Abstract
Attention Deficit Hyperactivity Disorder (ADHD) is characterized by inattention, impulsivity and hyperactivity mediated by frontal-striatal-cerebellar dysfunction. These circuits support implicit learning of perceptual-motor sequences but not visual-spatial context. ADHD and control children performed the Alternating Serial Reaction Time (ASRT) task, a measure of sequence learning, and the Contextual Cueing (CC) task, a measure of spatial contextual learning. Relative to controls, children with ADHD showed inconsistent ASRT learning but did not differ on CC learning. Thus, implicit sequence learning, a cognitive process mediated by frontal-striatal-cerebellar circuitry that is not under executive control, was atypical in ADHD.
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is defined by symptoms of inattention, hyperactivity, and impulsivity that significantly disrupt voluntary control of behavior in cognitive, social, and emotional domains (Barkley, 2005). Indeed, neurocognitive models of ADHD have emphasized executive dysfunction reflected in ineffective inhibitory control of thoughts and actions and mediated by atypical development of frontal-striatal-cerebellar circuitry (Castellanos & Tannock, 2002). More recently, however, deficits in non-executive domains have been recognized in childhood ADHD (Castellanos, Sonuga-Barke, Milham, & Tannock, 2006). One non-executive domain of cognition is our ability to learn from environmental regularities (e.g., when or where events may occur) without intention or conscious awareness, termed implicit learning. Implicit learning comprises multiple neuroanatomically dissociable processes that support acquisition of visual, linguistic, perceptual-motor, and cognitive skills (Gabrieli, 1998). Whether implicit learning processes are intact in ADHD is currently unknown. This knowledge is necessary for specifying comprehensive models of ADHD.
The present study examined two forms of implicit learning known to be neuroanatomically dissociable in adults: sequence learning and spatial contextual learning. Implicit sequence learning involves repeated experience with invariant, sequential stimulus structure, which forms the basis for predicting subsequent responses to contiguous (e.g., Serial Reaction Time [SRT] task, Nissen & Bullemer, 1987) or non-contiguous (e.g., Alternating SRT [ASRT] task, Howard & Howard, 1997) stimuli. On these tasks, subjects respond faster to stimuli whose locations follow a repeating pattern than to stimuli whose locations are randomly determined. Learning is implicit because participants are unable to distinguish between repeated and novel sequences on subsequent recognition measures. Patients with striatal (Willingham & Koroshetz, 1993) and cerebellar (Pascual-Leone et al., 1993) damage from degenerative disease or stroke show impaired learning on SRT tasks. Neuroimaging studies of sequence learning in healthy subjects reveal frontal involvement in addition to striatal and cerebellar regions (for review see Doyon, 2008). Thus, implicit sequence learning appears to be mediated by frontal-striatal-cerebellar circuitry.
In contrast, implicit learning of spatial contextual information relies on the medial temporal lobes. This form of learning involves repeated experience with invariant spatial relationships, which provide predictive cues that guide attention during visual search tasks (e.g., Contextual Cueing [CC] task, Chun & Jiang, 1998). In this task, faster visual search occurs for targets among distractors whose spatial configuration covaries with target location across trials (Chun, 2000). Learning is implicit because participants are unable to distinguish between repeated and novel distractor configurations on subsequent recognition measures. Patients with extensive medial temporal lobe damage show impaired spatial contextual learning (Chun & Phelps, 1999). Neuroimaging studies of spatial contextual learning in healthy subjects show involvement of hippocampal (Greene, Gross, Elsinger, & Rao, 2007) and surrounding entorhinal/perirhinal cortex (Preston & Gabrieli, 2008). Learning appears to be mediated by the surrounding cortex rather than the hippocampus because damage confined to the hippocampus did not impair learning on the task (Manns & Squire, 2001).
Two lines of reasoning predict impaired implicit learning in ADHD. First, functional and structural neuroanatomical studies in subjects with ADHD have found atypical frontal, striatal and cerebellar regions that mediate sequence learning but inconsistent evidence regarding medial temporal regions that mediate spatial contextual learning. Neuroimaging studies revealed reduced activation in frontal and striatal regions during response inhibition (for review see Aron & Poldrack, 2005) and in cerebellar regions during working memory performance (Valera, Faraone, Biederman, Poldrack, & Seidman, 2005) in ADHD relative to control subjects. Further, meta-analysis of structural imaging studies indicated that the most consistent volumetric reductions in ADHD relative to control subjects were in the cerebellum and prefrontal cortex bilaterally and the right caudate (Valera, Faraone, Murray, & Seidman, 2007). In light of these functional and volumetric abnormalities, any learning processes mediated by those regions ought to also be disrupted in ADHD. The medial temporal lobes, in contrast, did not differ between the groups in that meta-analysis, although one study, published after the meta-analysis was conducted, reported larger bilateral hippocampus volumes in children and adolescents with ADHD (Plessen et al., 2006). Further, cognitive processes depending critically upon the medial temporal lobes such as recall and recognition memory are within normal limits in ADHD (Denckla, 1996). Thus, the neuroanatomical support for predicting reduced implicit learning is stronger for sequential than spatial contextual learning in ADHD.
