Abstract
Neuromuscular junction (NMJ) formation, occurring between motoneurons and skeletal muscle, is a complex multistep process involving a variety of signaling molecules and pathways. In vitro motoneuron-muscle co-cultures are powerful tools to study the role of different growth factors, hormones and cellular structures involved in NMJ formation. In this study, a serum-free culture system utilizing defined temporal growth factor application and a non-biological substrate resulted in the formation of robust NMJs. The system resulted in long-term survival of the co-culture and selective expression of neonatal myosin heavy chain, a marker of myotube maturation. NMJ formation was verified by colocalization of dense clusters of acetylcholine receptors visualized using alpha-bungarotoxin and synaptophysin containing vesicles present in motoneuron axonal terminals. This model will find applications in basic NMJ research and tissue engineering applications such as bio-hybrid device development for limb prosthesis and regenerative medicine as well as for high-throughput drug and toxin screening applications.
Keywords: In Vitro Test, Muscle, Nerve Tissue Engineering, Neural Cell, Surface Modification, Self Assembly
Introduction
The neuromuscular junction (NMJ), formed between motoneurons and skeletal muscle fibers, is one of the most studied synaptic structures [1]. In a mammalian vertebrate, whenever an action potential is fired by a motoneuron, pre-synaptic vesicles loaded with the neurotransmitter acetylcholine (ACh) are released in the synaptic cleft [2]. The released ACh diffuses across the synaptic cleft and binds to the post-synaptic terminals in the muscle enriched with receptors for acetylcholine (AChRs). This leads to muscle contraction. In this transmission process the electrical impulses (action potentials) generated by the motoneuron are converted to chemical signals, then the chemical signals are converted into a mechanical signal in the form of muscle contraction. Therefore, not only do NMJs represent an important system for studying synapse formation and maturation, but also for studying how cells interconvert messages between electrical, chemical and mechanical modalities.
In vivo, NMJ formation is a multistep process, requiring the spatial and temporal interaction of growth factors, hormones and cellular structures that results in a pre-synaptic axonal terminal interfaced with a region of the skeletal muscle membrane (post-synaptic) prepatterned with AChRs [3, 4]. In vitro culture models represent a powerful cell biology tool to study the role of these different growth factors, hormones and cellular structures involved in NMJ formation in a defined, controlled system. Consequently, the development of an in vitro system resulting in NMJ formation would facilitate investigations into the roles of specific factors involved in and required for the process to occur efficiently. However, limited success has been achieved in developing a long-term in vitro system for NMJ formation in the absence of serum containing media and biological substrates. These issues limit the reproducibility of in vitro studies and their translation to tissue engineering applications and high-throughput assay development. For example, the concentration and/or temporal application of medium components could be investigated to determine their influence on NMJ formation, maturation and maintenance. Such a system also benefits from the absence of factors that may be present in serum that would inhibit these processes. Employing a non-biological growth substrate such as trimethoxy-silylpropyl-diethylenetriamine (DETA) provides an additional measure of control. DETA is a silane molecule that forms a covalently bonded monolayer on glass coverslips, resulting in a uniform, hydrophilic surface for cell growth. The use of DETA surfaces is advantageous from a tissue engineering perspective because it can be covalently linked to virtually any hydroxolated surface, it is amenable to patterning using standard photolithography [5] and it promotes long-term cell survival because it is non-digestible by matrix metalloproteinases secreted by the cells [6, 7]. It is also possible that its structural relationship to the growth factor spermidine, which has recently been shown to prolong cell life [8], contribute to its unique ability to enable long-term healthy cell cultures.
Previously, we developed a defined in vitro model facilitating the short-term co-culture of motoneurons and skeletal muscle that resulted in NMJ formation [9]. This model also utilized a biocompatible silane substrate and a serum-free medium formulation. However, further improvements were necessary to enhance the physiological relevance of the NMJ development system. Limitations of the previous model were that it did not support long-term tissue engineering studies and therefore, could not mimic several of the muscle maturation processes observed in vivo by obtaining myotubes that more accurately represent mature extrafusal fibers.
In this study, we have demonstrated a co-culture system that enable sarcomere assembly in the skeletal muscle myotubes as evidenced by A band I band formation, increased NMJ density and selective myosin heavy chain (MHC) class switching. These results suggest we have discovered a group of biomolecules that act as molecular switches promoting NMJ formation and maturation as well as skeletal muscle fiber maturation to the extrafusal phenotype. This model system will be a powerful tool in basic NMJ research, tissue engineered NMJ systems, bio-hybrid device development for limb prosthesis and in regenerative medicine. It could also be useful in new screening modalities for drug development and toxicology investigations.
Materials and Methods
Surface modification and characterization
Glass coverslips (Thomas Scientific 6661F52, 22 × 22 mm No.1) were cleaned using an O2 plasma cleaner (Harrick PDC-32G) for 20 minutes at 100 mTorr. The DETA (United Chemical Technologies Inc. T2910KG) films were formed by the reaction of the cleaned glass surface with a 0.1% (v/v) mixture of the organosilane in freshly distilled toluene (Fisher T2904). The DETA coated coverslips were then heated to approximately 100°C, rinsed with toluene, reheated to approximately 100°C, and then oven dried [10]. Surfaces were characterized by contact angle measurements using an optical contact angle goniometer (KSV Instruments, Cam 200) and by X-ray photoelectron spectroscopy (XPS) (Kratos Axis 165). XPS survey scans, as well as high-resolution N1s and C1s scans utilizing monochromatic Al Kα excitation were obtained [10].
