Abstract
The development of new ROMP-based oligomeric benzyl phosphates (OBPn) is reported for use as soluble, stable benzylating reagents. These oligomeric reagents are readily synthesized from commercially available materials and conveniently polymerized and purified in a one-pot process, affording bench stable, pure white, free-flowing solids on multi-gram scale. Utilization in benzylation reactions with a variety of nucleophiles is reported.
The development of new immmobilized reagents for the production of libraries for high-throughput screening is an important element in drug discovery. In this regard, new and improved immobilized reagents with tunable properties have emerged as critical components in facilitated synthetic protocols, particularly within the arena of combinatorial and green technologies.1,2 Consequently, this has driven the development of an array of polymer-bound supports, reagents and scavengers for streamlining synthetic methods into simple mix, filter and evaporate protocols.1,2 Despite many salient attributes of current immobilized platforms, limitations in load capacity, reaction kinetics, means of delivery and stability continue to warrant efforts in this area. 3 Among these, ring-opening metathesis (ROM) polymerization of functionalized norbornenes has surfaced as a powerful tool in the generation of high-load, immobilized reagents with tunable properties.4,5,6 In this regard, we report the development of new ROMP-based oligomeric benzyl phosphates (OBPn) for use as soluble, stable benzylating reagents.
The innate properties of phosphates as leaving groups have inspired the current study aimed at developing oligomeric phosphate-based reagents. While phosphates have been uniquely tailored to play vital roles in nature,7 only recently have they found widespread use in synthetic methodology and total synthesis. 8 This resurgence is primarily attributed to their stability, facile assembly and ideal monoanionic pKa profiles.9 Despite these attributes, synthetic oligomeric-based phosphates and other phosphorous-containing materials have primarily found applications in the production of flame-retardant materials10 with limited use in novel therapeutic applications.11 To the best of our knowledge, the literature is void of immobilized phosphate-based alkylating/benzylating agents.
The benzylation of amines and alcohols continues to serve as one of the most utilized protecting group strategies in organic synthesis due to its ease of incorporation and removal.12,13,7b In addition, benzylation has emerged as a key diversification reaction in medicinal/combinatorial chemistry approaches as well as diversity-oriented synthesis.14 This continued use has spurred development of a number of alternative approaches to benzylation. 15, 16 Among these, two ROMP-derived benzylating agents were recently developed in our laboratory.5c,16b Interest in further improvements17 of such protocols has lead to the study reported herein.
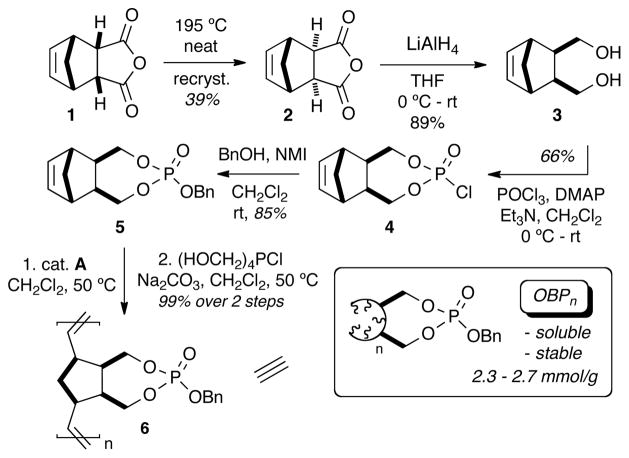
The synthesis of oligomeric benzyl phosphate 6 was envisioned to occur via reduction of endo norbornenyl anhydride 1 to the corresponding diol, followed by phosphorylation and subsequent condensation with benzyl alcohol. However, repeated attempts to polymerize the endo isomer of monomer 5 with a variety of metathesis catalysts resulted in incomplete conversions. Plausibly, both steric and electronic interactions of the P=O bond of the resulting endo isomer could interfere with catalyst/olefin activation or the subsequent propagation step.
Attention was next directed towards synthesis of the thermodynamic exo isomer (Scheme 1). Several thermal isomerization reactions of the inexpensive endo carbic anhydride 1 were performed on large scale using classical methods.18 Sequential recrystallizations in toluene yielded exo product 2 with diastereomeric ratios progressively increasing and yields decreasing with each recrystallization, i.e., dr = 15:1 and 39% yield after three recrystallizatons, dr = 29:1 and 34% yield after four, up to dr = 84:1 and 20% yield after six. Reduction of 2 with LiAlH4 yielded diol 3 as a clear, viscous oil with good yield. Phosphorylation of the exo diol 3 was performed using distilled POCl3 and Et3N in the presence of catalytic DMAP to yield phosphorochloridate 4 as a white solid in moderate yields.
