Abstract
A long-term cell culture system utilizing normal adult hippocampal neurons would represent an important tool that could be useful in research on the mature brain, neurological disorders and age-related neurological diseases. Historically, in vitro neuronal systems are derived from embryonic rather than mature brain tissue, a practice predicated upon difficulties in supporting regeneration, functional recovery and long-term survival of adult neurons in vitro. A few studies have shown that neurons derived from the hippocampal tissue of adult rats can survive and regenerate in vitro under serum-free conditions. However, while the adult neurons regenerated morphologically under these conditions, both the electrical activity characteristic of in vivo neurons as well as long-term neuronal survival was not consistently recovered in vitro. In this study, we report on the development of a defined culture system with the ability to support functional recovery and long-term survival of adult rat hippocampal neurons. In this system, the cell-adhesive substrate, N-1 [3-(trimethoxysilyl) propyl]-diethylenetriamine, supported neuronal attachment, regeneration, and long-term survival of adult neurons for more than 80 days in vitro. Additionally, the excitatory neurotransmitter glutamate, applied at 25 μM for 1 to 7 days after morphological neuronal regeneration in vitro, enabled full recovery of neuronal electrical activity. This low concentration of glutamate promoted the recovery of neuronal electrical activity but with minimal excitotoxicity. These improvements allowed electrically active adult neurons to survive in vitro for several months, providing a stable test-bed for the long-term study of regeneration in adult derived neuronal systems, especially for traumatic brain injury (TBI).
Keywords: Adult, neuron, culture, hippocampal, electrophysiology
INTRODUCTION
As people live longer, neurological disorders and diseases that affect the aging brain have become more common, however success to date for reversing these diseases has been limited. One of the reasons this research has been hindered has been the flawed, limited, or non-existent research models of aged or diseased human brain tissue. Neuronal cell culture, one of the most commonly used research models, is generally derived from embryonic rat or mouse tissue. Although this system has been used effectively in many studies, a neuronal culture system derived from adult brain tissue could be a much more relevant and effective model system. Such a system could be used to study the function of neurons, neuronal interactions, aging and neurodegenerative disease but from a new perspective where the different ion channels, receptors and other cellular components matured in vivo instead of in vitro (Ginsberg 2005). It also would be useful in drug studies, neuroprosthetic devices, neurocomputing and biorobotics for the same rational (Heiduschka and Thanos 1998; Simpson et al. 2001; Harms et al. 2006), and has a unique application to studying regeneration of injured adult neurons, especially in traumatic brain injury (TBI). These applications were the goal behind an earlier attempt at creating an adult hippocampal culture system, developed using a serum-free culture media with a biological surface (Brewer 1997). While this system supported the morphological recovery of adult hippocampal neurons in vitro, issues with the support of both long-term survival and full recovery of electrical activity of neurons in this culture system appears to have prevented its widespread use as a research tool (Bousse 1996; Heiduschka and Thanos 1998; Simpson et al. 2001).
To overcome these issues, modifications were made to a defined neuronal culture system previously developed for embryonic hippocampal neurons (Schaffner et al. 1995). First, culture surfaces were modified with the chemical substrate N-1 [3-(trimethoxysilyl) propyl]- diethylenetriamine (DETA), creating a covalently modified interface with exposed cell-adhesive amine groups, which has previously been shown to promote the attachment, regeneration and long-term survival of embryonic neurons in vitro (Stenger et al. 1993; Hickman et al. 1994; Schaffner et al. 1995; Ravenscroft et al. 1998; Das et al. 2003). Second, a neurotransmitter was added to the adult hippocampal culture media, an approach found to successfully trigger electriophysiological recovery in cultured adult spinal cord neurons (Das et al. 2008). This neurotransmitter, glutamate, conveys fast excitatory neurotransmission in vivo, primarily acting via the activation of ionotropic and metabotropic receptors (Verderio et al. 1999). In addition, activation of these receptors plays a major role in neuronal differentiation, CNS development, long-term potentiation and memory formation in vivo (Verderio et al. 1999; Zhu et al. 2005; Balazs 2006). In most embryonic hippocampal cultures, microMolar concentrations of glutamate are incorporated into the culture media in order to replicate these effects in vitro (Mattson 1988; Mattson et al. 1988; Brewer et al. 1993). However, issues with excitotoxicity led to the removal of glutamate from the media gradually beginning at day 4 in earlier attempts to culture adult hippocampal neurons (Brewer 1997; Brewer 1998; Brewer et al. 2005). Our hypothesis was that, lacking this vital neurotransmitter, the adult neurons in vitro could not fully recover the characteristic electrical activity found for neurons in vivo in the previous system.
In this study, the earlier adult hippocampal cell culture technique was modified to include silane-modified DETA surfaces and the application of 25 μM glutamate for 1 to 7 days, which was introduced after 21 DIV to minimize excitotoxicity. These changes promoted long-term neuronal survival and full recovery of electrical activity, providing a system for studying neurodegenerative diseases and disorders such as Alzheimer’s disease and especially neuroregeneration of injured adult tissue.
MATERIALS AND METHODS
Surface Modification of the Cover Slips
Glass cover slips (Thomas Scientific 6661F52, 22 × 22 mm2 no. 1) were cleaned by acid washing using a 50/50 mixture of concentrated hydrochloric acid and methanol. The cover slips were washed three times, 30 minutes per wash, and were rinsed in distilled de-ionized water between each washing. The DETA (N-1 [3-(trimethoxysilyl) propyl]-diethylenetriamine, United Chemical Technologies Inc., Bristol, PA, T2910KG) monolayer was formed by the reaction of the cleaned surface with a 0.1% (v/v) mixture of the organosilane in freshly distilled toluene (Fisher T2904) (Ravenscroft et al. 1998). The DETA-coated cover slips were heated to just below the boiling point of toluene, rinsed with toluene, reheated to just below the boiling temperature, and then oven dried. The DETA formed a reaction site limited monolayer on the surface of the cover slip (Ravenscroft et al. 1998).
Surface Characterization of the Cover Clips after DETA Monolayer Formation
The DETA cover slips were characterized to authenticate the monolayer formation. First, contact angle measurements were taken using an optical contact angle goniometer (KSV Instruments, Monroe, CT, Cam 200). The contact angle for the DETA-coated cover slips was 54.2 +/- 0.2, which was previously shown to be acceptable for neuronal hippocampal culture (Ravenscroft et al. 1998). Second, X-ray Photoelectron Spectroscopy (XPS) (FISONS ESCALab 220i-XL) was used to characterize the elemental and chemical state of the DETA-coated cover slip surfaces. The XPS survey scans as well as high-resolution N 1s and C 1s scans, using monochromatic Al Kα excitation, were obtained, similar to previously reported results (Spargo et al. 1994; Ravenscroft et al. 1998; Das et al. 2005).