Skeletal muscle culture in serum free medium
Skeletal muscle was dissected from the thighs of the hind limbs of fetal rat (17–18 days old). Briefly, rats were anaesthetized and killed by inhalation of an excess of CO2. This procedure was in agreement with the Animal Research Council of University of Central Florida, which adheres to IACUC policies. The tissue was collected in a sterile 15 mL centrifuge tube containing 1 mL of phosphate-buffered saline (calcium- and magnesium-free) (Gibco 14200075). The tissue was enzymatically dissociated using 2 mL of 0.05% of trypsin-EDTA (Gibco 25300054) solution for 30 minutes in a 37°C water bath at 50 rpm. After 30 minutes the trypsin solution was removed and 4 mL of Hibernate E + 10% fetal bovine serum (Gibco 16000044) was added to terminate the trypsin reaction. The tissue was then mechanically triturated with the supernatant being transferred to a 15 mL centrifuge tube. The same process was repeated two times by adding 2 mL of L15 + 10% FBS each time. The 6 mL cell suspension obtained after mechanical trituration was suspended on a 2 mL, 4% BSA (Sigma A3059) (prepared in L15 medium) cushion and centrifuged at 300×g for 10 minutes at 4°C. The pellet obtained was washed 5 times with L15 medium then resuspended in 10 mL of L15 and plated in 100 mm uncoated dishes for 30 minutes. The non-attached cells were removed and then centrifuged on a 4% BSA cushion [10]. The pellet was resuspended in serum-free medium according to the protocol illustrated in Figure 1 and plated on the coverslips at a density of 700–1000 cells/mm2. The serum-free medium containing different growth factors and hormones was added to the culture dish after one hour. The final medium was prepared by mixing medium one (Table 1) and medium two (Table 2) in a 1:1 v/v ratio. Figure 1 indicates the flowchart of the culture protocol. Tables 1 and 2 list the growth factor and hormone supplement compositions of medium one and medium two. The cells were maintained in a 5% CO2 incubator (relative humidity 85%). The entire medium was replaced after four days with NBactiv4 medium according to the protocol in Figure 1 [11]. Thereafter three-fourths of the medium was changed every three days with NbActiv4.
Figure 1.
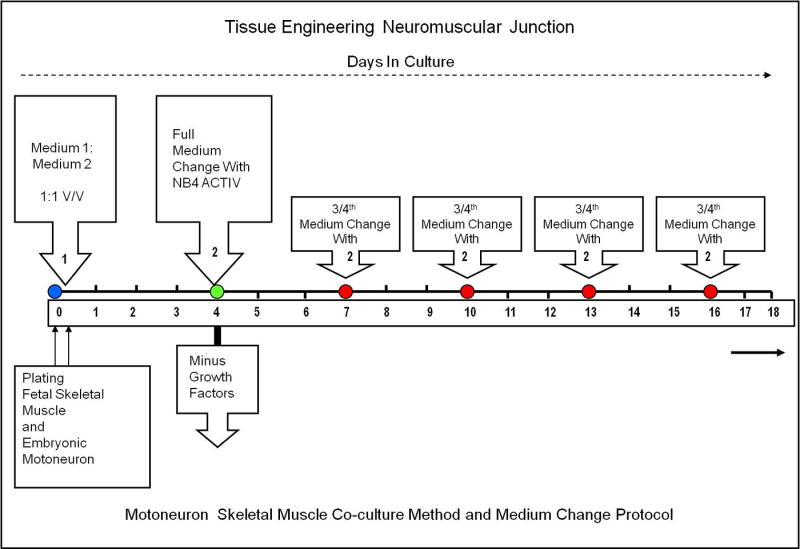
Protocol for long-term NMJ formation in a motoneuron and skeletal muscle co-culture.
Table 1.
Composition of medium 1.
| S. No | Component | Amount | Catalogue # | Source | References |
|---|---|---|---|---|---|
| 1. | Neurobasal | 500 ml | 10888 | Gibco/Invitrogen | [21] |
| 2. | Antibiotic-Antimycotic | 5 ml | 15240-062 | Gibco/Invitrogen | |
| 3. | Glutamax | 5 ml | 35050-061 | Gibco/Invitrogen | |
| 4. | B27 Supplement | 10 ml | 17504-044 | Gibco/Invitrogen | [6, 21] |
| 5. | G5 Supplement (100X) | 5 ml | 17503-012 | Gibco/Invitrogen | [22] |
| 6. | VEGF 165 r Human | 10 μg | P2654 | Gibco/Invitrogen | [23] |
| 7. | Acidic FGF | 12.5 μg | 13241-013 | Gibco/Invitrogen | [24, 25] |
| 8. | Heparin Sulphate | 50 μg | D9809 | Sigma | [24, 25] |
| 9. | LIF | 10 μg | L5158 | Sigma | [26–28] |
| 10. | Vitronectin (Rat Plasma) | 50 μg | V0132 | Sigma | [29, 30] |
| 11. | CNTF | 20 μg | CRC 401B | Cell Sciences | [31, 32] |
| 12. | NT 3 | 10 μg | CRN 500B | Cell Sciences | [33] |
| 13. | NT 4 | 10 μg | CRN 501B | Cell Sciences | [34] |
| 14. | GDNF | 10 μg | CRG 400B | Cell Sciences | [35, 36] |
| 15. | BDNF | 10 μg | CRB 600B | Cell Sciences | [37] |
| 16. | CT-1 | 10 μg | CRC 700B | Cell Sciences | [38, 39] |
Table 2.
