Abstract
Food intake behaviour and energy homeostasis are strongly regulated by a complex system of humoral factors and nerval structures constituting the brain-gut-axis. To date the only known peripherally produced and centrally acting peptide that stimulates food intake is ghrelin, which is mainly synthesized in the stomach. Recent data indicate that the orexigenic effect of ghrelin might be influenced by other gastrointestinal peptides such as cholecystokinin (CCK), bombesin, desacyl ghrelin, peptide YY (PYY), as well as glucagon-like peptide (GLP). Therefore, we will review on the interactions of ghrelin with several gastrointestinal factors known to be involved in appetite regulation in order to elucidate the interdependency of peripheral orexigenic and anorexigenic peptides in the control of appetite.
1. Introduction
According to the current state of knowledge, control of food intake behaviour and energy homeostasis particularly relies on the complex interactions between various humoral components indicating the actual metabolic state of the organism. As a well-established hypothesis in the context of appetite regulation, the glucostatic theory suggests an important role of metabolic substrates (e.g., blood glucose levels) for the regulation of food intake [1]. Also, the assumed modulation of food intake by signals reflecting upon energy storage [2] has been validated by the discovery of the adipose tissue hormone leptin [3].
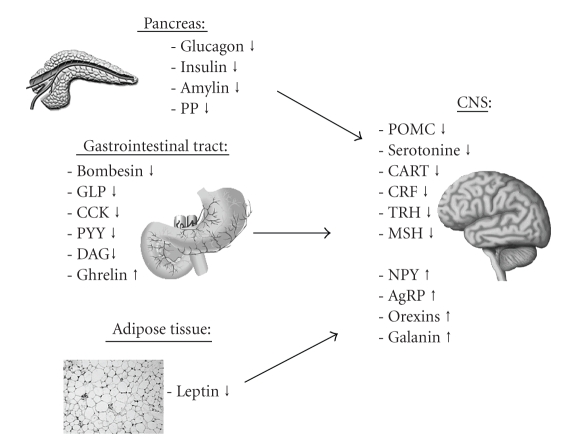
During the past decades these theories were complemented by the discovery of several additional mechanisms involved in the control of energy homeostasis. Numerous studies revealed that diverse gastrointestinal peptides are particularly responsible for the control of hunger and satiety [4]. Serving as the most important gateway connecting the endocrine with the central nervous system (CNS), the hypothalamus has been found to comprise and integrate the humorally mediated information, which reflect the metabolic state of the organism [4]. This interaction between the central nervous system and the intestinal tract by humoral factors and neuronal pathways has been named brain-gut-axis [4]. As a part of the brain-gut-axis gastrointestinal neuropeptides as cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), peptide YY (PYY), and many other humoral components are mainly involved in short-term regulation of energy homeostasis. Figure 1 provides an overview of the sites of synthesis as well as of the effects exhibited by these peripheral and central peptidergic factors responsible for the regulation of hunger and satiety.
Figure 1.
Peripheral and central peptides reducing (↓) or stimulating (↑) food intake (modified after Arora et al. [5]). AgRP: agouti-related peptide; CART: cocaine and amphetamine regulated transcript; CCK: cholecystokinin; CRF: corticotropin releasing factor; DAG: desacyl ghrelin; GLP: glucagon-like peptide; MSH: melanocyte stimulating hormone; NPY: neuropeptide Y; POMC: proopiomelanocortin; PP: pancreatic polypetide; PYY: peptide YY; TRH: thyreotropin releasing hormone.
2. Ghrelin
So far the only known peripherally produced peptide exerting a stimulating effect on food intake behaviour is ghrelin [6]. In 1999 this peptide was discovered by Kojima et al. as the first endogenous ligand of the Growth Hormone Secretagogue Receptor (GHS-R) [6]. Ghrelin is a 28 amino acid peptide, which exhibits an esterification with an octanoyl chain at the serine residue on position three as an unique modification [6]. The acylation is catalyzed by the ghrelin-O-acyltransferase (GOAT) and converts the peptide to the biologically active form [7]. Moreover, the fatty acid residue has been found to be essential for the directed transfer via the blood-brain-barrier [8].
Ghrelin is mainly produced by mucosal X/A-cells of the stomach and in much smaller shares also in the pancreas, duodenum, small intestine, and coecum as well as in the heart and aorta [6, 9, 10]. Additionally, studies indicate that also regions of the brain are involved in the ghrelin synthesis as ghrelin-containing neurons were identified in the pituitary gland as well as in the arcuate nucleus of the hypothalamus [11, 12]. Moreover, ghrelin-immunopositive neurons have been described in a hypothalamic region located nearby the third ventricle [13].
Blood ghrelin levels rise preprandially, after weight loss and in the fasted state [14, 15]. Moreover, plasma ghrelin levels have been found elevated in mammals after H. pylori infection [16] as well as in patients suffering from peptic ulcers [17]. In addition, Masaoka et al. found an increase in plasma ghrelin levels and gastric preproghrelin mRNA expression in diabetic rats, whereas gastric ghrelin levels were decreased compared to nondiabetic animals [18]. In this context, zinc supplementation significantly reduced the density of ghrelin-producing cells in the fundic mucosa in diabetic animals in comparison to untreated nondiabetic controls [19].
In addition to a significant elevation of GH-secretion [6], exogenous ghrelin strongly stimulates food intake behaviour in rodents [14, 20–24] as well as in humans [25]. Likewise, elevated endogenous plasma levels of ghrelin in patients suffering from Prader-Willi-syndrome result in distinct hyperphagia [26]. In addition to its impact in the context of energy homoestasis ghrelin is also involved in the regulation of several intestinal functions, such as gastric acid secretion [27, 28] or extraintestinal actions, which are summerized in Table 1.
Table 1.
Physiological effects of ghrelin.
| Reference | Physiological effect |
|---|---|
| Masuda 2000, Dornoville 2004 [56, 57] | Increased gastrointestinal motility |
| Masuda 2000, Date 2001 [56, 58] | Influence on gastric acid secretion |
| Broglio 2001, Dezaki 2004, Yada 2008 [59–61] | Reduction of insulin secretion |
| Nagaya 2001 [62] | Decreased blood pressure |
| Baldanzi 2002 [63] | Inhibition of apoptosis in cardiomyocytes |
| Cassoni 2001 [64] | Inhibition of proliferation in breast cancer |
| Weikel 2003 [65] | Extension of slow-wave sleep |
| Asakawa 2001, Carlini 2002 [66, 67] | Anxiogenesis and memory consolidation |
Studies suggest that the orexigenic effect of ghrelin is mediated via central mechanisms located in the arcuate nucleus (ARC) of the hypothalamus. It has been shown that intracerebroventricular (icv.) injection of ghrelin leads to a significant increase of neuronal activity within ARC as well as in the paraventricular nucleus (PVN), dorsomedial nucleus of the hypothalamus (DMH), in lateral hypothalamic areas (LHA), in the nucleus of the solitary tract (NTS), and in the area postrema (AP) [29, 30]. Interestingly, intraperitoneal (ip.) injection of ghrelin has been found to induce neuronal activity in the ARC and PVN also, but yet failed to do so in the NTS and AP [31, 32]. However, after intravenous (iv.) ghrelin injection an increase in neuronal activity in the ARC, PVN, as well as in the NTS and AP [33] or activity within ARC, NTS, and AP but not in the PVN and DMH [34] has been reported.