Scheme 1.
Synthesis of the oligomeric benzyl phosphate (OBP)
This was conveniently stored as a solid over argon in a dessicator for use in preparing the various reagents for up to three months.
Addition of 4 into a solution containing benzyl alcohol, NMI, and CH2Cl2 at room temperature cleanly afforded the benzyl phosphate 5 in good yields and purity. Polymerization of 5 and other phosphate analogs of this type in the presence of (IMesH2)(PCy3)(Cl)2Ru=CHPh (cat. B)19 occurred rapidly at room temperature resulting in formation of insoluble and unusable gels. However, polymerization with RuCl2(PCy3)2=CHPh (cat. A), 20 cleanly afforded the oligomeric reagent with desirable characteristics.21 Following polymerization, the reaction was quenched with ethyl vinyl ether (EVE) and stirred for 30 minutes. A basic workup involving the Pederson protocol22 was applied in the same pot until cat. A was visibly removed as indicated by precipitate formation and lack of coloration. The resulting solution was washed several times with water, dried over MgSO4 and concentrated to critical viscosity. 23 Precipitation via dropwise addition into anhydrous Et2O afforded oligomeric benzyl phosphate (OBPn) 6 as a free-flowing white solid where n = relative lengths of 20, 50, and 100-mers – each displaying slightly different solubility profiles. 24
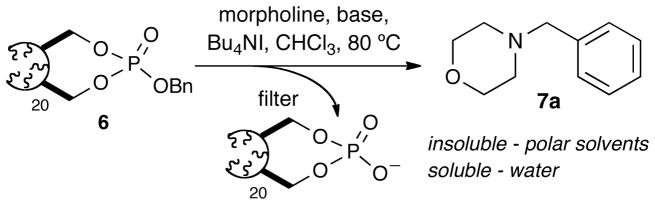
The oligomeric benzyl phosphate 20-mer (OBP20) was then investigated for benzylation of various amines (Scheme 2, Table 1). The reagent was delivered either as a free-flowing powder or as a stock solution in anhydrous CHCl3 alongside a catalytic amount of tetrabutylammonium iodide. 25 During the reaction, precipitation of the resulting oligomeric phosphate monoanion typically occured within a 0.5 – 2 hour period after addition of the nucleophile. The mother liquor was subsequently concentrated over silica or precipitated into Et2O, filtered via silica SPE and concentrated under vacuum to afford the corresponding the benzylated analog(s) in good to excellent yields and high purity. The resulting monoanionic oligomeric phosphate was found to be water soluble at elevated temperatures and remained soluble on cooling to room temperature. This observation would be of particular importance in potential large-scale applications for the removal of spent oligomer.
Scheme 2.
Benzylation of morpholine
Table 1.
Benzylation of N-, O-, and S-nucleophiles using OBP20
| nucleophile | pdt | yield (%)[a] | purity (%)[b] |
|---|---|---|---|
| morpholine | 7a | 99 | 98 |
| thiomorpholine | 7b | 93 | 98 |
| N-phenylpiperizine | 7c | 98 | 99 |
| piperizine | 7d | 95 | 97 |
| pyrrolidine | 7e | 80 | 99 |
| piperidine | 7f | 73 | 99 |
| dihydroindole | 7g | 98 | 85 |
| 7h | 69 | 97 | |
| phenol | 7i | 80 | 95 |
| lithium thiophenolate[e] | 7j | 98 | 96 |
| Bn-NH2 | 7k/l | 99[c] | 4:1[d] |
| Ph-NHEt | 7m | 81 | 89 |
Isolated yields after filtration through a silica SPE.
Purities calculated using GC and further confirmed by 1H NMR.
Percent conversion and ratio of mono to dibenzylated amine as found by GC/MS.
Reaction was performed w/OBP50.
