Abstract
The role of adult hippocampal neurogenesis in contextual fear conditioning (CFC) is debated. Several studies demonstrated that blocking adult hippocampal neurogenesis in rodents impairs CFC, while several other studies failed to observe an impairment. We sought to determine whether different CFC methods vary in their sensitivity to the arrest of adult neurogenesis. Adult neurogenesis was arrested in mice using low-dose, targeted x-irradiation, and the effects of irradiation were assayed in conditioning procedures that varied in the use of a discrete conditioned stimulus, the number of trials administered, and the final level of conditioning produced. We demonstrate that irradiation impairs CFC in mice when a single-trial CFC procedure is used but not when multiple-trial procedures are used, regardless of the final level of contextual fear produced. In addition, we show that the irradiation-induced deficit in single-trial CFC can be rescued by providing pre-exposure to the conditioning context. These results indicate that adult hippocampal neurogenesis is required for CFC in mice only when brief training is provided.
Keywords: dentate gyrus, memory, learning, hippocampus, postnatal neurogenesis, contextual fear conditioning
The dentate gyrus (DG) is one of a small number of brain regions that retains the ability to generate neurons in adulthood. Adult-born neurons in the DG become granule cells that form functional synapses (Toni et al., 2008; Toni et al., 2007) and exhibit activity-related plasticity (Ge, Yang, Hsu, Ming, & Song, 2007; Schmidt-Hieber, Jonas, & Bischofberger, 2004; Wang, Scott, & Wojtowicz, 2000). There is a growing consensus that adult-born hippocampal neurons make functionally significant contributions to hippocampal physiology and behavior, based on evidence that these cells are activated in situations that evoke hippocampus-dependent learning (Kee, Teixeira, Wang, & Frankland, 2007; Ramirez-Amaya, Marrone, Gage, Worley, & Barnes, 2006), reports that arresting adult hippocampal neurogenesis impairs performance in some hippocampus-dependent behavioral tasks (e.g., Clelland et al., 2009; Saxe et al., 2006; Shors et al., 2001; Snyder, Hong, McDonald, & Wojtowicz, 2005; Zhang, Zou, He, Gage, & Evans, 2008), and neural network models positing plausible mechanisms through which adult-born neurons might contribute to learning and memory (Aimone, Wiles, & Gage, 2009; Becker, 2005; Becker, Macqueen, & Wojtowicz, 2009; Meltzer, Yabaluri, & Deisseroth, 2005; Wiskott, Rasch, & Kempermann, 2006). Despite the consensus that adult-born neurons are functionally significant, there is little or no agreement on which behavioral tasks are sensitive to the disruption of adult neurogenesis and on which underlying psychological processes are altered when adult neurogenesis is arrested.
The literature on contextual fear conditioning is characteristic of the disagreement in the field. Contextual fear conditioning (CFC) is a form of learning produced by pairing an aversive stimulus, such as a footshock, with a distinctive context. Several studies in mice and rats demonstrated that blocking adult hippocampal neurogenesis with low-dose irradiation (Hernandez-Rabaza et al., 2009; Ko et al., 2009; Saxe et al., 2006; Warner-Schmidt, Madsen, & Duman, 2008; Winocur, Wojtowicz, Sekeres, Snyder, & Wang, 2006; Wojtowicz, Askew, & Winocur, 2008) or inducible genetic systems (Imayoshi et al., 2008; Saxe et al., 2006) impairs CFC. When neurogenesis was arrested prior to conditioning in these studies, mice exhibited less conditioned fear of the shock-paired context than did control mice in a post-conditioning test session. However, several other studies failed to observe any effect of arresting adult neurogenesis on CFC (Clark et al., 2008; Dupret et al., 2008; Pollak et al., 2008; Shors, Townsend, Zhao, Kozorovitskiy, & Gould, 2002; Zhang et al., 2008). Some of the discrepancies between studies may be attributable to differences in the ablation methods, which vary in the extent of the ablation, the cell types targeted, and the side-effect profile. However, differences in the ablation method do not explain all of the inconsistencies, as conflicts exist even among studies using the same method. For instance, several studies reported that arresting neurogenesis with low-dose irradiation impaired CFC (Saxe et al., 2006; Warner-Schmidt et al., 2008; Winocur et al., 2006; Wojtowicz et al., 2008), whereas another study found no effect of irradiation (Clark et al., 2008).
Another critical variable is likely to be the CFC methodology, which has varied considerably in the neurogenesis literature. For instance, some studies (Farioli-Vecchioli et al., 2008; Imayoshi et al., 2008; Saxe et al., 2006; Warner-Schmidt et al., 2008; Winocur et al., 2006; Wojtowicz et al., 2008; Zhang et al., 2008) trained subjects with pairings between a discrete stimulus and a footshock (a procedure sometimes termed “background” context conditioning), while other studies (Clark et al., 2008; Hernandez-Rabaza et al., 2009; Ko et al., 2009) used context-shock pairings without a discrete cue (sometimes termed “foreground” context conditioning). Studies have also differed in the number of conditioning trials administered, the shock intensity, and the final level of conditioned fear produced.