Although the complete central mechanism of action remains to be elucidated, it is well established that the orexigenic effect of ghrelin is mediated via central pathways involving neuropeptide Y (NPY) and agouti-related peptide (AgRP) in the ARC [21, 35–39]. Accordingly, ghrelin does not effect food intake behaviour in NPY-/AgRP-deficient mice [38]. These findings and the colocalization of NPY and ghrelin receptor GHS-R1a in neurons of the ARC suggest that NPY- and AgRP-positive neurons are a basic prerequisite for the ghrelin-induced orexigenic effect [12, 36]. However, taking into account that the GHS-R1a is widely distributed in the brain [40], many other brain regions have been also found activated after ghrelin injection [32–34, 41, 42]. Therefore, it can be assumed, that there are further—yet unknown—mechanisms mediating the various effects of ghrelin. It is furthermore noteworthy that the effects of exogenous as well as endogenous ghrelin seem to be influenced by other factors of the brain-gut-axis. Therefore, some recent studies focused on the interaction between ghrelin and other humoral factors known to regulate hunger and satiety. These findings and their impact on the role of ghrelin in the hypothalamic system of food intake behaviour and energy homeostasis will be discussed in the following.
3. Interaction between Ghrelin and Peripheral Anorexigenic Peptides
3.1. Cholecystokinin
Cholecystokinin (CCK) was the first gut hormone found to reduce food intake [43]. CCK is secreted by I-cells located in the proximal small intestine as a mixture of peptides with varying numbers of amino acids, each of which possessing the required epitope for bioactivity [44]. It is widely accepted that CCK-induced satiation is mainly mediated by binding to CCK-1 receptors located on the vagus nerve [45, 46].
As the orexigenic effect of ghrelin is also partly mediated by vagal afferents, Date et al. found that peripheral injection of CCK curbs the decreased activity of gastric vagal afferents induced by ghrelin [23]. Besides, exogenous ghrelin significantly inhibits CCK-stimulated pancreatic protein secretion—even after acute subdiaphragmatic vagotomy [47]. Furthermore, it has been shown that elevated food intake after peripheral ghrelin administration is antagonized by pre- or simultaneous injection of CCK [48, 49]. Accordingly, the markedly increased neuronal activation of the hypothalamic ARC in response to peripheral ghrelin application is diminished by pre- or coapplication of CCK [48, 50]. However, peripheral ghrelin had no effect on CCK-induced neuronal activity in the PVN and the NTS [50]. Thus, it has been hypothesized that CCK inhibits the effect of ghrelin via vagal projections to hypothalamic pathways involving the ARC [50].
Interestingly, CCK-1 and -2 receptor deficient mice display a lower response to exogenous ghrelin and lower plasma ghrelin levels after fasting as compared to their wild-type littermates [51]. Moreover, intraduodenal infusion of ghrelin has been found to increase CCK secretion [52]. However, there are conflicting data concerning the influence of CCK on ghrelin release. Two studies indicated that exogenous CCK suppresses ghrelin release in healthy subjects, whereas after ingestion of lipids CCK seems to act on CCK-1 receptors to decrease ghrelin secretion [53, 54]. In contrast, it has been shown that CCK perfusion of isolated stomachs increases ghrelin secretion by ~200% [55].
In summary, there is good evidence for the functional antagonism of ghrelin and CCK on food intake whilst the exact interplay concerning the secretion of both peptides remains to be elucidated.
3.2. Bombesin
Bombesin is an anorexigenic tetradecapeptide initially isolated from the amphibian skin of Bombina bombina [68]. Since initial discovery, several mammalian bombesin-like peptides with structural homology to bombesin, such as gastrin-releasing peptide, neuromedin B, and neuromedin C, have been described [69]. Peripheral as well as central injection of bombesin reduces food intake mediated by bombesin receptors (BB1 and BB2) which are widely spread in the gastrointestinal tract as well as in the central nervous system [69–71]. Within the CNS, in particular the nucleus of the solitary tract of the brainstem has been shown to play a crucial role in the mediation of the anorexigenic effect of bombesin [72].
Concerning a possible interaction with ghrelin, evidence has been provided that coinjection of bombesin inhibits the orexigenic effect of intraperitoneal ghrelin [73]. In addition, simultaneous injection of bombesin and ghrelin significantly increased neuronal activity of CRF-immunoreactive neurons in the PVN compared to vehicle and to single ghrelin application while it did not alter ghrelin-induced neuronal activity in the ARC [73]. Therefore, it can be assumed that peripheral bombesin inhibits ghrelin-induced food intake and increases activation of CRF neurons in the PVN [73].
In addition, in goldfish (Carassius auratus) peripheral injection of bombesin diminished ghrelin expression levels in the gut [74]. Furthermore, while exhibiting opposing effects on food intake, application of exogenous bombesin and ghrelin both stimulated growth hormone release. However, the two peptides exerted different effects on somatostatin production, whereas peripheral ghrelin blocks the effects of bombesin on synthesis of the somatostatin mRNA [74]. Thus, the interactions between bombesin and ghrelin might account for postprandial variations found in serum GH levels and the forebrain expression of somatostatin mRNA [74].
In summary, bombesin directly interferes with sundry effects of ghrelin, most likely via central mechanisms.
3.3. Desacyl Ghrelin
The gastrointestinal peptide desacyl ghrelin (DAG) displays the identical amino acid sequence as ghrelin, however lacking the fatty acid residue [6]. Therefore, DAG—in contrast to ghrelin—does not interact with the GHS-R1a and thus was initially considered to be a degradation product of ghrelin without any biological effect [6]. However, recent literature indicates numerous actions of DAG (e.g., concerning cell proliferation and adipogenesis) [63, 64, 75–77]. In this context, it was found that transgenic mice over-expressing DAG showed a reduced food intake and a lower body weight compared to wild-type mice suggesting a role in the regulation of energy homeostasis [78, 79]. Also, exogenous DAG led to a significantly reduced cumulative body weight gain in adult male rats after one week of chronic infusion [80].
In addition, there is inconsistent data concerning a potentially anorexigenic effect of exogenous DAG [41, 79, 81, 82] that might be mediated by central pathways involving Urocortin and Cocaine and Amphetamine Regulated Transcript (CART) in the hypothalamic ARC and PVN [8, 41, 79]. However, data remain inconclusive.
Concerning a possible interaction between DAG and ghrelin, DAG was found to abrogate the metabolic effects of ghrelin after coadministration of both peptides [83]. More precisely, in rodents as well as in goldfish intraperitoneally administered ghrelin significantly increased food intake whereas simultaneously injected DAG abolished the stimulatory effect of ghrelin on feeding behaviour [83, 84]. Accordingly, the effect on neuronal activity in the ARC induced by ghrelin was significantly reduced when injected simultaneously with DAG [83]. As nesfatin-1 immunoreactive neurons in the ventromedial part of the ARC were activated by simultaneous injection of ghrelin and DAG, one might speculate that DAG suppresses ghrelin-induced food intake by curbing ghrelin-induced increased neuronal activity in the ARC and recruiting nesfatin-1 immunoreactive neurons [83].