Isolation and Culture of Adult Hippocampal Neurons
Hippocampi were removed from 3-6 month-old Sprague Dawley rats, which were purchased from Charles River. Hippocampal cells were extracted and isolated based upon a previously described method (Brewer 1997). In brief, adult rats were euthanized by exposure to CO2 according to practices that adhered to IACUC policies and the hippocampal regions of the brain were removed. The hippocampi were sliced into small pieces, collected in a mixture of Hibernate A (http://www.brainbitsllc.com) and an antibiotic/antimycotic (Ab/Am, Invitrogen, 15240-062), and enzymatically digested in a papain solution (2 mg/ml Hibernate A) (Worthington, LS003119). Next, the tissue was triturated in 6 ml of fresh Hibernate A - Ab/Am to dissociate the tissue into a cell suspension. This 6 ml cell suspension was then layered over a 4 ml step gradient (Optiprep diluted 0.505:0.495 [v/v] with Hibernate A-Ab/Am and made to 15, 20, 25 and 35% [v/v] in Hibernate A-Ab/Am) and centrifuged at 800 × g for 15 minutes, 4°C. Hippocampal neurons were collected from the second and third layers within the gradient. These layers were collected, diluted with 5 ml of fresh Hibernate A-Ab/Am and centrifuged at 500 × g for 7 minutes. The supernatant was removed and the cell pellet resuspended in culture media, composed of Neurobasal A (Gibco, 10888), B27 supplement (Invitrogen, 17504-044), glutamax (Invitrogen, 35050-061), antibiotic/antimycotic (Invitrogen, 15240-062) and basic FGF (5 ng/ml, Invitrogen, 13256-029). 500 μl of the cell suspension was applied to each cover slip for 1 hour, the cells adhered during this time, and then an additional 2 ml of media was added to each cover slip. After four days, the existing media was replaced by fresh culture media. Thereafter, every four days half the media was removed and replaced with fresh media. Remaining cultures were discarded after 90 days.
Application of Glutamate
This study utilized the excitatory neurotransmitter glutamate, N-Acetyl-L-glutamic acid (Sigma, 855642), which is essential for normal brain function (Auditore et al. 1966). It was applied at different dosages, where the initial concentrations were derived from previous glutamate excitotoxicity studies (Mattson 1988; Mattson et al. 1988; Balazs 2006). Glutamate doses, 10 μM (Group G21-10), 25 μM (Group G21-25) and 100 μM (Group G21-100), were separately applied to adult hippocampal neurons after 21 DIV. After incubating for different time periods, neuronal viability and electrical activity were evaluated and compared to cultured neurons not exposed to glutamate. Short-, medium-, and long-term effects from the application of 25 μM glutamate to adult hippocampal neurons 21 DIV were evaluated after 1 hr (Group G21-1h), 1 day (Group G21-1d), 7 days (Group G21-7d) and 14 days (Group G21-14d). The optimal parameter for in vitro glutamate application was used to comprehensively study the effect of this neurotransmitter on neurons cultured for different periods. 25 μM glutamate was added to neurons 14 DIV (Group G14-25), 21 DIV (Group G21-25), 31 DIV (Group G31-25) and 38 DIV (Group G38-25). After 24 hours, neuronal electrical activity and viability were evaluated. Control cultures, where glutamate was not applied, were also evaluated after 14 DIV (Group C14), 21 DIV (Group C21), and 31 DIV (Group C31). The neurotransmitter solution was freshly prepared for each experiment. The underlying mechanism behind the glutamate-mediated improvement in neuronal electrical properties was investigated through the use of cycloheximide (CHX, Sigma, C4859), which is a protein synthesis inhibitor shown to block preconditioning in neurons (Barone et al. 1998). CHX was first applied at concentrations of 10 μg/ml (Group CHX10), 20 μg/ml (Group CHX20), and 80 μg/ml (Group CHX80) to neurons 21 DIV, with cell viability in each group assessed after 24 hours. Next, 20 μg/ml CHX was applied to neurons 21 DIV for 1 hour before 25 μM glutamate was introduced (Group G21CHX20). The neurons were evaluated for changes in viability and electrical properties after 1 day.
Immunocytochemistry
Cover slips were rinsed with 1X PBS two times. Cells on these cover slips were fixed with 10% glacial acetic acid and 90% ethanol for 20 minutes at room temperature. Cover slips were again rinsed 3 times with 1X PBS. Cells were permeabilized for 5 min with a permeabilizing solution (5 mM Lysine + 0.5% Triton X-100 + 100 ml of 1X PBS), and were then blocked for 2 hr (5% normal donkey serum in permeabilizing solution). Anti-neurofilament M polyclonal antibody (Chemicon, AB1981, diluted 1:1000), anti-MAP 2A/2B (MAB364, Chemicon, diluted 1:1000), anti-nestin (MAB5326, Chemicon, diluted 1:1000) and anti-GFAP monoclonal antibody (MAB360, Chemicon, diluted 1:400) were added in blocking solution for 12 hr at 4°C. After 3X washes with 1X PBS, fluorescently labeled secondary antibodies were applied for 2 hours. Vectashield mounting medium (H1000, Vector Laboratories, Burlingame, CA) was used to mount the cover slips onto slides. The cover slips were observed and photographed with an Ultra VIEWTM LCI confocal imaging system (Perkin Elmer).
Electrophysiology
Whole-cell, patch-clamp recordings were performed in a recording chamber that was placed on the stage of a Zeiss Axioscope, 2 FS Plus, upright microscope in Neurobasal culture medium (pH was adjusted to 7.3 with N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid [HEPES]) at room temperature. Patch pipettes (6-8 MΩ) were filled with intracellular solution (K-gluconate 140 mM, ethylene glycol-bis[aminoethylether]-tetraacetic acid 1 mM, MgCl2 2 mM, Na2ATP 5 mM, HEPES 10 mM; pH 7.2). Voltage clamp and current clamp experiments were performed with a Multiclamp 700A (Axon, Union City, CA) amplifier. Signals were filtered at 3 kHz and digitized at 20 kHz with an Axon Digidata 1322A interface. Data recordings and analysis were performed with pClamp 8 (Axon) software. Inward currents that had the characteristics of fast sodium currents (ISCs), and outward currents that had the characteristics of potassium currents (OPCs) were measured in voltage clamp mode using voltage steps of 10 mV from a −70 mV holding potential. Whole-cell capacitance and series resistance were compensated and a p/6 protocol was used. The access resistance was less than 22 MΩ and tight seals were measured to be above 1 GΩ. Action potentials (APs) were measured with 1 s depolarizing current injections from the − 70 mV holding potential.
Selection of cells for electrophysiological characterization was based upon morphology. Phase bright pyramidal neurons with large branching apical dendrites and small basal dendrites were selected. Cells with this morphology stained positive for neurofilament and negative for GFAP. Neurons cultured for 14, 21, 28 and 38 days were electrically characterized for evaluation of glutamate-mediated electrical recovery. Additionally, neurons cultured between 1-14 DIV and 39-87 DIV were electrically characterized to evaluate recovery and maintenance of electrical activity.
