Abstract
Objective
To determine if variations in common treatments for benign paroxysmal positional vertigo (BPPV) affected efficacy.
Study Design
Prospective, pseudo-randomized study.
Setting
Out-patient practice in a tertiary care facility
Subjects and Methods
Patients (n=118) with unilateral BPPV of the posterior canal, including 13 patients with BPPV of the lateral canal were tested at a tertiary care center on one of five interventions: canalith repositioning maneuver (CRP), CRP plus home exercise, modified CRP, CRP for patients with involvement of two semicircular canals, self-CRP home exercise. Self-CRP was also compared to previously published data on efficacy of the Brandt Daroff exercise. Main outcome measures were vertigo intensity and frequency, presence/ absence of Dix-Hallpike responses, Vestibular Disorders Activities of Daily Living Scale (VADL), computerized dynamic posturography.
Results
Vertigo intensity and frequency and Dix-Hallpike responses decreased significantly and posturography and VADL improved significantly from pre- to post tests. No other significant changes were found. The groups did not differ significantly. Vertigo intensity and frequency were not strongly related at pre-test but were related at post-test. Length of illness and age did not influence the results.
Conclusions
However the head is moved, as long as it is moved rapidly enough and through the correct planes in space repositioning treatments are likely to be effective. Therefore clinicians have a range of choices in selecting the treatment best suited for each patient’s unique needs.
Keywords: vertigo, dizziness, vestibular rehabilitation, repositioning maneuvers, benign positional nystagmus, vestibular
Introduction
Benign paroxysmal positional vertigo (BPPV) is a common problem. 1 Since exercises and passive maneuvers to reposition the putatively displaced otoconial matter were first described 2, 3, 4 several treatments have been described, mostly variations on Epley’s maneuver (CRP). 5,6,7,8,9 A self-CRP exercise has been described 10 but only one study has reported its effectiveness.11 In that study subjects had either a single out-patient CRP or home self-CRP three times daily for one week. Results were better in the home exercise group but the greater amount of treatment may have been the cause. No studies have yet compared the effectiveness of self-CRP to the older, Brandt Daroff exercise.
Recently, Rajguru et al, at University of Utah, modeled the ideal CRP based on the predicted mechanics of otoconial matter within the vestibular labyrinth. 12 They suggested that CRP would be more effective if the head was moved in a slightly different pattern than the classic CRP. 13 Faldon and Bronstein’s work suggests that as long as the head is moved with sufficient acceleration a maneuver should be successful. 14
We compared CRP without medication, mastoid vibration or post-treatment head restrictions 15 to groups treated with variations of repositioning treatments. The goal was to determine if any variation was more efficacious than our standard CRP.
Materials Methods
Subjects
The 118 subjects (28 males, 90 females; aged 26.2 to 83.0 yrs; mean age 56.9, SD 13.0) had unilateral BPPV for at least one week (median 8.7 wks, range 1 week to 15 years) and were referred to the senior author for vestibular rehabilitation. No subjects had been treated for BPPV previously. Most subjects had involvement of only the posterior semicircular canal. Some subjects also had lateral semicircular canal BPPV. (Table 1.)