Moreover, there is evidence indicating that DAG may counteract the role of ghrelin in the control of glucose metabolism. In humans exogenous ghrelin induced rapid changes in blood glucose and insulin levels, whereas DAG prevented the acylated ghrelin-induced effect when coadministered with acylated ghrelin [85, 86]. Furthermore, Gauna et al. found that glucose output by primary hepatocytes is time- and dose-dependently increased by incubation with ghrelin whilst this effect is counteracted by DAG coincubation [87]. Additionally, ghrelin-decreased insulin sensitivity has been reported to be prevented by intravenous coinjection of DAG [86, 88]. Besides interference with insulin secretion, in vitro DAG also abolished the effect of ghrelin on glucagon, pancreatic polypeptide, and somatostatin release [89].
Therefore, it can be summarized that DAG counteracts the effect of ghrelin on food intake, hypothalamic neuronal activation, glucagon, as well as on pancreatic polypeptide and somatostatin release. Furthermore, also opposing effects of DAG have been found on the effects of ghrelin covering insulin levels, sensitivity to insulin, as well as on blood glucose concentration.
3.4. Peptide YY
As a member of the pancreatic polypeptide family, peptide YY (PYY) is postprandially released from L-cells located in the distal gastrointestinal tract and has been reported to inhibit food intake via NPY-2 receptors expressed by neurons of the ARC [90, 91]. In addition to neurons of the ARC also vagal afferents projecting to the NTS have been found to be involved in the anorexigenic effect of PYY [92]. Based on the evidence that peripherally injected ghrelin acts via the N. vagus inducing neuronal activity in the ARC [24] a possible interaction of both peptides may be assumed theoretically.
However, recent data are conflicting as one study showed PYY infusion to significantly reduces plasma ghrelin levels in humans [93] while other reports failed to find an influence on ghrelin concentrations in mice [94] and pigs [95]. Furthermore, in mice the anorexigenic effect of intraperitoneal PYY injection has not been found to be regulated by prevailing endogenous plasma ghrelin concentrations or coinjection of ghrelin [94]. However, in contrast Chelikani et al. reported peripheral ghrelin injections in rats to attenuate PYY-induced inhibition of food intake and gastric emptying [96]. In support of these results, Riediger et al. observed in rats that subcutaneous PYY directly inhibited ghrelin-activated neurons of the ARC [97].
Taken together, available data remain inconclusive concerning the interactions of ghrelin and PYY with a need for further investigation.
3.5. Glucagon-Like Peptide
The 31 amino acid hormone glucagon-like peptide (GLP) belongs to the incretins and is postprandially secreted by L-cells in the ileum [98, 99]. The peptide has been found to significantly reduce energy intake, gastric emptying rate, and energy consumption in humans [100].
In the context of interaction, it has been shown that icv. injection of GLP-1 significantly inhibited ghrelin-induced stimulation of food intake [101]. Vice versa, also intravenous coinfusion of ghrelin has been found to significantly attenuate the GLP-1-induced reduction of food intake and its inhibitory effect on gastric emptying [96].
Moreover, it is noteworthy that GLP-1 administration has been found to prevent the initial postprandial decline in ghrelin levels, possibly due to delayed gastric emptying [102]. Furthermore, exogenous GLP-1 significantly decreased ghrelin secretion after meal ingestion in healthy man [102] as well as during vagal prestimulation in isolated rat stomachs [103]. Also, application of “the closely related peptide” GLP-2 has been reported to reduce ghrelin concentrations in humans [104]. However, Brennan et al. observed that intravenous GLP-1 injection did not exhibit any effect on ghrelin concentrations in healthy humans [53].
In conclusion, there is some evidence that GLP might diminish ghrelin-triggered effects on food intake and gastric emptying and lead to a reduction of ghrelin release.
3.6. Amylin
Amylin is an anorexigenic peptide hormone composed of 37 amino acids, which is cosecreted with insulin from pancreatic islet β-cells in response to nutrient ingestion, incretin hormones, and neural input [105, 106]. Acute as well as chronic administration of amylin has been found to reduce food intake and body weight, which is predominantly mediated by neurons located in the area postrema [107, 108].
Initially, it has been shown that coadministration of amylin did not alter ghrelin-induced hyperphagia in rats [73]. In accordance, Osto et al. observed that the anorexigenic effect of amylin injection remained unchanged by simultanous ghrelin application in rats [109]. Thus it may be hypothesised that the metabolic state—ad libitum fed [73] or fasted [109] —of the animals might determine whether effects of ghrelin or amylin are predominant.
However, in conclusion interaction between ghrelin and amylin seems to be unlikely.
3.7. Pancreatic Polypeptide
The 36 amino acid peptide pancreatic polypeptide (PP) is mainly produced by cells located in the periphery of endocrine pancreatic islets. Secretion of PP is stimulated postprandially and peripheral injection of PP in rodents as well as in humans has been shown to reduce food intake and body weight, most likely mediated via indirect effects on the hypothalamic ARC involving the area postrema [110, 111].
Arosio et al. reported that peripheral injection of ghrelin in humans leads to a significant increase of PP levels in healthy subjects but to have a variable effect on PP release in acromegalic patients [112, 113]. In contrast, Qader and colleagues observed a dose-dependent inhibitory effect of ghrelin perfusion on PP secretion of rodents' isolated islet cells [89].
Due to this conflicting data and the lack of studies investigating coinjection of both peptides the interplay between ghrelin and PP remains to be further elucidated.
3.8. Insulin
The 51 amino acid peptide insulin is produced by pancreatic beta islet cells and is commonly recognized as the most important hormone regulating glucose homeostasis. Central injection of insulin has been shown to reduce food intake as well as body weight [114], most likely mediated via insulin receptors expressed on ARC neurones [115]. High blood glucose levels increase insulin release and likewise ghrelin treatment in rats has been shown to stimulate insulin secretion from isolated pancreas tissue [116, 117] as well as in vivo [118]. In contrast, in experiments conducted by other investigators ghrelin perfusion of isolated rodents pancreas suppressed insulin release in response to glucose and other secretagogues [89, 119–121] and portal vein infusion of ghrelin inhibited the glucose-induced insulin secretion [122]. In line with these results, ghrelin administration decreased insulin serum levels in rats in vivo [59, 60, 123]. Accordingly, ghrelin infusion likewise significantly suppressed C-peptide levels in gastrectomized humans [124].
However, in growth hormone-deficient humans, peripheral ghrelin induced a rapid increase in plasma insulin levels, a stimulation of lipolysis, and a reduced peripheral insulin sensitivity [86, 125]. Interestingly, in ghrelin knockout mice the usually displayed high-fat diet-induced glucose intolerance was largely prevented [126] and also ghrelin receptor knockout mice were found to have an increased insulin sensitivity [127]. Also in ob/ob mice an improvement of the diabetic phenotype has been observed after the ablation of ghrelin [128].
