Abstract
Objective
Macrophages are critical components of diverse microenvironments (ME) in adulthood as well as during embryogenesis. Their role in development precludes the use of gene-targeting and knock-out approaches for studying their function. Hence, we proposed to create a macrophage-specific inducible transgenic mouse where genes can be turned on or off at will.
Methods
A transgenic mouse in which the reverse tetracycline activator (rtTA-M2) is expressed under the hCD68 promoter for macrophage-specific gene induction was developed and crossed with a second transgenic reporter mouse strain in which the gene for green fluorescent protein (GFP) is under the control of tetracycline responsive element (TRE) promoter. After doxycycline induction of the double transgenic animals (designated CD68-rtTA-tet-GFP), inducible expression of GFP was characterized by multi-color flow cytometric analysis of blood, marrow, and spleen cells and by demonstration of GFP expression in fresh frozen sections in diverse tissues.
Results
In bone marrow, inducible GFP expression was not confined to, or inclusive of, all cells expressing the classical macrophage markers such as F4/80. However, GFP expressing cells in thioglycollate-elicited peritoneal macrophages were also positive for F4/80 and monocyte macrophage specific antigen (MOMA-2). Interestingly, flow analysis also indicated little overlap between the F4/80 and CSF-1R positive populations. Fresh frozen samples of tissues known to contain macrophages revealed GFP-expressing cells with variable morphologies.
Conclusion
Our results show that the hCD68 promoter directs gene expression in a macrophage population distinct from that defined by classical monocyte-macrophage markers or promoters. Whether this population is functionally distinct remains to be established.
Keywords: macrophage, monocyte, CD68 promoter, tetracycline, transgenic, F4/80, CSF-1R, MOMA-2
INTRODUCTION
Monocytes and macrophages are hematopoietic in origin but found in diverse tissues where they are presumed to have homed from the circulation. Apart from their role in antigen presentation and “non-specific” immune responses, they are hypothesized to contribute to the regulation of normal as well as tumor microenvironments (ME). Their precise role in ME regulation has been difficult to define given the complexity of these tissue systems [1]. Standard approaches using knock-out mice to define the macrophage role have proven difficult since these cells have a sufficiently important role in embryogenesis to render the knock-outs embryonic lethals. Fortunately after Bujard et al. adapted the prokaryotic tetracycline operon for use in eukaryotes, investigators developed a variety of in vitro and in vivo models in which inducible and reversible control of gene expression is possible [2], thereby overcoming several limitations of traditional knock-out mice.
Combining inducible systems of gene expression with tissue-specific promoters has generated powerful transgenic mouse models where spatial and temporal regulation of specific gene expression can be attained [3]. Several promoter elements have been described as restricting gene expression to the monocyte-macrophage lineage. These include the promoters for c-fms (CSF-1R) [4], CD11b [5], Scavenger Receptor-A (SR-A) [6], the human lysozyme [7], and more recently, human CD68 or macrosialin, a scavenger receptor [8-13]. In transgenic mice, the human CD68 promoter (2.9 kb genomic fragment upstream of the start site along with the 83 bp first intron) has been shown to direct gene expression in a macrophage-specific fashion that differs from the tissue distribution specified by the murine CD68 promoter. Unlike the well-described macrophage-specific promoter, c-fms, which is active in placental trophoblasts) [4], the human CD68 (hCD68) promoter appears to restrict gene expression to mature macrophages.
In this report, we describe a macrophage-specific inducible transgenic mouse using the hCD68 promoter to drive GFP. The results show that the CD68 promoter has a distinct distribution compared to other reported macrophage-specific promoters. CD68 overlaps, but is not inclusive, with other commonly used macrophage markers such as F4/80 and MOMA-2. Whether this subset of myeloid cells is functionally distinct from those defined by the above antigens remains to be determined.
MATERIALS AND METHODS
The CD68-rtTA-M2 construct was made by cloning the modified reverse tetracycline activator rtTA-M2 downstream of the hCD68 promoter. The 4.3 Kb fragment containing the promoter, rtTA-M2 and bovine growth hormone poly A tail (Figure 1A) was purified and microinjected into the male pronuclei of two-cell zygotes and implanted in pseudo-pregnant recipient females; the resultant litter were analyzed for integration of the transgene by Southern blotting and PCR. Out of four lines positive for the transgene, one was chosen for further characterization.
Figure 1.
A. The hCD68-rtTA-M2 construct. The 4.3 kbp construct includes the 2.9 Kbp genomic DNA immediately upstream of the start site, followed by the 83 bp fist intron (IVIS), the rtTA-M2, and the bovine growth hormone poly A tail.
