Abstract
Background
Critically ill patients admitted to intensive care units (ICUs) are thought to gain an added survival benefit from management by critical care physicians, but evidence of this benefit is scant.
Objective
To examine the association between hospital mortality in critically ill patients and management by critical care physicians.
Design
Retrospective analysis of a large, prospectively collected database of critically ill patients.
Setting
123 ICUs in 100 U.S. hospitals.
Patients
101 832 critically ill adults.
Measurements
Through use of a random-effects logistic regression, investigators compared hospital mortality between patients cared for entirely by critical care physicians and patients cared for entirely by non–critical care physicians. An expanded Simplified Acute Physiology Score was used to adjust for severity of illness, and a propensity score was used to adjust for differences in the probability of selective referral of patients to critical care physicians.
Results
Patients who received critical care management (CCM) were generally sicker, received more procedures, and had higher hospital mortality rates than those who did not receive CCM. After adjustment for severity of illness and propensity score, hospital mortality rates were higher for patients who received CCM than for those who did not. The difference in adjusted hospital mortality rates was less for patients who were sicker and who were predicted by propensity score to receive CCM.
Limitation
Residual confounders for illness severity and selection biases for CCM might exist that were inadequately assessed or recognized.
Conclusion
In a large sample of ICU patients in the United States, the odds of hospital mortality were higher for patients managed by critical care physicians than those who were not. Additional studies are needed to further evaluate these results and clarify the mechanisms by which they might occur.
The extent of involvement and supervision by critical care physicians varies somewhat in U.S. intensive care units (ICUs) (1–6). Some ICUs are organized as strictly closed services, in which critical care physicians, or intensivists, assume control and decision-making ability over all aspects of patient care, whereas in some “hybrid” ICUs, mandated consultation and management by critical care physicians is the primary administrative model. Most ICUs, however, are structured as completely open units, in which the admitting physicians retain full clinical and decisional responsibility and thus have the option to care for their patients with or without input from critical care physicians.
Evidence from several settings suggests improved outcomes when critical care physicians assume substantial responsibility over the care and triage of ICU patients (1, 7–22). These studies, however, have methodological limitations and limited generalizability. Most are small, use historical controls or before–after study designs, and are limited to specific ICUs (for example, medical or surgical) in 1 or 2 centers. They have the usual risks for confounding by illness severity commonly seen in cross-sectional studies (7, 8, 14–21) and retrospective analyses of administrative databases that were limited to certain diagnostic categories (12, 13).
Recognizing the limitations of previously published studies and considerable variability in critical care management (CCM) in the United States, we examined data from 123 ICUs across the United States to assess the relationship between management by critical care physicians and hospital mortality rates of critically ill patients. These data were derived from a large national project that examined resource use in intensive care (2). At the beginning of our analysis, we hypothesized that CCM would be associated with improved outcomes in critically ill patients.
Methods
Patients
Patients were identified through Project IMPACT (Cerner, Bel Air, Maryland), a national database of ICU patients. The Project IMPACT database is a large administrative database originally developed by the Society of Critical Care Medicine in 1996. Participation is voluntary. All data are collected at each institution by on-site data collectors who are certified in advance by Project IMPACT to assure standardization and uniformity in data definitions and database definitions and entry. The database for 2000 to 2004 included 142 392 patients admitted to 123 ICUs in 100 U.S. hospitals. We excluded patients with missing data for variables of interest from our analysis, leaving 111 907 patients. We included only the first ICU admission, reducing the number of patients to 106 623, and then excluded patients who were managed only part time during their ICU stay, reducing the total observations to 101 832.
Variables
Our primary outcome variable was hospital mortality. Our key exposure or “risk factor” was the same regardless of whether a patient was managed by a critical care physician during his or her ICU stay. This was ascertained in Project IMPACT by using the survey question, “Was the patient managed by a critical care physician/team?” Trained data entry personnel for Project IMPACT define CCM as treatment occurring when the physician is asked to take responsibility for the overall management of a patient in the critical care unit without having to first provide expertise about a single organ system. A physician should meet 1 or more of the following criteria to be considered a critical care physician: 1) be recognized by the institution as a critical care specialist within a specialty unit, even without a specialty board certification (such as burn or neurointensivist), and must treat the total patient and not a single organ system; 2) have passed critical care medicine board examinations or be qualified to take the examination; and 3) be trained in an accredited critical care fellowship.
When a patient received CCM, it was documented, regardless of whether the treatment was for all or part of the ICU stay. Covariates included patient characteristics, such as demographic characteristics, diagnosis, and clinical condition at ICU admission. We also controlled for ICU and hospital characteristics. Severity of illness was measured by the Simplified Acute Physiology Score (SAPS) II. Through use of recently published work on SAPS (23), we added additional variables to SAPS II and modified coefficients in the logit model to derive a better fit. These included the patient’s age (<40 years, 40 to 59 years, 60 to 69 years, 70 to 79 years, and >79 years), sex, duration of hospital stay before ICU admission (<24 hours, 1 day, 2 days, 3 to 9 days, >9 days), patient’s location before ICU (transfer from outside emergency department, rehabilitation or skilled nursing facility, wards, or another hospital), clinical category (medical patient or other), and intoxication (yes or no). For this expanded SAPS II, the Hosmer–Lemeshow goodness-of-fit P value was 0.38. (The Appendix, available at www.annals.org, provides more detail on the expanded SAPS II.)
Statistical Analysis
We divided ICUs into 3 groups based on the percentage of patients receiving CCM for the entire stay: 95% of patients or more, 5% to 95% of patients, and 5% of patients or fewer.