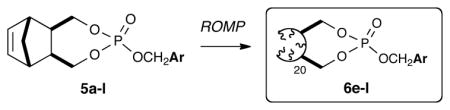
A number of cyclic and acyclic amines as well as O, and S nucleophiles were subjected to the established benzylation protocol and were found to proceeed smoothly to afford the desired benzylated products in excellent yields and purities (Table 1). A number of monomeric analogs of OBP were also prepared in good yields using several substituted benzyl alcohols. Subjection of the monomers to the established ROM polymerization protocol afforded the desired oligmeric products in excellent yields as free-flowing white solids. Interestingly, efforts towards production of monomeric phosphates 5a-d did not afford the desired products. This is likely due to a combination of the substituent mesomeric effect and/or eliminative degradation pathways of these phosphates (Table 2). The corresponding oligomers 6e-l were subjected to established benzylation conditions utilizing morpholine as a test substrate and conveniently afforded the desired benzylated products in moderate to good yields and purities (Table 3).
Table 2.
Synthesis of various OBP analogs
 | |||||
|---|---|---|---|---|---|
| monomer | Ar | yield (%) | monomer | Ar | yield (%) |
| 5a | 23% | 5e | o-CH3Ph | 75% | |
| 5f | 3,5-(OCH3)2Ph | 70% | |||
| 5b | 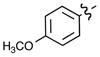 |
21% | 5g | p-BrPh | 79% |
| 5h | p-ClPh | 76% | |||
| 5c | <10% | 5i | p-FPh | 80% | |
| 5j | p-NO2Ph | 70% | |||
| 5d | 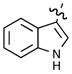 |
<10% | 5k | m-N(CH3)2Ph | 73% |
| 5l | p-CF3Ph | 77% | |||
Yields correspond to monomeric phosphates. Quantitative conversions were obtained for oligomers 6e-l. Monomers 5a-d were not polymerized.
Table 3.
Benzylation of amines using various OBP analogs
| entry | SM | product | yield(%) | purity(%)[a] |
|---|---|---|---|---|
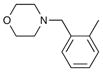
| 1 | 6e |
 8e 8e
|
64 | 94 |
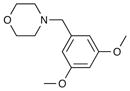
| 2 | 6f |
 8f 8f
|
54 | 89 |
| 3 | 6g |
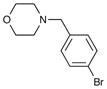 8g 8g
|
82 | 93 |
| 4 | 6h |
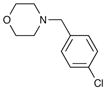 8h 8h
|
67 | 97 |
| 5 | 6i |
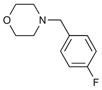 8i 8i
|
70 | 96 |
| 6 | 6j |
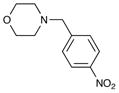 8j 8j
|
74 | 93 |
| 7 | 6k |
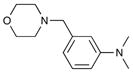 8k[b] 8k[b]
|
78 | 98 |
| 8 | 6k |
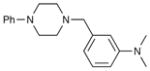 8k′[c] 8k′[c]
|
93 | 98 |
| 9 | 6l |
|
68 | 95 |
Purities calculated using GC and further confirmed by 1H NMR.
Despite their simplicity, each compound is classified as a NCE.
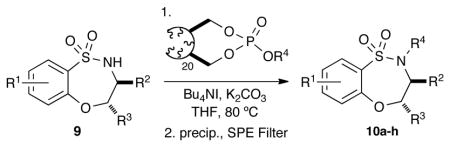
The 20-mer of OBP was tested on a select benzofused sultam scaffold for benzylation (Table 4). The reagent was added to a THF solution containing benzothiaoxazepine-1,1-dioxide (9a) in the presence of K2CO3 and Bu4NI and stirred at 80 °C overnight. The resulting mother liquor was precipitated from a Et2O/EtOAc mixture. Subsequent filtration employing a silica SPE cartridge, and evaporation of solvent afforded the desired benzylated product 10a in excellent yield and high purity. With this result in place, sultams 9a-d were subjected to benzylation employing OBP derivatives utilizing the conditions established above to afford the desired products (10b-h) in good to excellent yields.
Table 4.
Benzylation of benzothiaoxazepine-1,1-dioxides
 | ||||||
|---|---|---|---|---|---|---|
| SM | R1 | R2 | R3 | R4 | pdt | yield (%)[a] |
| 9a | 4-Br | Ph | H | Bn | 10a | 99 |
| 9a | 4-Br | Ph | H | 3,5-diMeO-Bn | 10b | 72 |
| 9a | 4-Br | Ph | H | 4-F Bn | 10c | 85 |
| 9b | 4-Br | iBu | H | 4-F Bn | 10d | 97 |
| 9b | 4-Br | iBu | H | 2-Me Bn | 10e | 81 |
| 9c | 3-Cl | iBu | H | 4-Cl Bn | 10f | 76 |
| 9d | 5-Cl | Me | Ph | 2-Me Bn | 10g | 78 |
| 9d | 3-Cl | Me | Ph | 2-Me Bn | 10h | 83 |
Yields after filtration through a SiO2 SPE.
