Abstract
Autophagy is an important cellular process by which ATG5 initiates the formation of double membrane vesicles (DMVs). Upon infection, DMVs have been shown to harbor the replicase complex of positive-strand RNA viruses such as MHV, poliovirus, and equine arteritis virus. Recently, it has been shown that autophagy proteins are proviral factors that favor initiation of hepatitis C virus (HCV) infection. Here, we identified ATG5 as an interacting protein for the HCV NS5B. ATG5/NS5B interaction was confirmed by co-IP and metabolic labeling studies. Furthermore, ATG5 protein colocalizes with NS4B, a constituent of the membranous web. Importantly, immunofluorescence staining demonstrated a strong colocalization of ATG5 and NS5B within perinuclear regions of infected cells at 2 days postinfection. However, colocalization was completely lacking at 5 DPI, suggesting that HCV utilizes ATG5 as a proviral factor during the onset of viral infection. Finally, inhibition of autophagy through ATG5 silencing blocks HCV replication.
Abbreviations: HCV, hepatitis C virus; ATG5, autophagy-related gene 5; DMV, double membrane vesicle
Keywords: HCV, Autophagy, ATG5, RdRp, NS5B, siRNA
Introduction
There is compelling evidence that replication of all positive-stranded RNA viruses requires the formation of virus-induced membrane vesicles and that DMVs are the sites of genome replication for some positive-stranded RNA viruses such as PV, MHV, EAV, dengue virus, coronavirus, and coxsackievirus (Gosert et al., 2003, Lee et al., 2008, Pedersen et al., 1999, Schlegel et al., 1996, Suhy et al., 2000, Wong et al., 2008). In cells, DMVs derived from the endoplasmic reticulum membranes can be produced through the ubiquitous autophagy pathways (Klionsky and Emr, 2000). Autophagy, which has been well characterized in yeast, is an essential process by which bulk protein degradation and organelle turnover take place (Kim et al., 2001, Mizushima et al., 1998).
In response to a limited supply of amino acids due to environmental depletion, the autophagy process will generate a new pool of amino acids required for cellular homeostasis. Recently, the contribution of an autophagy protein, ATG5, to viral replication has been demonstrated (Prentice et al., 2004). Indeed, production of MHV particles is profoundly reduced (> 99%) in ATG5−/− knockout (KO) embryonic stem cells (Prentice et al., 2004). In this system, the expression of ATG5 in ATG5−/− cells restores virus production. The relationship between ATG5 and MHV replication may come from the ability of ATG5 to initiate the formation of DMVs, which have been observed in MHV-infected cells (Gosert et al., 2003, Mizushima et al., 2001). As seen for dengue virus-2 (DV2), when autophagy is blocked by ATG5-KO MEF cells, extracellular DV2 virus titers are reduced by 3-fold compared to those from wild-type MEF cells (Lee et al., 2008).
Altered vesicles, called the membranous web, have been observed in cells harboring HCV replicons (Egger et al., 2002, Gosert et al., 2002). Because HCV replicons replicate autonomously in Huh-7, it has been proposed that the membranous web that contain the HCV replication complexes represents the genuine site of viral replication (Gosert et al., 2002, Moradpour et al., 2003). In HCV-infected cells, accumulation of lipid droplets, shown to be essential for HCV replication, has been observed in the proximity of the membranous web (Miyanari et al., 2007). Given the involvement of autophagy at the site of replication of other positive-stranded RNA viruses, are the autophagy proteins or structure involved in HCV replication? In that regard, Tanida et al. (2009) recently showed that Atg7 silencing decreased the levels of infectious HCVcc by about 40%, whereas intracellular HCV RNA and protein levels remained unchanged. At the same time, another group demonstrated that autophagy proteins (ATG4b and Beclin-1) are required only for the initiation of incoming HCV RNA translation/replication (Dreux et al., 2009).
To identify novel cellular factors that may play an essential role in HCV RNA replication, we have previously screened a human liver cDNA library for proteins interacting with the HCV NS5B RNA-dependent RNA polymerase (RdRp). Here we report that ATG5, a protein required for the formation of DMV in embryonic stem cell (Mizushima et al., 2001), specifically interacts with HCV NS5B. We propose that the NS5B/ATG5 interaction may be required for the initial onset of HCV replication.
Results
ATG5 interacts specifically with HCV NS5B protein
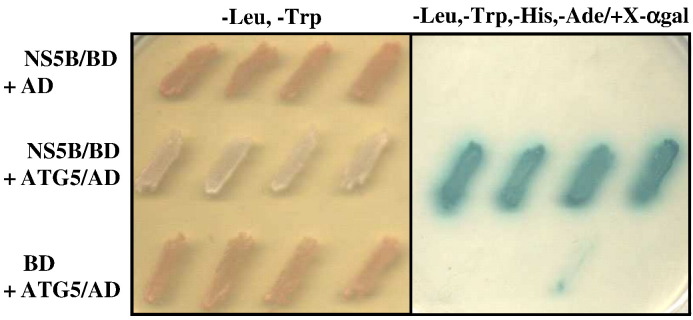
Using HCV NS5BΔ21 protein as a bait in the yeast two-hybrid system, we identified positive clones from a human liver library. The clones that showed the strongest blue color (MEL 1 gene activation) on SD (−Leu, −Trp, −His, −Ade/+X α-gal) plates were chosen for further characterization. Two of these positive clones matched the sequence of the human eukaryotic initiation factor 4AII (eIF4AII), recently identified as an interacting protein for HCV NS5B (Kyono et al., 2002). Two more clones corresponded to the EMBL/GenBank/DDBJ accession number Y711588a. This gene is highly homologous to the Saccharomyces cerevisiae ATG5 gene (Hammond et al., 1998). ATG5/NS5B interaction was confirmed in the yeast two-hybrid system using the full-length human ATG5 gene amplified from a human liver cDNA library (Clontech) (Fig. 1 ). As a control, a panel of proteins (RAR-β, RAR-α, HCV core, and nonstructural protein: NS3prot, NS3hel, NS4A, and NS4B) cloned in the GAL4 DNA binding domain was tested for interaction with ATG5. From this panel, only HCV NS3prot protein gave a weak signal, but the interaction was not characterized further (data not shown).