We sought to determine whether different CFC methods vary in their sensitivity to the arrest of adult neurogenesis. We arrested adult neurogenesis using low-dose, targeted x-irradiation, and then assayed the effects of irradiation in conditioning procedures that varied in the use of a discrete conditioned stimulus, the number of trials administered, and the final level of conditioning produced. Irradiation was selected because it is a common method of arresting neurogenesis employed by several laboratories, produces a complete and permanent arrest of adult neurogenesis, and leaves other neurogenic niches intact when applied focally (Meshi et al., 2006; Santarelli et al., 2003). Although side-effects of low-dose x-irradiation have been identified (e.g., Monje, Mizumatsu, Fike, & Palmer, 2002), behavioral or physiologic correlates of these side-effects have not been detected (Wojtowicz, 2006). Moreover, in studies employing multiple ablation strategies, the behavioral effects of irradiation have been identical to those of inducible genetic strategies (Clelland et al., 2009; Saxe et al., 2006; Saxe et al., 2007).
Here, we demonstrate that irradiation impairs CFC in mice when a single-trial CFC procedure is used but not when multiple-trial procedures are used, regardless of the final level of contextual fear produced. In addition, we show that the irradiation-induced deficit in single-trial CFC can be rescued by providing pre-exposure to the conditioning context. These results indicate that adult neurogenesis is necessary in mice for CFC only when brief training is provided.
Method
Subjects
A total of 345 male 129S6/SvEvTac mice were purchased from Taconic (Germantown, NY) and arrived at 7-9 weeks of age. Mice were housed 5 per cage with ad libitum food and water. Irradiation was performed at 9-10 weeks of age and behavior testing occurred between 15 and 21 weeks of age.
Apparatus
Fear conditioning was conducted in chambers obtained from Med Associates (St. Albans, VT), with internal dimensions of approximately 20 cm wide × 16 cm deep × 20.5 cm high. The chambers had metal walls on each side, clear plastic front and back walls and ceilings, and stainless steel bars on the floor. A house light (CM1820 bulb, 28v, 100mA) mounted directly above the chamber provided illumination. Each chamber was located inside a larger, insulated, plastic cabinet that provided protection from outside light and noise. Each cabinet contained a ventilation fan that was operated during the sessions. A paper towel dabbed with mint solution was placed underneath the chamber floor. Mice were held outside the experimental room in their home cages prior to testing and transported to the conditioning apparatus individually in standard mouse cages. Chambers were cleaned with 70% ethanol between each set of mice.
The training and context test sessions were conducted with the conditioning chambers configured exactly as described above. For the tone test sessions (described below), the chambers and handling procedure were changed in several ways so fear of the tone CS could be assessed in the absence of contextual cues associated with shock. The floor and walls of the chamber were covered by plastic inserts; the chamber was scented with lemon; the ventilation fan was not operated; chambers were cleaned with a non-alcohol disinfectant between runs; the room lighting was altered; and mice were transported to the apparatus in a different type of cage.
The behavior of mice was recorded with digital video cameras mounted above the conditioning chamber. Video recordings were analyzed using FreezeFrame software from Actimetrics (Evanston, IL). This software assesses freezing by measuring changes in the intensity of each pixel between successive frames of the video file. Each file was examined individually, and statistical analysis of the pixel-change distribution was used to detect motion and distinguish it from background pixel noise. We have determined that the scores obtained with this software are highly correlated with the scores assigned by human observers.
To assess the unconditioned response to shock, we measured the distance travelled by mice during the shock. Videos of the shock response (filmed from above the conditioning chamber) were converted to a sequence of still frames using VirtualDub software (http://www.virtualdub.org/), and then imported into ImageJ (http://rsb.info.nih.gov/ij/) as a stack. The path of the mouse was traced manually using the segmented line tool, and the length of the path in centimeters was obtained using the “measure” function.
Procedure
Irradiation was performed as described previously (Meshi et al., 2006; Santarelli et al., 2003; Saxe et al., 2006). Briefly, mice were x-irradiated three times (5 Gy per dose) in the course of one week, for a cumulative dose of 15 Gy. Sham mice were treated identically but did not receive irradiation. Mice were anesthetized with ketamine and xylazine (105 mg/kg, 6 mg/kg IP), placed in a stereotaxic frame, and exposed to cranial irradiation. A lead shield covered the entire body but left unshielded a treatment field above the hippocampus.
Experiment 1: Tone-Shock Fear conditioning
The fear conditioning procedure took place over three consecutive days. On day 1, mice (Sham n = 15, Irrad. n = 15) were placed in the conditioning chamber and received 3 pairings between a tone (20 s, 80dB, 2KHz) and a co-terminating shock (1 s, 0.5 mA). The tones commenced at 120, 290, and 400 s after the start of the session.
The tone test was conducted on day 2. Mice were placed into the altered conditioning chambers (described above), and the tone was presented twice for 20 s at 120 and 290s into the session. No shocks were administered. Freezing was scored for the 1 min prior to the first tone presentation (pre-tone freezing) and during each tone presentation (tone-elicited freezing).
On day 3, mice were tested for conditioned fear of the training context. The testing procedure and context were identical to those used on day 1, except the CS and shocks were not given. Mice were placed into the chambers for 3 min. The entire session was scored for freezing.