Composition of medium 2.
| S. No | Component(s) | Amount | Catalogue | Source | References |
|---|---|---|---|---|---|
| 1. | NBActiv4 | 500 ml | NBActiv4-500 | BrainBits | |
| 2. | Glutamax | 5 ml | 35050-061 | Invitrogen/Gibco | |
| 3. | Antibiotic-antimycotic | 5 ml | 15240-062 | Invitrogen/Gibco | |
| 4. | B27 supplement | 10 ml | 17504-044 | Invitrogen/Gibco | [6, 21] |
| 5. | Cholesterol (250X) | 5 ml | 12531 | Invitrogen/Gibco | |
| 6. | TNF-alpha, human | 10 μg | T6674 | Sigma-Aldrich | [40, 41] |
| 7. | PDGF BB | 50 μg | P4056 | Sigma-Aldrich | [26, 42] |
| 8. | Vasoactive intestinal peptide (VIP) | 250 μg | V6130 | Sigma-Aldrich | [43] |
| 9. | Insulin-like growth factor 1 | 25 μg | I2656 | Sigma-Aldrich | [27, 40] |
| 10. | NAP | 1 mg | 61170 | AnaSpec, Inc. | [44] |
| 11. | Recombinant Apolipoprotein E2 | 50 μg | P2002 | Panvera, Madison, WI | [45] |
| 12. | Laminin, mouse purified | 2 mg | 08-125 | Millipore | [46] |
| 13. | Beta amyloid (1–40) | 1 mg | AG966 | Millipore | [47, 48] |
| 14. | Human Tenascin-C protein | 100 μg | CC065 | Millipore | [49] |
| 15. | rr- Sonic hedgehog, Shh N-terminal | 50 μg | 1314-SH | R&D Systems | [15, 50, 51] |
| 16. | rr (Agrin C terminal) | 50 μg | 550-AG-100 | R&D Systems | [52] |
Rat embryonic motoneuron isolation and co-culture
Rat spinal motoneurons were purified from ventral cords of embryonic day 14 (E14) embryos. Briefly, rats were anaesthetized and killed by inhalation of an excess of CO2. This procedure was in agreement with the Animal Research Council of University of Central Florida, which adheres to IACUC policies. Ventral spinal cord cells from the embryo were collected in cold Hibernate E + GlutaMAX™ + antibiotic-anti-mycotic + B27. The cells were dissociated with 0.05% trypsin-EDTA (Invitrogen) treatment for 15 minutes. The dissociated cells were layered over a 4 mL step gradient (Optiprep diluted 0.505: 0.495 (v/v) with Hibernate E + GlutaMAX™ + antibiotic anti-mycotic + B27 and then made to 15%, 20%, 25% and 35% (v/v) in Hibernate E + GlutaMAX™ + antibiotic-anti-mycotic + B27 followed by centrifugation for 15 min, using 200×g at 4°C. After centrifugation, four bands of cells were obtained. The motoneurons with large somas constituted the uppermost band. These cells present in the uppermost band were collected in fresh Hibernate E + GlutaMAX™ + antibiotic-anti-mycotic + B27 and centrifuged for 5 minutes at 200×g and 4°C. The pelleted motoneurons were resuspended in plating medium then plated on top of muscle cells at a density of 100 cells/mm2. Motoneuron plating was performed 30 minutes after plating of the muscle cells.
Immunocytochemistry
Neonatal myosin heavy chain (neonatal MHC)
Coverslips were rinsed with PBS, fixed in 20°C meth anol for 5–7 min, washed in PBS, incubated in PBS supplemented with 1% BSA and 0.05% saponin (permeabilization solution), and blocked for 30 min in a permeabilization solution + 10% goat serum (blocking solution). Cells were incubated overnight with primary antibody against neonatal MHC (N3.36, IgG, Developmental Studies Hybridoma Bank) diluted (1:5) in the blocking solution. Cells were washed with PBS and incubated with AlexaFluor secondary antibody (Invitrogen) (diluted in PBS) for 2 h. The secondary antibody solution was removed and the cells were rinsed using PBS. The coverslips were dried and mounted on glass slides using VectaShield+DAPI mounting medium (Vector Laboratories H-1200) and viewed on a confocal microscope (UltraVIEW™ LCI, PerkinElmer).
Double staining with Neurofilament 150 and Neonatal myosin heavy chain
Co-cultures were processed for immunocytochemistry as described above. Next, cells were incubated overnight at 4°C with rabbit a nti-neurofilament M polyclonal antibody, 150 kD, (Chemicon, AB1981, diluted 1:2000) and neonatal MHC (N3.36, IgG, Developmental Studies Hybridoma Bank diluted 1:5). After overnight incubation, the coverslips were rinsed three times with PBS and then incubated with the AlexaFluor secondary antibodies (Invitrogen) for 2h. After rinsing three times in PBS, the coverslips were mounted with Vectashield+DAPI mounting medium onto glass slides. The coverslips were visualized and images collected using a confocal microscope (UltraVIEW™ LCI, PerkinElmer). Controls without primary antibody were negative.
AChR + synaptophysin co-staining
AChRs were labeled as described previously by incubating cultures with 5 × 10−8 M of α-bungarotoxin, Alexa Fluor® 488 conjugate (Molecular Probes, B-13422) for 1.5 hr at 37°C before observation [9]. Labeled cultures were fixed with glacial acetic acid and ethanol, washed with PBS, dried, mounted and examined by confocal microscopy. The coverslips which were used for double staining with AChR + synaptophysin for locating the NMJ were processed further. After 1.5 hr of α-bungarotoxin labeling of the AChR receptors, the coverslips were fixed, blocked, permeabilized and incubated overnight with synaptophysin antibody (MAB368, diluted 1:1000; Millipore/Chemicon), the pre-synaptic marker present in motoneuron axonal terminals.
Data Analysis
The statistics shown in Table 3 were calculated using the following procedure. One coverslip was randomly selected from each experiment (typically, there are six coverslips per experiment). 25 non-overlapping fields of view were used to characterize each coverslip. At the magnification used, 25 fields covers over 40% of the surface area of the coverslip. The values of n shown in the table indicate the number of experiments used to obtain each result.
Table 3.
