Abstract
There is increasing evidence that exposure to high magnetic fields of 4 tesla and above perturbs the vestibular system of rodents and humans. Performance in a swim test is a sensitive test of vestibular function. In order to determine the effect of magnet field exposure on swimming in mice, mice were exposed for 30-min within a 14.1 tesla superconducting magnet and then tested at different times after exposure in a 2-min swim test. As previously observed in open field tests, mice swam in tight counter-clockwise circles when tested immediately after magnet exposure. The counter-clockwise orientation persisted throughout the 2-min swim test. The tendency to circle was transient, because no significant circling was observed when mice were tested at 3 min or later after magnet exposure. However, mice did show a decrease in total distance swum when tested between 3 and 40 min after magnet exposure. The decrease in swimming distance was accompanied by a pronounced postural change involving a counter-clockwise twist of the pelvis and hindlimbs that was particularly severe in the first 15 s of the swim test. Finally, no persistent difference from sham-exposed mice was seen in the swimming of magnet-exposed mice when tested 60 min, 24 h, or 96 h after magnet exposure. This suggests that there is no long-lasting effect of magnet exposure on the ability of mice to orient or swim. The transient deficits in swimming and posture seen shortly after magnet exposure are consistent with an acute perturbation of the vestibular system by the high magnetic field.
Keywords: vestibular system, posture, magnetic resonance imaging, inner ear
Introduction
There is increasing evidence that static high magnetic fields of 4 tesla (T) and above interact with the vestibular system of rodents [1] and humans [2–5], although the mechanism of interaction is unknown. Following exposure to static magnetic fields of 7 T and above, rats walk in tight head-to-tail circles and have decreased levels of rearing [6, 7]. If magnetic field exposure is paired with a novel taste solution (e.g. a saccharin or glucose-saccharin solution), the rats acquire a conditioned taste aversion (CTA) [6, 8]. Multiple pairings produce a stronger CTA that extinguishes more slowly than single-pairing CTA [6, 9]. Magnet exposure also activates neurons in the visceral and vestibular relays of the brainstem, as revealed by c-Fos induction [10]. All of these effects are consistent with the consequences of vestibular stimulation or perturbation in rats. For example, whole-body rotation can mediate CTA [11–13] and induces brainstem c-Fos [14]; unilateral labyrinthectomy causes locomotor circling and induces c-Fos in vestibular nuclei, especially during the period of vestibular compensation (e.g. [15]). Furthermore, all of the effects of magnet exposure are abolished in rats after bilateral chemical labyrinthectomy, demonstrating that the peripheral vestibular apparatus of the inner ear is necessary for magnet-induced perturbation [16].
The most obvious acute effect of magnetic field exposure is the induction of locomotor circling in rats and mice. Although not every animal circles after exposure to a high magnetic field, the greater the intensity of the magnet field (i.e. from 7 T to 17 T) the greater the average number of circles [6, 7]. Similarly, the longer the duration of exposure in a 14.1 T magnet at 5 min or above, the more circles are observed on average [6].
Intriguingly, the direction of circling is dependent on the orientation of the rat within the magnetic field. Rats placed head-up in a 14.1 T superconducting magnet facing the positive pole of the magnet (B+) walk in counter-clockwise circles [6]. Rats placed head-down in the 14.1 T magnet facing the negative pole (B−) walk in clockwise circles [6]. The response depends on the relative orientation of the magnetic field, and not the fact that the rat is head-up or head-down. Thus, rats placed head-up in a resistive magnet with the field orientation reversed (i.e., so that the head is towards the negative pole) also walk in clockwise circles [7]. The reason for the asymmetrical effect of the magnetic field is unknown, but presumably it reflects an asymmetry of the rat’s vestibular apparatus such that opposite forces are experienced during head-up vs. head-down exposure to the magnetic field.
We hypothesize that locomotor circling reflects either an acute perturbation of the vestibular system, or a compensatory response to sustained vestibular stimulation during the exposure period. (For example, if the animal perceives a clockwise turning during magnet exposure, immediately after the exposure it might walk in a counter-clockwise direction in an attempt to compensate, as in podokinetic after-rotation [17]). It would be helpful to determine the intensity and duration of the magnet’s effect on locomotor orientation. A rapid decay of the locomotor circling would indicate a transient effect on the animal’s vestibular system, consistent with acute perturbation or short-term compensation. A more prolonged deficit would indicate a long-lasting effect of the high magnetic field, perhaps including permanent damage.
In previous studies the circular walking induced by magnet exposure was only apparent for 2–3 minutes in an open field test chamber. The duration of the magnet’s effect on locomotor orientation is difficult to assess in the open field, however. There is variability in the amount of locomotion produced by individual animals. Locomotion in general may be suppressed, which would mask a continuing effect on orientation; for example, magnet exposure suppresses rearing and drinking behavior [18]. The effects of the magnet at later time points after exposure have also not been assessed.
To better quantify the effects of the magnetic field on orientation, we evaluated the swimming behavior of mice at various time points after exposure to a 14.1 T magnetic field. The path and distance traveled was determined by computerized tracking of the swimming mice. Because position of the mice was precisely determined, angular velocity could be calculated as a measure of the mouse’s tendency to circle.
