Abstract
There is increasing evidence that high magnetic fields interact with the vestibular system of humans and rodents. In rats, exposure to high magnetic fields of 7T or above induces locomotor circling and leads to a conditioned taste aversion if paired with a novel taste. Sex differences in the behavioral responses to magnetic field exposure have been found, such that female rats show more locomotor circling and enhanced conditioned taste aversion compared to male rats. To determine if estrogen modulates the neural response to high magnetic fields, c-Fos expression after 14T magnetic field exposure was compared in ovariectomized rats and ovariectomized rats with estradiol replacement. Compared to sham exposure, magnetic field exposure induced significantly more c-Fos positive cells in the nucleus of the solitary tract and the parabrachial, medial vestibular, prepositus, and supragenualis nuclei. Furthermore, there was a significant asymmetry in c-Fos induction between sides of the brainstem in several regions. In ovariectomized rats, there was more c-Fos expressed in the right side compared to left side in the locus coeruleus and parabrachial, superior vestibular, and supragenualis nuclei; less expression in the right compared to left side of the medial vestibular; and no asymmetry in the prepositus nucleus and the nucleus of the solitary tract. Chronic estradiol treatment modulated the neural response in some regions: less c-Fos was induced in the superior vestibular nucleus and locus coeruleus after estradiol replacement; estradiol treatment eliminated the asymmetry of c-Fos expression in the locus coeruleus and supragenualis nucleus, created an asymmetry in the prepositus nucleus and reversed the asymmetry in the parabrachial nucleus. These results suggest that ovarian steroids may mediate sex differences in the behavioral responses to magnetic field exposure at the level of visceral and vestibular nuclei of the brainstem.
Keywords: Conditioned taste aversion, Locomotor circling, Magnetic resonance imaging, Motion sickness, Ovariectomy
Introduction
Advances in magnetic resonance imaging (MRI) are leading to the development of more powerful MRI machines capable of producing higher resolution images. There is increasing evidence that high magnetic fields (>= 4 T) interact with the vestibular system of rodents and humans, although the mechanism of interaction is unknown. Magnetic field exposure under 2 T does not appear to be detectable by humans (Schenck, 1992; Winther et al., 1999) and has been reported to have no effect on a variety of behavioral tasks in rats (Innis et al., 1986; Messmer et al., 1987; Ossenkopp et al., 1986). Surveys of workers employed and within a 4 T MRI magnet (Schenck, 1992) or 9.4 T MRI magnet (Patel et al., 2008) reported sensations of vertigo, nausea, and illusions of movement that have been attributed to vestibular perturbations. Subjects undergoing MRI scans at 7 T (Theysohn et al., 2008) or 8 T (Kangarlu et al., 1999) reported vertigo while being moved in or out of the MRI machine, but not when positioned in the center of the machine for the duration of the scan. In a psychophysical study, subjects in a 7 T MRI machine experienced sensations of motion while moving into the magnet, or when moving their heads, but not when stationary (Glover et al., 2007). When positioned at the homogeneous center of the magnetic field, movement of the head (e.g. head nodding or rolling) generated mild to severe vertigo (Glover et al., 2007). Theoretical models of the mechanisms by which a static magnetic field could interact with the human vestibular system have been proposed (Glover et al., 2007; Schenck, 1992).
Our laboratory has discovered that a 30-min exposure to a static magnetic field of 7 T to 17 T has behavioral and neural effects in male and female rats and male mice (Cason et al., 2006; Houpt et al., 2003; Houpt and Smith, 2009; Nolte et al., 1998; Snyder et al., 2000). At the behavioral level, magnetic field exposure suppressed normal rearing and induced tight locomotor circling for the first few minutes following magnet exposure. The direction of circling was dependent on the orientation of the magnetic field, such that when the rat was exposed head-up towards the positive pole it circled in a counterclockwise direction in the horizontal plane when viewed from the dorsal perspective. Exposure head-down towards the negative pole induced horizontal clockwise circling as viewed dorsally (Houpt et al., 2003). Furthermore, we observed that when the magnetic field exposure was paired with a novel taste solution, a conditioned taste aversion (CTA) was induced (Cason et al., 2006; Houpt et al., 2003; Lockwood et al., 2003; Nolte et al., 1998), similar to that seen after a taste stimulus is paired with off-axis rotation or motion sickness (Arwas et al., 1989; Braun and McIntosh, 1973; Fox et al., 1984; Green and Rachlin, 1973). At the neural level, magnetic field exposure induced significant c-Fos immunoreactivity, a marker of neuronal activation, in specific vestibular and visceral nuclei within the rat brainstem (Cason et al., 2009; Snyder et al., 2000).
The circling seen in rats immediately after magnetic field exposure is strikingly similar to the locomotor circling seen after unilateral hemilabyrinthectomy (Kaufman et al., 1999). The suppression of rearing behavior following magnet exposure is similar to the suppression of rearing seen after horizontal whole-body rotation (Ossenkopp et al., 1994). Magnetic field exposure and vestibular stimulation, e.g., by rotation, unilateral labyrinthectomy, or galvanic stimulation induce similar c-Fos patterns in the rat brainstem (Kaufman et al., 1991; Kaufman et al., 1992; Kaufman et al., 1993; Kaufman et al., 1999; Marshburn et al., 1997). Because these results demonstrate parallel responses to vestibular activation and magnet exposure, they suggest that rats may be experiencing a vestibular disturbance similar to the self-reports of vertigo and subjective motion from humans after magnetic field exposure.
We also found significant sex differences in the response of rats to high magnetic field exposure that were modulated by estrogen (Cason et al., 2006). Female Sprague-Dawley rats exhibited more locomotor circling than male rats. Estrogen levels modulated the response to the high magnetic field: female rats in estrus with low circulating levels of endogenous estrogens and ovariectomized females with the endogenous source of estrogen removed showed more circling than female rats with high levels of estrogen. Females also showed a more persistent CTA than male rats, which was not dependent upon the phase of estrous cycle or influenced by ovariectomy or hormone replacement. A second study utilizing gonadectomized and testosterone-treated male rats showed that the sex difference in CTA was not dependent on testosterone (unpublished data). Taken together, these studies suggest that there is an activational effect of estrogen to depress circling behavior, and also an organizational effect of sex on CTA that is not influenced by activational effects of estrogen or testosterone.