We excluded 4793 patients who received CCM for only part of the ICU stay from the analysis, leaving 2 patient management types: CCM for the entire stay and no CCM. For each of the 6 categories defined by the combination of patient management type and ICU group, we computed expected and actual mortality rates. Expected mortality was the mean SAPS II probability of mortality. Actual mortality was the percentage of patients who did not survive the hospital stay. We computed the standardized mortality ratio and its 95% CI, based on an exact Poisson distribution, as the ratio of actual to expected mortality.
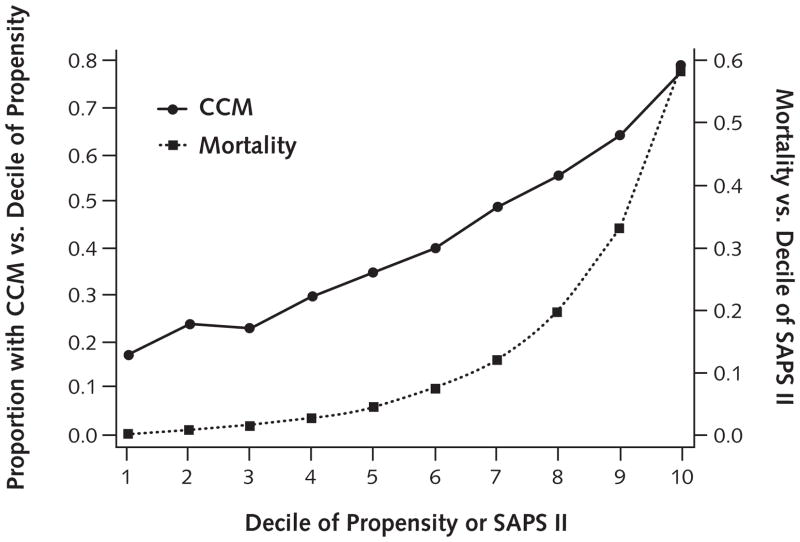
We developed a score to measure the propensity that a patient would be selected for CCM. We derived our score from a logistic regression model, with CCM as the dependent variable. The model was estimated on patients only from ICUs not mandating CCM. We screened all available patient characteristics known at the time of ICU admission and ICU characteristics for inclusion in the model. A propensity score was then estimated for each patient. Variables used to create the propensity score were age, Glasgow Coma Score, number of licensed hospital beds, insurance (commercial, Medicaid or Medicare, or self-pay), ventilation at ICU admission, tracheostomy at ICU admission, gastrointestinal bleeding, noninvasive ventilation at ICU admission, cerebrovascular event, chronic immunosuppression, chronic respiratory disease, acute renal failure, hospital location (rural, suburban, or urban), continuous sedation, and admission source (emergency department, another hospital, invasive procedures, or other non-ICU location). Figure 1 shows the proportion of patients managed by critical care physicians. Hospital mortality rates tend to increase from the first decile to the last decile of propensity and SAPS II. More details of the score and the sensitivity of results to changes in the propensity score are shown in the Appendix (available at www.annals.org).
Figure 1. Critical care management (CCM) and mortality.
SAPS = Simplified Acute Physiology Score.
We performed random-effects logistic regressions on the entire sample, using hospital death as the dependent variable. This method uses the within- and between-ICU variability inherent in the nesting of the patients into 123 ICUs. The crude model included only the risk factor “CCM for the entire stay” versus no CCM. Severity of illness (as measured by the expanded SAPS II score) and likelihood of selection for CCM (as measured by the propensity score) were then added to the model as control variables, along with all interactions of the control variables and risk factor. Where a statistically significant interaction term indicated that a control variable was an effect modifier, the regression was estimated within each quartile of the control variable.
We repeated random-effects logistic regression analysis of mortality on several subsamples. The “no-choice” sub-sample included 2 groups of patients: those from ICUs in which 95% or more or 5% or fewer patients received CCM. In addition, the following subsamples were examined: patients not transferred from another hospital, patients with a respiratory diagnosis with ventilator support at ICU admission, patients with respiratory diagnosis without ventilator support at ICU admission, patients with ventilator support at ICU admission, patients with a diagnosis other than respiratory and no ventilator at ICU admission, patients with a circulatory diagnosis, patients with a diagnosis of infection, patients with at least 1 ICU procedure, and patients with no ICU procedures. The Appendix (available at www.annals.org) presents additional details of regression analyses.
Role of the Funding Source
Eli Lilly and the Department of Bioethics at the National Institutes of Health Clinical Center funded the study. The funding services had no role in the design, conduct, and analysis of the study and did not participate in the decision to submit the manuscript for publication.
Results
Table 1 shows that ICUs that manage 95% or more of their patients with critical care physicians for the entire stay were, on average, larger and in larger hospitals than other ICUs. A greater percentage of ICUs had academic affiliation or activity and were only medical, surgical, or trauma, as opposed to a mixed model. A smaller percentage had staffing policies that permitted either licensed practical nurses or registered nurses.
Table 1.
Characteristics of Critical Care Management in Intensive Care Units*
| Characteristic | Critical Care Management for Patients† |
||
|---|---|---|---|
| ≥95% | 5%–95% | ≤5% | |
| ICUs, n | 23 | 79 | 21 |
| ICU beds, n Mean |
17.3 |
17.5 |
13.5 |
| Median | 16 | 16 | 12.9 |
| Minimum–maximum | 10–32 | 7–66 | 4–25 |
| Hospital beds, n Mean |
668 |
468 |
404 |
| Median | 570 | 430 | 375 |
| Minimum–maximum | 257–1389 | 70–1049 | 130–774 |
| Urban location, % | 61 | 47 | 43 |
| Academic hospital, % | 52 | 5 | 0 |
| Primary medical school hospital, % | 61 | 6 | 0 |
| Primary hospital for critical care fellowship, % | 87 | 6 | 5 |
| Critical care fellows rotate, % | 48 | 8 | 19 |
| ICU type, % | |||
| Medical only |
30 |
8 |
10 |
| Surgical or trauma only | 22 | 9 | 0 |
| Mixed or other | 48 | 83 | 90 |
| Nursing policy, % RN or LPN |
4 |
15 |
14 |
| RN only | 96 | 81 | 86 |
| RN with CCRN | 0 | 4 | 0 |
CCRN = critical care registered nurse; ICU = intensive care unit; LPN = licensed practical nurse; RN = registered nurse.