Second, a hypothesis about the etiology of ADHD has posited that impaired prediction of environmental events may lead to the behavioral phenotype seen in children with ADHD (Nigg & Casey, 2005). Specifically, Nigg and Casey argue that impaired predictions about when or what environmental events are likely to occur are mediated by frontal-cerebellar and frontal-striatal dysfunction, respectively, in children with ADHD. Deficits in these learning mechanisms interact with other cognitive and emotional processes over the course of development, adversely impacting the maturation of cognitive control (Nigg & Casey, 2005). Importantly, there are no hypothesized deficits in learning where environmental events are likely to occur. Thus, this hypothesis would predict deficits in implicit sequence learning but not spatial contextual learning.
We examined implicit learning of temporal sequences using the ASRT task (Howard & Howard, 1997) and of spatial context using the CC task (Chun & Jiang, 1998), in children with ADHD versus age-, gender-, and IQ-matched controls. On the ASRT task, participants respond to the location of a visual stimulus via keypress. Unbeknownst to participants, the stimulus location is varied in a fixed sequence involving alternate trials (i.e., item n predicts item n +2); in other words, randomly determined stimulus locations alternate with sequence trials. Context-dependent learning is indexed by faster responses on sequence compared to random trials. On the CC task, participants search for a target (left/right oriented “T”) among distractors (rotated “Ls”) whose spatial configuration is repeated on some trials and novel on others. Context-dependent learning is indexed by faster responses on trials with repeated, rather than novel, distractor configurations. We predicted that learning would be reduced on the ASRT task but not the CC task in children with ADHD relative to controls.
Method
Participants
Twenty children with ADHD (15 males) aged 7 to 12 years (M = 10.20, SD = 1.61) with normal IQ (M = 113.15, SD = 13.60) and twenty control children (16 males) aged 7 to 14 years (M = 10.50, SD = 1.82) with normal IQ (M = 115.20, SD = 12.78) were recruited from the Washington DC area through advertisements in the Children's National Medical Center's newsletter and on-hold phone messages and were paid for participation. Both the ADHD and control samples were racially diverse (ADHD: 85% White, 5% Hispanic, 5% other, 5% no report; control: 60% White, 10% Black, 10% Hispanic, 5% other, 15% no report). The groups did not differ in age (p = .58), IQ (p = .63), or in the proportion of white and minority (i.e., non-white) participants (p = .15). Parents and children gave informed consent and assent, respectively. Data from fourteen control participants was described in Barnes et al. (2008). All participants with ADHD had to provide documentation from the professional who made the diagnosis (either a physician or a psychologist). All documentation was reviewed by a clinical psychologist to confirm DSM-IV criteria were met (i.e., presence of 6 out of 9 symptoms of inattention (for inattentive subtype) and 6 out of 9 symptoms of inattention and 6 out of 9 symptoms of hyperactivity/impulsivity (for combined subtype) in more than one setting, present before age 7 years and causing impairment). An ADHD Rating Scale-IV (DuPaul, Power, Anastopoulos, & Reid, 1998), as completed by the parent, was collected on participants with ADHD to confirm that diagnostic criteria (i.e., presence of 6 out of 9 symptoms of inattention (for inattentive subtype) and 6 out of 9 symptoms of inattention and 6 out of 9 symptoms of hyperactivity/impulsivity (for combined subtype) were met (Total Score ADHD Rating Scale, out of a possible 27: Inattention: M = 14.05, SD = 5.36, Hyperactivity: M = 10.55, SD = 5.85. Of the 20 children with ADHD, 13 were diagnosed with ADHD-Combined Subtype and 7 with ADHD-Inattentive Subtype. The Behavior Assessment System for Children (BASC) (Reynolds & Kamphaus, 1992) was administered to confirm symptom presentation (BASC Hyperactivity-impulsivity: M = 61.83, SD = 10.50; BASC Attention Problems: M = 64.00, SD = 8.73) and to screen for co-morbid neurological or psychiatric disorders (e.g., anxiety or depression) in children with ADHD. The Behavior Rating Inventory of Executive Function (BRIEF) (Gioia, Isquith, Guy & Kenworthy, 2000) was used an additional descriptive measure (BRIEF Global Executive Composite, M = 65.84, SD = 12.86). Control children were screened for psychiatric conditions with the Child Behavior Checklist (Achenbach, 1991) (n =14, Attention Problems: all scores ≤ 50) or the BASC (n = 7, Hyperactivity: M = 42.83, SD = 8.08; Attention Problems: M = 43.83, SD = 3.37). All children were screened for reading disorder using the Woodcock Johnson Tests of Achievement Third Edition (III) Letter Word Identification and Word Attack subtests. Children with ADHD were excluded if they had co-morbid neurological or psychiatric disorders or learning disabilities except Oppositional Defiant Disorder (n = 2). ADHD children participated following withdrawal of stimulant medications for at least 24 hours (methylphenidate: n = 8; dextroamphetamine: n = 6); 6 children were unmedicated.