Cell Survival Study
Survival of the cultured adult hippocampal neurons was evaluated following the addition of glutamate (10, 25, 100 μM), cycloheximide (10, 20, 80 μg/ml), or simultaneous addition of 25 μM glutamate and 20 μg cycloheximide. In the first method, the total number of neurons on each cover slip was approximated both before and after the application of each factor(s). Using a phase contrast microscope at 20X magnification, neuronal cell counts were made from 20 random spots on each cover slip, an area equal to 4% of the total area of the cover slip (22 × 22 mm). The total number of living neurons on each cover slip was mathematically determined. In the second method, the number of living versus dead neurons was quantified using a Live/Dead Assay kit (Molecular Probe, L-3224). The percentage of living neurons on each cover slips was calculated from total cells counted, both living and dead. Cell counts were made using a phase-contrast microscope with epiflourescent light source. Total cells were approximated after cells, both living and dead, were counted on 20 randomly selected spots at 20X magnification. Neurons in vitro were also morphologically evaluated throughout the culture period.
Statistical Analysis
Statistical analysis included performing a two-sample t-test on the morphological, immunocytochemical, live/dead assay and electrophysiological data. Parameters obtained from the DETA-plated adult hippocampal neurons treated with glutamate ± cycloheximide were compared with DETA-plated hippocampal control neurons which were not treated. Numerical summary results are reported as a mean, plus or minus the sample standard error of the mean (± SEM). For two-group comparisons, the student’s t-test was used.
RESULTS
Regeneration of Adult Hippocampal Neurons with Limited Recovery of Electrical Activity in a Defined System
After deposition onto DETA surfaces, the adult hippocampal neurons adhered to the surface within 60 minutes (Figure 1A). Immediately following attachment, most neurons displayed characteristics indicative of recovery from the trauma of cell culture, with some regeneration occurring in all viable neurons by the end of the initial 24 hours. After 2 DIV, recovery of axonal and dendritic processes was clearly visible (Figure 1 B, C) and by 14 DIV the vast majority of adult neurons had typical neuronal morphology (Figure 1 B, C), which was maintained up to 80 DIV (Figure 1, D-I). These adult neurons exhibited phase-bright, smooth-appearing somas, with one or more small dendrites and a large apical dendrite with second-order dendritic branching. While few non-neuronal cells were present after 14 DIV, a small population of glial cells (astrocytes, oligodendrocytes, microglia) remained for the duration of the cultures. Terminally differentiated, mature neurons were distinguished from immature neurons and progenitor cells through immunocytochemical analysis with antibodies against MAP-2, a protein specific to mature neurons (Coceres et al. 1984), and nestin, a protein found specifically in immature and progenitor cell populations (Stemple and Anderson 1992). During the first 14 DIV, recovering adult neurons displayed MAP2 as well as nestin expression, possibly pointing to the regression of mature neurons to a more immature phenotype during regeneration (Das 2008, Biomaterials). After 14 DIV, nestin expression was not observed. The majority of the cells after 21 DIV and 38 DIV (90% and 94% respectively) displayed expression of the neuronal structural proteins neurofilament and/or MAP-2, while the remaining cells expressed the glial-specific structural protein GFAP (Figure 1 J, K, L). The low percentage of non-neuronal cells may be attributed to the poor support of glial growth and survival provided by the Neurobasal-A/B27 media (Brewer 1997).
Figure 1.
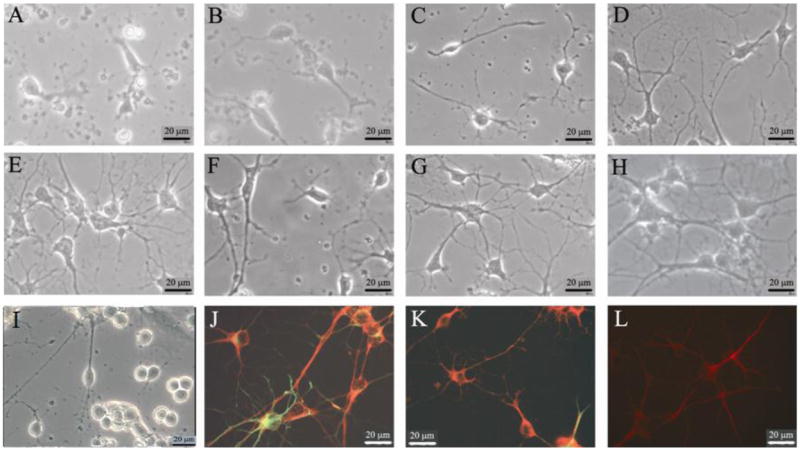
Representative phase-contrast and anti-neurofilament/anti-GFAP immunostained pictures of neurons cultured from adult hippocampal tissue. This figure ilustrates both the recovery of the hippocampal neurons in this defined in vitro system as well as the purity of the neuronal culture, neurons versus glial cells. A-I illustrates phase-contrast images of living cultures taken during different culture ages, immediately following cell culture through 28 days in vitro, 40x view. (A) Phase picture of neurons in vitro 1 hour following the attachement of neurons onto a silane-modified coverslip. (B) 6 hours post-attachment (C) 2 days post-attachment. Note the rapid recovery of axons as well as the phase bright cell soma. (D) Phase picture of the neurons after 7 days in vitro. (E) Phase picture of the neurons after 14 days in vitro. Morphologically these adult-derived hippocampal neurons are fully recovered. (F) Phase picture of neurons after 22 days in vitro. (G) Phase picture of neurons after 22 days in vitro, with a prior 1-day exposure to 25 μM glutamate added to the culture media on day 21. (H) Phase picture of neuron after 78 days in vitro. (I) Phase picture of neuron after 78 days in vitro, after incubation with 25 μM glutamate between days 21 to 28, further visualized after application of antibodies against neurofilament (red) and GFAP (green) antibodies (J, K) and (red) MAP-2 (L)
In a previous study of in vitro adult neuronal electrical properties, both embryonic and adult hippocampal neurons were evaluated based upon recovery of electrical activity and the ability to fire action potentials (Evans et al. 1998). The embryonic hippocampal neurons were able to fire action potentials in approximately 84% of the neurons studied at 14 DIV in this earlier work. However, the percentage of neurons obtained from the hippocampus of adult rats that were able to fire action potentials was much lower, between 25% and 55%. In the current study, whole-cell patch-clamp experiments were used to evaluate in vitro electrical activity of cultured adult hippocampal neurons using the same conditions and the results obtained were similar to those found in the previous study. Additionally, adult neurons were selected for 4 different periods for the initial electrical property study: 14 DIV (Group C14), 21 DIV (Group C21), 28 DIV (Group C28) and 38 DIV (Group C38). The current flow (inward and outward) in these control populations of neurons was limited or non-existent in many cases. The number of neurons with induced inward sodium currents ranged from 38.5% (Group C14) to 53.8% (Group C38), while the number with induced outward currents ranged from 38.5% (Group C14) to 76.9% (Group C38) (Table 1, rows 1-4). The percentage of these neurons that fired action potentials was similar to the previously reported results (Table 1, rows 1-4: 23.1% to 38.5%).
Table 1.
