Abstract
Glomerulonephritides represent a diverse array of diseases that have in common immune cell-mediated effector mechanisms that cause organ damage. The contribution of neutrophils to the pathogenesis of proliferative glomerulonephritis (GN) is not well recognized. Most equate neutrophils with killing pathogens and causing collateral tissue damage during acute inflammation. However, these phagocytes are endowed with additional characteristics that have been traditionally reserved for cells of the adaptive immune system. They communicate with other cells, exhibit plasticity in their responses and have the potential to coordinate and inform the subsequent immune response, thus countering the notion that they arrive, destroy and then disappear. Therefore, neutrophils, which are the first to arrive at a site of inflammation, are potential game changers in GN.
Introduction
Glomerulonephritides collectively are the third leading cause of end-stage renal disease in the USA after diabetes and hypertension (http://www.usrds.org/adr.htm), and increased in prevalence by 55% between 1990 and 2001 to over 50,000 existing cases [1]. Despite this, treatments for these diseases are largely nonspecific (e.g. immunosuppressive or cytotoxic) and have moderate efficacy [1]. Glomerulonephritis (GN) can be categorized into two groups: proliferative forms associated with increased glomerular cellularity resulting from immune cell influx and the proliferation of intrinsic cells, and nonproliferative forms. At several levels, leukocytes are central players in the progression of proliferative GN, from influencing the development of the humoral and adaptive immune responses to affecting local effector mechanisms directly responsible for glomerular damage. Several excellent reviews have focused on the role of T and B cells as well as macrophages in proliferative GN [2–5]; here, we focus on neutrophils. Neutrophils are well-established players in host defense and acute inflammation. However, owing to the fact that they are short-lived and terminally differentiated cells with no immunological memory, their participation in chronic disease has been largely neglected. This is despite the fact that in partnership with the adaptive immune response, neutrophils have the potential to coordinate every stage of inflammation from induction to resolution and tissue repair.
Clinical examples of GN associated with glomerular neutrophil accumulation
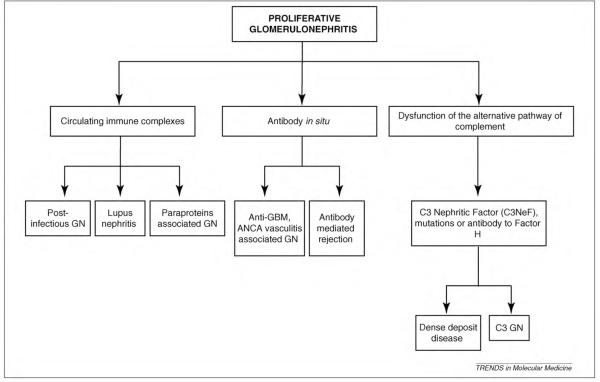
The term “proliferative” is classically used in the histo-pathological description and classification of GN and refers to glomerular hypercellularity often resulting from an increase in leukocyte accumulation. A summary of proliferative GN is presented in Figure 1. Neutrophil accumulation is observed with proliferative GN in many clinical settings. These include proliferative GN owing to the deposition of circulating immune complexes (i.e. in infections, autoimmune disease and paraproteinemia), the generation of immune complexes in situ (i.e. antiglomerular basement membrane antibodies – anti-GBM, antineutrophil cytoplasmic antibodies – ANCA, antibody-mediated rejection in a transplant) or complement dysfunction (Figure 1) [6]. Studies have documented neutrophil infiltration in renal biopsies from patients with membranoproliferative GN (MPGN), lupus and crescentic GN [7–9] that coincide with strong macrophage infiltration [8]. The ongoing presence of neutrophils in these chronic renal diseases strengthens the notion that these cells play significant roles in progression of not only acute but also chronic GN. Specific examples of human GN associated with neutrophil infiltration are discussed below.
Figure 1.
Proliferative GN. Summary of proliferative GN subtypes categorized by the primary mechanism underlying disease.
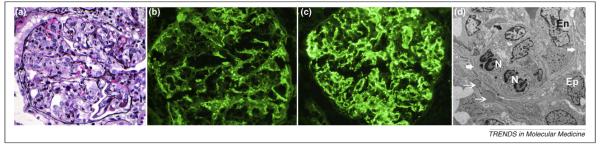
Postinfectious GN is the prototypic diffuse proliferative GN typically seen following bacterial infection, such as streptococcal throat infection, but can occur in the setting of other bacterial, viral, fungal or protozoal infections [10]. Immune complex deposition along the glomerular capillary walls resulting in prominent endocapillary proliferation associates with extensive neutrophil infiltration within the glomerular capillaries [6]. Immunofluorescence studies demonstrate immunoglobulins, usually IgG and C3, in a granular appearance along the capillary walls. Electron microscopy shows subendothelial electron-dense deposits along with characteristic dome-shaped subepithelial electron-dense deposits termed “humps”, which aid the diagnosis of postinfectious proliferative GN [11] (Figure 2).
Figure 2.
Representative figures of a case of postinfectious diffuse proliferative glomerulitis. (a) Histology showing marked endocapillary proliferation with numerous neutrophils. (b, c) Immunofluorescence microscopy showing capillary wall staining for (b) granular IgG and (c) C3. (d) Electron microscopy showing subepithelial deposits “humps” (thin white arrows) and infiltrating neutrophils (N). Glomerular basement membrane is marked by thick white arrows. Abbreviations: Ep– visceral epithelial cell or podocytes and En– endothelial cell.
Infiltrating mononuclear leukocytes and neutrophils also contribute to the glomerular hypercellularity of MPGN (characterized by diffuse mesangial cell proliferation), for instance secondary to systemic immune complex diseases (such as systemic lupus erythematosus, SLE; or mixed cryoglobulinemia, see Glossary) or in the context of infections (hepatitis B and hepatitis C) [12]. The sustained production of nephritogenic immune complexes and their specific deposition in the mesangium and the subendothelial space triggers complement activation and the recruitment of effector immune cells including neutrophils [13]. Finally, although not immune complex or antibody mediated, proliferative GN can also result from dysfunction of the alternative pathway (AP) of complement. The AP is constitutively active at low levels. However, progression of the cascade is strictly controlled at each level by multiple complement regulators and inhibitors such as Factor H. In some types of MPGN, glomerular inflammation is induced by the uncontrolled activation of the alternative complement pathway, owing to autoantibody directed against the C3 convertase (C3 nephritic factor, C3NeF) that prevents degradation of the convertase, or a genetic defect or a functional deficiency of Factor H. Complement proteins accumulate along the capillary walls. This is observed as dense deposit by electron microscopy and is, therefore, referred to as the “dense deposit disease.” If immuno-type deposits are composed of only C3, it is referred to as C3 GN. Proliferative GN develops in both cases and is associated with neutrophil influx [14–16].
Rapidly progressive GN (RPGN) (with severe acute renal failure developing within a few days) can have many causes, but often results from autoantibody-mediated injury and typically occurs in two settings: autoantibodies against the GBM or autoantibodies directed against ANCA (antiproteinase 3 – anti-PR-3 or antimyeloperoxidase – anti-MPO) or another neutrophil antigen, Lamp2 [17–19]. Human lysosomal-associated membrane protein 2 (Lamp-2) is an autoantigenic target in patients with active necrotizing and crescentic ANCA GN. Lamp-2 is located on the membranes of neutrophil intracellular vesicles that contain PR-3 and MPO and on the surface of endothelial cells [20]. In both anti-GBM and ANCA associated RPGN, the GN is very aggressive and results in necrotizing and crescentic GN, often with neutrophil infiltration into the glomerular capillaries, crescent formation and areas of necrosis. Similarly, in kidney transplantation, antibodies to human leukocyte antigen can result in acute (and chronic) antibody-mediated graft rejection characterized by neutrophil infiltration into the glomerular capillaries (glomerulitis) and peritubular capillaries (capillaritis) with endothelial injury. Antibody-mediated injury associates with the peritubular capillaries of the graft staining positive for C4d, a degradation product of complement component 4 [21]. A summary of renal biopsy findings of proliferative GN is presented in Table 1.
Table 1.