We hypothesize that the sex differences in behavioral responses of rats to magnetic fields are modulated by estrogen at the level of the vestibular and visceral relays of the brainstem. Therefore, we examined c-Fos immunohistochemistry as a marker of neural activation after 30-min 14T magnetic field or sham exposure in ovariectomized female rats with and without chronic estradiol treatment. c-Fos is an immediate early gene that is often induced by acute sensory stimulation; it has been widely used to map central pathways of sensory processing, including brain stem circuitry activated by vestibular and visceral stimulation. For example, c-Fos is induced in brainstem vestibular relays by whole-body rotation (Kaufman et al., 1991; Kaufman et al., 1992; Kaufman et al., 1993; Marshburn et al., 1997) and unilateral labyrinthectomy (Kaufman et al., 1999). c-Fos is induced in visceral relays by toxic or stressful stimuli such as lithium chloride injection (Houpt et al., 1994; Koh et al., 2003; Spencer and Houpt, 2001) or whole body restraint (Cullinan et al., 1995; Ostrander et al., 2003). Similarly, we have also found that c-Fos is induced in both vestibular and visceral relays after 30-min exposure to a 9.4T magnetic field in male rats (Snyder et al., 2000) and to a 14T magnetic field in female rats (Cason et al., 2009).
Ovariectomy and estrogen treatment have been shown to influence the amount of c-Fos expression in other models. In feeding, for example, ovariectomy increases meal size and decreases sensitivity to cholecystokinin; chronic estrogen (estradiol treatment) reverses these effects. In parallel with these behavioral responses, estrogen treatment increased the number of c-Fos-positive cells induced by food intake (Eckel and Geary, 2001) or cholecystokinin (Eckel et al., 2002) in the caudal and intermediate (but not rostral) nucleus of the solitary tract (NTS), the paraventricular nucleus of the hypothalamus, and the central nucleus of the amygdala. Thus the estrogen-modulated change in feeding behavior was accompanied by a change in neural sensitivity as revealed with c-Fos. In a parallel fashion, we hypothesized that females treated with estradiol would show a different magnitude or pattern of c-Fos expression after high magnetic field exposure, compared to females without estrogen.
Results
To evaluate the effect of estradiol on c-Fos expression induced by magnetic-field exposure, ovariectomized female rats (OVX) and ovariectomized female rats with estradiol implants (OVX-E) were restrained within a 14.1 T magnet for 30 min, and then processed for c-Fos 60 min after the end of exposure. Control rats were restrained and sham-exposed for 30 min.
Locomotor Activity
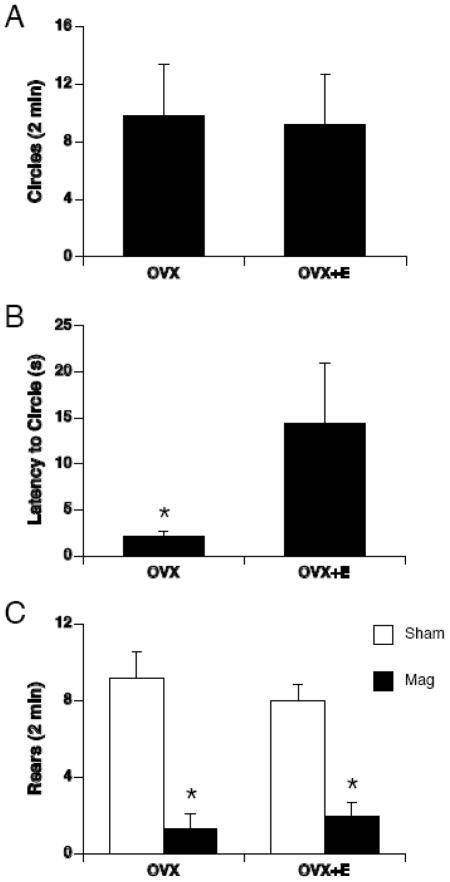
Magnetic field exposure, but not sham exposure, induced locomotor circling (Figure 1A). Magnet-exposed rats walked in tight, counterclockwise circles as previously reported. None of the sham-exposed rats circled. Therefore, the number of circles and the latency to circle was compared between magnet-exposed groups only. There was no significant difference in the number of circles between OVX and OVX +E magnet-exposed rats. However, the latency to circle was greater in OVX+E compared to OVX magnet-exposed rats [t(10) = 2.25, p < 0.05] (see Figure 1B).
Figure 1.
Open-field locomotor behavior was scored for tight circling and rearing following 30-min magnetic field-exposure or sham-exposure. A. Only magnet-exposed rats circled and there was no difference in the number of circles between OVX and OVX+E rats. B. The latency to circle was significantly lower in OVX compared to OVX+E rats, * p < 0.01. C. Magnetic field-exposure (black bars) suppressed rearing in both OVX and OVX+E rats compared to sham-exposed rats (white bars); * p < 0.001.
There was also a significant effect of exposure on rearing [F(1,19) = 48.02, p < 0.001] (Figure 1B). Magnetic field exposure almost completely suppressed rearing behavior in both OVX and OVX+E rats. Sham-exposed rats regardless of group reared more than magnet-exposed rats.
c-Fos expression
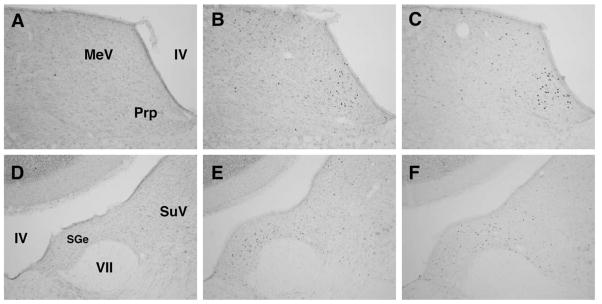
Example photomicrographs of c-Fos induction after sham or magnet exposure can be seen in Figures 2 and 3.
Figure 2.
Example photomicrographs of c-Fos induction in vestibular relays after sham exposure (A,D) or after 30-min magnetic field exposure in OVX rats (B,E) or OVX+E rats (C,F). IV, fourth ventricle; MeV, medial vestibular nucleus; Prp, prepositus nucleus, VII, genu of the 7th cranial nerve; SGe, supragenulis nucleus; SuV, superior vestibular nucleus.
Figure 3.
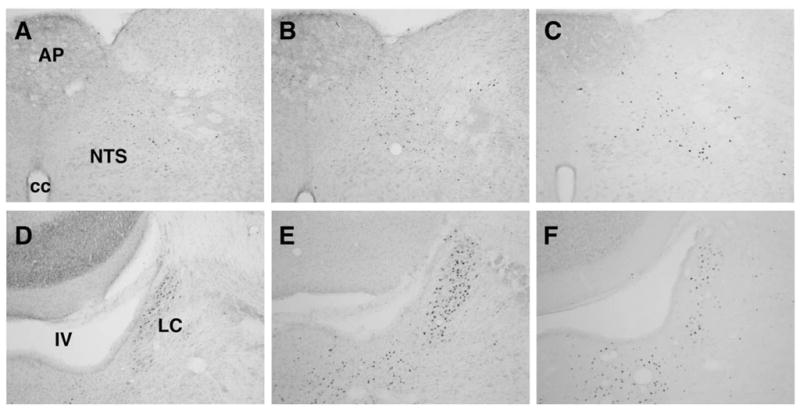
Example photomicrographs of c-Fos induction in visceral relays after sham exposure (A,D) or after 30-min magnetic field exposure in OVX rats (B,E) or OVX+E rats (C,F). IV, fourth ventricle; cc, central canal; AP, area postrema; NTS, nucleus of the solitary tract; LC, locus coeruleus.