Comparison of the culture following temporal application of the growth factor and continuous application of the growth factors
| Temporal application of growth factors | Continuous application of growth factors | |
|---|---|---|
| Quality of the coculture based on visual observations | Visually individual myotubes and motoneurons could be distinctly seen in the culture by day 10. Myotube formation is initiated on day 4 and continued through day 12. Large, multipolar motoneurons with long processes were clearly seen by day 10. The maturation and appearance of striated myotubes were seen after day 15, and by day 25 a large population of myotubes exhibited striation patterns. | When growth factors were applied continuously, the cells proliferated uncontrollably. The uncontrolled growth of different cell types in the culture prevented the myotube formation process. This resulted in detachment of the cells from the substrate by day 9 and the culture to die out by day 12. The formation of very few myotubes was observed in the culture during this 10–12 day period. |
| Survival of the culture | Cultures survived for more than 5 weeks with a maximum up to 7 weeks (n>25); where n was the total number of trials. | Cultures survived for 10–12 days (n>7); where n was the total number of trials. |
| Fraction of myotubes indicating evidence of neuromuscular junction (NMJ) formation between day 12–15 in the co-culture | % Myotubes with NMJ: 20.36 ± 3.75 (mean ± standard error, n=11) | Since the cultures died by day 10–12 and there were hardly any myotube formation, no NMJ quantification was done on these cultures. |
| Fraction of myotubes exhibiting characteristic striation patterns in in pure muscle culture as well as in co-culture between day 20–25 |
In pure muscle culture: 40.95 ± 3.06 (mean ± standard error, n=8) In coculture: 70.33 ± 2.17 (mean ± standard error, n=8) |
Since there was little evidence of myotube formation, and the cultures did not survive beyond day 10, no estimation was possible. |
| Fraction of myotubes exhibiting N3.36 protein expression in pure muscle culture as well as in co- culture between day 20–25 |
In pure muscle culture: 29.95 ± 2.96 (mean ± standard error, n=12) In coculture: 60.33 ± 3.79 (mean ± standard error, n=12) |
No N3.36 expression was observed in any of the surviving myotubes. |
Results
DETA surface modification and characterization
Static contact angle and XPS analysis were used for the validation of the surface modifications and for monitoring the quality of the surfaces. Stable contact angles (40.64° ± 2.9/mean ± SD) throughout the study indicated high reproducibility and quality of the DETA surfaces and these characteristics were similar to previously published results [6, 7, 9, 10, 12]. Based on the ratio of the N 1s (401 and 399 eV) and the Si 2p3/2 peaks, XPS measurements indicated that a reaction-site limited monolayer of DETA was formed on the coverslips [13].
Temporal growth factor application
The formation of the maximal number of neuromuscular junctions was observed using the temporal growth factor application technique (Table 3) described in Figure 1. Upon plating of the motoneurons and skeletal muscle, the cells were treated with medium containing factors that promoted both growth and survival as well as enhancement of NMJ formation (Table 1, Table 2 and Table 3). After 3 days in culture, the entire medium was removed and switched to a minimal formulation, NBactiv4, which facilitated both long-term survival and further development of the NMJs (Figure 1). Further 3/4th of the NB4Activ medium per well was removed and replaced with an equal volume of fresh Nb4Activ medium. When compared to the continuous application of growth factors, the timed application resulted in cultures that lasted for up to 7 weeks as opposed to 10–12 days.
Culture morphology of motoneuron and skeletal muscle myotube interactions
Phase contrast microscopy was used to visualize motoneuron axons appearing to interact with skeletal muscle myotubes between days 12–15 (Table 3, Figure 2, A–D). Some of the axonal processes appear to branch and terminate on the myotubes. Furthermore, many of the myotubes exhibited characteristic striation patterns (Table 3) observed after sarcomere formation when the fibers reached approximately 25–30 days in culture (Figure 3, A–D). Quantification of the appearance of striations after this time indicated that the co-cultures contained about twice the number of myotubes showing striations.
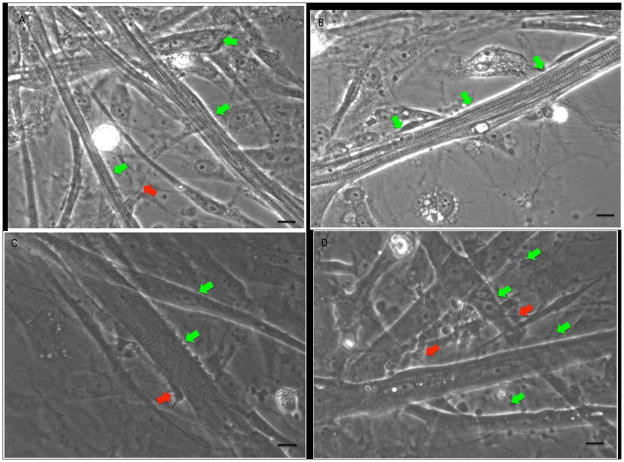
Figure 2. Phase contrast micrographs of the motoneuron and skeletal muscle co-culture between days 12–15.
(A–D) Red arrows indicated the distinct morphology of the motoneuron and its processes. Green arrows indicate the myotubes. Scale bar = 25 μm.
Figure 3. Phase contrast pictures of the co-cultures between days 25–30.
(A, B) The myotubes exhibited characteristic striations. (C, D) Myotubes with striations and myotubes without striations. Red arrows indicate the motoneuron cell body and the processes. The green arrows indicate the myotubes. The scale bar for A, B = 40 μm. The scale bar for C, D = 25 μm.