Table 1.
Demographic data (mean age (yrs) and SD, percent females, and median length of illness), and equilibrium scores (EQT) and falls on posturography, SOT condition 5, eyes closed with sway-referenced force platform motion. Mean equilibrium scores, standard deviations and ranges in parentheses. Median falls, ranges in parentheses. At time 4, Group 2 had 1 subject. The historical Brandt Daroff group is reported for comparison.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Brandt Daroff |
|
|---|---|---|---|---|---|---|
| Mean age (yrs) (SD) |
56.4(13.8) | 55.4(12.4) | 57.5 (12.9) | 56.7 (13.5) | 58.4(12.2) | 56.3 (14.0) |
| % female | 68% | 66% | 77% | 92% | 73% | 56% |
| Median LOI (wks) |
9.6 | 9.6 | 9.4 | 11.3 | 7.9 | 17 |
| T1 EQT score |
45.9 (21.4, 0 to 84) |
52.0 (10.8, 34 to 65) |
41.5 (22.7, 0 to 77) |
42.8 (19.1, 0 to 73) |
36.9 (24.8, 0 to 74) |
40.6 (22.4, 0 to 72) |
| T2 EQT score |
59.1 (17,0 to 84) |
58.8 (9.7, 42 to 71) |
55.4 (12.9, 19 to 75) |
56.3 (16.1, 23 to 80) |
46.1 (24.1, 0 to 69) |
49.3 (15.0, 27 to 75) |
| T3 EQT score |
51.1 (20.3, 0 to 89) |
55.8 (9.2, 48 to 67) |
55.6, (19.3, 41 to 74) |
51.2 (17.9, 16 to 76) |
43.8 (20.0, 17 to 68) |
47.9 (19.4, 0 to 70) |
| T4 EQT score |
59.6 (12.4, 34 to 83) |
64.0 (n = 1) |
60.6 (10.9, 39 to 80) |
58.5 (11.0, 47 to 74) |
45.0 (25.1, 0 to 78) |
49.6 (21.4, 6 to 75) |
| T1 falls | 0 (0 to 3) | 0 (0 to 1) | 0 (0 to 3) | 0 (0 to 3) | 0 (0 to 3) | 0 (0 to 3) |
| T2 falls | 0 (0 to 3) | 0 (0) | 0 (0 to 1) | 0 (0 to 1) | 0 (0 to 3) | 0 (0 to 2) |
| T3 falls | 0 (0 to 3) | 0 (0) | 0 (0 to 1) | 0 (0 to 1) | 0 (0 to 2) | 0 (0 to 3) |
| T4falls | 0 (0 to 1) | 0 (0) | 0 (0 to 1) | 0 (0 to 1) | 0 (0 to 3) | 0 (0 to 2) |
Referring physicians, board-certified otolaryngologists and neurologists, at this institution and in the community, made all diagnoses based on their clinical examinations and objective diagnostic testing. Every subject had a unilaterally positive Dix-Hallpike test with nystagmus beating upward and ipsilaterally.16 Subjects with lateral canals involved also had unilateral, positive responses to positional tests lying on the involved side. 16 During Dix-Hallpike and positional tests, eye movements were recorded with infrared video-oculography and analyzed using software from Micromedical Technologies (Spectrum Software version S3.0.1). At the discretion of their physicians some subjects also had a full battery of bi-thermal caloric tests, low-frequency harmonic acceleration tests, and vestibular-evoked myogenic potentials. Every subject was tested by one of three experienced technicians who, collectively, had 51 to 55 years of experience, and were blinded to subjects’ group assignments.
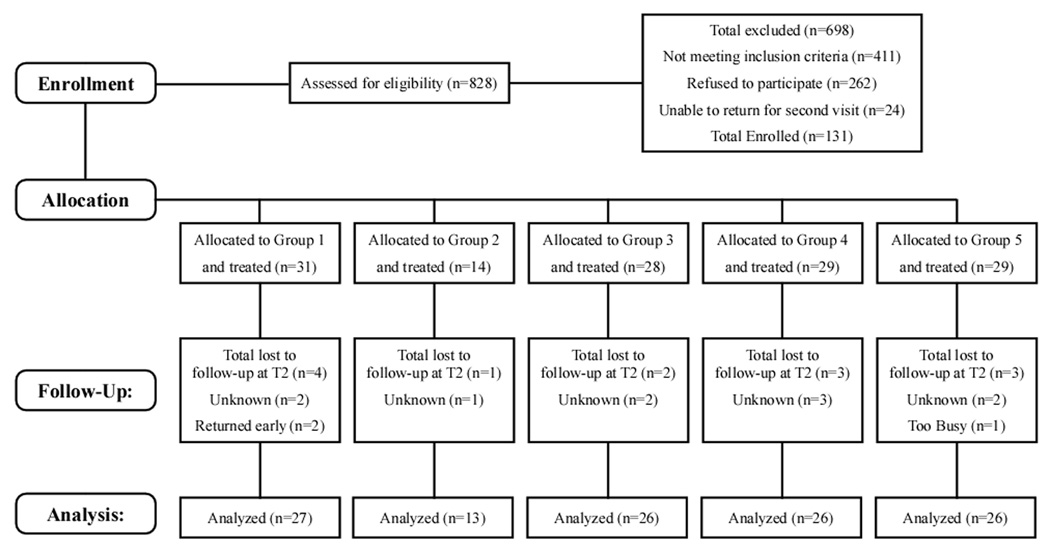
All subjects had cervical range of motion within functional limits; age-appropriate strength and motor coordination; no significant orthopedic, neurologic, or other otologic disorders; weight within range for computerized dynamic posturography, i.e. less than 136 kg, and were competent to give informed consent. All patients who met the inclusion criteria were invited to participate. (Figure 1.)
Figure 1.
Consort flow diagram of screening, recruitment and treatment.