For the entire stay for patients in the ICU.
Table 2 shows patient characteristics by ICU category and CCM status. Among the 123 ICUs, 23 (18 618 patients) had at least 95% of patients managed for the entire stay by critical care physicians, whereas 21 (22 870 patients) had 5% or fewer managed by critical care physicians. These 2 groups together make up the “no-choice” group. The remaining 60 344 patients were treated in the 79 ICUs in which 5% to 95% of patients received CCM for the entire stay (the “choice” group).
Table 2.
Patient Characteristics*
| Characteristic | Critical Care Management† |
No Critical Care Management† |
||||
|---|---|---|---|---|---|---|
| ≥95% | 5%–95% | ≤5% | ≥95% | 5%–95% | ≤5% | |
| Patients, n | 18 601 | 23 324 | 261 | 17 | 37 020 | 22 609 |
| Mean age, y | 59.9 | 59.6 | 63.2 | 65.7 | 61 | 63.6 |
| Men, % | 54.3 | 53.1 | 50.2 | 58.8 | 52.4 | 51.9 |
| Race, % Black |
16.6 |
13.5 |
18.0 |
5.9 |
12.1 |
16.5 |
| Other or unknown | 5.5 | 8.5 | 5.0 | 5.9 | 9.6 | 6.4 |
| White | 77.9 | 78.0 | 77.0 | 88.2 | 78.3 | 77.1 |
| Full code, % | 95.6 | 94.9 | 97.3 | 94.1 | 94.4 | 93.6 |
| ICU procedure, % | 80.5 | 72.5 | 72.4 | 64.7 | 52.4 | 60.8 |
| Intravenous drugs, % | 93.2 | 94.7 | 96.2 | 100.0 | 89.3 | 93.1 |
| Surgery during ICU, % | 51.3 | 42.5 | 59.8 | 64.7 | 43.8 | 44.2 |
| Ventilation during the first 24 h in ICU, % | 36 | 33.5 | 28 | 11.8 | 13.9 | 17.6 |
| Postoperative, % | 40.2 | 30.3 | 37.9 | 58.8 | 33.6 | 35.3 |
| Continuous sedation, % | 11.9 | 18.7 | 11.9 | 5.9 | 10 | 6.8 |
| Origin, % Another hospital |
8.8 |
5.3 |
3.4 |
0 |
5.7 |
5.1 |
| Emergency department | 30.2 | 42.1 | 29.5 | 11.8 | 42.3 | 39.6 |
| Invasive procedure | 36.3 | 27.2 | 45.6 | 64.7 | 33.1 | 34.3 |
| Other unit (not ICU) | 20.7 | 21.4 | 17.6 | 23.5 | 15.4 | 17.1 |
| Major disease category, % Circulatory system |
19.4 |
20.9 |
32.6 |
52.9 |
30.1 |
26.1 |
| Digestive system | 14.4 | 12 | 14.9 | 17.6 | 13.5 | 15.4 |
| Nervous system | 15.2 | 12.9 | 9.2 | 11.8 | 19.1 | 16.9 |
| Respiratory system | 27.3 | 28.9 | 25.7 | 11.8 | 16.2 | 19.7 |
| Infection | 7.8 | 8.1 | 7.7 | 0 | 5.2 | 6 |
| Trauma | 6.6 | 5.1 | 5.7 | 0 | 2.5 | 2.8 |
| Payment, % Medicare |
49.5 |
50.2 |
60.2 |
58.8 |
52.9 |
59.8 |
| Medicaid | 7.8 | 7.5 | 3.4 | 0 | 6.6 | 7.2 |
| Self-pay | 8.3 | 10.2 | 7.3 | 11.8 | 8.6 | 5.3 |
| Commercial or managed care | 34.4 | 32.1 | 29.1 | 29.4 | 32 | 27.7 |
ICU = intensive care unit.
For the entire stay for patients in the ICU.
Comparison of patients managed for the entire stay by critical care physicians versus those managed by other physicians shows that more patients treated by critical care physicians received interventions, such as ICU procedures, intravenous drugs, mechanical ventilation, and continuous sedation. They were less likely to be postoperative patients or to receive surgery while in the ICU. Respiratory system disease, infections, and trauma occurred more among patients treated by critical care physicians. Intensive care units managing 95% or more of patients with critical care physicians had somewhat more admissions from other hospitals and from invasive procedures and somewhat fewer admissions from the emergency department than other ICUs did.
Table 3 provides the discharge destination according to CCM status of patients who survived their hospital stay. More than 59% of patients who did not receive CCM were discharged home (including those with home health care and those leaving against medical advice) compared with 58% of patients who received CCM. About 16% of patients were discharged to an extended care facility for CCM and no CCM, whereas 13% and 9% were discharged to a rehabilitation center for CCM and no CCM, respectively. More than 13% of patients who did not receive CCM were discharged to an unknown location compared with about 9% of those who received CCM.
Table 3.