Experiment 2: Context-shock conditioning
The procedure was based on that of Wiltgen, Sanders, Anagnostaras, Sage, & Fanselow (2006). Mice receiving the delayed shock (Sham n = 17, Irrad. n = 13) were placed in the conditioning chambers and 3 min later received one shock (2 s, 0.75 mA). Mice were removed 15 s following the shock. Mice in the no-shock condition (Sham n = 7, Irrad. n = 7) were placed in the chamber for 197 s and no shocks were delivered. Mice in the immediate shock condition (Sham n = 7, Irrad. n = 6) received a shock 10 s after placement in the chamber and were removed 15 s after the shock ended. The next day all mice were returned to the conditioning chamber for 3 min for a test of context-elicited fear.
Experiment 3: Tone-Shock fear conditioning with varying shock intensities
Conditioning was conducted as described in Experiment 1, except the shock intensity was varied between groups: 0.3 mA (Sham n = 25, Irrad. n = 25), 0.4 mA (Sham n = 31, Irrad. n = 33), or 0.7 mA (Sham n = 13, Irrad. n =12).
Experiment 4: Effect of additional context exposure or shocks
One group of mice (Sham n = 15, Irrad. n = 17) received context-shock conditioning as described above (shock at 180 s, 2 s duration, 0.75 mA). A second group of mice (Sham n = 24, Irrad. n = 24) also received context-shock conditioning except they received two shocks commencing at 90 and 180s after the start of the session. A third group of mice (Sham n = 13, Irrad. n = 14) received context-shock training as described above (one shock at 180 s) but were given pre-exposure to the conditioning context 24 h prior to conditioning. For the pre-exposure, mice were transported to the chambers as on the conditioning day, placed in the conditioning chamber for 197 s, and then returned to their home cages. To control for possible effects of context exposure on freezing, we included a fourth group of mice (Sham n = 6, Irrad. = 6) that was treated the same as the previous group except that no shock was delivered. All mice received a 3-min context test session 24 h after conditioning.
Doublecortin (DCX) immunohistochemistry was used to confirm the irradiation-induced arrest of neurogenesis. Mice (N = 12) were killed by transcardial perfusion following fear conditioning 6.5 or 12.5 weeks after irradiation. Immunohistochemistry was conducted as described previously (Meshi et al., 2006), and sections were counterstained with Gill's hematoxylin.
Data analysis
The primary measure of interest was percent time freezing. Freezing data from the tone test sessions were subjected to a 2 (Treatment: Irrad. v. Sham) X 2 (Period: Pre-Tone v. Tone) ANOVA with Period as a repeated measure. The freezing score for the tone period was a mean taken across the two 20-s tone presentations. Data from the context test sessions were initially subjected to 2 (Treatment) X 3 (Minute) ANOVA with Minute as a repeated measure, and with additional factors as described below. Although the effect of Minute reached significance in some analyses, it never interacted significantly with other variables. Therefore, to simplify reporting of the results, we removed Minute from the analyses reported below; the analyses were thus performed on mean freezing across minutes 1 through 3. Interactions were probed using t-tests with the Bonferroni correction. Alpha was set to .05 for all analyses.
Results
Experiment 1: No effect of irradiation on tone-shock fear conditioning.
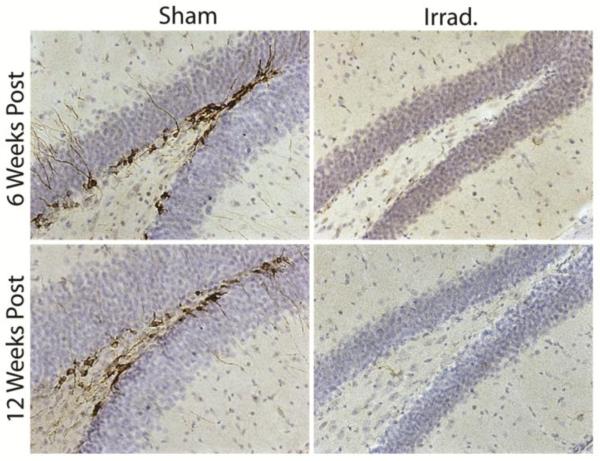
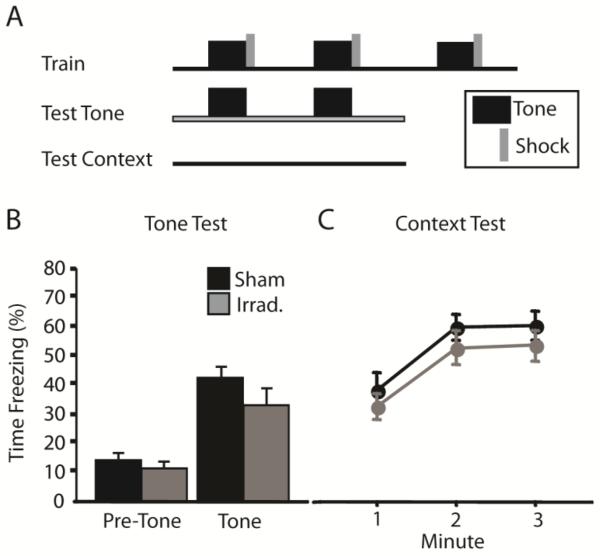
Immunohistochemical labeling of DCX was completely absent in the DG of irradiated mice at 6 and 12 weeks post irradiation (Fig. 1), indicating that neurogenesis was halted for the duration of behavioral testing. We began by testing the effects of irradiation on CFC by using a tone-shock conditioning protocol. We previously reported that irradiation impairs CFC produced via multiple tone-shock pairings (Saxe et al., 2006). However, another recent study found no effect of arresting neurogenesis in this procedure (Zhang et al., 2008). We trained irradiated and sham mice as described by Saxe et al. (2006; Fig. 2A): mice were given 3 tone-shock pairings, and then context- and tone-elicited fear were assayed separately. Nevertheless, both irradiated and sham mice acquired robust conditioned fear of the tone and context (Fig. 2B and 2C). In the tone test session, freezing during the tones was significantly higher than during the pre-tone period [F(1,28) = 102.8, p < .001]. There was no effect of irradiation [F(1,28) = 1.3, p = .267] or of the interaction [F(1,28) = 1.84, p = .186] on freezing during the tone test. Context-elicited freezing was also robust in both groups of mice. Although context- and tone-elicited freezing appeared to be slightly lower in irradiated mice, these effects did not approach statistical significance [F(1,28) < 1].