Renal biopsy findings in proliferative GN
| Type of proliferative GN | Light microscopy | Immunofluorescence microscopy | Electron microscopy (electron-dense deposits) |
|---|---|---|---|
| Postinfectious GN | Diffuse proliferative GN, 3+ neutrophils in gc |
IgG, occasionally IgA or IgM, C3, granular, CW |
Subendothelial deposits, subepithelial humps |
| Lupus nephritis (class III/IV) | Focal or diffuse proliferative GN, 1–3+ neutrophils in gc |
IgG, IgA, IgM, C1q, C3, granular, CW and mesangial |
Subendothelial and mesangial deposits |
| Paraproteins/monoclonal gammopathy |
Focal or diffuse proliferative GN, 1–3+ neutrophils in gc |
IgG, IgA, or IgM with κ or λ light chain restriction, or light chains only, CW |
Subendothelial and mesangial deposits |
| Rapidly progressive GN | Crescentic and necrotizing GN, 1–3+ neutrophils in gc, in crescents and necrotizing lesions |
IgG linear staining along CW in anti-GBM GN, negative in ANCA vasculitis |
No deposits in anti-GBM or ANCA-associated GN |
| Antibody-mediated rejection | Glomerulitis with neutrophils, 1–3+ neutrophils in gc |
Negative, positive C4d staining of post-transplant capillaries |
Negative for immune deposits |
| Alternative complement pathway dysfunction |
Proliferative GN, 1–3+ neutrophils in gc |
C3, granular, CW negative for Ig | Dense deposits of complement proteins in mesangium and along CW |
Abbreviations: gc, glomerular capillaries; CW, capillary wall.
Scoring: 1+ defined as neutrophils in less than 25% of gc, 2+ as neutrophils involving up to 50% gc and 3+ as neutrophils involving more than 50% of gc.
Despite consistent reports of neutrophil infiltration in human GN, their involvement in disease pathogenesis remains speculative in most cases. An exception is ANCA GN wherein neutrophils are the targets of antibodies that recognize neutrophil antigens. ANCA bind MPO and PR-3 expressed on the surface of activated neutrophils. This in turn induces cytoskeletal rigidity and intracellular signaling, which promotes degranulation and the release of chemoattractants and cytotoxic reactive oxygen species (ROS) that together cause tissue damage [22,23]. ANCA-induced GN is associated with a paucity of immunoglobulin in glomerular capillaries and vessel walls and is frequently referred to as pauci-immune GN. The number of activated neutrophils in renal biopsies from patients with ANCA-associated vasculitis correlates with the ANCA titer and the renal damage [24]. Moreover, the level of neutrophil expression of membrane PR-3 in patients with Wegener granulomatosis as well as the anti-PR-3 titer might correlate with the risk of relapse of the disease [25].
Mouse models of GN: leading roles for neutrophils
Studies in genetically engineered mice in combination with neutrophil-depletion approaches and GN models developed in mice have accelerated our understanding of the role of neutrophils, their receptors and associated molecules in GN. Neutrophils play leading roles in the pathogenesis of anti-GBM thrombotic microangiopathies and ANCA-induced GN, which are relatively acute models, as well as accelerated nephrotoxic nephritis, lupus nephritis and MPGN, which are more chronic models of GN. It is noteworthy that the relative contribution of neutrophils compared to other leukocyte subsets in murine models probably underestimates their involvement in human GN as the median value of circulating neutrophil counts in humans is approximately 3- to 3.5-fold higher than in mice [26].
Anti-GBM GN in mice induced by injection of nephrotoxic serum is an acute, rapidly resolving nephritis that recapitulates aspects of post-streptococcal GN in humans. Glomerular neutrophil accumulation is observed early in the course of disease and is well-recognized as the effector cell responsible for the observed proteinuria [27,28]. Thrombotic GN in mice, induced by the sequential injection of lipopolysaccharide (LPS) and anti-GBM, resembles thrombotic microangiopathies, recapitulate features observed in clinical settings including thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Microangiopathies in humans are also present secondary to malignant hypertension, antiphospholipid syndrome (frequently associated with SLE), some cancers, therapeutic drug use (e.g. immunosuppressive agents) or infection (e.g. HIV) [29]. In a murine model of thrombotic GN, neutrophils are essential for glomerular damage and the accompanying thrombosis [30]. ANCA GN can be partially reproduced in mice by inducing an immune reaction (antibody or cell mediated) to MPO. Several studies in these animal models demonstrate that neutrophils are important effectors of ANCA GN [20,31,32].
In contrast to these relatively acute models, the role of neutrophils in more chronic models that require an adaptive immune response is less well understood. A murine model of nephrotoxic serum nephritis, induced by preimmunizing mice with IgG followed by the injection of nephrotoxic serum, replicates aspects of MPGN. The selective expression of human FcγRs (receptors for complexed IgG) on neutrophils is sufficient to promote susceptibility to proliferative GN. Notably, neutrophil influx precedes the accumulation of macrophages [33], which play a key role in development of crescentic GN [34].
Several murine models of SLE that spontaneously develop GN have been used to understand effector mechanisms of lupus nephritis [35]. In particular, the MRL/lpr strain of mice spontaneously develops a diffuse proliferative GN associated with immune complex deposition and infiltration of neutrophils, T cells and monocytes in the glomerulus [36]. There is indirect evidence that neutrophils promote lupus nephritis. Blockade of C5a, a powerful neutrophil and monocyte chemoattractant, decreases the influx of these cells and prevents the development of kidney injury [37]. Moreover, double-negative T cells (CD4-, CD8-) in MRl/lpr mice produce high amounts of interleukin 17 (IL-17), a potent proinflammatory cytokine that functions mainly by recruiting neutrophils to sites of inflammation. Indeed, these T cells infiltrate the kidney and upon adoptive transfer can cause nephritis in a non-autoimmune mouse model [38]. Finally, MRL/lpr mice with a genetic deficiency in leukocyte adhesion molecules ICAM-1, LFA-1 or all CD18 integrins exhibit a prolonged survival and reduced severity of GN as compared to their wild-type MRL/lpr counterparts; these outcomes correlate with decreased glomerular neutrophil accumulation[39,40]. Although these adhesion molecules would be expected to directly mediate neutrophil migration, the phenotype in the knockout mice could also be attributed to decreased autoantibody production [39,40].
Uncontrolled complement activation associates with the development of MPGN. Similar to observations in patients, Factor H deficiency in mice results in unhindered C3 activation and the spontaneous development of MPGN over a period of 12 months with C3 and C9 depositing within the renal capillary wall [41]. Glomerular neutrophil accumulation is observed in mice coincident with disease development and C5 is implicated in neutrophil accumulation and the induction of renal lesions [42]. The effects of C5 might be direct or they might be indirect, as C5a and/or membrane attack complex formation at sublytic concentrations activates the endothelium and stimulates chemokine IL-8 and MCP-1 production [43,44]. MPGN can also be induced in mice by the intraperitoneal injection of murine hybridoma cells producing a cryoglobulinemic IgG3 anti-IgG2a. The severe GN reported in these mice associates with extensive glomerular neutrophil infiltration [45] and neutrophil immunodepletion prevents development of GN [46].
Mobilization of foot soldiers
Neutrophils are the first leukocyte subset to arrive at a site of inflammation and are recruited via a multistep process. The sequence of events in nonantibody-mediated inflammation is well known. Cells tether and roll on the activated endothelium in postcapillary venules. In response to inflammatory mediators that upregulate integrin activity, the rolling velocity slows, cells adhere and crawl on the endothelium and then transmigrate either through or between adjacent endothelial cells. The molecules responsible for these steps have been delineated primarily by intravital microscopy (IVM), which allows the direct visualization of the vasculature in a living animal. Rolling is promoted by selectins and their ligands, which are cell surface glycans, and in some cases α4 integrin, which interacts with VCAM-1 on the activated endothelium. The CD18 integrins and their cognate receptor on the endothelium, ICAM-1, slow the rolling velocity and promote cell adhesion and intravascular crawling. Molecules at the endothelial junctions, PECAM-1 and JAM-C, support transmigration [47]. Whether these steps are required for neutrophil recruitment in the unique fenestrated vasculature of glomerular capillaries is beginning to be elucidated. The anatomic location of glomeruli precludes analysis by conventional IVM. Experiments in a hydronephrotic kidney model, which provides the tissue transparency needed to visualize the glomerular vasculature by IVM suggests that as in other capillaries, neutrophils deform to move through the narrow space of glomerular capillaries under high pressure. In response to anti-GBM treatment, cells do not roll in the glomerular capillaries but undergo immediate arrest [48]. Although P-selectin, required for leukocyte rolling in many vasculatures is largely absent in the capillaries of a normal glomerulus [49], P-selectin on platelets deposited in the nephritic kidney provides a platform for leukocyte arrest [48]. One caveat of this model is that hydronephrosis might alter the steady state of the tissue and thus potentially alter the requirements for adhesion receptors. Multiphoton-fluorescence microscopy systems capable of collecting optical sections from deep within the normal kidney have been used for real-time analysis of kidney function, such as glomerular permeability and microvascular blood flow [50]. However, to our knowledge this method has not yet been exploited for visualizing leukocyte interactions in the glomerular vasculature.
