Abstract
Background
Hepsin is a cell surface protease that is over-expressed in more than 90% of human prostate cancer cases. The previously developed Probasin-hepsin/Large Probasin-T antigen (PB-hepsin/LPB-Tag) bigenic mouse model of prostate cancer demonstrates that hepsin promotes primary tumors that are a mixture of adenocarcinoma and neuroendocrine (NE) lesions, and metastases that are neuroendocrine in nature. However, since the majority of human prostate tumors are adenocarcinomas, the contribution of hepsin in the progression of adenocarcinoma requires further investigation.
Methods
We crossed the PB-hepsin mice with PB-Hi-myc transgenic mouse model of prostate adenocarcinoma and characterized the tumor progression in the resulting hepsin/myc bigenic mice.
Results
We report that PB-hepsin/PB-Hi-myc bigenic mice develop invasive adenocarcinoma at 4.5 months. Further, histological analysis of the 12-17 month old mice revealed that the PB-hepsin/PB-Hi-myc model develops a higher grade adenocarcinoma compared with age-matched tumors expressing only PB-Hi-myc. Consistent with targeting hepsin to the prostate, the PB-hepsin/PB-Hi-myc tumors showed higher hepsin expression as compared to the age matched myc tumors. Furthermore, endogenous expression of hepsin increased in the PB-Hi-myc mice as the tumors progressed.
Conclusions
Although we did not detect any metastases from the prostates in either the PB-hepsin/PB-Hi-myc or the PB-Hi-myc mice, our data suggests that hepsin and myc co-operate during the progression to high grade prostatic adenocarcinoma.
Keywords: adenocarcinoma, hepsin, mouse model, myc, progression, prostate
Introduction
Following the development of prostatic intraepithelial neoplasia (PIN), the natural course of progression in human prostate cancer typically involves the development of adenocarcinoma with local invasion, and the final development of distal metastases. Transgenic mouse models provide an excellent opportunity to identify genes that contribute to the development of prostate adenocarcinoma, and to elucidate the molecular basis of prostate cancer progression in vivo. However, several transgenic mouse models develop androgen independent neuroendocrine cancer, and neuroendocrine pathophysiology does not reflect the predominant androgen dependent adenocarcinoma phenotype observed in human prostate cancer (1-3). Thus, there is a need to develop and characterize novel transgenic models that better mimic prostate adenocarcinoma in humans.
Proteolytic activity associated with both secreted and cell surface proteases are thought to play a critical role in progression of cancer to an advanced stage (4,5). Cell surface proteases have been hypothesized to play a role in the cleavage of extracellular matrix proteins that are components of the basement membrane, thus allowing tumor cells to invade and metastasize (6). Several studies have shown that hepsin, a type II transmembrane serine protease (TTSP), is upregulated at both the mRNA and protein levels in more than 90% of human prostate cancers. For example, one study reported that hepsin is up-regulated by 34 fold in Gleason grades 4 and 5. (7,8). Hepsin levels have been correlated positively with disease aggressiveness with highest hepsin expression levels present in tumors of Gleason grade 4/5 (9). This suggests a role for hepsin in aggressive prostate cancers. A transgenic mouse model over-expressing hepsin in the prostate was created by using the prostate-specific probasin promoter (1). Prostates in these mice showed normal cell proliferation and differentiation but the basement membrane showed disorganization (1). Further, these hepsin transgenic mice when crossed with the LPB-Tag 12T-7f model showed significant tumor progression and metastases to the bone making it the only mouse model to develop bone metastases (1). This study indicated that hepsin plays a key role in the progression of prostate cancer to a metastatic phenotype, consistent with the high levels of hepsin found in patients with advanced prostate cancer. However, these bigenic mice developed adenocarcinoma and neuroendocrine tumors at the primary site but the metastatic lesions are NE cancer, a rare type of human prostate cancer.
To determine the role of hepsin in the progression of adenocarcinoma of the prostate, we crossed the PB-hepsin mice with PB-Hi-myc mice, the probasin directed myc mouse model of prostate adenocarcinoma. The PB-Hi-myc model (hereafter referred as myc mice) expresses high levels of myc and develops adenocarcinoma by 6 months of age (10). Herein we provide evidence that over-expression of hepsin in the myc tumors decreases the time required for development of adenocarcinoma from 6 months to 4.5 months. Further, with aging, the PB-hepsin/PB-Hi myc mice (hereafter referred to as hepsin/myc mice) developed a pathologically higher grade of tumor as compared to the tumors of the age-matched myc transgenic mice. In short, our data confirms that hepsin co-operates with myc in the progression of adenocarcinoma in a mouse model of prostate cancer and underscores the relevance of hepsin during prostate cancer progression indicating that enzymatic inhibition of hepsin activity may have therapeutic benefit.
Materials and Methods
Generation of hepsin/myc mice
Mice were housed in the animal care facility at Vanderbilt University Medical Center in accordance with the National Institutes of Health (NIH) and institutional guidelines for laboratory animals. The PB-Hi-myc and PB-hepsin mouse models have been described elsewhere (1,10). These models were generated by utilizing the probasin promoter (ARR2PB) to target the myc and hepsin genes, respectively, to the mouse prostate. The ARR2PB sequence consists of the original probasin sequence PB (-286/+28) combined with an additional androgen response region (11). PB-hepsin and PB-Hi-myc mice were maintained in CF7BL/6J and FVB backgrounds respectively. All the mice were F1 offspring so the PB-Hi-myc mice were age matched and genetically matched to the hepsin/myc mice, therefore the Pb-Hi-myc and hepsin/myc bigenic mice were 50% C57BL/6J and 50% FVB. Mice were analyzed starting at 3 months of age.