Figure 1.
X-irradiation produces a complete and lasting arrest of adult hippocampal neurogenesis. Doublecortin (DCX) immunohistochemistry labels immature neurons lining the inner granule cell layer of sham mice. DCX-labeled cells are absent in irradiated mice, indicating that neurogenesis was ablated.
Figure 2.
Arrest of hippocampal neurogenesis does not impair contextual fear conditioning (CFC) produced via tone-shock pairings. (A) Irradiated and sham mice were given 3 tone-shock pairings. Tone-elicited fear was assessed by presenting the tones in an alternate context. Context-elicited fear was tested by returning mice to the shock-paired context. Irradiation failed to affect freezing in the tone test session (B) or in the context test session (C).
Experiment 2: Irradiation impairs context fear conditioning produced via a single context-shock pairing
Next, we trained mice using a context-shock (“foreground”) conditioning protocol (Fig. 3A) that was reported to be highly sensitive to pre-training hippocampal lesions (Wiltgen et al., 2006) and to the arrest of adult hippocampal neurogenesis (Hernandez-Rabaza et al., 2009; Ko et al., 2009). Mice were placed in the conditioning chamber and given a single footshock 180 s later. Context-elicited fear was tested on the following day. We included two control groups: an immediate shock group, which received a footshock 10 s after being placed in the chamber; and a no-shock group, which was placed in the chamber for the same amount of time as the conditioned group but did not receive a footshock. The immediate shock condition was included to control for possible unconditioned effects of shock. An immediate shock typically does not produce fear conditioning, putatively because subjects do not have adequate time to acquire a mental representation of the context prior to receiving the shock (Fanselow, 1986; Landeira-Fernandez, DeCola, Kim, & Fanselow, 2006). As expected, both control groups showed very little freezing in the context test session (Fig. 3B), indicating that exposure to an immediate shock or to the context alone was not sufficient to generate conditioned fear of the context. Because two control groups did not differ in their levels of context-elicited freezing [Effect of procedure: F(1,24) < 1] or in their sensitivity to irradiation [Procedure X Treatment interaction: F(1,24) < 1], the groups were combined into single control group in the following analyses.
Figure 3.
Irradiation impairs CFC produced via a single context-shock pairing. (A) Fear conditioning was produced by placing the mouse in the conditioning chamber and delivering one footshock 180 s later (Delayed shock). One control group received context exposure but no shock. The other control group received a footshock within 10 s of being placed in the chamber (Immed. shock). Mice were returned to the conditioning chamber on the following day to assess context-elicited fear. The two control groups exhibited negligible levels of freezing in the context test (B) and did not differ from each other, and, therefore, were combined as one control group. There was no effect of irradiation in the control groups, but among mice receiving the delayed shock, irradiated mice exhibited significantly less context-elicited fear than sham mice. Neither irradiation nor the shock latency (immediate versus delayed) affected the unconditioned response to shock, operationalized as the mouse's velocity of locomotion during the shock (C). *p < .01
To test for an effect of irradiation in this procedure, we subjected the context test data to 2 (Treatment) X 2 (Procedure: Control v. Conditioned) ANOVA, which yielded a significant interaction effect [F(1,58) = 5.1, p = .028]. The interaction was probed by separately testing for effects of irradiation in the conditioned (shock at 180 s) and control groups. Among conditioned mice, irradiated mice exhibited significantly less context-elicited freezing than did sham mice [F(1,32) = 6.0, p = .020]. Among control mice, there was no effect of irradiation [F(1,26) < 1]. These results indicate that irradiation impairs CFC produced by a single context-shock pairing, and the impairment is not explained by unconditioned effects of context exposure or shock alone.
One advantage of the tone-shock training protocol over the context-shock protocol is that tone-elicited freezing can serve as an embedded positive control. That is, tone-elicited freezing can demonstrate that a deficit in context-elicited fear is not caused by changes in unconditioned processes such as shock sensitivity. Because the context-shock protocol does not offer such an embedded positive control, we explicitly assessed the unconditioned response to shock in this experiment. We measured the distance traveled by the mice during the shock, and used this information to calculate the mouse's velocity, which has been previously shown to be a measure of shock sensitivity (Wiltgen et al., 2006). As shown in Figure 3C, velocity was similar in all groups of mice. The data were subjected to a 2 (Treatment) X 2 (Shock Latency: 10s or 180s) ANOVA. There was no effect of Treatment [F(1,43) < 1], Shock Latency [F(1,43) < 1], or the interaction [F(1,43) = 1.2, p = .286], indicating that differences in shock sensitivity do not explain the reduced context fear in irradiated mice or in mice receiving the immediate shock.
