Abstract
While much is known about the attachment of the chlamydiae to the host cell and intracellular events during the developmental cycle, little is known about the mechanism(s) by which elementary bodies exit the cell. In this report, we use the guinea pig conjunctival model of Chlamydia caviae infection to present in vivo ultrastructural evidence supporting two mechanisms for release of chlamydiae from the mucosal epithelia. Four days after infection, histopathologic observation shows an intense infiltration of polymorphonuclear leukocytes (PMN) in the conjunctival epithelium. By transmission electron microscopy, a gradient-directed PMN response to chlamydiae-infected epithelial cells was observed. As PMN infiltration intensifies, epithelial hemidesmosome/integrin/focal adhesion adherence with the basal lamina is disconnected and PMNs literally lift off and release infected superficial epithelia from the mucosa. Many of these infected cells appear to be healthy with intact microvilli, nuclei, and mitochondria. While lysis of some infected cells occurs with release of chlamydiae into the extracellular surface milieu, the majority of infected cells are pushed off the epithelium. We propose that PMNs play an active role in detaching infected cells from the epithelium and that these infected cells eventually die releasing organisms but, in the process move to new tissue sites via fluid dynamics.
Keywords: Chlamydia, guinea pigs, neutrophils, conjunctiva, transmission electron microscopy
Introduction
An important question regarding the pathogenesis of chlamydial infections, and more specifically with respect to chlamydial genital infections, is how does this non-motile organism spread from one cell to another and, more importantly, how does the organism ascend the genital tract from the initial site of infection in the cervix to the Fallopian tubes? Because the primary strategy for an effective vaccine is to prevent ascending infection, an understanding of this mechanism assumes great relevance in the ultimate design of a vaccine.
The developmental cycle of chlamydiae has been studied at length over many years. While the molecular signaling events that dictate the conversion of the elementary body (EB) to the reticulate body (RB) and back again to the EB are still not completely understood, there is a plethora of visual images of the various stages of the cycle at both the light and ultra microscopic level. However, the vast majority of the published images are of chlamydiae grown in tissue culture with only a few reports actually showing chlamydiae directly in situ in animal tissue. There are many images, both in vitro and in vivo, of the entry of EB into the host cell, and that of course has spawned a continuing controversy of what exactly is the molecular mechanism and/or moieties that initiate the entrance. In contrast, there has been very little attention paid to the end stage of the developmental cycle, i.e. the mechanism by which the newly formed EBs exit the cell. An elegant in vitro cinematographic study by Neeper et al (Neeper et al., 1990) showed the dramatic bursting of an infected cell to release the EBs into the surrounding milieu. Perhaps the most realistic in vitro model for culture of chlamydiae was primary human endometrial gland epithelial cells grown in a polarized state (Wyrick et al., 1993). In this study, it was observed that the terminal stage of the cycle could reflect the polarity of the infectious process, i.e., luminal C. trachomatis serovar E were released at the apical domain whereas for the more invasive and lymphotrophic biovar Lymphogranuloma venereum (LGV) serovar L2, EBs and RBs exited via rupture of the infected cell basal domain to spread into the submucosa. In some cases, the genital serovar E infected cell was extruded intact from the polarized monolayer into the apical/luminal milieu. More recently, the application of confocal microscopy for fluorescence imaging in real time of live GFP-transfected HeLa cells has revealed that egress of serovar L2 chlamydial progeny can occur by both rapid (10–30 minutes) host cell lysis as well as by slow (~3 hours) extrusion of the intact inclusion out of the host epithelial cell (Hybiske & Stephens, 2007).
As might be expected, it is appreciably more difficult to detect chlamydiae in tissue samples taken directly from a biopsy or tissue collected from an animal. The majority of the images published show single host cells and inclusions with representative developmental forms, but there is little showing how the organisms actually exit the cell in vivo. The only pictures providing any evidence for a possible mechanism were published by Soloff et al (Soloff et al., 1985) and Doughri et al (Doughri et al., 1972) in which the authors observed neutrophils in contact with epithelial cells from guinea pig cervical cells infected with Chlamydia caviae and bovine intestinal epithelial cells infected with C. psittaci respectively. The suggestion was that the neutrophils participated in the detachment of intact epithelial cells. Dougrhi also presented evidence for lysis of host cells and extrusion of inclusions from infected cells. In this report, we have taken advantage of the guinea pig conjunctival model of C. caviae infection to present in vivo ultrastructural evidence that support two mechanisms for the release of chlamydiae from the mucosal epithelia in vivo and the implications of this mechanism for the spread of the infection to other tissue sites.
Materials and Methods
Experimental Animals
Female Hartley strain guinea pig, weighing 500–550 g were obtained from Charles River Laboratories, Boston, MA. All animals were housed individually in cages with fiber glass filter tops in an environmentally controlled room with a 12:12 light:dark cycle and were fed food and water ad libitum.
Infection of animal and collection of tissue
A guinea pig was inoculated ocularly by depositing 25 µl of inoculum, containing 106 inclusion-forming units of the C. caviae agent of guinea pig inclusion conjunctivitis (GPIC), on the eye and lifting the upper and lower conjunctiva to allow the fluid to contact the inner conjunctival surface. The inoculating dose is clearly unnaturally high but was necessary to produce a higher level of infection to facilitate detection at the ultrastructural level.
At four days after infection, the animal was euthanized and the conjunctival tissue collected. A portion of the tissue was placed in formalin and processed by standard histopathological technique for staining with hemotoxylin and eosin. Immunohistochemistry on the paraffin section used a monoclonal mouse anti-chlamydial LPS antibody (a kind gift of Dr. You-xun Zhang, Boston University). An additional portion was placed in 2% glutaraldehyde-0.5% paraformaldehyde, in 0.1M Cacodylate buffer (pH 7.2), and a portion was placed in 2% paraformaldehyde – 0.25% glutaraldehyde, in 0.1M Sorensen’s buffer, both pre-fixatives for subsequent standard processing, infiltration and embedding in Epon 812 resin for high contrast transmission electron microscopy (TEM) or Lowicryl K4M resin for post-embedding labeling immunoelectron microscopy, respectively (Wyrick et al., 1994).
Transmission electron microscopy
Prefixed conjunctival tissue was cut into two halves: one half was processed at room temperature and embedded in Epon-Aradlite 812 resin for high contrast transmission electron microscopy; the other half was processed at 4°C and −20C and embedded in Lowicryl resin for immuno-electron microscopy (Wyrick et al., 1994). Primary antibodies included Chlamydia genus-specific LPS polyclonal antibodies generated in rabbits (10 µg/ml; Cortex Biochem Inc.) or mouse monoclonal antibodies (10 µg/ml; Virostat). Important controls always included duplicate thin sections exposed to an irrelevant primary antibody as well as gold-conjugated second-affinity antibody alone to determine background cross-reactivity.
