Abstract
The efficient migration of mesenchymal stem cells (MSCs) to diseased tissues is required for the fulfillment of their regenerative potential. Recruitment of circulating cells into the damaged tissues is regulated by a complex network, which includes the non-neural cholinergic system. We found that human MSCs (hMSCs) express nicotinic acetylcholine receptor subunits alpha 7, beta 2 and beta 4. The receptor agonist nicotine caused calcium (Ca++) influx into hMSCs suggesting that the calcium ion channel alpha 7 homopolymer mediated this response. While high concentrations of nicotine (10−5M) induced hMSC apoptosis, physiological concentrations (10−7M) did not interfere with cell survival. At non-toxic concentrations, nicotine increased spontaneous migration of hMSCs, whereas chemotaxis of hMSCs toward C3a and bFGF in vitro and migration of intravenously infusion hMSCs into bone marrow and spleen in vivo were inhibited. The antagonist for the alpha 7 homopolymer, bungarotoxin, blocked the inhibitory effect of nicotine on chemotactic factor-induced migration of hMSCs. These findings reveal an involvement of the non-neural cholinergic system in regulation of hMSC migration.
Introduction
Human mesenchymal stem cells (hMSCs) possess a great capacity for tissue regeneration. Systemic intravascular delivery of hMSCs would be desirable for treatment of multifocal disorders including muscular and skeletal diseases. The efficacy of the intravascular administration of hMSCs depends on the ability of circulating hMSCs to migrate into the damaged areas (Khaldoyanidi 2008; Karp and Leng Teo 2009). Over the past decade several molecular pathways that regulate hMSC homing were identified (Chamberlain, Fox et al. 2007). However, the role of the non-neural cholinergic system in regulation of hMSCs migration has not been investigated.
The existence and functional relevance of a non-neural cholinergic system that is independent of cholinergic nerves is now a well established concept (Wessler, Kirkpatrick et al. 1998; Gahring and Rogers 2005; Grando, Kawashima et al. 2007). Elements of the cholinergic system including acetyltransferase, acetylcholinesterase and acetylcholine receptors (AChRs) are expressed in a large array of non-neural cells including epithelial and endothelial cells, mature blood cells, hematopoietic progenitors, osteoblasts, fibroblasts and mesenchymal stem cells (Grando 1997; Heeschen, Jang et al. 2001; Walker, Preston et al. 2001; Deutsch, Pick et al. 2002; Kawashima and Fujii 2004; Serobyan, Schraufstatter et al. 2005; Fujii, Takada-Takatori et al. 2008; Hoogduijn, Cheng et al. 2008; Rothem, Rothem et al. 2009). The non-neural cholinergic system regulates cell proliferation, migration, and the expression of adhesion molecules, ECM molecules, cytokines and chemokines (Tomek, Rimar et al. 1994; Tipton and Dabbous 1995; Carty, Soloway et al. 1996).
The effects of the cholinergic system on cell function are mediated by AChRs that have been divided into 2 major subfamilies: the muscarinic and nicotinic. Receptors of the muscarinic type are G-protein coupled receptors, whereas the nicotinic acetylcholine receptors (nAChR) are ion channel (ionotrophic) pentameric proteins that respond to nicotine as the agonist. The subunits are arranged in a circle to create a transmembrane pore, and the complete receptor complexes generally have two ligand binding sites. At the entrance and exit of these pore complexes are rings of charged side chains that can help explain ion selectivity (Hogg, Raggenbass et al. 2003; Kalamida, Poulas et al. 2007). In humans the α-subfamily consists of 9 members (α1-α10 with the α8 apparently absent), and the β-subfamily of 4 members (β1-β4). Most nAChR are heteropolymers, but the α7, α9, and α-10 nAChRs are homopolymers (Gotti and Clementi 2004; Gotti, Moretti et al. 2007; Millar and Harkness 2008). While nAChRs typically provide transmembrane channels for potassium and sodium ions, the nAChR consisting of a homopolymer of α7 provides gating to calcium ions (Kalamida, Poulas et al. 2007). Interestingly, the α7 nAChR has been assigned a role as a receptor that mediates the effects of the cholinergic system in non-neural tissues (Wang, Yu et al. 2003; Gallowitsch-Puerta and Tracey 2005; de Jonge and Ulloa 2007; Fujii, Fujigaya et al. 2007).