Treatment Groups
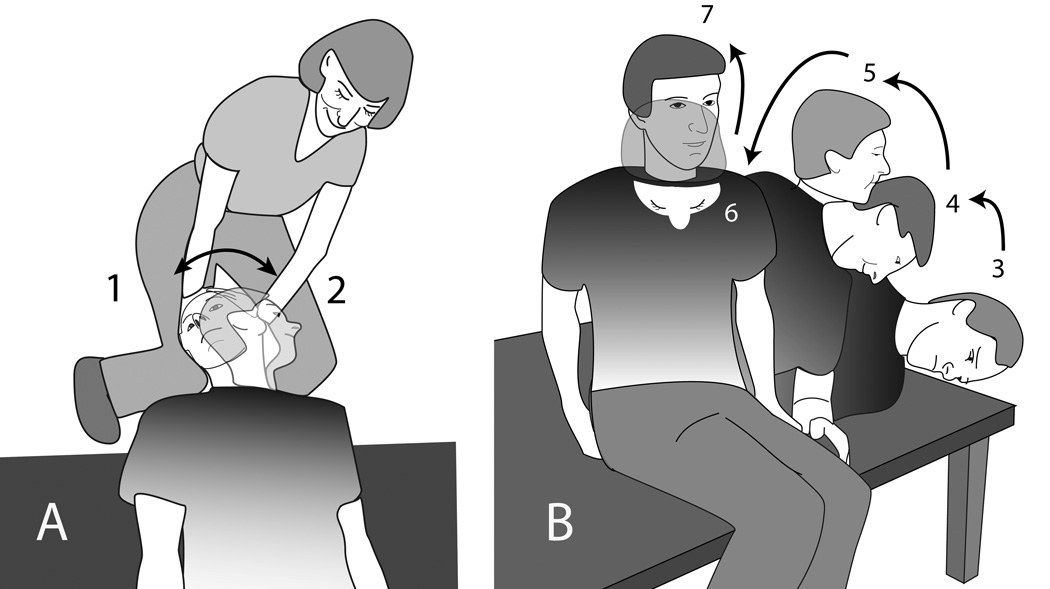
Group 1 received 3 trials of a standard CRP described previously: 15 1) Dix-Hallpike maneuver, 2) head turned 90° contralaterally (Figure 2A), 3) torso and head turned 30° further contralaterally (Figure 2B.3), 4) sit up, head turned contralaterally (not shown). The head was maintained in each position for 10 seconds after vertigo ceased or for at least 30 seconds if no vertigo was elicited. This inter-position interval was used in all groups. No premedication, mastoid vibration or home instructions for head position were used.
Figure 2.
Diagram of modified CRP used in Group 4. A. Position 1 is the Dix-Hallpike maneuver. In position 2 the head is rotated but the torso remains stationary. Positions 1 and 2 are the same as in CRP. B. In Positions 3 to 7 the head moves and the torso moves from side lying to upright. Position 3 is the same as in CRP.
In Group 2, which had involvement of 2 semicircular canals, subjects also received 3 trials of CRP as in Group 1. Group 2 was smaller because fewer patients present with two-canal involvement. The inter-trial interval was 2 to 3 minutes.
Group 3 received 3 trials of CRP and practiced a home program using the Brandt Daroff exercise 17 modified to keep the head turned so that the nose pointed approximately 30° away from the involved side throughout the exercise (approximately, because controlling the exact head position used at home was impossible), beginning by sitting on the side of her bed: 1) the subject briskly laid down on the ipsilateral side, 2) the subject briskly laid on the contralateral side, 3) the subject sat up. They performed 5 trials per session, 3 times daily: before breakfast, mid-day or upon returning home from work, and at bedtime.
Group 4 received 3 trials of CRP modified from the CRP used for Group 1, based on the computational model of CRP (Utah CRP). 12 Steps 1 to 3 were the same. Step 4) sit-up approximately 45° with head rotated −15° toward the ipsilateral side in yaw rotations, 5) sit up with nose turned to midline, 6) pitch the head downward 30°, 7) sit with head erect. (Figure 2A and B.) Group 5 practiced a self-CRP exercise at home, 10 3 trials per session, 3 times daily: before breakfast, mid-day or upon returning home from work, and at bedtime.
Pre- and Post-treatment Tests
Subjects were pre-tested when they were recruited to the study (T1). They were post-tested 1 week after treatment (T2) and were tasked to return 3 months (T3) and 6 months (T4) later. Actual T3 and T4 dates varied based on subjects’ personal schedules. All subjects returned at T2. The sample size decreased at later follow-up periods, however, perhaps because some subjects felt no need to return if they felt better. (Table 2). Thus results at T3 and T4 may be less robust than at T2. All available data for every subject were used in the statistical analyses.
Table 2.
VADL means with groups collapsed. (Standard deviations, ranges in parentheses).
| T1 (n=118) | T2 (n=118) | T3 (n=75) | T4 (n=53) | |
|---|---|---|---|---|
| Total | 2.4 (1.4,1.0–7.3) | 1.5 (1.0,1.0–7.0) * (p<0.0001) |
1.5 (0.9,1.0– 6.0) | 1.6 (1.2,1.0–6.0) |
| Functional | 2.4 (1.3,1.0–5.8) | 1.5 (0.7,1.0–5.4) *(p<0.0001) |
1.4 (0.7,1.0– 4.0) | 1.5 (1.0,1.0– 5.5) |
| Ambulation | 2.3 (1.4,1.0–8.8) | 1.6 (1.0,1.0– 7.1) * (p<0.0001) |
1.6 (1.1,1.0– 7.0) | 1.5 (1.1,1.0– 5.4) |
| Instrumental | 2.6 (2.1,1.0–10.0) | 1.6 (1.4,1.0 –9.0) * (p<0.0001) |
1.5 (1.0,1.0– 6.3) | 1.7 (1.6,1.0– 8.1) |
= significant change compared to previous test.