Tissue preparation and histopathologic analysis
Mice were sacrificed using the cervical dislocation technique following inhalation of an anesthetic agent in accordance with the guidelines of Vanderbilt University Animal Care committee. Generally, the prostates were dissected into four different lobes (dorsal, ventral, lateral and anterior) with the aid of a dissecting microscope. In some instances it was not possible to separate the dorsal and lateral lobes, then tissues were taken together as the dorsolateral lobe. Liver, lungs, kidney, spleen, seminal vesicles, epididymis, bladder, femur bone, jaw bone and inguinal lymph nodes were also harvested for histologic examination. Tissues were fixed in 10% formalin and processed and embedded using standard techniques. Paraffin embedded tissue was cut at 5 μm sections and were used for H&E staining and immunohistochemistry. Histology was classified in a blinded manner by a pathologist (MW) based on the definitions from the Prostate Pathology committee of the National Cancer Institute Mouse Models of Human Cancer Consortium (12). Generally 3-4 sections were reviewed per specimen.
Immunohistochemistry
Immunohistochemistry was performed on 5 μm thick paraffin sections following deparaffinization and rehydration using standard techniques. For Foxa1, AR and Myc immunostaining, antigen retrieval was achieved by microwaving in 1 M urea for 30 minutes (min) and the slides were then cooled to room temperature for 1 hour (h). Endogenous peroxidase activity was blocked by Dako peroxidase blocking reagent (Dako, CA, USA) 30 min followed by washing in PBS (pH 7.4). After rinsing with PBS, the slides were placed in blocking solution (goat or horse serum) for 20 min to block nonspecific binding of antibody to the tissues or cells. Sections were incubated with primary antibody overnight at 4°C. The following primary antibodies were used (with the indicated dilutions in PBS): AR N-20 Santa Cruz Biotechnology Inc., sc-816 (1:1000); Foxa1 C-20 sc-6553 (1:1000), Hepsin Cayman Chemical cat# 100022 (1 μg/ml) and Myc N terminal antibody Epitomics cat #1472-1 (13) (dilution 1:1000). Staining was visualized using Vectastain ABC kit (Vector Laboratories Inc, Burlingame, CA, USA) and 3,3′-diaminobenzidine tetrahydrochloride (Dako). Slides were counterstained with hematoxylin, dehydrated, and cover-slipped.
Quantitative real-time reverse transcriptase polymerase chain reaction (q RT-PCR)
RNA was extracted from tissue samples fixed in RNA later using the RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol including treatment with DNase. RNA concentration was measured using a spectrophotometer and RNA quality was assessed by agarose gel electrophoresis. Reverse transcription and quantitative real-time q RT-PCR used Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and iQ SYBR green supermix (Biorad, Hercules, CA, USA) respectively. Relative quantitation of hepsin was performed by ΔΔct method normalized to 18s r RNA. The hepsin primers were generated based on the mouse sequence of hepsin. The forward hepsin primer (5′ CTCTAGCTCCCTGCCTCTCA 3′) and reverse hepsin primer (3′ CGTTGCTTATGATGGGAACC 5′) generated a 170 bp amplimer. 18S forward primer used was (5′ CAAGAACGAAAGTCGGAGGTTC) and Reverse (5′ GGACATCTAAGGGCATCACAG generated a 488 bp amplimer.
Statistical analysis
Statistical analysis was performed using SPSS version 17 (Chicago, IL). The proportions of negative outcomes (formation of adenocarcinoma) for both control (myc) and test (hepsin/myc) groups were compared using Fisher exact tests, with p values < 0.05 taken as significant.
Results
Characterization of hepsin/myc bigenic mice
Since both myc and hepsin genes are over-expressed in human prostate tumors (14-24), it was essential that we specifically drove expression of these transgenes to the luminal epithelial cells of the prostate in a temporal manner. Immunohistochemical analyses revealed the presence of myc expression in the epithelial cells of the hepsin/myc and myc mice (Fig. 1, Panels A through F). Immunohistochemical analyses revealed that hepsin expression was low in the myc transgenic mice and, as expected, was higher in the hepsin/myc mice at all ages (Fig. 1, Panels G through L). Notably, the myc transgenic mice spontaneously developed a low level of endogenous hepsin that increased as the myc tumors progressed, consistent with the elevated levels of hepsin found in advanced human prostate cancer (7,8). Our results indicate that forced hepsin and myc transgene expression was specifically targeted to the epithelial compartment of bigenic mice and that as the transgenic myc mouse ages, hepsin is spontaneously expressed.
Fig. 1. Immunohistochemical staining of myc and hepsin in the transgenic animals showing progression from 12 to 17 months.

(Panels A & G) Myc staining and Hepsin staining respectively of 12 month old myc mice; (Panels D & J) Myc staining and Hepsin staining respectively of 12 month old hepsin/myc mice; (Panels B & H) Myc staining and Hepsin staining respectively of 15 month old myc mice; (Panels E & K) Myc staining and Hepsin staining respectively of 15 month old hepsin/myc mice; (Panels C & I) Myc staining and Hepsin staining respectively of 17 month old myc mice; (Panels F & L) Myc staining and Hepsin staining respectively of 17 month old hepsin/myc mice
Histological examination of prostates from the myc and hepsin/myc mice showed progressive tumor development with age (Figs. 2 and 3; Supplementary Table 1). The prostates were characterized by a pathologist in a blinded manner as either containing no histologic abnormality, low grade prostatic intraepithelial neoplasia (LGPIN), high grade prostatic intraepithelial neoplasia (HGPIN) or adenocarcinoma with various grades (Figs. 2 and 3; Supplementary Table 1). A detailed mouse-by-mouse description of the histopathological findings has been provided in Supplementary Table 1. LGPIN and HGPIN were described by crowding of epithelial cells within a gland but still bound by the basement membrane combined with cytologic abnormalities such as nuclear enlargement. HGPIN (Fig 2, Panel B) differed from LGPIN (Fig 2, Panels A & D) based on pronouncement of these features including nuclear atypia, increased number of heterochromatic nuclei and higher mitotic rates.. Adenocarcinoma was characterized by invasive lesions that lacked glandular prostate differentiation and absence of a clear basement membrane contour (Fig 2, Panels C, E &F; Fig 3, Panels A through F; Supplementary Table 1). This data indicates that hepsin/myc bigenic mice develop histopathological hallmarks associated with adenocarcinoma.