Sequential semi-thin sections of sagittal views of the conjunctival tissue were first cut, placed on a glass side, stained with Epoxy Tissue Stain (EM Sciences, Hatfield, PA), which is a Toluidine Blue O/Basic Fuchsin in a water/alcohol solution, and examined by brightfield microscopy for orientation. When the section depth revealed areas of early inflammation, indicating the likely presence of chlamydial infection, the area was isolated, re-trimmed, and silver-gold thin sections were cut with a diamond knife on a Reichert Ultracut S ultramicrotome (Leica Microsystems Inc., Bannockburn, IL, USA), collected on gold grids and examined in a Philips Tecnai-10 electron microscope (FEI) at 80 kV. Repetition of these steps permitted an assessment of the chronology of the inflammatory events occurring in vivo to GPIC-infected conjunctival tissue.
Results
Characteristically, early in the course of chlamydial infection, one routinely finds a heavy infiltration of polymorphonuclear leukocytes (PMNs) into the epithelium, and these are almost invariably, but not surprisingly, associated with the portion of the epithelium that is infected with chlamydiae (Figure 1). Some mononuclear cells may be found in the submucosa as well as lymphoid aggregates which are characteristic of normal and infected conjunctiva in the guinea pig. GPIC is not an invasive organism, and one finds inclusions primarily restricted to the superficial epithelial cell layer (Figure 2). Interestingly, when examined at higher magnifications, it is not unusual to find PMNs in direct contract with infected epithelial cells (Figure 1). The close association of PMNs with infected cells is to be expected because of chemokines and cytokines released from the cells as a result of chlamydial infection, and it makes perfect sense that PMNs would be attempting to kill chlamydiae and resolve the infection.
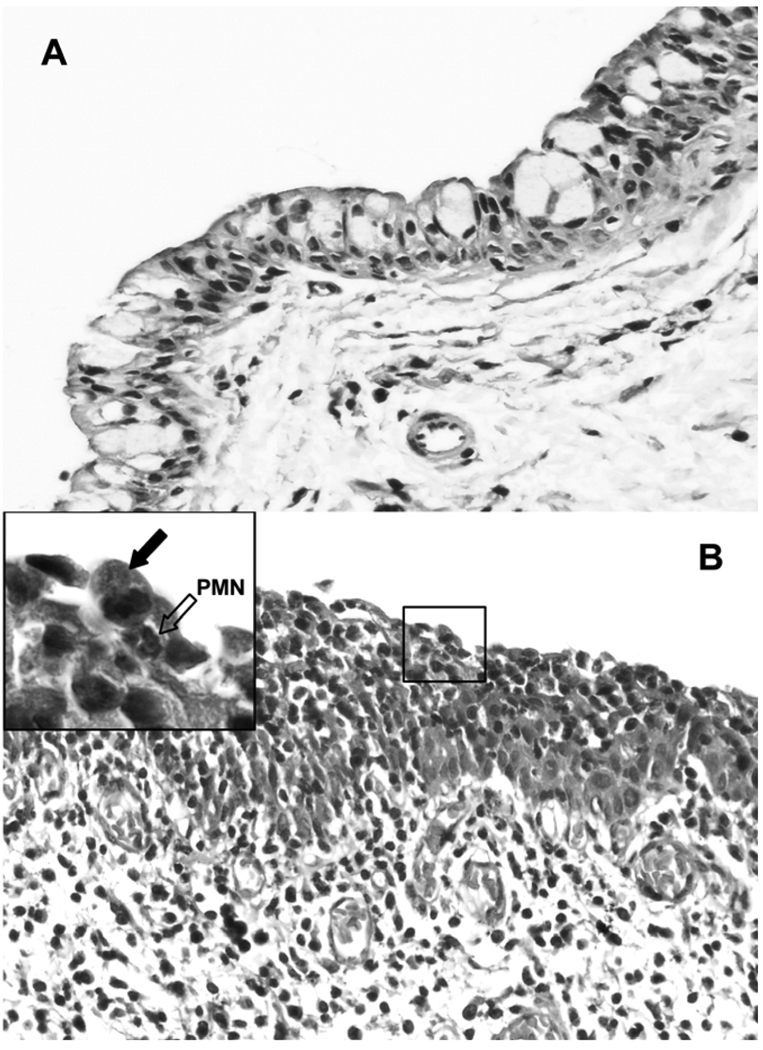
Figure 1.
Hematoxylin and eosin-stained section of guinea pig conjunctiva showing the acute inflammatory response. (A) Control uninfected conjunctival epithelium showing goblet cells and a epithelium only a few cells in depth. (B) Inflamed epithelium showing heavy infiltration of the epithelium by PMNs. Occasional mononuclear cells are also seen in the submucosa. 40 ×. (Insert) High-powered view showing a chlamydia-infected cell (arrow) with a PMN (open arrow) directly underneath and in contact with the cell that is being dislodged from the epithelium.
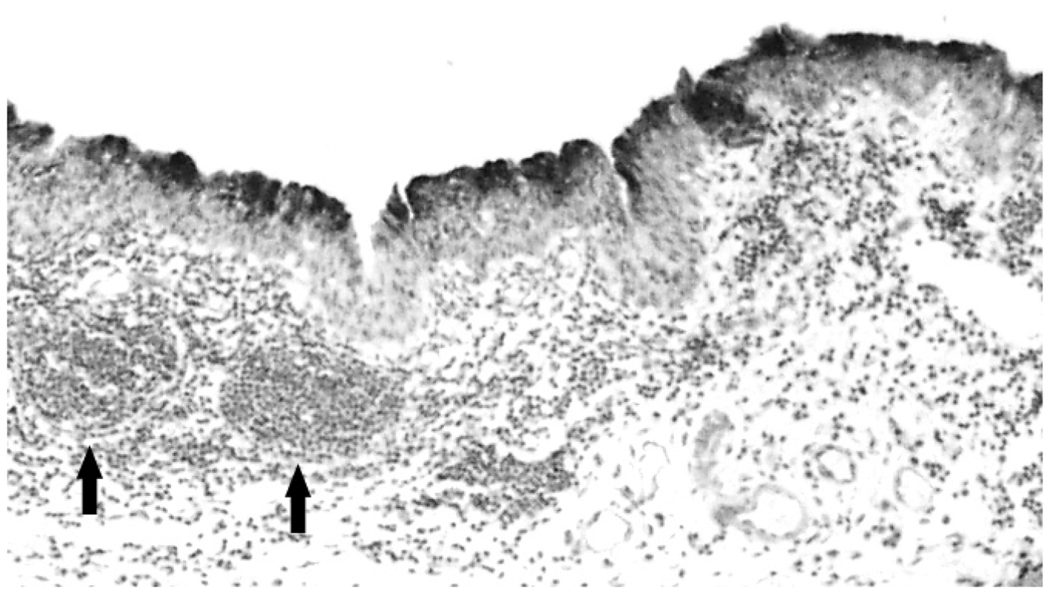
Figure 2.