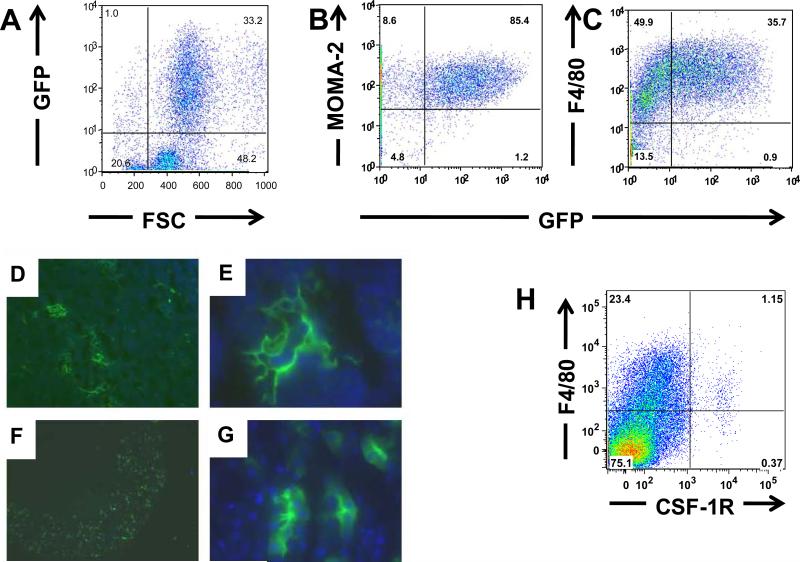
B. Demonstration of GFP in hematopoietic tissue of double transgenic animals induced with doxycycline (induced) or without doxycycline (control). BM – Bone Marrow, PB-Peripheral Blood, SPLN- Spleen. About 10% of all bone marrow cells were positive for GFP, while only 0.2 % of cells were positive in peripheral blood and 1% in spleen. Negligible numbers of cells were GFP-positive in the control animals. When GFP-positive cells were superimposed (green) onto FSC-SSC contour plots, they distributed to a pattern expected of monocytes-macrophages.
Double transgenic mice were created by crossing the CD68-rtTA line with the tet-SNARE/GFP mice (Philip Haydon, University of Pennsylvania) [14], double transgenic animals were identified by PCR for both rtTA and GFP. Adult animals 6-8 weeks in age were induced by doxycycline in the feed for 3-5 days before harvesting tissues for functional analysis of induction of GFP by flow cytometry and frozen sections of fresh tissue. Flow cytometry was performed on peripheral blood, bone marrow spleen and thioglycollate-elicited peritoneal macrophages with the following antibodies: APC conjugated anti-F4/80 conjugated to APC; PE-conjugated anti-CSF-1R(CD115 or c-fms) anti-CD68, anti-CD206 and anti-MOMA-2 (all Abd Serotec, Oxford, UK); and PE-Cy7 conjugated to anti-Gr-1, CD11b, Ter-119, B220 and Thy1.2 (all Ebiosciences, San Diego, CA) and appropriate isotype controls. Fixation and permeabilization when required was attained by use of Cytofix-Cytoperm kit from BD Biosciences (San Jose, CA) per manufacturer's instructions. For MOMA-2 staining, fixation time was reduced to 5 minutes to preserve GFP fluorescence. Sytox Blue or Vital Violet stain (Invitrogen, Carlsbad, CA) were used to distinguish between dead and live cells. Flow cytometry was performed on LSR II or FACS CALIBUR (BD Biosciences). Frozen sections of various tissues were made from lightly fixed tissue (4 % paraformaldehyde or PFA) for 2 hours frozen in sucrose and OCT compound; GFP fluorescence was demonstrated by direct visualization of 10 μm sections in a Nikon E800 microscope.
RESULTS AND DISCUSSION
From a total of 15 transgenic mice (CD68-rtTA) born after microinjection, 4 were found to have stable expression of the transgene. One line (termed CD68-MP3) was selected for further characterization based on strength of transgene expression, stability over several subsequent generations, and ease of breeding. To characterize the inducible and reversible expression of transgene in this transgenic mouse and to determine the tissue specificity of the CD68-rtTA mouse, double transgenic mice were generated by crossing CD68-MP3 with the tet-SNARE/GFP line; this line has the second component of the tetracycline system (tet O promoter driving a reporter gene GFP, along with a dominant negative transport protein SNARE (VAMP-2 or vesicle-associate membrane protein-2). This is less than ideal as the effect of dominant negative VAMP-2 on macrophage function in the short term is unknown. Nevertheless the tet-SNARE/GFP line was used as no other suitable reporter strains were commercially available.