Design and Stimulus Materials
ASRT
A 2 × 2 × 5 mixed design was used with Group (ADHD vs. Control) as a between-subjects factor and Trial type (Pattern vs. Random) and Epoch (1 – 5) as within-subjects factors.
Each trial began with three empty circles displayed horizontally across a screen, each spatially mapped to a keyboard key (“M” and the adjacent symbol keys < and > which were marked with stickers) upon which participants placed their index, middle, and ring finger, respectively. On each trial, one circle was filled and remained filled until participants pressed the correct key. The circles remained empty for 120 ms between trials. A pattern was randomly assigned to each participant (either A-r-B-r-C-r or A-r-C-r-B-r, where A, B, and C denote the left, central and right positions and r denotes a random element, constrained so that all locations appeared with equal frequency). The assigned pattern repeated throughout the experiment.
CC
A 2 × 2 × 6 mixed design was used with Group (ADHD vs. Control) as a between-subjects factor and Configuration (Repeated vs. Novel) and Epoch (1 – 6) as within-subject factors.
Each trial consisted of a 12-element stimulus array of a single target and 11 distractors presented in white on a gray background. The target was a horizontal “T” rotated left or right by 90°, to which subjects responded by pressing a keyboard key (“Z” for left, “/” for right which were marked with stickers) with the index fingers of their left and right hands. The distractors were “L”s randomly rotated by 0°, 90°, 180°, or 270°. Arrays were generated by randomly placing the 12 items into cells of an invisible grid (6 rows × 8 columns). Target location was balanced for distance from the screen's center and screen half (left/right); no target appeared in the four center or corner cells. Every element was randomly repositioned by ± 2 pixels along each axis to avoid colinearity. Each block consisted of 24 trials: 12 unique distractors configurations (Novel) and 12 distractors configurations that repeated across the experiment (Repeated). Target location, but not orientation (left/right), was fixed for each Repeated configuration. Following the task, 24 configurations (12 Novel, 12 Repeated) were tested for recognition memory.
Procedure
Participants performed the ASRT and CC tasks within a single session in counterbalanced order. Both tasks were self-paced. Participants took short breaks between blocks, approximately every 60 – 90 s. Including breaks, total time on the ASRT task ranged from 20 – 25 minutes and total time on the CC task ranged from 30 – 45 minutes. The experimenter confirmed that children rested their hands over the relevant response keys throughout the task.
ASRT Task
Stimuli were presented via E-Prime with instructions to press the key that matched the filled-in circle's location. Participants completed 20 blocks of 60 trials each. Blocks were grouped into 5 epochs of 4 blocks (e.g., Blocks 1 – 4 comprised Epoch 1). Each block began with 8 practice trials and ended with feedback encouraging speed and accuracy. We did not test for recognition memory for learned sequences, following the procedure used in Barnes et al. (2008). Adding these tests would have unacceptably increased testing time.
CC Task
Stimuli were presented via Matlab with instructions to locate the “T” as quickly and accurately as possible. Following 24 practice trials, participants completed 30 blocks of 24 trials each. Trials were randomized within blocks. Blocks were grouped into 6 epochs of 5 blocks (e.g., Blocks 1 – 5 comprised Epoch 1). On each trial, a fixation dot appeared for 1 s followed by a stimulus, which remained until a response was made. If no response was made within 6 s, the trial timed-out following an error-tone. Feedback tones were high-pitched for correct responses and low-pitched for errors. The recognition memory test was administered at the end of the task. Participants viewed one block of 24 recognition trials and pressed a key for familiar configurations.
Results
Percentage of correct responses (accuracy) and median Reaction Times (RTs) for correct trials were computed for each condition and epoch for each participant. Intra-individual variability was examined by computing coefficients of variation (Mean RT/Standard Deviation). Cohen's d and ηp2 effect sizes are reported for t-tests and Analyses of Variance (ANOVAs), respectively.