Pre- and post-test visits used the same measures. Each subject was asked to rate vertigo intensity and frequency using 10-point scales, where 1 was none and 10 was extreme vertigo (intensity) and where 1 was none and 10 was constant vertigo (frequency). For each scale, the subject viewed a 13 × 18 cm card showing each scale and selected the number that most closely matched her experience. Balance was evaluated using computerized dynamic posturography (EquiTest, Neurocom). Subjects also competed the Vestibular Disorders Activities of Daily Living Scale (VADL). 18,19
Medication Use
All subjects were instructed to avoid taking Antivert, generic meclizine, or other vestibular-suppressant medications for at least 24 hours before their first visit and to avoid taking them for the duration of participation in the study. No subjects received medication, special instructions about sleeping position, or mastoid vibration during treatment.
Informed consent, pseudorandomization, blinding
This study was approved by the Institutional Review Board for Human Subject Research for our institution. Subjects gave informed consent before administration of pretreatment study assessments. The sample size was calculated for power = 0.8, based on previous data.15 With alpha set to 0.05, a sample size of 26 per group was adequately powered (80%) to detect a difference in average change of 1.4 units in vertigo intensity or frequency (on a 10-point vertigo scale) between any two groups when standard deviation (SD) at baseline was 1.6 or less. For group 2 (N=13) comparisons, difference in average change detectable was about 1.7 units, using SD at baseline of 1.6 or lower.
Subjects completed study questionnaires, were tested on computerized dynamic posturography, and were assigned to groups. Except for subjects with involvement of two semicircular canals, who were assigned to Group 2, the senior investigator assigned groups from a spreadsheet in which the groups had been pseudo-randomized a priori, to maintain the sample size per group. If a subject in a group dropped out, i.e. did not return for the post-test at T2 despite reminders, the next subject who was recruited to the study was placed in that group, to maintain the group size. Tests on T1 were performed before group assignments were made.
Statistical Methods
Multilevel statistical methods 20 were used to describe changes in primary outcomes of interest between the five study groups. A separate model was produced and fitted to each outcome. Within each model, we examined significance of within subject effect over time as well as differences among the 5 study groups. Interaction effects were included in each model and tested. Changes over time were compared between study groups by using a likelihood ratio statistic, which follows a chi-square distribution. Adjustments were made for multiple comparisons. P <.05 was considered statistically significant. All analyses were performed using SAS Statistical software (SAS, Carry, NC).
Results
Vertigo measures
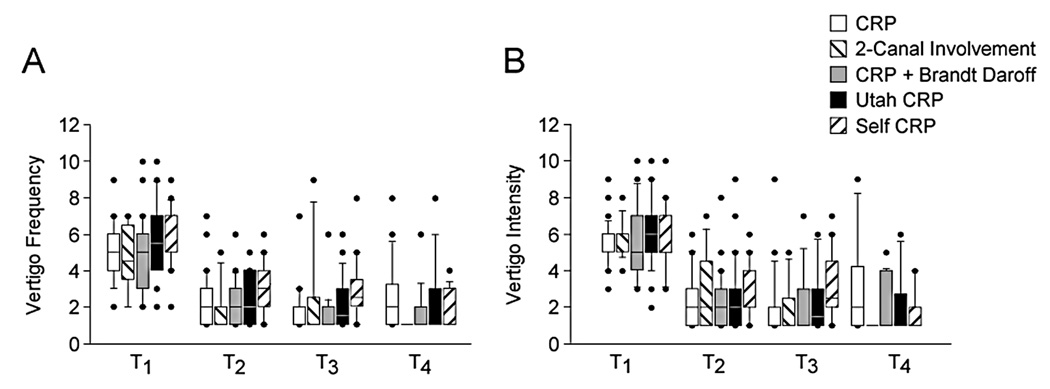
For all groups vertigo intensity and frequency decreased significantly from pre-test to the one-week post-test and plateaued after that. Vertigo frequency (mean (SD/range) at pre-test was 5.3 (1.8/2–10), at T2 was 2.4 (1.4/1–7), at T3 was 2.2 (1.7/1–9) and at T4 was 2.1 (1.7/ 1–8). Vertigo intensity mean (SD/range) at pre-test was 5.7 (1.7/2–10), at T2 was 2.6 (1.7/1–9), at T3 was 2.4 (1.9/1–9) and at T4 was 2.3 (2.0/ 1–9). The groups did not differ significantly. (Figure 3.)
Figure 3.
