Abstract
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a secreted protein which reduces endoplasmic reticulum (ER) stress and has neurotrophic effects on dopaminergic neurons. Intracortical delivery of recombinant MANF protein protects tissue from ischemic brain injury in vivo. In this study, we examined the protective effect of adeno-associated virus serotype 7 encoding MANF in a rodent model of stroke. An AAV vector containing human MANF cDNA (AAV-MANF) was constructed and verified for expression of MANF protein. AAV-MANF or an AAV control vector was administered into three sites in the cerebral cortex of adult rats. One week after the vector injections, the right middle cerebral artery (MCA) was ligated for 60 min. Behavioral monitoring was conducted using body asymmetry analysis, neurological testing, and locomotor activity. Standard immunohistochemical and western blotting procedures were conducted to study MANF expression. Our data showed that AAV-induced MANF expression is redistributed in neurons and glia in cerebral cortex after ischemia. Pretreatment with AAV-MANF reduced the volume of cerebral infarction and facilitated behavioral recovery in stroke rats. In conclusion, our data suggest that intracortical delivery of AAV-MANF increases MANF protein production and reduces ischemic brain injury. Ischemia also caused redistribution of AAV-mediated MANF protein suggesting an injury-induced release.
Keywords: gene therapy, stroke, AAV, MANF, neurotrophic factor, Mesencephalic astrocyte-derived neurotrophic factor, ARMET, CDNF
Introduction
The pathophysiological mechanisms of acute stroke involve multiple processes including oxygen and glucose deprivation, excessive glutamate release with corresponding excitoxicity, reactive oxygen species formation, protein and lipid modifications, Ca++ dysregulation, mitochondrial dysfunction, endoplasmic reticulum (ER) stress and apoptosis. These processes lead to neurodegeneration in the ischemic area which results in disruption of neural circuits that can affect many behavioral and cognitive functions. Evidence from animal models show that neuronal rewiring and synapse strengthening occurs during recovery from ischemic brain injury (for review see/(Murphy and Corbett, 2009). Thus, three therapeutic strategies for treating ischemic brain injury are: 1) to minimize the acute molecular mechanisms related to neuronal death e.g. endoplasmic reticulum (ER) –stress, mitochondrial dysfunction, excitoxicity and apoptosis; 2) to promote functional enhancement of remaining circuitry; and 3) to increase behavioral and cognitive recovery through “neural rewiring’ by promoting new functional neural connections. These latter compensations include neural precursor proliferation and migration, axonal pathfinding, neuritic outgrowth and synaptogenesis. Neurotrophic factors have demonstrated capacity for both of these therapeutic strategies. Viral vectors have been shown to provide sustained neurotrophic factor expression throughout the brain. However, the use of viral vectors encoding neurotrophic factors has not been extensively studied in the stroke literature (for review see/(Lim, et al., 2009).
Mesencephalic astrocyte-derived neurotrophic factor (MANF) was initially characterized as a trophic factor for cultured embryonic dopaminergic neurons (Petrova, et al., 2003). MANF and its homologue, cerebral dopamine neurotrophic factor (CDNF) have been shown to promote survival and recovery of midbrain dopamine neurons (Lindholm, et al., 2007, Petrova, et al., 2003, Voutilainen, et al., 2009). MANF is endogenously expressed in neurons and in non-neuronal tissues (Lindholm, et al., 2008, Mizobuchi, et al., 2007). In the brain, the highest levels of MANF are detected in cerebral cortex, hippocampus and cerebellar Purkinje cells (Lindholm, et al., 2008). MANF expression has been shown to be upregulated by ischemia (Apostolou, et al., 2008, Lindholm, et al., 2008, Tadimalla, et al., 2008, Yu, et al.) as well as by endoplasmic reticulum (ER) - stress (Apostolou, et al., 2008, Lee, et al., 2003, Mizobuchi, et al., 2007). We have recently shown that intra- cortical delivery of recombinant MANF protein reduces cerebral infarction and ischemia-mediated apoptosis in stroke animals (Airavaara, et al., 2009). Additionally, MANF has been shown to protect cells against glucose deprivation and tunicamycin, a inhibitor of protein glycosylation that induces ER-stress (Apostolou, et al., 2008). Thus, increased MANF levels after injury may be a result of activation of endogenous protective processes against protein misfolding.
Adeno-associated viral (AAV) vectors are currently the predominant viral vector used in clinical trials for neurodegenerative diseases (Lim, et al., 2009). Although the transgene capacity of single (i.e., 4-5 kb) or double (2-3 kb) stranded (McCarty, et al., 2003, Wang, et al., 2003) AAV vectors is limited, the vector genome is amenable to relatively small cDNA sizes which makes them ideal for expressing functional neurotrophic factors such as MANF (<1 kb). The advantage of using dsAAV vectors or self-complementary vectors is higher transduction efficiency and more rapid onset of transgene expression compared to single-stranded AAV vectors (McCarty, et al., 2003, Wang, et al., 2003).
In this study, we generated a serotype 7, dsAAV vector expressing human MANF cDNA for intracortical expression of MANF in a rat model of stroke. We devised a delivery scheme to provide wide spread transduction of cortical cells in the area affected by middle cerebral artery occlusion in rats. AAV-MANF reduced infarction volume and increased post-stroke recovery of locomotor activity and neurological score. Ischemic injury caused a redistribution of MANF in the AAV-MANF treated animals which was consistent with injury-induced secretion of MANF. Overall, our findings show that AAV-mediated delivery of MANF improves the outcome following ischemic brain injury in rats.
MATERIAL AND METHODS
Animals
Adult male Sprague-Dawley rats (250-350 g, Charles River Laboratory) were maintained under a 12-h light–dark cycle. Food and water were freely available in the home cage. Experimental procedures followed the guidelines of the “Principles of Laboratory Care” (National Institutes of Health publication No. 86-23, 1996) and were approved by the NIDA Animal Care and Use Committee.