ASRT Task
Accuracy and median RTs for correct trials were computed for each participant and were analyzed in separate Group (ADHD vs. Control) × Trial type (Pattern vs. Random) × Epoch (1 – 5) repeated measures ANOVAs. On this task, sequence learning is defined by a Trial type × Epoch interaction, indicating greater sensitivity to sequential information with practice. Analysis of accuracy revealed no significant main effects or interactions except higher accuracy on Pattern than Random trials (main effect of Trial type), F (1, 38) = 65.14, p < .0001, ηp2 = .63 (other ps > .23, ηp2 < .04). Overall accuracy was high in both ADHD (Pattern: M = 93.62%, SD = 4.05; Random: M = 91.18%, SD = 4.33) and control (Pattern: M = 94.06%, SD = 3.60; Random: M = 91.28%, SD = 4.82) groups.
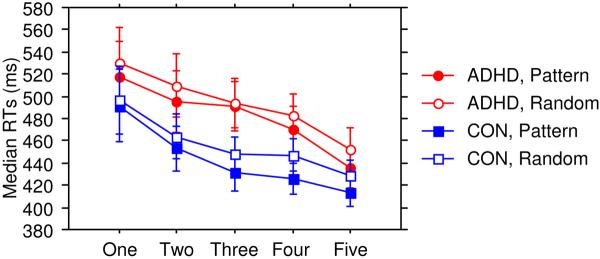
Overall RTs did not differ between ADHD (M = 487.86 ms, SD = 111.57) and control (M = 449.97 ms, SD = 92.47) children (main effect of Group), p = .19, ηp2 = .04 (Figure 1). Participants exhibited improvement in perceptual-motor skill, as responses were faster with practice (main effect of Epoch), F (4, 152) = 13.53, p < .0001, ηp2 = .26. Overall, participants were sensitive to sequential information, as responses were faster on Pattern than Random trials (main effect of Trial Type), F (1, 38) = 40.27, p < .0001, ηp2 = .52. While the Trial type × Epoch interaction was not significant, p = .11, ηp2 = .05, the Group × Trial Type × Epoch interaction was, F (4, 152) = 2.89, p = .02, ηp2 = .07. No other interactions reached significance (other ps > .62, ηp2 < .02). We examined the three-way interaction in two ways: 1) We determined whether significant sequence learning was obtained in each group with separate Trial type × Epoch ANOVAs; and 2) We determined whether sensitivity to sequential information differed between groups during practice with separate Group × Trial type ANOVAs for each epoch. Sequence learning was significant in control children (Trial type × Epoch interaction), F (4, 76) = 2.71, p = .04, ηp2 = .13, but was not significant in children with ADHD, p = .09, ηp2 = .10. Further, sensitivity to sequential information was reduced in ADHD relative to control children at the midpoint of practice, in Epoch 3 (Group × Trial type interaction), F (1, 38) = 5.52, p = .02, ηp2 = .13, but not in other epochs (ps > .11, ηp2 < .07). Thus, the progression of learning was inconsistent in ADHD relative to control children.
Figure 1.
Median response time (in milliseconds) on the ASRT task as a function of epoch and trial type for the ADHD and control groups.
Analysis of coefficients of variation did not reveal group differences in intra-individual variability (ADHD: M = .34, SD = .14; CON: M = .34, SD = .23) as no main effects or interactions with group reached significance (all ps > .13, ηp2 < .06).
CC Task
One subject with ADHD was excluded for failing to comply with task instructions. Following Chun & Jiang (1998), trials without a response within 6 s were excluded from analysis. The mean number of trials without a response was small and did not differ between groups (ADHD: M = 1.37, SD = 1.98, control: M = 1.75, SD = 4.47, p = .73, d = .11).
Accuracy and median RTs for correct trials were computed for each participant and were analyzed in separate Group (ADHD vs. Control) × Configuration (Repeated vs. Novel) × Epoch (1 – 6) repeated measures ANOVAs. On this task, spatial contextual learning is defined by a Configuration × Epoch interaction indicating greater sensitivity to repeated spatial context with practice. Accuracy was not sensitive to contextual learning, as the Configuration × Epoch interaction was not significant, F (5, 185) = 1.95, p = .09, ηp2 = .05 (other ps > .13, ηp2 < .06); no other main effects or interactions reached significance. Overall accuracy was high in the ADHD (Repeated: M = 96.39%, SD = 2.27; Novel: M = 96.55%, SD = 2.38) and control (Repeated: M = 96.79%, SD = 2.35; Novel: M = 96.35%, SD = 2.77) groups.