Vice versa, most studies revealed an inhibitory effect of exogenous insulin on ghrelin levels in humans [129–132], rats [133, 134] as well as in isolated rat stomachs [55, 103, 135]. Moreover, Murdolo et al. observed that insulin seems to be essential for the prandial suppression of ghrelin levels in humans [136]. However, challenging these results Caixas et al. found that parenteral insulin does not influence blood levels of ghrelin in humans [137], while Toshinai and colleagues even observed increased ghrelin mRNA levels in the stomach after insulin administration [138].
Furthermore, during ghrelin infusion, insulin-dependent suppression of endogenous glucose production in mice has been reported to be less effective [88]. However, coadministration of ghrelin stimulated the insulin-induced glucose uptake in adipocytes [139]. Additionally, in hepatoma cells ghrelin has been identified to regulate downstream molecules of insulin signalling [140]. As antighrelin antibodies abolished the insulin-induced neuronal activation within the nucleus tractus solitarii of the brainstem, Solomon et al. concluded that this brain area might participate in peripheral ghrelin hunger signalling mediated by insulin [141].
Taken together, ghrelin and insulin obviously interfere in the reciprocal secretion regulation in a very complex manner.
4. Summary
Discovered in 1999, investigation of ghrelin as well as ghrelin-dependent effects and interactions is a quite novel field of research. However, during the last decade effects of ghrelin have been subject to intensive investigation. As obesity is a challenging problem worldwide, especially the orexigenic effect of ghrelin has been extensively explored. In this context, various possibilities to curb the stimulating effect on food intake behaviour have been investigated with more or less promising results [142, 143]. However, so far no substance has been identified to reliably inhibit food intake during long-term treatment. Nevertheless, it has been shown that the stimulatory effect of ghrelin on food intake is diminished by several anorexigenic peptides such as CCK, bombesin, desacyl ghrelin, PYY, insulin, and GLP but not by amylin. Some of these peptides inhibit ghrelin secretion and exert opposite effects on hypothalamic neuronal activity or gastric emptying. Thus, interaction between ghrelin and these anorexigenic gastrointestinal hormones might be an auspicious approach in the context of pharmacological obesity treatment.
Moreover, in addition to the previously introduced peptides originating from the gastrointestinal tract, also the satiety factor leptin, which is primarily synthesized in the adipose tissue, interacts with ghrelin. In this context, it has been described that leptin and ghrelin diminish each others' effects on food intake via oppositional influence on NPY-positive neurons within the ARC [37, 39]. Furthermore, as summarized in Table 2, both peptides interfere in various other ways [154, 155].
Table 2.
Interference between ghrelin and leptin.
| Reference | Interaction |
|---|---|
| Barazzoni 2003 [144] | Leptin injection reduces starvation-induced ghrelin secretion. |
| Toshinai 2001 [138] | Leptin administration increases ghrelin mRNA level in the stomach |
| Dixit 2004 [145] | Ghrelin inhibits leptin-induced cytokine expression |
| Nakazato 2001, Kim 2004 [21, 146] | Ghrelin reverses leptin-induced feeding reduction |
| Shintani 2001, Kohno 2003, Kohno 2007 [37, 39, 147] | Leptin suppresses Ghrelin-induced activation of NPY neurons within the ARC |
| Rosicka 2003, Park 2005 [148, 149] | Ghrelin and leptin levels are reversely correlated and depend on the BMI |
| Bagnasco 2002, Beretta 2002, Dube 2002, Bagnasco 2003 [150–153] | Central transgenic leptin expression elevates serum ghrelin levels |
Taken together, during the last decade many aspects of appetite regulation associated with ghrelin have been elucidated. However, the brain-gut-axis—including ghrelin as the only peripheral orexigenic peptide—is a very complex system, for which our understanding to date remains limited. Thus, we can be curious for the next decades of ghrelin and its role in appetite regulation.
Acknowledgments
This work was supported by grants from the German Research Foundation to P.Kobelt (DFG KO 3864/2-1) and from the Charité-Universitätsmedizin Berlin to P.Kobelt (UFF 09/41730 and 09/42458).
References
- 1.Mayer J. Glucostatic mechanism of regulation of food intake. The New England Journal of Medicine. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proceedings of the Royal Society of London. Series B. 1953;140:578–596. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 4.Konturek SJ, Konturek JW, Pawlik T, Brzozowki T. Brain-gut axis and its role in the control of food intake. Journal of Physiology and Pharmacology. 2004;55(1):137–154. [PubMed] [Google Scholar]
- 5.Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity—a review. Neuropeptides. 2006;40(6):375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. Journal of Pharmacology and Experimental Therapeutics. 2002;302(2):822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 9.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 10.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochemical and Biophysical Research Communications. 2000;279(3):909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 11.Korbonits M, Bustin SA, Kojima M, et al. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. Journal of Clinical Endocrinology and Metabolism. 2001;86(2):881–887. doi: 10.1210/jcem.86.2.7190. [DOI] [PubMed] [Google Scholar]
- 12.Mondal MS, Date Y, Yamaguchi H, et al. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regulatory Peptides. 2005;126(1-2):55–59. doi: 10.1016/j.regpep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 14.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 15.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Masaoka T, Hasoda H, et al. Helicobocter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut. 2004;53(2):187–194. doi: 10.1136/gut.2003.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H, Masaoka T, Nomoto Y, et al. Increased levels of plasma ghrelin in peptic ulcer disease. Alimentary Pharmacology and Therapeutics. 2006;24(supplement 4):120–126. [Google Scholar]
- 18.Masaoka T, Suzuki H, Hosoda H, et al. Enhanced plasma ghrelin levels in rats with streptozotocin-induced diabetes. FEBS Letters. 2003;541(1–3):64–68. doi: 10.1016/s0014-5793(03)00306-5. [DOI] [PubMed] [Google Scholar]
- 19.Bolkent S, Yanardag R, Bolkent S, et al. The effect of zinc supplementation on ghrelin-immunoreactive cells and lipid parameters in gastrointestinal tissue of streptozotocin-induced female diabetic rats. Molecular and Cellular Biochemistry. 2006;286(1-2):77–85. doi: 10.1007/s11010-005-9095-1. [DOI] [PubMed] [Google Scholar]