Changes in vertigo over test time. A. Vertigo frequency. B. Vertigo intensity. Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, circles are outliers.
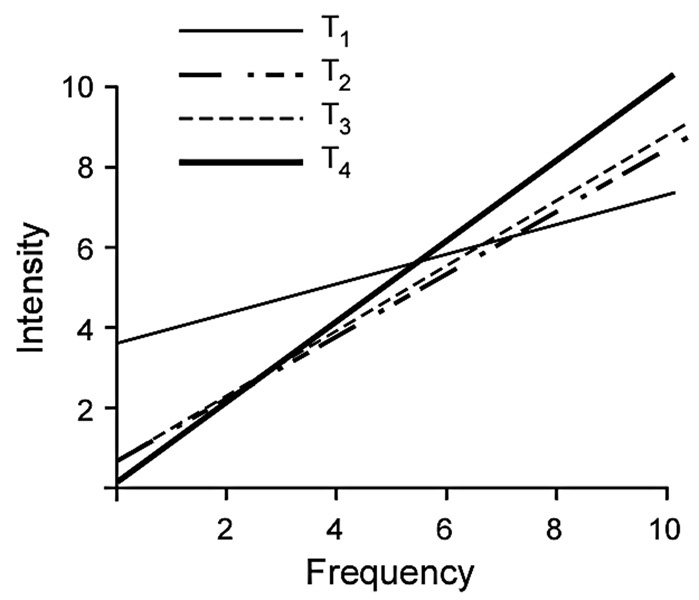
The relationship between vertigo intensity and frequency over time was tested with Spearman rank order correlations with the groups collapsed. At T1 the relationship was weak: r=0.3, p= 0.0002. At T2 the relationship was stronger: r=0.8, p< 0.0001. At T3 the relationship was even stronger: r=0.95, p< 0.0001. At T4 the relationship remained very strong: r=0.95, p< 0001. (Figure 4.)
Figure 4.
Correlations between intensity and frequency over test dates. The figure shows the actual regression lines.
Posturography scores
Equilibrium scores on Condition 5 (eyes closed, sway-referenced force platform motion) increased from T1 to T2, with no further changes at later tests. Group 5 had significantly lower (worse) equilibrium scores at than Group 1, CRP, at T2. No other differences were found among groups. (Table 1.) The number of falls decreased from preto post-test, with no further changes at later tests. Group 5, self-CRP, had more falls than other groups at pre-test but other groups did not differ significantly. (Table 1.)
VADL scores
The VADL scale was normed using the median score. We tested the scores using the means and the medians. Overall similar results were obtained with means and medians. On the mean total score, the groups did not differ significantly. With the groups collapsed total scores decreased (improved) significantly from T1 to T2 (F=13.3, p< 0.0001), but did not change significantly after T2. Functional, ambulation and instrumental subscores all decreased significantly from T1 to T2 (Functional: F=23.96, p< 0.0001; Ambulation: F = 8.7, p< 0.0001; Instrumental: F = 10.9, p< 0.0001) and then did not change significantly at T3 and T4. (Table 2.)
Dix-Hallpike responses
We tested presence or absence of nystagmus with a classical response pattern, i.e. horizontal and torsional quick phases beating ipsilaterally, upward-beating vertical quick phases. All subjects had nystagmus at T1. The groups did not differ significantly at T2, T3 or T4. The presence of nystagmus decreased significantly from T1 (when all subjects had nystagmus) to T2 (39.8%) (p<0.0001), and remained significant at T3 (37.3%) and T4 (39.6%) (p<0.0001). After T2 nystagmus did not decline significantly.
Length of illness
We tested the relationship between the length of time subjects had vertigo when they enrolled in the study, i.e. length of illness (LOI) and vertigo intensity, vertigo frequency, age, and presence or absence of response to the Dix-Hallpike maneuver at T1. Without the subject who reported having had vertigo for 15 years, which may have been inaccurate, regression showed that neither vertigo intensity, vertigo frequency, nor age were related to LOI: intensity/LOI, F=2.37, p=0.13; frequency/LOI, F=2.43, p=0.12; age/LOI, F=0.12, p=0.73. The relationship between Dix-Hallpike response and LOI was analyzed with an odds ratio; that test was also nonsignificant: odds ratio = 1.0, p=0.96. This finding confirms previous results. 15
Self-CRP group compared to previous Brandt Daroff group
Previous work suggested that the modified Brandt Daroff exercise was significantly more effective than a sham treatment and slightly less effective than CRP. 15 Those data were collected under comparable conditions, using the same staff. Therefore, we compared the present self-CRP group (Group 5) to the previous data on the Brandt Daroff exercise. Unlike the present Group 3 the previous Brandt Daroff group subjects did not also receive CRP. Thus, this comparison pairs two groups of similar subjects that only practiced a home exercise.