Hospital Discharge Destination*
| Hospital Discharge Destination | No Critical Care Management, % | Critical Care Management, % | Total, % |
|---|---|---|---|
| Extended-care facility | 15.78 | 16.41 | 16.03 |
| Another hospital | 1.50 | 1.53 | 1.41 |
| Rehabilitation center | 8.55 | 13.21 | 10.36 |
| Home | 49.29 | 44.12 | 47.26 |
| Home with home health care | 9.43 | 13.04 | 10.85 |
| Home (against medical advice) | 0.38 | 0.48 | 0.42 |
| Hospice | 1.01 | 1.25 | 1.10 |
| Long-term acute care | 0.06 | 0.29 | 0.15 |
| Other | 0.66 | 0.88 | 0.75 |
| Unknown | 13.33 | 8.80 | 11.55 |
| Total | 100.00 | 100.00 | 100.00 |
Percentages exclude patients who died in the hospital.
Table 4 shows that patients managed by critical care physicians for the entire stay had a higher mean severity of illness (SAPS II probability of mortality) than patients who did not receive CCM. These patients also had higher hospital mortality. The standardized mortality ratio for patients who received CCM in ICUs that managed 95% or more patients was 1.09 (95% CI, 1.05 to 1.13) compared with a standardized mortality ratio of 0.91 (CI, 0.88 to 0.94) for patients who did not receive CCM in ICUs in which critical care physicians managed 5% or fewer patients. Among patients who received CCM in ICUs that managed 5% to 95% of patients, the standardized mortality ratio was 1.09 (CI, 1.05 to 1.12) for patients who received CCM for the entire stay compared with 0.91 (CI, 0.88 to 0.94) for patients who did not receive CCM.
Table 4.
Expected and Actual Hospital Mortality*
| Variable | Critical Care Management† |
No Critical Care Management† |
||||
|---|---|---|---|---|---|---|
| ≥95% | 5%–95% | ≤5% | ≥95% | 5%–95% | ≤5% | |
| Patients, n | 18 601 | 23 324 | 261 | 17 | 37 020 | 22 609 |
| Mean SAPS II probability | 0.1650 | 0.1733 | 0.1511 | 0.0585 | 0.1102 | 0.1368 |
| Mean mortality rate | 0.1800 | 0.1884 | 0.1801 | 0.0588 | 0.1004 | 0.1244 |
| SMR (95% CI) | 1.09 (1.05–1.13) | 1.09 (1.05–1.12) | 1.19 (0.88–1.58) | 1.01 (0.03–5.60) | 0.91 (0.88–0.94) | 0.91 (0.88–0.94) |
SAPS = Simplified Acute Physiology Score; SMR = standardized mortality ratio.
For the entire stay for patients in the intensive care unit.
A random-effects logistic regression model including only CCM as a predictor of hospital mortality produced a crude odds ratio (OR) of 2.13 (P < 0.001). The addition of SAPS II to this model reduced this OR to 1.42 (P < 0.001). Further inclusion of the propensity score decreased the OR to 1.40 (P < 0.001). For additional regression results, see the Appendix (available at www.annals.org).
Interaction terms were statistically significant, indicating that severity and propensity were acting as effect modifiers. Models were estimated for each quartile of severity and propensity score (Table 5). For 11 of 16 resulting groups, the OR for mortality was statistically significant (P < 0.05). All statistically significant ORs were greater than 1.0, ranging from 2.83 (severity quartile 1 and propensity quartile 1) to 1.18 (severity quartile 4 and propensity quartile 4). Within each severity quartile, ORs tended to decrease as propensity quartiles increased.
Table 5.
Random-Effects Logistic Regression Odds Ratio for Mortality, Stratified by SAPS II and Propensity Score*
| Quartile of SAPS II Probability† | Propensity Score Quartile‡ | No CCM Count | CCM Count | CCM Odds Ratio (95% CI)§ | P Value | ρ|| |
|---|---|---|---|---|---|---|
| 1 | 1 | 6011 | 1200 | 2.83 (1.28–6.27) | 0.010 | 0.08 |
| 1 | 2 | 5974 | 2013 | 1.98 (1.07–3.66) | 0.028 | 0.06 |
| 1 | 3 | 3664 | 2586 | 1.45 (0.82–2.58) | 0.21 | 0.05 |
| 1 | 4 | 1224 | 3005 | 1.11 (0.52–2.37) | 0.79 | 0.21 |
| 2 | 1 | 6335 | 1428 | 2.12 (1.48–3.05) | <0.001 | 0.05 |
| 2 | 2 | 4749 | 1989 | 1.88 (1.40–2.55) | <0.001 | 0.02 |
| 2 | 3 | 3189 | 2576 | 1.25 (0.93–1.69) | 0.143 | 0.03 |
| 2 | 4 | 1580 | 3671 | 0.86 (0.63–1.18) | 0.34 | 0.07 |
| 3 | 1 | 5135 | 1457 | 2.26 (1.78–2.87) | <0.001 | 0.06 |
| 3 | 2 | 4078 | 1876 | 1.76 (1.42–2.19) | <0.001 | 0.05 |
| 3 | 3 | 3320 | 2967 | 1.50 (1.24–1.81) | <0.001 | 0.04 |
| 3 | 4 | 2147 | 4342 | 1.19 (0.99–1.43) | 0.064 | 0.05 |
| 4 | 1 | 2874 | 1201 | 1.53 (1.27–1.83) | <0.001 | 0.05 |
| 4 | 2 | 2770 | 1959 | 1.36 (1.16–1.58) | <0.001 | 0.04 |
| 4 | 3 | 3487 | 3621 | 1.36 (1.19–1.54) | <0.001 | 0.04 |
| 4 | 4 | 3109 | 6295 | 1.18 (1.05–1.32) | <0.001 | 0.04 |
CCM = critical care management; ICU = intensive care unit; SAPS = Simplified Acute Physiology Score.
Quartile 1 includes the lowest SAPS II probabilities of death, whereas quartile 4 includes the highest probabilities.