- 20.Wren AM, Small CJ, Ward HL, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141(11):4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 21.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 22.Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50(7–12):2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 23.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123(4):1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Saint-Pierre DH, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y—synthesizing neurons in mouse hypothalamic arcuate nucleus. Neuroscience Letters. 2002;325(1):47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- 25.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. Journal of Clinical Endocrinology and Metabolism. 2001;86(12):5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 26.DelParigi A, Tschop M, Heiman ML, et al. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader-Willi syndrome. Journal of Clinical Endocrinology and Metabolism. 2002;87(12):5461–5464. doi: 10.1210/jc.2002-020871. [DOI] [PubMed] [Google Scholar]
- 27.Mori M, Suzuki H, Masaoka T, et al. Intravenous ghrelin administration enhances gastric acid secretion—evaluation using wireless pH capsule. Alimentary Pharmacology and Therapeutics. 2006;24(supplement 4):96–103. [Google Scholar]
- 28.Suzuki H, Nishizawa T, Hibi T. Therapeutic strategies for functional dyspepsia and the introduction of the Rome III classification. Journal of Gastroenterology. 2006;41(6):513–523. doi: 10.1007/s00535-006-1847-5. [DOI] [PubMed] [Google Scholar]
- 29.Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. Journal of Neuroendocrinology. 2000;12(11):1047–1049. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence CB, Snape AC, Baudoin FM-H, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143(1):155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 31.Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. Journal of Neuroendocrinology. 2002;14(7):580–586. doi: 10.1046/j.1365-2826.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- 32.Ruter J, Kobelt P, Tebbe JJ, et al. Intraperitoneal injection of ghrelin induces Fos expression in the paraventricular nucleus of the hypothalamus in rats. Brain Research. 2003;991(1-2):26–33. doi: 10.1016/j.brainres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto H, Fujihara H, Kawasaki M, et al. Centrally and peripherally administered ghrelin potently inhibits water intake in rats. Endocrinology. 2007;148(4):1638–1647. doi: 10.1210/en.2006-0993. [DOI] [PubMed] [Google Scholar]
- 34.Takayama K, Johno Y, Hayashi K, Yakabi K, Tanaka T, Ro S. Expression of c-Fos protein in the brain after intravenous injection of ghrelin in rats. Neuroscience Letters. 2007;417(3):292–296. doi: 10.1016/j.neulet.2007.02.089. [DOI] [PubMed] [Google Scholar]
- 35.Asakawa A, Inui A, Kaga T, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120(2):337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 36.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50(7–12):2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 37.Shintani M, Ogawa Y, Ebihara K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50(2):227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 38.Chen HY, Trumbauer ME, Chen AS, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145(6):2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 39.Kohno D, Gao H-Z, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52(4):948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- 40.Guan X-M, Yu H, Palyha OC, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Molecular Brain Research. 1997;48(1):23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 41.Chen C-Y, Inui A, Asakawa A, et al. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129(1):8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Kobelt P, Wisser A-S, Stengel A, et al. Peripheral injection of ghrelin induces Fos expression in the dorsomedial hypothalamic nucleus in rats. Brain Research. 2008;1204:77–86. doi: 10.1016/j.brainres.2008.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. Journal of Comparative and Physiological Psychology. 1973;84(3):488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 44.Rehfeld JF, Sun G, Christensen T, Hillingso JG. The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. Journal of Clinical Endocrinology and Metabolism. 2001;86(1):251–258. doi: 10.1210/jcem.86.1.7148. [DOI] [PubMed] [Google Scholar]
- 45.MacLean DB. Abrogation of peripheral cholecystokinin-satiety in the capsaicin treated rat. Regulatory Peptides. 1985;11(4):321–333. doi: 10.1016/0167-0115(85)90204-6. [DOI] [PubMed] [Google Scholar]
- 46.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. American Journal of Physiology. 1997;272(4):R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Chen M, Chen X, Segura BJ, Mulholland MW. Inhibition of pancreatic protein secretion by ghrelin in the rat. Journal of Physiology. 2001;537(1):231–236. doi: 10.1111/j.1469-7793.2001.0231k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Date Y, Toshinai K, Koda S, et al. Peripheral interaction of hrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146(8):3518–3525. doi: 10.1210/en.2004-1240. [DOI] [PubMed] [Google Scholar]
- 49.Kobelt P, Tebbe JJ, Tjandra I, et al. CCK inhibits the orexigenic effect of peripheral ghrelin. American Journal of Physiology. 2005;288(3):R751–R758. doi: 10.1152/ajpregu.00094.2004. [DOI] [PubMed] [Google Scholar]
- 50.Kobelt P, Paulitsch S, Goebel M, et al. Peripheral injection of CCK-8S induces Fos expression in the dorsomedial hypothalamic nucleus in rats. Brain Research. 2006;1117(1):109–117. doi: 10.1016/j.brainres.2006.08.092. [DOI] [PubMed] [Google Scholar]
- 51.Sakurai C, Ohta M, Kanai S, Uematsu H, Funakoshi A, Miyasaka K. Lack of ghrelin secretion in response to fasting in cholecystokinin-A (-1), -B (-2) receptor-deficient mice. Journal of Physiological Sciences. 2006;56(6):441–447. doi: 10.2170/physiolsci.RP003306. [DOI] [PubMed] [Google Scholar]
- 52.Nawrot-Porabka K, Jaworek J, Leja-Szpak A, et al. The effect of luminal ghrelin on pancreatic enzyme secretion in the rat. Regulatory Peptides. 2007;143(1–3):56–63. doi: 10.1016/j.regpep.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Brennan IM, Otto B, Feltrin KL, Meyer JH, Horowitz M, Feinle-Bisset C. Intravenous CCK-8, but not GLP-1, suppresses ghrelin and stimulates PYY release in healthy men. Peptides. 2007;28(3):607–611. doi: 10.1016/j.peptides.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 54.Degen L, Drewe J, Piccoli F, et al. Effect of CCK-1 receptor blockade on ghrelin and PYY secretion in men. American Journal of Physiology. 2007;292(4):R1391–R1399. doi: 10.1152/ajpregu.00734.2006. [DOI] [PubMed] [Google Scholar]
- 55.Shrestha YB, Wickwire K, Giraudo SQ. Direct effects of nutrients, acetylcholine, CCK, and insulin on ghrelin release from the isolated stomachs of rats. Peptides. 2009;30(6):1187–1191. doi: 10.1016/j.peptides.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masuda Y, Tanaka T, Inomata N, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochemical and Biophysical Research Communications. 2000;276(3):905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 57.Dornonville de la Cour C, Lindstrom E, Norlen P, Hakanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regulatory Peptides. 2004;120:23–32. doi: 10.1016/j.regpep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochemical and Biophysical Research Communications. 2001;280(3):904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- 59.Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. Journal of Clinical Endocrinology and Metabolism. 2001;86(10):5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 60.Dezaki K, Hosoda H, Kakei M, et al. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: implication in the glycemic control in rodents. Diabetes. 2004;53(12):3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 61.Yada T, Dezaki K, Sone H, et al. Ghrelin regulates insulin release and glycemia: physiological role and therapeutic potential. Current Diabetes Reviews. 2008;4(1):18–23. doi: 10.2174/157339908783502352. [DOI] [PubMed] [Google Scholar]