Primary cortical cell cultures
Neocortical tissue from E15 embryos of timed-pregnant Sprague-Dawley rats were used to prepare neuronal cultures as described previously with modifications (Howard, et al., 2008). Cells (6×104 viable cells/well) were plated in 96 well plates coated with poly-D lysine and placed in 37°C humidified incubator with 5.5% CO2. Cells were fed by 50% media exchange on 4th day in vitro (DIV4) with feed media (plating media without serum or glutamate). Cells were transduced with dsAAV-GFP or dsAAV-MANF on DIV6 using a multiplicity of infection (MOI) of 1-4×104. Cells were fed every 2-3 days by 50% media exchange and fixed on DIV13 for immunofluorescent detection of MANF. For hypoxia experiments, plates were placed into to hypoxia chambers (Billups-Rothenberg Inc., Del Mar, CA, USA) on DIV12 and flushed with nitrogen gas for 1 minute then sealed. Hypoxia chambers were then placed into humidified incubators for 8 hours at 37°C. Plates were removed from hypoxia chambers and cells were allowed to reoxygenate for 24 hours. Cells were fixed by treating with 4% PFA for 1 hour then returned to PBS for immunostaining.
Construction, Packaging, Purification, and Titering of AAV Vectors
Viral construction, packaging and characterization: The cDNA for eGFP was removed from dsAAV-GFP (Wang, et al., 2003) using the restriction enzymes KpnI and XbaI and replaced with the human MANF cDNA using KpnI and XbaI digestion of PCR3.1-MANF (Lindholm, et al., 2008) to create pdsAAV-MANF. The plasmid was sequence verified and transfected into HEK293 cells. Twenty-four hours after transfection, cell lysates were made and analyzed by western blot using a rabbit anti-human MANF antibody (Lindholm, et al., 2008). Viral stocks of AAV-MANF were prepared using the triple-transfection method (Howard, et al., 2008, Xiao, et al., 1998). Briefly, twenty 15 cm dishes containing HEK293 cells at 85-95% confluency were transfected by the CaCl2 method with pHelper (Stratagene, La Jolla, CA), pdsAAV-GFP or pdsAAV-MANF and a plasmid containing rep/cap genes for serotype7, pAAV7 (Gao, et al., 2002). Plasmids used for packaging AAV were generously provided by Dr. Xiao Xiao (UNC, Chapel Hill, NC). Approximately 48 hours post-transfection, cells were harvested, lysed by freeze/thaw, and purified by centrifugation on a CsCl gradient. Final samples were dialyzed in PBS containing 12.5 mM MgCl2 to ~1013vg/ml, aliquoted and stored at −80°C until use. All vectors were titered by quantitative PCR using the CMV promoter as the target sequence. Viral titers are recorded as viral genome/ml.
Intracerebral AAV injections
Animals were anesthetized with chloral hydrate (0.4 g/kg, i.p.). AAV-MANF was given intracerebrally into three cortical sites in the distribution of the middle cerebral artery (Fig. 1). The stereotaxic coordinates were AP +1.2 (site 1), −0.3 (site 2), −1.8 (site 3); ML +5.5; DV −3.5 from the skull. Two microliters of AAV-MANF or AAV-GFP (serotype 7, titer ~ 1013 vg/mL) were injected using a 10 ul Hamilton syringe with a 30 G blunt needle and the needle was lifted by 2 mm at the middle of the injection. The rate of infusion (0.5 μl/min per site) was controlled using a microprocessor controlled injector mounted to a stereotaxic frame (UMP4; World Precision Instruments, Sarasota, FL, USA). The needle was slowly removed 2 minutes after completion of each injection.
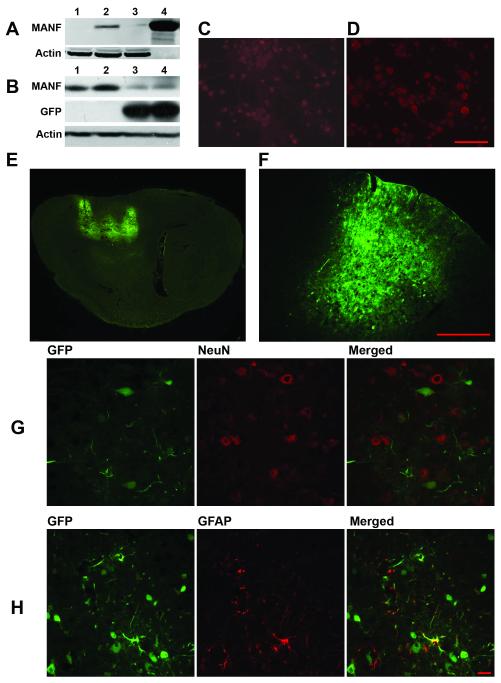
Figure 1. Characterization of AAV-MANF and AAV-GFP serotype 7 transduction in rat cortex.
(A) HEK293 cells were transfected with the AAV-MANF packaging plasmid for 24 hours and then protein extracts were analyzed by western blot (A1-untreated cells, 2-AAV-MANF, 3-AAV-GFP, 4-recombinant MANF protein, 2 ug). Rat primary cortical cultures were transduced with AAV-GFP (C) or AAV-MANF (D) virus for 48 hours then fixed and immunostained for MANF (red). (B) AAV-MANF and AAV-GFP transduction in rat cortex was verified with western blot analysis (B1 and B2 – AAV-MANF; B3 and B4 – AAV-GFP). Delivery of AAV-GFP (serotype 7) into three cortical sites produced wide-spread GFP expression at 1 week post-injection as shown in sagittal (E) and coronal (F) brain sections. AAV-induced GFP expression is detected in NeuN+(G, neuronal marker) and GFAP+ (H, glial marker) cells. Scale bars: C,D = 100μm, F = 500μm, G,H = 10μm.
Immunofluorescent staining
Seven days after viral injections, rats were perfused transcardially with saline followed by 4% paraformaldehyde (PFA) in 0.1 M PBS. Brains were stored in 18% sucrose and sectioned coronally or sagittally (40 μm) using a Leica cryostat. Sections were rinsed three times with phosphate buffer (PB) for 10 min and incubated for 1h with blocking solution (4% BSA in 0.3% Triton X-100 in PB). Sections were incubated overnight with a rabbit anti-MANF antibody (1:100, / (Lindholm, et al., 2008)) and mouse anti-GFAP (glial fibrillary acidic protein, 1:300, Chemicon, Temecula, CA, USA) or mouse anti-NeuN (neuronal nuclei, 1:200, Chemicon). The sections were rinsed three times in PBS for 10 min. The bound primary antibody was visualized using the AlexaFluor 488 goat anti-rabbit or AlexaFluor 568 goat anti-mouse secondary antibody (Invitrogen, Carlsbad, CA, USA). Sections were rinsed three times with PB and incubated with DAPI (4,6-diamidino-2-phenylindole, 1:2000; Invitrogen) for 15 min. Brain sections were examined with a Nikon eclipse 80i microscope with Q-imaging camera or with Nikon D-eclipse C1 confocal imaging system equipped for epifluorescence.