Fig. 1.
The NS5BΔ21 protein interacts with the full-length human ATG5 in yeast two-hybrid assay. NS5B and ATG5 fused to the Gal4 DNA binding and activation domains, respectively, were double-transformed into AH109 cells. Four independent colonies of recombinant AH109 cells were allowed to grow for a few days on −Leu, −Trp SD medium (left panel), after which they were replica-plated onto −Leu, −Trp, −His, −Ade + X-αgal plates (right panel).
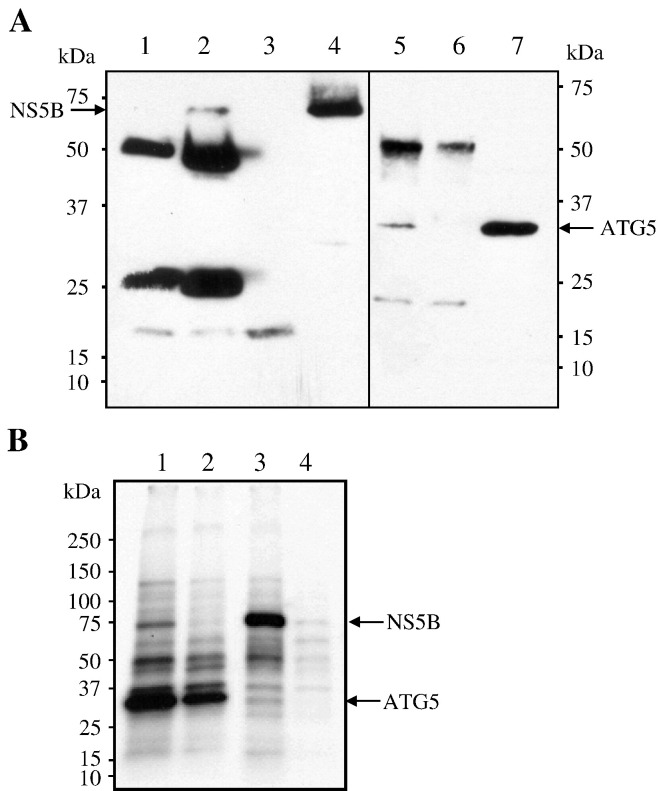
To demonstrate a physical interaction between ATG5 and NS5B proteins, we performed co-IP on radio-inert or metabolically labeled yeast cells coexpressing ATG5 and NS5B. We used yeast cells in this study because cotransfection of Huh-7 cells with c-myc-NS5B and HA-tagged ATG5 resulted in a low level expression of ATG5 protein. Yeast extracts were immunoprecipitated with monoclonal antibody against either the c-myc or the HA tag, followed by separation on SDS–PAGE. As shown in Fig. 2A, an immunoreactive band (lane 2) that corresponds to the size of the NS5B protein (68 kDa; lane 4) was coimmunoprecipitated with ATG5. This band was not present when the extracts were immunoprecipitated with monoclonal anti-5A antibody (lane 1) or without any antibody (Protein A/G sepharose beads alone) (lane 3). The bands corresponding to the 50- and 25-kDa molecular weight are the heavy and light chains, respectively, of the antibody. Conversely, ATG5 was coimmunoprecipitated with NS5B when the same extract was incubated with monoclonal anti-c-myc antibody. Indeed, a distinct immunoreactive band (lane 5) that corresponds to the size of ATG5 (lane 7) was detected. However, this band could not be immunoprecipitated by the monoclonal anti-5A antibody (lane 6). This protein–protein interaction was further substantiated by the metabolic labeling experiments shown in Fig. 2B in which ATG5 was constitutively expressed and NS5B expression was under a copper-inducible promoter. As expected, when the labeled extracts were immunoprecipitated with monoclonal anti-HA, a prominent band corresponding to the size of ATG5 was detected in yeast extracts under induced (lane 1) and noninduced (lane 2) conditions. However, the band corresponding to NS5B was only detected in lane 1 (induced condition). When the same extracts were immunoprecipitated with monoclonal anti-c-myc antibody, an intense band corresponding to the size of NS5B was apparent under the induced condition (lane 3). As expected, a band corresponding to the size of ATG5 was also detected in the extracts under the induced condition (lane 3) but not under the noninduced condition (lane 4). Taken together, these observations indicate that ATG5 and NS5B interact.
Fig. 2.
Specific interaction of HCV NS5BΔ21 protein with ATG5 as observed by co-IP. A. Soluble yeast extracts containing NS5BΔ21 (N-terminal c-myc tag) and ATG5 (N-terminal HA tag) were incubated with different monoclonal antibodies and the immunoprecipitates were pulled down using protein A/G beads. Precipitated proteins were revealed by Western blot using anti-c-myc (left panel) or anti-HA (right panel) monoclonal antibodies. Note that IP of ATG5 using anti-HA monoclonal antibody coprecipitated NS5BΔ21 (lane 2). Anti-HCV NS5A (lane 1) or no antibody (beads only, lane 3) did not precipitate NS5BΔ21. Soluble extract loaded on the gel was used as the size marker for NS5BΔ21. IP of NS5BΔ21 using anti-c-myc antibody precipitated ATG5 (lane 5) but not anti-HCV NS5A antibody (lane 6). Soluble extract loaded on the gel was used as the size marker for ATG5 (lane 7). B. Soluble [35S]Met-labeled protein extract was immunoprecipitated using anti-HA (lanes 1 and 2) or anti-c-myc (lanes 3 and 4). Extracts were incubated in the presence (lanes 1 and 3) or absence (lanes 2 and 4) of cupric sulfate, which induced NS5BΔ21 expression. Precipitated bands were analyzed by SDS–PAGE followed by autoradiography.