In this study, we demonstrated that hMSCs express a panel of α- and β-subunits of nAChR. A biological activity of α7 sub-units was determined by measuring nicotine-induced calcium influx in hMSCs. We further found that nicotine-induced activation of α7 subunit of nAChRs inhibits C3a- and bFGF-induced migration of hMSCs.
Materials and Methods
Cell Culture and Reagents
Human mesenchymal stem cells (hMSCs) were provided by Dr. D. Prockop (Tulane University, New Orleans, LA). The stem cell properties of the provided samples of hMSCs were verified by the providers prior to the banking and shipping of the cells. The cells were cultured in alpha-MEM (Invitrogen, Carlsbad, CA) with 16.5% fetal calf serum (Atlanta Biologicals) and used up to passage 5. Where indicated, hMSCs were stimulated with 10−5M, 10−6M, 10−7M nicotine (Sigma), 10–100 nM C3a produced as previously described (Schraufstatter, Discipio et al. 2009), 20 ng/ml bFGF (Santa Cruz Biotechnologies), and 100 nM α-bungarotoxin (Calbiochem), a selective antagonist for the α7 sub-unit of nAChR. For in vivo homing studies, GFP-transfected hMSCs provided by Dr. D. Prockop were used.
RT-PCR
RNA from cultured hMSCs was isolated with the RNeasy kit (Qiagen, Valencia, CA). Complementary DNA was synthesized from MSC RNA using Omniscript reverse transcriptase (Qiagen). The detailed description of primers used for the PCR is provided in Supplementary Table 1. Hot start RT-PCR was performed using Qiagen’s Taq PCR master mix kit. Amplification was performed for 40 cycles. DNA derived from the PCR reactions was cloned into the plasmid pCR II (Invitrogen, Carlsbad, CA) and was sequenced using a capillary ABI 3730 sequencer (Retrogen, San Diego, CA).
Western Blot Analysis
Monolayers of cultured hMSCs were lysed with modified RIPA buffer (50 mM Tris-HCl, pH 7.4, 10% glycerol, 1% NP-40, 150 mM NaCl, 5 mM MgCl2, 2 mM EDTA, 0.2 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 2 mM sodium pyrophosphate, 2 mM sodium vanadate and 10 mM NaF) and clarified by centrifugation. The cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membranes, blocked with 4% dry milk in TBS-Tween, and exposed to specific primary antibodies as described for each experiment. Antibody binding was detected using horseradish peroxidase (HRP)-conjugated goat anti-rabbit or anti-mouse secondary antibodies and enhanced chemi-luminescence (ECL Plus, GE Lifesciences).
Intracellular Calcium Ion Changes
Calcium mobilization induced by nicotine was performed with hMSCs using the Fluo-4 NW Calcium assay kit (Molecular Probes/Invitrogen, Carlsbad, CA). Human MSC were grown to confluence on Falcon 24 well plates followed by washing with the assay buffer. The calcium sensitive dye, Fluo-4 AM (2 μM) was added to the cultured hMSC and allowed to incorporate for 30 min. The relative fluorescence was measured before and after the addition of 2 μM nicotine or a buffer control. Data were subtracted from the background and are presented as delta (Δ) between control- and nicotine-treated samples. Where indicated, EDTA (5 mM) was added to the buffer prior to the nicotine addition to chelate free calcium ions.
Measurements were made in a Wallac/Perkin Elmer Victor V2 fluorescent plate reader by taking data points every 1 sec over a period of 90 seconds. Data were subtracted from background and presented as the mean of two separate experiments.