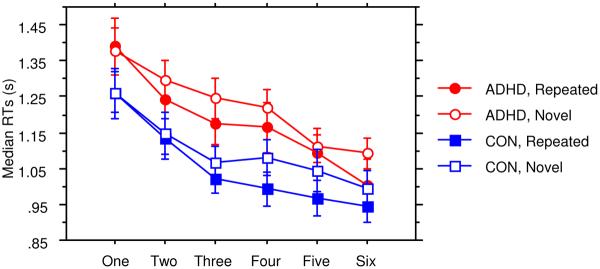
Analysis of RTs failed to reveal significant differences in response speed for ADHD (M = 1.20 s, SD = .27) and control (M = 1.05 s, SD = .23) children (main effect of Group), F (1, 37) = 3.05, p = .09, ηp2 = .08 (Figure 2). Participants exhibited improvement in visual search skill, as responses were faster with practice (main effect of Epoch) F (5, 185) = 61.11, p < .0001, ηp2 = .62. While overall responses were faster to Repeated than Novel configurations (main effect of Configuration), F (1, 37) = 15.84, p = .003, ηp2 = .30, children exhibited context-dependent learning, as the benefit of repetition increased with practice (Configuration × Epoch interaction), F (5, 185) = 2.52, p = .03, ηp2 = .06. No other interactions reached significance (all ps > .24, ηp2 < .04). Thus, in contrast to the ASRT task, spatial contextual learning did not differ between groups.
Figure 2.
Median response time (in seconds) on the CC task as a function of epoch and configuration for the ADHD and control groups.
In light of nominally slower visual search in ADHD relative to control children, we determined whether differences in learning were apparent on a measure that expressed learning as a proportion of one's baseline speed (i.e., Novel – Repeated/Novel calculated per epoch). Proportional learning scores computed for each participant were analyzed in a Group × Epoch ANOVA. Similar to the analysis of RTs, proportional measures of learning did not differ between groups (Group × Epoch interaction), p = .22, ηp2 = .04. Thus, the absence of group differences in learning cannot be attributed to baseline speed differences.
Analysis of coefficients of variation failed to reveal significant differences in response variability for ADHD (M = .45, SD = .05) relative to control (M = .42, SD = .05) children, F (1, 37) = 3.15, p = .08, ηp2 = .08. No other main effects or interactions reached significance (other ps > .19, ηp2 < .04).
For the recognition memory test, the percentage of repeated trials correctly identified as old (i.e., Hits) and the percentage of novel trials incorrectly identified as old (i.e., False Alarms) were computed for each participant and analyzed in a Group (ADHD vs. Control) × Trial (Hits vs. False Alarms) repeated measures ANOVA. Overall, the percentage of Hits (M = 58.33%, SD = 19.44) was greater than the percentage of False Alarms (M = 44.14%, SD = 18.83) (main effect of Trial), F (1, 35) = 6.23, p = .02, ηp2 = .15. However, recognition memory for repeated configurations did not differ between groups (main effect of Group, p = .13, ηp2 = .07, Group × Trial interaction, p = .57, ηp2 = .01). Thus, there were no group differences in recognition memory on the CC task.
Discussion
Characteristics of learning differed between children with ADHD and controls on one measure of implicit learning: Implicit sequence learning on the ASRT task progressed inconsistently in ADHD children such that it was similar early (Epochs 1 and 2) and later (Epochs 4 and 5) in learning but reduced at the midpoint (Epoch 3) relative to controls. In contrast, no characteristics of implicit spatial contextual learning on the CC task differed between children with ADHD and controls. Inconsistent sequence learning in ADHD children was not an artifact of atypical perceptual-motor performance because response latencies and variability did not differ between ADHD and control groups. These results suggest a variable rate of learning in childhood ADHD.
The present CC results differ from past studies in that recognition memory for repeated configurations was above chance. Bennett et al. (2009) recently reported that 4 out of 29 adults noticed that certain arrays were repeated and accurately described one of the repeated arrays. Thus, having a few participants in a sample develop explicit memory of some of the spatial regularities embedded within the task is not unprecedented. Inspection of individual children's recognition memory data revealed these results were due to superior recognition in a few control children. Specifically, high recognition accuracy in three control children (Hits: M = 100.0%, SD = 0; False Alarms: M = 2.78%, SD = 4.81) accounted for the significant difference between Hits and False Alarms. However, the control children with high recognition accuracy on the CC task did not influence CC learning results for the following reasons. First, their CC learning (M = .28, SD = .40) was within the 95% Confidence Interval of the remaining control children (CI = .27 ± .11). Second, and most importantly, lack of group difference in CC learning persisted after excluding those three control participants (Group × Epoch × Configuration interaction for RTs, p = .39). Thus, superior recognition memory in a subset of control children did not account for the lack of group differences in implicit spatial contextual learning.