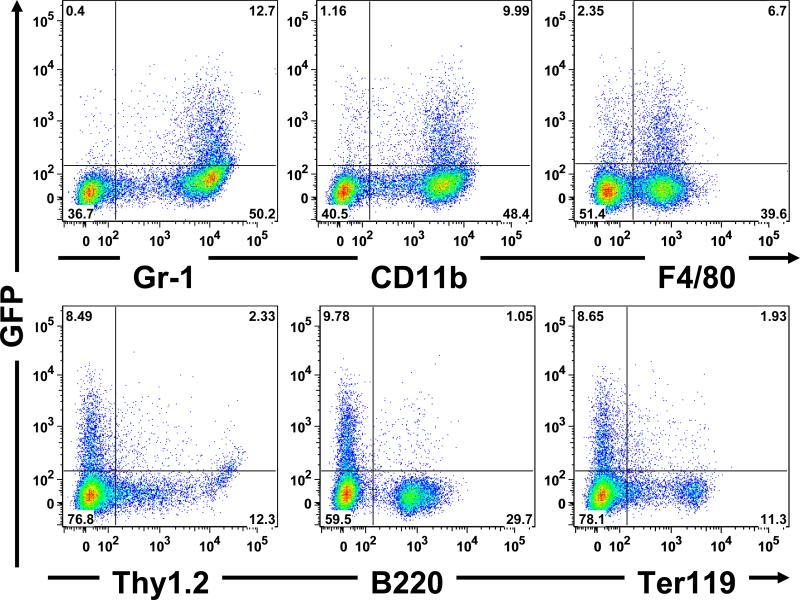
Adult double transgenic mice thus generated were induced by doxycycline and analyzed for GFP expression by flow-cytometry and microscopy of fresh frozen sections. GFP-expression in hematopoietic tissues (bone marrow, peripheral blood and spleen) in double-transgenic mice induced with doxycycline was analyzed by flow cytometry along with uninduced controls, representative results are shown in Figure 1B. GFP-positive cells were most present in the bone marrow (10%), and a smaller proportion in spleen (1%) and peripheral blood (0.2%). In comparison, the uninduced control animals had minimal GFP-positive cells, suggesting minimal leakiness and robust induction of the tet-system in the context of the double transgenic mice. GFP-positive cells appeared to have the forward and side light scattering characteristics of monocyte-macrophages. Although the hCD68 promoter along with the 83 bp first intron has been reported to be specific to macrophages when used in transgenic mice, the specificity of the promoter has not been evaluated using simultaneous labeling with hematopoietic lineage markers. As shown in Figure 2, we used lineage-specific markers for determining the lineage of the GFP-expressing cells in the bone marrow including: CD11b and GR-1(myeloid); B-220 (B-lymphoid); Thy1.2 (T-lymphoid) and Ter-119 (erythroid) and F4/80 (monocyte-macrophage). To further determine the lineage specificity of the GFP expressing cells, we analyzed GFP-expressing thioglycollate-elicited peritoneal macrophages for co-expression of F4/80 [15], and Monocyte Macrophage Antigen 2 (MOMA-2) [16]. Other macrophage specific antigens such as CD68, CSF-1R (CD115) and CD206 required prolonged fixation and permeabilization for detection and therefore could not be visualized with GFP. As shown in Figure 2A-C, elicited GFP-expressing peritoneal macrophages were almost completely contained in the F4/80 and MOMA-2 positive populations. In contrast, although the majority of the GFP-expressing cells in the bone marrow were also F4/80 positive, a distinct subpopulation was negative for F4/80 (Figure 1B). In contrast, the majority of F4/80 cells did not express GFP. These observations of distinct CD68 and F4/80 populations prompted an analysis of F4/80 and CSF-1R co-expression in wild-type cells. As shown in Figure 2H, these expression patterns also did not correlate, underscoring the problems inherent with using single markers or promoters to define cells of the monocyte-macrophage lineage [17-20].
Figure 2. Identifying the lineage of the GFP-expressing cells in bone marrow by flow-cytometry.
Gr-1 and Cd11b are markers of the myeloid lineage; the GFP-expressing cells were mostly contained in the Gr-1 and Cd11b fraction of cells. While most of the GFP-expressing cells also expressed F4/80, about 25% of GFP-expressing cells were F4/80-negative, suggesting that some mature macrophages are negative for F4/80 in hematopoietic tissue. The GFP-expressing cells were also analyzed for markers of other lineages including Thy1.2 (T-lymphocyte), B220 (B-lymphocyte) and Ter-119 (erythrocyte).