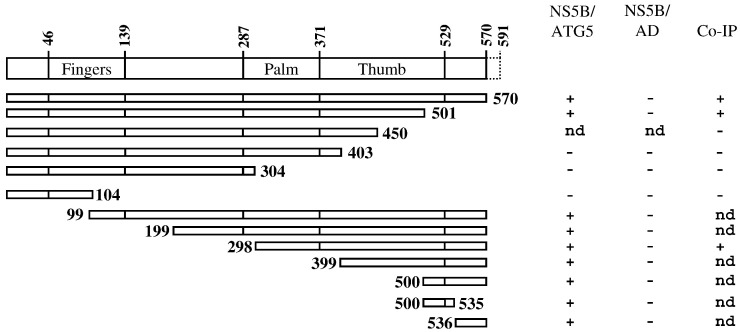
To map the interaction domains between NS5B and ATG5, N- and C-terminally truncated mutants of both proteins were assessed by a yeast two-hybrid system. For that purpose, the truncated fragments were inserted into a pGBKT7 or pGADT7 plasmid. As illustrated in Fig. 3 , all constructs containing the C-terminal end of the NS5B displayed interaction with ATG5, while truncation of the NS5B C-terminus completely abrogated such interaction. To confirm the data obtained by yeast two-hybrid screening, co-IP of the putative interacting domains was performed. Both techniques were able to identify the C-terminal end (aa 450–570) of NS5B as the interacting domain for ATG5 (Fig. 3). The NS5B may have several amino acids involved in this interaction since the fragments 1–501, 500–535, and 536–570 independently interacted with ATG5. Thus, the binding domain of NS5B to ATG5 corresponds to the back surface of the thumb domain which has been proposed to be a premium site for protein-protein interaction due to the presence of a highly conserved patch of basic amino acids (Bressanelli et al., 1999).
Fig. 3.
Mapping of the NS5BΔ21 and ATG5 binding domains. Results are indicated in the column on the right and were obtained by yeast two-hybrid assay (first two columns) or by co-IP (third column). Deletion mutants of NS5B were assessed for interaction with full-length ATG5. The binding area covered the N-terminal amino acids 450–570 as indicated by yeast two-hybrid assay and co-IP. Note that none of the deletion mutants of NS5BΔ21 self-activated in the yeast two-hybrid screen, as shown in the second column. nd indicates not done.
ATG5 is localized to the membranous web
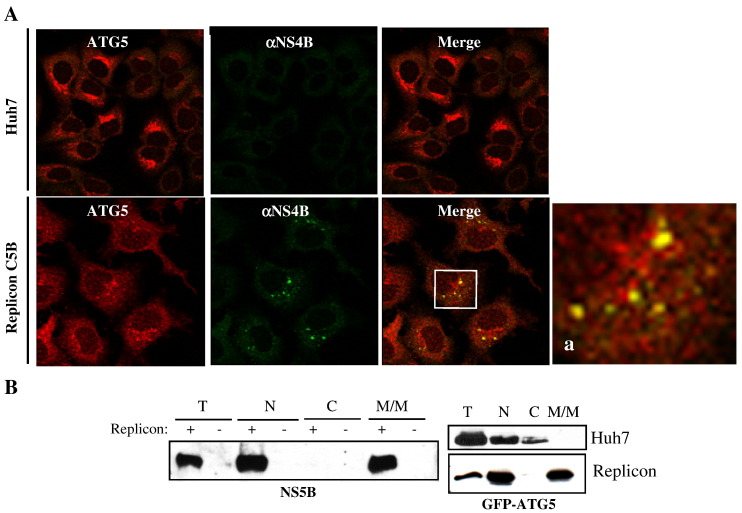
The interaction observed between ATG5 and the HCV NS5B RdRp suggests that ATG5 might be involved in HCV replication. In cells replicating HCV RNA, NS5B has been localized to the membranous web (Egger et al., 2002, Gosert et al., 2002). Therefore, we analyzed the localization of ATG5 and NS4B (a well-recognized marker of the membranous web) in genome-length C5B replicon cells (Aligo et al., 2009, Konan et al., 2003). The results presented in Fig. 4A indicate that NS4B indeed colocalized with the endogenous ATG5 protein. It is known that ATG5 and its conjugated form, ATG5-APG12, are mostly cytoplasmic in mouse embryonic stem cells but that their association with the membrane increases upon starvation (Mizushima et al., 2001). Thus, we looked at the subcellular membrane association of ATG5 in replicon cells. As expected, both NS5B and ATG5 proteins were excluded from the cytoplasmic fraction (Fig. 4B). Indeed, both proteins appeared to reside in the microsomal/mitochondrial fractions, suggesting that ATG5 is directed to membranes in replicon cells. Note that the nuclear fractions contain both proteins most likely through contamination from the single-step purification protocol of the nuclear fraction.
Fig. 4.
A. Huh7 and C5B cells were grown for 48 h and costained with antibodies specific to ATG5 and NS4B protein. All samples were examined via confocal microscopy. Magnified areas (a) are indicated by rectangles. Notice the colocalization of NS4B with ATG5 in C5B cells. B. Subcellular fractionation of clone A cells (subgenomic replicon) transfected with GFP-ATG5. Proteins were resolved on SDS–PAGE and visualized by Western blot using a polyclonal rabbit anti-NS5B (left) or a monoclonal anti-GFP (right). N, nucleus; C, cytoplasm; M/M, microsome and mitochondria.