Immunocytochemical characterization of motoneuron and skeletal muscle co-culture
The characteristic protein expression patterns of the motoneurons and myotubes in co-culture were evaluated at day 25. Immunocytochemistry was used to visualize the neurofilament protein expression in the motoneurons and neonatal myosin heavy chain (MHC) expression for the myotubes (Figure 4, A–B). Motoneuron processes were clearly indicated interacting with the skeletal muscle myotubes. A band/I band formation was more visible in the myotubes after staining with the neonatal myosin heavy chain antibody (Table 3). The immunocytochemical analysis supported the morphological analysis, which had indicated the presence of striations in double the number of myotubes as observed with the muscle only controls.
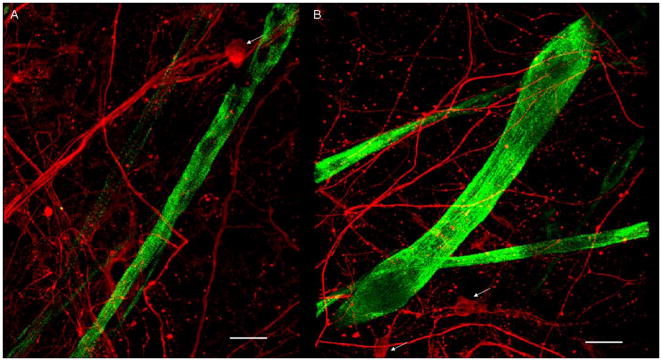
Figure 4. Immunocytochemistry of co-cultures at day 25.
(A–B) NF-150 (red) indicates the large motoneurons and their processes (white arrows). The striated myotubes (green) stained for nMHC (N3.36). Scale bar = 50 μm.
Neuromuscular junction formation
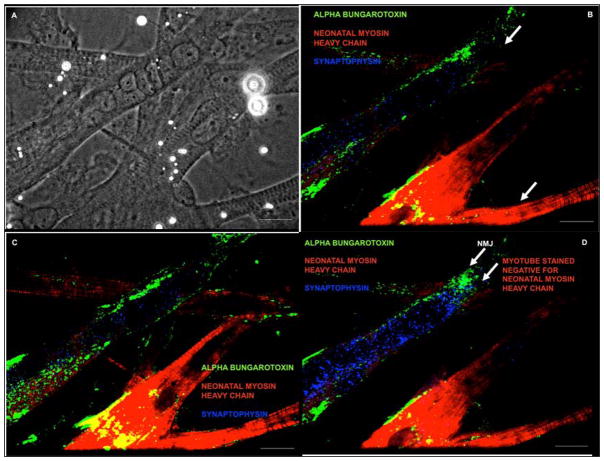
In order to determine neuromuscular junction formation using this novel medium formulation, the clustering of AChRs using alpha bungarotoxin and their colocalization with synaptophysin vesicles was analyzed immunocytochemically. The colocalization of these two synaptic markers indicated the proximity of pre-synaptic and post-synaptic structures and was a positive indication of NMJ formation. This technique was used to identify the colocalization of synaptophysin vesicles with the AChR clusters (Figure 5, A–D). The axon + myotube interactions that did not result in the colocalization of pre-synaptic and post-synaptic structures were also identified (Figure 6, A–B). The observation of the negative result defines the difference between colocalization and non-colocalization and emphasizes the positive result observed in this system. Figure 7 illustrates NMJ formation between a myotube in culture that did not stain for neonatal myosin heavy chain and a motoneuron (Table 3).
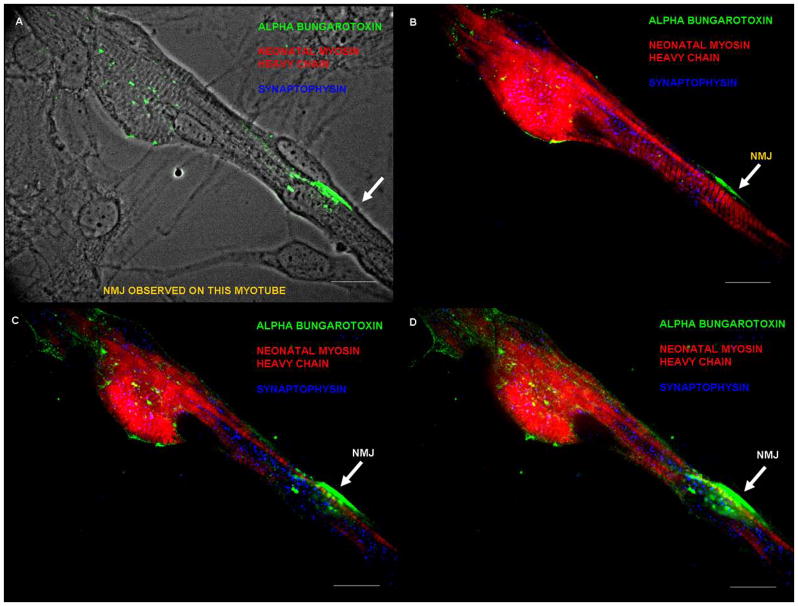
Figure 5. Neuromuscular junction (NMJ) formation between day 30–40.
(A) Phase picture of the myotube indicating the alpha-bungarotoxin staining in green, (B) Triple stain, showing the close proximity of alpha-bungarotoxin (green) and synaptophysin (blue) indicating synapse formation at a specific confocal plane and myotube striations are indicated in red (nMHC), (C–D) NMJ observed at two different planes using confocal microscopy. A much more dense clustering of synaptophysin and alpha-bungarotoxin was observed in these planes.
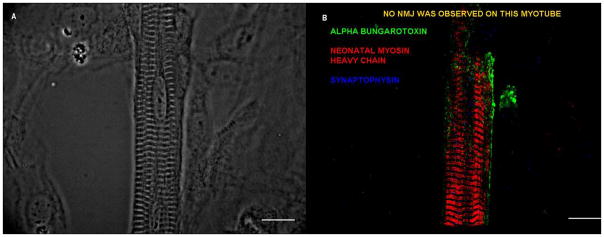
Figure 6. Striated myotube development in the absence of NMJ formation.