Comparison of the total number of neurons patched after exposure to different culture conditions. The groups were designed to find the optimal conditions under which glutamate induced maximal improvements to the electrical activity of the cultured, adult hippocampal neurons while causing the least amount of neuronal cell death. These various conditions resulted in different groups which measured the effect of neuronal electrical activity and cell death of the following conditions: no glutamate application – controls (day 14 (C14), day 21 (C21), day 28 (C28), day 38 (C38)); different concentrations of glutamate applied to day 21 neurons (10 μM (G21-10), 25 μM (G21-25), 100 μM (G21-100)); different durations for the application of 25 μM glutamate to day 21 neurons (1 hour (G21-1h), 1 day (G21-1d), 7 days (G21-7d), 14 days (G21-14d)), 25 μM glutamate applied for 24 hours to different aged cultures (day 14 (G14-25), day 21 (G21-25), day 31 (G31-25), day 38 (G38-25)), different concentrations of cycloheximide (CHX) (10 μg/ml (CHX10), 20 μg/ml (CHX20), 80 μg/ml (CHX80)), and inhibition that protein synthesis (20 μg CHX / ml media) has on glutamate (25 μM, 24 hour duration, day 21 culture) induced improvement (G21CX20). Cell death was measured both through a live/dead assay and through cell counts. The live neuron percentage value is the fraction of neurons that are live versus the total (live and dead).
| Groups (timing, duration, and dosages for glutamate application) | Total number of cells patched | Number of cells with induced inward sodium current (ISC) | Number of cells with induced outward potassium current (OPC) | Number of cells which fired single action potentials (SAP) | Average fraction of cells alive (vs total number of cells, live + dead) |
|---|---|---|---|---|---|
| C14 | 13 | 5 (38.5%) | 5 (38.5%) | 3 (23.1%) | 94% |
| C21 | 17 | 8 (47.1%) | 9 (52.9%) | 6 (35.3%) | 96% |
| C28 | 14 | 7 (50.0%) | 8 (57.1%) | 5 (35.7%) | 95% |
| C38 | 13 | 7 (53.8%) | 10 (76.9%) | 5 (38.5%) | 95% |
| G21-10 | 19 | 10 (52.6%) | 12 (63.2%) | 10 (52.6%) | 88% |
| G21-25 | 34 | 24 (70.6%) | 29 (85.3%) | 24 (70.6%) | 84% |
| G21-100 | 17 | 15 (88.2%) | 15 (88.2%) | 12 (70.6%) | 69% |
| G21-1h | 15 | 7 (46.7%) | 10 (66.7%) | 4 (26.7%) | 93% |
| G21-1d | 34 | 24 (70.6%) | 29 (85.3%) | 24 (70.6%) | 84% |
| G28-7d | 15 | 10 (66.7%) | 12 (80.1%) | 11 (73.3%) | 78% |
| G35-14d | 14 | 11 (78.6%) | 11 (78.6%) | 11 (78.6%) | 65% |
| G14-25 | 13 | 7 (53.8%) | 9 (69.2%) | 7 (53.6%) | 86% |
| G21-25 | 34 | 24 (70.6%) | 29 (85.3%) | 24 (70.6%) | 84% |
| G31-25 | 18 | 12 (66.7%) | 14 (77.8%) | 13 (72.2%) | 82% |
| G38-25 | 16 | 11 (68.8%) | 11 (68.8%) | 10 (62.5%) | 83% |
| CHX10 | - | - | - | - | 89% |
| CHX20 | - | - | - | - | 84% |
| CHX80 | - | - | - | - | 52% |
| G21CX20 | 23 | 8 (34.8%) | 12 (52.2%) | 5 (21.7%) | 66% |
Lower Concentrations of Glutamate Trigger Excitotoxicity in Recovering Neurons versus Morphologically Recovered Neurons in Vitro
To test our theory that neurotransmitter treatment enables functional recovery of injured neuronal cells, adult hippocampal neurons were challenged with various concentrations of glutamate during the following periods: 1) recovery, during which the neurons recovered from the trauma of cell culture (0-3 DIV), 2) regeneration, during which morphology characteristic of in vivo neurons, was recovered (3-14 DIV), and 3) after long-term survival (14-x DIV). 10 μM, 25 μM or 100 μM aliquats of glutamate were added to the adult neuronal culture medium during each of these culture periods, and the effect upon neuronal health and viability was assessed after 1 hr, 1 day, and 4 days. Excitotoxicity caused by each concentration of glutamate (10 μM, 25 μM, 100 μM) and application period (1 h, 1 d, 4 d) was measured, as well as on the in vitro neuronal viability during each culture period (0-3 DIV, 3-14 DIV, 14-x DIV).
The different concentrations of glutamate evoked significantly different levels of excitotoxicity in the neurons for each culture period examined. When glutamate was added to the culture media after the initial 1 h plating period (0-3 DIV), even low glutamate concentrations were strongly excitotoxic; damaging and killing the majority of the cultured neurons within 1 day. During the regeneration period (3-14 DIV), although a longer incubation period was required, glutamate damaged or killed the majority of neurons within 4 days. However, in the third period (14-x DIV), minimal excitotoxicity was observed after the adult neurons were incubated with low concentrations of glutamate (10 μM, 25 μM). Higher concentrations of glutamate (≥ 100 μM) remained significantly excitotoxic.
In the next step of the investigation, glutamate was applied after the cultured neurons appeared to be completely regenerated morphologically in the culture system. First, adult hippocampal neurons 21 DIV were incubated with 25 μM glutamate for 1 h, 1 d, 7 d, and 14 d. Short-term application of glutamate was found to cause very little excitotoxicity in these neurons, while long-term incubation of glutamate provoked excitotoxicity to a greater degree (Table 1). Next, 21 DIV adult hippocampal neurons were incubated for 1 d with 10 μM, 25 μM or 100 μM glutamate. Glutamate at concentrations of 10 μM and 25 μM was minimally excitotoxic, causing very little damage and death in these neurons. However, at 100 μM, glutamate evoked significant neuronal death, with only 69% of the neurons surviving after the incubation period (Table 1). Finally, 14, 21, 31, and 38 DIV adult hippocampal neurons were incubated with 25 μM glutamate for 1 day. A small percentage of neurons in each of these cultures died in response to glutamate, with toxicity the same in each culture. Overall, glutamate at low μM concentrations, and with a short incubation time, was minimally excitotoxic to adult neurons that were morphologically recovered in vitro, and this provided the baseline culture system conditions for the electrophysiological characterization.
Glutamate Applied for a Minimum of 24 Hours Increases the Number of Electrically Active Adult Hippocampal Neurons in Vitro
After morphological recovery of the adult hippocampal neurons (≥ 14 DIV), recovery of in vitro neuronal electrical activity was investigated to determine if the introduction of glutamate to the neurons through the culture medium had a positive effect on functional recovery. In order to determine the parameters that produced the best electrical recovery, glutamate was applied for periods of 1 h to 14 days at concentrations of 10, 25, or 100 μM to the hippocampal neurons at 14, 21, 31 or 38 DIV. After the application of glutamate under each set of conditions, the electrical properties of these neurons were fully evaluated by whole cell patch-clamp electrophysiology and compared to the electrical activity of neurons without the glutamate treatment. In addition, the electrical activity of adult neurons up to 80 DIV was measured in cultures where 25 μM glutamate had been applied from 21 to 28 DIV as shown in Figure 1-I and detailed in Figure 3.