- 62.Nagaya N, Kojima M, Uematsu M, et al. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. American Journal of Physiology. 2001;280(5):R1483–R1487. doi: 10.1152/ajpregu.2001.280.5.R1483. [DOI] [PubMed] [Google Scholar]
- 63.Baldanzi G, Filigheddu N, Cutrupi S, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. Journal of Cell Biology. 2002;159(6):1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cassoni P, Papotti M, Ghe C, et al. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. Journal of Clinical Endocrinology and Metabolism. 2001;86(4):1738–1745. doi: 10.1210/jcem.86.4.7402. [DOI] [PubMed] [Google Scholar]
- 65.Weikel JC, Wichniak A, Ising M, et al. Ghrelin promotes slow-wave sleep in humans. American Journal of Physiology. 2003;284(2):E407–E415. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]
- 66.Asakawa A, Inui A, Kaga T, et al. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74(3):143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- 67.Carlini VP, Monzon ME, Varas MM, et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochemical and Biophysical Research Communications. 2002;299(5):739–743. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- 68.Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, two analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971;27(2):166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- 69.Moody TW, Merali Z. Bombesin-like peptides and associated receptors within the brain: distribution and behavioral implications. Peptides. 2004;25(3):511–520. doi: 10.1016/j.peptides.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 70.Gibbs J, Fauser DJ, Rowe EA, Rolls BJ, Rolls ET, Maddison SP. Bombesin suppresses feeding in rats. Nature. 1979;282(5735):208–210. doi: 10.1038/282208a0. [DOI] [PubMed] [Google Scholar]
- 71.Gibbs J, Kulkosky PJ, Smith GP. Effects of peripheral and central bombesin on feeding behavior of rats. Peptides. 1981;2(supplement 2):179–183. doi: 10.1016/0196-9781(81)90028-0. [DOI] [PubMed] [Google Scholar]
- 72.Ladenheim EE, Ritter RC. Caudal hindbrain participation in the suppression of feeding by central and peripheral bombesin. American Journal of Physiology. 1993;264(6):R1229–R1234. doi: 10.1152/ajpregu.1993.264.6.R1229. [DOI] [PubMed] [Google Scholar]
- 73.Kobelt P, Goebel M, Stengel A, et al. Bombesin, but not amylin, blocks the orexigenic effect of peripheral ghrelin. American Journal of Physiology. 2006;291:R903–R913. doi: 10.1152/ajpregu.00681.2005. [DOI] [PubMed] [Google Scholar]
- 74.Canosa LF, Unniappan S, Peter RE. Periprandial changes in growth hormone release in goldfish: role of somatostatin, ghrelin, and gastrin-releasing peptide. American Journal of Physiology. 2005;289(1):R125–R133. doi: 10.1152/ajpregu.00759.2004. [DOI] [PubMed] [Google Scholar]
- 75.Bedendi I, Alloatti G, Marcantoni A, et al. Cardiac effects of ghrelin and its endogenous derivatives des-octanoyl ghrelin and des-Gln14-ghrelin. European Journal of Pharmacology. 2003;476(1-2):87–95. doi: 10.1016/s0014-2999(03)02083-1. [DOI] [PubMed] [Google Scholar]
- 76.Cassoni P, Ghe C, Marrocco T, et al. Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. European Journal of Endocrinology. 2004;150(2):173–184. doi: 10.1530/eje.0.1500173. [DOI] [PubMed] [Google Scholar]
- 77.Thompson NM, Gill DAS, Davies R, et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145(1):234–242. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- 78.Ariyasu H, Takaya K, Iwakura H, et al. Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology. 2005;146(1):355–364. doi: 10.1210/en.2004-0629. [DOI] [PubMed] [Google Scholar]
- 79.Asakawa A, Inui A, Fujimiya M, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54(1):18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martini AC, Fernandez-Fernandez R, Tovar S, et al. Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology. 2006;147(5):2374–2382. doi: 10.1210/en.2005-1422. [DOI] [PubMed] [Google Scholar]
- 81.Toshinai K, Yamaguchi H, Sun Y, et al. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147(5):2306–2314. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- 82.Neary NM, Druce MR, Small CJ, Bloom SR. Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect. Gut. 2006;55(1):p. 135. [PMC free article] [PubMed] [Google Scholar]
- 83.Inhoff T, Mönnikes H, Noetzel S, et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29(12):2159–2168. doi: 10.1016/j.peptides.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsuda K, Miura T, Kaiya H, et al. Regulation of food intake by acyl and des-acyl ghrelins in the goldfish. Peptides. 2006;27(9):2321–2325. doi: 10.1016/j.peptides.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 85.Broglio F, Gottero C, Prodam F, et al. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. Journal of Clinical Endocrinology and Metabolism. 2004;89(6):3062–3065. doi: 10.1210/jc.2003-031964. [DOI] [PubMed] [Google Scholar]
- 86.Gauna C, Meyler FM, Janssen JAMJL, et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. Journal of Clinical Endocrinology and Metabolism. 2004;89(10):5035–5042. doi: 10.1210/jc.2004-0363. [DOI] [PubMed] [Google Scholar]
- 87.Gauna C, Delhanty PJD, Hofland LJ, et al. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. Journal of Clinical Endocrinology and Metabolism. 2005;90(2):1055–1060. doi: 10.1210/jc.2004-1069. [DOI] [PubMed] [Google Scholar]
- 88.Heijboer AC, van den Hoek AM, Parlevliet ET, et al. Ghrelin differentially affects hepatic and peripheral insulin sensitivity in mice. Diabetologia. 2006;49(4):732–738. doi: 10.1007/s00125-006-0138-2. [DOI] [PubMed] [Google Scholar]
- 89.Qader SS, Hakanson R, Rehfeld JF, Lundquist I, Salehi A. Proghrelin-derived peptides influence the secretion of insulin, glucagon, pancreatic polypeptide and somatostatin: a study on isolated islets from mouse and rat pancreas. Regulatory Peptides. 2008;146(1–3):230–237. doi: 10.1016/j.regpep.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 90.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 91.Batterham RL, Bloom SR. The gut hormone peptide YY regulates appetite. Annals of the New York Academy of Sciences. 2003;994:162–168. doi: 10.1111/j.1749-6632.2003.tb03176.x. [DOI] [PubMed] [Google Scholar]