(A, B) No NMJs were observed on this striated myotube. (A) A phase picture of the myotube, (B) Immunostained picture of the same myotube with alpha-bungarotoxin, N3.36 and synaptophysin. Scale bar = 50 μm.
Figure 7. NMJ formation on a N3.36 (−) myotube.
(A) Phase picture showing the different morphologies of myotubes in the co-culture, (B–D) NMJ formation was observed on a myotube that was negative for N3.36. Culture stained with alpha-bungarotoxin, N3.36 and synaptophysin. Possibly the myotube on which NMJ was formed was immature and had not yet expressed the neonatal myosin heavy chain marker (N3.36).
Discussion
This work documents the substantial improvement of an in vitro model system for NMJ formation. Specifically, we observed enhanced survivability of the culture resulting in our ability to conduct long-term studies on the motoneuron-skeletal muscle co-cultures. This increased survivability resulted in maturation of the skeletal muscle myotubes and a significant improvement in the number of NMJs formed in culture.
Previously, we developed the first defined culture model to co-culture embryonic motoneuron and fetal skeletal muscle, however this model was not suitable for long-term tissue engineering studies and the myotubes in the culture only expressed an early muscle marker, i.e. fetal myosin heavy chain and none of the myotubes exhibited characteristic striations. In this study, significant improvement over our previous motoneuron-skeletal muscle co-culture model system was documented. This new culture model supported long-term co-culture of both motoneuron and muscle, resulted in a more adult-like morphology of the muscle and a higher density of neuromuscular junctions (NMJ). Our findings were supported by morphological and immunocytochemical data and results were summarized in Table 3.
We developed this serum-free medium, supplemented with growth factors that supported the survival, proliferation and fusion of fetal rat myoblasts into contractile myotubes, in a semi-empirical fashion. The rational for selecting the growth factors was based on the distribution of their cognate receptors in the developing myotubes in rat fetus [14–16]. Tables 1 and 2 reference the literature where these individual growth factors, hormones and neurotransmitters were observed to support muscle and neuromuscular junction development. The composition in Table 1 is the formulation used for a previously published medium utilized for motoneuron-muscle co-culture and adult spinal cord neuron culture [9, 17–19]. Table 2 lists the twelve additional factors identified in muscle development and neuromuscular junction formation that enabled the increased survivability of the system. Further addition of the factors in Table 2 promoted formation of characteristic striation in the muscle in the culture. The use of NBactiv4 for the maintenance of the cells significantly improved the survival of the skeletal muscle derived myotubes despite the fact that the original purpose of the development of NBactiv4 was for the long-term maintenance and synaptic connectivity of fetal hippocampal neurons in vitro [11].
In our previous co-culture model, we did not observe the expression of neonatal MHC proteins in the myotubes. Interestingly, when this same medium and protocol was used to culture pure skeletal muscle we observed certain striking differences. The pure muscle culture survived longer, exhibited characteristic striations, but only a very small percentage of myotubes expressed N3.36 [20]. To the best of our understanding, the N3.36 expression in skeletal muscle in culture is influenced by the motoneurons either physically or by certain trophic factors secreted in the presence of this modified medium and Nbactiv4. This observation needs further studies in order to dissect the molecular pathways regulating N3.36 expression in pure skeletal muscle culture and in skeletal muscle-motoneuron co-culture. Also, the potential regulation of MHC class switching independent of neuronal innervation/denervation represents an interesting topic for further study and would have application in therapies for muscle-nerve diseases such as ALS, spinal muscular atrophy, spinal cord injury and myasthenia gravis.
Conclusions
The development of robust NMJ formation, long-term survival of motoneuron+skeletal muscle co-cultures and selective MHC class switching is documented in this research. This improved system supports the goal of creating a physiologically relevant tissue engineered motoneuron+skeletal muscle construct and puts within reach the goal of developing functional bio-hybrid devices to analyze NMJ activity. This defined model can also be used to map the developmental pathway regulating NMJ formation and MHC class switching. Furthermore, we believe this serum-free culture system will be a powerful tool in developing advanced strategies for regenerative medicine in ALS, stretch reflex arc development and integrating motoneuron+skeletal muscle with bio-hybrid prosthetic devices. Due to the use of a serum-free defined culture system this also has applications for new high-throughput screening systems for use in drug discovery research and toxicology investigations.