Fig. 2. Histopathology (H&E) of transgenic animals showing progression from 3 to 6 months.
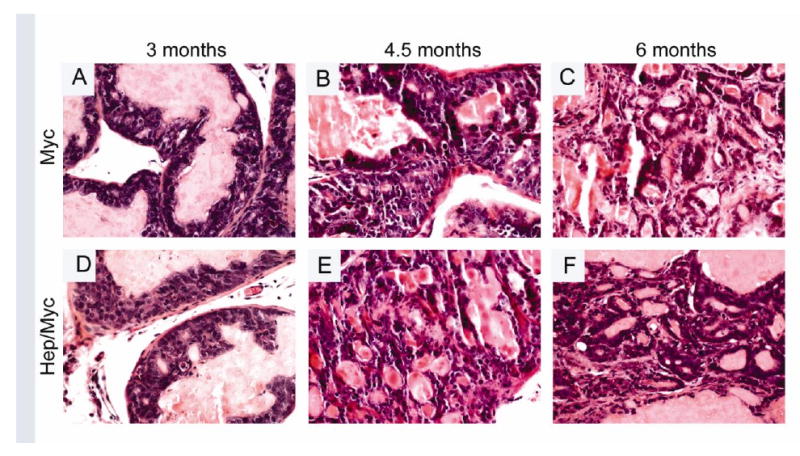
Panels A & D showing LGPIN in 3 month old myc and hepsin/myc mice respectively; Panel B showing HGPIN & Panel E showing adenocarcinoma in 4.5 month old myc and hepsin/myc mice respectively; Panel C & F showing adenocarcinoma in 6 month old myc and hepsin/myc mice respectively.
Fig. 3. Histopathology (H&E) of transgenic animals showing progression of adenocarcinoma from 12 to 17 months.
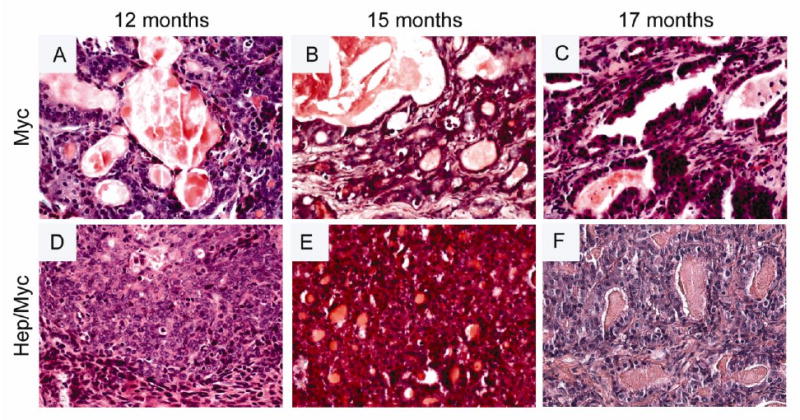
(Panels A & D) H&E of 12 month old myc and hepsin/myc mice respectively; (Panels B & E) H&E of 15 month old myc and hepsin/myc mice respectively; (Panels C & F) H&E of 17 month old myc and hepsin/myc mice respectively.
Hepsin/myc bigenic mice display accelerated tumor progression
The PB-hepsin transgenic mice when crossed with the LPB-Tag mice has been shown to display significant progression from HGPIN to adenocarcinoma and NE cancers (1). Further, these NE cancers metastasized to the liver, lungs, and bone (1). Our model tested if the overexpression of hepsin causes progression of adenocarcinoma in the myc model. Since adenocarcinoma develops first in the dorsolateral prostate of the myc mouse, the histology of these lobes was carefully examined in the hepsin/myc bigenic mice. We found that at 3 months, both myc and hepsin/myc mice displayed PIN lesions (Fig. 2, Panels A & D; Supplementary Table 1). But notably, in contrast to the myc mice, the hepsin/myc mice displayed adenocarcinoma at 4.5 months (5 out of 6 mice) since all the PIN lesions had advanced to adenocarcinoma (Fig 2, Panel E; Table 1; Supplementary Table 1) whereas none of the myc mice displayed adenocarcinoma (0 out of 6 mice) but instead developed HGPIN (Fig. 2, Panel B; Table 1; Supplementary Table1; p=0.015). At 6 months, both hepsin/myc and myc mice displayed invasive adenocarcinoma (Fig. 2, Panels C & F; Table1). Since Foxa1 is an established marker to detect prostate luminal epithelial cells, invasion of the tumor cells into the surrounding stroma can be visualized by Foxa1 staining. (Fig. 4, Panels A through F; Fig. 5, Panels A through F). It has been previously shown that hepsin causes significant progression of prostate cancer in the LPB-Tag model including metastases to the bone (1). These tumors were shown to express neuroendocrine markers including synaptophysin (1). In our model of the hepsin/myc mouse, neuroendocrine differentiation, as detected by synaptophysin staining was negative (Supplementary Fig. 1). Further, to check the status of the androgen receptor in the tumors, we performed immunohistochemistry with androgen receptor (Fig. 4, Panels G through L; Fig. 5, Panels G through L). Both myc and hepsin/myc tumors at all ages stained positive for the androgen receptor. This data indicates that when compared to myc transgenic mice, bigenic hepsin/myc mice develop adenocarcinoma at an accelerated rate and that both myc and hepsin/myc tumors are adenocarcinomas devoid of any neuroendocrine (NE) differentiation.