Given our interest in tissue macrophages, we next prepared fresh frozen sections from tissue samples harvested after doxycycline induction. We found that scattered GFP expressing cells with variable morphologies; representative images from thymus and kidney are shown in Figure 3D-G. Ideally, the tissue-specificity of these GFP expressing cells should be validated by staining the GFP-positive cells with a macrophage-specific antibody (as was performed by flow-cytometry). Unfortunately, this was not technically feasible by frozen sections, the fixation and permeabilization required for detecting the intracellular antigens as CD68, CSF-1R or MOMA-2 would hinder the direct visualization of vital fluorescent proteins like GFP.
Figure 3.
Panels A to C: Demonstration of GFP-expressing putative macrophages in thioglycollate elicited macrophages. These cells also express MOMA-2 (Panel B) as well as F4/80 (Panel C). Brief fixation (5 minutes) followed by permeabilization was performed prior to staining with MOMA-2. The fixation and permeabilization likely depleted the peritoneal cells of the neutrophils, and hence the proportion of GFP-expressing cells in Figures 3B (which were fixed and permeabilized for MOMA-2 staining) is higher than those shown in Figures 3A and 3C which are unfixed.
Panels D to F: Demonstration of GFP-expressing putative macrophages in freshly frozen sections. Panels D and E: Thymus sections at 10X and 60X magnification, respectively. Thymic macrophages have intricate processes surrounding other macrophages.
F and G: Kidney sections at 2X and 60X magnifications. Most of the GFP-expressing cells were found in the renal cortex and seen intercalated between renal tubules.
Panel H: F4/80 and CSF-1R expression in murine bone marrow mononuclear cells (BMMNC). Wild-type C57/Bl6 mouse were analyzed for expression of F4/80 antigen and CSF-1R by multi-color flow cytometry results indicate that only a small proportion of BMMNC (1.5%) are CSF-1R-positive, while about 25% of cells are positive for F4/80. Amongst the CSF-1R-positive cells, 25% are negative for F4/80 antigen.
However, considering that flow-cytometry of elicited peritoneal macrophages were consistent with all GFP-expressing cells being macrophages and that tissue macrophages are known to be heterogeneous in morphology, function, and surface phenotype [20,23,24], we believe these GFP-expressing cells in tissue sections are likely to be tissue macrophages.
Given that the c-fms promoter is known to be active in placental trophoblasts [4], we examined near-term fetuses (E17.5) for GFP expressing cells in the placenta. Only scattered GFP-expressing cells were found in the placenta, while hematopoietic tissue, such as liver had an impressive amount of GFP expressing cells (data not shown). Taken together, we conclude that the human CD68 promoter directs gene expression in a subset of macrophages that overlaps with, but is nevertheless distinct from that defined by F4/80 or the c-fms promoter. Macrophage-specific tetracycline-inducible transgenic mouse models utilizing the c-fms reporter [21] and the scavenger receptor A (SRA) [22] have recently been described. This growing repertoire of transgenic mouse models using different promoter elements should prove useful as complimentary models for identifying distinct subsets of macrophages and defining their roles in diverse tissue microenvironments. Given that there are clear differences in the specificity of CD68 promoter from the other macrophage-specific promoters, we feel that this tetracyclineinducible mouse model is an important addition to this repertoire.
ACKNOWLEDGMENTS
We thank Dr Herman Bujard (University of Heidleberg, Germany) for the pUHRT-62-1 plasmid, and Dr, Elaine Raines (University of Washington, Seattle, WA) for the hCD68 promoter plasmid; Dr. Philip Haydon (University of Pennsylvania, Philadelphia, PA) for the tet-SNARE transgenic mouse line; Nanyan Jiang of the transgenic core for performing the microinjections to produce the CD68-rtTA-M2 mouse; GM Venkataraman for guidance with molecular biology and cloning; and Bonnie Larson and Helen Crawford for preparing the manuscript (all at FHCRC). We would also like to acknowledge the core facilities of Animal Health Resources (AHR), Flow Cytometry, Genomics and Scientific imaging at FHCRC for their help in care of animals. Supported by the National Institutes of Health (grant K08 DK073701 to M.M.P and grants R01HL62923 and P30DK56465 to B.T-S). The core facilities used in performing the above experiments are funded by P30CA015704 (Comprehensive Cancer Center Support Grant), also from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Scientific Section: General Hematopoiesis
Conflict of Interest Disclosure: The Authors have no conflict of interest to declare.