In vitro immunofluorescent staining of rat primary cortical cultures was conducted as described previously (Howard, et al., 2008). Briefly, cells were permeabilized for 15 min then blocked for 1 hour with PBS containing 0.1% Triton X-100, 2% BSA, and 5% serum. Cells were incubated over night with rabbit anti-MANF antibody (Lindholm, et al., 2008) , 1:250, in PBS containing 0.1% Triton X-100 and 5% goat serum at 4°C with gentle shaking. Cells were washed 3 times with PBS for 3 minutes and then incubated for 1h with AlexaFluor 568 goat anti-rabbit secondary antibody (Invitrogen) and washed again with PBS. Immunofluorescence was visualized and imaged using Nikon Elements 3.0 software controlling a Nikon TE2000 inverted microscope and CoolSnap HQ2 camera.
Middle cerebral artery occlusion (MCAo)
One week after the AAV injections, ligation of the right MCA and common carotids (CCAs) bilaterally was performed as described previously (Chen, et al., 1986). Briefly, the bilateral CCAs were identified and isolated through a ventral midline cervical incision. Rats were placed in stereotaxic apparatus and a craniotomy was made in the right hemisphere. The right (MCA) was ligated with a 10-0 suture and bilateral common carotids (CCA) were ligated with non-traumatic arterial clamps for 60 minutes. After sixty minutes of ischemia, the suture around the MCA and arterial clips on CCAs were removed to introduce a reperfusional injury. After recovery from anesthesia, the rats were returned to their home cage. Body temperatures during and after surgery were maintained at 37°C.
Behavioral monitoring
1) Elevated Body asymmetry analysis
Body asymmetry was analyzed using an elevated body swing test (Borlongan, et al., 1998). Rats were examined for lateral movements/turning when their bodies were suspended 20 cm above the testing table by lifting their tails. The frequency of initial turning of the head or upper body contralateral to the ischemic side was counted in 20 consecutive trials. The maximum impairment in body asymmetry in stroke animals is 20 contralateral turns/20 trials. In normal rats, the average body asymmetry is 10 contralateral turns/20 trials (i.e. the animals turn in each direction with equal frequency).
2) Neurological test
Neurological deficits were evaluated using Bederson’s score (Bederson, et al., 1986). In a postural reflex test, rats were examined for the degree of abnormal posture when suspended by 20-30 cm above the testing table. They were scored according to the following criteria: 0, Rats extend both forelimbs straight and no observable deficits; 1, Rats keep the one forelimb to the breast and extend the other forelimb straight; 2, rats show decreased resistance to lateral push in addition to behavior in score 1 without circling; 3, rats twist the upper half of their body in addition to behavior in score 2.
3) Locomotor behavior
Locomotor activity was measured using an infrared activity monitor (Accuscan, Columbus, OH). Animals were individually placed in a 42×42×31 cm plexiglass open box which contained horizontal infrared sensors spaced 2.5 cm apart. In the recovery experiment after MCAo locomotor activity was measured for 1h at 2, 7 and 14 days after MCAo. In the experiment to study the effect of AAV-MANF on locomotor behavior 24-h behavior was conducted the day before AAV injections and 7, 14 and 24 days afterwards.
Triphenyltetrazolium chloride (TTC) staining
Two days after MCAo, the infarction area was measured by TTC staining as described previously (Shen, et al., 2009). Rats were decapitated and the brains were removed and sliced into 2.0-mm-thick sections using an acrylic rat brain block. The brain slices were incubated in a 2% TTC solution (Sigma, St. Louis, MO, USA) for 15 min at room temperature and then transferred into a 4% paraformaldehyde solution for fixation. The area of infarction in each slice was measured with a digital scanner and Imagetools software (University of Texas Health Sciences Center). The volume of infarction in each animal was obtained from the product of average slice thickness (2 mm) and sum of infarction areas in all brain slices examined.
Immunoblotting from samples
For in vitro characterization of the AAV-MANF packaging plasmid, HEK293 cells were plated at 2.0×105 cell/well seeding density in 24-well tissue culture plates. After 24 hours, dsAAV vector packaging plasmids containing GFP, control, or MANF cDNAs were transfected using Lipofectamine 2000 (Invitrogen). Cells were maintained for 24-48 hours at 37°C in 5% CO2. Cell lysates were harvested in RIPA buffer (50mM Tris-HCl pH 7.4, 1% NP40, 0.25% deoxycholic acid, 150 mM NaCl, 1 mM EDTA) with 1× protease inhibitors (Sigma) and media was collected. Protein concentrations were determined by DCA assay using bovine serum albumin (BSA) as a standard curve. Cell lysates and media were separated by 4-12% SDS-PAGE and transferred onto nitrocellulose membranes. Western blot analysis was performed using rabbit polyclonal anti-Actin (Sigma) and polyclonal anti-MANF (Lindholm, et al., 2008) primary antibodies. Following primary incubation, anti-Rabbit IgG secondary antibody labeled with IR-700nm conjugate was applied and membranes were scanned using an Odyssey Infrared Imager (Li-Cor, Lincoln, NE, USA).
For in vivo samples, brain cortices containing the three AAV injection sites were isolated and frozen with isopentane on dry ice. Cortices were homogenized in microfuge tubes with a electric homogenizer on ice in RIPA lysis buffer (50mM Tris/HCl, pH 8.0, 150mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, PIC cocktail, and 1mM phenylmethylsulfonyl fluoride).The lysates were centrifuged for 15min at 14000rpm at 4°C and the supernatants were aspirated and transferred in fresh tubes kept on ice. Total protein content of the supernatants was assayed by the BCA method. Equal amount of the total proteins from samples were subjected to immunoblot assay as described with indicated antibodies for MANF, GFP and actin (Fang, et al., 2001).
Statistical analysis
Two tailed Student’s t-test and one or two way ANOVA were used for statistical comparison. Bonferroni tests or Newman-Keuls tests were used for post-hoc analysis. Data are presented as mean ± s.e.m; p-values of <0.05 were considered significant.
RESULTS
Characterization of AAV-MANF in vitro
We successfully constructed a dsAAV vector packing plasmid containing the human MANF cDNA. Transfection of HEK293 cells with the AAV-MANF packaging plasmid for 24 hours resulted in increased MANF protein as analyzed by western blotting (Fig.1A). Neuronal MANF immunoreactivity was found in rat primary cortical cultures transduced with AAV-MANF virus for 48 hours and immunostained for MANF (Fig.1D). Minimal MANF-immunoreactivity was seen in no-virus treated cultures (1C) or cultures transduced by AAV-GFP (not shown).