c-Fos expression in vestibular nuclei
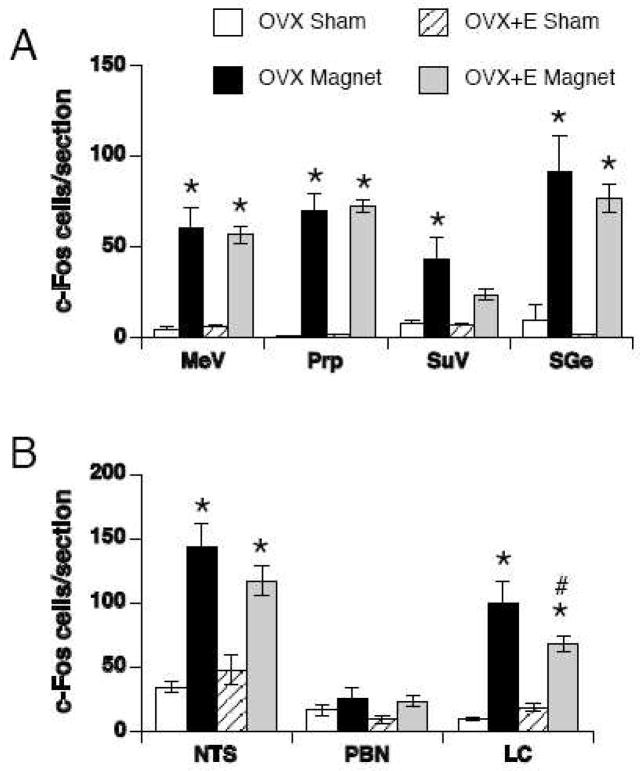
Magnetic field exposure induced significantly more bilateral c-Fos than sham-exposure in the MeV [F(1,19) = 69.90, p < 0.001], Prp [F(1,19) = 149.31, p < 0.001], SuV [F(1,19) = 13.86, p < 0.01] and SGe [F(1,19) = 44.92, p < 0.001] (see Figures 2 and 4A). In particular, c-Fos positive cells were densely induced by magnetic field exposure in the Prp and SGe. Within the MeV, the pattern of c-Fos-positive cells was diffuse. In the SuV, c-Fos was sparse and scattered.
Figure 4.
Quantification of c-Fos positive cells in sham-exposed (white and striped bars) or magnet-exposed (black and gray bars) rats in vestibular (A) and visceral relays (B). The bilateral counts (mean ± SEM) through each section are shown. MeV = medial vestibular nucleus, Prp = prepositus nucleus, SuV = superior vestibular nucleus, SGe = supragenualis nucleus, NTS = nucleus of the solitary tract, PBN = parabrachial nucleus, LC = locus coeruleus. * p < 0.05 within group v. sham-exposed rats; # p < 0.05 within magnet-exposed rats, OVX v. OVX+E.
There were no significant effect of estradiol and no significant interactions of exposure and estradiol on bilateral c-Fos counts within the vestibular relays. In the SuV of OVX+E rats, however, magnetic field exposure did not induce significantly more c-Fos than sham exposure, with a trend towards a decrease compared to magnet-exposed OVX rats (p = 0.06).
c-Fos expression in visceral relays
Magnetic field exposure induced significantly more total number of c-Fos-positive cells (counted bilaterally) compared to sham-exposure in the NTS [F(1,19) = 49.11, p < 0.001], PBN [F(1,19) = 4.46, p < 0.05], and LC [F(1,19) = 50.94, p < 0.01] (see Figures 3 and 4B). In the NTS, abundant c-Fos was observed in the medial, intermediate, subpostremal, and caudal NTS while little or no c-Fos was observed in the lateral or rostral (gustatory) NTS. In the PBN, c-Fos was observed only in the lPBN. Specifically, c-Fos was concentrated in the external lateral, central lateral and ventrolateral regions of the lPBN. In the LC, c-Fos was abundant along the border of the ventricle.
There was a significant interaction of exposure and estradiol [F(1,19) = 4.37, p = 0.05] on bilateral c-Fos counts in the LC. OVX rats displayed significantly more c-Fos-positive cells than OVX+E rats in the LC following magnetic field exposure (p < 0.001). There was no significant effect of estradiol and no interaction of exposure and estradiol in the NTS or PBN.
Asymmetries in c-Fos induction
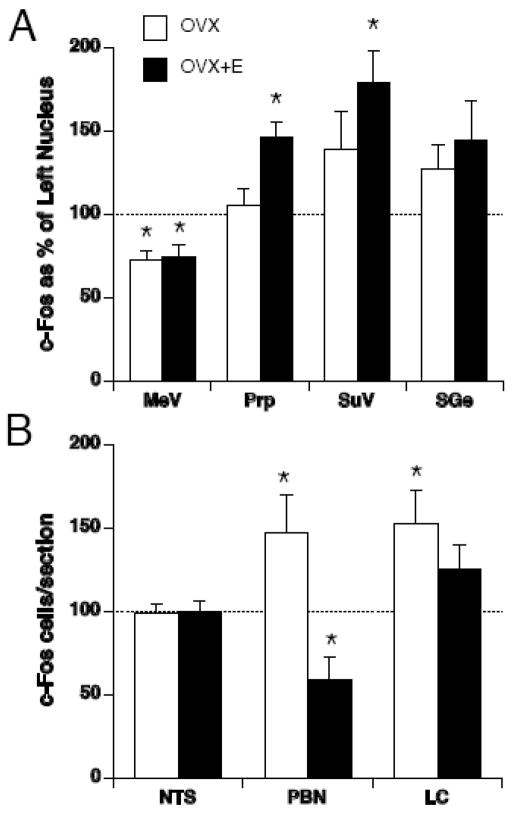
Pronounced asymmetries were seen in c-Fos expression after magnetic field exposure in several brain regions (Figure 5). In OVX rats, paired t-tests revealed that there was more c-Fos expression in the right nucleus compared to left nucleus of the PBN, LC, SuV and SGe. Conversely, there was less c-Fos in the right nucleus compared to the left nucleus of the MeV. No asymmetry of c-Fos expression was seen in the Prp and NTS.
Figure 5.
Number of c-Fos positive cells after magnetic field exposure in the right side of each brain region, expressed as a percentage of c-Fos-positive cells in the left side of each brain region, for OVX (white bars) and OVX+E (black bars) rats in vestibular (A) and visceral relays (B). A significant difference from 100% indicates an asymmetrical induction of c-Fos. * p < 0.05 left v. right side, # p < 0.05 OVX vs. OVX+E.