There are several advantages of a swimming test vs. an open field test chamber. Rodents are motivated to escape the water, e.g. by rapidly swimming in a straight line from the center to the edge of the pool. Thus all the subjects are likely to swim, and locomotion will be continuous throughout the swim test. The use of a swimming pool minimizes distractions, such as odors, that might be found in an open field test chamber. Importantly for vestibular studies, a swim test minimizes gravitational and surface cues that aid in orientation. Swimming is frequently used as a test of vestibular function, e.g. in animals with unilateral or bilateral labyrinthectomy [19, 20], with mutations of the vestibular system [21, 22], after exposure to low gravity environments [23], or after vestibular stimulation by whole-body rotation [24].
Materials and Methods
Animals
Adult male C57BL/J mice (20–25 g; Jackson Laboratories, Bar Harbor, ME) were housed individually in polycarbonate cages (28 cm × 18 cm × 12cm height) in a temperature-controlled colony room (22 ± 2 °C, 30–40% humidity) at the US National High Magnetic Field Laboratory at The Florida State University. The mice were maintained on a 12 h light/dark cycle with lights-on at 7:00 A.M. All procedures were conducted during the light cycle. The mice had ad libitum access to Purina Rodent Chow and deionized-distilled water. All procedures were approved by the Florida State University animal care and use committee.
Magnet
Exposure to the high magnetic field was conducted in a superconducting magnet with a vertical bore designed for biochemical nuclear magnetic resonance (NMR) studies. The 14.1 T magnet was a 600 MHz Bruker Cryo magnet with an 89 mm bore and fixed field strength of 14.1 T. It contained a shim magnet extending along the magnet bore for approximately ± 15 cm from the magnet’s core, which was used to stabilize the magnetic field and give a central core field of uniform strength. The magnetic field was oriented vertically so that the positive pole was at the top of the magnet. The magnet was operated without radiofrequency pulses, so rats were exposed only to static magnetic fields.
Magnet exposure and sham exposure
Prior to exposure to the magnetic field (“magnet exposure”) or sham exposure, mice were restrained by placing them in a plastic tube made from a 50 ml conical centrifuge tube, with the head of the mouse positioned at the cone end [25]. A hole in the tip of the cone allowed for breathing. A plastic plug with a hole to allow for the tail of the mouse at the caudal end of the tube restrained the mouse from moving. Two or three restrained mice in these tubes were fitted into a plastic collar that was inserted vertically into the bore of the magnet and exposed to the 14.1 T magnetic field. Mice were inserted head-up into the bottom of the vertical bore of the magnet, then quickly raised through the magnet until the mice were in the core of the magnet. Mice remained in the 14.1 T magnetic field for 30 min.
To control for restraint and handling, additional mice were sham-exposed. Sham-exposed mice were inserted into identical restraint tubes. Then, the sham-exposed mice were inserted vertically into an opaque polyvinyl-chloride pipe with dimensions and conditions similar to those of the bore of the 14.1 T magnet. The sham apparatus was located in the same room as the 14.1 T magnet, but placed outside the 5 gauss field line.
Test of Swimming
At varying time points after magnet exposure or sham exposure, the swimming behavior of the mice was assessed. Individual mice were placed in the center of a circular swimming pool (1 m diameter and 30 cm depth) and allowed to swim for 2 min. Mice were videotaped from above with a digital camcorder (Sony DCR-TRV530) mounted above the swimming pool. After the swim test mice were returned to their home cage, which was positioned under a heat lamp until dry. All mice were tested only once.
For quantitative analysis, the videotapes of mice swimming were later digitized onto a Macintosh computer using the iMovie program (Apple, Inc.) at a rate of 30 frames/s. Each frame of the digitized video was 640 pixels wide by 480 pixels tall, giving a spatial resolution of 4 pixels / cm. A custom software program was used to determine automatically the position of the mouse every 1/3 s (i.e. in every tenth video frame). Thus, a total of 360 position points were collected for each 2-min test. From every successive pair of position points [x,y]i and [x,y]i +1 the tracking program derived the distance traveled (cm) and the bearing between the two points. Angular velocity (degrees/s) was calculated as the change in bearing between two successive pairs of points (i.e. the angle defined by [x,y]i, [x,y]i +1, and [x,y]i +2) divided by the elapsed time. The average number of circles was calculated from the cumulative angle traversed by the mice while swimming.
Statistical analysis
All data were analyzed using Kaleidagraph software (Synergy Software) as described below. When ANOVA revealed a significant difference, Tukey's HSD test was performed to determine significant differences between specific groups. Data are presented as mean +/− s.e.m.
Experiment 1: Short-term effects of magnetic field exposure
Mice (n=42) were exposed within the bore of the 14.1 T magnet for 30 min. After exposure, one group of mice (at the 0 min time point) was immediately placed in the swimming pool for the 2-min test. The remaining magnet-exposed mice were returned to their home cages until they were removed and placed in the swimming pool for the 2-min test at 3, 6, 10, 20, 40 or 60 minutes after the end of the magnet exposure. Six mice were tested at each time point and individual mice were tested only once at one time point. In addition, a control group of mice (n=6) were sham-exposed for 30 min, and then immediately tested in the swimming pool.
Experiment 2: Long-term effects of magnetic field exposure
Mice (n=12) were exposed to the 14.1 T magnetic field for 30-min, then returned to their home cages. Twenty-four hours or 96 h after magnet-exposure (n=6 at each time point), the mice were removed from their home cage and placed in the swimming pool for a 2-min swim test. In addition, control mice (n= 12) were sham-exposed for 30-min, returned to their home cages, and then tested in the swimming pool at 24 or 48 hours after sham-exposure (n= 6 at each time point).