AAV serotype 7 transduction of rat cortex in vivo
At 8 days post-injection of AAV-GFP, overexpression of GFP is widely expressed in cortex (Fig. 1E-F). Immunolabeling brain sections with the neuronal marker NeuN, and with glial marker, glial fibrillary acidic protein (GFAP), showed that AAV-GFP conferred GFP expression by detectable fluorescence in both neurons and glial cells in rat cortex (Fig. 1G-H). Western blot analysis of cortical tissue at 8 days post-injection revealed a significant increase (p<0.001, Student’s t-test) in MANF protein production in AAV-ANF injected animals compared to AAV-GFP (Fig 1B).
AAV-MANF does not alter locomotor activity or weight gain in non-stroke rats
A total of 16 rats were injected with AAV-GFP (n=8) or AAV-MANF (n=8) into three cortical sites. Locomotor activity was monitored on 7, 14 and 24 days after viral injection. There is no significant difference (p >0.05 ) between AAV-GFP and AAV-MANF in locomotor activity during 1 hour sessions for parameters such as horizontal activity (Suppl. Fig 1A), vertical activity (Suppl. Fig 1A), distance travelled and stereotypy (data not shown) measured up to 24 days after injection (Supplemental Figure 1). Total distance travelled over 24 hours measured in 2-h intervals did not show significant difference between the two viral treatments but did show similar diurnal patterns of activity (Suppl. Fig 1D). No significant difference in weight gain was observed between AAV-MANF and AAV-GFP treatments up to 24 days post-injection (Suppl. Fig 1A).
Intracortical delivery of AAV-MANF reduced cerebral infarction after MCAo
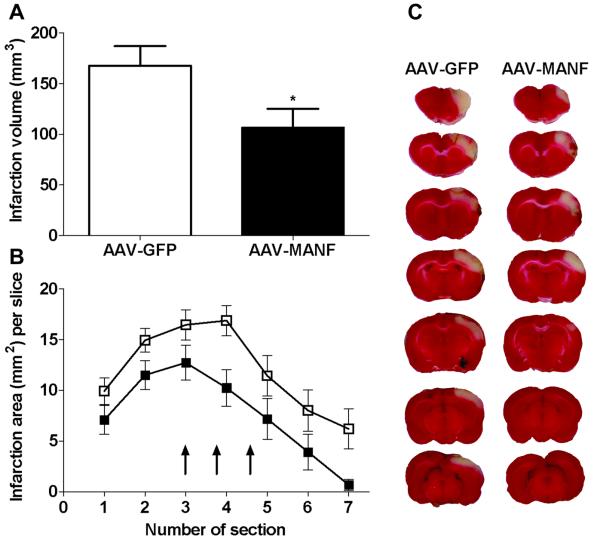
A total of 23 rats were used to examine cortical infarction 2 days after MCAo. Fourteen of these rats were injected with AAV-GFP and nine with AAV-MANF into three cortical sites, as shown in Fig. 1E, seven days before MCAo. Administration of AAV-MANF significantly decreased infarction volume, as compared to the control AAV-GFP group (Fig. 2A, p<0.05, Student’s t-test). To further examine the topographic protection, the area of infarction in each slice was further analyzed. Using a two-way ANOVA, we found a significant decrease in infarction per slice in rats receiving AAV-MANF treatment (Fig. 2B, p<0.001, two-way ANOVA). The biggest difference between the two treatment groups occurred on the 4th 2-mm section (Fig. 2B-C), which is central to the region encompassing the three AAV injection sites (Shen, et al., 2009, Wang, et al., 2001, Wang, et al., 1997).
Figure 2. AAV-MANF decreases infarction volume in stroke rats.
Rats received three intracortical injections of AAV-MANF or AAV-GFP one week prior to a 60 minute MCAo. Tissue was sectioned (2 mm) and stained with TTC at 48 hours post-stroke. (A) AAV-MANF significantly reduced infarction volume compared to AAV-GFP (*p=0.0447, Student’s t-test). (B) Area of infarction per 2-mm section (7 sections/brain; anterior to posterior) was significantly reduced for all sections in the AAV-MANF group with the most notable difference in section 4 (two-way ANOVA, p<0.0001). Three cortical injections of AAV-MANF or AAV-GFP were made between sections 3 to 5 (arrows). C) Representative TTC stained sections.
AAV-MANF promoted behavioral recovery after MCAo
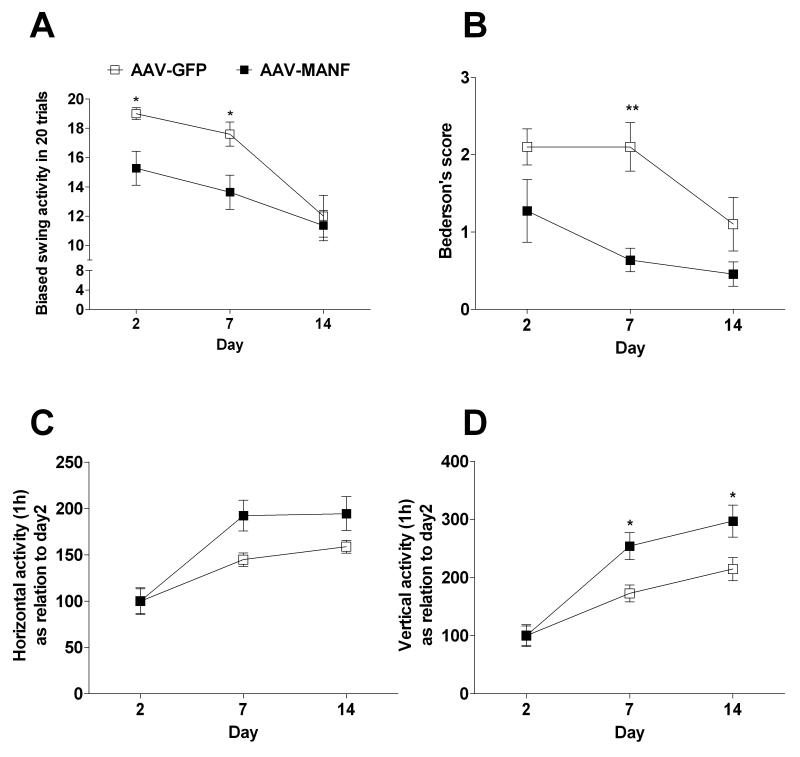
We analyzed biased body swing, Bederson’s score, 1-h horizontal activity and vertical activity on days 2, 7 and 14 after ischemic brain injury from an additional AAV-GFP (n=10) and AAV-MANF (n=11) treated rats. For all locomotor parameters that were analyzed, there was a significant time effect (p<0.001, 2-way ANOVA), which is consistent with previous studies showing spontaneous recovery (Chang, et al., 2003, Chou, et al., 2006, Ren, et al., 2000, Yonemori, et al., 1999). AAV-MANF, compared to AAV-GFP, treatment significantly increased behavioral recovery based on the biased body swing assay (Fig. 3A, AAV treatment effect, p<0.05, Time × AAV Treatment interaction p<0.05, 2-way ANOVA). AAV-MANF treatment decreased neurological deficits assessed by Bederson’s test (Fig. 3B, AAV Treatment effect, p<0.01, 2-way ANOVA) and increased 1-h horizontal activity (Fig. 3C, AAV Treatment × Time interaction, p<0.05) and 1-h vertical activity (Fig. 3D, AAV Treatment effect p<0.05, Time × AAV Treatment interaction, p<0.05).