Immune-Fluorescence Staining and Confocal Microscopy
MSC were grown on collagen-coated glass cover slips until 50% confluent. The cells were fixed with 4% paraformaldehyde in phosphate buffered saline for 30 min, and permeabilized with 0.02% Triton X100. After washing and blocking with 2% FCS in PBS for 2 h at room temperature, the cells were treated with diluted solutions (1:200) of primary antibody (rabbit anti-human-AChR β2, β4, α7, a gift from Dr. Skok, Palladin Institute for Biochemistry, Kiev, Ukraine) overnight at 4° C. After washing, the cells were incubated with anti-rabbit IgG conjugated with Alexa-488 in PBS containing 2% FCS for 1 h at room temperature. Negative controls were without primary antibody and with a rabbit IgG control. After washing and staining the nuclei with DAPI (4′-6-diamidino-2-phenylindole) (Sigma Chemical Co.) for 10 min, the cells were washed and covered with a drop of AntiFade (Molecular Probes). Images were taken on an Olympus Fluoview FV1000 confocal microscope.
Apoptosis Assays
Human MSC were grown in the wells of a 24 well plate until 50% confluent. Following exposure to nicotine (10−5, 10−6, 10−7M) or 100μM H2O2, the cells were incubated for 24 hrs in a 37°C tissue culture incubator. Cells were collected by trypsinization, washed twice and re-suspended in fresh media. An aliquot of cells was used to determine cell viability by trypan blue exclusion. The remaining cells were stained with 0.6μg/ml propidium iodide (PI) and immediately examined using a ASI Nikon Fluorescent Microscope.
Apoptotic/necrotic cells were identified as PI-stained cells and calculated as the fraction of PI-positive cells of the total cell number counted with phase contrast. Where indicated, cell viability was assessed at 24 hrs using a FITC-Annexin V/PI staining kit (Calbiochem) followed by FACS analysis.
Transmigration Assay
A single cell suspension of cultured hMSCs was loaded into the upper wells of 0.15% gelatin-coated Transwells (Costar, 8μm pore-size, 2 × 104cells/insert) in triplicates. The lower wells contained culture media containing 0.2% BSA, but no growth factors, or the same media supplemented with 100nM C3a or 20ng/ml bFGF (Santa Cruz Biotechnologies).
The assembled wells were incubated for 16 hrs in a tissue culture incubator, cells in the upper compartment were carefully removed, the filters were stained with DAPI and the transmigrated cells were counted on a Nikon Eclipse TE200 inverted fluorescence microscope (Nikon Instruments, Melville, NY) with a Spot camera system (Diagnostics Instruments).
In vivo Homing of hMSCs
Eight-week-old female NOD/SCID mice were obtained from Jackson Laboratory (Bar Harbor, Maine, USA) and kept under standard pathogen-free conditions. Cultured GFP-transfected hMSCs (GFP-hMSCs) were injected into NOD/SCID mice through the tail vein at a concentration of 3×105cells per mouse (3 mice per group). Where indicated, GFP-hMSCs were pretreated with 300 nM C3a.
Following cell injection, mice were intravenously treated with 10−8M nicotine. After 24 hours, mice were sacrificed, blood, bone marrow, spleen, liver, lungs and brain were collected, a single cell suspension was prepared and examined for the number of migrated GFP-hMSCs using FACS analysis. All experiments were conducted in agreement with the institutional policy on animal use and approved by the Institutional Animal Care and Use Committee (IACUC).
Results
nAChR Subunits Are Expressed by Human MSCs
RT-PCR was used to detect the expression of α and β sub-units of nAChR in hMSCs on the mRNA level. Supplementary Table 1 shows the sequence of primers used for the detection of each individual nAChR sub-unit. Correctly sized RT-PCR products of the correct size for the nAChR subunits α1, α2, α3, α4, α5, α7 and α9 were detected in hMSCs (Figure 1A). Although a band was seen for the α6 sub-units, it was larger than the expected 301 bp, and therefore was identified as an artifact. While a smear of bands for α10 was detected on the gel, no product of the expected size of 234 bp was observed.