Experiment 3: Altering the shock intensity does not increase the sensitivity of tone-shock fear conditioning to irradiation
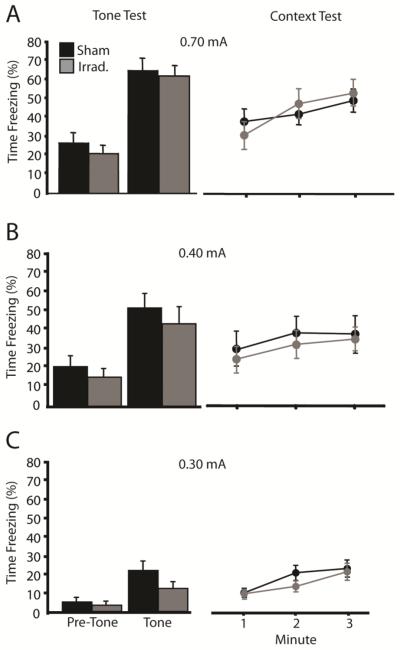
Our tone-shock conditioning procedure produced somewhat more conditioned fear than did our context-shock conditioning procedure. In this experiment, we asked whether the final level of context fear affects the sensitivity of tone-shock fear conditioning to irradiation. To manipulate the level of context fear, we used the same tone-shock conditioning procedure as in Experiment 1 but varied the shock intensity between subjects (0.3, 0.4, or 0.7 mA). As shown in Figure 4, shock intensity affected the overall levels of conditioned fear in the tone and context test sessions, but did not affect the sensitivity to irradiation. Data from the tone test sessions were subjected to a 2 (Treatment) X 3 (Shock Intensity) X 2 (Period: Pre-Tone v. Tone) ANOVA with Period as a repeated measure. There was a significant effect of shock intensity [F(2,133) = 44.8, p < .001] and a significant Intensity X Period interaction [F(2,133) = 17.3, p < .001], indicating that the variation in shock intensity affected freezing in the tone test session. However, there was no main effect of irradiation treatment [F(1,133) = 3.2, p = .077], and irradiation treatment did not interact with any other variable (F's < 1). Data from the context sessions were subjected to a 2 (Treatment) X 3 (Shock Intensity) ANOVA. Again, there was a significant effect of shock intensity [F(1,133) = 31.0, p < .001], but no effect of irradiation [F(1,133) < 1] or of the interaction [F(2,133) < 1]. The results indicate that the tone-shock conditioning procedure is insensitive to irradiation across a broad range of conditioned fear levels.
Figure 4.
Manipulating the final level of conditioned fear via changes in shock intensity fails to increase the sensitivity of tone-shock fear conditioning to irradiation. The figures depict freezing during the tone and context test sessions for mice given tone-shock fear conditioning with shocks of 0.7, 0.4, or 0.3 mA. Shock intensity strongly influenced tone- and context-elicited freezing, and irradiation failed to affect tone- or context-elicited fear at any shock intensity level.
Experiment 4: The irradiation-induced impairment in context-shock fear conditioning is rescued by context pre-exposure or by an additional shock
The differential sensitivity to irradiation of tone-shock versus context-shock conditioning might be explained by the different amounts of training provided in each procedure. In the tone-shock procedure, mice were given 3 tone-shock pairings, but in the context-shock procedure mice were given only a single context-shock pairing. If the amount of training affects the sensitivity to irradiation, the deficit in context-shock conditioning should be rescued by additional training. We hypothesized that additional training could be provided in two ways: through additional shocks or through additional pre-shock exposure to the conditioning context. In this experiment, we asked whether these manipulations would rescue the CFC deficit in irradiated mice.
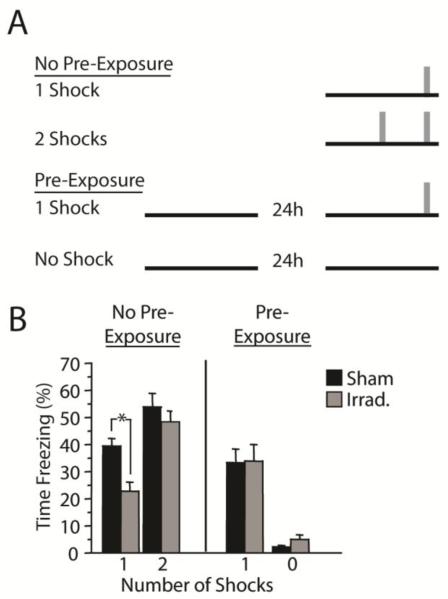
Mice were given either (1) context-shock fear conditioning as described in Experiment 2 (shock at 180 s), (2) context-shock fear conditioning with two shocks rather than one shock (shocks at 90 and 180 s), or (3) context-shock fear conditioning (one shock) with pre-exposure to the conditioning chamber prior to conditioning Figure 5A. Because activity tends to habituate over time in an environment, and the absence of activity can be confused with freezing, we included a control group that received the same amount of context exposure as group 3, but with no shock.
Figure 5.