Low power (10×) view of a section of infected epithelium stained with mouse anti-chlamydial LPS. The staining is restricted to the epithelium, demonstrating that the infection is restricted to superficial epithelial cells. Note two lymphoid aggregates (arrows) which are characteristic of the guinea pig conjunctiva. These are routinely found in infected animals as well.
However, at the ultrastructural level, the well-known chronological events of the primary inflammatory response, usually described from a histological viewpoint, take on a more clear perspective with high definition. From an ultrastructural perspective, GPIC infection of guinea pig conjunctiva in vivo at 4 days after infection reveals several interesting features (Figure 3). First, as has been reported using light microscopy, chlamydial infection appears restricted to the superficial epithelial cells, including mucous secreting epithelial cells (Figure 3C). Second, the number of infected epithelial cells is numerous, suggesting that more than one round of chlamydial replication and progeny release have probably occurred in 4 days. This is further supported by the fact that the chlamydiae in various epithelial cells seem to be in different stages of development (Figure 4). Chlamydial inclusions in mucosal epithelia in vivo develop in the apical cytoplasmic domain. As the inclusion enlarges, there is disruption of the actin cytoskeletal apical ring and the actin microfilaments, which form the core of the microvillus protrusions; as such, the microvilli often retract, resulting in fewer and shorter microvilli.
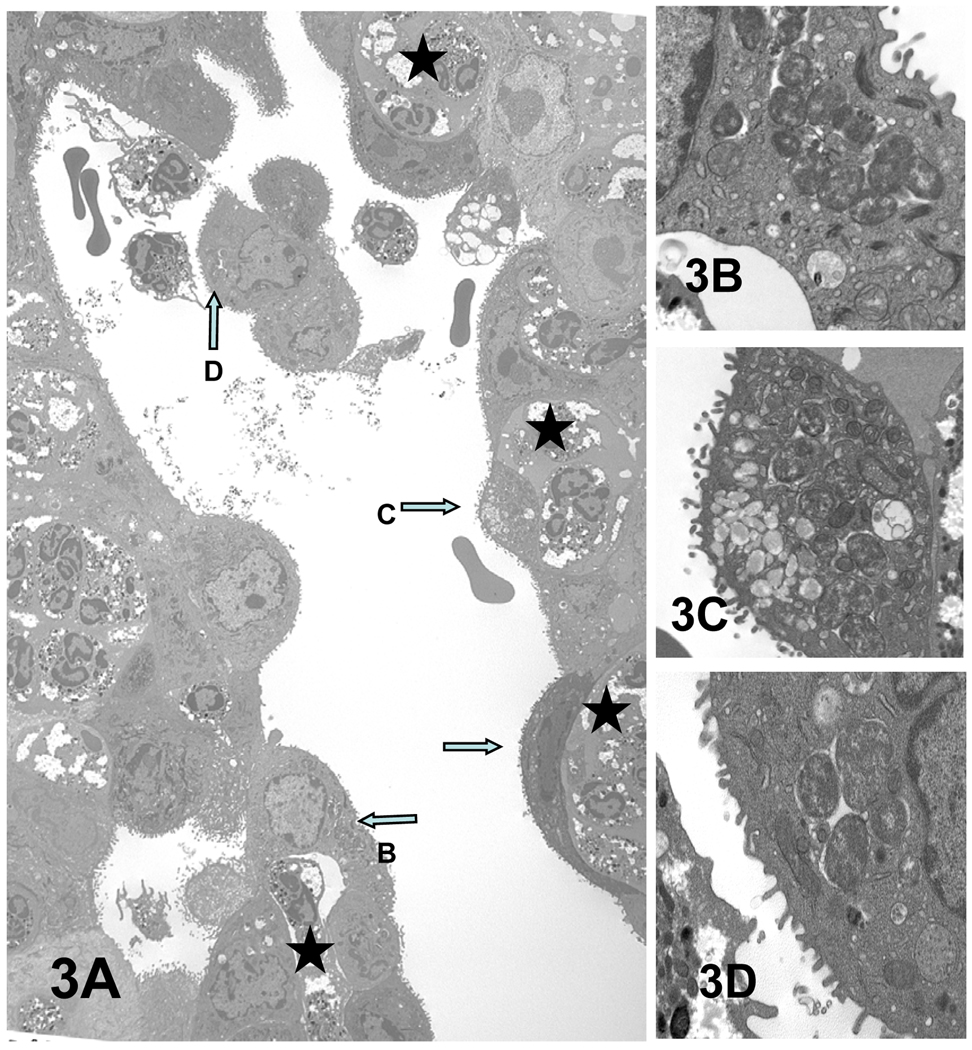
Figure 3.
PMN response to C. caviae infection of superficial epithelia cells of guinea pig conjunctiva. (A) Some of the prominent chlamydial inclusions are indicated by arrows and enlarged in panels B–D. (D) The infected epithelial cell cluster has apparently been released from the conjunctiva into the lumen. Serial sections of this cell confirmed that it is not attached to the epithelium. Stars denote the PMNs juxtaposed to some chlamydiae infected epithelial cells. Magnifications: A = × 2,500; B = × 17,900; C and D = × 12,500.