Table 1.
Incidence of PIN / adenocarcinoma in Hep/Myc and Myc mice with age progression
| Age (months) | No. of mice | PIN / Adenocarcinoma / | Higher grade (if applicable) | ||
|---|---|---|---|---|---|
| 4.5 | Myc | 6 | PIN in all 6 mice | ||
| Hep/Myc | 6 | PIN in 1, Adenocarcinoma in 5 mice | |||
| 6-10 | Myc | 2 | Adenocarcinoma in both mice | ||
| Hep/Myc | 2 | Adenocarcinoma in both mice | |||
| 12-17 | Myc | 6 | Adenocarcinoma in all 6 mice | ||
| Hep/Myc | 6 | Adenocarc. in all 6 mice, | Higher grade in 4/6 mice compared with Mycalone | ||
Fig. 4. Immunohistochemical staining of Foxa1 and AR in the transgenic animals showing progression from 3 to 6 months.
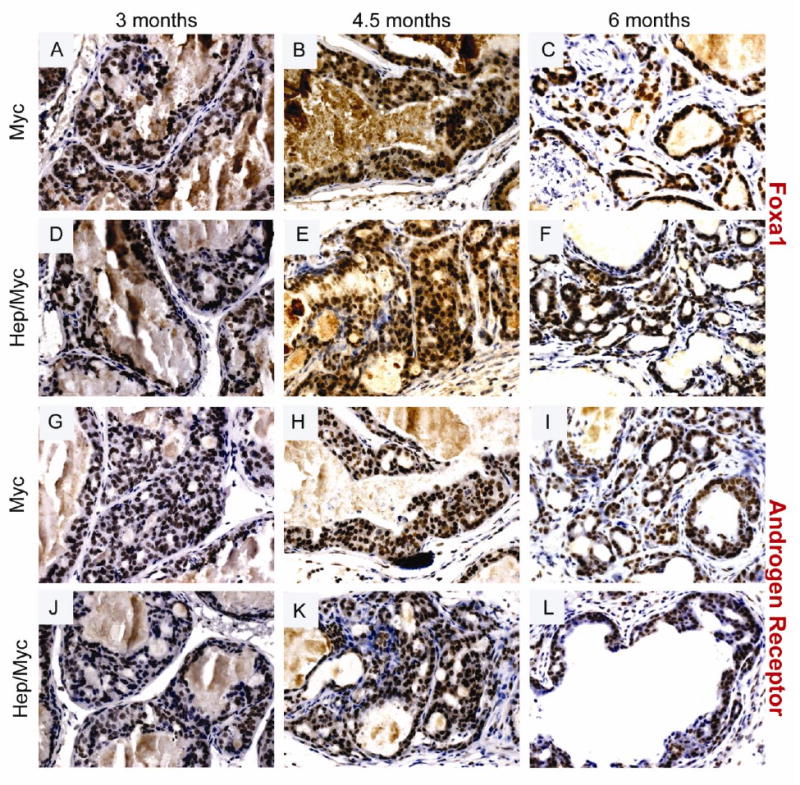
(Panels A & G) Foxa1 and AR staining respectively of 3 month old myc mice; (Panels D & J) Foxa1 and AR staining respectively of 3 month old hepsin/myc mice; (Panels B & H) Foxa1 and AR staining respectively of 4.5 month old myc mice; (Panels E & K) Foxa1 and AR staining respectively of 4.5 month old hepsin/myc mice; (Panels C & I) Foxa1 and AR staining respectively of 6 month old myc mice; (Panels F & L) Foxa1 and AR staining respectively of 6 month old hepsin/myc mice.
Fig. 5. Immunohistochemical staining of Foxa1 and AR in the transgenic animals showing progression from 12 to 17 months.
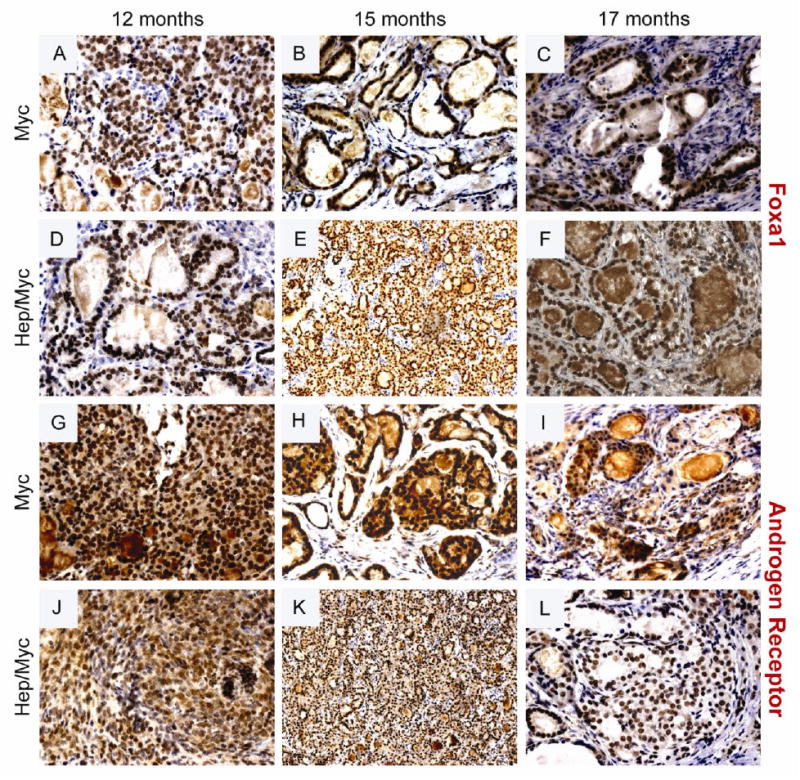
(Panels A & G) Foxa1 and AR staining respectively of 12 month old myc mice; (Panels D & J) Foxa1 and AR staining respectively of 12 month old hepsin/myc mice; (Panels B & H) Foxa1 and AR staining respectively of 15 month old myc mice; (Panels E & K) Foxa1 and AR staining respectively of 15 month old hepsin/myc mice; (Panels C & I) Foxa1 and AR staining respectively of 17 month old myc mice; (Panels F & L) Foxa1 and AR staining respectively of 17 month old hepsin/myc mice.