Many glomerulonephritides result from the generation of immune complexes (ICs) in situ by the binding of IgG to glomerular planted or self antigen or via the deposition of soluble immune complexes that form in circulation (Figure 1). Thus, a fuller understanding of IgG-mediated responses is required to appreciate the molecular mechanisms recruiting neutrophils in GN. This has been partially accomplished by developing models of soluble IC deposition in the cremaster muscle, which has the requisite tissue transparency for real-time analysis of leukocyte interactions within the vasculature [33,51–53]. Classical neutrophil adhesion receptors (CD18) and endothelial ligands (P-selectin, ICAM-1), platelets and chemokines, together with components of the humoral immune system (FcγRs and complement) support neutrophil recruitment in these models; details of these studies can be found in another recent review [54]. The individual molecular requirements appear to be dictated by the location of immune complexes (i.e. primarily intravascular versus extravascular), which in turn determines the repertoire of inflammatory mediators generated and the intensity of the inflammatory response. Mechanisms of neutrophil recruitment have also been inferred by immunohistological examination of renal tissue harvested from mice subjected to GN. These studies have produced some surprises. For example, P-selectin deficiency [49,55], Mac-1 deficiency [40] or chemokine blockade [56] paradoxically adversely affect glomerular inflammation. These unexpected results are explained by different processes. P-selectin deficiency depletes soluble P-selectin, an immunomodulatory molecule that interferes with neutrophil interaction with the endothelium in nephrotoxic serum nephritis. The absence of the C–C chemokine receptor CCR5 enhances leukocyte recruitment to glomeruli following induction of nephrotoxic serum nephritis and worsens the damage by inducing increased expression of the chemokines CCL3 and CCL5 that interact with CCR1. Although Mac-1 deficiency prevents neutrophil recruitment in an acute model of anti-GBM nephritis [57], in the context of SLE [40], it enhances disease. The mechanisms that grant Mac-1 a protective role remain unclear.
ANCA-induced neutrophil recruitment has also been studied by IVM. Anti-MPO serum-induced neutrophil adhesion in the glomerulus of the hydronephrotic kidney occurred through α4 integrin [58]. In the cremaster, high doses of anti-MPO also led to α4 integrin-dependent neutrophil recruitment [58]. By contrast, lower doses of anti-MPO IgG in combination with local treatment of the cremaster muscle with cytokines or LPS resulted in neutrophil adhesion that depended on CD18 [58,59] and FcγRs [59].
In summary, leukocytes appear not to roll in glomerular capillaries and, therefore, do not require the selectins such as P-selectin in their traditional role as tethering receptors. By contrast, as shown in postcapillary venules of the nonrenal vasculature, the interaction of integrins (i.e. α4β1 and the CD18) with their adhesion molecule ligands on the activated glomerular endothelium promote leukocyte arrest. In addition, FcγRs have emerged as important regulators of leukocyte recruitment in the context of IgG deposition, as assessed by IVM and murine models of GN.
Ready to serve with an arsenal of weapons
Neutrophils, once activated, can release several inflammatory mediators, including ROS and reactive nitrogen species, proteinases, cationic proteins, lipid mediators and cytokines [60].
ROS are essential but might also promote tissue injury [60]. The amount and localization of ROS (extracellular versus intracellular) are dictated by the activating stimulus that, in turn, defines whether the ROS are cytotoxic, induce neutrophil apoptosis [61] or aid in communication between cells. With regard to the latter, ROS might modify the environment of other cells (e.g. by protein oxidation and cleavage) and/or influence intracellular signaling pathways as ROS are membrane-permeant [62]. Enhanced ROS generation in GN in humans and rodents [63] associates with the accumulation of neutrophils and macrophages [64,65], suggesting that these cell types contribute to oxidative stress. Moreover, ROS generated by FcγR-engaged neutrophils perpetuate neutrophil recruitment and glomerular injury [66]. Recent studies have highlighted the capacity of ROS to regulate mitogenic pathways in the glomerulus. For example, oxidative stress induces mesangial proliferation and promotes fibrosis[63,67,68]. Nitric oxide (NO), produced in neutrophils by inducible nitric oxide synthase (iNOS), can react with superoxide anion to generate peroxynitrate, which can modify protein function by nitrosylation. In an anti-MPO model, iNOS expression and ROS are observed in infiltrating neutrophils and monocytes and correlate with nitrotyrosine generation in these cells [64]. In a rat model of mesangial proliferative GN, iNOS strongly associates with neutrophil infiltration [69]. The role specifically of iNOS in GN has not been evaluated.
Neutrophil proteases are stored in specialized intracellular granules that have specific conditions for release, which allow a rapid and graded response to an offending stimulus. These proteases are used against microbes in neutrophil phagosomes but if a stimulus is overwhelming or if a target is too large to be engulfed (i.e. “frustrated” phagocytosis), they can be released into the extracellular space. Neutral proteases, elastases, collagenases and cathepsin G are active at extracellular neutral pH and are, therefore, particularly damaging to tissue: they can cause structural damage to components of the GBM and amplify the local inflammatory response. Alpha-1 proteinase inhibitor protects tissue from inflammatory cell proteases especially elastase. Oxidation of specific methionines of α1PI by oxidative agents (that might include ROS released by neutrophils) and its cleavage by neutrophil-derived metalloproteinases (MMPs) reduce the elastase inhibitory capacity of α1PI. Thus, neutrophil activity might contribute to the glomerular damage by triggering an imbalance in the elastase to α1PI ratio [70].
Elastase influences both thrombosis and inflammation. It can activate platelets through limited proteolytic cleavage of GpIIb/IIIa [71], degrade collagen and laminin to generate fragments with high neutrophil–chemoattractant potential [72] and can remain bound to the cell surface to directly serve as a ligand for the leukocyte adhesion receptor Mac-1 [73]. In a murine model of thrombotic GN, elastase and Mac-1 are required for neutrophil accumulation [30]. In other models, elastase affects the filtration barrier downstream of neutrophil recruitment [74]. Consistent with this, human elastase perfused into the glomeruli of animals produces a dose-dependent induction of proteinuria in parallel with a reduction in heparan sulfate proteoglycans. This perturbs the anionic charge and antithrombotic properties of the capillary permeability barrier [22].
PR-3 (another neutral proteinase) and MPO, apart from being targets of ANCA also manifest proinflammatory functions. PR-3 proteolytically modifies chemokines, thus enhancing their activity (i.e. cleavage of IL-8 increases its neutrophil chemoattractant potential) and induces the production of inflammatory cytokines by monocytes (tumor necrosis factor-α-TNFα- and IL-1) [75]. Although in vivo confirmation of these activities has yet to be reported. In the presence of hydrogen peroxide, MPO forms highly reactive intermediates including hypochlorous acid that have profound biological properties [76]. These intermediates can induce permeability in the isolated glomerulus [77] and facilitate the activation of polymorphonuclear neutrophil (PMN)-derived MMP9 (type IV collagenase) [78], thus potentially rendering the GBM more susceptible to degradation. In vivo, MPO-deficient mice initially exhibit reduced glomerular damage in a neutrophil-mediated model of anti-GBM GN despite exacerbation of neutrophil recruitment. However, unexpectedly, in the autologous (Th1 T cell macrophage-mediated) phase of the disease, MPO deficiency increases the adaptive immune response [79]. These studies highlight the multiple roles of MPO in the immune response [76]. As MPO is present in both neutrophils and monocyte/macrophages, the relative contribution of MPO from each of these cell types to the observed phenotypes in GN models requires further study.
Neutrophils in areas of inflammation also engage in biosynthesis of lipid mediators such as arachidonate derived prostaglandins and leukotriene B4 (LTB4) through pathways involving cyclooxygenases and lipooxygenases. As the inflammation resolves, neutrophils can generate proresolving lipid mediators by switching biosynthesis from one class of lipid mediator to another (i.e. from LTB4 to lipoxins). Lipoxins, including aspirin triggered lipoxins then promote the resolution of inflammation by inhibiting further neutrophil recruitment, reducing vascular permeability and promoting the recruitment of monocytes [80]. With regard to GNs, lipoxins reduce leukocyte rolling and adhesion and decrease neutrophil recruitment [81].