ATG5 transiently interacts with NS5B during HCV infection
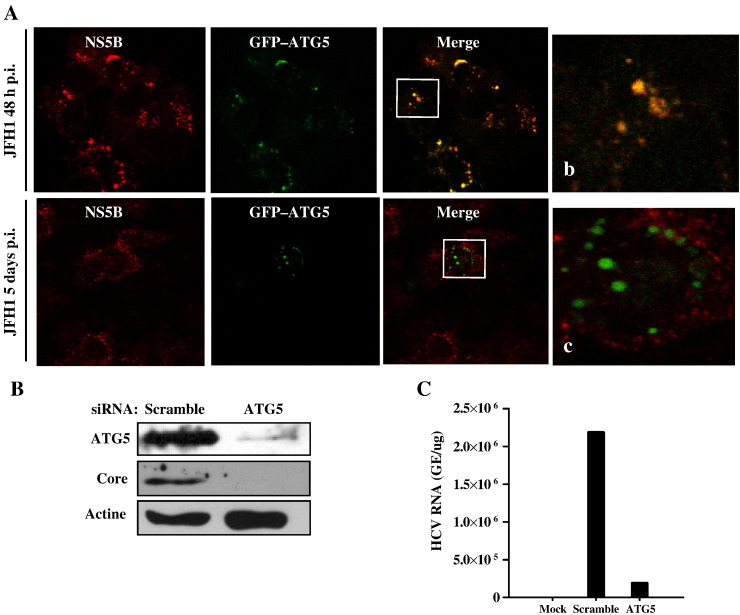
Previously, we and others have unsuccessfully attempted to identify a HCV protein involved in the modulation of the autophagic response that occurs upon HCV infection (Ait-Goughoulte et al., 2008, Dreux et al., 2009). It has recently been suggested that autophagy is required only early in infection (Dreux et al., 2009). Therefore, we evaluated the colocalization of ATG5 and NS5B in infected Huh7 cells at 2 days postinfection (2 DPI). The selection of 2 DPI, as our earliest time point, was based on the slow replication of JFH1 in Huh7 cells for the first few days of infection (Wakita et al., 2005, Zhong et al., 2005). Indeed, at 1 DPI, HCV core, NS5B, and NS5A were undetectable by immunofluorescence staining (data not shown). Therefore, infected Huh7 cells were transfected with pEGFP-ATG5 and analyzed at 2 or 5 DPI for the presence of NS5B and ATG5 (Fig. 5A). To our surprise, a strong colocalization of GFP-ATG5 and NS5B was evident in approximately 80% of infected cells at 2 DPI, but completely disappeared at 5 DPI. This result suggests that ATG5–NS5B interaction occurs only during the initial onset of HCV replication and may explain why this interaction has not been detected previously.
Fig. 5.
A. Subcellular distribution of NS5B and ATG5 in HCVcc-infected cells. JFH1-infected Huh7 cells were transfected with the GFP-ATG5 DNA construct and analyzed at 2 or 5 DPI. Colocalization of ATG5 and NS5B was observed at 2 DPI (magnified area in b) but not at 5 DPI (magnified area in c). B. Silencing ATG5 reduced viral replication in Huh7 cells. Huh7 cells were transfected with siRNA targeting ATG5 or with a scramble siRNA as control. The cells were then infected for 2 days and analyzed for the presence of HCV core protein by Western blot. As expected, scramble siRNA had no effect on HCV replication, whereas ATG5 greatly reduced HCV protein expression. C. Quantification of intracellular HCV genome from samples in panel B. Mock, mock-infected cells.
ATG5 silencing inhibits HCV replication
ATG5 silencing is known to disrupt autophagy (Matsushita et al., 2007, Mizushima et al., 2001). Thus, we used ATG5 siRNA to evaluate the importance of ATG5–NS5B interaction on HCV replication. As controls, a scramble siRNA was used. The results indicate that silencing ATG5 up to 2 DPI results in undetectable HCV core protein (Fig. 5B) and in a marked reduction in intracellular viral replication as observed by qRT–PCR (Fig. 5C). This results suggest that ATG5 is required for proper viral replication and this requirement is likely through ATG5–NS5B interaction.
Discussion
Recent reports suggest a role for autophagic proteins in HCV replication and/or secretion (Ait-Goughoulte et al., 2008, Dreux & Chisari, 2009, Dreux et al., 2009, Tanida et al., 2009). However, these reports are conflicting and no consensus has yet been reached. Here we provide for the first time a link between a HCV protein, NS5B, and the autophagy machinery. The specific interaction observed between ATG5 and NS5B was through the thumb domain of the polymerase, a region with numerous basic amino acids that could favor protein–protein interaction.
Using Huh7 cells harboring HCV replicon, we showed that ATG5 is associated with the membrane and colocalizes with the membranous web constituent, NS4B. We then used HCVcc to better define the subcellular distribution of ATG5 and NS5B during the course of viral replication. Interestingly, strong colocalization between the two proteins was only seen early in infection and was completely absent late in infection (Fig. 5A). This result may imply that the ATG5–NS5B interaction is required for the onset of the viral replication. Since the primary known function of ATG5 is the formation of the crescent shape DMV, one could argue that HCV requires membrane import during the early stages of viral infection. Indeed, ATG5 is involved in other positive-strand RNA virus replication, probably through the formation of DMV (Khakpoor et al., 2009, Lee et al., 2008, Prentice et al., 2004). However, we were unsuccessful in visualizing these crescent shaped vesicles or autophagosomes in HCV-infected cells.
Although unconjugated ATG5 can be found on the crescent-shaped autophagosome precursor (Mizushima et al., 2001), maturation into the autophagosome requires the conjugate ATG5–APG12 as well as a series of specific interactions with autophagy proteins (George et al., 2000, Kim & Klionsky, 2000, Mizushima et al., 2001). Because viruses such as MHV and perhaps HCV may utilize ATG5 to initiate DMV formation but may not require further maturation of the DMV into autophagosomes, the function of APG12 in virus-induced DMVs remains to be determined.