Results
Experiment 1: Short-term effects of magnetic field exposure
Description of Swimming
Sham-exposed mice placed in the center of the swimming pool swam without difficulty and quickly oriented towards the side of the pool (see example trace in Figure 1A). Upon reaching the side, sham-exposed mice followed it in either direction, with occasional forays towards the center of the pool. With the exception of occasional turns in either clockwise or counter-clockwise directions, the angular velocity of the sham-exposed mice was generally close to 0 degrees/s (see example in Figure 2A). All sham-exposed mice swam for the full duration of the 2-min test.
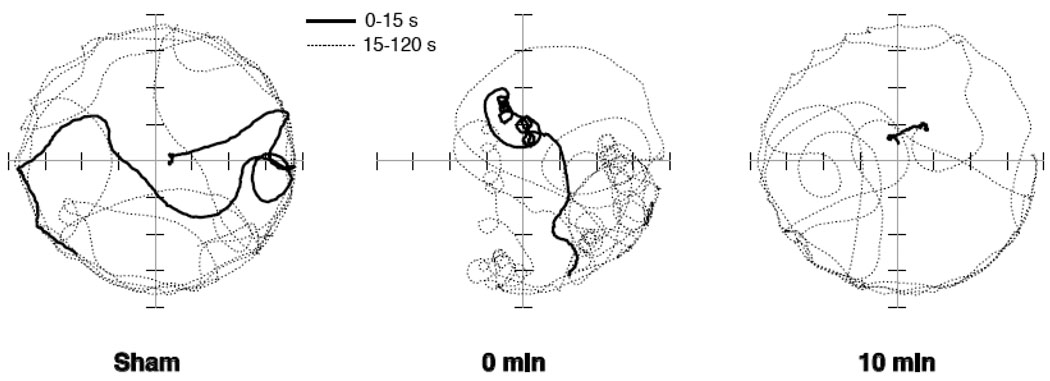
Figure 1.
Traces of paths taken by mice during 2-min swim test after 30-min sham exposure (left), immediately after 30-min exposure to 14.1 T (center), or 10 min after the end of 30-min magnet exposure (right). Mice were placed in the center of the 1-m diameter swimming pool. The solid line shows swimming for the first 15-s of each swim test, and the dotted line shows swimming for the remaining 105 s. The sham-exposed mouse swam in straight lines or broad curves with only occasional circles. The mouse tested immediately after magnet exposure showed intense counter-clockwise circling during the first 15 s, and the swim path continued to be interrupted with bouts of tight circling. In contrast, the mouse placed in the swimming pool 10 min after magnet exposure was nearly motionless for the first 15 s in the water, and showed no circling when swimming for the remainder of the test.
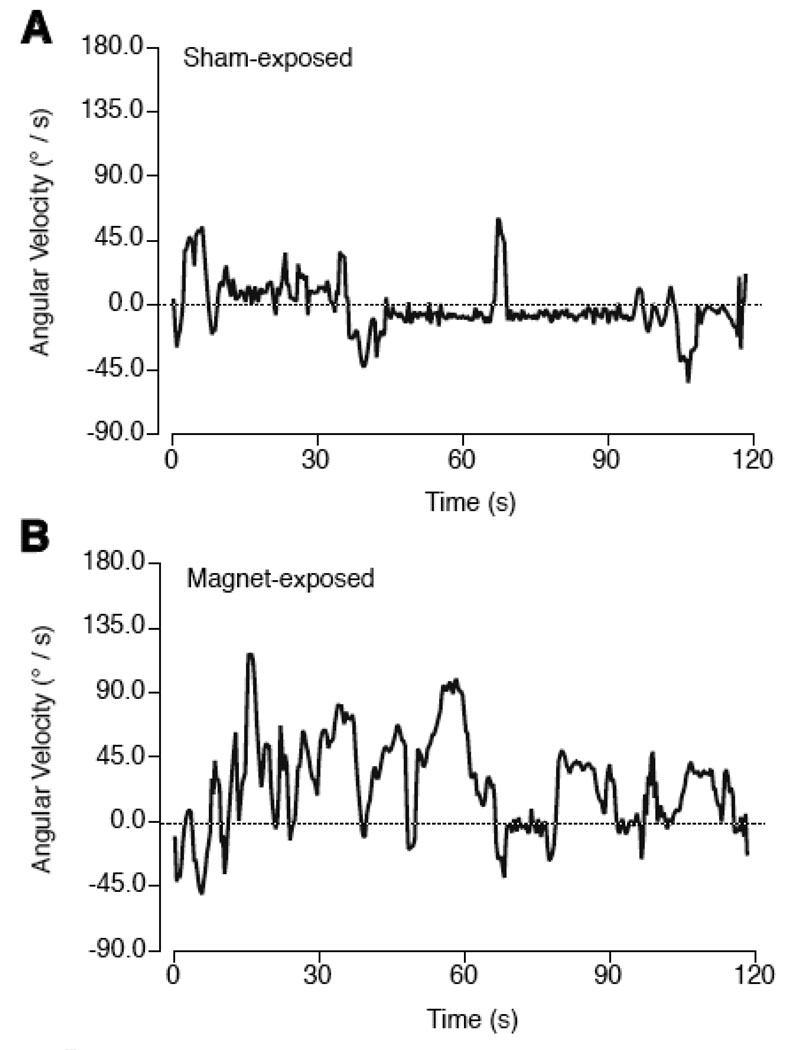
Figure 2.