Figure 1.
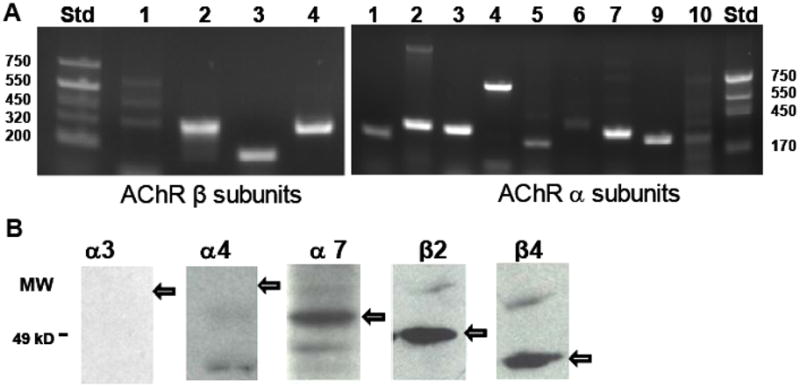
Expression of nAChR subunits in hMSCs. A: The expression of sub-unit specific mRNA in hMSCs was detected by RT-PCR. RNA was purified from three separate sets of hMSCs cultures and reverse transcribed as described in the Method section. The sequences of primers used to amplify mRNA from each sub-unit are shown in Supplemental Table 1. Products for the nAChR subunits were consistently detected as shown on representative gels for α (left gel) and β (right gel) sub-units out of three similar experiments. B: The nAChR subunit protein expression was detected by Western blotting. Cell lysates were prepared from hMSCs and immunoblotted as described in the Method section. Arrows indicate the location of the anticipated protein band. One experiment representative of two similar experiments is shown.
We detected clear bands for the nAChR subunits β2, β3 and β4. In the case of the β1 sub-unit, three bands were detected after RT-PCR, but these are likely artifacts as the smallest band is 320 bp being somewhat larger than the expected 282 bp product (Figure 1A). DNA sequencing verified that the correct size products for α7, β2 and β4 were indeed the respective nAChR subunits.
We next analyzed the expression of nAChR subunits in hMSCs on the protein level. Western blotting (Figure 1B) was performed on a limited set of nAChR sub-units, namely α3, α4, α7, β2 and β4. Specific bands were observed only for α7, β2, and β4. Although these bands ran at a lower molecular weight than anticipated, previous reports had shown that natural as well as recombinant nAChR behave in this fashion on SDS gels (Chen and Patrick 1997; Beckel, Kanai et al. 2006).
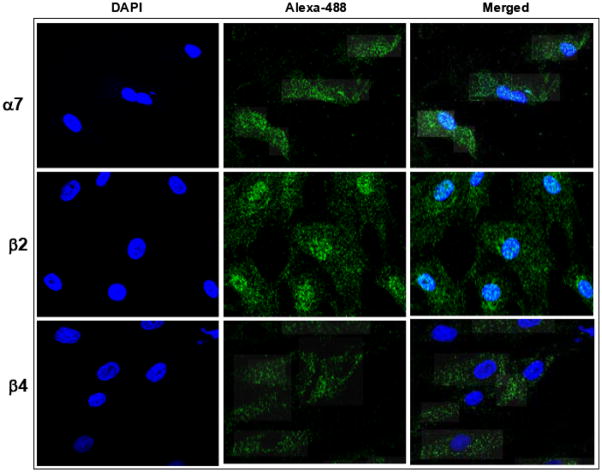
Several weak cross-reacting protein bands were noted for α4, however none of these were of a previously reported molecular weight. By confocal microscopy we detected strong labeling for the nAChR α7, β2, and β4 subunits (Figure 2) present on a great majority of MSCs, but not for the α3 or the α4 sub-units (not shown). α7 subunits were distributed on the cell surface of the whole population of hMSCs. Curiously, β2 subunit labeling tended to concentrate around the nucleus. In contrast, no nucleus-associated staining was observed for the β4.