Prostate tumors from aged hepsin/myc mice exhibit a higher grade of adenocarcinoma compared with myc mice
In an effort to determine if increased hepsin expression in the tumors would lead to further progression, mice were aged up to 17 months. Staining with the GFP antibody [since the mouse PB-hepsin construct is tagged with GFP (1)] was used to detect potential metastasis in the lymph nodes, femur and jaw bones, kidney, lung, liver etc. No metastases were detected. Notably, hepsin/myc tumors (12-17 months) exhibited a higher grade adenocarcinoma as compared with the myc tumors (4 out of 6 mice) (Fig. 3; Table 1; Supplementary Table 1). (A detailed mouse-by-mouse description of the histopathological findings along with the grades of the respective tumors has been provided in Supplementary Table 1. The tumor size was not significantly different between hepsin/myc and myc mice. As expected, hepsin levels were significantly higher (p<0.05) in the tumors of hepsin/myc mice compared with age matched myc tumors, as measured by quantitative real-time, qRT-PCR (Fig. 6) and immunohistochemistry (Fig. 1, Panels G through L). Notably, hepsin/myc tumors displayed less stroma as compared with the myc alone tumors. To determine if tumors in the myc mice reflected the pathobiology of human adenocarcinoma, we analyzed hepsin expression of myc tumors during progression from PIN to higher grade cancer. Notably, with aging, the prostates of the myc mice begin to spontaneously express hepsin. Further, hepsin expression in the myc tumors increased significantly (p<0.05) in the 12, 15 and 17 month time points compared to 6 month prostate tumor from myc mice (Fig. 6). This change in hepsin expression also was detected by immunohistochemistry (Fig. 1, Panels G through I). This data indicates that hepsin expression in the hepsin/myc tumors causes the tumors to progress to a higher grade and that endogenous hepsin expression in the myc tumors increases with age progression.
Fig. 6. Quantitative real-time q RT-PCR of hepsin expression in hep/myc and myc mice.
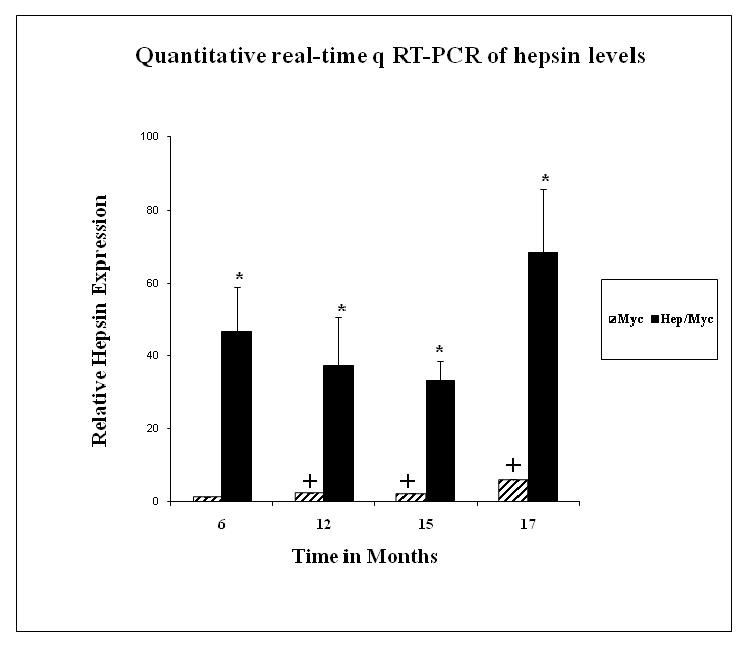
Hepsin expression in 6, 12, 15 and 17 month hep/myc and myc mice. (*) represents significant difference (p<0.05) in hepsin expression between myc and hep/myc mice. (+) represents significant difference (p<0.05) in hepsin expression between 6 month old myc mice as compared with 12, 15 and 17 month old myc mice respectively.