Examples of angular velocity (degrees/s) calculated from successive bearings of mice during the 2-min swim test, from a sham-exposed mouse (A) or a mouse tested immediately after 30-min magnet exposure (B). Positive values indicated counter-clockwise turning, and 0 indicates straight swimming (dashed line). Although sham-exposed mice showed occasional turns in either counter-clockwise or clockwise directions, their mean angular velocity stayed close to 0. Magnet-exposed mice, when tested immediately after exposure, showed persistent bouts of counter-clockwise turning throughout the 2-min test.
The swimming of magnet-exposed mice was very different. When tested immediately after magnet-exposure (0-min group), mice swam in tight, counter-clockwise circles with occasional intervals of straight swimming (see example trace of swimming in Figure 1B and example trace of angular velocity in Figure 2B.). An example video can be seen at http://www.magnet.neuro.fsu.edu/mouseswimtest.mov. Although mice swam in tight circles, we did not see any “corkscrewing” by the mice, i.e. very intense circling in place without any forward motion. Also, all mice were able to keep their heads above water and no diving into the water was observed.
When tested at later time points after exposure, magnet-exposed mice did not swim in circles. (Mice were observed to walk in counter-clockwise circles, however, in their home cages during the interval after exposure.) Instead, magnet-exposed mice placed in the swimming pool at 3–40 min after exposure did not swim immediately, but rather floated almost motionless for approximately 15 s (see example trace in Figure 1C). When floating, the mice showed a stereotyped posture with apparent rightward (counter-clockwise) flexion of the pelvis, with the right hindlimb extended out of the water (see photograph Figure 3).
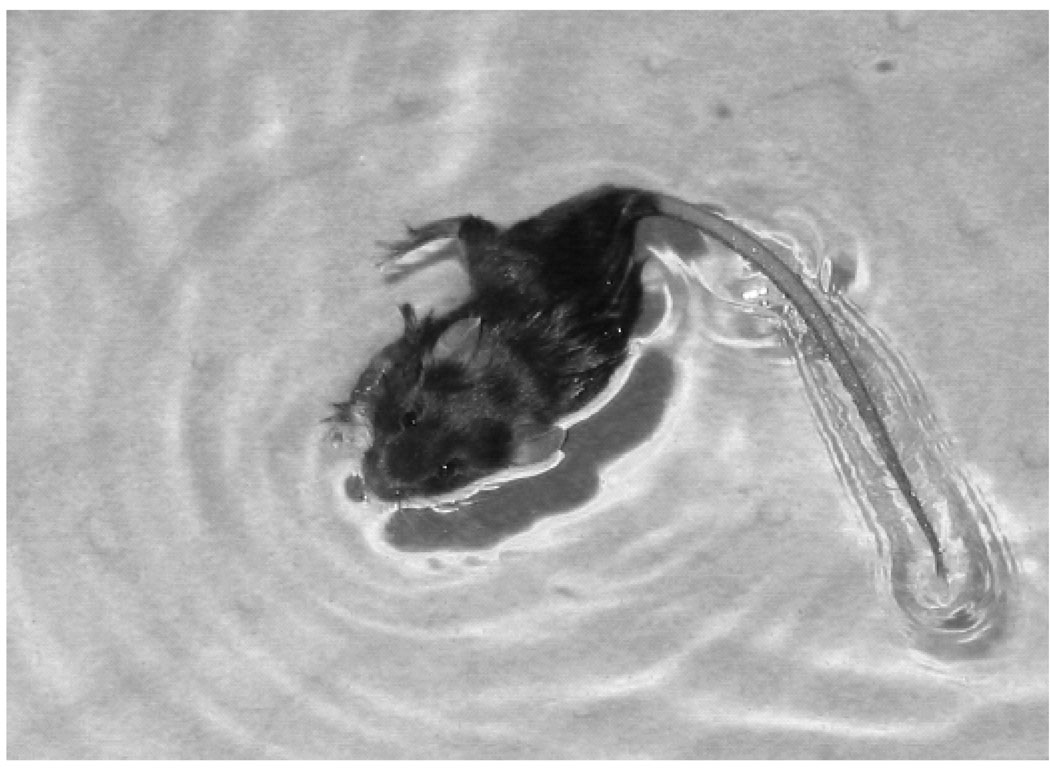
Figure 3.
Photograph of a mouse placed in the swimming pool at 10 min after a 30-min exposure to the high magnetic field, show characteristic floating with turning of the pelvis to the right and extension of the rear right leg out of the water. After ~ 15 s, mice typically recovered from this posture and swam without much circling.
Mice tested 60-min after magnet exposure appeared to swim similar to sham-exposed mice, with no circling or floating.