Chronic estradiol treatment altered the pattern of c-Fos expression. Compared to OVX rats, OVX+E reduced the asymmetry in the LC and SGe, induced a significant asymmetry in the Prp (right side > left side) and reversed the asymmetry of activation within the PBN (i.e. left > right). These changes resulted in a significant difference between OVX and OVX+E groups in the right-to-left ratio of c-Fos counts in the Prp and PBN. Thus, although estradiol treatment did not change the overall number of c-Fos positive cells counted bilaterally in Prp, SGe, and PBN, it did alter the relative pattern of expression across the two sides of the brainstem.
Correlation of c-Fos Induction and Circling
Pearson correlation coefficients were calculated to determine if there was a correlation between locomotor circling and c-Fos induction in the magnet-exposed groups. Number of circles was examined across both OVX and OVX+E groups. Number of circles was positively correlated with the number of c-Fos-positive cells in the bilateral Prp [r(10) = 0.592, p < 0.05] and bilateral MeV [r(10) = 0.596, p < 0.05].
Latency to circle was examined only in the OVX+E group, because all the rats in the OVX group circled almost immediately (i.e. they had a uniformly low latency to circle). Latency to circle in the OVX+E group was negatively correlated with c-Fos induction in the left MeV [r(4)=−0.836, p < 0.05] and left SGe [r(4)=−0.975 p < 0.05], but positively correlated with c-Fos in the right LC [r(4) = 0.816, p < 0.05].
No correlation was found between number of circles or latency to circle and c-Fos induction in the NTS or PBN. This may be consistent with the role of the NTS and PBN in processing visceral responses rather than specific vestibular sensation.
Discussion
In this study, we found that estradiol modulated the neural response of the brainstem to magnetic field exposure in several ways. While magnetic field exposure induced significant amounts of c-Fos in both visceral and vestibular nuclei of the ovariectomized rat as previously found in male rats, chronic estradiol replacement significantly reduced the amount of c-Fos positive cells in the LC and prevented a significant induction in the SuV. Importantly, significant lateral asymmetries in c-Fos induction were quantified. Estradiol replacement also changed these patterns of left-right c-Fos expression.
As in previous studies, magnetic field exposure also induced counterclockwise locomotor circling and suppressed rearing (Cason et al., 2006; Houpt et al., 2003; Houpt et al., 2005; Lockwood et al., 2003). Previously, Cason et al. found that female rats showed increased counterclockwise circling compared to male rats, especially on the day of estrus when endogenous estrogen levels were low. Furthermore, ovariectomy increased circling and estradiol replacement decreased circling to the level of intact females following magnetic field exposure (Cason et al., 2006). Thus, both endogenous and exogenous estrogen could exert an inhibitory effect on responsiveness to magnetic fields.
In this study, although there was no difference in total number of circles after magnetic field exposure, OVX+E rats had a significantly longer latency to begin circling compared to OVX rats. The lack of an estradiol effect here on absolute number of circles might be due to methodological differences between this study and the previous report. The only obvious differences were that in the previous study, rats underwent water restriction and were conditioned with 10-min saccharin access prior to magnet exposure, whereas in the present study rats were not water deprived. It is difficult to explain how these differences could contribute to circling behavior. There is evidence that chronic water restriction can affect levels of locomotor activity (Seiden et al., 1985). However there is no particular reason to believe that estradiol modulation would be specifically altered by water restriction or saccharin consumption.
c-Fos Induction
Magnetic field exposure induced more c-Fos expression compared to sham treatment in all areas examined in OVX rats, including both visceral (NTS, PBN, LC) and vestibular relays (MeV, Prp, SuV and SGe). These results are consistent with previous findings (Cason et al., 2009; Snyder et al., 2000). The induction of c-Fos was not due to restraint alone because sham-exposed rats, which were also restrained, expressed little or no c-Fos. The induction of c-Fos in magnet-exposed rats is not due to the locomotor circling seen in magnet-exposed rats per se, because c-Fos has been observed in magnet-exposed rats that remain restrained for 15 min after exposure and do not circle upon release (unpublished results).
We hypothesized that decreased behavioral responsiveness to magnetic fields would be correlated with decreased neuronal activation in OVX+E rats. While the bilateral magnitude of activation in most regions of the brainstem was unaffected by estradiol, OVX+E rats showed less activation in the LC and the SuV. This suggests that estradiol can modulate responsiveness of the LC and SuV (directly or indirectly). The LC and SuV are therefore candidate sites mediating the reduced responsiveness of female rats with elevated estrogen levels to high magnetic fields. The lack of effect of estradiol on other nuclei examined may reflect the potency of the 14T stimulus (i.e. a ceiling effect), or suggest that small focal changes in c-Fos expression can be correlated with integrated behavioral responses.
While it is clear that there is an association between c-Fos activation and circling, it is difficult to correlate precisely levels of c-Fos activation in the brainstem with behavioral responses of the vestibular system. Examples can be found, however. The vestibular system of mutant head-tilt (het) mice, which lack macular otoconia, are practically insensitive to linear acceleration; consequently, centrifugation induces almost no c-Fos in the brainstem of head-tilt mice, although it is a potent stimulus in wild-type mice (Fuller et al., 2004). Furthermore, drug treatments that alter vestibular responses also alter c-Fos expression. For example, MK801 (an NMDA receptor antagonist) administered to rats shortly after unilateral labyrinthectomy inhibits vestibular compensation while increasing c-Fos expression in the contralateral MeV nucleus (Kim et al., 1997).
Asymmetrical c-Fos Induction
An significant aspect of the effects of high magnetic fields is the induction of an asymmetric behavior: rats and mice walk in counterclockwise circles after magnetic field exposure with their heads towards the positive pole of the magnet. Thus suggests an underlying asymmetry of the neural responsive in the brainstem. When laterality of c-Fos expression was examined, it was found that c-Fos expression in OVX rats was greater in the right side compared to left side of the SuV, SGe, PBN, and LC; in only the MeV was the left side greater than the right side. Thus magnetic fields cause asymmetrical activation of the vestibular and visceral relays. The observed pattern of asymmetric activation (with asymmetry in the MeV opposite of other vestibular relays) is very similar to the pattern of c-Fos induced by unilateral labyrinthectomy (Kaufman et al., 1999) and suggests that magnetic fields may cause asymmetrical stimulation of the vestibular system (or equivalently, asymmetrical inhibition), perhaps at the level of the inner ear.
Chronic estradiol replacement changed the left-right pattern of c-Fos induction, either by increasing the ratio of right to left c-Fos (Prp), decreasing the ratio of right to left c-Fos (PBN), or abolishing asymmetry altogether (SGe, LC). If the asymmetric distribution of neuronal activation represented by c-Fos expression determines the integrated behavioral response to a vestibular stimulus, then the estradiol-induced changes in c-Fos distribution may represent a substrate for modulation by estrogens.