The self-CRP and Brandt Daroff groups did not differ significantly on vertigo intensity or frequency at any test dates. At T1 the self-CRP group had significantly lower VADL Total scores than the Brandt Daroff group (t=2.0, p=0.04), and significantly lower Ambulation subscores (t=2.10, p=0.03). The two groups did not differ significantly on Functional and Instrumental subscores. On VADL Total and Ambulation scores the self-CRP group continued to have lower scores than the Brandt Daroff group but the percent change from T1 to T4 did not differ significantly between the two groups. Likewise, VADL Functional and Instrumental subscores declined over tests for both groups with similar percent changes. (Table 3.)
Table 3.
Comparison between self-CRP group and historical Brandt Daroff group for median vertigo intensity and frequency (ranges in parentheses), percent of positive Dix-Hallpike responses, VADL medians (ranges in parentheses).
| Self CRP group | Historical Brandt Daroff group | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | |
| Vertigo Intensity |
5(3–10) | 2(1–6) | 2.5(1–7) | 1(1–4) | 7(4–10) | 5(2–8) | 3(1–9) | 3.5 (1–7) |
| Frequency | 6 (2–9) | 3(1–6) | 2.5(1–8) | 1(1–4) | 5(2–8) | 4(1–10) | 3(1–10) | 2.5 (1–7) |
| Dix-Hallpike response |
100% | 34.6% | 43.7% | 45.5% | 100% | 48% | 30% | 18.8% |
| VADL Total |
2(1–4) | 1(1–4) | 1(1–4) | 1(1–3) | 3(1–5) | 2(1–6) | 1(1–5) | 1(1–5) |
| Functional | 2(1–4) | 1(1–4) | 1(1–4) | 1(1–4) | 2.5(1–9) | 2(1.5) | 1(1–6) | 1(1–9) |
| Ambulation | 1(1–4) | 1(1–4) | 1(1–4) | 1(1–4) | 3(1–10) | 2(1–7) | 1(1–7) | 2(1–6) |
| Instrumental | 2(1–10) | 1(1–4) | 1(1–3) | 1(1–3) | 3(1–10) | 2(1–4) | 1(1–4.5) | 1(1–10) |
At T2 both groups had significantly decreased Dix-Hallpike responses. At T3 and T4 the self-CRP group did not differ significantly different from T2. The Brandt Daroff group, however, decreased further at T3 and T4 so that the change at T4 differed significantly from T2. The change at T3 was intermediate and did not differ from either T2 or T4. The Brandt Daroff group trended toward differing from the self-CRP group at T4 but the sample sizes were small at those times so differences are probably not meaningful. Larger and longer-term trials are needed to examine this issue.
On posturography, SOT Condition 5, both groups had decreased scores and some falls at pre-test, but improved significantly over time. The groups did not differ significantly. (Table 1.)
Discussion
The lack of differences among groups indicates that some variations in treatment methodology do not reduce the effectiveness of care. CRP has been shown to be significantly more effective than a sham treatment and slightly more effective than Brandt Daroff exercise. 15 This study shows that modifying the CRP, performing CRP plus a home program, or having involvement of a second semicircular canal does not change treatment effectiveness. One week of the self-CRP exercise is as effective as the modified Brandt Daroff exercise or three trials of CRP and its variations. Thus, self-CRP is an effective home program. It may be preferred for patients who have limited ability to sit up quickly.
This study replicated previous findings showing that CRP is effective in reducing vertigo and responses to the Dix-Hallpike maneuver. 8,15,21,22 and improving posturography and VADL scores.15 Previous work 23, 24 supports the finding that CRP plus a home program does not improve the outcome. By contrast, Tanimoto et al found that CRP, alone, was less effective than CRP plus self-CRP at home, 11 perhaps due to paradigm differences.
Consistent with previous research15 subjects in Groups 1,2,3 and 4 were all treated at T1 and did not receive CRP at subsequent dates. Since some patients may need a second or third treatment 25 the finding that some subjects were still symptomatic at T2 is not surprising. Also, some patients may have had subclinical Dix-Hallpike responses without concomitant vertigo. The underlying pathophysiology in such cases may have been residual otoconial matter in the semicircular canals. Anatomical work has shown that otoconial matter may be present in the semicircular canals of people who had not complained of vertigo in life. 26 Groups 3 and 5 practiced home exercise for a week. With the availability of the Internet some patients may try to treat themselves with instructions for exercises that they find on-line. Therefore, an important area for future research might be to determine the optimal treatment for subjects who self-treat with exercise.
The relationship between vertigo frequency and intensity changes over time. Before treatment vertigo frequency and intensity are weakly related. After treatment vertigo frequency and intensity are strongly related, suggesting that they co-vary with improvement after care. This finding provides indirect support for the theory that BPPV is caused by otoconial matter displaced into the semicircular canal. If the particles become relocated to the utricle then the frequency of episodes and the intensity of sensation should decrease as fewer particles remain in the involved canal.