Figure 2.
Expression of nAChR proteins in hMSCs. The nAChR subunits were visualized by immune fluorescence. Nuclei were stained with DAPI (blue). To visualize binding of nAchR sub-unit specific antibodies, secondary Alexa-488 labeled antibodies were used (green). Merged images are shown in the right panel. The images are representative of five separately stained slides with hMSC.
Since α7 subunits of nAChRs play an important regulatory role in non-neural cells (Gallowitsch-Puerta and Tracey 2005; de Jonge and Ulloa 2007), and because a specific antagonist of the α7 subunit, α-bungarotoxin (Pugh and Berg 1994), is available, the following studies are focused on the function of the nAChRs α7 homopolymer.
nAChRs Expressed in hMSCs are Functionally Active
Since α7 nAChRs are calcium ion channels (Kalamida, Poulas et al. 2007), functional activity of the α7 homopolymer can be measured by the detection of calcium influx. We used nicotine, a chemically stable compound, as the receptor agonist to stimulate nAChRs. To test the functional activity of the α7 nAChRs, hMSCs were loaded with the calcium sensitive dye Fluo-4 followed by incubation in culture medium containing Ca++ or in the same media supplemented with 5 mM EDTA, which chelates Ca++ and prevents its cellular uptake. The relative fluorescence was measured before and after the addition of 2 μM nicotine or a buffer control (control). The results indicate that the of fluorescence between control and nicotine samples was significantly higher in the presence of free calcium ions suggesting that calcium is transported from the media into cells treated with nicotine (Figure 3). No difference between control and nicotine samples was detected when Ca++ was bound by EDTA indicating that the increase in intracellular calcium was due to influx from the extracellular medium rather than mediated by release from intracellular stores. These findings suggest that nicotine activated α7 nAChRs expressed by hMSCs are functionally active since only the α7 nAChR forms a calcium ion channel.
Figure 3.
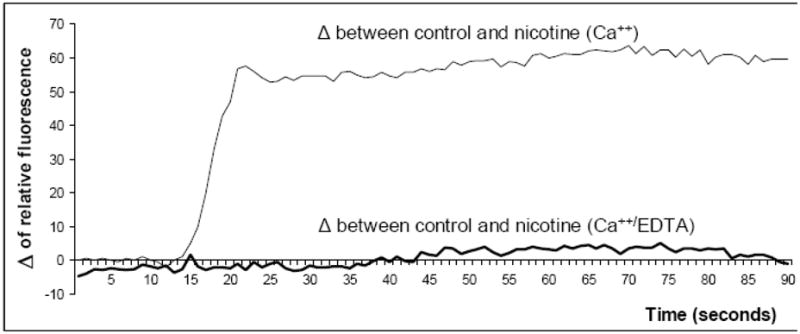
Functional activity of nAChRs in hMSCs determined by calcium flux. The effect of nicotine on calcium influx by MSC was tested. hMSCs were loaded with Fluo-4, a fluorescent calcium indicator, and incubated in phenol red free culture medium containing 1.26 mM calcium ions (Ca++) or in the same culture medium and 5 mM EDTA (Ca++/EDTA). The relative fluorescence was measured immediately after the addition of 2 μM nicotine or the buffer control (control samples). Data were subtracted from the background and are presented as a delta (Δ) of relative fluorescence between control and nicotine-treated samples. The depicted results are the mean of two separate experiments.