Quantitation of Angular Velocity and Distance
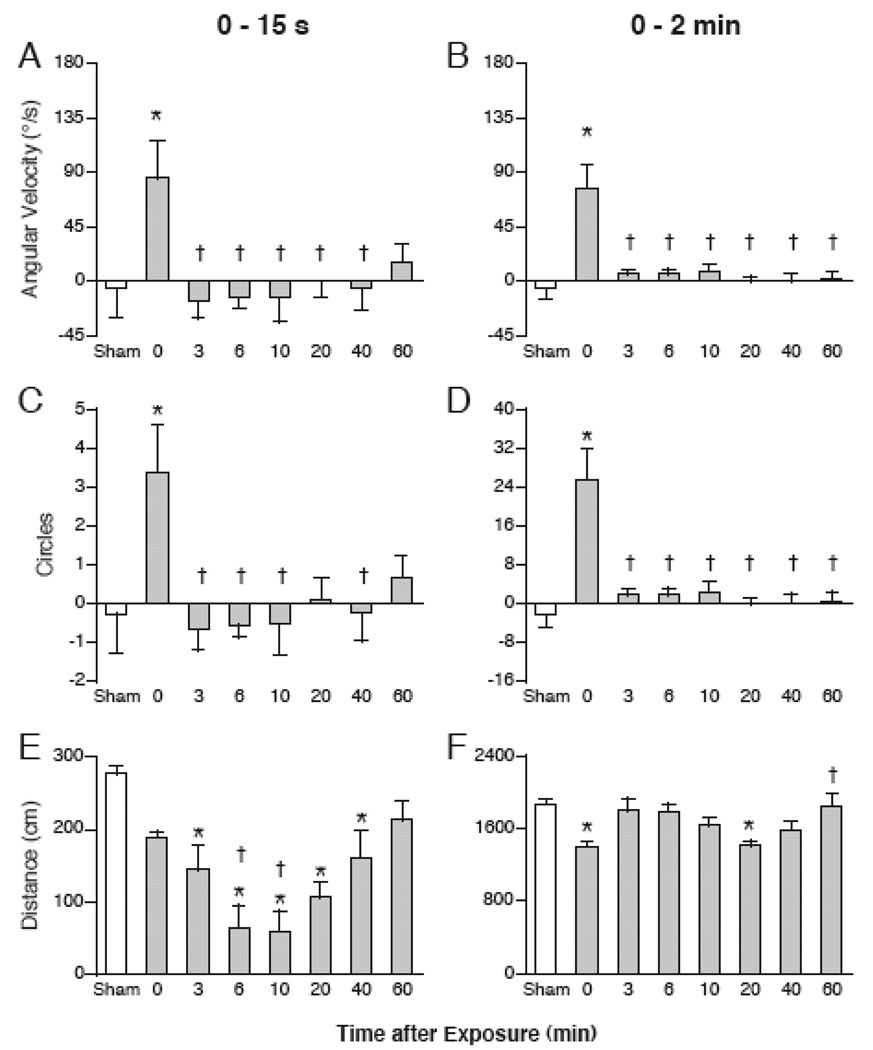
There was a significant effect of treatment on average angular velocity (Figure 4 A and B) during the first 15 s (F(7,47) = 3.46, p < 0.01) as well as across the entire 2 min of the swim test (F(7,47) = 10.67, p < 0.0001). Parallel to the angular velocity of swimming, there was a significant effect of treatment on the average number of circles (Figure 4 C and C) that mice traversed during the first 15 s (F(7,47) = 3.11, p < 0.05) as well as across the entire 2 min of the swim test (F(7,47) = 10.67, p < 0.0001). In particular, sham-exposed mice swam, on average, with an angular velocity close to 0 degrees/s (i.e. in straight lines), while mice tested immediately after magnet exposure (0 min) swam with an angular velocity of 90 degrees/s in the counter-clockwise direction. As a result, magnet-exposed mice in the 0-min group circled on average more than 24 times during the 2-min swim test. The effect of magnet exposure on circling was transient, as mice tested 3 – 60 min after magnet exposure did not differ from sham-exposed mice in their direction of swimming.
Figure 4.
Quantification of swimming in mice tested at 0–60 min after magnet exposure, or after sham-exposure. Values are shown for the first 15 s (left panels), or the entire 2 min of the swim test (right panels). (A,B) Angular velocity in degrees/s calculated from successive bearings of mice. (C,D) Total number of circles was calculated from the cumulative angular velocity. Positive values of angular velocity and circling are in the counter-clockwise direction. Mice tested at 0 min after magnet exposure showed significant more counter-clockwise swimming and circles than sham-exposed mice or mice tested at later time points. (E,F) Cumulative distance swum in cm. Mice tested at 3 – 40 min after magnet exposure swam significantly shorter distance in the first 15 s of the swim test. * p < 0.05 vs sham-exposed; † p < 0.05 vs. 0 min.
There was a significant effect of treatment on cumulative distance traveled (Figure 4 E and F) within the first 15 s (F(7,47) = 8.34, p < 0.0001) as well as across the entire 2 min of the swim test (F(7,47) = 4.8, p < 0.005). Mice tested 3–40 min after magnet exposure swam significantly less distance than sham-exposed mice during the first 15 s. When tested 60 min after magnet exposure, mice did not swim significantly less than sham-exposed mice.