Acknowledgments
We would like to acknowledge funding support from NIH grant number 5RO1-NS050452.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Witzemann V. Development of the neuromuscular junction. Cell Tissue Res. 2006;326(2):263–71. doi: 10.1007/s00441-006-0237-x. [DOI] [PubMed] [Google Scholar]
- 2.Chow I, Poo MM. Release of acetylcholine from embryonic neurons upon contact with muscle cell. J Neurosci. 1985;5(4):1076–82. doi: 10.1523/JNEUROSCI.05-04-01076.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colomar A, Robitaille R. Glial modulation of synaptic transmission at the neuromuscular junction. Glia. 2004;47(3):284–9. doi: 10.1002/glia.20086. [DOI] [PubMed] [Google Scholar]
- 4.English AW. Cytokines, growth factors and sprouting at the neuromuscular junction. J Neurocytol. 2003;32(5):943–60. doi: 10.1023/B:NEUR.0000020634.59639.cf. [DOI] [PubMed] [Google Scholar]
- 5.Ravenscroft MS, Bateman KE, Shaffer KM, Schessler HM, Jung DR, Schneider TW, et al. Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane- modified surfaces. J Am Chem Soc. 1998;120(47):12169–77. [Google Scholar]
- 6.Das M, Molnar P, Gregory C, Riedel L, Hickman JJ. Long-term culture of embyonic rat cardiomyocytes on an organosilane surface in a serum free medium. Biomaterials. 2004;25(25):5643–7. doi: 10.1016/j.biomaterials.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Das M, Wilson K, Molnar P, Hickman JJ. Differentiation of skeletal muscle and integration of myotubes with silicon microstructures using serum-free medium and a synthetic silane substrate. Nat Protoc. 2007;2(7):1795–801. doi: 10.1038/nprot.2007.229. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305–14. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 9.Das M, Rumsey JW, Gregory CA, Bhargava N, Kang JF, Molnar P, et al. Embryonic motor neuron-skeletal muscle co-culture in a defined system. Neuroscience. 2007;146:481–8. doi: 10.1016/j.neuroscience.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 10.Das M, Gregory CA, Molnar P, Riedel LM, Hickman JJ. A defined system to allow skeletal muscle differentiation and subsequent integration with silicon microstructures. Biomaterials. 2006;27(24):4374–80. doi: 10.1016/j.biomaterials.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 11.Brewer GJ, Boehler MD, Jones TT, Wheeler BC. NbActiv4 medium improvement to Neurobasal/B27 increases neuron synapse densities and network spike rates on multielectrode arrays. J Neurosci Methods. 2008;170(2):181–7. doi: 10.1016/j.jneumeth.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das M, Molnar P, Devaraj H, Poeta M, Hickman J. Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnol Prog. 2003;19:1756–61. doi: 10.1021/bp034076l. [DOI] [PubMed] [Google Scholar]
- 13.Stenger DA, Georger JH, Dulcey CS, Hickman JJ, Rudolph AS, Nielsen TB, et al. Coplanar molecular assemblies of aminoalkylsilane and perfluorinated alkylsilane - characterization and geometric definition of mammalian-cell adhesion and growth. J Am Chem Soc. 1992;114(22):8435–42. [Google Scholar]
- 14.Arnold HH, Winter B. Muscle differentiation: more complexity to the network of myogenic regulators. Curr Opin Genet Dev. 1998;8(5):539–44. doi: 10.1016/s0959-437x(98)80008-7. [DOI] [PubMed] [Google Scholar]
- 15.Brand-Saberi B. Genetic and epigenetic control of skeletal muscle development. Ann Anat. 2005;187(3):199–207. doi: 10.1016/j.aanat.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992;154(2):261–72. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- 17.Das M, Bhargava N, Bhalkikar A, Kang J-F, Hickman JJ. Temporal neurotransmitter conditioning restores the functional activity of adult spinal cord neurons in long-term culture. Exp Neurol. 2008;209:171–80. doi: 10.1016/j.expneurol.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das M, Bhargava N, Gregory C, Riedel L, Molnar P, Hickman JJ. Adult rat spinal cord culture on an organosilane surface in a novel serum-free medium. In Vitro Cell Dev Biol Anim. 2005;41(10):343–8. doi: 10.1007/s11626-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 19.Das M, Patil S, Bhargava N, Kang J-F, Riedel L, Seal S, et al. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28(10):1918–25. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. Skeletal muscle tissue engineering: an improved model promoting long term survival of myotubes, structural development of e-c coupling apparatus and neonatal myosin heavy chain (MHC) expression. Biomaterials. 2009;30:5392–402. doi: 10.1016/j.biomaterials.2009.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal-neurons in b27-supplemented neurobasal(tm), a new serum-free medium combination. J Neurosci Res. 1993;35(5):567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 22.Bottenstein JE. Advances in vertebrate cell culture methods. Science. 1988;239(4841 Pt 2):G42–G8. [PubMed] [Google Scholar]
- 23.Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, et al. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10(5):844–54. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Dusterhoft S, Pette D. Evidence that acidic fibroblast growth factor promotes maturation of rat satellite-cell-derived myotubes in vitro. Differentiation. 1999;65(3):161–9. doi: 10.1046/j.1432-0436.1999.6530161.x. [DOI] [PubMed] [Google Scholar]
- 25.Motamed K, Blake DJ, Angello JC, Allen BL, Rapraeger AC, Hauschka SD, et al. Fibroblast growth factor receptor-1 mediates the inhibition of endothelial cell proliferation and the promotion of skeletal myoblast differentiation by SPARC: a role for protein kinase A. J Cell Biochem. 2003;90(2):408–23. doi: 10.1002/jcb.10645. [DOI] [PubMed] [Google Scholar]
- 26.Husmann I, Soulet L, Gautron J, Martelly I, Barritault D. Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev. 1996;7(3):249–58. doi: 10.1016/s1359-6101(96)00029-9. [DOI] [PubMed] [Google Scholar]