Activation of nAChRs Induces Cell Death
Increased intracellular calcium can cause cell death (Berridge, Bootman et al. 1998), and it was therefore important to determine whether various concentrations of nicotine could diminish cell survival of hMSCs prior to testing the role of nAChRs on hMSCs function. The viability of hMSCs treated with nicotine (10−5M – 10−7M) was measured by trypan blue uptake or by AnnexinV staining. At a concentration of 10−7M, nicotine did not induce statistically significant changes in cell viability, but a 10−5M concentrations of nicotine induced cell death comparable to that from oxidative damage by 100μM H2O2 (Figure 4).
Figure 4.
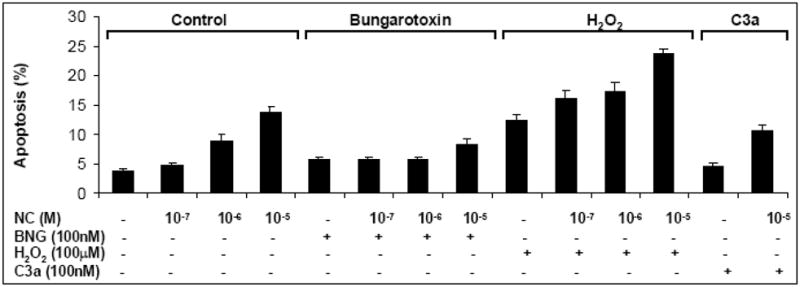
The effect of nicotine on hMSC death. MSC were treated with various concentration of nicotine as described under Methods and cell viability was assessed by detecting the percentage of Annexin V positive cells by FACS. H2O2 (100μM) was used as a positive control for the assay. Where indicated, 100 nM α-bungarotoxin was added into the hMSC cultures. Mean and SD of two similar experiments is shown. Statistical differences are indicated by * for p values of <0.05 (Student’s t test). Results of one out of four similar experiments are shown.
The threshold dose of nicotine toxicity was around 10−6M. These effects of nicotine on hMSC survival appeared to be mediated by α7 nAChRs since the effects of nicotine were blocked by α-bungarotoxin. Our findings suggest that at a concentration of less than 10−6M, nicotine does not interfere with hMSC survival and thus can be used for studying the physiological relevance of nAChRs.
Interestingly, a combination of nicotine with H2O2 further increased cell death. Since our previous findings demonstrate that anaphylatoxins protect hMSCs from oxidative damage (Schraufstatter, Discipio et al. 2009), we tested the effect of C3a on nicotine-induced cell death. We found that similar to the hydrogen peroxide-induced cell death, C3a ameliorated nicotine-induced damage (Figure 4).
Effect of Nicotine on Migration of MSCs
Since our previously published results demonstrated that nicotine inhibits chemokine-mediated transmigration of hematopoietic stem/progenitor cells (Serobyan, Jagannathan et al. 2007), we further tested the role of nAChRs in regulating the migration of hMSCs. nAChRs were stimulated with 10−6M nicotine because this concentration did not significantly influence hMSCs survival. The number of spontaneously migrating hMSCs in chemokine-free cultures was increased from 52.8±7 in control to 75±19.5 migrated cells per visual field in nicotine supplemented cultures (p=0.025). When C3a or bFGF were added into the lower wells of Transwells, the number of migrated cells was 5-fold and 4-fold higher as compared to control. Nicotine at a concentration of 10−6M significantly inhibited both C3a- (p=0.003) and bFGF-mediated (p=0.002) chemotaxis of hMSCs (Figure 5). To investigate whether these effects are mediated by nAChRs, we tested the effect of the selective nAChR antagonist α-bungarotoxin, which blocks the α7 subunit, on nicotine-induced chemotaxis. While α-bungarotoxin alone did not have an effect on hMSC migration, it attenuated the nicotine-induced inhibition of hMSC chemotaxis toward both C3a and bFGF (Figure 5A).
Figure 5.