Figure 3. AAV-MANF promotes recovery after cerebral ischemia.
Rats received three intracortical injections of AAV-MANF or AAV-GFP one week prior to a 60 minute MCAo. Behavioral tests were carried out at 2, 7 and 14 days after the MCAo and analyzed with 2-way ANOVA. AAV-MANF injection significantly reduced body asymmetry in an elevated body swing test (A) and neurological abnormality scores in Bederson’s test (B). AAV-MANF increased horizontal activity (C) and vertical activity (D). Results of Bonferroni post-hoc tests are indicated by an asterisk. X-axis shows days after the MCAo.
MCAo causes redistribution of MANF immunoreactivity in vivo
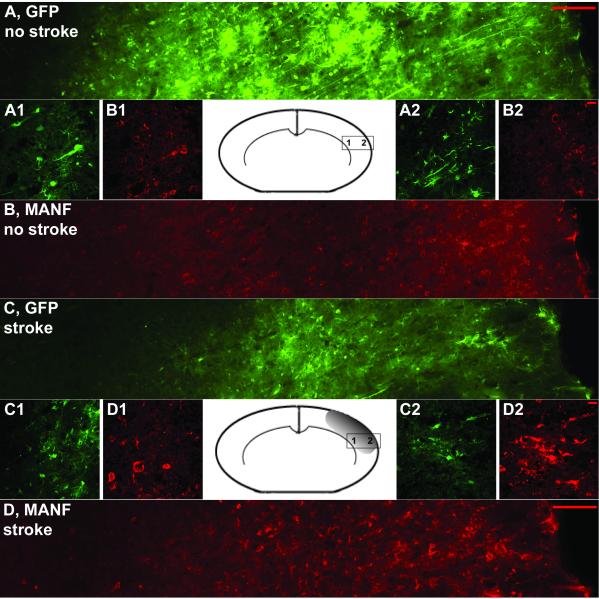
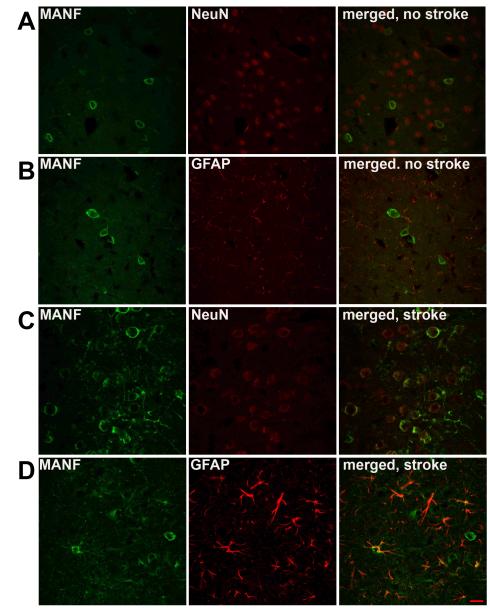
We examined MANF protein expression in ischemic and non-ischemic brains at 8 days after AAV-MANF injection and observed altered MANF immunoreactivity in ischemic brains compared to non-ischemic brains. To determine whether the altered MANF immunoreactivity was specific to MANF or general to the overexpressed AAV transgene, we injected a mixture of AAV-MANF and AAV-GFP (1:1) into cortex 7 days before MCAo or sham surgery. The expression of MANF and GFP was analyzed on coronal sections at 6 hours after MCAo surgery. In non-stroke controls, GFP and MANF expressions were found to be equivalently distributed on coronal sections near the injection sites in the cortex. GFP fluorescence was present throughout the cell body as well as extensively labeling processes of neurons and glial cells. In contrast, MANF immunoreactivity (IR) was primarily detected in the perinuclear cell soma of NeuN- positive cells (Fig 4A and B; Fig 5A). MANF-IR was also found in some GFAP cells (Fig. 5B). Following MCAo, GFP and MANF expression was found in the lesioned cortex. There was a broader distribution of MANF immunoreactivity, compared to GFP, in the ischemic cortex (Fig. 4C and D). In the area near the ischemic penumbra (based on TTC staining from other animals), the distribution of MANF-IR was similar to that in non-stroke rats treated with MANF (Fig 4B and 4D1). However, in the region of the ischemic core, MANF-IR was detectable in the cell soma as well as the processes of both NeuN-positive and GFAP-positive cells (Fig. 5C and 5D), suggesting the translocation of MANF to the processes of neurons and glia. The punctate pattern of MANF-IR not associated with NeuN or GFAP cells was also observed (Fig 4D1 and Fig 4D2). These patterns of distribution were not found in stroke animals pre-treated AAV-GFP (Fig 4C1 and Fig 4C2). Additionally, the presence of MANF-IR in cortex of naïve animals or AAV-GFP treated animals was minimal and did not appear to change following ischemia suggesting the observed change in MANF-IR reflected changes in human MANF produced by AAV-MANF transduction.
Figure 4. MCAo alters MANF-IR in AAV-MANF injected animals.