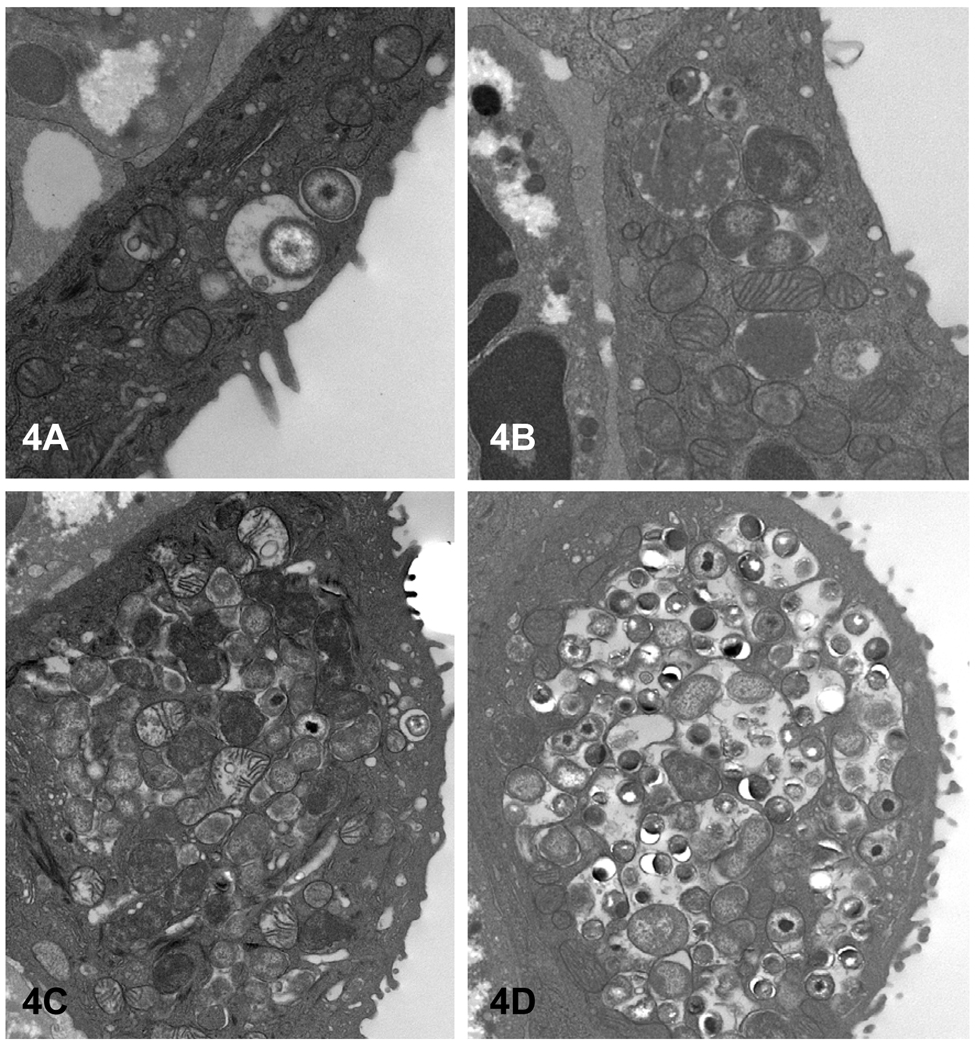
Figure 4.
All stages of the chlamydial developmental cycle can be found in various infected conjunctival epithelial cells. (A) An EB and intermediate body; (B) an early inclusion containing a few RB; (C) a larger inclusion filled with RB and EB. The latter inclusion more clearly depicts the tendency of C. caviae to initially form multiple small inclusions, which may later fuse to form larger inclusions. Magnifications: A = × 25,100; B = × 17,900; C and D = × 12,500.
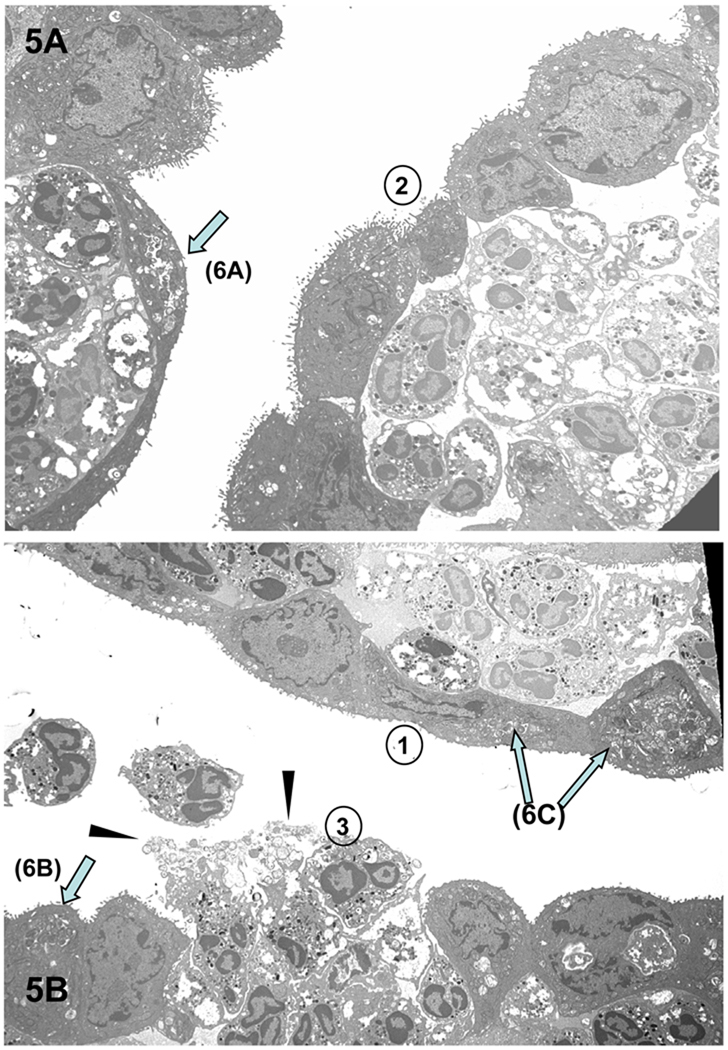
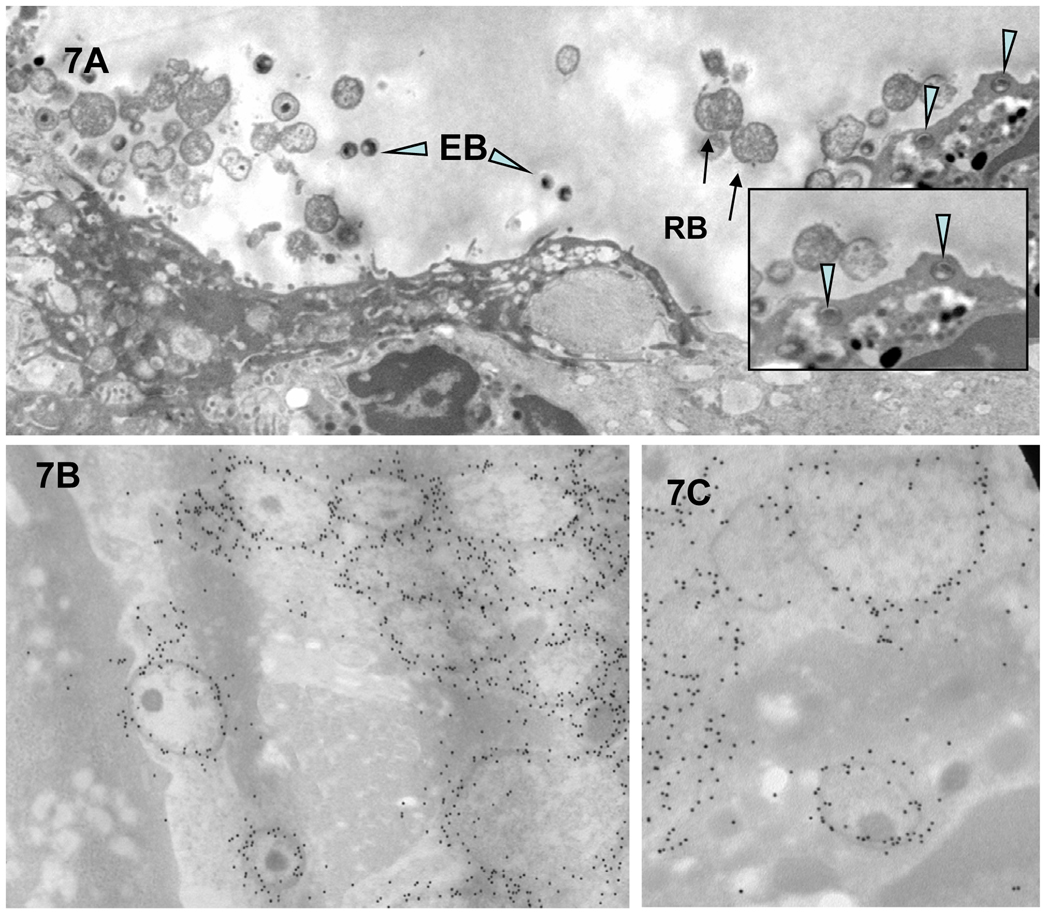
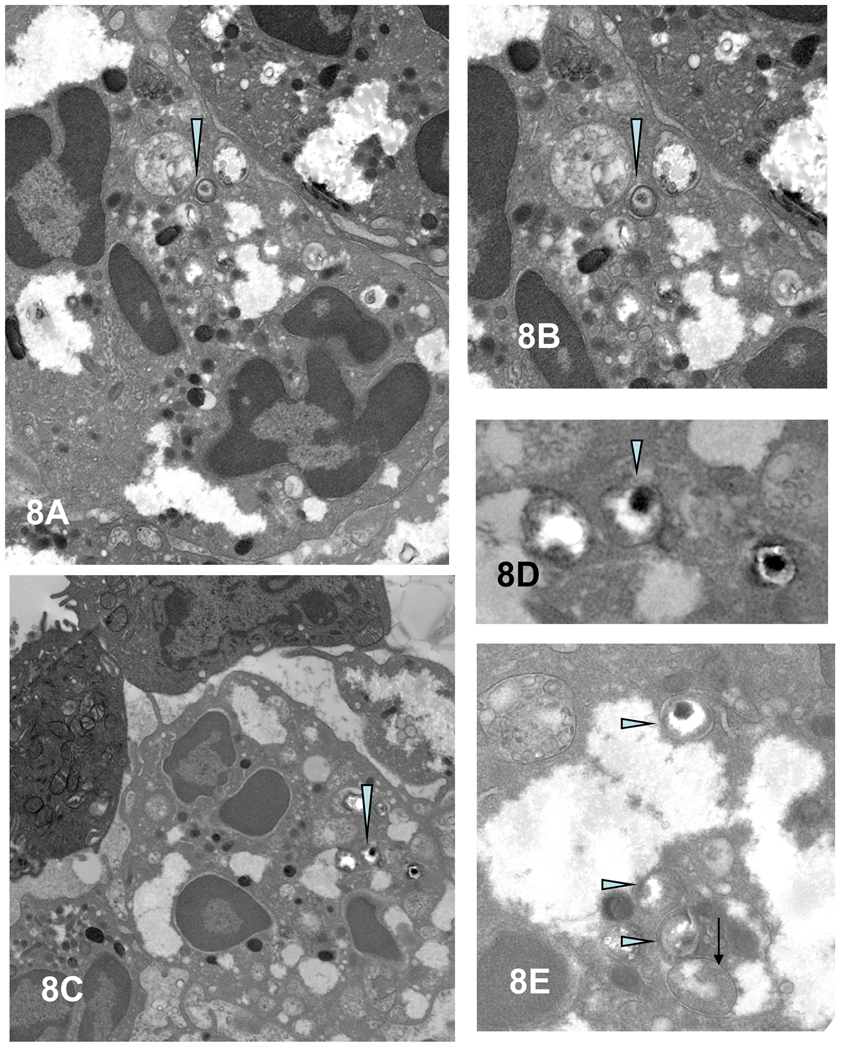
Third, with time, there is an increased accumulation of PMNs to the chlamydiae-infected epithelial cells (Figure 3A). We have observed that virtually every cell infected with chlamydiae has PMNs in the immediate proximity, generally in direct contact with the infected cell. Fourth, as PMN infiltration intensifies, epithelial tight junctions, desmosomes and junctional complexes are weakened and disrupted, followed by the disconnection of hemidesmosome/integrin/focal adhesion adherence with the basal lamina; thus, the PMNs seem to literally lift off and release infected superficial epithelia from the mucosa (Figures 3A [arrow D] & 5 and 6D). Chlamydial infection in epithelial cells has also been reported to disrupt the cadherin-dependent epithelial cell lateral junctional complexes, which leads to host cell rounding and retraction from neighboring epithelia (Figure 5A, top monolayer) (Prozialeck et al., 2002) and more release of intact infected epithelial cells into the lumen. In addition, lysis of some infected