In summary, the contents of neutrophil intracellular granules together with ROS and lipid products released by activated neutrophils clearly play major cytotoxic and regulatory roles in tissue injury. Identification of the mechanisms of actions of neutrophil derived inflammatory mediators and the upstream receptors that trigger their generation are being delineated in vitro. However, for the most part, the relative in vivo relevance of these mechanisms in glomerular inflammation remains to be demonstrated.
Orchestrators of leukocyte influx
PMNs synthesize, store and release chemokines and cytokines that influence the recruitment of leukocytes. The storage and regulated release of specific chemokine receptors from intracellular granules to the cell surface [82] allows a neutrophil to fine-tune its response to chemokine gradients. A given inflammatory stimulus dictates the magnitude and pattern of cytokine release [83]. These properties endow neutrophils with the ability to tightly regulate inflammation by tailoring the leukocytic infiltrate to cues encountered in the inflamed tissue. Although per cell neutrophils produce fewer chemokines than their mononuclear cell counterparts, they far outnumber monocytes/macrophages and, therefore, their overall impact is probably as powerful [84]. The capacity of neutrophil-derived cytokines to influence macrophage and lymphocyte accumulation in vivo is inferred from studies in which neutrophil depletion in models of infection, contact hypersensitivity and autoimmune disease resulted in a significant reduction in lymphocyte and macrophage accumulation [83].
Activated neutrophils secrete cytokines such as TNFα, IL-1β and IL-12 and chemokines such as IL-8, macrophage inflammatory protein (MIP)-1α, MIP-1β, interferon-γ (IFNγ)-inducible protein of 10 kDa (IP-10) and monokine induced by IFNγ (MIG) [83]. IL-8 is the most abundantly produced chemokine by neutrophils and might serve as a feedback mechanism to increase PMN influx. Neutrophils from patients with lupus have an impaired ability to produce IL-8, which could predispose these patients to infection [84]. Neutrophil MIP-1α promotes the chemotaxis of monocytes [85], whereas IP-10 and MIG trigger adhesion of activated T cells [86–88]. Finally, human neutrophils release CCL19, CCL20 and β-defensins that recruit dendritic cells (DCs), which in turn promote neutrophil accumulation by producing high amounts of IL-8 [89]. The impact of these chemokines could be amplified by the simultaneous presence of neutrophil-derived ROS and proteinases that affect chemokine activity. Post-transcriptional modifications of IL-8 regulate neutrophil dynamics. In particular, citrullination protects IL-8 from being processed into a more active form and reduces neutrophil extravasation to the peritoneal cavity, compared to the uncitrullinated form [90]. Neutrophils could regulate IL-8 citrullination and thus limit the inflammatory response.
Collaborators with platelets
Neutrophil interaction with platelets plays a central role in inflammation and also provides an important link between inflammation and thrombosis. This interaction can occur directly through platelet P-selectin/neutrophil PSGL-1, platelet Gp1bα/neutrophil Mac-1 or platelet JAM-3/neutrophil Mac-1 interaction or through a bridging molecule such as fibrinogen, which binds both platelet GpIIb/IIIa and Mac-1 [91]. Following the induction of thrombotic GN or immune complex-induced nephritis, platelets are observed within minutes followed by neutrophil influx[49,92,93]. In some models, platelet accumulation, which is P-selectin dependent, is required for glomerular neutrophil accumulation and thrombosis [92,94]. Conversely, neutrophils are required for platelet accumulation in other models [30,95]. Surprisingly, in a mouse model of thrombotic GN, initial glomerular platelet deposition protects the vessel wall from neutrophil Mac-1-mediated sequelae [30]. Subsequent glomerular injury depends on Mac-1 interaction with platelet Gp1bα, as blocking this interaction does not affect neutrophil influx but attenuates thrombosis [30]. Interestingly, in the acute phase of immune complex-mediated nephritis in rats, blocking fibrinogen interaction with its receptor GpIIb/IIIa on platelets does not affect neutrophil accumulation but completely blocks subsequent proteinuria [95]. Thus, neutrophil interaction with Gp1bα or GpIIb/IIIa on platelets might play a critical role in directing glomerular injury. A neutrophil–platelet partnership could also be important in the resolution phase of GN. Contact between these two cell types triggers a transcellular pathway for the production of lipoxins, which are lipoxygenase-derived eicosanoids that potentially inhibit the recruitment of neutrophils to the glomerulus [81]. P-selectin is important in generating this “stop signal”, which suggests potentially opposing roles for P-selectin in leukocyte recruitment. Neutrophils might also aid in the resolution of GN by promoting phagocytic clearance of activated platelets [96], although the relevance of this pathway to renal disease remains to be assessed.
Shaping adaptive immunity: neutrophil interaction with T cells and dendritic cells (DCs)
Neutrophils shape the adaptive immune response by communicating with T cells and DCs. This could have direct consequences for proliferative GN; polarization towards a Th1 T-cell response associates with susceptibility to crescentic GN [5]. Furthermore, recent research in a mouse model of proliferative GN that probes the role of effector T cells in renal injury demonstrated a critical role for Th1 and Th17 cells in disease progression [97]. The recipients of Th17 cells exhibit glomerular neutrophil influx [98].In turn, neutrophils can orient the immune response towards a Th1-type response [99,100] and increase the severity of CD8+ T-cell-mediated disease [101]. PMNs can also interact with T cells via several other pathways. They might function as antigen presenting cells: major histocompatibility complex (MHC) class II, CD83 and CD86 are synthesized de novo in neutrophils stimulated with IFNγ [88] and are detected on PMNs from patients with active Wegener’s granulomatosis [102] and rheumatoid arthritis[103]. PMNs can also have immunosuppressive effects on T cells. Liberation of arginase from activated or dying PMNs leads to the depletion of extracellular L-arginine and the subsequent inhibition of T-cell proliferation. High levels of arginase are found in human pus and a cell-free exudate completely suppresses T-cell proliferation in an arginase I-dependent manner [104]. Neutrophils can also inhibit the proliferation of IFNγ producing T cells through a NO-dependent mechanism [105].
Signals delivered by DCs to T cells direct immune responses, tolerance or the induction of anergy. Immune outcomes depend on the DC status, which is dictated by the nature of stimuli DCs encounter prior to interaction with T cells. Human neutrophils form clusters with monocyte-derived DCs and upregulate MHC class II and costimulatory molecules CD80 and CD86 that correlate with DC maturation. CD18 integrins and TNF generated by neutrophils are required for this maturation. DCs exposed to neutrophils are more potent stimulators of naïve T cells and secrete IL-12 to polarize helper T-cell differentiation towards the Th1 type [89,106]. Indeed, there is compelling evidence that neutrophils instruct Th1 differentiation in vivo. In models of infection that elicit a strong Th1-dependent T-cell response required for pathogen clearance, neutrophil depletion associates with a reversal of the Th1 response into a primarily Th2 response [100,107,108].
Self destruction that is not futile
Neutrophils are short-lived cells with a half-life in circulation of 8–15 h. Their lifespan can be extended to 1–4 days in inflamed tissues by mediators such as granulocyte macrophage-colony stimulating factor, IFNγ and LPS. Neutrophil survival is modulated in autoimmune glomerular disease. For example and paradoxically, neutrophil survival is decreased in ANCA-associated vasculitis and SLE[109]. Given that 0.8–1.6 billion neutrophils per kg of body weight are generated daily, mechanisms for their safe elimination are required, as release of their intracellular contents can lead to significant tissue damage. Neutrophils undergo apoptosis and are subsequently removed by macrophages and DCs. Neutrophils also undergo necrosis and autophagic cell death [110]. More recently neutrophils were reported to undergo a process of cell death referred to as netosis. This route of cell death serves as an antimicrobial defense mechanism but might also increase the risk for autoimmunity.
Neutrophils undergo apoptosis in the absence of extra-cellular stimuli (spontaneous apoptosis) but this process can be markedly accelerated by cytokines, Fas Ligand and phagocytosis of complement or IgG-opsonized targets [61]. LPS-activated monocytes produce the immunosuppressive cytokines IL-10 and TGFβ in the presence of apoptotic neutrophils [111]. Phagocytosis of apoptotic neutrophils by macrophages and DCs triggers anti-inflammatory signals. In particular, the uptake of apoptotic neutrophils promotes macrophage IL-23 secretion, which curbs granulopoiesis[112] and leads to the generation of TGFβ [113,114]. Uptake by DCs reduces the ability of immature DCs to stimulate T-cell proliferation [115,116].