- 27.Malm C, Sjodin TL, Sjoberg B, Lenkei R, Renstrom P, Lundberg IE, et al. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J Physiol. 2004;556(Pt 3):983–1000. doi: 10.1113/jphysiol.2003.056598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vakakis N, Bower J, Austin L. In vitro myoblast to myotube transformations in the presence of leukemia inhibitory factor. Neurochem Int. 1995;27(4–5):329–35. doi: 10.1016/0197-0186(95)00014-y. [DOI] [PubMed] [Google Scholar]
- 29.Biesecker G. The complement SC5b-9 complex mediates cell adhesion through a vitronectin receptor. J Immunol. 1990;145(1):209–14. [PubMed] [Google Scholar]
- 30.Gullberg D, Sjoberg G, Velling T, Sejersen T. Analysis of fibronectin and vitronectin receptors on human fetal skeletal muscle cells upon differentiation. Exp Cell Res. 1995;220(1):112–23. doi: 10.1006/excr.1995.1297. [DOI] [PubMed] [Google Scholar]
- 31.Cannon JG. Intrinsic and extrinsic factors in muscle aging. Ann N Y Acad Sci. 1998;854:72–7. doi: 10.1111/j.1749-6632.1998.tb09893.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Mao Z, Liu S, Liu H, Wang X, Wu H, et al. Dedifferentiation of adult human myoblasts induced by ciliary neurotrophic factor in vitro. Mol Biol Cell. 2005;16(7):3140–51. doi: 10.1091/mbc.E05-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oakley RA, Lefcort FB, Clary DO, Reichardt LF, Prevette D, Oppenheim RW, et al. Neurotrophin-3 promotes the differentiation of muscle spindle afferents in the absence of peripheral targets. J Neurosci. 1997;17(11):4262–74. doi: 10.1523/JNEUROSCI.17-11-04262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrasco DI, English AW. Neurotrophin 4/5 is required for the normal development of the slow muscle fiber phenotype in the rat soleus. J Exp Biol. 2003;206(Pt 13):2191–200. doi: 10.1242/jeb.00412. [DOI] [PubMed] [Google Scholar]
- 35.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266(5187):1062–4. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 36.Yang LX, Nelson PG. Glia cell line-derived neurotrophic factor regulates the distribution of acetylcholine receptors in mouse primary skeletal muscle cells. Neuroscience. 2004;128(3):497–509. doi: 10.1016/j.neuroscience.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 37.Mousavi K, Parry DJ, Jasmin BJ. BDNF rescues myosin heavy chain IIB muscle fibers after neonatal nerve injury. Am J Physiol Cell Physiol. 2004;287(1):C22–9. doi: 10.1152/ajpcell.00583.2003. [DOI] [PubMed] [Google Scholar]
- 38.Oppenheim RW, Wiese S, Prevette D, Armanini M, Wang S, Houenou LJ, et al. Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J Neurosci. 2001;21(4):1283–91. doi: 10.1523/JNEUROSCI.21-04-01283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peroulakis ME, Forger NG. Ciliary neurotrophic factor increases muscle fiber number in the developing levator ani muscle of female rats. Neurosci Lett. 2000;296(2–3):73–6. doi: 10.1016/s0304-3940(00)01649-9. [DOI] [PubMed] [Google Scholar]
- 40.Al-Shanti N, Saini A, Faulkner SH, Stewart CE. Beneficial synergistic interactions of TNF-alpha and IL-6 in C2 skeletal myoblasts--potential cross-talk with IGF system. Growth Factors. 2008;26(2):61–73. doi: 10.1080/08977190802025024. [DOI] [PubMed] [Google Scholar]
- 41.Caratsch CG, Santoni A, Eusebi F. Interferon-alpha, beta and tumor necrosis factor-alpha enhance the frequency of miniature end-plate potentials at rat neuromuscular junction. Neurosci Lett. 1994;166(1):97–100. doi: 10.1016/0304-3940(94)90849-4. [DOI] [PubMed] [Google Scholar]
- 42.Jin P, Sejersen T, Ringertz NR. Recombinant platelet-derived growth factor-BB stimulates growth and inhibits differentiation of rat L6 myoblasts. J Biol Chem. 1991;266(2):1245–9. [PubMed] [Google Scholar]
- 43.Gold MR. The effects of vasoactive intestinal peptide on neuromuscular transmission in the frog. J Physiol. 1982;327:325–35. doi: 10.1113/jphysiol.1982.sp014234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aracil A, Belmonte C, Calo G, Gallar J, Gozes I, Hoyer D, et al. Proceedings of Neuropeptides 2004, the XIV European Neuropeptides Club meeting. Neuropeptides. 2004;38(6):369–71. doi: 10.1016/j.npep.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Robertson TA, Dutton NS, Martins RN, Taddei K, Papadimitriou JM. Comparison of astrocytic and myocytic metabolic dysregulation in apolipoprotein E deficient and human apolipoprotein E transgenic mice. Neuroscience. 2000;98(2):353–9. doi: 10.1016/s0306-4522(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 46.Foster RF, Thompson JM, Kaufman SJ. A laminin substrate promotes myogenesis in rat skeletal muscle cultures: analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Dev Biol. 1987;122(1):11–20. doi: 10.1016/0012-1606(87)90327-7. [DOI] [PubMed] [Google Scholar]
- 47.Akaaboune M, Allinquant B, Farza H, Roy K, Magoul R, Fiszman M, et al. Developmental regulation of amyloid precursor protein at the neuromuscular junction in mouse skeletal muscle. Mol Cell Neurosci. 2000;15(4):355–67. doi: 10.1006/mcne.2000.0834. [DOI] [PubMed] [Google Scholar]
- 48.Wang P, Yang G, Mosier DR, Chang P, Zaidi T, Gong YD, et al. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. J Neurosci. 2005;25(5):1219–25. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22(2):138–47. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 50.Elia D, Madhala D, Ardon E, Reshef R, Halevy O. Sonic hedgehog promotes proliferation and differentiation of adult muscle cells: Involvement of MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta. 2007;1773(9):1438–46. doi: 10.1016/j.bbamcr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Norris W, Neyt C, Ingham PW, Currie PD. Slow muscle induction by Hedgehog signalling in vitro. J Cell Sci. 2000;113 ( Pt 15):2695–703. doi: 10.1242/jcs.113.15.2695. [DOI] [PubMed] [Google Scholar]
- 52.Bandi E, Jevsek M, Mars T, Jurdana M, Formaggio E, Sciancalepore M, et al. Neural agrin controls maturation of the excitation-contraction coupling mechanism in human myotubes developing in vitro. Am J Physiol Cell Physiol. 2008;294(1):C66–73. doi: 10.1152/ajpcell.00248.2007. [DOI] [PubMed] [Google Scholar]