cells occurs with release of chlamydiae into the extracellular milieu (Figures 5B and 7A). That these morphologically-appearing RBs and EBs are chlamydiae was confirmed by immunoelectron microscopy, employing post-embedding labeling of Lowicryl thin sections with a Chlamydia genus-specific primary anti-LPS polyclonal antibody and gold-conjugated second affinity antibodies (Figure 7B and C). The physical and mechanical forces of PMNs for dislodging epithelial lateral junctional complexes, normally held together tightly by the adhesive forces of the cadherin family of molecules, evidently results in breeches of the monolayer (Figure 5B, bottom monolayer) and migration of PMNs onto the epithelial surface (Figures 3D and 5B). In areas where there was clear cellular destruction, chlamydial EBs and RBs could be detected inside PMNs (Figure 7A inset and 8A–E)).
Figure 5.
Results of the PMN response to chlamydiae-infected conjunctival epithelial cells. (A) PMNs apparently in the act of “pushing” the infected epithelial cells off the mucosal lining, causing a breech in the barrier with (B) subsequent release of intact, infected epithelial cells, damaged epithelial cells, chlamydiae (arrowheads) and PMNs. One can discern a progression in events (as denoted by the circled numbers) in which 1) PMNs accumulate under an intact infected epithelium; 2) the epithelial cell layer begins to lose its integrity; and 3) the epithelium has been breached, releasing PMNs onto the surface. Arrows denote examples of chlamydiae-infected superficial epithelial cells, which are more clearly evident in enlarged images in Figures 6A–C. Magnification: × 3,760.