Replicase proteins of positive-stranded RNA viruses are localized in virus-induced membrane vesicles. In HCV replicon-harboring cells, a membranous structure that contains both viral proteins and RNA, called the membranous web, has been identified (Moradpour et al., 2003). It has been shown that the formation of the membranous web can be induced by NS4B alone (Konan et al., 2003). Another report has shown physical interaction between NS5B (or NS5A) and the SNARE-like protein, hVAP-33 (Tu et al., 1999), leading to the localization of the HCV replicase complex on lipid rafts (Aizaki et al., 2006, Gao et al., 2004). Despite these findings, the role of the host factors in the formation and function of the HCV replication complex needs to be better defined. In that regard, we propose that autophagic proteins, and perhaps the resulting membranes, are indispensable during the onset of HCV replication.
Materials and methods
Yeast two-hybrid screening
Yeast strains for yeast two-hybrid screening were obtained from Clontech (Mountain View, CA, USA) as components of the pretransformed MATCHMAKER cDNA libraries and the MATCHMAKER Two-Hybrid System 3. S. cerevisiae Y187 (MATα), which contained the pretransformed human cDNA library (complexity > 2–4 × 106 independent clones) cloned into the Gal4 activation domain vector (pACT2) was allowed to mate with S. cerevisiae AH109 (MATa), which had been transformed with a Gal4 DNA-binding domain vector (pGBKT7) containing HCV NS5B as a bait. To construct the bait, the HCV NS5B gene lacking the region encoding the C-terminal 21 amino acid residues was amplified by PCR using the HCV-BK (genotype 1b) genomic cDNA as a template and NS5B-H1 and NS5B-R1700 as primers (Table S1). The resulting NS5BΔ21 gene containing unique EcoRI and BamHI sites at the N- and C-termini, respectively, was cloned into the pGBKT7 expression plasmid to generate an in-frame fusion protein with a Gal4 DNA binding domain. The resulting plasmid, pGBKT7-5BΔ21 was sequenced and subsequently used to transform the AH109 yeast strain. Following mating, the diploid yeast strain (Y187-AH109-NS5B) was selected on SD medium in the absence of leucine, tryptophan, and histidine (−LTH). Eight hundred potential positive yeast clones were obtained from two million screenable clones and were replica-plated onto X-α Gal indicator plates in the absence of leucine, tryptophan, histidine, and adenine. Three hundred blue colonies (positive for X-α Gal as a result of MEL1 gene activation) were selected by prototrophy for histidine and adenine. These clones were retested for positive interaction, and pACT2/cDNA plasmids were isolated from 20 strong positive clones as reflected by the intensity of the blue color formed on X-α Gal indicator plates. These plasmids were retransformed into yeast strains carrying the bait construct, pGBKT7-5BΔ21 or pGBKT7, to confirm true interactions. Clones that gave a positive signal when cotransformed with pGBKT7-5BΔ21 were chosen for DNA sequencing. The DNA sequences of the positive pACT2/cDNA clones were translated and compared with a nonredundant sequence database using the BLAST program through the National Center for Biotechnology Information network service. CLUSTAL_X program was used to analyze statistically significant matches.
Full-length hAPG5–NS5BΔ21 interaction by yeast two-hybrid assay
To obtain the full-length coding region of the hATG5 gene (825 bp), the primers ATG5-H1 and ATG5-R800 (Table S1) were prepared for PCR amplification using a human liver cDNA library (Clontech) as a template. PCR products with unique NdeI and BamHI at the N- and C-termini, respectively, were cloned into the pGADT7 vector. The sequence of the resulting plasmid was confirmed by DNA sequencing. The integrity of the hATG5 and NS5BΔ21 genes inserted into the yeast plasmids (pGBKT7 and pGADT7) was confirmed in vitro by expressing the two proteins using the TNT® T7 Coupled Reticulocite Lysate System (Promega, Madison, WI, USA) as described by the manufacturer. Final confirmation of hAPG5/NS5BΔ21 interactions by yeast two-hybrid experiments in AH109 cells was carried out as described previously.
Coimmunoprecipitation (Co-IP)
For co-IP, N-terminal tag HA-hAPG5 and c-myc-NS5BΔ21 proteins were expressed in the BJ2168 yeast strain. Briefly, the hAPG5 and NS5BΔ21 genes were amplified using the Tag-HA/APG5-R800-Trp and Tag-MYC/NS5B-R1700-Leu primer sets, respectively (Table S1). The PCR products were then cloned into the YEpc (NS5B) and YEpTDH (hAPG5) plasmids using unique restriction sites (Table S1). The resulting expression plasmids, YEpc-NS5BΔ21 (copper-inducible) and YEpTDH-hAPG5, were used to transform a protease-deficient yeast strain (BJ2168). The double transformant yeast strain, YEpc-NS5BΔ21/YEpTDH-hAPG5 was grown in SD medium depleted of tryptophan and leucine (−LT). For the induction of c-myc-NS5BΔ21, cupric sulfate (1 μM) was added to the −LT medium and incubated overnight at 30 °C.