Netosis is a recently described process of neutrophil cell death. Neutrophil activation by stimuli that generate an oxidative burst leads to the release of neutrophil extracellular traps (NETs) composed of DNA containing histones and granule proteins, including neutrophil elastase, MPO and bactericidal permeability increasing protein. Netosis is distinct from apoptosis and necrosis as it associates with disintegration of the nuclear envelope, intermixing of cytoplasmic and nuclear compartments and the loss of internal membranes and cytoplasmic organelles[110]. A recent study showed that NETs might also be produced by the release of mitochondrial but not nuclear DNA [117], which suggest that NETs do not lead to cell death under all conditions. Intriguingly, NETs trap several pathogens and thus serve as an antimicrobial defense mechanism. Stimuli that lead to netosis can also result in the deimination of arginine to citrulline in histones, which changes histone structure [118]. The presence of a modified major human autoantigen in NETs suggests that netosis could promote chronic autoimmunity by serving as a source of antigen–chromatin complexes. Thus, NETS could provide a link between infection and autoimmunity. Importantly, a recent report indicates that NETs containing the ANCA antigens MPO and PR-3 are released by anti-ANCA stimulation and NETs are observed in kidney biopsies of patients with small-vessel vasculitis [119].
Concluding remarks and future directions
Despite the significant morbidity and mortality associated with glomerulonephritides, treatments remain relatively toxic owing to their broad mechanisms of action [1,48]. Greater delineation of cellular and molecular mechanisms, with the hope of identifying game changers in glomerular disease, is needed to develop more targeted therapeutics. This is a challenge as human GN, which can occur either as a primary renal disorder or secondary to systemic imbalances, encompass a broad range of diseases that are classified according to their histopathologic features but differ in etiology and in many cases are idiopathic in origin. However, there is mounting evidence that the inflammation responsible for end-organ tissue damage is a common entity that links these diseases. Thus, targeting immune cells is an attractive strategy for attenuating GN even if the mechanisms initiating disease remain elusive.
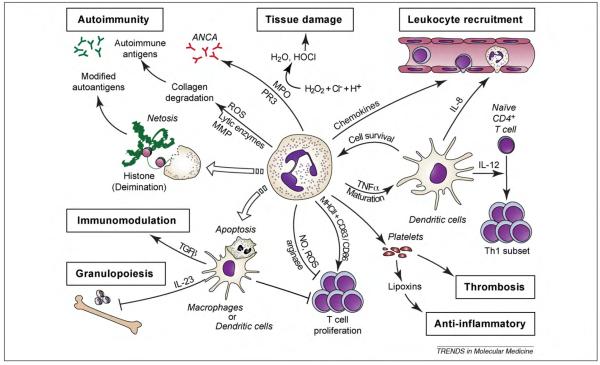
Here, we reviewed data that suggest neutrophils can directly damage the glomerulus and also trigger a cascade of events that reverberate to inform the adaptive immune response. The latter in turn regulates neutrophil production, activation and recruitment and thus mobilizes a whole cadre of new recruits. Data in human biopsies indicate that neutrophils are present in various glomerulonephritides. Indeed, neutrophils are probably underrepresented in biopsies as they are short-lived, and at the time of sampling their influx could have subsided in comparison to macrophages and T cells that are long-lived and can proliferate in situ. Although long considered to be relatively transcriptionally inert, neutrophils can generate de novo several cytokines, chemokines and lipid mediators in response to inflammatory mediators. These molecules shape the subsequent immune response by regulating the influx of leukocyte subsets and the activity of components of the adaptive immune system and other hematopoietic cells such as platelets. Neutrophil-derived cytotoxic elements might also increase the risk for autoimmunity, as they can be targets of the immune response or they can generate epitopes that are autoantigenic. Even the death of neutrophils is tailored to mold the immune landscape; apoptosis and the subsequent phagocytosis of the corpses by macrophages deliver anti-inflammatory signals. By contrast, in netosis, neutrophils extrude nuclear material to form “NETs” that trap bacteria but could also present modified autoantigens that increase the risk of autoimmunity (Figure 3).
Figure 3.
Neutrophils shape the immune landscape. Neutrophils can impact several immune related functions from leukocyte recruitment and T-cell regulation to thrombosis and autoimmunity. Neutrophil effects are communicated by direct contact with other cells and/or the secretion of inflammatory mediators. Abbreviations: ANCA, anti-neutrophil cytoplasmic antigen; MHC, major histocompatibility complex; MPO, myeloperoxidase; NO, nitric oxide; PR-3, proteinase 3; ROS, reactive oxygen species; H2O, water; HOCl, hypochlorous acid; H2O2, hydrogen peroxide; Cl−; chloride anion; H+, hydrogen ion.
We hope that the data reviewed herein will stimulate research into the contribution of and mechanisms by which neutrophils promote GN, as these cells are not only capable of directly instigating glomerular damage but also of collaborating with members of “specific immunity” to shape the immune landscape. There is much to be done in this area and the potential for novel discoveries is significant. Areas of interest include (i) a detailed analysis of mechanisms of leukocyte recruitment in the specialized micro-vasculature of the glomerulus under normal and pathological states using real-time imaging techniques; (ii) development of animal models that more faithfully model GN of differing etiologies; (iii) the analysis of clinical samples to correlate neutrophil influx and activation with adaptive immune cells and markers; and (iv) understanding the relative importance of neutrophil-derived components (e.g. cytokines, ROS and proteinases) in glomerular injury that can be achieved by studying mice with neutrophil-specific knockdown of the molecules of interest [120]. Results from these studies could direct therapeutic strategies to limit the scope of renal inflammation and subsequent glomerular damage in proliferative GN. Possible targets include the adhesion molecules that promote neutrophil recruitment in the glomerulus, the immunomodulators of neutrophil function or the intracellular signaling molecules that promote neutrophil activation and the generation of inflammatory mediators responsible for glomerular injury. For example, a pharma-cological inhibitor of the tyrosine kinase, Spleen tyrosine kinase (Syk) protects against experimentally induced arthritis [121,122] and SLE [123] in mice and its therapeutic benefit has been shown in clinical trials for human arthritis [124,125]. Syk is expressed primarily in hematopoietic cells and signals via Immunoreceptor Tyrosine Activating Motif-containing receptors such as FcγRs. Small chemical inhibitors of FcγRIIA, a FcγR present on neutrophils, macrophages, platelets, mast cells and basophils, inhibit destructive autoimmune arthritis in mice engineered to express the human FcγRIIA [126]. These are encouraging leads that could translate into antineutrophil therapeutic strategies in GN as the inhibitors appear to be effective in diseases initiated by ICs, known pathogenic entities in many types of GN. They also probably target neutrophils as Syk and FcγRIIIA are important mediators of IC-induced neutrophil responses. However, such single-point interdiction strategies might not be successful in the complex setting of clinical human GN where neutrophils are one of many components involved in disease pathogenesis. Moreover, the window of therapeutic opportunity is also limited given that neutrophil involvement is probably most prominent at the onset of disease. Nonetheless, given the paucity and relative toxicity of current treatments and the emerging role of neutrophils in proliferative GN, it is essential that the potential role of this largely ignored leukocyte subset in GN is fully explored, as they have the potential to be game changers in disease pathogenesis.
Glossary
- Alternate complement pathway
complement is activated by three pathways, alternative, classical (complement protein C1 binding to antigen-antibody complexes) and lectin (binding of plasma lectin to mannose residues on microbes). C3 cleavage to C3b and C3a is a central event in complement activation and the steps leading to this event differs between the pathways. The alternative pathway (AP) of C3 cleavage is initiated by the slow, spontaneous hydrolysis of a thioester in plasma C3 that leads to the formation of a fluid-phase C3 convertase and generation of C3a and C3b (“tick-over” mechanism). In fluid phase, C3b is inactivated by hydrolysis. If it becomes cell-associated, it binds factor B and forms the AP C3 convertase. This convertase cleaves C3 to produce additional C3b and aids in the formation of C5 convertase, which cleaves C5 into C5a and C5b. The remaining steps of complement activation are the same. The activity of C3b is inhibited by regulatory proteins such as Factor H present on host cells.