Figure 3.
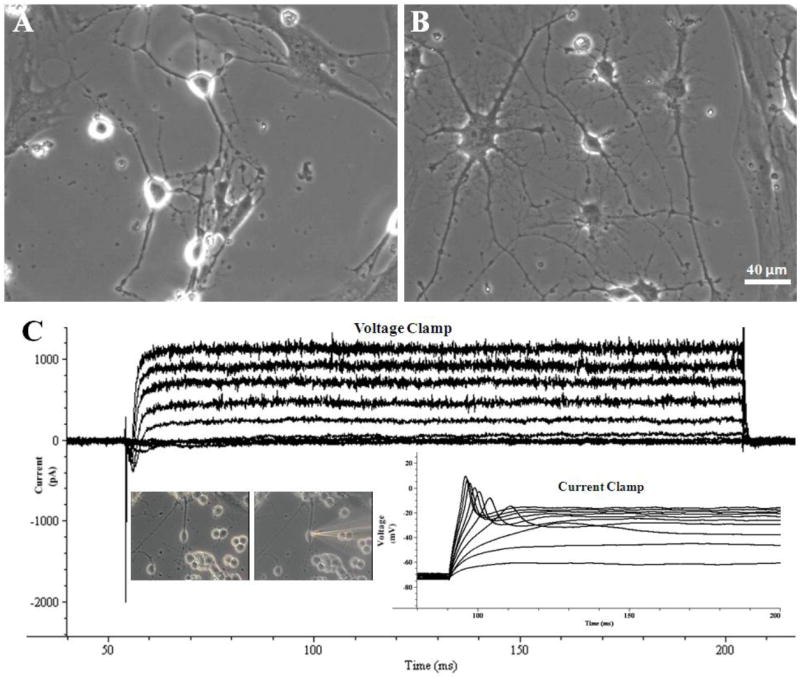
Representative phase-contrast pictures and electrophysiological recordings of adult hippocampal neurons after approximately 80 DIV. (A, B) Phase contrast pictures of neurons 80 DIV. (C) Representative traces for voltage and current clamp of an adult neuron 78 DIV. Neurons retain the ability to move current into and out of cells through the voltage-gated ion channels (voltage clamp trace) as well as to fire single action potentials after electrical stimulation (current clamp trace). These traces originated from adult hippocampal neurons after 78 days in vitro, where 25 μM glutamate had been applied to the culture medium between days 21 and 28.
Initially, the shortest and best incubation time that induced improved activity was evaluated. 25 μM glutamate was applied to 21-day-old adult, hippocampal neuronal cultures for periods of 1 h, 1 d, 7 d or 14 d, after which neuronal electrical activity was immediately analyzed. After an incubation period of 1 h with glutamate, the cultured neurons exhibited no improvement in activity, both in the number of neurons with current flow (ISCs, OPCs) and action potentials (APs) (Table 1, Groups G21-1h vs. C21). However, after glutamate was applied for longer durations (1 d, 7 d, 14 d), the number of neurons with ISCs and/or OPCs increased significantly, to 71% and 85% respectively in those neurons exposed for 24 hours (Table 1, Group G21-1d). In addition, a significant increase in the number of neurons with the ability to fire APs was triggered by exposure to glutamate for 1 or more days. After 25 μM glutamate was applied for 1 d, 7 d, or 14 d, 70-80% of the adult neurons were able to fire APs, a 35-40% improvement versus untreated neurons (Table 1, Groups G21-1d vs. C21; Groups G21-7d vs. C28; Groups G21-14d vs. C38).
Efficacy versus excitotoxicity of glutamate at different concentrations was re-examined to find the least excitotoxic level of glutamate that still triggered the recovery of neuronal electrical activity in vitro. 10 μM, 25 μM, or 100 μM glutamate was added to neurons at 21 DIV for 1 day. After incubation with 10 μM glutamate, ISCs, OPCs and APs were observed in 51%, 63% and 53% of patched neurons respectively, a very slight improvement in electrical activity versus untreated neurons. After incubation with 25 μM glutamate, only slightly excitotoxic under these in vitro conditions, 71% of neurons had ISCs, 85% had OPCs and 71% fired APs. This was an improvement of 24, 32 and 35% respectively versus untreated neurons. The 100 μM dose of glutamate indicated similar efficacy, but triggered a significant excitotoxic response (Table 1, Groups G21-10; G21-25; G21-100; C21). Finally, a dose of 25 μM glutamate was applied to the neurons at 14, 21, 31, and 38 DIV for 24 hours. When compared to untreated control cultures of similar age, a higher percentage of neurons in each treated culture exhibited ISCs (15-24%), OPCs (20-30%) and APs (24-36%). However, approximately 20% fewer neurons displayed electrical activity when glutamate treatment was initiated after 14 DIV versus 21, 31, and 38 DIV.
The best experimental conditions for improving adult neuronal cultures were found by minimizing glutamate excitotoxicity while maximizing glutamate-mediated recovery of neuronal electrical activity in vitro. Challenging cultured adult neurons at 21 DIV with 25 μM glutamate for 1 to 7 days caused manageable levels of excitotoxicity, while significantly improving the electrical potential of these cultured neurons. This combination of culture age, glutamate concentration and incubation time yielded significantly improved electrical activity with minimal neuronal cell loss. Representative voltage and current clamp traces (21 and 78 DIV) are displayed in Figure 2.
Figure 2.
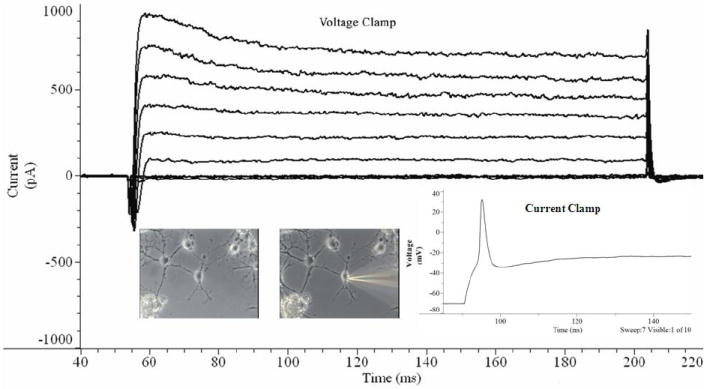
Representative traces for voltage and current clamp of an adult neuron 21 DIV. Neurons retain the ability to move current into and out of cells through the voltage-gated ion channels (voltage clamp trace) as well as to fire single action potentials after electrical stimulation (current clamp trace). These traces originated from adult hippocampal neurons after 21 days in vitro, where 25μM glutamate had been applied to the culture medium between days 20 and 21.