Although it is difficult to interpret these complex patterns and their modulation by estradiol, there are some examples in which the degree or pattern of asymmetry reflects the integrated behavioral response to a vestibular challenge. For example, c-Fos expression in brainstem nuclei following unilateral labyrinthectomy varies during the period of vestibular compensation. During vestibular compensation, there are changes in the overall amount of c-Fos expression as well as changes in pattern of asymmetry; thus the pattern of asymmetry can reflect both the degree of behavioral deficit and behavioral recovery (Kaufman et al., 1993; Kaufman et al., 1999; Kim et al., 2002; Kitahara et al., 1995).
Interestingly, most of the asymmetric activation occurred in the side of the brainstem contralateral to the direction of locomotor circling (i.e. rats circled counterclockwise to their left, but the right side of the brainstem showed more activation.) We have recently begun to observe the head movements of rats during magnetic field exposure. After being placed in the center of the 14.1 T magnet, rats immediately tilt their heads to their right side, and they maintain this posture throughout the 30-min exposure. Thus, the lateralized c-Fos expression may reflect ipsilateral neuronal activation by a rightward vestibular stimulus during magnetic field exposure, while the counterclockwise circling after exposure may reflect a contralateral, leftward compensatory response when the magnetic stimulus is removed. The reasons for an asymmetrical effect of the homogenous magnetic field are unknown.
We have recently found using labyrinthectomized rats that the inner ear is required for the behavioral and neural response to magnetic field exposure, which suggests that the magnetic field interacts with the labyrinth (Cason et al., 2009; Houpt et al., 2007). Because the mechanism of physical interaction of the magnetic field with the peripheral vestibular apparatus is unknown, the magnetic field could be either stimulating or inhibiting to the inner ear. The vestibular system processes and compares inputs from the left and right inner ears. Asymmetrical stimulation of the vestibular system can cause motion sickness and locomotor circling (Cohen et al., 2003; Kaufman et al., 1999). The CTA acquired by rats in previous studies suggests that magnetic field exposure may interact with the vestibular system to cause motion sickness (Cason et al., 2006; Houpt et al., 2003; Lockwood et al., 2003; Nolte et al., 1998).
Patterns of c-Fos expression cannot distinguish direct vs. indirect actions of magnetic field exposure on the vestibular system. The magnetic field could be directly acting on the peripheral vestibular apparatus (i.e. the inner ear) or influencing processing within central vestibular nuclei. Studies from our laboratory using labyrinthectomized rats as well as current studies using mutant mice which lack critical components of the external vestibular apparatus suggest that the inner ear is necessary for magnetic sensitivity. In particular, otoconia-deficient head-tilt and tilted-head mutant mice do not appear to respond to high magnetic field exposure, suggesting that the normal otolith function is critical. The magnetic field could also be acting on other sensory systems including the visual and proprioceptive systems that provide convergent information to the central vestibular network to induce the changes observed in c-Fos expression. Visual and proprioceptive sensory inputs could be removed to test this possibility.
Estradiol could be modulating the effect of magnetic field exposure on c-Fos expression at the level of the external vestibular apparatus, within the central vestibular network, or in other areas of the central network that send information to the central vestibular system. In order for estradiol to modulate any of these effects, estrogen receptors (ER) would need to be localized within one of the aforementioned sites. ER and immunoreactivity has been reported in the nuclei of inner and outer hair cells of mouse and rat, suggesting that estrogens might affect the inner ear (Stenberg et al., 1999), but there has been little characterization of the ER in the rat brainstem. Several studies have demonstrated that the ER is present within the rat brainstem (Creutz and Kritzer, 2002; Curran-Rauhut and Petersen, 2002; Haywood et al., 1999; Lee et al., 2000; Lu et al., 2001; Reyes et al., 2001; Simerly et al., 1990; Spary et al., 2009; Tsukahara and Yamanouchi, 2002), but no precise work has been done to characterize estrogen receptors throughout the central vestibular relays of the brainstem network.
In conclusion, these results extend our observation of sex differences in magnetic field responses at the behavioral level to the level of neural responses in the brainstem of the rat. Sex differences in magnetic field responses in rats may be relevant to the vestibular responses of women. Although there is no research demonstrating human sex differences in responses to high-strength magnetic field exposure, there is some evidence that women are more sensitive than men to other types of vestibular stimulation. Women are more prone to orthostatic intolerance (Fritsch-Yelle et al., 1996; Ray, 2000; Robertson, 1999) and motion sickness than men (Darlington and Smith, 1998; Park and Hu, 1999). Furthermore, symptoms of vestibular disturbance have been demonstrated to depend on ovarian hormone status in women. Women report a higher level of vertigo and dizziness during the premenstrual period (Abdel Nabi et al., 1984). Thus, as magnetic fields in clinical MRI machines increase in strength, it is possible that women might experience more vestibular side effects than men.
Methods
Subjects
Female Sprague Dawley rats (n=23, 200–225 g; Charles River Laboratories, Wilmington, MA) were housed individually in polycarbonate cages in a temperature-controlled colony room at the National High Magnetic Field Laboratory at The Florida State University. The rats were maintained on a 12-h light/dark cycle with lights-on at 7:00 am. All procedures were conducted during the light cycle. The rats had ad libitum access to Purina Rat Chow and deionized-distilled water. All procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
Surgical Procedures
Bilateral ovariectomy was performed through an intra-abdominal approach under halothane anesthesia in 23 female rats. A midline 2-cm incision was made and the uterine horns externalized and clamped with a hemostat below the fallopian tubes. The ovaries were excised by scalpel, and the incision sutured closed. During the same surgery, silastic capsules containing crystalline estradiol (E) were inserted subcutaneously in the neck of 11 rats (OVX+E rats). The E capsules were 5 mm in length with i.d. 1.57 mm and o.d. 2.41 mm. Identical implants produced serum E concentrations of 157±80 pg/ml and P concentrations of 6±1 ng/ml in previous studies using rats of the same strain and size (Gerhold et al., 2001). The remaining 12 ovariectomized rats did not receive silastic capsules (OVX rats). At 7 days after surgery, OVX rats had gained significantly more weight (+40.0 ± 9.3 g) than OVX+E rats (+ 11.5 ± 6.0 g; t(22) = 8.8, p < 0.001), thus verifying activity of the estradiol implant. Rats were tested 14 days after surgery.
Magnet
Magnetic field exposure was conducted in a superconducting magnet with a vertical bore designed for biochemical nuclear magnetic resonance (NMR) studies. The 14T magnet was a 600Mhz Bruker Cryo magnet with an 89mm bore and fixed field strength of 14.1 T. It contained a shim magnet extending along the magnet bore for approximately ±15 cm from the magnet core, which was used to stabilize the magnetic field and give a central core field of uniform strength. The magnetic field was oriented vertically so that the positive pole was at the top of the magnet. The magnet was operated without radiofrequency pulses, so rats were exposed only to static magnet fields.