- Cryoglobulinemia
a group of disorders commonly characterized by the presence in the serum of cryoglobulins, blood proteins precipitating at temperatures lower than 37 °C. Cryoglobulinemia is also often used to specifically refer to small-to-medium vasculitis involving cryoglobulins containing immune complexes. Cryoglobulins consist of immunoglobulins (Ig) and complement components and are categorized into three types depending on their composition: type I – monoclonal Ig (e.g. multiple myeloma), type II – mixture of polyclonal Ig associated with a monoclonal Ig (e.g. chronic viral infection such as hepatitis C) and type III – polyclonal Ig (usually associated with connective tissue diseases)
- Eicosanoids
a group of autocrine or paracrine molecules derived from the metabolism of omega-3 or omega-6 essential fatty acids. This group includes the metabolites of the arachidonic acid such as prostaglandins, leukotrienes, prostacyclins and thromboxanes, which are involved in immunity and inflammatory processes. Eicosanoids are frequently the targets of anti-inflammatory drugs (such as nonsteroidal anti-inflammatory drugs)
- Hydronephrosis
dilatation of the renal collecting system secondary to a urinary tract obstruction
- Paraproteinemias
also called monoclonal gammopathies and refer to a group of disorders characterized by the pathologic clonal proliferation of a plasma cell producing a monoclonal protein termed paraprotein or M-protein, which can be detected in the serum and/or in the urine. This includes among others multiple myeloma and monoclonal gammopathy of undetermined significance.
- Proteinuria
pathological urinary protein excretion above 150 mg/day (physiological threshold). Protein excretion above 3 g/day is considered heavy proteinuria and is a sign of prominent renal injury
- TTP and hemolytic uremic syndrome (HUS)
acute systemic disorders associating thrombocytopenia and microangiopathic hemolytic anemia (non-immune hemolysis with red blood cells fragmentation) without any apparent cause. TTP is commonly the diagnosis when neurologic abnormalities are present with minimal renal involvement. HUS usually refers to patients with prominent acute renal failure without neurologic abnormalities. When both neurologic and renal involvements are present, the mixed term TTP–HUS can be used as the pathologic changes of both are similar (thrombi deposition mainly composed of platelets in the microvessels of the affected organs).
References
- 1.Javaid B, Quigg RJ. Treatment of glomerulonephritis: will we ever have options other than steroids and cytotoxics? Kidney Int. 2005;67:1692–1703. doi: 10.1111/j.1523-1755.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 2.Kurts C, et al. Role of T cells and dendritic cells in glomerular immunopathology. Semin. Immunopathol. 2007;29:317–335. doi: 10.1007/s00281-007-0096-x. [DOI] [PubMed] [Google Scholar]
- 3.Clatworthy MR, Smith KG. B cells in glomerulonephritis: focus on lupus nephritis. Semin. Immunopathol. 2007;29:337–353. doi: 10.1007/s00281-007-0092-1. [DOI] [PubMed] [Google Scholar]
- 4.Bagavant H, Fu SM. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2009;21:489–494. doi: 10.1097/BOR.0b013e32832efff1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tipping PG, Holdsworth SR. T cells in crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2006;17:1253–1263. doi: 10.1681/ASN.2005091013. [DOI] [PubMed] [Google Scholar]
- 6.Holdsworth SR, Tipping PG. Leukocytes in glomerular injury. Semin. Immunopathol. 2007;29:355–374. doi: 10.1007/s00281-007-0097-9. [DOI] [PubMed] [Google Scholar]
- 7.Camussi G, et al. The polymorphonuclear neutrophil (PMN) immunohistological technique: detection of immune complexes bound to the PMN membrane in acute poststreptococcal and lupus nephritis. Clin. Nephrol. 1980;14:280–287. [PubMed] [Google Scholar]
- 8.Segerer S, et al. Expression of the chemokine receptor CXCR1 in human glomerular diseases. Kidney Int. 2006;69:1765–1773. doi: 10.1038/sj.ki.5000337. [DOI] [PubMed] [Google Scholar]
- 9.Hooke DH, et al. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987;31:964–972. doi: 10.1038/ki.1987.93. [DOI] [PubMed] [Google Scholar]
- 10.Kanjanabuch T, et al. An update on acute postinfectious glomerulonephritis worldwide. Nat. Rev. Nephrol. 2009;5:259–269. doi: 10.1038/nrneph.2009.44. [DOI] [PubMed] [Google Scholar]
- 11.Nasr SH, et al. Acute postinfectious glomerulonephritis in the modern era: experience with 86 adults and review of the literature. Medicine (Baltimore) 2008;87:21–32. doi: 10.1097/md.0b013e318161b0fc. [DOI] [PubMed] [Google Scholar]
- 12.Alchi B, Jayne D. Membranoproliferative glomerulonephritis. Pediatr. Nephrol. 2010;25:1409–1418. doi: 10.1007/s00467-009-1322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith KD, Alpers CE. Pathogenic mechanisms in membranoproliferative glomerulonephritis. Curr. Opin. Nephrol. Hypertens. 2005;14:396–403. doi: 10.1097/01.mnh.0000172729.60122.f9. [DOI] [PubMed] [Google Scholar]
- 14.Servais A, et al. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J. Med. Genet. 2007;44:193–199. doi: 10.1136/jmg.2006.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith RJ, et al. New approaches to the treatment of dense deposit disease. J. Am. Soc. Nephrol. 2007;18:2447–2456. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethi S, et al. Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int. 2009;75:952–960. doi: 10.1038/ki.2008.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer EG, Lager DJ. Anti-glomerular basement membrane glomerulonephritis: a morphologic study of 80 cases. Am. J. Clin. Pathol. 2006;125:445–450. doi: 10.1309/nptp-4ukv-7ju3-elmq. [DOI] [PubMed] [Google Scholar]
- 18.Xiao H, et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am. J. Pathol. 2005;167:39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennette JC, Falk RJ. Pathogenesis of the vascular and glomerular damage in ANCA-positive vasculitis. Nephrol. Dial. Transplant. 1998;13(Suppl. 1):16–20. doi: 10.1093/ndt/13.suppl_1.16. [DOI] [PubMed] [Google Scholar]
- 20.Kain R, et al. Pathogenesis of small vessel vasculitis associated with autoantibodies to neutrophil cytoplasmic antigens: new insights from animal models. Curr. Opin. Rheumatol. 2010;22:15–20. doi: 10.1097/BOR.0b013e328332c9e4. [DOI] [PubMed] [Google Scholar]
- 21.Sis B, et al. Banff ‘09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am. J. Transplant. 2010;10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 22.Harper L, Savage CO. Pathogenesis of ANCA-associated systemic vasculitis. J. Pathol. 2000;190:349–359. doi: 10.1002/(SICI)1096-9896(200002)190:3<349::AID-PATH524>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.Jennette JC, Falk RJ. New insight into the pathogenesis of vasculitis associated with antineutrophil cytoplasmic autoantibodies. Curr. Opin. Rheumatol. 2008;20:55–60. doi: 10.1097/BOR.0b013e3282f16c0a. [DOI] [PubMed] [Google Scholar]
- 24.Brouwer E, et al. Neutrophil activation in vitro and in vivo in Wegener’s granulomatosis. Kidney Int. 1994;45:1120–1131. doi: 10.1038/ki.1994.149. [DOI] [PubMed] [Google Scholar]
- 25.Rarok AA, et al. Neutrophil membrane expression of proteinase 3 (PR3) is related to relapse in PR3-ANCA-associated vasculitis. J. Am. Soc. Nephrol. 2002;13:2232–2238. doi: 10.1097/01.asn.0000028642.26222.00. [DOI] [PubMed] [Google Scholar]
- 26.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J. Immunol. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayadas TN, et al. Glomerular inflammation: use of genetically deficient mice to elucidate the roles of leukocyte adhesion molecules and Fc-gamma receptors in vivo. Curr. Opin. Nephrol. Hypertens. 1999;8:293–298. doi: 10.1097/00041552-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Cochrane CG, et al. A role of polymorphonuclear leukocytes and complement in nephrotoxic nephritis. J. Exp. Med. 1965;122:99–116. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franchini M. Thrombotic microangiopathies: an update. Hematology. 2006;11:139–146. doi: 10.1080/10245330600667583. [DOI] [PubMed] [Google Scholar]
- 30.Hirahashi J, et al. Mac-1 (CD11b/CD18) links inflammation and thrombosis after glomerular injury. Circulation. 2009;120:1255–1265. doi: 10.1161/CIRCULATIONAHA.109.873695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jennette JC, et al. Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J. Am. Soc. Nephrol. 2006;17:1235–1242. doi: 10.1681/ASN.2005101048. [DOI] [PubMed] [Google Scholar]