Yeast extracts containing soluble hAPG5 and NS5BΔ21 proteins were prepared according to the protocol of Mizushima et al. (2001). Briefly, yeast cells were washed and resuspended in ice-cold TES buffer (50 mM Tris, 5 mM EDTA, and 150 mM NaCl, pH 7.5). Yeast cell walls were disrupted with acid-washed glass beads by vortexing vigorously for 10 minutes. After centrifugation at 3000 × g for 5 minutes, the supernatant (cytoplasmic and microsomal fractions) was mixed with 0.1 volume of 10% NP40 and incubated for 15 minutes before centrifugation at 10,000 × g for 15 minutes. Aliquots of the resulting supernatant (500–600 μg of protein) were incubated with or without 1 μl of monoclonal anti-Myc antibody (9E10: Santa Cruz, CA, USA) or anti-HA antibody (F6: Santa Cruz) for 2 hours. A protein A/G sepharose bead mixture (Pierce, Rockford, IL, USA) (10 μl) was added, and samples were incubated for an additional 2 hours. The sepharose beads were washed three times with TES buffer, and the bound proteins were eluted with 30 μl of Laemmli buffer. Samples (20 μl) were analyzed by SDS–PAGE and immunoblotting. Proteins were detected by monoclonal anti-c-Myc (9E10) or anti-HA (F2) and visualized by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech; Baie d'Urfé, QC, Canada).
Alternatively, co-IP was performed using metabolically labeled cell extract. In brief, BJ2168 yeast cells carrying the YEpc-NS5BΔ21 and YEpTDH-hAPG5 plasmids were grown in SD (−LT) medium overnight at 30 °C. When the cells reached mid-log phase, they were washed and incubated for 1 hour in SD (−LT) medium depleted in methionine. Subsequently, 10 μM of cupric sulfate and 35S-labeled methionine (50 μCi) were added to the culture, and incubated for 3 hours. After washing the cells twice with SD medium, yeast extracts were prepared for co-IP study as described previously. Immunoprecipitated proteins were separated on SDS–PAGE followed by autoradiography. The negative control included yeast extracts prepared from the yeast strain without cupric sulfate induction (i.e., expressing only HA-hAPG5) and subjected to the same analysis.
Mapping of hAPG5–NS5BΔ21 interaction domain
Deletion mutants of NS5BΔ21 were generated by PCR using the primers indicated in Table S1. PCR fragments were inserted in pGBKT7 or pGADT7 and the interactions were analyzed by yeast two-hybrid assay. To identify the interaction domains, we performed co-IP of the deletion mutants expressed in the BJ2168 yeast strain using the YEpc and YEpTDH plasmids.
Preparation of viral stock and infection
JFH-1 virus was generated in Huh7 cells by transfection of in vitro-transcribed full-length JFH1 RNA (MEGAscript, Ambion, Streetville, Ontario, Canada), and viral stocks were produced by infection of Huh7 cells at a multiplicity of infection (MOI) of 0.01, as described previously (Guevin et al., 2009).
Subcellular fractionation analysis
For subcellular fractionation, the hAPG5 gene was cloned into the pEGFP-C1 plasmid (Clontech). The resulting pEGFP-hAPG5 plasmid was then transfected into Clone-A and naïve Huh7 cells using Lipofectamine as suggested by the manufacturer (Invitrogen; Burlington, Ontario, Canada). The engineering and characterization of the clone-A cells, which constitutively expressed the HCV nonstructural proteins (NS3, 4A, 4B, 5A, and 5B), have been reported elsewhere (Howe et al., 2006). At 48 hours after transfection, cells were trypsinized and washed twice with PBS. After washing, 2 × 107 cells were homogenized in a hypotonic buffer containing 10 mM Tris–HCl, pH 7.5, and 2 mM MgCl2, followed by centrifugation at 1000 × g for 5 min to yield the nuclear fraction. The supernatant was then centrifuged at 14,000 × g for 40 min to pellet the microsomal/mitochondrial (mit/mic) fraction. The nuclear and the mit/mic pellets were resuspended in the same volume as the final supernatant using the hypotonic buffer, and 20 μl of each extract was resolved on SDS–PAGE and immunoblotting using either a rabbit polyclonal antiserum directed against the HCV NS5BΔ21 or a mouse monoclonal anti-GFP (GFP-20; Sigma). Proteins were visualized by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech).
Indirect immunofluorescence
For indirect immunofluorescence, cells were transfected with the pEGFP-hATG5 and infected with HCVcc JFH1 at a MOI of 0.01. At 24 hours after transfection, the cells were trypsinized and grown on glass coverslips for another 24 hours. The coverslips were then fixed in PBS containing 4% formaldehyde for 10 min, washed three times in PBS, and incubated for 1 h at 4 °C in blocking buffer (PBS, 3% bovine serum albumin, 0.1% Triton X-100). After three washes in PBS, the coverslips were incubated with a rabbit polyclonal antibody directed against the HCV NS5B protein (generously provided by Dr Takaji Wakita, National Institute of Infectious Diseases, Tokyo, Japan) (dilution 1:200) in blocking buffer for 1 hour at room temperature (RT). The coverslips were then washed three times in PBS and incubated for 1 hour at RT with Alexa Fluor 488 or 568-conjugated secondary antibody goat anti-mouse or anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) (dilution 1:500). Coverslips were washed four times in PBS and mounted on glass slides with Prolong™ Antifade (Molecular Probes), and cells were examined with a laser scanning confocal BioRad Radiance 2000 microscope.
SiRNA transfection
SiRNA duplexes targeting human were purchased from Ambion (siRNA ATG5 no. AM16708A and scramble siRNA no. 4611G). SiRNA duplexes (150 pmol) were transfected into 1 × 105 Huh7 cells using the RNAiMAX transfection reagent (Invitrogen) and infected 6 hours later with HCV JFH-1. Protein knockdown was usually analyzed 48 hours after transfection.