Extracellular Glutamate Applied for a Minimum of 24 Hours to Fully Recovered Adult Hippocampal Neurons in vitro Resulted in Increased Inward and Outward Current Flow in Electrically Active Neurons
The effect from glutamate at various concentrations and incubation times to neurons at different days in vitro was measured in relation to the changes in mean resting membrane potential, membrane resistance, membrane capacitance, peak inward current, peak outward current and action potential amplitude. These results are summarized as mean ± SE (Table 2). Statistical analysis performed on the results between the different group’s resting membrane potential, membrane resistance, membrane capacitance and action potential height indicated no significant change due to adult neuronal exposure to glutamate. However, incubation with glutamate triggered significant changes in the peak inward and outward currents (Table 2). The amplitude of current flow into the cultured neurons after application of glutamate on average doubled as compared to the current flow observed in the control neurons, from − 188.3 ± 72.4 (Group C21) to − 403.6 ± 98.5 (Group G21-25). Likewise, the amplitude of current flowing out of the glutamate-treated cultured neurons increased on average 2.5 to 3 times versus the control neurons, from 304.2 ± 144.2 (Group C21) to 923.5 ± 201.2 (Group G21-25). In the different cultures incubated with 25 μM glutamate for at least 1 d (1 d, 7 d, 14 d), no significant amplitude differences in mean peak ISCs or OPCs were evident between these cultures.
Table 2.
Comparison of the electrical properties of those neurons that exhibited action potentials. The different glutamate application conditions resulted in different groups, which measured the effect of neuronal electrical properties in the following conditions: no glutamate application – controls (day 14 (C14), day 21 (C21), day 28 (C28), day 38 (C38)); different concentrations of glutamate applied to day 21 neurons (10 μM (G21-10), 25 μM (G21-25), 100 μM (G21-100)); different duration for the application of 25 μM glutamate to day 21 neurons (1 hour (G21-1h), 1 day (G21-1d), 7 days (G21-7d), 14 days (G21-14d)), 25 μM glutamate applied for 24 hours to different aged cultures (day 14 (G14-25), day 21 (G21-25), day 31 (G31-25), day 38 (G38-25)), and inhibition that protein synthesis (20 μg CHX / ml media) has on glutamate (25 μM, 24 hour duration, day 21 culture) induced improvement (G21CX20).
| C14 | C21 | C28 | C38 | G21-10 | G21-25 | G21-100 | G21-1h | G21-7d | G21-14d | G14-25 | G31-25 | G38-25 | G21CX20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days in vitro before addition of glutamate | n/a | n/a | n/a | n/a | 20 | 20 | 20 | 21 | 21 | 21 | 13 | 30 | 37 | 20 |
| Total days in vitro | 14 | 21 | 28 | 38 | 21 | 21 | 21 | 21 + 1 hour | 28 | 35 | 14 | 31 | 38 | 21 |
| Number of neurons (n) | 13 | 17 | 14 | 13 | 19 | 34 | 17 | 15 | 15 | 14 | 13 | 18 | 16 | 23 |
| Resting potential (mV) | -70 | -69±1.56 | -69.05±1.05 | -69.63±0.45 | -70.25±1.92 | -71.4±0.88 | -70.9±0.54 | -71.4±1.36 | -69±1.24 | -69.1±1.42 | -70.3±0.84 | -69.75±1.06 | -69.5±0.92 | -68.1±0.53 |
| Input resistance (mΩ) | 23.1 | 23.1±9.62 | 23.1±2.34 | 22.9±1.74 | 23±14.15 | 25.74±10.06 | 19.11±5.64 | 23.1±11.49 | 22.75±2.21 | 23.1±6.3 | 26.86±9.51 | 22.3±11.15 | 27.44±6.06 | 16.11±8.34 |
| Capacitance (pF) | 15.41 | 15.77±8.1 | 14.57±6.5 | 14.67±4.8 | 12.86±11.69 | 10.55±4.96 | 8.9±3.88 | 13.14±9.28 | 11.22±2.7 | 10.63±5.76 | 11.13±6.75 | 12.13±3.65 | 10.96±7.62 | 9.35±7.45 |
| Peak inward current (pA) | -172.4 | -188.3±72.4 | -165.2±57.3 | -178.1±66.3 | -237.2±77.3 | -403.6±98.5 | -388.3±97.4 | -173.3±88.9 | -402.2±77.5 | -343.7±87.4 | -302.5±93.6 | -378.8±73.4 | -379.8±43.9 | -255.6±92.4 |
| Peak outward current (pA) | 285.4 | 304.2±144.2 | 298.6±74.9 | 285.6±71.4 | 479.3±177.7 | 923.5±201.2 | 788.3±243.6 | 276.3 ±81.4 | 802.4±344.5 | 734.0±133.2 | 723.4±174.3 | 893.2±202.4 | 933.1±355.3 | 402.5±166.3 |
| Action potential height (mV) | 28.2 | 29.7±2.1 | 31.8±3.1 | 30.3±2.8 | 34.2±4.2 | 30.6±2.8 | 27.1±4.3 | 34.2±4.4 | 31.7±1.9 | 32.6±3.2 | 26.4±2.1 | 29.1±3.2 | 28.4±1.1 | 31.2±2.3 |
In addition, cycloheximide (CHX), an inhibitor of protein synthesis that had previously been used to block preconditioning in neurons (Barone et al. 1998), was used to block all possible changes in protein synthesis induced by incubation of the neurons with glutamate. When hippocampal neurons (21 DIV) were pre-incubated for 1 h with CHX (20 mg/ml) prior to the introduction of 25 mM glutamate, all improvements in neuronal electrical activity were completely blocked (Tables 1 & 2).
DISCUSSION
In this study, a defined culture system for adult hippocampal neurons was developed that included a cell-adhesive silane surface and a serum-free media containing glutamate. This culture system allowed adult neurons to recover full electrical activity and survive long-term in vitro for more than 80 DIV. First, culture surfaces, in this case glass cover slips, were modified with the chemical substrate DETA to create stable surfaces with exposed cell-adhesive amine groups, that has been shown to be stable for longer periods in culture because the silanes are covalently linked to the surface as opposed to PDL and poly-ornithine which are physisorbed. These chemically modified surfaces not only promoted neuron attachment, regeneration and long-term culture compared to PDL and poly-ornithine (Schaffner et al. 1995; Das et al. 2003), but were also stable, reproducible and can be further modified to create high-resolution patterns, which could be useful for the creation of engineered networks of these adult hippocampal neurons (Stenger et al. 1993; Ravenscroft et al. 1998; Stenger et al. 1998). Second, the optimal glutamate concentration, culture age for glutamate application and duration of glutamate exposure were determined such that excitotoxicity was minimized and neuronal electrical improvements was maximized, which resulted in the long-term survival of electrophysiologically functional adult neurons. Based on the experimental results, challenging 21 DIV adult neurons with 25 μM glutamate for 1 to 7 d caused minimal excitotoxicity, while inducing recovery of full electrical activity in vitro. Together, these improvements allowed adult neurons to functionally recover and to survive for several months in vitro, providing a stable test-bed for long-term study of the mature mammalian brain.