Magnetic field and sham-exposure
Prior to magnetic field or sham exposure, rats were placed in a Plexiglas restraint tube that had an inside diameter of 56 mm and an outside diameter of 64 mm. A cone shaped plug with a 1cm hole at the apex was inserted in the rostral end of the restraint tube to accommodate the head of the rat and to allow fresh air for breathing. A second plug was inserted in the caudal end of the restraint tube and could be adjusted to restrain the movement of the rat. It had a 1 cm hole in the center to accommodate the rat’s tail. When in the tube, the rat was almost completely immobile. Restrained animals were carried individually to the 14 T magnet where the rat was inserted head-up into the bottom of the vertical bore of the magnet. The rat was quickly raised through the magnet until the head of the rat was in the core of the magnetic field. Rats remained in the 14 T magnetic field for 30 min (magnet-exposed rats; n=6, OVX; n=6, OVX+E)
To control for restraint and handling, additional rats were sham-exposed. Sham-exposed rats (n=6, OVX, n=5, OVX+E) were inserted into identical restraint tubes. Then, the sham-exposed rats were inserted vertically into an opaque polyvinylchloride pipe with dimensions and conditions similar to those of the bore of the 14 T magnet. The “sham magnet” was located in the same room as the 14 T magnet, but placed outside the 5 gauss field.
One magnet-exposed and one sham-exposed rats were exposed simultaneously. In order to allow time for tissue collection, rats were exposed individually at 30-min intervals, alternating between magnetic field and sham exposures.
Locomotor activity
Following magnetic field or sham exposure, the rostral plug of the restraint tube was removed; and the rat was allowed to emerge into an open polycarbonate cage (37cm wide × 47cm long × 20cm high). The floor of the cage was covered with chip bedding. The locomotor behavior of each rat was recorded on videotape for 2 min after release into the cage. Then, the rat was returned to its home cage and left undisturbed until perfusion. An observer blind to the rats’ treatment scored the videotapes later. Instances of tight circling behavior and rearing behavior (both forepaws on the side of the cage) were quantified. Circles were counted if the rat moved continuously around a full circle with a diameter less than the length of the rats’ body. Partial circles or circles interrupted by stationary pauses were not counted.
Tissue Processing
One hour following magnetic field exposure or sham restraint, rats were overdosed with sodium pentobarbital. Once completely unresponsive, rats were transcardially perfused, first with 100ml heparinized isotonic saline containing 0.5% sodium nitrite, then with 400 ml 4% paraformaldehyde in 0.1M sodium phosphate buffer. Brains were, removed, blocked and post-fixed for 2h and transferred into 30% sucrose for cryoprotection. The right side of each brain was marked with a hypodermic needle puncture hole. Coronal sections were cut at 40 μm on a freezing, sliding microtome. Sections were cut through the medulla from the caudal subpostremal end of the nucleus of the solitary tract (NTS; bregma –13.8mm) to the rostral extent of the medial vestibular nucleus (MeV; bregma –11.0mm). In addition, sections were cut from the pons from the caudal supragenualis nucleus (SGe; bregma –10.52mm), through the locus coeruleus (LC) to the rostral tip of the lateral parabrachial nucleus (lPBN; bregma –9.3mm; coordinates from Paxinos and Watson).
Alternative tissue sections were processed for c-Fos immunohistochemistry. Free-floating sections were washed twice in 0.1M sodium phosphate-buffered saline (PBS), then permeabilized in 0.2% Triton, 1% bovine serum albumin (BSA) in PBS for 30 min. After two washes in PBS-BSA, sections were incubated overnight with a rabbit anti-c-Fos polyclonal antiserum raised against human c-Fos residues 4-17 (Oncogene Sciences Ab-5, 1:20,000 dilution). After incubation for 1 h with a biotinylated anti-rabbit goat antibody bound secondary antibody was amplified with a Vector Elite ABC kit. Antibody complexes were visualized by a 5-min reaction with diaminobenzidine.
c-Fos data analysis
Images of brain regions (720 × 540 μm, 1.1 pixels/μm) were digitized with a MTI CCD72S grayscale camera mounted on an Olympus AX70 microscope. Cells expressing positive c-Fos immunohistochemistry were counted automatically by a Macintosh image analysis program (MindsEye). Cells with dark nuclear staining were automatically detected and counted by the software across each image, based on the relative pixel darkness and circular symmetry of the nuclei relative to surrounding background tissue in the digitized image. To insure consistent criteria for the automatic counting, the same threshold parameters were used for all images from all treatments.
Cells were counted in several visceral nuclei, including NTS (mean of 11 sections per rat), LC (3 sections), and lPBN (8 sections). Stained cells were also quantified in the MeV (20 sections per rat), nucleus prepositus (Prp; 20 sections), nucleus supragenualis (SGe; 9 sections) and superior vestibular nucleus (SuV; 9 sections). Unilateral cell counts were then averaged for each rat; the mean counts were then averaged across rats in each experimental group.
Statistical Analysis
Data are presented as mean +/− standard error of the mean. A t-test was calculated to compare the number of circles and latency to circle in OVX and OVX+E rats following 30 min magnetic field-exposure. A 2-way ANOVA was calculated to determine differences in rearing behavior and c-Fos expression using exposure (magnet or sham) and group (OVX or OVX+E) as the main factors. When ANOVAs were significant, Fisher’s LSD post-hoc analyses were performed.
Asymmetries between the left and right sides of the brainstem were detected by paired samples t-test of individual brain regions. Significant differences between magnet-exposed groups in asymmetrical c-Fos induction were detected by comparing the ratio of right to left side cell counts by students t-test for each brain region. Pearson’s correlation coefficient was calculated to determine correlations between c-Fos induction and latency to circle or number of circles across magnet-exposed groups.