CRP is a robust treatment technique. As performed in this study CRP is as effective as the liberatory maneuver, 4 CRP without the second 90° turn, and CRP with additional “shaking” head motions, 15, 27 with other variations on CRP with medication and compared to a no-treatment control group, 28 compared to a different sham group than that used by Cohen and Kimball 21 for more than 3 trials, 8 or with variations in the duration of each position. 21 Some investigators have used cervical collars, 22 but CRP is effective without them. Vibration and postural restrictions do not influence effectiveness. 29, 30 Repositioning treatments appear to be efficacious, regardless of minor differences among maneuvers. If the head is moved in the appropriate motions, and rapidly enough -- approximately 55°/second to 75°/second, 14 the treatment is likely to be effective. Thus, minor variations in technique may not substantially influence the outcome of treatment and the clinician can be confident in using repositioning treatments with a wide variety of patients.
The clinician has several options for care and may tailor the treatment plan to meet the needs of the individual patient as long as the basic requirements for head movement are met. Several trials of CRP given in the out-patient clinic may be preferable because CRP takes only a few minutes but a home program takes several days. Therefore, the clinician need not feel obligated to recommend a home program, especially when musculoskeletal limitations make a home exercise program contraindicated.
The home-based repositioning exercises described here are effective, but with a caveat. The patient must be instructed properly. Patients do not learn exercises from printed directions. In this study, the senior author explained the premise of the exercise, demonstrated it herself, had the patient practice it, reiterated the instructions, give the patient written instructions to take home, and than had the patient repeat the instructions and ask questions. This process takes time. During treatment planning the clinician should decide if she has time to instruct the patient in home exercise or if office-based CRP would be as effective and more efficient. The recurrence rate for BPPV is high 24 so some patients may have recurrences. The clinician could use CRP in the office and then give a home exercise for use initially in the case of a recurrence. The patient could be encouraged to return for out-patient care if the home-based exercise does not resolve the symptoms.
Acknowledgements
We thank the staff of the Center for Balance Disorders, Baylor College of Medicine, and Ondrej Cakrt, MSc, PT, Second Medical Faculty, Charles University, for their invaluable technical assistance.
Source of support
Supported by NIH grant R01 DC003602.
Footnotes
Financial Disclosure
Conflict of interest: none
References
- 1.von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo. A population based study. J Neurol Neurosurg Psychiatry. 2007;78:710–715. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt T, Daroff RB. Physical therapy for benign paroxysmal positional vertigo. Arch Otolaryngol Head Neck Surg. 1980;106:484–485. doi: 10.1001/archotol.1980.00790320036009. [DOI] [PubMed] [Google Scholar]
- 3.Epley JM. The canalith repositioning procedure: for treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1992;107:399–404. doi: 10.1177/019459989210700310. [DOI] [PubMed] [Google Scholar]
- 4.Semont A, Freyss G, Vitte E. Curing the BPPV with a liberatory maneuver. Adv Oto Rhino Laryngol. 1988;42:290–293. doi: 10.1159/000416126. [DOI] [PubMed] [Google Scholar]
- 5.Massoud ES, Ireland DJ. Post-treatment instructions in the nonsurgical management of benign paroxysmal positional vertigo. J Otolaryngol. 1996;25:121–125. [PubMed] [Google Scholar]
- 6.Parnes LS, Price-Jones RG. Particle repositioning maneuver for benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol. 1993;102:325–331. doi: 10.1177/000348949310200501. [DOI] [PubMed] [Google Scholar]
- 7.Welling DB, Barnes DE. Particle repositioning maneuver for benign paroxysmal positional vertigo. Laryngoscope. 1994;104:946–949. doi: 10.1288/00005537-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Yimtae K, Srirompotong S, Srirompotong S, Sae-seaw P. A randomized trial of the canalith repositioning procedure. Laryngoscope. 2003;113:828–832. doi: 10.1097/00005537-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Harvey SA, Hain TC, Adamiec LC. Modified liberatory maneuver: effective treatment for benign paroxysmal positional vertigo. Laryngoscope. 1994;104:1206–1212. doi: 10.1288/00005537-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Radtke A, Neuhauser H, von Brevern M, Lempert T. A modified Epley's procedure self-treatment of benign paroxysmal positional vertigo. Neurology. 1999;53:1358–1360. doi: 10.1212/wnl.53.6.1358. [DOI] [PubMed] [Google Scholar]