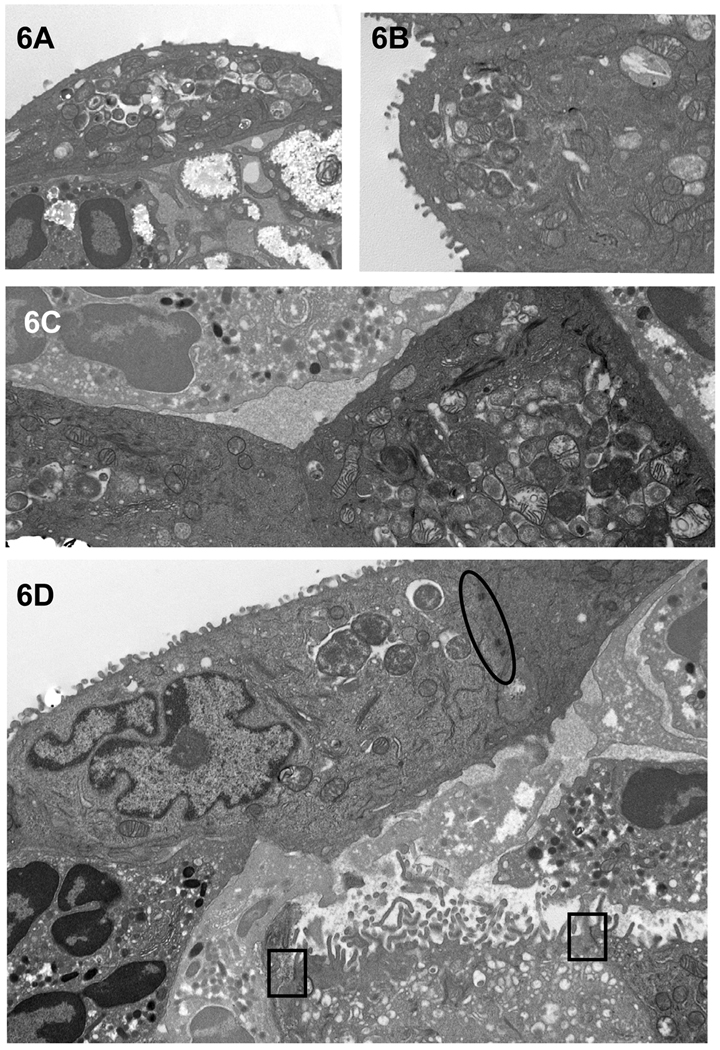
Figure 6.
Chlamydiae in inclusions in infected guinea pig conjunctival superficial epithelial cells abutted by PMNs. (A–C) Representative enlarged electron photomicrographs of portions of infected epithelial cells, designated by arrows in Figure 5, to more clearly reveal chlamydial morphology. (D) Enlarged electron photomicrograph of another example of intact infected epithelial cells seemingly being lifted off the conjunctival surface by underlying PMNs. Note that the desmosomes (black oval) are still intact at the epithelial cell lateral membranes. Tight junctions (black squares) are visible between the epithelial cells of the secondary layer; however, the inter-epithelial cell PMN has disrupted the left-most tight junction of the sub-epithelial cell. Furthermore, note that the detaching of the superficial epithelial cells has now exposed the microvilli of the underlying cells of the secondary layer. Magnifications: A–B = 7,530; C = 10,500; D = 12,500.
Figure 7.
Chlamydiae can also be found in the extracellular environment and inside infiltrating PMNs. (A and inset) Released EBs/IBs (arrowheads) and RBs (arrows) were observed extracellularly as well as in apical PMNs. (B and C) A strong and specific immunogold labeling of EBs and RBs was detected when anti-chlamydial LPS polyclonal antibodies were exposed to the Lowicryl sections of infected epithelial cells.
Magnifications: A = 7530; B = 25,100; C = 35,800; D = 17,900; E = 30,400.
Figure 8.
Intact chlamydiae in vesicles within PMNs. (A,C) EB/IB (arrowheads) in inter-epithelial PMNs, enlarged, respectively, in (C and D). (E) Higher magnification of EBs (arrowheads) and an RB (arrow) in separate vesicles in a PMN. Magnifications: A and C = 7,000; B = 17,900; D = 25,000; and E = 30,400.
It should be noted that we observed no morphological evidence of apoptotic cells, even of those cells which were completely detached from the epithelium. With the exception of those obviously destroyed cells, the vast majority of infected epithelial cells were apparently healthy with intact nuclei, mitochondria and microvilli. This is of interest as it has been proposed, based on in vitro studies, that at the end of the developmental cycle, chlamydiae up-regulate apoptosis of the host cells (Ojcius et al., 1998).
Discussion
The developmental cycle of chlamydiae has been the subject of numerous investigations over the years, ranging from the mechanism of attachment and infection to the regulatory events in the conversion of the organism from EB to RB and RB to EB. Until recently, the “end stage” of the developmental cycle has not been explored in depth but was based on in vitro studies in which the host cell bursts, releasing EBs (Neeper et al., 1990). It has been assumed that this is the primary mechanism of EB release. However, recently Hybiske and Stephens published an elegant study demonstrating that, in addition to chlamydiae exiting the cell via destruction of the cell, the inclusion could also be extruded from the cell by the slow pinching and protrusion of the inclusion out of the cell while still encased in a cell membrane compartment, eventually detaching from the cell (Hybiske & Stephens, 2007). Nevertheless, despite the high quality of these studies, the question remains as to whether these mechanisms actually occur in vivo since the in vitro studies are obviously lacking in the complete repertoire of cytokines, chemokines and cellular components found in the epithelial milieu of a living animal and, thus, may not present a true picture of the events involved in the release of the organisms.
The only accurate way to determine the molecular and cellular events surrounding the release of organisms from the host cell is to make the observations in vivo, in the tissue in which the infection is occurring in the animal. This has been difficult to accomplish because of the scarcity of organisms in tissue. However, the guinea pig conjunctival model lends itself to such a study because (i) one can inoculate the conjunctiva with a large number of organisms to facilitate visualization microscopically; (ii) one can evaluate the gross pathologic response to determine the optimal time to collect the tissue, and (iii) the tissue of interest is small and readily obtainable.