In conclusion, we have demonstrated the synthesis and utilization of a ROMP-based oligomeric phosphate for facilitated benzylation of cyclic amines and have applied it towards simple diversification pathways in relevant scaffolds. These oligomeric reagents are readily synthesized from commercially available materials and are conveniently polymerized and purified in a one-pot process affording pure reagent on multi-gram scale. Efforts to widen the scope of this reagent, improvement in synthesis and scale-up and its continued integration into diversity-oriented synthetic protocols is underway. The results of these endeavors will be reported in due course.
Supplementary Material
Acknowledgments
This investigation was generously supported by the National Institute of General Medical Sciences (NIH P050-GM069663 and NIH-STTR R41 GM076765) with additional funds from the State of Kansas. We would like to also thank Materia, Inc. for supplying catalyst and helpful discussions.
Footnotes
Supporting Information Available. Detailed experimental procedures and tabulated 1H NMR, 13C NMR, 31P NMR, FTIR, and mass data, and 1H NMR spectra of crude products obtained by the described benzylation method.
References
- 1.(a) Booth RJ, Hodges JC. Acc Chem Res. 1999;32:18–26. [Google Scholar]; (b) Ley SV, Baxendale IR, Bream RN, Jackson PS, Leach AG, Longbottom DA, Nesi M, Scott JS, Storer RI, Taylor SJJ. Chem Soc, Perkin Trans 1. 2000:3815–4195. [Google Scholar]; (c) Kirschning A, Monenschein H, Wittenberg R. Angew Chem Int Ed. 2001;40:650–679. [PubMed] [Google Scholar]; (d) Eames J, Watkinson M. Eur J Org Chem. 2001:1213–1224. [Google Scholar]; (e) Strohmeier GA, Kappe CO. Angew Chem Int Ed. 2004;43:621–624. doi: 10.1002/anie.200352731. [DOI] [PubMed] [Google Scholar]; (f) Lesch B, Thomson DW, Lindell SD. Comb Chem High T Scr. 2008;11:31–36. doi: 10.2174/138620708783398359. [DOI] [PubMed] [Google Scholar]
- 2.For reviews concerning soluble polymers, see: Gravert DJ, Janda KD. Chem Rev. 1997;97:489–509. doi: 10.1021/cr960064l.Toy PH, Janda KD. Acc Chem Res. 2000;33:546–554. doi: 10.1021/ar990140h.Dickerson TJ, Reed NN, Janda KD. Chem Rev. 2002;102:3325–3344. doi: 10.1021/cr010335e.Haag R. Chem Eur J. 2001;7:327–335. doi: 10.1002/1521-3765(20010119)7:2<327::aid-chem327>3.0.co;2-m.Haag R, Sunder A, Hebel A, Roller S. J Comb Chem. 2002;4:112–119. doi: 10.1021/cc010058p.Bergbreiter DE. Chem Rev. 2002;102:3345–3384. doi: 10.1021/cr010343v.Bergbreiter DE, Tian J, Hongfa C. Chem Rev. 2009;109:530–582. doi: 10.1021/cr8004235.