- 32.Gan PY, et al. Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J. Am. Soc. Nephrol. 2010;21:925–931. doi: 10.1681/ASN.2009070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuboi N, et al. Human neutrophil Fcgamma receptors initiate and play specialized nonredundant roles in antibody-mediated inflammatory diseases. Immunity. 2008;28:833–846. doi: 10.1016/j.immuni.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duffield JS, et al. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am. J. Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu K, Mohan C. What do mouse models teach us about human SLE? Clin. Immunol. 2006;119:123–130. doi: 10.1016/j.clim.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Teramoto K, et al. Microarray analysis of glomerular gene expression in murine lupus nephritis. J. Pharmacol. Sci. 2008;106:56–67. doi: 10.1254/jphs.fp0071337. [DOI] [PubMed] [Google Scholar]
- 37.Bao L, et al. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur. J. Immunol. 2005;35:2496–2506. doi: 10.1002/eji.200526327. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, et al. The role of IL-23/IL-17 axis in lupus nephritis. J. Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bullard DC, et al. Intercellular adhesion molecule-1 deficiency protects MRL/MpJ-Fas(lpr) mice from early lethality. J. Immunol. 1997;159:2058–2067. [PubMed] [Google Scholar]
- 40.Kevil CG, et al. Loss of LFA-1, but not Mac-1, protects MRL/MpJ-Fas(lpr) mice from autoimmune disease. Am. J. Pathol. 2004;165:609–616. doi: 10.1016/S0002-9440(10)63325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickering MC, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat. Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 42.Pickering MC, et al. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9649–9654. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilgore KS, et al. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am. J. Pathol. 1996;149:953–961. [PMC free article] [PubMed] [Google Scholar]
- 44.Kilgore KS, et al. Enhancement by the complement membrane attack complex of tumor necrosis factor-alpha-induced endothelial cell expression of E-selectin and ICAM-1. J. Immunol. 1995;155:1434–1441. [PubMed] [Google Scholar]
- 45.Pastore Y, et al. An experimental model of cryoglobulin-associated vasculitis in mice. Springer Semin. Immunopathol. 2001;23:315–329. doi: 10.1007/s002810100075. [DOI] [PubMed] [Google Scholar]
- 46.Fulpius T, et al. Polymorphonuclear leukocytes play a key role in the generation of “wire-loop” lesions induced by a murine IgG3 rheumatoid factor. Kidney Int. 1996;49:647–655. doi: 10.1038/ki.1996.93. [DOI] [PubMed] [Google Scholar]
- 47.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 48.Kitching AR, et al. Targeting leukocytes in immune glomerular diseases. Curr. Med. Chem. 2008;15:448–458. doi: 10.2174/092986708783503230. [DOI] [PubMed] [Google Scholar]
- 49.Rosenkranz AR, et al. P-selectin deficiency exacerbates experimental glomerulonephritis: a protective role for endothelial P-selectin in inflammation. J. Clin. Invest. 1999;103:649–659. doi: 10.1172/JCI5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunn KW, et al. Live-animal imaging of renal function by multiphoton microscopy. Curr. Protoc. Cytom. 2007 doi: 10.1002/0471142956.cy1209s41. Chapter 12, Unit 12.9. [DOI] [PubMed] [Google Scholar]
- 51.Stokol T, et al. C1q governs deposition of circulating immune complexes and leukocyte Fcgamma receptors mediate subsequent neutrophil recruitment. J. Exp. Med. 2004;200:835–846. doi: 10.1084/jem.20040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lauterbach M, et al. Role of TNF priming and adhesion molecules in neutrophil recruitment to intravascular immune complexes. J. Leukoc. Biol. 2008;83:1423–1430. doi: 10.1189/jlb.0607421. [DOI] [PubMed] [Google Scholar]
- 53.Lister KJ, et al. Immune complexes mediate rapid alterations in microvascular permeability: roles for neutrophils, complement, and platelets. Microcirculation. 2007;14:709–722. doi: 10.1080/10739680701404879. [DOI] [PubMed] [Google Scholar]
- 54.Mayadas TN, et al. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation. 2009;120:2012–2024. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He X, et al. Deficiency of P-selectin or P-selectin glycoprotein ligand-1 leads to accelerated development of glomerulonephritis and increased expression of CC chemokine ligand 2 in lupus-prone mice. J. Immunol. 2006;177:8748–8756. doi: 10.4049/jimmunol.177.12.8748. [DOI] [PubMed] [Google Scholar]
- 56.Anders HJ, et al. Questions about chemokine and chemokine receptor antagonism in renal inflammation. Nephron Exp. Nephrol. 2010;114:e33–e38. doi: 10.1159/000254389. [DOI] [PubMed] [Google Scholar]
- 57.Tang T, et al. A role for Mac-1 (CDIIb/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcgamma receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J. Exp. Med. 1997;186:1853–1863. doi: 10.1084/jem.186.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuligowski MP, et al. Antimyeloperoxidase antibodies rapidly induce alpha-4-integrin-dependent glomerular neutrophil adhesion. Blood. 2009;113:6485–6494. doi: 10.1182/blood-2008-12-192617. [DOI] [PubMed] [Google Scholar]
- 59.Nolan SL, et al. Mechanisms of ANCA-mediated leukocyte-endothelial cell interactions in vivo. J. Am. Soc. Nephrol. 2008;19:973–984. doi: 10.1681/ASN.2007111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 61.Mayadas TN, Cullere X. Neutrophil beta2 integrins: moderators of life or death decisions. Trends Immunol. 2005;26:388–395. doi: 10.1016/j.it.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Fialkow L, et al. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 63.Gaertner SA, et al. Glomerular oxidative and antioxidative systems in experimental mesangioproliferative glomerulonephritis. J. Am. Soc. Nephrol. 2002;13:2930–2937. doi: 10.1097/01.asn.0000034908.43113.5d. [DOI] [PubMed] [Google Scholar]
- 64.Heeringa P, et al. Expression of iNOS, eNOS, and peroxynitrite-modified proteins in experimental anti-myeloperoxidase associated crescentic glomerulonephritis. Kidney Int. 1998;53:382–393. doi: 10.1046/j.1523-1755.1998.00780.x. [DOI] [PubMed] [Google Scholar]
- 65.Poelstra K, et al. Intraglomerular platelet aggregation and experimental glomerulonephritis. Kidney Int. 1990;37:1500–1508. doi: 10.1038/ki.1990.141. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki Y, et al. Pre-existing glomerular immune complexes induce polymorphonuclear cell recruitment through an Fc receptor-dependent respiratory burst: potential role in the perpetuation of immune nephritis. J. Immunol. 2003;170:3243–3253. doi: 10.4049/jimmunol.170.6.3243. [DOI] [PubMed] [Google Scholar]
- 67.Budisavljevic MN, et al. Oxidative stress in the pathogenesis of experimental mesangial proliferative glomerulonephritis. Am. J. Physiol. Renal Physiol. 2003;285:F1138–1148. doi: 10.1152/ajprenal.00397.2002. [DOI] [PubMed] [Google Scholar]
- 68.Bondi CD, et al. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J. Am. Soc. Nephrol. 2010;21:93–102. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goto S, et al. Expression and localization of inducible nitric oxide synthase in anti-Thy-1 glomerulonephritis. Am. J. Pathol. 1995;147:1133–1141. [PMC free article] [PubMed] [Google Scholar]
- 70.Kuzniar J, et al. Elastase deposits in the kidney and urinary elastase excretion in patients with glomerulonephritis – evidence for neutrophil involvement in renal injury. Scand. J. Urol. Nephrol. 2007;41:527–534. doi: 10.1080/00365590701430893. [DOI] [PubMed] [Google Scholar]
- 71.Afshar-Kharghan V, Thiagarajan P. Leukocyte adhesion and thrombosis. Curr. Opin. Hematol. 2006;13:34–39. doi: 10.1097/01.moh.0000190107.54790.de. [DOI] [PubMed] [Google Scholar]
- 72.Mydel P, et al. Neutrophil elastase cleaves laminin-332 (laminin-5) generating peptides that are chemotactic for neutrophils. J. Biol. Chem. 2008;283:9513–9522. doi: 10.1074/jbc.M706239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai TQ, Wright SD. Human leukocyte elastase is an endogenous ligand for the integrin CR3 (CD11b/CD18, Mac-1, alpha M beta 2) and modulates polymorphonuclear leukocyte adhesion. J. Exp. Med. 1996;184:1213–1223. doi: 10.1084/jem.184.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schrijver G, et al. Antiglomerular basement membrane nephritis in beige mice. Deficiency of leukocytic neutral proteinases prevents the induction of albuminuria in the heterologous phase. J. Exp. Med. 1989;169:1435–1448. doi: 10.1084/jem.169.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soehnlein O. An elegant defense: how neutrophils shape the immune response. Trends Immunol. 2009;30:511–512. doi: 10.1016/j.it.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Klebanoff SJ. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 77.Li JZ, et al. Polymorphonuclear leukocytes increase glomerular albumin permeability via hypohalous acid. Kidney Int. 1994;46:1025–1030. doi: 10.1038/ki.1994.363. [DOI] [PubMed] [Google Scholar]