- 92.Koda S, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146(5):2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 93.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. New England Journal of Medicine. 2003;349(10):941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 94.Adams SH, Won WB, Schonhoff SE, Leiter AB, Paterniti JR., Jr. Effects of peptide YY[3-36] on short-term food intake in mice are not affected by prevailing plasma ghrelin levels. Endocrinology. 2004;145(11):4967–4975. doi: 10.1210/en.2004-0518. [DOI] [PubMed] [Google Scholar]
- 95.Ito T, Thidarmyint H, Murata T, Inoue H, Neyra RM, Kuwayama H. Effects of peripheral administration of PYY3-36 on feed intake and plasma acyl-ghrelin levels in pigs. Journal of Endocrinology. 2006;191:113–119. doi: 10.1677/joe.1.06855. [DOI] [PubMed] [Google Scholar]
- 96.Chelikani PK, Haver AC, Reidelberger RD. Ghrelin attenuates the inhibitory effects of glucagon-like peptide-1 and peptide YY(3-36) on food intake and gastric emptying in rats. Diabetes. 2006;55(11):3038–3046. doi: 10.2337/db06-0730. [DOI] [PubMed] [Google Scholar]
- 97.Riediger T, Bothe C, Becskei C, Lutz TA. Peptide YY directly inhibits ghrelin-activated neurons of the arcuate nucleus and reverses fasting-induced c-Fos expression. Neuroendocrinology. 2004;79(6):317–326. doi: 10.1159/000079842. [DOI] [PubMed] [Google Scholar]
- 98.Vaillant CR, Lund PK. Distribution of glucagon-like peptide I in canine and feline pancreas and gastrointestinal tract. Journal of Histochemistry and Cytochemistry. 1986;34(9):1117–1121. doi: 10.1177/34.9.3755450. [DOI] [PubMed] [Google Scholar]
- 99.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2(8571):1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 100.Flint A, Raben A, Rehfeld JF, Holst JJ, Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. International Journal of Obesity. 2000;24(3):288–298. doi: 10.1038/sj.ijo.0801126. [DOI] [PubMed] [Google Scholar]
- 101.Schusdziarra V, Zimmermann J-P, Erdmann J, Bader U, Schick RR. Differential inhibition of galanin- and ghrelin-induced food intake by i.c.v. GLP-1(7-36)-amide. Regulatory Peptides. 2008;147(1–3):29–32. doi: 10.1016/j.regpep.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 102.Hagemann D, Holst JJ, Gethmann A, Banasch M, Schmidt WE, Meier JJ. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regulatory Peptides. 2007;143(1–3):64–68. doi: 10.1016/j.regpep.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 103.Lippl F, Kircher F, Erdmann J, Allescher H-D, Schusdziarra V. Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regulatory Peptides. 2004;119(1-2):93–98. doi: 10.1016/j.regpep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Banasch M, Bulut K, Hagemann D, et al. Glucagon-like peptide 2 inhibits ghrelin secretion in humans. Regulatory Peptides. 2006;137(3):173–178. doi: 10.1016/j.regpep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 105.Morley JE, Flood JF. Amylin decreases food intake in mice. Peptides. 1991;12(4):865–869. doi: 10.1016/0196-9781(91)90148-i. [DOI] [PubMed] [Google Scholar]
- 106.Butler PC, Chou J, Carter WB, et al. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990;39(6):752–756. doi: 10.2337/diab.39.6.752. [DOI] [PubMed] [Google Scholar]
- 107.Lutz TA, Senn M, Althaus J, Del Prete E, Ehrensperger F, Scharrer E. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides. 1998;19(2):309–317. doi: 10.1016/s0196-9781(97)00292-1. [DOI] [PubMed] [Google Scholar]
- 108.Riediger T, Schmid HA, Lutz T, Simon E. Amylin potently activates AP neurons possibly via formation of the excitatory second messenger cGMP. American Journal of Physiology. 2001;281(6):R1833–R1843. doi: 10.1152/ajpregu.2001.281.6.R1833. [DOI] [PubMed] [Google Scholar]
- 109.Osto M, Wielinga PY, Alder B, Walser N, Lutz TA. Modulation of the satiating effect of amylin by central ghrelin, leptin and insulin. Physiology and Behavior. 2007;91(5):566–572. doi: 10.1016/j.physbeh.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 110.Track NS, McLeod RS, Mee AV. Human pancreatic polypeptide: studies of fasting and postprandial plasma concentrations. Canadian Journal of Physiology and Pharmacology. 1980;58(12):1484–1489. doi: 10.1139/y80-223. [DOI] [PubMed] [Google Scholar]
- 111.Batterham RL, Le Roux CW, Cohen MA, et al. Pancreatic polypeptide reduces appetite and food intake in humans. Journal of Clinical Endocrinology and Metabolism. 2003;88(8):3989–3992. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- 112.Arosio M, Ronchi CL, Gebbia C, Cappiello V, Beck-Peccoz P, Peracchi M. Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels. Journal of Clinical Endocrinology and Metabolism. 2003;88(2):701–704. doi: 10.1210/jc.2002-021161. [DOI] [PubMed] [Google Scholar]
- 113.Arosio M, Ronchi CL, Gebbia C, et al. Ghrelin administration affects circulating pituitary and gastro-entero-pancreatic hormones in acromegaly. European Journal of Endocrinology. 2004;150(1):27–32. doi: 10.1530/eje.0.1500027. [DOI] [PubMed] [Google Scholar]
- 114.Woods SC, Lotter EC, McKay LD, Porte D., Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282(5738):503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 115.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nature Neuroscience. 2002;5(6):566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 116.Adeghate E, Ponery AS. Ghrelin stimulates insulin secretion from the pancreas of normal and diabetic rats. Journal of Neuroendocrinology. 2002;14(7):555–560. doi: 10.1046/j.1365-2826.2002.00811.x. [DOI] [PubMed] [Google Scholar]
- 117.Date Y, Nakazato M, Hashiguchi S, et al. Ghrelin is present in pancreatic α-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51(1):124–129. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 118.Lee H-M, Wang G, Englander EW, Kojima M, Greeley GH., Jr. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143(1):185–190. doi: 10.1210/endo.143.1.8602. [DOI] [PubMed] [Google Scholar]
- 119.Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. European Journal of Endocrinology. 2002;146(2):241–244. doi: 10.1530/eje.0.1460241. [DOI] [PubMed] [Google Scholar]
- 120.Reimer MK, Pacini G, Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144(3):916–921. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- 121.Qader SS, Lundquist I, Ekelund M, Hakanson R, Salehi A. Ghrelin activates neuronal constitutive nitric oxide synthase in pancreatic islet cells while inhibiting insulin release and stimulating glucagon release. Regulatory Peptides. 2005;128(1):51–56. doi: 10.1016/j.regpep.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 122.Cui C, Ohnuma H, Daimon M, et al. Ghrelin infused into the portal vein inhibits glucose-stimulated insulin secretion in Wistar rats. Peptides. 2008;29(7):1241–1246. doi: 10.1016/j.peptides.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 123.Dezaki K, Kakei M, Yada T. Ghrelin uses Gα i2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes. 2007;56(9):2319–2327. doi: 10.2337/db07-0345. [DOI] [PubMed] [Google Scholar]
- 124.Damjanovic SS, Lalic NM, Pesko PM, et al. Acute effects of ghrelin on insulin secretion and glucose disposal rate in gastrectomized patients. Journal of Clinical Endocrinology and Metabolism. 2006;91(7):2574–2581. doi: 10.1210/jc.2005-1482. [DOI] [PubMed] [Google Scholar]