Rats were injected with mixture of AAV-MANF and AAV-GFP (1:1) and MANF (red) and GFP (green) expression were analyzed before and after MCAo. Figure 4A and 4B shows the expression patterns induced by the AAV vector before MCAo. After 1h MCAo and 6h reperfusion, there was broad change in the pattern of MANF expression in the ischemic cortex (Fig. 4D) which was not seen for GFP expression. Left and right sides of the figure are proximal to the corpus callosum and infarction core, respectively. Panels A/B and C/D are from the same brain section of non-stroke and stroke animals, respectively. Inset pictures (1 and 2) are high magnification images taken from indicated regions on the inset diagram of the coronal brain section. Note the change in MANF-IR is more prominent in the ischemic core (D2 versus D1). Scale bars: A,B, C, D = 100μm, A1-2, B1-2, C1-2, D1-2 = 10μm.
Figure 5. MANF-IR colocalizes to both neurons and astrocytes after MCAo.
Before MCAo, MANF-IR in AAV-MANF injected cortical tissue colocalizes to NeuN+ (A, neuronal marker) cells and to a lesser extent GFAP+ (B, glial marker) cells. Six hours after reperfusion MANF is co-localized with both NeuN+ (C) and GFAP+ (D) cells ,with more prominent labeling of the cellular processes. A punctate pattern not associated with cell bodies is observed following stroke suggesting an extracellular location. Scale bar = 10μm.
Hypoxia causes redistribution of MANF immunoreactivity in primary cortical culture
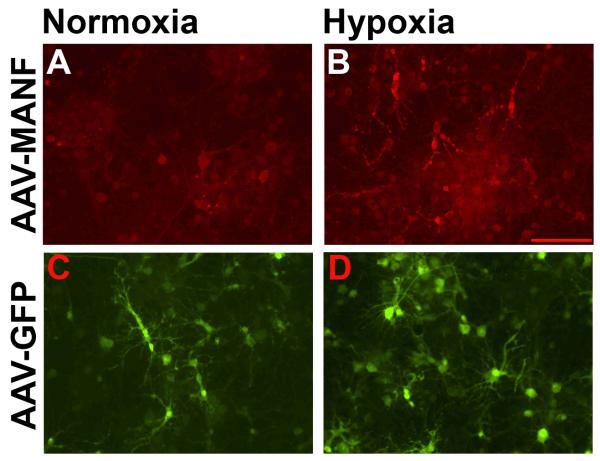
To further explore the ischemia-related changes in MANF-IR, we examined the effects of hypoxia on primary cortical cells transduced with AAV-MANF or AAV-GFP. In AAV-MANF transduced cultures under normoxic conditions, MANF-IR was present in cell soma and processes, (Fig 6A); however, after 8 hours of hypoxia and 24 hours reoxygenation, MANF-IR was clearly outlined in cellular processes (primarily GFAP-positive cells, data not shown) and exhibited a punctate pattern not associated with cell bodies (Fig 6B). Under normoxic conditions, AAV-GFP extensively labeled cellular processes of neurons and glia and the GFP fluorescence pattern was similar in hypoxic cultures.
Figure 6. Hypoxia causes redistribution of MANF-IR in primary cortical neurons transduced with AAV-MANF.
Primary cortical neurons were transduced with AAV-MANF (A,B) or AAV-GFP (C,D) on DIV6 and given 8 hours hypoxia and 24 hours normoxia on DIV12. MANF-IR in AAV-MANF transduced cultures associates with cellular processes following hypoxia (A vs. B). No gross changes in GFP fluorescence is observed in AAV-GFP transduced cultures after hypoxia (C vs. D). Scale bars = 100μm.
DISCUSSION
We report the first in vivo gene therapy study using AAV-mediated delivery of the human MANF in a rodent model of stroke. We show that an AAV vector (serotype 7) can be used to achieve widespread transduction of cells throughout the ischemic region in rats. Increased human MANF protein levels by AAV-MANF in ischemic rodent cortex reduces brain injury and promotes behavioral recovery in rats.
Increased expression of human MANF protein via AAV-MANF delivery to rat cortex did not alter locomotor behavior or weight gain in non-stroke animals. These findings suggest there are no overt changes in neural function. With other proteins identified as neurotrophic factors, such as nerve growth factor and glial cell line-derived neurotrophic factor, weight loss in rodents and in human clinical trials has been reported when recombinant protein has been injected (Manfredsson, et al., 2009, Nutt, et al., 2003, Olson, 1993, Pizzo and Thal, 2004, Tumer, et al., 2006). In the case of GDNF, weight loss occurs when GDNF is delivered to the hypothalamus or nigra but not striatum indicating these effects are region specific (Eslamboli, et al., 2003, Manfredsson, et al., 2009, Tumer, et al., 2006). Our results suggest that AAV serotype 7 is suitable for a wide-spread cortical expression of GFP or MANF without altering weight or normal locomotor behavior. In our previous study (Airavaara, et al., 2009), MANF protein was applied locally to the cortex using the same injection paradigm as the AAV-MANF and did not alter blood pressure, body temperature, serum electrolytes or blood gases suggesting that local increases of MANF protein in the cortex do not alter these physiological parameters.
AAV-MANF reduced infarction volume compared to AAV-GFP with the greatest reduction of the infarction area occurring on the 4th 2-mm brain section which is centered at the site of AAV injection. These results are consistent with our previous data showing localized administration of recombinant MANF protein in cortex reduces infarction in the region of protein delivery (Airavaara, et al., 2009). Compared to our previous study, AAV-MANF conferred a larger area of protection and better improved the behavioral recovery which correlates with increased distribution of MANF protein by AAV-MANF compared to direct injection of protein. In our previous study (Airavaara, et al., 2009) we showed that MANF decreases TUNEL labeling in the ischemic cortex, suggesting a MANF-induced a decrease in apoptosis/necrosis. Stroke-induced neuronal damage involves activation of a complex cascade of cellular events than can persist long-term resulting in increased cell death. Thus, chronic MANF expression may be more efficient at reducing the damage than acute protein injection. The demonstrated neurorestorative/neuroregenerative properties in animal models of Parkinson’s disease (Voutilainen et al 2009) further supports the notion that chronic MANF expression may promote neuronal recovery following stroke. Further studies examining the long term consequences of MANF over-expression and its role in neuromodulation and neuroregeneration are needed.