Experiment 2: Long-term effects of magnetic field exposure
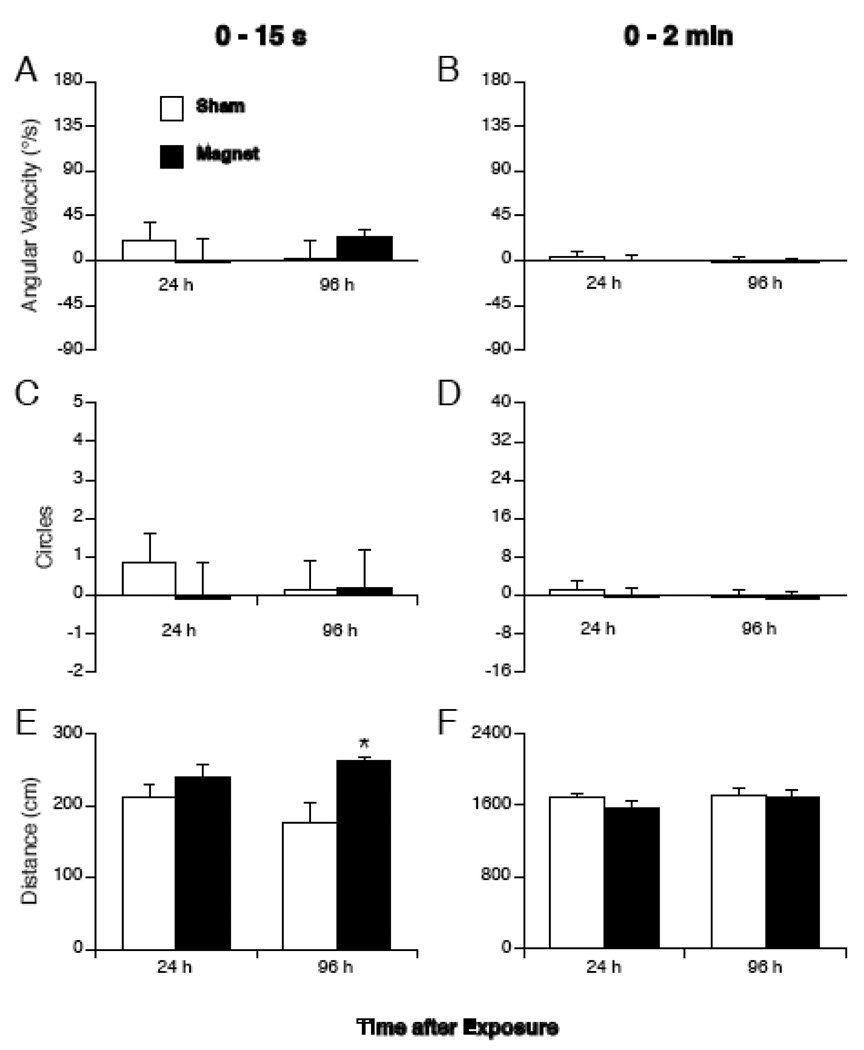
Two-way ANOVA with treatment (sham vs. magnet exposure) and time (swim test at 24 h or 96 h after exposure) revealed a significant effect of treatment on distance that mice swam in the first 15 s, such that magnet-exposed mice swam further than sham-exposed mice at 96 h (Figure 5A). With the exception of this time point, however, no other significant differences were detected between sham-exposed mice and magnet-exposed mice in terms of distance travelled, angular velocity, or number of circles (Figure 5 B–D). No qualitative difference was observed in the swimming behavior or posture of the magnet-exposed mice in the long-term tests.
Figure 5.
Quantification of swimming in mice tested at 24 h or 96 h after sham exposure (white bars) or magnet exposure (black bars). Values are shown for the first 15 s (left panels), or the entire 2 min of the swim test (right panels). (A,B) Angular velocity in degrees/s calculated from successive bearings of mice. (C,D) Total number of circles was calculated from the cumulative angular velocity. Positive values of angular velocity and circling are in the counter-clockwise direction. (E,F) Cumulative distance swum in cm. With the exception of one comparison (E), there was no significant difference in swimming between sham- and magnet-exposed mice tested days after exposure. * p < 0.05 vs sham-exposed mice.
Discussion
Immediately after 30-min exposure to a 14.1 T magnetic field, mice swam in tight counter-clockwise circles. The counter-clockwise orientation persisted throughout the 2-min swim test. The tendency to circle was transient, because no significant circling was observed when mice were tested at 3 min or later after magnet exposure. However, mice did show a decrease in total distance swum when tested between 3 and 40 min after magnet exposure. The decrease in swimming distance was accompanied by a pronounced postural change involving a counter-clockwise twist of the pelvis and hindlimbs that was particularly severe in the first 15 s of the swim test. Finally, no persistent difference from sham-exposed mice was seen in the swimming of magnet-exposed mice when tested 60 min, 24 h, or 96 h after magnet exposure. This suggests that there is no long-lasting effect of magnet exposure on the ability of mice to orient or swim.
Swimming is a sensitive test of vestibular function. In previous studies using an open field chamber, only a fraction of mice were observed to circle [25]. In contrast, all of the magnet-exposed mice tested in the swimming pool immediately after magnet exposure were observed to circle. Circling by mice in the swimming pool was more intense than circling by rats observed in the open field (e.g. 24 vs. 8 circles / 2 min, respectively; [6]).
Deficits in swimming, and swimming in circles in particular, is symptomatic of rodents with vestibular mutations, damage, or perturbation. For example, the circling rat mutant [21], manganese-deficient rats [26], unilateral labyrinthectomized guinea pigs [19], and bilaterally labyrinthectomized voles [20] all tend to swim in circles or have problems maintaining correct orientation and posture in the water. The transient induction of circular swimming after magnet exposure is consistent with an acute perturbation, much as circular swimming is observed transiently after whole-body rotation of intact rats [24]. There was no evidence that mice had a gravitometric deficit, however, because none of the mice showed any difficulty keeping their head above water, and no “somersaulting” behavior was observed, e.g. as seen in mutant mice that lack otoconia which pitch forward headfirst into the water [22].