In fast excitatory neurotransmission, the neurotransmitter glutamate primarily acts via the activation of ionotropic and metabotropic receptors. In addition to conveying fast excitatory neurotransmission, activation of these channels appears to play a major role in neuronal differentiation and CNS development, as well as in processes that give rise to long-term potentiation and memory formation (Verderio et al. 1999). Recovery of the neuronal electrical activity in this culture system, induced by the application of glutamate, illustrates a role for glutamate beyond simple neurotransmission. The application of glutamate triggered this change either through transient or persistent cellular changes. Transient changes in activity could have occurred as a direct result from the presence of glutamate, triggering changes in passive membrane properties (capacitance and resistance), alteration in the magnitude of the voltage-activated sodium, potassium and calcium currents (Daoudal et al. 2002; Zhang and Linden 2003; Walmsley et al. 2006; Sjostrom et al. 2008), or alteration in the activity levels of the ion channels. Persistent changes to neuronal excitability would be dependent upon the induction of gene expression to cause long-lasting changes in the adult neurons. Results from this study point to the induction of persistent changes in these cultured adult neurons as improved electrical activity was not evident in the adult neurons after only one hour of glutamate application.
In this culture system, glutamate appears to activate gene transcription through the same mechanism found in vivo. Through activation of ionotropic and metabotropic receptors in vivo, glutamate has been shown to trigger gene activity in immature, newborn and mature hippocampal neurons. In immature neurons, glutamate regulates cell proliferation, neuronal differentiation and survival responses primarily through the N-methyl-D-aspartate receptor (NMDAR) channels activation (Dalva et al. 2000; Husi et al. 2000; Kornhauser et al. 2002). However, in mature neurons glutamate regulates the expression of adaptive response genes as well as genes that regulate more complex neural functions, such as learning and memory, though the activation of L-type voltage gated calcium channels (L-VGCC) rather than NMDAR channels (Kornhauser et al. 2002). Activation of these channels triggers calcium influx, thus activating a number of signaling molecules. These molecules potentiate the signals through the activation of downstream signaling proteins such as protein kinase A (PKA), Ca2+/calmodulin dependent protein kinase type IV (CaMK-IV), regulated S6 protein kinase (Rsk) and other pathways to amplify the calcium signal and carry it to the nucleus. In the nucleus these kinase phosphorylate transcription factors, such as cyclic AMP response element binding protein (CREB) or myocyte enhancer factor 2 (MEF2), make them competent to mediate gene transcription (West et al. 2001). Although glutamate activates transcription factors like CREB in both mature and immature neurons, different genes are activated in each case. Overall, different signaling pathways trigger the expression of genes that regulate the survival, differentiation and function of immature neurons (cAMP-CREB pathway) (Nakagawa et al. 2002; Balazs 2006), neurite outgrowth (Mattson 1988; Mattson et al. 1988; Verderio et al. 1999) or activity-dependent synaptic plasticity and trophic factor-dependent neuronal survival (West et al. 2001; Tominaga-Yoshino et al. 2008). In this adult neuronal culture system, glutamate appeared to induce gene activation, resulting in increased numbers of neurons firing APs and in significantly increased sodium and potassium current amplitude. The initiation of protein synthesis by the glutamate treatment is supported by the experiments where CHX addition blocked any functional recovery of electrical activity due to glutamate addition. These differences in the activated signaling pathways in the adult neurons from that observed in development may give insight to pathways necessary for neuroregeneration, specifically for recovery in traumatic brain injury (TBI).
Another explanation from a mechanistic perspective is the possible conditioning in the mature brain to the reliance of electrical potential between neurons and supporting cells such as astrocytic recycling of glumamate. Without this feedback between the supporting cells and neurons, low concentrations of glutamate may not be enough to be able to reestablish the membrane potential. Instead, lack of glutamate recycling in the synaptic cleft limits the amount of available glutamate for stimulation of the recovery of the neuronal electrical properties. Thus, increasing the levels of glutamate in vitro restores the natural concentration of glutamate to post-synaptic terminals, restoring the natural “conditioning” of neurons to glutamate and the cellular changes resulting from this level of glutamate. These changes (i.e. gene expression changes) can result in increased expression of ion channels in the neuronal cell membranes. This results in a more “normal” electrical potential as compared to in vivo membrane potential results due to the recovery of in vivo like ion channel expression levels.
In this study, silane-modified DETA surfaces and the transient application of glutamate have been incorporated into an adult, hippocampal cell culture system in order to sustain long-term neuronal survival as well as to promote full recovery of electrical activity in vitro. Together, these improvements allowed electrically active adult neurons to survive for several months in vitro, providing a stable system with potential for a wide range of applications. Promising applications include the long-term study of the mature brain, neurological disorders, and diseases affecting the aging brain. In addition, because DETA monolayers can be applied not only to glass cover slips but also to electrodes, this system can be extended to integrate living and electronic systems. Overall, potential uses for such a system range from research into the function of neurons, neuronal interactions, aging, and neurodegenerative disease (Ginsberg 2005) as well as drug studies, neuroprosthetic devices, neurocomputing, and biorobotics (Heiduschka and Thanos 1998; Simpson et al. 2001; Harms et al. 2006), or most importantly, neuroregeneration, especially in TBI.