The size of infarction caused by MCAo positively correlates with the impairment in locomotor activity, specifically vertical activity parameter (Shen and Wang, 2009). We observed enhanced recovery of MCAo-related impairments in vertical activity for the AAV-MANF treated animals compared to the AAV-GFP group. Although AAV-MANF significantly improved the recovery of locomotor activity compared to AAV-GFP, the AAV-GFP group did show improved locomotor function over time. A similar pattern of behavioral recovery was observed for two additional behavioral assessments used in our current study, body asymmetry (Borlongan, et al., 1998) and Bederson’s test (Bederson, et al., 1986) which further support that AAV-MANF increased behavioral recovery compared to AAV-GFP. Consistent with our observation of improved behavioral recovery over time in the AAV-GFP group, previous studies have shown a spontaneous recovery of abnormal neurological scores (Chou, et al., 2006), step through latency (Yonemori, et al., 1999), paw placing (Ren, et al., 2000) and locomotor activity (Chang, et al., 2003) after experimental stroke. Taken together, our results show that MANF overexpression in cortex significantly reduced the infarction volume and improved the behavioral and neurological recovery over the 2 weeks after MCAo compared with AAV-GFP controls.
In rat cortex, AAV serotype 7 transduced both neurons (NeuN+ cells) and astrocytes (GFAP+ cells); however, there was a marked difference in the distribution of transgene following ischemia. The overall MANF protein levels at 6 hours post-MCAo of AAV-MANF treated animals were similar to non-MCAo controls (data not shown). The AAV-GFP extensively labeled the cells and their processes with GFP fluorescence whereas AAV-MANF increases MANF-IR primarily in perinuclear cytoplasm of neurons. Ischemic injury did not alter the distribution pattern of GFP at 6 hours after MCAo. In contrast, in the same brain section from the same animal co-injected with AAV-MANF, the MANF-IR in the ischemic area labeled cellular processes such as axons and dendrites of neurons and astrocytic ramifications. We postulate that ischemic injury induced redistribution of MANF-IR from perinuclear cytoplasm to cell surface. MANF has been shown to be localized to the endoplasmic reticulum and Golgi apparatus (Apostolou, et al., 2008, Mizobuchi, et al., 2007, Tadimalla, et al., 2008) and is induced by ER-stress (Apostolou, et al., 2008, Lee, et al., 2003, Mizobuchi, et al., 2007), ischemia (Apostolou, et al., 2008, Lindholm, et al., 2008, Tadimalla, et al., 2008) and status epilepticus (Lindholm, et al., 2008). We observed perinuclear MANF-IR which is consistent with an ER localized pattern. Agents that induce ER stress (Apostolou, et al., 2008, Mizobuchi, et al., 2007, Tadimalla, et al., 2008, Yu, et al.)) and ischemia (Tadimalla, et al., 2008) also cause secretion of MANF.
The extracellular, punctate pattern of MANF-IR suggests that MANF protein is released from the cell following ischemia. Although we can not rule out that MANF is being released in a non-regulated manner resulting from cellular damage near the core of the infarction, the observation of ischemia-induced release of MANF has also been made in cardiac myocytes (Tadimalla, et al., 2008). Crystal structure analysis of MANF and CDNF identifies two putative domains and it has been suggested that the C-terminal domain is involved in ER-function and the N-terminal domain resembles a saposin-like lipid binding domain (Parkash, et al., 2009). Once released from the ER (C-terminal domain function), the N-terminal domain may interact with cell membranes via lipids (Lindholm and Saarma, 2010) to exert neurotrophic effects such as those observed in other studies (Airavaara, et al., 2009, Palgi, et al., 2009, Voutilainen, et al., 2009, Yu, et al.). It is also possible that MANF exerts its neuroprotective effects after internalization into the cell. Further studies are needed to confirm the movement of MANF protein following cellular stressors as well as the mechanism of neuroprotection.
Before MANF treatment can be considered for clinical applications there are several questions that need to be addressed. Prophylactic treatment capable of dampening the pathological responses to ischemia may be therapeutically important for only small number of patients who are highly prone to ischemic attacks. Before preclinical post-stroke experiments are conducted there are “proof of principle” questions that need to be addressed. Delivery to the intact brain via the ventricular system, vascular system or intranasal route needs further development. The increased permeability of blood brain barrier (BBB) after the onset of stroke, provides a window to target ischemic brain tissue for compounds that normally do not cross BBB. Other problems that need to be studied include the ability of post-stroke MANF delivery to promote recovery, timing of administration after stroke, and efficacy of coadministration of MANF with other treatments, like physical therapy or exercise, to promote recovery. Also, pre-clinical studies of MANF in other animal models of ischemic brain injury are needed. After these issues are addressed, safety, tolerance and efficacy of delivery should be evaluated in humans. Ultimately, a clinical study in a small number of stroke patients could be considered.
The possible systemic administration of AAV, may lead to over-expression of MANF in other than the target brain area, which may have side effects. The unwanted over-expression can be controlled using cell-specific or synthetically regulated (e.g. small molecule regulation) promoters. Together with ruptured BBB, AAV containing regulated promoters may enable a less invasive means to deliver and control transgene expression in the penumbra of the infarcted brain. Future studies are needed to confirm the feasibility of this approach.
In conclusion, in the present study we used AAV-mediated gene delivery and characterized AAV-MANF for protection against ischemia. The methods used establish a paradigm for evaluating gene function during stroke.