At 3–40 minutes after magnet exposure, mice showed a pronounced alteration in their posture during the first 15 s of the swim test. While floating, the hindquarters of the mice were rotated counter-clockwise resulting in an extension of the right rear leg out of the water. This postural change was not observed in mice that swam in circles immediately after magnet exposure. It is possible that the change in posture was masked by the circular swimming, or it may be that the induction of circling and the alteration of posture occur independently with different time courses. We also cannot rule out fatigue as a contributor to the postural change, because even mice tested in the swimming pool at later time points were observed to walk in circles in their home cages. Because the vestibular system is intimately involved in the maintenance of posture, the postural change induced by magnet exposure is consistent with an interaction of the high magnetic field with the vestibular system. Interestingly, the postural change observed after magnet exposure is similar to the postural change seen in rats after hypogravity. When placed in water, rats reared in low-earth orbit from postnatal day 14 to postnatal day 30 showed a 60° rotation of their hindquarters around their rostral-caudal axis [23].
The fact that we saw almost no difference in swimming between sham- and magnet-exposed mice when tested at 24 h and 96 h suggests that there is no long-term effect of magnet exposure. (Mice did swim faster in the first 15 s when tested 96-h after magnet exposure, so we cannot rule out a subtle cognitive or stress effect that would bolster performance in the swim test.) A more sensitive assay to test more than simple swimming, e.g. the Morris water maze test of navigation and memory, might reveal other consequences of magnetic field exposure. It is also possible, of course, that a stronger long-term effect might be observed if mice were exposed for a longer duration, or if they were repeatedly exposed. Furthermore, we have observed long-term habituation in rats of the circling response and induction of conditioned taste aversion after repeated exposures to the 14.1 T magnetic field [27]. Rats were pre-exposed for 30 min within the magnet two times, and then after a third exposure they circled less in the open field test. The attenuated response was seen after pre-exposures at 24-h intervals, and even 36 days after the second pre-exposure. Thus, there appear to be long-term effects of high magnetic field exposure, although they may not manifest as deficits in orientation.
Our previous work points to the inner ear as the locus of high magnetic field transduction. Rats showed the highest response in terms of circling and CTA magnitude after magnetic field exposure encompassed the head [28]; chemical labyrinthectomy abolishes all responses to the magnetic field [16]. The susceptibility of mice to the effects of high magnetic fields will allow the use of vestibular mutant strains to dissect the contribution of different elements of the inner ear. For example, epistatic circler mice have a defect in their lateral semicircular canal[29], and there are several mutant lines that lack otoconia and hence have non-responsive otolith organs, such as head-tilt and tilted-head mice [22]. Preliminary results indicate that head-tilt and tilted-head mice do not show increased locomotor circling and do not acquire CTA after magnetic field exposure. Thus, we hypothesize that high magnetic fields interact with the otoconia of normal rodents to cause vestibular perturbations.
Acknowledgements
Supported by National Institute on Deafness and Other Communication Disorders Grant RO1DC4607. We thank Drs. Timothy Cross and Zhehong Gan of the United States National Magnetic Field Laboratory for providing access to the magnet.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Houpt TA, Smith JC. Conditioned taste aversion induced by exposure to high-strength static magnetic fields. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. NY: Oxford University Press; 2009. pp. 422–441. [Google Scholar]
- 2.Schenck JF. Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Ann. NY Acad. Sci. 1992;649:285–301. doi: 10.1111/j.1749-6632.1992.tb49617.x. [DOI] [PubMed] [Google Scholar]
- 3.Glover PM, Cavin I, Qian W, Bowtell R, Gowland PA. Magnetic-field-induced vertigo: a theoretical and experimental investigation. Bioelectromagnetics. 2007:28. doi: 10.1002/bem.20316. [DOI] [PubMed] [Google Scholar]
- 4.de Vocht F, Stevens T, Glover P, Sunderland A, Gowland P, Kromhout H. Cognitive effects of head-movements in stray fields generated by a 7 Tesla whole-body MRI magnet. Bioelectromagnetics. 2007;28:247–255. doi: 10.1002/bem.20311. [DOI] [PubMed] [Google Scholar]
- 5.Patel M, Williamsom RA, Dorevitch S, Buchanan S. Pilot study investigating the effect of the static magnetic field from a 9.4-T MRI on the vestibular system. J. Occup. Environ. Med. 2008;50:576–583. doi: 10.1097/JOM.0b013e318162f5d6. [DOI] [PubMed] [Google Scholar]
- 6.Houpt TA, Pittman DM, Barranco JM, Brooks EH, Smith JC. Behavioral effects of high strength magnetic fields on rats. J. Neurosci. 2003;23:1498–1505. doi: 10.1523/JNEUROSCI.23-04-01498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houpt TA, Pittman DW, Riccardi C, Cassell JA, Lockwood DR, Barranco JM, Kwon BS, Smith JC. Behavioral effects on rats of high strength magnetic fields generated by a resistive electromagnet. Physiol. Behav. 2005;86:379–389. doi: 10.1016/j.physbeh.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Nolte CM, Pittman DW, Kalevitch B, Henderson R, Smith JC. Magnetic field conditioned taste aversion in rats. Physiol. Behav. 1998;63:683–688. doi: 10.1016/s0031-9384(97)00526-x. [DOI] [PubMed] [Google Scholar]