- 3.(a) Studer A, Curran DP. Tetrahedron. 1997;53:6681–6696. [Google Scholar]; (b) Hjerten S, Li Y-M, Liao JL, Mankazato K, Mohammad J, Pettersson G. Nature. 1992;356:810–811. [Google Scholar]; (c) Baumann M, Baxendale IR, Ley SV, Nikbin N, Smith CD. Org Biomol Chem. 2008;6:1577–1586. doi: 10.1039/b801631n. [DOI] [PubMed] [Google Scholar]
- 4.(a) Barrett AGM, Hopkins BT, Kobberling J. Chem Rev. 2002;102:3301–3324. doi: 10.1021/cr0103423. [DOI] [PubMed] [Google Scholar]; (b) Harned AM, Probst DA, Hanson PR. In: Handbook of Metathesis. Grubbs RH, editor. Vol. 2. Wiley-VCH; Weinheim: 2003. pp. 361–402. [Google Scholar]; (c) Flynn DL, Hanson PR, Berk SC, Makara GM. Curr Opin Drug Discov Devel. 2002;5:571–579. [PubMed] [Google Scholar]; (d) Harned AM, Zhang M, Vedantham P, Mukherjee S, Herpel RH, Flynn DL, Hanson PR. Aldrichim Acta. 2005;38:3–16. [Google Scholar]
- 5.(a) Rolfe A, Probst D, Volp K, Omar I, Flynn D, Hanson PR. J Org Chem. 2008;73:8785–8790. doi: 10.1021/jo801578f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Stoianova DS, Yao L, Rolfe A, Samarakoon T, Hanson PR. Tetrahedron Lett. 2008;49:4553–4555. doi: 10.1016/j.tetlet.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang M, Flynn DL, Hanson PR. J Org Chem. 2007;72:3194–3198. doi: 10.1021/jo0620260. [DOI] [PubMed] [Google Scholar]; (d) Roberts RS. J Comb Chem. 2005;7:21–32. doi: 10.1021/cc049915q. [DOI] [PubMed] [Google Scholar]; (e) Harned AM, Sherrill WM, Flynn DL, Hanson PR. Tetrahedron. 2005;61:12093–12099. [Google Scholar]; (f) Arstad E, Barrett AGM, Tedeschi L. Tetrahedron Lett. 2003;44:2703–2707. [Google Scholar]; (g) Barrett AGM, Hopkins BT, Love AC, Tedeschi L. Org Lett. 2004;6:835–837. doi: 10.1021/ol049915z. [DOI] [PubMed] [Google Scholar]
- 6.Vedantham P, Zhang M, Gor PJ, Huang M, Georg GI, Lushington GH, Mitscher LA, Ye QZ, Hanson PR. J Comb Chem. 2008;10:195–203. doi: 10.1021/cc7000869. [DOI] [PubMed] [Google Scholar]
- 7.(a) Westheimer FH. Science. 1987;235:1173–1178. doi: 10.1126/science.2434996. [DOI] [PubMed] [Google Scholar]; (b) Paquette LA. Encyclopedia of Reagents for Organic Synthesis. 1. John Wiley and Sons; 1995. pp. 316–318. [Google Scholar]
- 8.(a) Yanagisawa A, Nomura N, Noritake Y, Yamamoto H. Synthesis. 1991;12:1130–1136. [Google Scholar]; (b) Torneiro M, Fall Y, Castedo L, Mourino A. J Org Chem. 1997;62:6344–6352. [Google Scholar]; (c) Murphy KE, Hoveyda AH. J Am Chem Soc. 2003;125:4690–4691. doi: 10.1021/ja0300618. [DOI] [PubMed] [Google Scholar]; (d) Williams DR, Heidebrecht RW., Jr J Am Chem Soc. 2003;125:1843–1850. doi: 10.1021/ja0279803. [DOI] [PubMed] [Google Scholar]; (e) Fuwa H, Sasaki M. Heterocycles. 2008;76:521–539. [Google Scholar]; (f) Thomas CD, McParland JP, Hanson PR. Eur J Org Chem. 2009:5487–5500. doi: 10.1002/ejoc.200900560. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Smith AG, Johnson JS. Org Lett. 2010;12:1784–1787. doi: 10.1021/ol100410k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Nicolaou KC, Shi GQ, Gunzner JL, Gaertner P, Yang Z. J Am Chem Soc. 1997;119:5467–5468. [Google Scholar]; (b) La Cruz TE, Rychnovsky SD. J Org Chem. 2007;72:2602–2611. doi: 10.1021/jo0626459. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lapointe D, Fagnou K. Org Lett. 2009;11:4160–4163. doi: 10.1021/ol901689q. [DOI] [PubMed] [Google Scholar]