Discussion
Hepsin is consistently up-regulated in more than 90% of human prostate tumors, including one report which provided evidence of increases of 34-fold when compared to non-tumor controls (7,8). Additionally, hepsin expression levels have been shown to positively correlate with disease progression in human prostate cancer (9,22,25). A number of well characterized models for mouse prostate adenocarcinoma exist; however, in most of these models hepsin levels are low or nonexistent (26,27). Transgenic mice expressing hepsin under the control of the prostate specific probasin promoter do not display changes in cell proliferation or differentiation but do show a disorganization of the basement membrane (1). Further, when PB-hepsin transgenic mice are crossed with a transgenic line expressing the SV40 Large-T antigen in the prostate (LPB-Tag 12-7f line), the resulting bigenic offspring displayed dramatic acceleration in tumor progression and metastasis to the bone making it the only transgenic prostate cancer mouse model displaying reproducible bone metastasis (1). It was recently reported that in vitro, hepsin cleaves Laminin-332, a component of the basement membrane and that cleaved hepsin increased the in vitro migration of DU145 human prostate cancer cells (28). These studies indicated that hepsin plays a key role in prostate cancer progression, as opposed to the initiation of prostate tumorigenesis. However, previously developed LPB-Tag/PB-hepsin mice develop invasive adenocarcinoma as well as neuroendocrine cancer in the primary tumor. Further, the metastatic lesions in these LPB-Tag/PB-hepsin mice were neuroendocrine in nature. In human prostate cancer, the vast majority of primary tumors are adenocarcinomas (29). However, in several human prostate tumors, following androgen deprivation therapy, the adenocarcinoma progresses to more aggressive androgen independent tumors that develop varying degrees of neuroendocrine differentiation. Primary human prostate tumors that are neuroendocrine in nature are extremely rare and akin to NE cancers in other tissues are highly aggressive (30-36). Therefore, a more desirable mouse model for prostate cancer would incorporate the predominant features of human prostate cancer including reliable and faster progression to invasive adenocarcinoma, absence of neuroendocrine differentiation, and development of adenocarcinoma metastases to the bone. In order to directly test the role of increased hepsin expression in prostate cancer progression, we introduced high levels of hepsin expression into the prostate of the Hi-myc mouse model of prostate adenocarcinoma. This resulting hepsin/myc bigenic mouse model showed that the increased expression of hepsin results in earlier development of prostate adenocarcinoma, and eventually a higher grade tumor when compared to tumors derived from the myc mice.
The myc protooncogene has been shown to cause increased cell proliferation in multiple studies. Several reports have demonstrated increased myc copy number in up to 30% of human prostate tumors (14-17,37). The myc transgenic mouse model utilizing the prostate specific probasin promoter displays a reliable penetrance to HGPIN lesions and progression to invasive adenocarcinoma in 6 months (10). The myc transgenic mice develop PIN with progression to an adenocarcinoma that is devoid of any neuroendocrine differentiation. However, the myc model of prostate cancer spontaneously expresses low hepsin levels suggesting that progression of the cancer in this model is paralleling events that occur in human prostate cancer. However, these low levels of hepsin expression may not accurately reflect the physiological consequences that the widespread over-expression of hepsin causes in human prostate tumors.
To investigate the effect of hepsin over-expression in a mouse model of prostate adenocarcinoma, we generated the hepsin/myc bigenic mouse. Hepsin up-regulation in the myc model accelerates the incidence of adenocarcinoma from 6 months to 4.5 months. As mice were aged up to 17 months, the prostate tumors of hepsin/myc mice displayed a higher pathological grade of cancer when compared to age matched mice expressing myc alone in the prostate. This finding further supports an important role for increased hepsin expression and activity during progression of adenocarcinoma. Also, the tumors in our bigenic mice did not develop features of neuroendocrine differentiation and were negative for synaptophysin, a neuroendocrine marker. Further, we found that endogenous hepsin levels, although low when compared to the probasin targeted hepsin, were present in the myc mice and increased significantly (p <0.05) as the myc tumors progressed to 12, 15 and 17 months when compared with the 6 month myc tumor. This finding demonstrates that expression of hepsin in the myc tumors occurs spontaneously and reflects similar events seen during the progression of human prostate cancer.
However, unlike the LPB-Tag/PB-hepsin mice, no metastasis was detected in either myc alone or hepsin/myc bigenic mice. It has been argued that while hepsin may play an important role in the primary tumors by degrading basement membrane molecules, the low levels of hepsin in the metastatic lesions and metastasis-derived human prostate cell lines indicates a paradoxical nature of hepsin as a metastasis promoting gene. It is plausible to hypothesize that hepsin may play varying roles depending on the prostate tumor type (adenocarcinoma or neuroendocrine tumor) and also depending on its expression at specific time points in the tumor progression cascade. We speculate that the absence of metastasis in this model is linked to the relatively less aggressive adenocarcinoma that occurs in the myc mice as compared to the LPB-Tag/PB-hepsin mice that develop aggressive neuroendocrine cancers. This data suggests that hepsin expression in the tumor is not sufficient to induce metastasis from the slowly developing adenocarcinoma of the myc mouse prostate; however, hepsin expression does cause adenocarcinoma to appear earlier and tumor grade to increase at a faster rate than when myc alone is expressed in the mouse prostate.
Our hepsin/myc model develops cancer as early as 4.5 months and only the knock out (KO) of Pten in the prostate and the TRAMP models develop cancer at such an early age (38,39). However, TRAMP mice develop neuroendocrine cancer rather than adenocarcinoma (40). Both the hepsin/myc and Pten models develop primary tumors that are adenocarcinoma. However, prostates of Pten KO mice have limited adenocarcinoma cells that show neuroendocrine differentiation that further increased in castrated mice (41). Further, no neuroendocrine differentiation is seen in our hepsin/myc model.
Conclusions
In summary, our hepsin/myc bigenic model shows rapid progression to prostate adenocarcinoma and mimics multiple features of human prostate cancer progression by the over-expression of hepsin as well as myc. The use of this new bigenic model will aid in the identification of novel hepsin substrates that contribute to prostate adenocarcinoma progression and more importantly aid in the testing of compounds that inhibit hepsin activity, thus identifying compounds for prostate cancer therapy in humans.