It is unknown whether consistent progression of implicit sequence learning in control children was mediated by superior explicit memory for sequential information. It is unlikely that children would recognize the regularity in the ASRT task given that college students do not become aware of the regularity despite use of multiple and highly sensitive assessments including recognition and sequence generation tests (Howard et al., 2004). While explicit memory on the CC task may not necessarily be a good proxy for explicit memory for sequences per se, recognition memory on the CC task provides an index of children's explicit memory ability. The control children with superior explicit memory, however, did not inflate the magnitude of ASRT learning because: 1) Reduced implicit sequence learning in ADHD persisted after excluding those three participants (Group × Epoch × Trial type interaction for RTs, p = .01) and 2) Implicit sequence learning on the ASRT task for those participants (M = 85.83, SD = 27.26) was within the 95% confidence interval of the group (CI = 65.33 ± 28.08). Thus, using recognition memory on the CC task as an index of children's explicit memory ability, it appears that high recognition accuracy on the CC task did not relate to superior implicit sequence learning in controls.
Atypicality in sequential but not spatial contextual learning suggests that a learning process dependent upon frontal-striatal-cerebellar circuitry was selectively disrupted in ADHD. In light of current models of ADHD, at least two characteristics of that learning process are suggested by the present findings: First, functional and structural pathology of dorsal striatal projections with prefrontal regions are thought to underlie impaired executive control of responses in ADHD (Castellanos et al., 2006). Impaired executive response control, however, is unlikely to mediate the observed reduction in implicit sequence learning because learning occurs without explicit memory and independently of working memory capacity (Gabrieli, 1998). Executive control, however, requires conscious awareness and relies heavily on working memory capacity (Miller & Cohen, 2001). Indeed, the magnitude of ASRT learning in ADHD children in the present study (i.e., sum of the difference between trial types across epochs) did not correlate with parental report of the extent of executive dysfunction (measured using BRIEF Global Executive Composite, r = −.01). Further, electrophysiological studies with non-human primates reveal dissociable time courses of neuronal activity during learning and executive control. During associative learning, activity in striatal neurons encodes probabilistic information and their firing rate precedes that of prefrontal neurons (Pasupathy & Miller, 2005), whereas during executive control, activity in prefrontal neurons encodes goal related information and their firing rate precedes that of striatal neurons (Muhammad, Wallis, & Miller, 2006). Thus, impairments in implicit sequence learning and executive control may reflect distinct deficits in bottom-up and top-down frontal-striatal-cerebellar signaling, respectively.
Second, the present results most likely reflect aberrant temporal processing evidenced by behavioral and neuroimaging studies with ADHD participants (Nigg & Casey, 2005). As the to-be-learned sequence unfolds over time, deficits in predicting when an event is likely to occur, as posited by Nigg and Casey, are likely to be manifested in the lack of consistent acquisition of sequential information in the course of the task. As discussed in the introduction, children and adolescents with ADHD showed a smaller difference in response speed and reduced cerebellar activation when target stimuli on a Go/No-Go task appeared at an unexpected rather than an expected time (Durston et al., 2007). However, that study did not examine whether these differences emerged later in the task, suggesting impaired learning, or were pervasive throughout the task, suggesting an atypical response to novelty. The observed pattern of learning in the present study could relate to the reduced response to expectancy violations in ADHD, but it does not exclude the possibility that an atypical response to novel information could also contribute to the results. Further work is needed to disentangle the relationship between learning, temporal processing, and novelty processing in childhood ADHD.