Abbreviations
- ANOVA
analysis of variance
- AP
area postrema
- BSA
bovine serum albumin
- cc
central canal
- CTA
conditioned taste aversion
- ER
estrogen receptor
- IV
fourth ventricle
- LC
locus coeruleus
- lPBN
lateral parabrachial nucleus
- MeV
medial vestibular nucleus
- MRI
magnetic resonance imaging
- NMR
nuclear magnetic resonance
- NTS
nucleus of the solitary tract
- OVX
ovariectomized
- E
estradiol
- PBN
parabrachial nucleus
- PBS
phosphate-buffered saline
- Prp
prepositus nucleus
- SGe
supragenualis nucleus
- SuV
superior vestibular nucleus
- T
tesla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel Nabi E, Motawee E, Lasheen N, Taha A. A study of vertigo and dizziness in the premenstrual period. J Laryngol Otol. 1984;98:273–5. doi: 10.1017/s0022215100146559. [DOI] [PubMed] [Google Scholar]
- Arwas S, Rolnick A, Lubow RE. Conditioned taste aversion in humans using motion-induced sickness as the US. Behav Res Ther. 1989;27:295–301. doi: 10.1016/0005-7967(89)90049-1. [DOI] [PubMed] [Google Scholar]
- Braun JJ, McIntosh H. Learned taste aversions induced by rotational stimulation. Physiol Psychol. 1973;1:301–304. [Google Scholar]
- Cason AM, DenBleyker MD, Ferrance K, Smith JC, Houpt TA. Sex and estrous cycle differences in the behavioral effects of high-strength static magnetic fields: role of ovarian steriods. Am J Physiol. 2006;290:R659–67. doi: 10.1152/ajpregu.00305.2005. [DOI] [PubMed] [Google Scholar]
- Cason AM, Kwon BS, Smith JC, Houpt TA. Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiol Behav. 2009;97:36–43. doi: 10.1016/j.physbeh.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Cohen B, Dai M, Raphan T. The critical role of velocity storage in production of motion sickness. Ann N Y Acad Sci. 2003;1004:359–76. doi: 10.1196/annals.1303.034. [DOI] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neurosci. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Curran-Rauhut MA, Petersen SL. The distribution of progestin receptor mRNA in rat brainstem. Brain Res Gene Expr Patterns. 2002;1:151–7. doi: 10.1016/s1567-133x(02)00011-x. [DOI] [PubMed] [Google Scholar]
- Darlington CL, Smith PF. Further evidence for gender differences in circularvection. J Vestib Res. 1998;8:151–3. [PubMed] [Google Scholar]
- Eckel LA, Geary N. Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol. 2001;281:R738–46. doi: 10.1152/ajpregu.2001.281.3.R738. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Houpt TA, Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol. 2002;283:R1378–85. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- Fox RA, Lauber AH, Daunton NG, Phillips M, Diaz L. Off-vertical rotation produces conditioned taste aversion and suppressed drinking in mice. Aviat Space Environ Med. 1984;55:632–5. [PubMed] [Google Scholar]
- Fritsch-Yelle JM, Whitson PA, Bondar RL, Brown TE. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. J Appl Physiol. 1996;81:2134–41. doi: 10.1152/jappl.1996.81.5.2134. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Jones TA, Jones SM, Fuller CA. Evidence for macular gravity receptor modulation of hypothalamic, limbic and autonomic nuclei. Neurosci. 2004;129:461–71. doi: 10.1016/j.neuroscience.2004.05.059. [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Res. 2001;919:48–56. doi: 10.1016/s0006-8993(01)02993-6. [DOI] [PubMed] [Google Scholar]
- Glover PM, Cavin I, Qian W, Bowtell R, Gowland PA. Magnetic-field-induced vertigo: a theoretical and experimental investigation. Bioelectromagnetics. 2007:28. doi: 10.1002/bem.20316. [DOI] [PubMed] [Google Scholar]
- Green L, Rachlin H. The effect of rotation on the learning of taste aversions. Bull Psychon Soc. 1973;1:137–192. [Google Scholar]
- Haywood SA, Simonian SX, van der Beek EM, Bicknell RJ, Herbison AE. Fluctuating estrogen and progesterone receptor expression in brainstem norepinephrine neurons through the rat estrous cycle. Endocrinology. 1999;140:3255–63. doi: 10.1210/endo.140.7.6869. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Philopena JM, Wessel TC, Joh TH, Smith GP. Increased c-Fos expression in the rat nucleus of the solitary tract after conditioned taste aversion formation. Neurosci Lett. 1994;172:1–5. doi: 10.1016/0304-3940(94)90648-3. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Pittman DM, Barranco JM, Brooks EH, Smith JC. Behavioral effects of high strength magnetic fields on rats. J Neurosci. 2003;23:1498–505. doi: 10.1523/JNEUROSCI.23-04-01498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houpt TA, Pittman DW, Riccardi C, Cassell JA, Lockwood DR, Barranco JM, Kwon BS, Smith JC. Behavioral effects on rats of high strength magnetic fields generated by a resistive electromagnet. Physiol Behav. 2005;86:379–89. doi: 10.1016/j.physbeh.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Cassell JA, Riccardi C, DenBleyker MD, Hood A, Smith JC. Rats avoid high magnetic fields: dependence on an intact vestibular system. Physiol Behav. 2007;92:741–7. doi: 10.1016/j.physbeh.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houpt TA, Smith JC. Conditioned taste aversion induced by exposure to high-strength static magnetic fields. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; NY: 2009. pp. 422–441. [Google Scholar]
- Innis NK, Ossenkopp KP, Prato FS, Sestini E. Behavioral effects of exposure to nuclear magnetic resonance imaging: II. Spatial memory tests. Magn Reson Imaging. 1986;4:281–4. doi: 10.1016/0730-725x(86)91037-4. [DOI] [PubMed] [Google Scholar]
- Kangarlu A, Burgess RE, Zhu H, Nakayama T, Hamlin RL, Abduljalil AM, Robitaille PML. Cognitive, cardiac, and physiological safety studies in ultra high field magnetic resonance imaging. Magn Reson Imag. 1999;17:1407–1416. doi: 10.1016/s0730-725x(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Kaufman GD, Anderson JH, Beitz A. Activation of a specific vestibulo-olivary pathway by centripetal acceleration in rat. Brain Res. 1991;562:311–317. doi: 10.1016/0006-8993(91)90637-b. [DOI] [PubMed] [Google Scholar]
- Kaufman GD, Anderson JH, Beitz AJ. Fos-defined activity in rat brainstem following centripetal acceleration. J Neurosci. 1992;12:4489–4500. doi: 10.1523/JNEUROSCI.12-11-04489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman GD, Anderson JH, Beitz AJ. Otolith-brain stem connectivity: evidence for differential neural activation by vestibular hair cells based on quantification of Fos expression in unilateral labyrinthectomized rats. J Neurophysiol. 1993;70:117–127. doi: 10.1152/jn.1993.70.1.117. [DOI] [PubMed] [Google Scholar]
- Kaufman GF, Shinder ME, Perachio AA. Correlation of Fos expression and circling asymmetry during gerbil vestibular compensation. Brain Res. 1999;817:246–255. doi: 10.1016/s0006-8993(98)01284-0. [DOI] [PubMed] [Google Scholar]