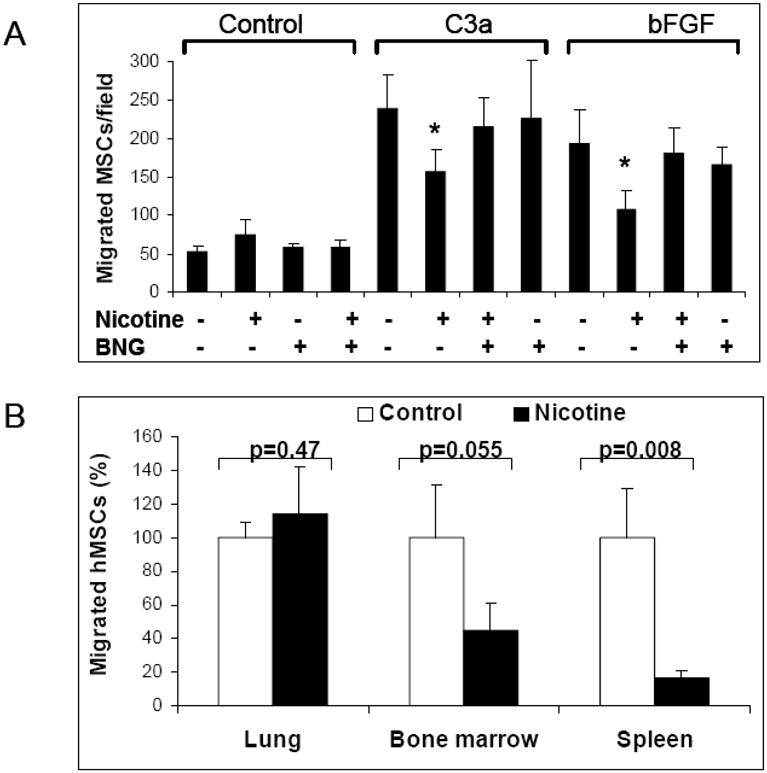
The effect of nicotine on chemokine-mediated migration of hMSCs. A: Chemotaxis of hMSCs towards plain culture media or media containing 0.2% BSA or the same media supplemented with 20 ng/ml bFGF or 100 nM C3a was evaluated using a Transwell assay. Where indicated, 10−6M nicotine and/or 100 nM α-bungarotoxin was added into the Transwell cultures. Each sample was assayed in triplicates. Statistical differences are indicated by * for p values of <0.05 (Student’s t test). Results of one out of two similar experiments are shown. B: SCID mice were irradiated with 6Gy followed by intravenous infusion of 3×105 hMSCs expressing GFP (GFP-hMSCs). After 24 hours, bone marrow, spleens and lungs were collected and the number of GFP-hMSCs was measured by FACS. The number of GFP-hMSCs in control animals is presented as 100%. The data shown are the average (±SD) of three independent measurements.
Effect of Nicotine on hMSC Homing
In vivo, nAChRs expressed in MSCs can be activated either by acetylcholine released from non-neural sources (Fujii, Fujigaya et al. 2007), or by nicotine present in the blood and tissues as a result of smoking (Hukkanen, Jacob et al. 2005).
Thus, we next tested whether nicotine at concentrations seen in chronic smokers (10−7M–10−8M) can influence homing of intravenously administered hMSCs. Mice were injected with nicotine according to recommended doses (Matta, Balfour et al. 2006). In the presence of nicotine the migration of hMSCs from peripheral blood to bone marrow and spleen was significantly decreased, whereas the migration to the lungs was not affected (Figure 5B).
Discussion
Our previously published results suggest that the non-neural cholinergic system contributes to the regulatory network in bone marrow and may regulate the function of the regulatory microenvironment (Khaldoyanidi, Sikora et al. 2001; Serobyan, Orlovskaya et al. 2005; Serobyan, Jagannathan et al. 2007). Since MSCs represent an important cellular element of the hematopoietic microenvironment, we investigated whether the function of these cells can also be regulated by the non-neural cholinergic system. In this study we demonstrated for the first time that hMSCs express functionally active nAChRs which negatively regulate migration of hMSCs towards chemoattractants.