- 78.Peppin GJ, Weiss SJ. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4322–4326. doi: 10.1073/pnas.83.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Odobasic D, et al. Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2007;18:760–770. doi: 10.1681/ASN.2006040375. [DOI] [PubMed] [Google Scholar]
- 80.Serhan CN, et al. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Meara YM, Brady HR. Lipoxins, leukocyte recruitment and the resolution phase of acute glomerulonephritis. Kidney Int. Suppl. 1997;58:S56–61. [PubMed] [Google Scholar]
- 82.Borregaard N, et al. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Scapini P, et al. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 84.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 85.Kasama T, et al. Expression and regulation of human neutrophil-derived macrophage inflammatory protein 1 alpha. J. Exp. Med. 1993;178:63–72. doi: 10.1084/jem.178.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gasperini S, et al. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J. Immunol. 1999;162:4928–4937. [PubMed] [Google Scholar]
- 87.Molesworth-Kenyon SJ, et al. A novel role for neutrophils as a source of T cell-recruiting chemokines IP-10 and Mig during the DTH response to HSV-1 antigen. J. Leukoc. Biol. 2005;77:552–559. doi: 10.1189/jlb.0904485. [DOI] [PubMed] [Google Scholar]
- 88.Muller I, et al. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30:522–530. doi: 10.1016/j.it.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 89.Boudaly S. Activation of dendritic cells by polymorphonuclear neutrophils. Front. Biosci. 2009;14:1589–1595. doi: 10.2741/3326. [DOI] [PubMed] [Google Scholar]
- 90.Proost P, et al. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J. Exp. Med. 2008;205:2085–2097. doi: 10.1084/jem.20080305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Semple JW, Freedman J. Platelets and innate immunity. Cell. Mol. Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zachem CR, et al. A role for P-selectin in neutrophil and platelet infiltration in immune complex glomerulonephritis. J. Am. Soc. Nephrol. 1997;8:1838–1844. doi: 10.1681/ASN.V8121838. [DOI] [PubMed] [Google Scholar]
- 93.Johnson RJ, et al. Mechanisms and kinetics for platelet and neutrophil localization in immune complex nephritis. Kidney Int. 1989;36:780–789. doi: 10.1038/ki.1989.263. [DOI] [PubMed] [Google Scholar]
- 94.Ito I, et al. Effects of a new synthetic selectin blocker in an acute rat thrombotic glomerulonephritis. Am. J. Kidney Dis. 2001;38:265–273. doi: 10.1053/ajkd.2001.26085. [DOI] [PubMed] [Google Scholar]
- 95.Wu X, et al. Fibrinogen mediates platelet-polymorphonuclear leukocyte cooperation during immune-complex glomerulonephritis in rats. J. Clin. Invest. 1994;94:928–936. doi: 10.1172/JCI117459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manfredi AA, et al. Dangerous connections: neutrophils and the phagocytic clearance of activated platelets. Curr. Opin. Hematol. 2010;17:3–8. doi: 10.1097/MOH.0b013e3283324f97. [DOI] [PubMed] [Google Scholar]
- 97.Summers SA, et al. Th1 and Th17 cells induce proliferative glomerulonephritis. J. Am. Soc. Nephrol. 2009;20:2518–2524. doi: 10.1681/ASN.2009030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Korn T, et al. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 99.Romani L, et al. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J. Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- 100.Romani L, et al. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J. Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 101.Grabie N, et al. Neutrophils sustain pathogenic CD8+ T cell responses in the heart. Am. J. Pathol. 2003;163:2413–2420. doi: 10.1016/S0002-9440(10)63596-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iking-Konert C, et al. Polymorphonuclear neutrophils in Wegener’s granulomatosis acquire characteristics of antigen presenting cells. Kidney Int. 2001;60:2247–2262. doi: 10.1046/j.1523-1755.2001.00068.x. [DOI] [PubMed] [Google Scholar]
- 103.Iking-Konert C, et al. Transdifferentiation of polymorphonuclear neutrophils to dendritic-like cells at the site of inflammation in rheumatoid arthritis: evidence for activation by T cells. Ann. Rheum. Dis. 2005;64:1436–1442. doi: 10.1136/ard.2004.034132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Munder M, et al. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108:1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 105.Zehntner SP, et al. Neutrophils that infiltrate the central nervous system regulate T cell responses. J. Immunol. 2005;174:5124–5131. doi: 10.4049/jimmunol.174.8.5124. [DOI] [PubMed] [Google Scholar]
- 106.van Gisbergen KP, et al. Close encounters of neutrophils and DCs. Trends Immunol. 2005;26:626–631. doi: 10.1016/j.it.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Ismail HF, et al. Depletion of neutrophils in IL-10(-/-) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J. Immunol. 2003;170:3782–3789. doi: 10.4049/jimmunol.170.7.3782. [DOI] [PubMed] [Google Scholar]
- 108.Tateda K, et al. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 2001;166:3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 109.Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am. J. Hematol. 2008;83:288–295. doi: 10.1002/ajh.21078. [DOI] [PubMed] [Google Scholar]
- 110.Cabrini M, et al. New insights into the mechanisms controlling neutrophil survival. Curr. Opin. Hematol. 2010;17:31–35. doi: 10.1097/MOH.0b013e3283333b29. [DOI] [PubMed] [Google Scholar]
- 111.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J. Immunol. 2002;168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 112.Stark MA, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 113.Huynh ML, et al. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lucas M, et al. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J. Immunol. 2003;171:2610–2615. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 115.Clayton AR, et al. Dendritic cell uptake of human apoptotic and necrotic neutrophils inhibits CD40, CD80, and CD86 expression and reduces allogeneic T cell responses: relevance to systemic vasculitis. Arthritis Rheum. 2003;48:2362–2374. doi: 10.1002/art.11130. [DOI] [PubMed] [Google Scholar]
- 116.Aleman M, et al. Spontaneous or Mycobacterium tuberculosis-induced apoptotic neutrophils exert opposite effects on the dendritic cell-mediated immune response. Eur. J. Immunol. 2007;37:1524–1537. doi: 10.1002/eji.200636771. [DOI] [PubMed] [Google Scholar]
- 117.Yousefi S, et al. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 118.Neeli I, et al. Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 119.Kessenbrock K, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cullere X, et al. Neutrophil-selective CD18 silencing using RNA interference in vivo. Blood. 2008;111:3591–3598. doi: 10.1182/blood-2007-12-127837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Braselmann S, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 122.Pine PR, et al. Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clin. Immunol. 2007;124:244–257. doi: 10.1016/j.clim.2007.03.543. [DOI] [PubMed] [Google Scholar]
- 123.Bahjat FR, et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum. 2008;58:1433–1444. doi: 10.1002/art.23428. [DOI] [PubMed] [Google Scholar]
- 124.Weinblatt ME, et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58:3309–3318. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]
- 125.Kyttaris VC, Tsokos GC. Syk kinase as a treatment target for therapy in autoimmune diseases. Clin. Immunol. 2007;124:235–237. doi: 10.1016/j.clim.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pietersz GA, et al. Inhibition of destructive autoimmune arthritis in FcgammaRIIa transgenic mice by small chemical entities. Immunol. Cell Biol. 2009;87:3–12. doi: 10.1038/icb.2008.82. [DOI] [PubMed] [Google Scholar]