Western blot analysis
Cells were washed three times in phosphate-buffered saline and lysed in RIPA buffer (50 mm Tris–HCl, pH 8.0, 1% (vol./vol.) Nonidet P40, 0.5% sodium deoxycholate, 150 mm NaCl and 0.1% (vol./vol.) SDS) with a Complete Protease Inhibitor Mixture (Roche Applied Science). After SDS–PAGE electrophoresis, protein samples were transferred to an Immuno-Blot PVDF membrane for protein blotting (Bio-Rad) for 45 min. Nonspecific binding sites were blocked for 1 hour in PBS–5% skimmed milk, and the membrane was stained for 1 hour with the primary antibodies. The antibodies used were HCV polyclonal anti-core (obtained from Dr Denis Leclerc, Laval University, Canada) (dilution 1:1000) and anti-ATG5 (FL-275) polyclonal antibody (Santa Cruz Biotechnology, CA, USA) (dilution 1:1000). After incubating with the primary antibody, the membranes were washed four times in PBS–0.1% Tween-20. Bound antibodies were detected by incubation for 45 min with a goat anti-rabbit HRP antibody (Jackson ImmunoResearch) (dilution 1:10,000). The signals were developed with SuperSignal™ West Pico chemiluminescent substrate (Pierce).
Quantitative RT–PCR
Total cellular RNA was prepared from siRNA-transfected cells by using the RNeasy Mini Kit (QIAGEN). The cDNA were prepared from 250 ng of total cellular RNA. Briefly, RNAs were incubated 3 min at 70 °C then cooled on ice for 2 min before the addition of 4 μl of RT-Buffer 5X (Invitrogen), 2 μl of DTT (0.1 M), 1 μl of random primer p(dN6) (100 ng/μl), 1 μl of dNTP (20 mM), 20 U of RNAsin, and 100 U of MMLV reverse transcriptase. Samples were incubated for 10 min at 25 °C and 1 h at 37 °C. To inactivate the MMLV, samples were incubated 15 min at 70 °C, and cDNAs were diluted to a final volume of 200 μl with Rnase-free water. Primers used for amplification were 5′UTR-R: 5′-GAGTGGGTTTA TCCAAGAAAG-3′ and 5′UTR-F: 5′-TCTGCGGAACCGGTGAGT-3′. The mixture consists of 2.5 μl of cDNA in a final volume of 25 μl of the reaction mixture containing 8.6 μl H2O, 0.5 μl of probe FAM-UTR (12.5 μM) CCGGAATTGCCGGGAAGACTG, and 0.25 μl (90 μM) of each HCV primers. For the internal control, the 18S Ribosomal RNA Kit was used as suggested by the manufacturer (Applied Biosystem). The mixture was completed with 12.5 μl of the TaqMan Universal Master Mix 2X (Applied Biosystem), and the amplification was performed as suggested by the manufacturer in a Rotor-Gene RG-3000 (Corbet Research).
Acknowledgments
We are grateful to Takaji Wakita and Denis Leclerc for Reagents and Nathalie Fournier for technical assistance. This work was supported by NSERC of Canada (grant no. 312225-05). C. G. and C. B. were supported by a fellowship from the Armand-Frappier Foundation (Canada).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.virol.2010.05.032.
Appendix A. Supplementary data
References
- Ait-Goughoulte M., Kanda T., Meyer K., Ryerse J.S., Ray R.B., Ray R. Hepatitis C virus genotype 1a growth and induction of autophagy. J. Virol. 2008;82(5):2241–2249. doi: 10.1128/JVI.02093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizaki H., Choi K.S., Liu M., Li Y.J., Lai M.M. Polypyrimidine-tract-binding protein is a component of the HCV RNA replication complex and necessary for RNA synthesis. J. Biomed. Sci. 2006;13(4):469–480. doi: 10.1007/s11373-006-9088-4. [DOI] [PubMed] [Google Scholar]
- Aligo J., Jia S., Manna D., Konan K.V. Formation and function of hepatitis C virus replication complexes require residues in the carboxy-terminal domain of NS4B protein. Virology. 2009;393(1):68–83. doi: 10.1016/j.virol.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressanelli S., Tomei L., Roussel A., Incitti I., Vitale R.L., Mathieu M., De Francesco R., Rey F.A. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Proc. Natl. Acad. Sci. U. S. A. 1999;96(23):13034–13039. doi: 10.1073/pnas.96.23.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux M., Chisari F.V. Autophagy proteins promote hepatitis C virus replication. Autophagy. 2009;5(8):1224–1225. doi: 10.4161/auto.5.8.10219. [DOI] [PubMed] [Google Scholar]
- Dreux M., Gastaminza P., Wieland S.F., Chisari F.V. The autophagy machinery is required to initiate hepatitis C virus replication. Proc. Natl. Acad. Sci. U. S. A. 2009;106(33):14046–14051. doi: 10.1073/pnas.0907344106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger D., Wolk B., Gosert R., Bianchi L., Blum H.E., Moradpour D., Bienz K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J. Virol. 2002;76(12):5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Aizaki H., He J.W., Lai M.M. Interactions between viral nonstructural proteins and host protein hVAP-33 mediate the formation of hepatitis C virus RNA replication complex on lipid raft. J. Virol. 2004;78(7):3480–3488. doi: 10.1128/JVI.78.7.3480-3488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M.D., Baba M., Scott S.V., Mizushima N., Garrison B.S., Ohsumi Y., Klionsky D.J. Apg5p functions in the sequestration step in the cytoplasm-to-vacuole targeting and macroautophagy pathways. Mol. Biol. Cell. 2000;11(3):969–982. doi: 10.1091/mbc.11.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosert R., Kanjanahaluethai A., Egger D., Bienz K., Baker S.C. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 2002;76(8):3697–3708. doi: 10.1128/JVI.76.8.3697-3708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosert R., Egger D., Lohmann V., Bartenschlager R., Blum H.E., Bienz K., Moradpour D. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J. Virol. 2003;77(9):5487–5492. doi: 10.1128/JVI.77.9.5487-5492.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevin C., Lamarre A., Labonte P. Novel HCV replication mouse model using human hepatocellular carcinoma xenografts. Antivir. Res. 2009;84(1):14–22. doi: 10.1016/j.antiviral.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Hammond E.M., Brunet C.L., Johnson G.D., Parkhill J., Milner A.E., Brady G., Gregory C.D., Grand R.J.A. Homology between a human apoptosis specific protein and the product of APG5, a gene involved in autophagy in yeast. FEBS Lett. 1998;425(3):391–395. doi: 10.1016/s0014-5793(98)00266-x. [DOI] [PubMed] [Google Scholar]