Supplementary Material
Acknowledgments
We would like to thank Dr. David DeGraff for critical reading of this manuscript. This research was supported by NIH NCI grant R01-CA76142 and the Frances Preston Laboratories of the T.J. Martel Foundation to RJM. VV is supported by RO1-CA102365. SRN is supported by Department of Defense pre-doctoral fellowship W81XWH-07-1-0155. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sources of Support: This research was supported by NIH NCI grant R01-CA76142 and the Frances Preston Laboratories of the T.J. Martel Foundation to RM. VV is supported by RO1-CA102365. SN is supported by Department of Defense pre-doctoral fellowship W81XWH-07-1-0155.
Abbreviations
- GFP
Green Fluorescent Protein
- HGPIN
High Grade Prostatic Intraepithelial Neoplasia
- KDa
Kilo Dalton
- KO
Knockout
- LGPIN
Low Grade Prostatic Intraepithelial Neoplasia
- mRNA
Messenger RiboNucleic Acid
- NE
Neuroendocrine
- qRT-PCR
Quantitative Real Time-Polymerase Chain Reaction
- TTSP
Type II transmembrane serine protease
References
- 1.Klezovitch O, Chevillet J, Mirosevich J, Roberts RL, Matusik RJ, Vasioukhin V. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell. 2004;6(2):185–195. doi: 10.1016/j.ccr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Masumori N, Thomas TZ, Chaurand P, Case T, Paul M, Kasper S, Caprioli RM, Tsukamoto T, Shappell SB, Matusik RJ. A probasin-large T antigen transgenic mouse line develops prostate adenocarcinoma and neuroendocrine carcinoma with metastatic potential. Cancer Res. 2001;61(5):2239–2249. [PubMed] [Google Scholar]
- 3.Perez-Stable C, Altman NH, Mehta PP, Deftos LJ, Roos BA. Prostate cancer progression, metastasis, and gene expression in transgenic mice. Cancer Res. 1997;57(5):900–906. [PubMed] [Google Scholar]
- 4.Woodward JK, Holen I, Coleman RE, Buttle DJ. The roles of proteolytic enzymes in the development of tumour-induced bone disease in breast and prostate cancer. Bone. 2007;41(6):912–927. doi: 10.1016/j.bone.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Noel A, Gilles C, Bajou K, Devy L, Kebers F, Lewalle JM, Maquoi E, Munaut C, Remacle A, Foidart JM. Emerging roles for proteinases in cancer. Invasion Metastasis. 1997;17(5):221–239. [PubMed] [Google Scholar]
- 6.Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995;7(5):728–735. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 7.Landers KA, Burger MJ, Tebay MA, Purdie DM, Scells B, Samaratunga H, Lavin MF, Gardiner RA. Use of multiple biomarkers for a molecular diagnosis of prostate cancer. Int J Cancer. 2005;114(6):950–956. doi: 10.1002/ijc.20760. [DOI] [PubMed] [Google Scholar]
- 8.Stephan C, Yousef GM, Scorilas A, Jung K, Jung M, Kristiansen G, Hauptmann S, Kishi T, Nakamura T, Loening SA, Diamandis EP. Hepsin is highly over expressed in and a new candidate for a prognostic indicator in prostate cancer. J Urol. 2004;171(1):187–191. doi: 10.1097/01.ju.0000101622.74236.94. [DOI] [PubMed] [Google Scholar]
- 9.Xuan JA, Schneider D, Toy P, Lin R, Newton A, Zhu Y, Finster S, Vogel D, Mintzer B, Dinter H, Light D, Parry R, Polokoff M, Whitlow M, Wu Q, Parry G. Antibodies neutralizing hepsin protease activity do not impact cell growth but inhibit invasion of prostate and ovarian tumor cells in culture. Cancer Res. 2006;66(7):3611–3619. doi: 10.1158/0008-5472.CAN-05-2983. [DOI] [PubMed] [Google Scholar]
- 10.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4(3):223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141(12):4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 12.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64(6):2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 13.Gurel B, Iwata T, Koh CM, Jenkins RB, Lan F, Van Dang C, Hicks JL, Morgan J, Cornish TC, Sutcliffe S, Isaacs WB, Luo J, De Marzo AM. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod Pathol. 2008;21(9):1156–1167. doi: 10.1038/modpathol.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18(19):3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins RB, Qian J, Lieber MM, Bostwick DG. Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res. 1997;57(3):524–531. [PubMed] [Google Scholar]
- 16.Qian J, Jenkins RB, Bostwick DG. Detection of chromosomal anomalies and c-myc gene amplification in the cribriform pattern of prostatic intraepithelial neoplasia and carcinoma by fluorescence in situ hybridization. Mod Pathol. 1997;10(11):1113–1119. [PubMed] [Google Scholar]
- 17.Sato K, Qian J, Slezak JM, Lieber MM, Bostwick DG, Bergstralh EJ, Jenkins RB. Clinical significance of alterations of chromosome 8 in high-grade, advanced, nonmetastatic prostate carcinoma. J Natl Cancer Inst. 1999;91(18):1574–1580. doi: 10.1093/jnci/91.18.1574. [DOI] [PubMed] [Google Scholar]
- 18.Luo J, Duggan DJ, Chen Y, Sauvageot J, Ewing CM, Bittner ML, Trent JM, Isaacs WB. Human prostate cancer and benign prostatic hyperplasia: molecular dissection by gene expression profiling. Cancer Res. 2001;61(12):4683–4688. [PubMed] [Google Scholar]
- 19.Halvorsen OJ, Oyan AM, Bo TH, Olsen S, Rostad K, Haukaas SA, Bakke AM, Marzolf B, Dimitrov K, Stordrange L, Lin B, Jonassen I, Hood L, Akslen LA, Kalland KH. Gene expression profiles in prostate cancer: association with patient subgroups and tumour differentiation. Int J Oncol. 2005;26(2):329–336. [PubMed] [Google Scholar]