Several limitations of the current study merit further attention. First, a subset of controls participated in a prior study (i.e., Barnes et al., 2008) that screened for the presence of any psychiatric or neurological disorders using the CBCL rather than the BASC. The CBCL and the BASC are highly correlated at this age (Doyle, Ostrander, Skare, Crosby, & August, 1997) and therefore, it is unlikely that the use of the two rating scales introduced heterogeneity into the screening of the control sample. Second, the mean IQs of the ADHD (M = 113.15) and control (M = 115.20) children were in the high-normal range. Further, IQ did not differ between groups, unlike studies with larger samples (e.g., Frazier, Demaree, and Youngstrom, 2004) that have reported significant differences, albeit of modest magnitude, in IQ between children with ADHD and control children. It is not known how level of intellectual function affects implicit learning. The task demands are low (i.e., visual search for a target for the CC task and pressing a key corresponding to target location for the ASRT task). Further, learning is unintentional and not dependent upon executive function. Therefore, IQ may affect magnitude of learning minimally. Nevertheless, it is important to examine these forms of learning in samples with a wider range of IQ to determine whether these findings generalize to lower IQ children. Third, we examined the status of implicit learning in children with either ADHD-combined subtype or ADHD-inattentive subtype. While the present sample size precludes direct investigation of subtype effects in the present study, this may be an important direction for future research, particularly in light of hypotheses that the two subtypes may reflect distinct disorders (Barkley, 2001; Diamond, 2005; Milich, Balentine, & Lynam, 2001). Prior studies have demonstrated that subtype effects are not significant for temporal processing (Barkley, Murphy& Bush, 2001). Given our speculation that the cognitive processes underlying ASRT task may overlap more with those underlying tasks of temporal processing than working memory or inhibition, we predict that there would not be subtype effects on the ASRT task. However, this question warrants direct investigation in future studies with larger sample sizes to improve statistical power to detect differences across subtypes and between children with ADHD and control children.
In sum, the present study extends atypical cognition in ADHD children beyond the domain of conscious processes to associative implicit learning. Our findings show atypical associative implicit learning mediated by frontal-striatal-cerebellar circuits but typical spatial contextual learning dependent upon the medial temporal lobes. Reduced implicit learning of sequences but not spatial-context has also been observed in dyslexia (Howard, Howard, Japikse, & Eden, 2006), another developmental disorder. However, dissociations between implicit sequence and spatial contextual learning are not ubiquitous in developmental disorders because both forms of learning were spared in autism spectrum disorders (ASD) (Barnes et al., 2008). While the symptomatology of ADHD, ASD, and dyslexia differs, they often share executive dysfunction and temporal processing deficits. Thus, exploring the relationship between cognitive processes mediated by frontal, striatal, and cerebellar regions (e.g., implicit sequence learning, executive control, and temporal processing) in a broader developmental context may ultimately yield the greatest insight into the neurocognitive basis of disordered development.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. University of Vermont; Burlington: 1991. [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. Third ed. The Guilford Press; New York: 2005. [Google Scholar]
- Barkley RA. The Inattentive Type of ADHD As a Distinct Disorder: What Remains To Be Done. Clinical Psychology. 2001;8:489–493. [Google Scholar]
- Barkley RA, Murphy KR, Bush T. Time perception and reproduction in young adults with attention deficit hyperactivity disorder. Neuropsychology. 2001;15:351–360. doi: 10.1037//0894-4105.15.3.351. [DOI] [PubMed] [Google Scholar]
- Barnes KA, Howard JH, Jr., Howard DV, Gilotty L, Kenworthy L, Gaillard WD, Vaidya CJ. Intact implicit learning of spatial context and temporal sequences in childhood Autism Spectrum Disorder. Neuropsychology. 2008;22:563–570. doi: 10.1037/0894-4105.22.5.563. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Barnes KA, Howard JH, Jr., Howard DV. An abbreviated implicit spatial context learning task that yields greater learning. Behavioral Research Methods. 2009;41:391–395. doi: 10.3758/BRM.41.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borger N, van der Meere J. Visual behaviour of ADHD children during an attention test: An almost forgotten variable. Attention Deficit Hyperactivity Disorder. Journal of Child Psychology and Psychiatry. 2000;41:525–532. doi: 10.1017/s0021963000005655. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends in Cognitive Science. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Chun MM. Contextual cueing of visual attention. Trends in Cognitive Science. 2000;4:170–178. doi: 10.1016/s1364-6613(00)01476-5. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Contextual cueing: Implicit learning and memory of visual context guides spatial attention. Cognitive Psychology. 1998;36:28–71. doi: 10.1006/cogp.1998.0681. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nature Neuroscience. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Diamond A. Attention-deficit disorder (attention-deficit/hyperactivity disorder without hyperactivity): A neurobiologically and behaviorally distinct disorder from attention-deficit/hyperactivity disorder (with hyperactivity) Development and Psychopathology. 2005;17:807–825. doi: 10.1017/S0954579405050388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denckla M. Biological correlates of learning and attention: What is relevant to learning disability and attention-deficit hyperactivity disorder. Developmental and Behavioral Pediatrics. 1996;17:114–119. [PubMed] [Google Scholar]