REFERENCES
- 1.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages (Review). J. Leukoc. Biol. 2001;70:163–170. [PubMed] [Google Scholar]
- 2.Furth PA, St Onge L, Boger H, et al. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. PNAS. 1994;91:9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Zheng T, Lee CG, Homer RJ, Elias JA. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling (Review). Seminars in Cell and Developmental Biology. 2002;13:121–128. doi: 10.1016/s1084-9521(02)00018-6. [DOI] [PubMed] [Google Scholar]
- 4.Sasmono RT, Oceandy D, Pollard JW, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 5.Dziennis S, van Etten RA, Pahl HL, et al. The CD11b promoter directs high-level expression of reporter genes in macrophages in transgenic mice. Blood. 1995;85:319–329. [erratum appears in Blood 1995 Apr 1;85(7):1983].
- 6.Horvai A, Palinski W, Wu H, Moulton KS, Kalla K, Glass CK. Scavenger receptor A gene regulatory elements target gene expression to macrophages and to foam cells of atherosclerotic lesions. PNAS. 1995;92:5391–5395. doi: 10.1073/pnas.92.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke S, Greaves DR, Chung LP, Tree P, Gordon S. The human lysozyme promoter directs reporter gene expression to activated myelomonocytic cells in transgenic mice. PNAS. 1996;93:1434–1438. doi: 10.1073/pnas.93.4.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gough PJ, Gordon S, Greaves DR. The use of human CD68 transcriptional regulatory sequences to direct high-level expression of class A scavenger receptor in macrophages in vitro and in vivo. Immunology. 2001;103:351–361. doi: 10.1046/j.1365-2567.2001.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavin AL, Duong B, Skog P, et al. deltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J Immunol. 2005;175:319–328. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- 10.Groux H, Cottrez F, Rouleau M, et al. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1999;162:1723–1729. [PubMed] [Google Scholar]
- 11.Gough PJ, Raines EW. Gene therapy of apolipoprotein E-deficient mice using a novel macrophage-specific retroviral vector. Blood. 2003;101:485–491. doi: 10.1182/blood-2002-07-2131. [DOI] [PubMed] [Google Scholar]
- 12.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holness CL, da Silva RP, Fawcett J, Gordon S, Simmons DL. Macrosialin, a mouse macrophage-restricted glycoprotein, is a member of the lamp/lgp family. J Biol Chem. 1993;268:9661–9666. [PubMed] [Google Scholar]
- 14.Pascual O, Casper KB, Kubera C, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science . 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 15.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 16.Kraal G, Rep M, Janse M. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. An immunohistochemical study. Scand. J. Immunol. 1987;26:653–661. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 17.Andreesen R, Gadd S, Costabel U, et al. Human macrophage maturation and heterogeneity: restricted expression of late differentiation antigens in situ. Cell Tissue Res. 1988;253:271–279. doi: 10.1007/BF00222281. [DOI] [PubMed] [Google Scholar]
- 18.Taylor PR, Brown GD, Geldhof AB, Martinez-Pomares L, Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 2003;33:2090–2097. doi: 10.1002/eji.200324003. [DOI] [PubMed] [Google Scholar]
- 19.Noel JG, Guo X, Wells-Byrum D, Schwemberger S, Caldwell CC, Ogle CK. Effect of thermal injury on splenic myelopoiesis. Shock. 2005;23:115–122. doi: 10.1097/01.shk.0000154239.00887.18. [DOI] [PubMed] [Google Scholar]
- 20.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity (Review). Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 21.Yan C, Lian X, Li Y, et al. Macrophage-specific expression of human lysosomal acid lipase corrects inflammation and pathogenic phenotypes in lal-/- mice. Am J Pathol. 2006;169:916–926. doi: 10.2353/ajpath.2006.051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan H, Mostoslavsky G, Eruslanov E, Kotton DN, Kramnik I. Dual-promoter lentiviral system allows inducible expression of noxious proteins in macrophages. J. Immunol. Methods. 2008;329:31–44. doi: 10.1016/j.jim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forster O, Landy M. Heterogeneity of mononuclear phagocytes. Academic Press; NewYork: 1981. [Google Scholar]
- 24.Hirsch S, Gordon S. The use and limitation of monoclonal antibodies against mononuclear phagocytes. Immunobiology. Apr. 1982;161(3-4):298–307. doi: 10.1016/S0171-2985(82)80086-7. [DOI] [PubMed] [Google Scholar]