- Howe A.Y., Cheng H., Thompson I., Chunduru S.K., Herrmann S., O'Connell J., Agarwal A., Chopra R., Del Vecchio A.M. Molecular mechanism of a thumb domain hepatitis C virus nonnucleoside RNA-dependent RNA polymerase inhibitor. Antimicrob. Agents Chemother. 2006;50(12):4103–4113. doi: 10.1128/AAC.00365-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpoor A., Panyasrivanit M., Wikan N., Smith D.R. A role for autophagolysosomes in dengue virus 3 production in HepG2 cells. J. Gen. Virol. 2009;90(Pt 5):1093–1103. doi: 10.1099/vir.0.007914-0. [DOI] [PubMed] [Google Scholar]
- Kim J., Klionsky D.J. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu. Rev. Biochem. 2000;69:303–342. doi: 10.1146/annurev.biochem.69.1.303. [DOI] [PubMed] [Google Scholar]
- Kim J., Huang W.P., Klionsky D.J. Membrane recruitment of Aut7p in the autophagy and cytoplasm to vacuole targeting pathways requires Aut1p, Aut2p, and the autophagy conjugation complex. J. Cell Biol. 2001;152(1):51–64. doi: 10.1083/jcb.152.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D.J., Emr S.D. Cell biology—autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konan K.V., Giddings T.H., Jr., Ikeda M., Li K., Lemon S.M., Kirkegaard K. Nonstructural protein precursor NS4A/B from hepatitis C virus alters function and ultrastructure of host secretory apparatus. J. Virol. 2003;77(14):7843–7855. doi: 10.1128/JVI.77.14.7843-7855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyono K., Miyashiro M., Taguchi I. Human eukaryotic initiation factor 4AII associates with hepatitis C virus NS5B protein in vitro. Biochem. Biophys. Res. Commun. 2002;292(3):659–666. doi: 10.1006/bbrc.2002.6702. [DOI] [PubMed] [Google Scholar]
- Lee Y.R., Lei H.Y., Liu M.T., Wang J.R., Chen S.H., Jiang-Shieh Y.F., Lin Y.S., Yeh T.M., Liu C.C., Liu H.S. Autophagic machinery activated by dengue virus enhances virus replication. Virology. 2008;374(2):240–248. doi: 10.1016/j.virol.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M., Suzuki N.N., Obara K., Fujioka Y., Ohsumi Y., Inagaki F. Structure of Atg5.Atg16, a complex essential for autophagy. J. Biol. Chem. 2007;282(9):6763–6772. doi: 10.1074/jbc.M609876200. [DOI] [PubMed] [Google Scholar]
- Miyanari Y., Atsuzawa K., Usuda N., Watashi K., Hishiki T., Zayas M., Bartenschlager R., Wakita T., Hijikata M., Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 2007;9(9) doi: 10.1038/ncb1631. 1089-U74. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Noda T., Yoshimori T., Tanaka Y., Ishii T., George M.D., Klionsky D.J., Ohsumi M., Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395(6700):395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Yamamoto A., Hatano M., Kobayashi Y., Kabeya Y., Suzuki K., Tokuhisa T., Ohsumi Y., Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 2001;152(4):657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradpour D., Gosert R., Egger D., Penin F., Blum H.E., Bienz K. Membrane association of hepatitis C virus nonstructural proteins and identification of the membrane alteration that harbors the viral replication complex. Antivir. Res. 2003;60(2):103–109. doi: 10.1016/j.antiviral.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Pedersen K.W., van der Meer Y., Roos N., Snijder E.J. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J. Virol. 1999;73(3):2016–2026. doi: 10.1128/jvi.73.3.2016-2026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice E., Jerome W.G., Yoshimori T., Mizushima N., Denison M.R. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 2004;279(11):10136–10141. doi: 10.1074/jbc.M306124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel A., Giddings T.H., Jr., Ladinsky M.S., Kirkegaard K. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 1996;70(10):6576–6588. doi: 10.1128/jvi.70.10.6576-6588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhy D.A., Giddings T.H., Kirkegaard K. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J. Virol. 2000;74(19):8953–8965. doi: 10.1128/jvi.74.19.8953-8965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I., Fukasawa M., Ueno T., Kominami E., Wakita T., Hanada K. Knockdown of autophagy-related gene decreases the production of infectious hepatitis C virus particles. Autophagy. 2009;5(7):937–945. doi: 10.4161/auto.5.7.9243. [DOI] [PubMed] [Google Scholar]
- Tu H., Gao L., Shi S.T., Taylor D.R., Yang T., Mircheff A.K., Wen Y., Gorbalenya A.E., Hwang S.B., Lai M.M. Hepatitis C virus RNA polymerase and NS5A complex with a SNARE-like protein. Virology. 1999;263(1):30–41. doi: 10.1006/viro.1999.9893. [DOI] [PubMed] [Google Scholar]
- Wakita T., Pietschmann T., Kato T., Date T., Miyamoto M., Zhao Z., Murthy K., Habermann A., Krausslich H.G., Mizokami M., Bartenschlager R., Liang T.J. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005;11(7):791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Zhang J., Si X., Gao G., Mao I., McManus B.M., Luo H. Autophagosome supports coxsackievirus B3 replication in host cells. J. Virol. 2008;82(18):9143–9153. doi: 10.1128/JVI.00641-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Gastaminza P., Cheng G., Kapadia S., Kato T., Burton D.R., Wieland S.F., Uprichard S.L., Wakita T., Chisari F.V. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 2005;102(26):9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.