Quartile 1 is the lowest propensity of being seen by a critical care physician, whereas quartile 4 is the highest propensity.
Random-effects logistic regression results, in which outcome is hospital mortality, adjusted for SAPS II probability of mortality and propensity to see a critical care physician.
ρ is the ratio of the between-ICU variance to the total variance. Zero indicates that all variability is within the ICU, and 1.0 indicates that all variability is between ICUs.
Table 6 shows results of subgroup analysis through use of a random-effects logistic regression. When interaction variables were not significant, the ORs reported for CCM are from a model adjusted for SAPS II and propensity score. When 1 or both of these are effect modifiers, we report results by quartiles of the relevant variables. All of the ORs reported for the subgroup analyses are greater than 1.0, with 4 of 22 not significantly greater than 1.0. These analyses are a respiratory diagnosis of patients with no ventilator in place when admitted to the ICU (OR, 1.11; P = 0.121) and the first 2 quartiles of no ICU procedures (ORs, 2.11 and 1.53; P = 0.129 and 0.101).
Table 6.
Subgroup Analysis: Random-Effects Logistic Regression Odds Ratios for Mortality*
| Group | Quartile of Propensity Score† or SAPS II Probability‡ | No CCM | CCM | CCM Odds Ratio (95% CI)§ | P Value |
|---|---|---|---|---|---|
| Respiratory diagnosis and ventilation at ICU admission | – | 3075 | 4822 | 1.22 (1.01–1.46) | 0.034 |
| Respiratory diagnosis and no ventilation at ICU admission | – | 7363 | 7074 | 1.11 (0.97–1.26) | 0.121 |
| Ventilation at ICU admission | – | 9121 | 14 581 | 1.28 (1.15–1.43) | <0.001 |
| Circulatory diagnosis | – | 17 035 | 8572 | 1.55 (1.37–1.77) | <0.001 |
| Infection diagnosis | – | 3258 | 3351 | 1.12 (0.96–1.31) | 0.140 |
| “No-choice” ICU|| | – | 22 624 | 18 862 | 1.47 (1.21–1.77) | <0.001 |
| Patients not transferred from another hospital¶ | 1 | 19 146 | 4779 | 1.70 (1.46–1.99) | <0.001 |
| 2 | 16 690 | 7235 | 1.50 (1.31–1.71) | <0.001 | |
| 3 | 12 896 | 11 026 | 1.33 (1.19–1.48) | <0.001 | |
| 4 | 7643 | 16 281 | 1.25 (1.12–1.39) | <0.001 | |
| Diagnosis other than respiratory and no ventilation at ICU admission** | 1 | 11 326 | 4617 | 2.39 (1.39–4.11) | 0.002 |
| 2 | 11 365 | 4552 | 1.95 (1.44–2.64) | <0.001 | |
| 3 | 11 026 | 4884 | 2.00 (1.67–2.39) | <0.001 | |
| 4 | 9444 | 6478 | 1.57 (1.43–1.73) | <0.001 | |
| ≥1 ICU procedures¶ | 1 | 12 180 | 4131 | 1.73 (1.47–2.02) | <0.001 |
| 2 | 9510 | 6800 | 1.16 (1.03–1.31) | 0.019 | |
| 3 | 7087 | 9222 | 1.28 (1.14–1.43) | <0.001 | |
| 4 | 4384 | 11 926 | 1.16 (1.02–1.32) | 0.021 | |
| No ICU procedures** | 1 | 6506 | 2647 | 2.11 (0.80–5.51) | 0.129 |
| 2 | 6714 | 2445 | 1.53 (0.92–2.52) | 0.101 | |
| 3 | 6692 | 2440 | 1.38 (1.01–1.87) | 0.042 | |
| 4 | 6572 | 2575 | 1.24 (1.06–1.45) | 0.006 | |
CCM = critical care management; ICU = intensive care unit; SAPS = Simplified Acute Physiology Score.
Quartile 1 is the lowest propensity of being seen by a critical care physician, whereas quartile 4 is the highest propensity.
Quartile 1 includes the lowest SAPS II probabilities of death, whereas quartile 4 includes the highest probabilities.
Random-effects logistic regression results, in which outcome is hospital mortality, adjusted for SAPS II probability of mortality and propensity to see a critical care physician.
“No choice” is defined as an ICU that manages ≥95% of its patients by a critical care physician or an ICU that manages ≤5% of its patients by a critical care physician.
Statistically significant interactions between propensity score and CCM variable; thus, the analysis is run individually over the propensity score quartiles.
Statistically significant interactions between SAPS II probability of mortality and CCM variable; thus, the analysis is run individually over the SAPS II probability quartiles.
We conducted 2 sensitivity analyses to determine whether transferring patients to another location (for example, a new hospital, rehabilitation center, hospice care, or extended care) determined the reduced mortality rate seen in the group that did not receive CCM (Table 7). In the first case, the operational definition of mortality included in-hospital mortality, transfer to another hospital, a rehabilitation center, extended care, hospice, or a long-term acute care facility versus home. Patients whose discharge destination was unknown were omitted from the sensitivity analysis. The second sensitivity analysis included only in-hospital mortality versus discharge to home. The crude OR, the OR adjusted for expanded SAPS II, and the OR adjusted for both expanded SAPS II and propensity for patients to receive CCM are similar. The ORs are greater for the group that received CCM than the group that did not for both sensitivity analyses, demonstrating the robustness of our results. The Appendix (available at www.annals.org) shows additional sensitivity analyses involving changes to the propensity score. Conditional logistic regression analyses for the 19 largest ICUs generated an OR greater than 1.0 in 18 of 19 ICUs. In 50% of these ICUs, the difference was statistically significant. In the remaining ICUs, the difference was not statistically significant because of the small sample size within the individual ICUs.
Table 7.