- Doyle A, Ostrander R, Skare S, Crosby RD, August GJ. Convergent and criterion-related validity of the Behavior Assessment System for Children-Parent Rating Scale. Journal of Clinical and Child Psychology. 1997;26:276–284. doi: 10.1207/s15374424jccp2603_6. [DOI] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Current Opinion in Neurology. 2008;21:478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD- Rating Scales DSM-IV for parents and teachers. Guilford; New York: 1998. [Google Scholar]
- Durston S, Davidson MC, Mulder MJ, Spicer JA, Galvan A, Tottenham N, Scheres A, Castellanos FX, van Engeland H, Casey BJ. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry. 2007;48:881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in Attention-Deficit/Hyperactivity Disorder. Neuropsychology. 2004;18:543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Frensch P, Buchner A, Lin J. Implicit learning of unique and ambiguous serial transitions in the presence and absence of a distractor task. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1994;20:567–584. [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annual Review of Psychology. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Child Neuropsychology. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Greene AJ, Gross WL, Elsinger CL, Rao SM. Hippocampal differentiation without recognition: an fMRI analysis of the contextual cueing task. Learning and Memory. 2007;14:548–553. doi: 10.1101/lm.609807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DV, Howard JH, Jr., Japikse K, DiYanni C, Thompson A, Somberg R. Implicit sequence learning: Effects of level of structure, adult age, and extended practice. Psychology and Aging. 2004;19:79–92. doi: 10.1037/0882-7974.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JH, Jr., Howard DV. Age differences in implicit learning of higher order dependencies in serial patterns. Psychology and Aging. 1997;12:634–656. doi: 10.1037//0882-7974.12.4.634. [DOI] [PubMed] [Google Scholar]
- Howard JH, Jr., Howard DV, Dennis NA, Yankovich H. Event Timing and age deficits in higher-order sequence learning. Aging, Neuropsychology, & Cognition. 2007;14:647–668. doi: 10.1080/13825580601186635. [DOI] [PubMed] [Google Scholar]
- Howard JH, Jr., Howard DV, Japikse KC, Eden GF. Dyslexics are impaired on implicit higher-order sequence learning, but not on implicit spatial context learning. Neuropsychologia. 2006;44:1131–1144. doi: 10.1016/j.neuropsychologia.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, Robertson IH. Response variability in attention deficit hyperactivity disorder: evidence for neuropsychological heterogeneity. Neuropsychologia. 2007;45:630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Kuhn D. Metacognitive development. Current Directions in Psychological Science. 2000;9:178–181. [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica (Amst) 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Manns JR, Squire LR. Perceptual learning, awareness, and the hippocampus. Hippocampus. 2001;11:776–782. doi: 10.1002/hipo.1093. [DOI] [PubMed] [Google Scholar]
- Milich R, Balentine AC, Lynam DR. ADHD combined type and ADHD predominately inattentive type are distinct and unrelated disorders. Clinical Psychology: Science & Practice. 2001;8:463–488. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Psychology. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Muhammad R, Wallis JD, Miller EK. A comparison of abstract rules in the prefrontal cortex, premotor cortex, inferior temporal cortex, and striatum. Journal of Cognitive Neuroscience. 2006;18:974–989. doi: 10.1162/jocn.2006.18.6.974. [DOI] [PubMed] [Google Scholar]
- Mulas F, Capilla A, Fernandez S, Etchepareborda MC, Campo P, Maestu F, Fernandez A, Castellanos FX, Ortiz T. Shifting-related brain magnetic activity in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2006;59:373–379. doi: 10.1016/j.biopsych.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Developmental Psychopathology. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer PT. Attentional requirements for learning: Evidence from performance measures. Cognitive Psychology. 1987;18:1–32. [Google Scholar]
- Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou JS, Hallett M. Procedural learning in Parkinson's disease and cerebellar degeneration. Annals of Neurology. 1993;34:594–602. doi: 10.1002/ana.410340414. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Preston AR, Gabrieli JD. Dissociation between Explicit Memory and Configural Memory in the Human Medial Temporal Lobe. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. The Behavior Assesment System for Children. American Guidance Services; Circle Pines, MN: 1992. [Google Scholar]
- Thomas KM, Hunt RH, Vizueta N, Sommer T, Durston S, Yang Y, Worden MS. Evidence of Developmental Difference in Implicit Sequence Learning: An fMRI Study of Children and Adults. Journal of Cognitive Neuroscience. 2004;16:1339–1351. doi: 10.1162/0898929042304688. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Huger M, Howard DV, Howard JH., Jr. Developmental differences in implicit learning of spatial context. Neuropsychology. 2007;21:497–506. doi: 10.1037/0894-4105.21.4.497. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Koroshetz WJ. Evidence for dissociable motor skills in Huntington's disease patients. Psychobiology. 1993;21:173–182. [Google Scholar]