Supplementary Material
Acknowledgements
This research was supported by the intramural research program at NIDA, NIH, and DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
References
- 1.Airavaara M, Shen H, Kuo CC, Peranen J, Saarma M, Hoffer B, Wang Y. Mesencephalic astrocyte-derived neurotrophic factor reduces ischemic brain injury and promotes behavioral recovery in rats. J Comp Neurol. 2009;515:116–124. doi: 10.1002/cne.22039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apostolou A, Shen Y, Liang Y, Luo J, Fang S. Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death. Exp Cell Res. 2008;314:2454–2467. doi: 10.1016/j.yexcr.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 4.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Cerebral ischemia and CNS transplantation: differential effects of grafted fetal rat striatal cells and human neurons derived from a clonal cell line. Neuroreport. 1998;9:3703–3709. doi: 10.1097/00001756-199811160-00025. [DOI] [PubMed] [Google Scholar]
- 5.Chang CF, Lin SZ, Chiang YH, Morales M, Chou J, Lein P, Chen HL, Hoffer BJ, Wang Y. Intravenous administration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–564. doi: 10.1161/01.str.0000051507.64423.00. [DOI] [PubMed] [Google Scholar]
- 6.Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- 7.Chou J, Harvey BK, Chang CF, Shen H, Morales M, Wang Y. Neuroregenerative effects of BMP7 after stroke in rats. J Neurol Sci. 2006;240:21–29. doi: 10.1016/j.jns.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Eslamboli A, Cummings RM, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Kirik D, Annett LE. Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6- OHDA lesion in the common marmoset monkey (Callithrix jacchus) Exp Neurol. 2003;184:536–548. doi: 10.1016/j.expneurol.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Fang S, Ferrone M, Yang C, Jensen JP, Tiwari S, Weissman AM. The tumor autocrine motility factor receptor, gp78, is a ubiquitin protein ligase implicated in degradation from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2001;98:14422–14427. doi: 10.1073/pnas.251401598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao GP, Lu F, Sanmiguel JC, Tran PT, Abbas Z, Lynd KS, Marsh J, Spinner NB, Wilson JM. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol Ther. 2002;5:644–649. doi: 10.1006/mthe.2001.0591. [DOI] [PubMed] [Google Scholar]
- 11.Howard DB, Powers K, Wang Y, Harvey BK. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 2008;372:24–34. doi: 10.1016/j.virol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim ST, Airavaara M, Harvey BK. Viral vectors for neurotrophic factor delivery: A gene therapy approach for neurodegenerative diseases of the CNS. Pharmacol Res. 2009 doi: 10.1016/j.phrs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindholm P, Peranen J, Andressoo JO, Kalkkinen N, Kokaia Z, Lindvall O, Timmusk T, Saarma M. MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol Cell Neurosci. 2008;39:356–371. doi: 10.1016/j.mcn.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Lindholm P, Saarma M. Novel CDNF/MANF family of neurotrophic factors. Dev Neurobiol. 2010;70:360–371. doi: 10.1002/dneu.20760. [DOI] [PubMed] [Google Scholar]
- 16.Lindholm P, Voutilainen MH, Lauren J, Peränen J, Leppänen VM, Andressoo JO, Lindahl M, Janhunen S, Kalkkinen N, Timmusk T, Tuominen RK, Saarma M. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature. 2007;448:73–77. doi: 10.1038/nature05957. [DOI] [PubMed] [Google Scholar]
- 17.Manfredsson FP, Tumer N, Erdos B, Landa T, Broxson CS, Sullivan LF, Rising AC, Foust KD, Zhang Y, Muzyczka N, Gorbatyuk OS, Scarpace PJ, Mandel RJ. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol Ther. 2009;17:980–991. doi: 10.1038/mt.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 19.Mizobuchi N, Hoseki J, Kubota H, Toyokuni S, Nozaki J, Naitoh M, Koizumi A, Nagata K. ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element. Cell Struct Funct. 2007;32:41–50. doi: 10.1247/csf.07001. [DOI] [PubMed] [Google Scholar]
- 20.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 21.Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr., Lozano AM, Penn RD, Simpson RK, Jr., Stacy M, Wooten GF. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 22.Olson L. NGF and the treatment of Alzheimer’s disease. Exp Neurol. 1993;124:5–15. doi: 10.1006/exnr.1993.1167. [DOI] [PubMed] [Google Scholar]
- 23.Palgi M, Lindstrom R, Peranen J, Piepponen TP, Saarma M, Heino TI. Evidence that DmMANF is an invertebrate neurotrophic factor supporting dopaminergic neurons. Proc Natl Acad Sci U S A. 2009;106:2429–2434. doi: 10.1073/pnas.0810996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkash V, Lindholm P, Peranen J, Kalkkinen N, Oksanen E, Saarma M, Leppanen VM, Goldman A. The structure of the conserved neurotrophic factors MANF and CDNF explains why they are bifunctional. Protein Eng Des Sel. 2009;22:233–241. doi: 10.1093/protein/gzn080. [DOI] [PubMed] [Google Scholar]
- 25.Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003;20:173–188. doi: 10.1385/jmn:20:2:173. [DOI] [PubMed] [Google Scholar]
- 26.Pizzo DP, Thal LJ. Intraparenchymal nerve growth factor improves behavioral deficits while minimizing the adverse effects of intracerebroventricular delivery. Neuroscience. 2004;124:743–755. doi: 10.1016/j.neuroscience.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 27.Ren J, Kaplan PL, Charette MF, Speller H, Finklestein SP. Time window of intracisternal osteogenic protein-1 in enhancing functional recovery after stroke. Neuropharmacology. 2000;39:860–865. doi: 10.1016/s0028-3908(99)00261-0. [DOI] [PubMed] [Google Scholar]
- 28.Shen H, Kuo CC, Chou J, Delvolve A, Jackson SN, Post J, Woods AS, Hoffer BJ, Wang Y, Harvey BK. Astaxanthin reduces ischemic brain injury in adult rats. FASEB J. 2009;23:1958–1968. doi: 10.1096/fj.08-123281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen H, Wang Y. Correlation of locomotor activity and brain infarction in rats with transient focal ischemia. J Neurosci Methods. 2009 doi: 10.1016/j.jneumeth.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tadimalla A, Belmont PJ, Thuerauf DJ, Glassy MS, Martindale JJ, Gude N, Sussman MA, Glembotski CC. Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ Res. 2008;103:1249–1258. doi: 10.1161/CIRCRESAHA.108.180679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tumer N, Scarpace PJ, Dogan MD, Broxson CS, Matheny M, Yurek DM, Peden CS, Burger C, Muzyczka N, Mandel RJ. Hypothalamic rAAV-mediated GDNF gene delivery ameliorates age-related obesity. Neurobiol Aging. 2006;27:459–470. doi: 10.1016/j.neurobiolaging.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Voutilainen MH, Back S, Porsti E, Toppinen L, Lindgren L, Lindholm P, Peranen J, Saarma M, Tuominen RK. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J Neurosci. 2009;29:9651–9659. doi: 10.1523/JNEUROSCI.0833-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Chang CF, Morales M, Chou J, Chen HL, Chiang YH, Lin SZ, Cadet JL, Deng X, Wang JY, Chen SY, Kaplan PL, Hoffer BJ. Bone morphogenetic protein-6 reduces ischemia-induced brain damage in rats. Stroke. 2001;32:2170–2178. doi: 10.1161/hs0901.095650. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Lin SZ, Chiou AL, Williams LR, Hoffer BJ. Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J Neurosci. 1997;17:4341–4348. doi: 10.1523/JNEUROSCI.17-11-04341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 36.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yonemori F, Yamaguchi T, Yamada H, Tamura A. Spatial cognitive performance after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:483–494. doi: 10.1097/00004647-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Yu YQ, Liu LC, Wang FC, Liang Y, Cha DQ, Zhang JJ, Shen YJ, Wang HP, Fang S, Shen YX. Induction profile of MANF/ARMET by cerebral ischemia and its implication for neuron protection. J Cereb Blood Flow Metab. 2010;30:79–91. doi: 10.1038/jcbfm.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.