Sensitivity Analysis on the Robustness of the Results to Changes in Mortality Definition*
| Variable | Hospital Mortality† | Hospital Mortality Combined with Other Discharge Locations‡ | Hospital Mortality versus Discharge to Home§ |
|---|---|---|---|
| Main-effects model|| | |||
| Crude OR (95% CI); P value | 2.13 (2.03–2.24); <0.001 | 1.80 (1.73–1.87); <0.001 | 2.42 (2.30–2.56); <0.001 |
| OR adjusted for expanded SAPS II (95% CI); P value | 1.42 (1.34–1.52); <0.001 | 1.31 (1.25–1.37); <0.001 | 1.59 (1.48–1.71); <0.001 |
| OR adjusted for expanded SAPS II and propensity score (95% CI); P value | 1.40 (1.32–1.49); <0.001 | 1.34 (1.28–1.40); <0.001 | 1.58 (1.47–1.70); <0.001 |
| Deaths, n (%) | |||
| Yes | 14 318 (14.1) | 39 890 (39.2) | 14 318 (14.1) |
| No | 87 514 (85.9) | 51 223 (50.3) | 51 223 (50.3) |
| Not used in analysis | 0 (0.0) | 10 719 (10.5) | 36 291 (35.6) |
OR = odds ratio; SAPS = Simplified Acute Physiology Score.
Hospital mortality (yes vs. no), as used in this study.
“Hospital mortality” is defined as patients who died in the hospital in combination with those discharged to another hospital, rehabilitation center, hospice care, or long-term acute care vs. those who were discharged home. If the discharge location was unknown, these participants were left out of the sensitivity analysis.
“Hospital mortality” is defined as patients who died in the hospital vs. those who were discharged home. All others were excluded from the analysis.
Random-effects logistic regression, in which expanded SAPS II probability and propensity score are added into the model as main effects without interaction terms.
Discussion
By using a database of more than 100 000 patients, we identified 3 types of ICUs: ICUs in which all patients are required to receive management by critical care physicians, ICUs in which no patients are managed by critical care physicians, and ICUs in which patients may or may not be managed by critical care physicians. Despite adjustment for severity of illness, we cannot demonstrate any survival benefit with management by critical care physicians. In fact, patients managed by critical care physicians had higher odds of mortality than patients managed by physicians not trained in critical care medicine.
Our results are surprising and completely contrary to previously published findings (7–21). Almost all published studies on the impact of critical care physicians have demonstrated decreased morbidity or mortality with management by critical care specialists (24–28).
To control for potential confounders by severity of illness and the tendency for sicker patients to be transferred to physicians trained in critical care, we used an expanded SAPS II (23) and developed a propensity score. The expanded SAPS II was designed to better estimate the probability of mortality of patients admitted to ICUs than was possible with the older SAPS II system. To explore the possibility that some subgroups of patients might benefit from CCM more than others, we conducted several subgroup analyses. For almost all of the subgroups analyzed, risk for mortality associated with management by critical care physicians statistically significant increased.
What could account for these unexpected results? Several possible explanations must be considered. First, there may be residual confounders of severity not covered by either the expanded SAPS II or the propensity score. Our data indicate that patients cared for by pulmonary or critical care physicians for their entire ICU stay were sicker, as evidenced by higher median SAPS II scores. Our results are based on the ability to adjust the increased severity in patients managed by pulmonary or critical care physicians. Despite our attempts to adjust for severity to match patients in both groups for the purposes of comparison, no severity adjustment is perfect, and thus, there may be substantial unrecognized markers of severity in patients cared for by critical care physicians that remain unaccounted for. Some examples of residual unrecognized confounding include comorbid conditions and additional diagnoses not reported in the Project IMPACT database; responses to therapy; presence of protocols in some ICUs; presence and responsibilities of nonintensivist physicians, nurses, and other clinicians; and the influence of where and how long the patient received treatment before ICU admission (lead-time bias).
Second, we must consider the possibility that, for the patients in the Project IMPACT database, management by critical care physicians was associated with worse outcomes. Despite compelling evidence in the literature that care provided by trained critical care physicians leads to better outcomes, our data raise an important point: Although we believe that critical care physicians are trained and expertly skilled in the management of critically ill patients, perhaps some routine critical care practices and procedures may not be beneficial or cumulative use of more interventions may take a negative toll. Although further analyses and studies are needed to understand the possibility that care from critical care physicians is associated with higher hospital mortality, we speculate that there may be several plausible explanations. First, critical care physicians may use their own judgment to manage patients instead of using standardized protocols that may be associated with better outcomes. Second, because of their familiarity and expertise with procedures, they may use more procedures that subsequently lead to more complications. Their use of more procedures, such as placement of catheters and other invasive devices, may make critically ill patients more susceptible to life-threatening infections. Third, patients who receive care from a critical care physician may be transferred to different, unfamiliar physicians, whereas patients who receive care from non–critical care physicians may be more likely to receive ongoing care from physicians already familiar with them. Transfers may, be associated with greater chances of disruption in management and medical orders and create a greater likelihood of miscommunication and errors, all of which can have adverse consequences. This last possible explanation would be more noticeable in patients whose illnesses require less critical care expertise.
We do not claim that this list is exhaustive, but each speculation could be explored by future studies that examine the rates of protocol use, procedures, drug-resistant infections, and care for large groups of patients among physicians who are trained in critical care and those who are not.
Our study has several limitations. First, hospital mortality, rather than 30-day mortality, is the end point. Project IMPACT measures only ICU and hospital mortality. No information on the patients was collected after they left the hospital. Thus, the database contains no information on 30-day mortality. This allows for the possibility that the outcome between the 2 groups may be different at 30 days compared with hospital discharge. If more patients managed by non–critical care physicians died between hospital discharge and 30 days, our results might be very different. For this to be the case, non–critical care physicians would have to routinely discharge patients when they are sicker and at higher risk for death. The fact that more patients were discharged home by non–critical care physicians, rather than to extended care facilities, would seem to argue against this possibility.