- 9.Cason AM, DenBleyker MD, Ferrance K, Smith JC, Houpt TA. Sex and estrous cycle differences in the behavioral effects of high-strength static magnetic fields: role of ovarian steriods. Am. J. Physiol. 2006;290:R659–R667. doi: 10.1152/ajpregu.00305.2005. [DOI] [PubMed] [Google Scholar]
- 10.Snyder D, Jahng JW, Smith JC, Houpt TA. c-Fos induction in visceral and vestibular nuclei of the rat brainstem by a 9.4 T magnetic field. NeuroReport. 2000;11:1681–1685. doi: 10.1097/00001756-200008210-00015. [DOI] [PubMed] [Google Scholar]
- 11.Green L, Rachlin H. The effect of rotation on the learning of taste aversions. Bull. Psychon. Soc. 1973;1:137–192. [Google Scholar]
- 12.Cordick N, Parker LA, Ossenkopp KP. Rotation-induced conditioned rejection in the taste reactivity test. NeuroReport. 1999;10:1557–1559. doi: 10.1097/00001756-199905140-00030. [DOI] [PubMed] [Google Scholar]
- 13.Ossenkopp KP, Parker LA, Limebeer CL, Burton P, Fudge MA, Cross-Mellor SK. Vestibular lesions selectively abolish body rotation-induced, but not lithium-induced, conditioned taste aversions (oral rejection responses) in rats. Behav. Neurosci. 2003;117:105–112. [PubMed] [Google Scholar]
- 14.Kaufman GD, Anderson JH, Beitz AJ. Fos-defined activity in rat brainstem following centripetal acceleration. J. Neurosci. 1992;12:4489–4500. doi: 10.1523/JNEUROSCI.12-11-04489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman GF, Shinder ME, Perachio AA. Correlation of Fos expression and circling asymmetry during gerbil vestibular compensation. Brain Res. 1999;817:246–255. doi: 10.1016/s0006-8993(98)01284-0. [DOI] [PubMed] [Google Scholar]
- 16.Cason AM, Kwon BS, Smith JC, Houpt TA. Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiol. Behav. 2009;97:36–43. doi: 10.1016/j.physbeh.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Weber KD, Fletcher WA, Gordon CR, Melvill Jones G, Block EW. Motor learning in the "podokinetic" system and its role in spatial orientation during locomotion. Exp. Brain Res. 1998;120:377–385. doi: 10.1007/s002210050411. [DOI] [PubMed] [Google Scholar]
- 18.Houpt TA, Cassell JA, Riccardi C, Kwon BS, Smith JC. Suppression of drinking by exposure to a high-strength static magnetic field. Physiol. Behav. 2007;90:59–65. doi: 10.1016/j.physbeh.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Petrosini L. Task-dependent rate of recovery from hemilabyrinthectomy: an analysis of swimming and locomotor performances. Physiol. Behav. 1984;33:799–804. doi: 10.1016/0031-9384(84)90050-7. [DOI] [PubMed] [Google Scholar]
- 20.Ossenkopp KP, Eckel LA, Hargreaves EL, Kavaliers M. Sodium arsanilate-induced vestibular dysfunction in meadow voles (Microtus pennsylvanicus): effects on posture, spontaneous locomotor activity and swimming behavior. Behav. Brain Res. 1992;47:13–22. doi: 10.1016/s0166-4328(05)80248-7. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser A, Fedrowitz M, Ebert U, Zimmermann E, Hedrich HJ, Wedekind D, Löscher W. Auditory and vestibular defects in the circling (ci2) rat mutant. Eur. J. Neurosci. 2001;14:1129–1142. doi: 10.1046/j.0953-816x.2001.01726.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones SM, Erway LC, Johnson KR, Yu H, Jones TA. Gravity receptor function in mice with graded otoconial deficiencies. Hear Res. 2004;191:34–40. doi: 10.1016/j.heares.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Walton KD, Benavides L, Singh N, Hatoum N. Long-term effects of microgravity on the swimming behaviour of young rats. J. Physiol. 2005;565:609–626. doi: 10.1113/jphysiol.2004.074393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenov LV, Bures J. Vestibular stimulation disrupts acquisition of place navigation in the Morris water tank task. Behav Neural Biol. 1989;51:346–363. doi: 10.1016/s0163-1047(89)90987-4. [DOI] [PubMed] [Google Scholar]
- 25.Lockwood DR, Kwon BS, Smith JC, Houpt TA. Behavioral effects of high strength static magnetic fields on restrained and unrestrained mice. Physiol. Behav. 2003;78:635–640. doi: 10.1016/s0031-9384(03)00040-4. [DOI] [PubMed] [Google Scholar]
- 26.Huygen PL, Fischer AJ, Kuijpers W. The vestibular functions of the manganese-deficient rat. Acta Otolaryngol. 1986;101:19–26. doi: 10.3109/00016488609108603. [DOI] [PubMed] [Google Scholar]
- 27.Houpt TA, Cassell JA, Hood A, DenBleyker M, Janowitz I, Mueller K, Ortega B, Smith JC. Repeated exposure attenuates the behavioral response of rats to static high magnetic fields. Physiol. Behav. 2009 doi: 10.1016/j.physbeh.2009.12.024. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houpt TA, Cassell JA, Cason AM, Reidell A, Golden GJ, Riccardi C, Smith JC. Evidence for a cephalic site of action of high magnetic fields on the behavioral responses of rats. Physiol. Behav. 2007;92:665–674. doi: 10.1016/j.physbeh.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cryns K, van Alphen AM, van Spaendonck MP, van de Heyning PH, Timmermans JP, de Zeeuw CI, van Camp G. Circling behavior in the Ecl mouse is caused by lateral semicircular canal defects. J. Comp. Neurol. 2004;468:587–595. doi: 10.1002/cne.10975. [DOI] [PubMed] [Google Scholar]