- 125.Vestergaard ET, Gormsen LC, Jessen N, et al. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes. 2008;57(12):3205–3210. doi: 10.2337/db08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dezaki K, Sone H, Koizumi M, et al. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006;55(12):3486–3493. doi: 10.2337/db06-0878. [DOI] [PubMed] [Google Scholar]
- 127.Longo KA, Charoenthongtrakul S, Giuliana DJ, et al. Improved insulin sensitivity and metabolic flexibility in ghrelin receptor knockout mice. Regulatory Peptides. 2008;150(1–3):55–61. doi: 10.1016/j.regpep.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 128.Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metabolism. 2006;3(5):379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 129.Saad MF, Bernaba B, Hwu C-M, et al. Insulin regulates plasma ghrelin concentration. Journal of Clinical Endocrinology and Metabolism. 2002;87(8):3997–4000. doi: 10.1210/jcem.87.8.8879. [DOI] [PubMed] [Google Scholar]
- 130.Flanagan DE, Evans ML, Monsod TP, et al. The influence of insulin on circulating ghrelin. American Journal of Physiology. 2003;284(2):E313–E316. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- 131.Leonetti F, Iacobellis G, Ribaudo MC, et al. Acute insulin infusion decreases plasma ghrelin levels in uncomplicated obesity. Regulatory Peptides. 2004;122(3):179–183. doi: 10.1016/j.regpep.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 132.Kim SW, Kim KW, Shin CS, et al. Acylated ghrelin secretion is acutely suppressed by oral glucose load or insulin-induced hypoglycemia independently of basal growth hormone secretion in humans. Hormone Research. 2007;67(5):211–219. doi: 10.1159/000097098. [DOI] [PubMed] [Google Scholar]
- 133.McCowen KC, Maykel JA, Bistrian BR, Ling PR. Circulating ghrelin concentrations are lowered by intravenous glucose or hyperinsulinemic euglycemic conditions in rodents. Journal of Endocrinology. 2002;175(2):R7–R11. doi: 10.1677/joe.0.175r007. [DOI] [PubMed] [Google Scholar]
- 134.Ueno M, Carvalheira JBC, Oliveira RLGS, Velloso LA, Saad MJA. Circulating ghrelin concentrations are lowered by intracerebroventricular insulin. Diabetologia. 2006;49(10):2449–2452. doi: 10.1007/s00125-006-0371-8. [DOI] [PubMed] [Google Scholar]
- 135.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regulatory Peptides. 2004;119(1-2):77–81. doi: 10.1016/j.regpep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 136.Murdolo G, Lucidi P, Di Loreto C, et al. Insulin is required for prandial ghrelin suppression in humans. Diabetes. 2003;52(12):2923–2927. doi: 10.2337/diabetes.52.12.2923. [DOI] [PubMed] [Google Scholar]
- 137.Caixas A, Bashore C, Nash W, Pi-Sunyer F, Laferrere B. Insulin, unlike food intake, does not suppress ghrelin in human subjects. Journal of Clinical Endocrinology and Metabolism. 2002;87:p. 1902. doi: 10.1210/jcem.87.4.8538. [DOI] [PubMed] [Google Scholar]
- 138.Toshinai K, Mondal MS, Nakazato M, et al. Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochemical and Biophysical Research Communications. 2001;281(5):1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 139.Patel AD, Stanley SA, Murphy KG, et al. Ghrelin stimulates insulin-induced glucose uptake in adipocytes. Regulatory Peptides. 2006;134(1):17–22. doi: 10.1016/j.regpep.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 140.Murata M, Okimura Y, Iida K, et al. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. Journal of Biological Chemistry. 2002;277(7):5667–5674. doi: 10.1074/jbc.M103898200. [DOI] [PubMed] [Google Scholar]
- 141.Solomon A, De Fanti BA, Martinez JA. The nucleus tractus solitari (NTS) participates in peripheral ghrelin glucostatic hunger signalling mediated by insulin. Neuropeptides. 2006;40(3):169–175. doi: 10.1016/j.npep.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 142.Kobelt P, Helmling S, Stengel A, et al. Anti-ghrelin Spiegelmer NOX-B11 inhibits neurostimulatory and orexigenic effects of peripheral ghrelin in rats. Gut. 2006;55(6):788–792. doi: 10.1136/gut.2004.061010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zigman JM, Elmquist JK. In search of an effective obesity treatment: a shot in the dark or a shot in the arm? Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):12961–12962. doi: 10.1073/pnas.0605959103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barazzoni R, Zanetti M, Stebel M, Biolo G, Cattin L, Guarnieri G. Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology. 2003;124(5):1188–1192. doi: 10.1016/s0016-5085(03)00281-6. [DOI] [PubMed] [Google Scholar]
- 145.Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. Journal of Clinical Investigation. 2004;114(1):57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim M-S, Namkoong C, Kim H-S, et al. Chronic central administration of ghrelin reverses the effects of leptin. International Journal of Obesity and Related Metabolic Disorders. 2004;28(10):1264–1271. doi: 10.1038/sj.ijo.0802647. [DOI] [PubMed] [Google Scholar]
- 147.Kohno D, Nakata M, Maekawa F, et al. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology. 2007;148(5):2251–2263. doi: 10.1210/en.2006-1240. [DOI] [PubMed] [Google Scholar]
- 148.Rosicka M, Krsek M, Matoulek M, et al. Serum ghrelin levels in obese patients: the relationship to serum leptin levels and soluble leptin receptors levels. Physiological Research. 2003;52(1):61–66. [PubMed] [Google Scholar]
- 149.Park HS, Lee K-U, Kim YS, Park CY. Relationships between fasting plasma ghrelin levels and metabolic parameters in children and adolescents. Metabolism. 2005;54(7):925–929. doi: 10.1016/j.metabol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 150.Bagnasco M, Dube MG, Kalra PS, Kalra SP. Evidence for the existence of distinct central appetite, energy expenditure, and ghrelin stimulation pathways as revealed by hypothalamic site-specific leptin gene therapy. Endocrinology. 2002;143(11):4409–4421. doi: 10.1210/en.2002-220505. [DOI] [PubMed] [Google Scholar]
- 151.Beretta E, Dube MG, Kalra PS, Kalra SP. Long-term suppression of weight gain, adiposity, and serum insulin by central leptin gene therapy in prepubertal rats: effects on serum ghrelin and appetite-regulating genes. Pediatric Research. 2002;52(2):189–198. doi: 10.1203/00006450-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 152.Dube MG, Beretta E, Dhillon H, Ueno N, Kalra PS, Kalra SP. Central leptin gene therapy blocks high-fat diet-induced weight gain, hyperleptinemia, and hyperinsulinemia: increase in serum ghrelin levels. Diabetes. 2002;51(6):1729–1736. doi: 10.2337/diabetes.51.6.1729. [DOI] [PubMed] [Google Scholar]
- 153.Bagnasco M, Dube MG, Katz A, Kalra PS, Kalra SP. Leptin expression in hypothalamic PVN reverses dietary obesity and hyperinsulinemia but stimulates ghrelin. Obesity Research. 2003;11(12):1463–1470. doi: 10.1038/oby.2003.196. [DOI] [PubMed] [Google Scholar]
- 154.Williams J, Mobarhan S. A critical interaction: leptin and ghrelin. Nutrition Reviews. 2003;61(11):391–393. doi: 10.1301/nr.2003.nov.391-393. [DOI] [PubMed] [Google Scholar]
- 155.Nogueiras R, Tschop MH, Zigman JM. Central nervous system regulation of energy metabolism: ghrelin versus leptin. Annals of the New York Academy of Sciences. 2008;1126:14–19. doi: 10.1196/annals.1433.054. [DOI] [PMC free article] [PubMed] [Google Scholar]