The irradiation-induced impairment in context-shock fear conditioning is rescued by context pre-exposure or by an additional shock. (A) Mice were given context-shock fear conditioning with one or two shocks, or with one shock plus pre-exposure to the conditioning context. A control group received context exposure but no shock. (B) Freezing during the context test session. Irradiation impaired CFC in mice given a single context-shock pairing but had no effect in mice that received two shocks or that received context pre-exposure. Freezing was minimal and unaffected by irradiation after context-alone exposure. *p < .05
Figure 5B shows mean percent time freezing in the context test sessions. Data were analyzed using planned comparisons (t-tests), which were justified by the a priori predictions derived from the results of Experiments 1 through 3. The predictions were as follows: (1) irradiated mice will display less context-elicited freezing than sham mice after a single context-shock pairing; (2) additional context exposure or (3) an additional shock will abolish the difference between sham and irradiated mice; and (4) freezing levels will not differ between sham and irradiated mice given context-alone exposure. All four predictions were confirmed. Irradiated mice froze less than sham mice after a single context-shock pairing [t(30) = 3.3, p = .003]. However, the difference between irradiated and sham mice was abolished in the groups receiving context pre-exposure [t(25) = 0.2, p = .735] or an additional shock [t(46) = 0.5, p = .594]. Among mice given only context exposure, freezing levels were very low, and there was no effect of irradiation [t(9) = 1.8, p = .103].
Discussion
We found that CFC with a single context-shock pairing was sensitive to the arrest of neurogenesis via irradiation, whereas conditioning with multiple tone-shock pairings was not. The difference in sensitivity to irradiation was not related to differences in the final level of conditioned fear produced, as tone-shock fear conditioning was insensitive to irradiation even when the final level of conditioned fear was equated with that produced by a single context-shock pairing. The difference in sensitivity to irradiation was instead attributable to the number of conditioning trials or to the amount of context exposure. The irradiation-induced impairment in context-shock conditioning was rescued when mice were given an additional shock or pre-exposure to the conditioning context. These results suggest that, in mice, adult neurogenesis is necessary for CFC only when brief training is provided.
The learning that underlies CFC is typically thought to include two distinct processes: the acquisition of a mental representation of the context and of an association between the context representation and the US representation (e.g., Rudy, Huff, & Matus-Amat, 2004). Our finding that the irradiation-induced deficit in context conditioning could be rescued by extra context exposure would seem to argue that arresting adult hippocampal neurogenesis impedes the acquisition of the context representation. However, the rescue by an additional shock suggests that arresting neurogenesis impedes the acquisition of the context-shock association. It may be that both of these learning processes are impeded, but not altogether halted, by the arrest of neurogenesis, and the extra context exposure or additional shock permits normal levels of memory strength to accrue. An alternative explanation is that arresting neurogenesis impedes only the acquisition of the context representation, and this acquisition process is ameliorated by extra context exposure or by the emotional arousal produced by the additional shock. Arousing stimuli can promote acquisition and consolidation of weak memories that might otherwise be forgotten (Anderson, Wais, & Gabrieli, 2006; McGaugh, 2006). Multiple shocks presumably produce greater arousal than a single shock, and this greater arousal may further strengthen context memory and thereby alleviate the deficit in irradiated mice.
These results begin to explain discrepancies in the neurogenesis literature. In the mouse, single-trial CFC is often impaired by manipulations that interfere with hippocampal neurogenesis (Farioli-Vecchioli et al., 2008; Ko et al., 2009), whereas multiple-trial CFC is usually not impaired (Clark et al., 2008; Dupret et al., 2008; Pollak et al., 2008; Zhang et al., 2008). Yet there are exceptions to this generalization. Two mouse studies, including one of our own, reported that neurogenesis-arrested mice are impaired in multiple-trial CFC (Imayoshi et al., 2008; Saxe et al., 2006). If arresting neurogenesis impedes acquisition of CFC but does not altogether stop it, as our data suggest, study-to-study variability could originate from at least two sources. First, a residual deficit may be present even after multiple conditioning trials, and this deficit may be detected on occasion due to sampling variability. Second, acquisition speed is likely to vary according to the particulars of the conditioning protocol. We predict that multiple-trial CFC procedures will be sensitive to the arrest of neurogenesis under conditions that prolong acquisition, such as increased task difficulty. For instance, acquiring a mental representation of a complex context likely requires more time and/or trials than does acquiring a representation of a simple context. When the conditioning context is complex, arresting neurogenesis may thus impair context conditioning even when more than one conditioning trial is given. Consistent with this hypothesis, two studies reporting effects of irradiation on multiple-trial CFC used conditioning chambers with transparent walls permitting views of a complex surrounding environment. Finally, mouse strains differ considerably in their rates of neuronal proliferation and survival (Kempermann, Kuhn, & Gage, 1997), cognitive ability, and behavioral performance (Crawley et al., 1997), and these factors are likely to modulate the behavioral effects of arresting neurogenesis (e.g., Holick, Lee, Hen, & Dulawa, 2008).