In the current study, as has been seen before, chlamydial infection was restricted to the superficial epithelial layer of cells amid an acute inflammatory response (Soloff et al., 1982; Patton et al., 1989). However, interestingly, we observed with high resolution electron microscopy that almost invariably, PMNs are in direct contact with infected host cells and appear to actually be dislodging the infected cell from the epithelium. In many cases, there was a space occupied by PMNs under the host cell that is clearly detached or is detaching from the epithelium. Perhaps the most surprising observation was that the majority of the infected cells being detached had immature or early inclusions with only RBs present. This suggests that chemokines and/or cytokines produced by the infected cell, even early in the developmental cycle, attract the PMNs to the site and the PMNs then release enzymes such as matrix metalloproteinases, which break down the extracellular matrix that holds cells in place. It was not uncommon to find apparently normal cells, albeit infected with GPIC, completely dislodged from the epithelium. One can surmise that, ultimately, these inclusions either mature and the organisms exit the cell by either cell lysis or extrusion of the inclusion or that the cell dies resulting in the death of the osmotically fragile RBs. This mechanism is not unique to the conjunctiva. In a previous study on GPIC genital tract infection in the guinea pig, Soloff et al. (Soloff et al., 1985) observed a similar phenomenon and commented that it appeared that PMNs had a role in the ultimate degradation or damaging of the host cells. Dourghri also observed this phenomenon in tissue from calves infected with C. psittaci (Doughri et al., 1972). Dourghri further illustrated an in vivo mechanism where the inclusion was apparently being extruded from the host cell into the lumen analogous to the mechanisms reported by Hybiske and Stephens (Doughri et al., 1972; Hybiske & Stephens, 2007). However, the more common event in the tissue we examined was the dislodging of infected cells rather than the in situ destruction of the cell.
This gradient-directed PMN response has been observed previously in a polarized cell model with C. trachomatis serovars E and L2 (Paul et al., 1997; Wyrick et al., 1999) and is certainly deduced by the presence of PMNs at the site of chlamydial infection in virtually all animal models of chlamydial infection. While there are several potential mechanisms by which PMNs are attracted to the site, it has been demonstrated that IL-8 is produced by the chlamydiae-infected epithelial cell approximately mid-developmental cycle (Rasmussen et al., 1997; Buchholz & Stephens, 2006). As PMNs begin transepithelial migration, they are activated to release matrix metalloproteinase-9 (MMP-9), a type 4 collagenase, which helps dissolve the extracellular matrix/basal lamina of both conjunctival and genital epithelial mucosal layers (Abu El-Asrar et al., 1998; Ramsey et al., 2005), aiding PMN inter-epithelial movement as well as eventual epithelial extrusion.
While many intact infected cells could be seen detached from the epithelium, we also observed that some cells were obviously destroyed and that free and phagocytized EBs could be seen with PMNs. Register et al. (Register et al., 1987) provided data indicating that the majority of C. psittaci and C. trachomatis serovar E EBs internalized in human peripheral blood PMNs in vitro were rendered non-infectious within 1 hr. Noteworthy, however, was that a small percentage of EBs (representing approximately 500 – 84,000 EBs per 105 PMNs) from both Chlamydia species did survive and maintained infectivity for several hours, perhaps enabling them to establish subsequent productive infection in permissive host cells.
One can view this process from both the standpoint of the host and or that of the organism. On the side of the host, the PMNs have managed to discard an infected cell before the inclusion has had an opportunity to mature. In some cases, we did observe lysed cells in the epithelium and phagocytosed EBs, indicating that PMNs were able to bring about the destruction of the infected host cell or that the inclusion matured and destroyed its host in order to escape. In support of this role of the PMN, Barteneva et al (Barteneva et al., 1996) treated mice with antibodies to PMNs and found that the vaginal infection with C. muridarum was significantly higher than in untreated animals. Based on our observations, phagocytosis of chlamydiae may not be required to provide a protective response. In the female genital tract, the shedding of the infected cells from the cervical epithelium results in their migration down the genital tract to the vagina. This was actually observed over and over when we collected secretions and cells from the vagina and quantified the percentage of cells infected with GPIC (Barron et al., 1979; Rank et al., 1979). We routinely observed apparently healthy cells with inclusions at all stages of the developmental cycle as well as large numbers of PMNs. Some of these epithelial cells and PMNs may have come from the vagina but it was not uncommon to find infected cells and sheets of cells suspended in the mucus obtained from the vagina. Such “shedding” was not observed in uninfected animals. In the male urethra, detached cells can be easily eliminated by urine flow, and in ocular infections, the action of tears may facilitate the elimination of infected cells. Thus, in each of these tissues, the shedding of infected cells through the action of PMNs may serve as an effective defense mechanism.
However, from the point of view of the chlamydiae, the epithelial cell with an intact inclusion has now been discarded, making it available for transmission, either sexually in genital infections or by mechanical means in ocular infections. The host cell can actually allow the organism to survive longer in the extracellular milieu, so that this may be a protective mechanism for the organism in order to facilitate transmission to new hosts. In addition, because of the intense inflammatory reaction at the local site of infection, there is a paucity of susceptible cells, so the dislodging of the infected cell may allow the organism to be more easily distributed to other areas of uninfected epithelium by fluid dynamics or tissue movement such as peristalsis in the genital tract or the “blinking” action of eyelids in the eye. As has been demonstrated previously, even those EBs which are phagocytized by PMNs may remain viable for a period of time, so that these cells, too, could be available for transmission (Register et al., 1986).
These observations have important implications for the disease process. The destruction of the epithelium appears to be, in part, the local destruction of cells but mostly the shedding of the superficial epithelial layer by the “pushing” action of the PMNs. Consequently, there are breeches in the epithelial barrier which allow the PMNs to flood through the barrier onto the surface, resulting in an inflammatory exudate. More significantly, this mechanism may also help to explain how organisms move up the genital tract from the cervix which is the initial site of infection in genital infection of the female. Intact infected cells dislodged into the lumen may survive longer and move via peristalsis up the genital tract. It has been well-documented that there are peristaltic contracts of the uterus to rapidly move material in the direction of the oviducts, presumably to aid sperm in their migration (Barnhart et al., 2001; Kunz et al., 2006). Recently, work from Ramsey’s laboratory demonstrated that mice in which MMP-9 was inhibited did not develop ascending infection to the oviducts although the number of organisms in the cervix was the same as intact control mice (Imtiaz et al., 2006). Thus, combined with our visual evidence for host cell-PMN interaction, it would appear that the PMN response is essential for ascending genital infection to occur.
The data presented in this study indicate that in vivo, chlamydiae may exit their host cells in either of two ways. In the first, the cells may lyse in situ, releasing new EBs to be available for infection of new cells or to be phagocytized by phagocytes. Secondly, the infected cell may be removed from the epithelium by PMNs so that ultimately, the cell dies or is destroyed, liberating the EBs. From our observations, the majority of the infected cells are pushed off the epithelium in contrast to lysis of cells. We have not seen any evidence for extrusion of inclusions in our study as has been seen in vitro, but that mechanism cannot be ruled out.
