. Microbiol. 2009;72:551–565. doi: 10.1111/j.1365-2958.2009.06665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. A Short Course in Bacterial Genetics, A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- Miyashiro T, Goulian M. Stimulus-dependent differential regulation in the Escherichia coli PhoQ PhoP system. Proc. Natl. Acad. Sci. USA. 2007;104:16305–16310. doi: 10.1073/pnas.0700025104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy MP, Herbert BR, Slade MB, Rabilloud T, Nouwens AS, Williams KL. Proteomic analysis of the Escherichia coli outer membrane. Eur. J. Biochem. 2000;267:2871–2881. doi: 10.1046/j.1432-1327.2000.01296.x. [DOI] [PubMed] [Google Scholar]
- Moon K, Gottesman S. A PhoQ/P-regulated small RNA regulates sensitivity of Escherichia coli to antimicrobial peptides. Mol. Microbiol. 2009;74:1314–1330. doi: 10.1111/j.1365-2958.2009.06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutalik V, Nonaka G, Ades S, Rhodius VA, Gross CA. Promoter Strength Properties of the Complete Sigma E regulon of E. coli and Salmonella. J. Bacteriol. 2009 doi: 10.1128/JB.01047-09. doi:10.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfort K, Pfeiffer V, Mika F, Luchhini S, Hinton JCD, Vogel J. sE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol. Microbiol. 2006;62:1674–1688. doi: 10.1111/j.1365-2958.2006.05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfort K, Pfeiffer V, Lucchini S, Sonawane A, Hinton JC, Vogel J. Systematic deletion of Salmonella small RNA genes identifies CyaR, a conserved CRP-dependent riboregulator of OmpX synthesis. Mol. Microbiol. 2008;68:890–906. doi: 10.1111/j.1365-2958.2008.06189.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen AA, Eriksen M, Gilany K, Udesen C, Franch T, Petersen C, Valentin-Hansen P. Regulation of ompA mRNA stability: the role of a small regulatory RNA in growth phase-dependent control. Mol. Microbiol. 2005;58:1421–1429. doi: 10.1111/j.1365-2958.2005.04911.x. [DOI] [PubMed] [Google Scholar]
- Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol. 2006;4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N, Silhavy TJ. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 2005;8:122–126. doi: 10.1016/j.mib.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Sharma CM, Darfeuille F, Plantinga TH, Vogel J. A small RNA regulates multiple ABC transporter mRNAs by targeting C/A-rich elements inside and upstream of ribosome-binding sites. Genes Dev. 2007;21:2804–2817. doi: 10.1101/gad.447207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma CM, Vogel J. Experimental approaches for the discovery and characterization of regulatory small RNA. Curr. Opin. Microbiol. 2009;12:536–546. doi: 10.1016/j.mib.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Svenningsen SL, Tu KC, Bassler BL. Gene dosage compensation calibrates four regulatory RNAs to control Vibrio cholerae quorum sensing. EMBO J. 2009;28:429–439. doi: 10.1038/emboj.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, Rhodius VA, Gottesman S. sE regulates and is regulated by a small RNA in Escherichia coli. J. Bacteriol. 2007;189:4243–4256. doi: 10.1128/JB.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui H-CT, Feng G, Winkler M. Negative regulation of mutS and mutH repair gene expression by the Hfq and RpoS global regulators of Escherichia coli K-12. J. Bacteriol. 1997;179:7476–7487. doi: 10.1128/jb.179.23.7476-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udekwu KI, Darfeuille F, Vogel J, Reimegard J, Holmqvist E, Wagner EG. Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA. Genes & Dev. 2005;19:2355–2366. doi: 10.1101/gad.354405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udekwu KI, Wagner EG. Sigma E controls biogenesis of the antisense RNA MicA. Nucleic Acids Res. 2007;35:1279–1288. doi: 10.1093/nar/gkl1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrandt ND, Newitt JA, Bernstein HD. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell. 1997;88:187–196. doi: 10.1016/s0092-8674(00)81839-5. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P, Eriksen M, Udesen C. The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol. Microbiol. 2004;51:1525–1533. doi: 10.1111/j.1365-2958.2003.03935.x. [DOI] [PubMed] [Google Scholar]
- Waters LS, Storz G. Regulatory RNAs in bacteria. Cell. 2009;136:615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Wassarman KM, Rosenow C, Tjaden BC, Storz G, Gottesman S. Global analysis of small RNA and mRNA targets of Hfq. Mol. Microbiol. 2003;50:1111–1124. doi: 10.1046/j.1365-2958.2003.03734.x. [DOI] [PubMed] [Google Scholar]
- Zwir I, Shin D, Kato A, NIshino K, Latifi T, Solomon F, Hare JM, Huang H, Groisman EA. Dissecting the PhoP regulatory network of Escherichia coli and Salmonella enterica. Proc. Natl. Acad. Sci. USA. 2005;102:2862–2867. doi: 10.1073/pnas.0408238102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.