An alternative view is that multiple-trial CFC is usually insensitive to the arrest of neurogenesis because the hippocampus is not recruited in this version of the procedure. Indeed, pre-training lesions of the hippocampus often fail to affect context conditioning when multiple context-shock (Wiltgen et al., 2006) or tone-shock pairings are used (Frankland, Cestari, Filipkowski, McDonald, & Silva, 1998; Maren, Aharonov, & Fanselow, 1997). However, these studies likely underestimate the role of the hippocampus in these learning paradigms, because when the hippocampus is lesioned prior to training, alternative learning systems can become engaged, masking the effect of the lesion. Post-training lesions preclude these compensatory processes and reliably impair CFC (Anagnostaras, Maren, & Fanselow, 1999; Frankland et al., 1998; Maren et al., 1997). Furthermore, subtle pre-training manipulations (e.g., NMDA blockade; Bast, Zhang, & Feldon, 2003; Young, Bohenek, & Fanselow, 1994) can more strongly impair CFC than do gross lesions, which has prompted the hypothesis that the intact hippocampus actively inhibits contextual learning by extra-hippocampal structures, namely the neocortex (Rudy et al., 2004). When the hippocampus is completely inactivated, this inhibitory function is compromised and extra-hippocampal structures can compensate. More subtle hippocampal manipulations may fail to block the inhibition of compensatory processes, and, as a result, the hippocampal contribution to behavior is more plainly revealed. In summary, the hippocampus appears to be recruited in multiple-trial CFC, even though traditional hippocampal lesions sometimes fail to produce a behavioral effect.
The dissociation between single- and multiple-trial CFC procedures that we observed in the mouse does not appear to generalize to the rat. In the rat, arrest of hippocampal neurogenesis impairs both single- (Hernandez-Rabaza et al., 2009) and multiple-trial CFC (Hernandez-Rabaza et al., 2009; Snyder et al., 2009; Warner-Schmidt et al., 2008; Winocur et al., 2006; Wojtowicz et al., 2008). This apparent species difference may reflect species differences in the functional integration of adult-generated neurons. It appears that a considerably larger proportion of young, adult-generated neurons are activated by spatial learning in the rat than in the mouse (Snyder et al., 2009), which suggests that young adult-generated neurons may have greater functional significance in rats than in mice. Based on this evidence, one might predict that adult-generated neurons are recruited under a broader range of conditions in the rat than in the mouse. If, as our data suggest, additional training precipitates a switch from neurogenesis-dependent to neurogenesis-independent processing mechanisms, then this switch may require more training in the rat than in the mouse.
The finding that adult hippocampal neurogenesis is necessary for single-trial CFC is consistent with research on the role of DG and CA3 in memory acquisition. CA3, the major target of DG granule cell projections, is thought to be specialized for rapid acquisition of spatial information. Blocking NMDA-mediated plasticity in CA3 impairs the acquisition of single-trial spatial learning, but does not impair multiple-trial learning (e.g., Nakazawa et al., 2003). Although blocking NMDA-mediated plasticity within DG does not impair single-trial acquisition of CFC, CFC acquired under this condition is less precise than in control animals, in that conditioned fear more readily generalizes to an alternate context (McHugh et al., 2007). This discrimination impairment is rescued with continued discrimination training, suggesting that the DG is particularly important for the rapid formation of precise spatial memories. Consistent with this hypothesis, it was recently shown that a DG-specific genetic manipulation is sufficient to accelerate acquisition of spatial information (Saab et al., 2009). Our data suggest that arresting neurogenesis impairs the DG-CA3 circuits that are involved in rapid memory acquisition.
Recent literature suggests several ways in which adult neurogenesis might contribute to mnemonic processes. One idea is that the addition of cells to the DG helps preserve the structure's ability to continually encode new memories without interfering with previous ones (Meltzer et al., 2005; Wiskott et al., 2006). According to this view, arresting neurogenesis might reduce the fidelity of new memories, because encoding would need to be mediated by cells whose plasticity is constrained to preserve older memories. An alternative view emphasizes the increased excitability and plasticity exhibited by young neurons relative to mature neurons. A characteristic feature of the DG is its sparse representation of input stimulation, meaning that relatively few DG granule cells become active in response to a given pattern of stimulation (e.g., Jung & McNaughton, 1993; Leutgeb, Leutgeb, Moser, & Moser, 2007). Highly excitable and plastic young neurons presumably reduce the sparseness of DG activity, increasing the degree to which different stimuli excite overlapping populations of cells. This decrease in sparseness may allow contemporaneous events to be integrated into temporally-specific episodic memories (Aimone, Wiles, & Gage, 2006; Aimone et al., 2009). According to this view, arresting neurogenesis might impair the ability of the hippocampus to bind together multiple percepts (e.g., contextual stimuli, footshock) into a single episodic memory.
A third hypothesis is based on the view that the DG acts a gate that limits or slows hippocampal activation by cortical input stimulation (Hsu, 2007). The DG gate tends to favor persistent or repeated patterns of stimulation, in that brief stimuli are halted at the level of the DG, whereas prolonged or repeated stimuli activate the DG as well as downstream hippocampal subfields (e.g., Iijima et al., 1996). It is possible that arresting neurogenesis, and thereby removing a population of highly excitable young granule neurons, further slows the gating of cortical inputs, meaning that even more sustained patterns of stimulation are required before hippocampal processing mechanisms become engaged. Thus, in the absence of neurogenesis, very brief training episodes, such as a single context-shock pairing, may fail to recruit hippocampal memory networks. More sustained training episodes, such as multiple tone-shock pairings or extended exposure to a context, open the DG gate and initiate plasticity in CA3, a subfield believed to mediate rapid encoding of spatial memories (Nakazawa et al., 2003; Steele & Morris, 1999).