Therefore, we propose that the acute inflammatory response has a two-fold role in chlamydial infection. First, it controls the chlamydial infection by killing EBs liberated from destroyed cells and by detaching infected cells from the epithelium so that those cells can be eliminated by the action of tears in ocular infection or movement of the cells away from the target tissue in genital tract infections or until the adaptive response can be activated. Secondly, the organisms may be benefited because detached infected cells may facilitate transmission to new hosts by mechanical means in the eye or sexual transmission in the genital tract. Movement of the detached cells may also help the chlamydiae to move to uninfected tissue sites while providing a level of protection against host defense mechanisms.
Acknowledgements
This study was supported in part by grants AI59650 (RGR) and AI13446 (PBW) from the NIAID of the NIH.
Reference List
- Abu El-Asrar AM, Geboes K, al Kharashi SA, Tabbara KF, Missotten L. Collagen content and types in trachomatous conjunctivitis. Eye. 1998;12(Pt 4):735–739. doi: 10.1038/eye.1998.179. [DOI] [PubMed] [Google Scholar]
- Barnhart KT, Stolpen A, Pretorius ES, Malamud D. Distribution of a spermicide containing Nonoxynol-9 in the vaginal canal and the upper female reproductive tract. Hum.Reprod. 2001;16:1151–1154. doi: 10.1093/humrep/16.6.1151. [DOI] [PubMed] [Google Scholar]
- Barron AL, White HJ, Rank RG, Soloff BL. Target tissues associated with genital infection of female guinea pigs by the chlamydial agent of guinea pig inclusion conjunctivitis. J.Infect.Dis. 1979;139:60–68. doi: 10.1093/infdis/139.1.60. [DOI] [PubMed] [Google Scholar]
- Barteneva N, Theodor I, Peterson EM, de la Maza LM. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infection and Immunity. 1996;64:4830–4833. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz KR, Stephens RS. Activation of the host cell proinflammatory interleukin-8 response by Chlamydia trachomatis. Cell Microbiol. 2006;8:1768–1779. doi: 10.1111/j.1462-5822.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- Doughri AM, Storz J, Altera KP. Mode of entry and release of Chlamydiae in infections of intestinal epithelial cells. J.Infect.Dis. 1972;126:652–657. doi: 10.1093/infdis/126.6.652. [DOI] [PubMed] [Google Scholar]
- Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci U S A. 2007;104:11430–11435. doi: 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz MT, Schripsema JH, Sigar IM, Kasimos JN, Ramsey KH. Inhibition of matrix metalloproteinases protects mice from ascending infection and chronic disease manifestations resulting from urogenital Chlamydia muridarum infection. Infection and Immunity. 2006;74:5513–5521. doi: 10.1128/IAI.00730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz G, Beil D, Huppert P, Leyendecker G. Control and function of uterine peristalsis during the human luteal phase. Reprod Biomed.Online. 2006;13:528–540. doi: 10.1016/s1472-6483(10)60641-4. [DOI] [PubMed] [Google Scholar]
- Neeper ID, Patton DL, Kuo C-C. Cinematographic observations of growth cycles of Chlamydia trachomatis in primary cultures of human amniotic cells. Infection and Immunity. 1990;58:2042–2047. doi: 10.1128/iai.58.7.2042-2047.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojcius DM, Souque P, Perfettini JL, Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J.Immunol. 1998;161:4220–4226. [PubMed] [Google Scholar]
- Patton DL, Landers DV, Schachter J. Experimental Chlamydia trachomatis salpingitis in mice: Initial studies on the characterization of the leukocyte response to chlamydial infection. J.Infect.Dis. 1989;159:1105–1110. doi: 10.1093/infdis/159.6.1105. [DOI] [PubMed] [Google Scholar]
- Paul TR, Knight ST, Raulston JE, Wyrick PB. Delivery of azithromycin to Chlamydia trachomatis-infected polarized human endometrial epithelial cells by polymorphonuclear leucocytes. J Antimicrob.Chemother. 1997;39:623–630. doi: 10.1093/jac/39.5.623. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Fay MJ, Lamar PC, Pearson CA, Sigar I, Ramsey KH. Chlamydia trachomatis disrupts N-cadherin-dependent cell-cell junctions and sequesters beta-catenin in human cervical epithelial cells. Infect Immun. 2002;70:2605–2613. doi: 10.1128/IAI.70.5.2605-2613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KH, Sigar IM, Schripsema JH, Shaba N, Cohoon KP. Expression of matrix metalloproteinases subsequent to urogenital Chlamydia muridarum infection of mice. Infection and Immunity. 2005;73:6962–6973. doi: 10.1128/IAI.73.10.6962-6973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank RG, White HJ, Barron AL. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infection and Immunity. 1979;26:573–579. doi: 10.1128/iai.26.2.573-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, Fierer J, Stephens RS, Kagnoff MF. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. Journal of Clinical Investigation. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register KB, Davis CH, Wyrick PB, Shafer WM, Spitznagel JK. Nonoxidative antimicrobial effects of human polymorphonuclear leukocyte granule proteins on Chlamydia spp. in vitro. Infection and Immunity. 1987;55:2420–2427. doi: 10.1128/iai.55.10.2420-2427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register KB, Morgan PA, Wyrick PB. Interaction between Chlamydia spp. and human polymorphonuclear leukocytes in vitro. Infection and Immunity. 1986;52:664–670. doi: 10.1128/iai.52.3.664-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff BL, Rank RG, Barron AL. Ultrastructural studies of chlamydial infection in guinea pig urogenital tract. Journal of Comparative Pathology. 1982;92:547–558. doi: 10.1016/0021-9975(82)90007-x. [DOI] [PubMed] [Google Scholar]
- Soloff BL, Rank RG, Barron AL. Electron microscopic observations concerning the in vivo uptake and release of the agent of guinea pig inclusion conjunctivitis (Chlamydia psittaci) in guinea pig exocervix. Journal of Comparative Pathology. 1985;95:335–344. doi: 10.1016/0021-9975(85)90037-4. [DOI] [PubMed] [Google Scholar]
- Wyrick PB, Choong J, Knight ST, Goyeau D, Stuart ES, MacDonald AB. Chlamydia trachomatis antigens on the surface of infected human endometrial epithelial cells. Immunology & Infectious Diseases. 1994;4:131–141. [Google Scholar]
- Wyrick PB, Davis CH, Knight ST, Choong J, Raulston JE, Schramm N. An in vitro human epithelial cell culture system for studying the pathogenesis of Chlamydia trachomatis. Sex.Transm.Dis. 1993;20:248–256. doi: 10.1097/00007435-199309000-00002. [DOI] [PubMed] [Google Scholar]
- Wyrick PB, Knight ST, Paul TR, Rank RG, Barbier CS. Persistent chlamydial envelope antigens in antibiotic-exposed infected cells trigger neutrophil chemotaxis. J.Infect.Dis. 1999;179:954–966. doi: 10.1086/314676. [DOI] [PubMed] [Google Scholar]