- 11.Tanimoto H, Doi K, Katata K, Nibu K-i. Self-treatment for benign paroxysmal positional vertigo of the posterior semicircular canal. Neurology. 2005;65:1299–1300. doi: 10.1212/01.wnl.0000180518.34672.3d. [DOI] [PubMed] [Google Scholar]
- 12.Rajguru SM, Ifediba MA, Rabbitt RD. Three-dimensional biomechanical model of benign paroxysmal positional vertigo. Ann Biomed Eng. 2004;32:831–846. doi: 10.1023/b:abme.0000030259.41143.30. [DOI] [PubMed] [Google Scholar]
- 13.Brandt T, Steddin S, Daroff RB. Therapy for benign paroxysmal positioning vertigo, revisited. Neurology. 1994;44:796–800. doi: 10.1212/wnl.44.5.796. [DOI] [PubMed] [Google Scholar]
- 14.Faldon ME, Bronstein AM. Head accelerations during particle repositioning manoeuvres. Audiol Neurotol. 2008;13:345–356. doi: 10.1159/000136153. [DOI] [PubMed] [Google Scholar]
- 15.Cohen HS, Kimball KT. Effectiveness of treatments for benign paroxysmal positional vertigo of the posterior canal. Otol Neurotol. 2005;26:1034–1040. doi: 10.1097/01.mao.0000185044.31276.59. [DOI] [PubMed] [Google Scholar]
- 16.Coats AC. Electronystagmography. In: Bradford LJ, editor. Physiological Measures of the Audio-Vestibular System. New York: Academic Press; 1975. pp. 37–85. [Google Scholar]
- 17.Radtke A, von Brevern M, Tiel-Wilck K, Mainz-Perchalla A, Neuhauser H, Lempert T. Self-treatment of benign paroxysmal positional vertigo: Semont maneuver vs Epley procedure. Neurology. 2004;63:150–152. doi: 10.1212/01.wnl.0000130250.62842.c9. [DOI] [PubMed] [Google Scholar]
- 18.Cohen HS, Kimball KT. Development of the Vestibular Disorders Activities of Daily Living Scale. Arch Otolaryngol Head Neck Surg. 2000;126:881–887. doi: 10.1001/archotol.126.7.881. [DOI] [PubMed] [Google Scholar]
- 19.Cohen HS, Kimball KT, Adams AD. Application of the Vestibular Disorders Activities of Daily Living Scale. Laryngoscope. 2000;110:1204–1209. doi: 10.1097/00005537-200007000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Snijders T, Bosker R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Thousand Oaks, CA: Sage Publications; 1999. [Google Scholar]
- 21.Froehling DA, Bowen JM, Mohr DN, et al. The canalith repositioning procedure for the treatment of benign paroxysmal positional vertigo: a randomized controlled trial. Mayo Clin Proc. 2000;75:695–700. doi: 10.4065/75.7.695. [DOI] [PubMed] [Google Scholar]
- 22.Lynn S, Pool A, Rose D, Brey R, Suman V. Randomized trial of the canalith repositioning procedure. Otolaryngol Head Neck Surg. 1995;113:712–270. doi: 10.1016/S0194-59989570010-2. [DOI] [PubMed] [Google Scholar]
- 23.Helminski JO, Janssen I, Hain TC. Daily exercise does not prevent recurrence of benign paroxysmal positional vertigo. Otol Neurotol. 2008;29:976–981. doi: 10.1097/MAO.0b013e318184586d. [DOI] [PubMed] [Google Scholar]
- 24.Helminski JO, Janssen I, Kotaspouikis D, et al. Strategies to prevent recurrence of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2005;131:344–438. doi: 10.1001/archotol.131.4.344. [DOI] [PubMed] [Google Scholar]
- 25.Cohen HS, Jerabek J. Efficacy of treatments for posterior canal benign paroxysmal positional vertigo. Laryngoscope. 1999;109:584–590. doi: 10.1097/00005537-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Schuknecht HF. Cupulolithiasis. Arch Otolaryngol Head Neck Surg. 1969;90:765–778. doi: 10.1001/archotol.1969.00770030767020. [DOI] [PubMed] [Google Scholar]
- 27.Cohen HS, Kimball KT. Treatment variations on the Epley maneuver for benign paroxysmal positional vertigo. Am J Otolaryngol. 2004;25:33–37. doi: 10.1016/j.amjoto.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Asawavichianginda S, Isipradit P, Snidvongs K, Spiyaphun P. Canalith repositioning for benign paroxysmal positional vertigo: a randomized, controlled trial. Ear Nose Throat J. 2000;79:732–737. [PubMed] [Google Scholar]
- 29.Hain TC, Helminski JO, Reis IL, Uddin MK. Vibration does not improve results of the canalith repositioning procedure. Arch Otolaryngol Head Neck Surg. 2000;126:617–622. doi: 10.1001/archotol.126.5.617. [DOI] [PubMed] [Google Scholar]
- 30.Casqueiro JC, Ayala A, Monedero G. No more postural restrictions in posterior canal benign paroxysmal positional vertigo. Otol Neurotol. 2008;29:706–709. doi: 10.1097/MAO.0b013e31817d01e8. [DOI] [PubMed] [Google Scholar]