Second, the process for identifying the management of patients has limitations. Data collectors at each institution decided, on the basis of training and instructions from Project IMPACT staff, whether to classify patients as managed by critical care physicians. Ultimately, this is a subjective process and may have led to unrecognized bias in the classification of patients.
Third, data elements for analysis are limited to those available in the Project IMPACT database. Limited information is available about the internal structure of each ICU in the database. For example, the presence of protocols, order sets, the length of experience of the nursing staff, the nurse–patient ratio on any particular day, and how many different groups of critical care physicians function within each ICU remain unknown. These and other factors may have had a strong, unrecognized influence on the outcomes of patients in a given ICU. In addition, the Project IMPACT database was not established to address the impact of critical care physician management on patient outcome.
Finally, the percentage of patients managed by full-time intensivists cannot be identified in the Project IMPACT database, and we therefore cannot assess the benefit of full-time, on-site management by ICU physicians. Treatment designated as “management entire stay by critical care physicians” includes all models of management in the ICU by board-certified or board-eligible critical care physicians, including full-time intensivists, office-based pulmonary critical care physicians seeing patients on rounds in the ICU once or twice a day, and private consulting groups with responsibility for critical care patients. Therefore, our study does not identify 1 particular model of critical care practice but rather a broad array of practice management styles provided by trained, board-certified or board-eligible critical care physicians. In the Project IMPACT database, we know little about the non–critical care physicians who manage patients in the ICU or the ICUs in which no patients are managed by critical care.
Future prospective studies should be designed to better answer the questions raised by our study, including characteristics that identify high-performing critical care units.
In conclusion, our study, which to our knowledge is based on the largest cohort ever analyzed to examine the relationship of CCM to survival of critically ill patients, found some unexpected results. Patients managed by critical care physicians for the entire ICU stay had a higher risk for death than patients managed by non–critical care physicians. Although all of the possible explanatory mechanisms we have mentioned may seem to portend badly for the practice of critical care medicine, we suggest that, if true, they are amenable to correction or mitigation through such efforts as guideline development and adherence, quality improvement, and systematic efforts to reduce errors. Given the complexity of critical illness, the need for dedicated critical care physicians seems inevitable, and strategies to assure best practices will help them to guarantee the best outcomes possible. Further research is needed to explain these findings and determine whether these results may be explained by unrecognized residual confounders of illness severity.
Context
Critical care physicians or physicians without specialized critical care training may manage patients in intensive care units.
Contribution
This study described 101 832 patients in 123 intensive care units in the United States. Patients managed by critical care physicians were sicker, had more procedures, and had higher hospital mortality rates than those managed by other physicians. Analyses that adjusted for severity of illness and the tendency for sicker patients to be managed by critical care specialists still showed higher mortality among patients managed by the specialists.
Caution
Unrecognized confounders might diminish or invalidate the unexpected finding of higher mortality among patients managed by critical care specialists.
—The Editors
Acknowledgments
The authors thank Rito Bergemann MD, PhD; Laura Katz, MPH; Lisa Siegartel, MPH; and J.J. Doyle, PhD, whose initial statistical support contributed to the initial observation, and Barbara Shott, who assisted in preparing the manuscript.
Grant Support: By the National Institutes of Health Clinical Center and an unrestricted educational grant from Eli Lilly.
Footnotes
Disclaimer: The opinions expressed in this paper are those of the authors and do not reflect policies of the National Institutes of Health or the U.S. Department of Health and Human Services.
Potential Financial Conflicts of Interest: Consultancies: D.B. Chalfin (Project IMPACT).
Reproducible Research Statement: Study protocol: Not available. Statistical code: Available from Mr. Phillips (gary.phillips@osumc.edu). Data set: Available for purchase from Cerner Corporation (www.cerner.com/piccm).
Author Contributions: Conception and design: J. Rapoport, D.B. Chalfin, M. Danis, M.M. Levy.
Analysis and interpretation of the data: J. Rapoport, S. Lemeshow, D.B. Chalfin, G. Phillips, M. Danis, M.M. Levy.
Drafting of the article: J. Rapoport, D.B. Chalfin, G. Phillips, M.M. Levy.
Critical revision of the article for important intellectual content: J. Rapoport, S. Lemeshow, D.B. Chalfin, G. Phillips, M. Danis, M.M. Levy.
Final approval of the article: J. Rapoport, S. Lemeshow, M. Danis, M.M. Levy.
Statistical expertise: J. Rapoport, S. Lemeshow, G. Phillips.
Obtaining of funding: M. Danis.
References
- 1.The Leapfrog Group. Leapfrog Hospital Quality and Safety Survey Results. Accessed at www.leapfroggroup.org/cp on 17 April 2008.