Acknowledgments
The authors would like to acknowledge funding support from the Department of Energy, grant number DE-FG-02-04ER 46171 and National Institute of Health, grant number 5R01EB005459.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auditore JV, Wade L, Olson EJ. Occurrence of N-acetyl-L-glutamic acid in the human brain. Journal of Neurochemistry. 1966;13(11):1149–1155. doi: 10.1111/j.1471-4159.1966.tb04272.x. [DOI] [PubMed] [Google Scholar]
- Balazs R. Trophic effect of glutamate. Curr Top Med Chem. 2006;6(10):961–968. doi: 10.2174/156802606777323700. [DOI] [PubMed] [Google Scholar]
- Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ, Rothwell NJ. Ischemic Preconditioning and Brain Tolerance: Temporal Histological and Functional Outcomes, Protein Synthesis Requirement, and Interleukin-1 Receptor Antagonist and Early Gene Expression. Stroke. 1998;29(9):1937–1951. doi: 10.1161/01.str.29.9.1937. [DOI] [PubMed] [Google Scholar]
- Bousse L. Whole cell biosensors. Sensors and Actuators B-Chemical. 1996;34(1-3):270–275. [Google Scholar]
- Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods. 1997;71(2):143–55. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Age-related toxicity to lactate, glutamate, and beta-amyloid in cultured adult neurons. Neurobiol Aging. 1998;19(6):561–568. doi: 10.1016/s0197-4580(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Lim A, Capps NG, Torricelli JR. Age-related calcium changes, oxyradical damage, caspase activation and nuclear condensation in hippocampal neurons in response to glutamate and beta-amyloid. Exp Gerontol. 2005;40(5):426–437. doi: 10.1016/j.exger.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized Survival of Hippocampal-Neurons in B27-Supplemented Neurobasal(Tm), a New Serum-Free Medium Combination. Journal of Neuroscience Research. 1993;35(5):567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Coceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Research. 1984;315:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103(6):945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Hanada Y, Debanne D. Bidirectional plasticity of excitatory postsynaptic potential (EPSP)-spike coupling in CA1 hippocampal pyramidal neurons. PNAS. 2002;99(22):14512–14517. doi: 10.1073/pnas.222546399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Bhargava N, Bhalkikar A, Kang J-F, Hickman JJ. Temporal neurotransmitter conditioning restores the functional activity of adult spinal cord neurons in long-term culture. Experimental Neurology. 2008;209:171–180. doi: 10.1016/j.expneurol.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Bhargava N, Gregory C, Riedel L, Molnar P, Hickman JJ. Adult rat spinal cord culture on an organosilane surface in a novel serum-free medium. In Vitro Cellular & Developmental Biology - Animal. 2005;41(10):343–348. doi: 10.1007/s11626-005-0006-2. [DOI] [PubMed] [Google Scholar]
- Das M, Molnar P, Devaraj H, Poeta M, Hickman J. Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnology Progress. 2003;19:1756–1761. doi: 10.1021/bp034076l. [DOI] [PubMed] [Google Scholar]
- Evans MS, Collings MA, Brewer GJ. Electrophysiology of embryonic, adult and aged rat hippocampal neurons in serum-free culture. Journal of Neuroscience Methods. 1998;79(1):37–46. doi: 10.1016/s0165-0270(97)00159-3. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD. Glutamatergic Neurotransmission Expression Profiling in the Mouse Hippocampus After Perforant-Path Transection. Am J Geriatr Psychiatry. 2005;13(12):1052–1061. doi: 10.1176/appi.ajgp.13.12.1052. [DOI] [PubMed] [Google Scholar]
- Harms H, Wells MC, van der Meer JR. Whole-cell living biosensors--are they ready for environmental application. Appl Microbiol Biotechnol. 2006;70(3):273–280. doi: 10.1007/s00253-006-0319-4. [DOI] [PubMed] [Google Scholar]
- Heiduschka P, Thanos S. Impantable bioelectronic interfaces for lost nerve functions. Prog Neurobiology. 1998;55(5):433–461. doi: 10.1016/s0301-0082(98)00013-6. [DOI] [PubMed] [Google Scholar]
- Hickman JJ, Bhatia SK, Quong JN, Shoen P, Stenger DA, Pike CJ, Cotman CW. Rational Pattern Design for in-Vitro Cellular Networks Using Surface Photochemistry. Journal of Vacuum Science & Technology a-Vacuum Surfaces and Films. 1994;12(3):607–616. [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3(7):661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Kornhauser J, Cowan C, Shaywitz A, Dolmetsch R, Griffith E, Hu L, Haddad C, Xia Z, Greenberg M. CREB Transcriptional Activity in Neurons Is Regulated by Multiple, Calcium-Specific Phosphorylation Events. Neuron. 2002;34:221–233. doi: 10.1016/s0896-6273(02)00655-4. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. 1988;472(2):179–212. doi: 10.1016/0165-0173(88)90020-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Dou P, Kater SB. Outgrowth-Regulating Actions of Glutamate in Isolated Hippocampal Pyramidal Neurons. Journal of Neuroscience. 1988;8(6):2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim J, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman R. Localization of Phosphorylated cAMP Response Element-Binding Protein in Immature Neurons of Adult Hippocampus. Journal of Neuroscience. 2002;22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenscroft MS, Bateman KE, Shaffer KM, Schessler HM, Jung DR, Schneider TW, Montgomery CB, Custer TL, Schaffner AE, Liu QY, Li YX, Barker JL, Hickman JJ. Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane- modified surfaces. Journal of the American Chemical Society. 1998;120(47):12169–12177. [Google Scholar]
- Schaffner AE, Barker JL, Stenger DA, Hickman JJ. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. Journal of Neuroscience Methods. 1995;62(1-2):111–9. doi: 10.1016/0165-0270(95)00063-1. [DOI] [PubMed] [Google Scholar]
- Simpson ML, Sayler GS, Fleming JT, Applegate B. Whole-cell biocomputing. Trends Biotechnol. 2001;19(8):317–323. doi: 10.1016/s0167-7799(01)01691-2. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Rancz EA, Roth A, Hausser M. Dendritic Excitability and Synaptic Plasticity. Physiol Rev. 2008;88(2):769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- Spargo BJ, Testoff MA, Nielsen TB, Stenger DA, Hickman JJ, Rudolph AS. Spatially controlled adhesion, spreading, and differentiation of endothelial cells on self-assembled molecular monolayers. Proc Natl Acad Sci U S A. 1994;91(23):11070–11074. doi: 10.1073/pnas.91.23.11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71(6):973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- Stenger DA, Hickman JJ, Bateman KE, Ravenscroft MS, Ma W, Pancrazio JJ, Shaffer K, Schaffner AE, Cribbs DH, Cotman CW. Microlithographic determination of axonal/dendritic polarity in cultured hippocampal neurons. J Neurosci Methods. 1998;82(2):167–73. doi: 10.1016/s0165-0270(98)00047-8. [DOI] [PubMed] [Google Scholar]
- Stenger DA, Pike CJ, Hickman JJ, Cotman CW. Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Res. 1993;630(1-2):136–47. doi: 10.1016/0006-8993(93)90651-3. [DOI] [PubMed] [Google Scholar]
- Tominaga-Yoshino K, Urakubo T, Okada M, Matsuda H, Ogura A. Repetitive induction of late-phase LTP produces long-lasting synaptic enhancement accompanied by synaptogenesis in cultured hippocampal slices. Hippocampus. 2008;18(3):281–293. doi: 10.1002/hipo.20391. [DOI] [PubMed] [Google Scholar]
- Verderio C, Coco S, Pravettoni E, Bacci A, Matteoli M. Synaptogenesis in hippocampal cultures. Cellular and Molecular Life Sciences. 1999;55:1448–1462. doi: 10.1007/s000180050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B, Berntson A, Leao RN, Fyffe REW. Activity-dependent regulation of synaptic strength and neuronal excitability in central auditory pathways. J Physiol. 2006;572(2):313–321. doi: 10.1113/jphysiol.2006.104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A, Chen W, Dalva M, Dolmetsch R, Kornhauser J, Shaywitz A, Takasu M, Tao X, Greenberg M. Calcium regulation of neuronal gene expression. PNAS. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4(11):885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- Zhu D, Wu X, Strauss KI, Lipsky RH, Qureshi Z, Terhakopian A, Novelli A, Banaudha K, Marini AM. N-methyl-D-aspartate and TrkB receptors protect neurons against glutamate excitotoxicity through an extracellular signal-regulated kinase pathway. J Neurosci Res. 2005;80(1):104–113. doi: 10.1002/jnr.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]