- Kim MS, Jin BK, Chun SW, Lee MY, Lee SH, Kim JH, Park BR. Effect of MK801 on cFos-like protein expression in the medial vestibular nucleus at early stage of vestibular compensation in uvulonodullectomized rats. Neurosci Lttrs. 1997;231:147–150. doi: 10.1016/s0304-3940(97)00550-8. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kim JH, Jin YZ, Kry D, Park BR. Temporal changes of cFos-like protein expression in medial vestibular nuclei following arsanilate-induced unilateral labyrinthectomy in rats. Neurosci Lett. 2002;319:9–12. doi: 10.1016/s0304-3940(01)02422-3. [DOI] [PubMed] [Google Scholar]
- Kitahara T, Saika T, Takeda N, Kiyama H, Kubo T. Changes in fos and jun expression in the rat brainstem in the process of vestibular compensation. Acta Otolaryngol. 1995;520:S401–S404. doi: 10.3109/00016489509125282. [DOI] [PubMed] [Google Scholar]
- Koh MT, Wilkins EE, Bernstein IL. Novel tastes elevate c-fos expression in the central amygdala and insular cortex: implication for taste aversion learning. Behav Neurosci. 2003;117:1416–1422. doi: 10.1037/0735-7044.117.6.1416. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Moore CT, Hosny S, Centers A, Jennes L. Expression of estrogen receptor-alpha and c-Fos in adrenergic neurons of the female rat during the steroid-induced LH surge. Brain Res. 2000;875:56–65. doi: 10.1016/s0006-8993(00)02622-6. [DOI] [PubMed] [Google Scholar]
- Lockwood DR, Kwon BS, Smith JC, Houpt TA. Behavioral effects of high strength static magnetic fields on restrained and unrestrained mice. Physiol Behav. 2003;78:635–40. doi: 10.1016/s0031-9384(03)00040-4. [DOI] [PubMed] [Google Scholar]
- Lu H, Ozawa H, Nishi M, Ito T, Kawata M. Serotonergic neurones in the dorsal raphe nucleus that project into the medial preoptic area contain oestrogen receptor beta. J Neuroendocrinol. 2001;13:839–45. doi: 10.1046/j.1365-2826.2001.00695.x. [DOI] [PubMed] [Google Scholar]
- Marshburn TH, Kaufman GD, Purcell IM, Perachio AA. Saccule contribution to immediate early gene induction in the gerbil brainstem with posterior canal galvanic or hypergravity stimulation. Brain Res. 1997;761:51–58. doi: 10.1016/s0006-8993(97)00030-9. [DOI] [PubMed] [Google Scholar]
- Messmer JM, Porter JH, Fatouros P, Prasad U, Weisberg M. Exposure to magnetic resonance imaging does not produce taste aversion in rats. Physiol Behav. 1987;40:259–261. doi: 10.1016/0031-9384(87)90217-4. [DOI] [PubMed] [Google Scholar]
- Nolte CM, Pittman DW, Kalevitch B, Henderson R, Smith JC. Magnetic field conditioned taste aversion in rats. Physiol Behav. 1998;63:683–688. doi: 10.1016/s0031-9384(97)00526-x. [DOI] [PubMed] [Google Scholar]
- Ossenkopp KP, Innis NK, Prato FS, Sestini E. Behavioral effects of exposure to nuclear magnetic resonance imaging: I. Open-field behavior and passive avoidance learning in rats. Magn Reson Imaging. 1986;4:275–80. doi: 10.1016/0730-725x(86)91036-2. [DOI] [PubMed] [Google Scholar]
- Ossenkopp KP, Rabi YJ, Eckel LA, Hargreaves EL. Reductions in body temperature and spontaneous activity in rats exposed to horizontal rotation: abolition following chemical labyrinthectomy. Physiol Behav. 1994;56:319–324. doi: 10.1016/0031-9384(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Richtand NM, Herman JP. Stress and amphetamine induce Fos expression in medial prefrontal cortex neurons containing glucocorticoid receptor. Brain Res. 2003;990:209–14. doi: 10.1016/j.brainres.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Park AH, Hu S. Gender differences in motion sickness history and susceptibility to optokinetic rotation-induced motion sickness. Aviat Space Environ Med. 1999;70:1077–80. [PubMed] [Google Scholar]
- Patel M, Williamsom RA, Dorevitch S, Buchanan S. Pilot study investigating the effect of the static magnetic field from a 9.4-T MRI on the vestibular system. J Occup Environ Med. 2008;50:576–583. doi: 10.1097/JOM.0b013e318162f5d6. [DOI] [PubMed] [Google Scholar]
- Ray CA. Effect of gender on vestibular sympathoexcitation. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1330–3. doi: 10.1152/ajpregu.2000.279.4.R1330. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Estacio MA, I’Anson H, Tsukamura H, Maeda KI. Glucoprivation increases estrogen receptor alpha immunoreactivity in the brain catecholaminergic neurons in ovariectomized rats. Neurosci Lett. 2001;299:109–12. doi: 10.1016/s0304-3940(01)01490-2. [DOI] [PubMed] [Google Scholar]
- Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–7. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Schenck JF. Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Ann NY Acad Sci. 1992;649:285–301. doi: 10.1111/j.1749-6632.1992.tb49617.x. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Pachman SH, Heffner TG, Shaughnessy RA, Vosmer G. The effect of water-deprivation on locomotor activity in rats treated with 6-hydroxydopamine. Brain Res. 1985;337:225–32. doi: 10.1016/0006-8993(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Snyder D, Jahng JW, Smith JC, Houpt TA. c-Fos induction in visceral and vestibular nuclei of the rat brainstem by a 9.4 T magnetic field. NeuroReport. 2000;11:1681–5. doi: 10.1097/00001756-200008210-00015. [DOI] [PubMed] [Google Scholar]
- Spary EJ, Maqbool A, Batten TF. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat. 2009;38:185–96. doi: 10.1016/j.jchemneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Houpt TA. Dynamics of c-Fos and ICER mRNA expression in rat forebrain following lithium chloride injection. Molec Brain Res. 2001;93:113–126. doi: 10.1016/s0169-328x(01)00173-5. [DOI] [PubMed] [Google Scholar]
- Stenberg AE, Wang H, Sahlin L, MH Mapping of estrogen receptors alpha and beta in the inner ear of mouse and rat. Hear Res. 1999;136:29–34. doi: 10.1016/s0378-5955(99)00098-2. [DOI] [PubMed] [Google Scholar]
- Theysohn JM, Maderwald S, Kraff O, Moenninghoff C, Ladd ME, Ladd SC. Subjective acceptance of 7 Tesla MRI for human imaging. MAGMA. 2008;21:63–72. doi: 10.1007/s10334-007-0095-x. [DOI] [PubMed] [Google Scholar]
- Tsukahara S, Yamanouchi K. Sex difference in septal neurons projecting axons to midbrain central gray in rats: a combined double retrograde tracing and ER-immunohistochemical study. Endocrinol. 2002;143:285–94. doi: 10.1210/endo.143.1.8588. [DOI] [PubMed] [Google Scholar]
- Winther FØ, Rasmussen K, Tvete O, Halvorsen U, Haugsdal B. Static magnetic field and the inner ear: a functional study of hearing and vestibular function in man after exposure. Scand Audiol. 1999;28:57–59. doi: 10.1080/010503999424914. [DOI] [PubMed] [Google Scholar]