- 20.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412(6849):822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 21.Magee JA, Araki T, Patil S, Ehrig T, True L, Humphrey PA, Catalona WJ, Watson MA, Milbrandt J. Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res. 2001;61(15):5692–5696. [PubMed] [Google Scholar]
- 22.Chen Z, Fan Z, McNeal JE, Nolley R, Caldwell MC, Mahadevappa M, Zhang Z, Warrington JA, Stamey TA. Hepsin and maspin are inversely expressed in laser capture microdissectioned prostate cancer. J Urol. 2003;169(4):1316–1319. doi: 10.1097/01.ju.0000050648.40164.0d. [DOI] [PubMed] [Google Scholar]
- 23.Ernst T, Hergenhahn M, Kenzelmann M, Cohen CD, Bonrouhi M, Weninger A, Klaren R, Grone EF, Wiesel M, Gudemann C, Kuster J, Schott W, Staehler G, Kretzler M, Hollstein M, Grone HJ. Decrease and gain of gene expression are equally discriminatory markers for prostate carcinoma: a gene expression analysis on total and microdissected prostate tissue. Am J Pathol. 2002;160(6):2169–2180. doi: 10.1016/S0002-9440(10)61165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, Tamayo P, Renshaw AA, D'Amico AV, Richie JP, Lander ES, Loda M, Kantoff PW, Golub TR, Sellers WR. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell. 2002;1(2):203–209. doi: 10.1016/s1535-6108(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 25.Stamey TA, Warrington JA, Caldwell MC, Chen Z, Fan Z, Mahadevappa M, McNeal JE, Nolley R, Zhang Z. Molecular genetic profiling of Gleason grade 4/5 prostate cancers compared to benign prostatic hyperplasia. J Urol. 2001;166(6):2171–2177. [PubMed] [Google Scholar]
- 26.Hu Y, Ippolito JE, Garabedian EM, Humphrey PA, Gordon JI. Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice. J Biol Chem. 2002;277(46):44462–44474. doi: 10.1074/jbc.M205784200. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Parry G. Hepsin and prostate cancer. Front Biosci. 2007;12:5052–5059. doi: 10.2741/2447. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi M, Nandana S, Yamashita H, Ganesan R, Kirchhofer D, Quaranta V. Laminin-332 is a substrate for hepsin, a protease associated with prostate cancer progression. J Biol Chem. 2008;283(45):30576–30584. doi: 10.1074/jbc.M802312200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grignon DJ. Unusual subtypes of prostate cancer. Mod Pathol. 2004;17(3):316–327. doi: 10.1038/modpathol.3800052. [DOI] [PubMed] [Google Scholar]
- 30.Ghali VS, Garcia RL. Prostatic adenocarcinoma with carcinoidal features producing adrenocorticotropic syndrome. Immunohistochemical study and review of the literature. Cancer. 1984;54(6):1043–1048. doi: 10.1002/1097-0142(19840915)54:6<1043::aid-cncr2820540619>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 31.Trias I, Algaba F, Condom E, Espanol I, Segui J, Orsola I, Villavicencio H, Garcia Del Muro X. Small cell carcinoma of the urinary bladder. Presentation of 23 cases and review of 134 published cases. Eur Urol. 2001;39(1):85–90. doi: 10.1159/000052417. [DOI] [PubMed] [Google Scholar]
- 32.di Sant'Agnese PA, de Mesy Jensen KL. Somatostatin and/or somatostatin-like immunoreactive endocrine-paracrine cells in the human prostate gland. Arch Pathol Lab Med. 1984;108(9):693–696. [PubMed] [Google Scholar]
- 33.Cohen RJ, Glezerson G, Haffejee Z, Afrika D. Prostatic carcinoma: histological and immunohistological factors affecting prognosis. Br J Urol. 1990;66(4):405–410. doi: 10.1111/j.1464-410x.1990.tb14963.x. [DOI] [PubMed] [Google Scholar]
- 34.Capella C, Heitz PU, Hofler H, Solcia E, Kloppel G. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch. 1995;425(6):547–560. doi: 10.1007/BF00199342. [DOI] [PubMed] [Google Scholar]
- 35.Azumi N, Shibuya H, Ishikura M. Primary prostatic carcinoid tumor with intracytoplasmic prostatic acid phosphatase and prostate-specific antigen. Am J Surg Pathol. 1984;8(7):545–550. doi: 10.1097/00000478-198407000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Capella C, Usellini L, Buffa R, Frigerio B, Solcia E. The endocrine component of prostatic carcinomas, mixed adenocarcinoma-carcinoid tumours and non-tumour prostate. Histochemical and ultrastructural identification of the endocrine cells. Histopathology. 1981;5(2):175–192. doi: 10.1111/j.1365-2559.1981.tb01776.x. [DOI] [PubMed] [Google Scholar]
- 37.Fleming WH, Hamel A, MacDonald R, Ramsey E, Pettigrew NM, Johnston B, Dodd JG, Matusik RJ. Expression of the c-myc protooncogene in human prostatic carcinoma and benign prostatic hyperplasia. Cancer Res. 1986;46(3):1535–1538. [PubMed] [Google Scholar]
- 38.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92(8):3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 40.Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR, Balmain A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008;172(1):236–246. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao CP, Zhong C, Saribekyan G, Bading J, Park R, Conti PS, Moats R, Berns A, Shi W, Zhou Z, Nikitin AY, Roy-Burman P. Mouse models of prostate adenocarcinoma with the capacity to monitor spontaneous carcinogenesis by bioluminescence or fluorescence. Cancer Res. 2007;67(15):7525–7533. doi: 10.1158/0008-5472.CAN-07-0668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