In summary, we found that arresting adult hippocampal neurogenesis via targeted, low-dose irradiation impairs CFC in mice when a single-trial conditioning procedure is used but not when multiple-trial procedures are used. The irradiation-induced deficit in single-trial CFC can be rescued by providing pre-exposure to the conditioning context. The data indicate that the contribution of adult neurogenesis to CFC in mice is revealed only when brief training is provided, suggesting that adult-generated hippocampal neurons may be integral for rapid acquisition of context memories.
ACKNOWLEDGEMENTS
C.A.D. was supported by the Ruth L. Kirschstein Research Service Awards for Individual Predoctoral Fellows F31MH084529. R.H. was supported by the National Alliance for Research on Schizophrenia and Depression (NARSAD), the New York Stem Cell Initiative, and the National Institues of Health Grant R01 MH068542. M.R.D. was supported by the National Institute of Mental Health Grant K99 MH083943, the Charles H. Revson Foundation Senior Fellowship in Biomedical Science, and NARSAD Young Investigator Award. Address correspondence to R.H. (rh95@columbia.edu).
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9(6):723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61(2):187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19(3):1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Wais PE, Gabrieli JD. Emotion enhances remembrance of neutral events past. Proc Natl Acad Sci U S A. 2006;103(5):1599–1604. doi: 10.1073/pnas.0506308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13(6):657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;15(6):722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- Becker S, Macqueen G, Wojtowicz JM. Computational modeling and empirical studies of hippocampal neurogenesis-dependent memory: Effects of interference, stress and depression. Brain Research. 2009;1299:45–54. doi: 10.1016/j.brainres.2009.07.095. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155(4):1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clelland C, Choi M, Romberg C, Clemenson G, Fragniere A, Tyers P, Jessberger S, Saksida L, Barker R, Gage F, Bussey T. A Functional Role for Adult Hippocampal Neurogenesis in Spatial Pattern Separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132(2):107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest J, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous D, Piazza P, Mayeux R. Spatial Relational Memory Requires Hippocampal Adult Neurogenesis. PLoS ONE. 2008;3(4):e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Associative Vs Topographical Accounts of the Immediate Shock Freezing Deficit in Rats - Implications for the Response Selection-Rules Governing Species-Specific Defensive Reactions. Learning and Motivation. 1986;17(1):16–39. [Google Scholar]
- Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cinà I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biology. 2008;6(10):e246. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112(4):863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG, Gomez-Pinedo U, Perez-Villalba A, Rosello J, Trejo JL, Barcia JA, Canales JJ. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159(1):59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33(2):406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res. 2007;163:601–613. doi: 10.1016/S0079-6123(07)63032-5. [DOI] [PubMed] [Google Scholar]
- Iijima T, Witter MP, Ichikawa M, Tominaga T, Kajiwara R, Matsumoto G. Entorhinal-hippocampal interactions revealed by real-time imaging. Science. 1996;272(5265):1176–1179. doi: 10.1126/science.272.5265.1176. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3(2):165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94(19):10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Jang D, Son J, Kwak C, Choi J, Ji Y, Lee Y, Son H, Kaang B. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2(1):1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeira-Fernandez J, DeCola JP, Kim JJ, Fanselow MS. Immediate shock deficit in fear conditioning: effects of shock manipulations. Behav Neurosci. 2006;120(4):873–879. doi: 10.1037/0735-7044.120.4.873. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88(2):261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Make mild moments memorable: add a little arousal. Trends Cogn Sci. 2006;10(8):345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317(5834):94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Meltzer LA, Yabaluri R, Deisseroth K. A role for circuit homeostasis in adult neurogenesis. Trends Neurosci. 2005;28(12):653–660. doi: 10.1016/j.tins.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9(6):729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38(2):305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. An animal model of a behavioral intervention for depression. Neuron. 2008;60(1):149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26(47):12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neuroscience and biobehavioral reviews. 2004;28(7):675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Saab BJ, Georgiou J, Nath A, Lee FJ, Wang M, Michalon A, Liu F, Mansuy IM, Roder JC. NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory. Neuron. 2009;63(5):643–656. doi: 10.1016/j.neuron.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A. 2007;104(11):4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster-maturing, and more involved in behavior in rats than in mice. Journal of Neuroscience. 2009;29(46):14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130(4):843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9(2):118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11(8):901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10(6):727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42(2):248–257. [PubMed] [Google Scholar]
- Warner-Schmidt JL, Madsen TM, Duman RS. Electroconvulsive seizure restores neurogenesis and hippocampus-dependent fear memory after disruption by irradiation. Eur J Neurosci. 2008;27(6):1485–1493. doi: 10.1111/j.1460-9568.2008.06118.x. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26(20):5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz J, Sekeres M, Snyder J, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16(3):296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16(3):329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- Wojtowicz J. Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus. 2006;16(3):261–266. doi: 10.1002/hipo.20158. [DOI] [PubMed] [Google Scholar]
- Wojtowicz J, Askew M, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27(6):1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav Neurosci. 1994;108(1):19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zou Y, He W, Gage FH, Evans R. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451(7181):1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]