- 2.Danis M, Chalfin DB, Doyle JJ, Bergemann R, Katz LM, Siegartel LR, et al. Development of model-based computer software for ICU resource allocation [Abstract] Crit Care Med. 2004;32:A94. [Google Scholar]
- 3.Groeger JS, Guntupalli KK, Strosberg M, Halpern N, Raphaely RC, Cerra F, et al. Descriptive analysis of critical care units in the United States: patient characteristics and intensive care unit utilization. Crit Care Med. 1993;21:279–91. doi: 10.1097/00003246-199302000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Groeger JS, Strosberg MA, Halpern NA, Raphaely RC, Kaye WE, Guntupalli KK, et al. Descriptive analysis of critical care units in the United States. Crit Care Med. 1992;20:846–63. doi: 10.1097/00003246-199206000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Groeger JS, Strosberg MA, Halpern NA, Raphaely RC, Kaye WE, Guntupalli KK, et al. Descriptive analysis of critical care units in the United States. Crit Care Med. 1992;20:846–63. doi: 10.1097/00003246-199206000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Kelley MA, Angus DC, Chalfin DB. The critical care crisis in the United States: a report from the profession. Crit Care Med. 2004;32:1219–1222. doi: 10.1378/chest.125.4.1514. [DOI] [PubMed] [Google Scholar]
- 7.Multz AS, Chalfin DB, Samson IM, Dantzker DR, Fein AM, Steinberg HN, et al. A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU. Am J Respir Critic Care Med. 1998;157:1468–73. doi: 10.1164/ajrccm.157.5.9708039. [DOI] [PubMed] [Google Scholar]
- 8.Carson SS, Stocking C, Podsadecki T, Christenson J, Pohlman A, MacRae S, et al. Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of ‘open’ and ‘closed’ formats. JAMA. 1996;276:322–8. [PubMed] [Google Scholar]
- 9.Carlson RW, Weiland DE, Srivathsan K. Does a full-time, 24-hour intensivist improve care and efficiency? Crit Care Clin. 1996;12:525–51. doi: 10.1016/s0749-0704(05)70260-8. [DOI] [PubMed] [Google Scholar]
- 10.Barie PS, Bacchetta MD, Eachempati SR. The contemporary surgical intensive care unit. Structure, staffing, and issues. Surg Clin North Am. 2000;80:791–804. vii. doi: 10.1016/s0039-6109(05)70096-7. [DOI] [PubMed] [Google Scholar]
- 11.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–62. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 12.Dimick JB, Pronovost PJ, Heitmiller RF, Lipsett PA. Intensive care unit physician staffing is associated with decreased length of stay, hospital cost, and complications after esophageal resection. Crit Care Med. 2001;29:753–8. doi: 10.1097/00003246-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Pronovost PJ, Jenckes MW, Dorman T, Garrett E, Breslow MJ, Rosenfeld BA, et al. Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery. JAMA. 1999;281:1310–7. doi: 10.1001/jama.281.14.1310. [DOI] [PubMed] [Google Scholar]
- 14.Brown JJ, Sullivan G. Effect on ICU mortality of a full-time critical care specialist. Chest. 1989;96:127–9. doi: 10.1378/chest.96.1.127. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds HN, Haupt MT, Thill-Baharozian MC, Carlson RW. Impact of critical care physician staffing on patients with septic shock in a university hospital medical intensive care unit. JAMA. 1988;260:3446–50. [PubMed] [Google Scholar]
- 16.Lima C, Levy MM. The impact of an on-site intensivist on patient charges and length of stay in the medical intensive care unit [Abstract] Crit Care Med. 1995;23:A238. [Google Scholar]
- 17.Ghorra S, Reinert SE, Cioffi W, Buczko G, Simms HH. Analysis of the effect of conversion from open to closed surgical intensive care unit. Ann Surg. 1999;229:163–71. doi: 10.1097/00000658-199902000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li TC, Phillips MC, Shaw L, Cook EF, Natanson C, Goldman L. On-site physician staffing in a community hospital intensive care unit. Impact on test and procedure use and on patient outcome. JAMA. 1984;252:2023–7. [PubMed] [Google Scholar]
- 19.Manthous CA, Amoateng-Adjepong Y, al-Kharrat T, Jacob B, Alnuaimat HM, Chatila W, et al. Effects of a medical intensivist on patient care in a community teaching hospital. Mayo Clin Proc. 1997;72:391–9. doi: 10.4065/72.5.391. [DOI] [PubMed] [Google Scholar]
- 20.Pollack MM, Katz RW, Ruttimann UE, Getson PR. Improving the outcome and efficiency of intensive care: the impact of an intensivist. Crit Care Med. 1988;16:11–7. doi: 10.1097/00003246-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Hanson CW, 3rd, Deutschman CS, Anderson HL, 3rd, Reilly PM, Behringer EC, Schwab CW, et al. Effects of an organized critical care service on outcomes and resource utilization: a cohort study. Crit Care Med. 1999;27:270–4. doi: 10.1097/00003246-199902000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Milstein A, Galvin RS, Delbanco SF, Salber P, Buck CR., Jr Improving the safety of health care: the Leapfrog initiative. Eff Clin Pract. 2000;3:313–6. [PubMed] [Google Scholar]
- 23.Varelas PN, Conti MM, Spanaki MV, Potts E, Bradford D, Sunstrom C, et al. The impact of a neurointensivist-led team on a semiclosed neurosciences intensive care unit. Crit Care Med. 2004;32:2191–8. doi: 10.1097/01.ccm.0000146131.03578.21. [DOI] [PubMed] [Google Scholar]
- 24.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J., Jr Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284:2762–70. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 25.Dara SI, Afessa B. Intensivist-to-bed ratio: association with outcomes in the medical ICU. Chest. 2005;128:567–72. doi: 10.1378/chest.128.2.567. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs RJ, Berenholtz SM, Dorman T. Do intensivists in ICU improve outcome? Best Pract Res Clin Anaesthesiol. 2005;19:125–35. [PubMed] [Google Scholar]
- 27.Higgins TL, McGee WT, Steingrub JS, Rapoport J, Lemeshow S, Teres D. Early indicators of prolonged intensive care unit stay: impact of illness severity, physician staffing, and pre-intensive care unit length of stay. Crit Care Med. 2003;31:45–51. doi: 10.1097/00003246-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Young MP, Birkmeyer JD. Potential reduction in mortality rates using an intensivist model to manage intensive care units. Eff Clin Pract. 2000;3:284–9. [PubMed] [Google Scholar]