- 10.(a) Morgan AB, Tour JM. J Appl Polym Sci. 1999;73:707–718. [Google Scholar]; (b) Wang J, Mao HQ, Leong KW. J Am Chem Soc. 2001;123:9480–9481. doi: 10.1021/ja016062m. [DOI] [PubMed] [Google Scholar]; (c) Iliescu S, Manoviciu I, Ilia G, Dehelean G. Roum Chem Q Rev. 1997;5:267–277. [Google Scholar]; (d) Kobayashi S, Tokunoh M, Saegusa T. Macro Mol. 1986;19:466–469. [Google Scholar]; (e) Allcock HR, de Denus CR, Prange R, Laredo WR. Macro Mol. 2001;34:2757–2765. [Google Scholar]; (f) Seeberger PH. Chem Soc Rev. 2008;37:19–28. doi: 10.1039/b511197h. [DOI] [PubMed] [Google Scholar]
- 11.Kane RR, Lee CS, Drechsel K, Hawthorne MF. J Org Chem. 1993;58:3227–3228. [Google Scholar]
- 12.March J. Advanced Organic Chemistry. 4. Wiley; New York: 1991. [Google Scholar]
- 13.Greene TW, Wuts PGM. Protective Groups in Organic Synthesis. 4. John Wiley and Sons; New York: 2007. pp. 102–148. [Google Scholar]
- 14.(a) Dolle RE, Le Bourdonnec B, Goodman AJ, Morales GA, Thomas CJ, Zhang W. J Comb Chem. 2009;11:739–790. doi: 10.1021/cc9000828. [DOI] [PubMed] [Google Scholar]; (b) Fenster E, Rayabarapu DK, Zhang M, Mukherjee S, Hill D, Neuenswander B, Schoenen F, Hanson PR, Aubé J. J Comb Chem. 2008;10:230–234. doi: 10.1021/cc700174c. [DOI] [PubMed] [Google Scholar]
- 15.(a) Loris A, Perosa A, Selvia M, Tundo P. J Org Chem. 2004;69:3953–3956. doi: 10.1021/jo049840c. [DOI] [PubMed] [Google Scholar]; (b) Shieh WC, Lozanov M, Loo M, Repic O, Blacklock TJ. Tetrahedron Lett. 2003;44:4563–4565. [Google Scholar]; (c) Shieh WC, Lozanov M, Repic O. Tetrahedron Lett. 2003;44:6943–6945. [Google Scholar]; (d) Huang W, He B. Chin J React Polym (Engl) 1992;1:61–70. [Google Scholar]; (e) Hunt JA, Roush WR. J Am Chem Soc. 1996;118:9998–9999. [Google Scholar]; (f) Rueter JK, Nortey SO, Baxter EW, Leo GC, Reitz AB. Tetrahedron Lett. 1998;39:975–978. [Google Scholar]; (g) Baxter EW, Rueter JK, Nortey SO, Reitz AB. Tetrahedron Lett. 1998;39:979–982. [Google Scholar]; (h) Takahashi T, Ebata S, Doi T. Tetrahedron Lett. 1998;39:1369–1372. [Google Scholar]
- 16.For recent benzylation via activation of benzyl alcohols see: Poon KWC, House SE, Dudley GB. Synlett. 2005:3142–3144.Zhang M, Moore JD, Flynn DL, Hanson PR. Org Lett. 2004;6:2657–2660. doi: 10.1021/ol049209y.Jha M, Enaohwo O, Marcellus A. Tetrahedron Lett. 2009;50:7184–7187.Martinez R, Ramon DJ, Yus M. Org Biomol Chem. 2009;7:2176–2181. doi: 10.1039/b901929d.Zhang C, Gao X, Zhang J, Peng X. Synlett. 2010:261–265.
- 17.(a) The synthesis of oligomeric benzylsulfonium salts requires 5 steps and the use of benzyl bromides. These reagents were not soluble in typical organic solvents (Reference 5c). (b) The oligomeric sulfonate esters were used in a “catch and release” protocol whereby benzyl alcohols were reacted after polymerization (Reference 16b).
- 18.Craig D. J Am Chem Soc. 1951;73:4889–4892. [Google Scholar]
- 19.Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 20.(a) Schwab P, Grubbs RH, Ziller JW. J Am Chem Soc. 1996;118:100–110. [Google Scholar]; (b) Schwab P, France MB, Ziller JW, Grubbs RH. Angew Chem, Int Ed Engl. 1995;34:2039–2041. [Google Scholar]
- 21.These characteristics include homogeneity and the ability to achieve the necessary critical viscocity for precipitation.
- 22.Pederson RL, Fellows IM, Ung TA, Ishihara H, Hajela SP. Adv Synth Catal. 2002;344:728–735. [Google Scholar]
- 23.Critical viscosity described in these terms is the optimum viscosity to achieve a free-flowing precipitate.
- 24.See Supporting Information for solubility tables of OBPn. For information regarding Gaussian distribution of oligomers see ref. 5a.
- 25.We have recently formulated ROMP-tabs, whereby premeasured OBP20 tablets can be conveniently added to the reaction.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.