Analysis of the reverse transcribed mRNA for all tested nAChR subunits detected the expression of α1, α2, α3, α4, α5, α7, α9, β2, β3 and β4 nAChRs, but not of α6, α10, and β1. The expression of proteins for the α7, β2 and β4 subunits was confirmed by Western blot and immune fluorescence. Although mRNA for α3 and α4 were detected in hMSCs, protein assays failed to show the presence of the corresponding proteins. Since the quality and specificity of these antibodies was confirmed by our collaborators ((Tsetlin, Shelukhina et al. 2007) and personal communication with Dr. Skok), we concluded that the corresponding proteins were either not expressed or expressed at a very low level. These findings are not completely in line with those of another laboratory (Hoogduijn, Cheng et al. 2008). Specifically, we detected both β2 and β4 subunits in contrast to the published negative findings of Hoogduijn and coworkers (Hoogduijn, Cheng et al. 2008). Presumably the intrinsic heterogeneity of MSC is responsible for these discrepancies. Another factor that may play a role is the difficulty associated with the generation of antibodies specific to nAChR subunits due to the highly conserved nature of these molecules across different species. However, in agreement with Hoogduijn et al., we did detected the α7 subunit expressed on hMSCs. Although Hoogduijn et al. reported that only about half of their MSCs expressed the α7 subunit, we detected α7 on the entire hMSC population.
The nAChRs typically provide transmembrane channels for potassium and sodium ions, but an nAChR consisting of a homopentamer of α7 atypically provides gating to calcium ions (Kalamida, Poulas et al. 2007). The determination that nicotine induced calcium influx from the extracellular medium, suggested that hMSCs express functionally active homopentamers of α7 nAChR. It has been previously reported that nicotine (at 10 μM) evoked transient calcium fluxes reaching a maximum in 30–45 sec in approximately half of the MSC examined (Hoogduijn, Cheng et al. 2008). However, the investigators did not distinguish between the release of calcium from internal pools and the uptake of calcium from the milieu. Our data show that calcium ions are entering hMSCs from the external medium as would be expected if it were mediated by a plasma membrane ion channel. Since the relevance and importance of α7 nAChR in the non-neural cholinergic system has been previously reported, including an anti-inflammatory effect of activation of α7 nAChR (Wang, Yu et al. 2003; de Jonge and Ulloa 2007; Tracey 2007), our further studies were focused on the α7 subunits.
At a concentration of 10−5 M, nicotine evoked apoptosis in about 15% of MSC. However at the “physiological” concentration of 10−7M, e.g. the concentration of nicotine seen in the blood of smokers (Hukkanen, Jacob et al. 2005), nicotine did not influence hMSC survival and therefore this concentration was further used to test physiological effects of nicotine on hMSC function.
We reported previously that - while spontaneous transmigration of hematopoietic progenitors in the presence of nicotine was slightly increased, SDF-1-mediated chemotaxis of hematopoietic progenitors was significantly decreased in the presence of nicotine (Serobyan, Schraufstatter et al. 2005), which correlated with a decreased expression of CXCR4 (Serobyan, Jagannathan et al. 2007). This finding suggests a role for the non-neural cholinergic system in the regulation of cell migration. Since chemotaxis of circulating MSCs into bone marrow is physiologically important during systemic therapeutic administration of MSCs or during the development, we investigated whether the non-neural cholinergic system is involved in regulation of this process. Nicotine was used as an agonist of nAChRs. Similar to our previously published findings (Serobyan, Schraufstatter et al. 2005), we found that spontaneous migration of cells across the membrane was slightly increased. However, the migration of hMSC toward the chemotactic factors bFGF and C3a was significantly inhibited when nicotine was added into the cultures. Importantly, the in vivo migration studies were in line with the in vitro observations. We found that stimulation of nAChRs with nicotine inhibits homing of hMSCs into bone marrow and spleen. The inhibitory effect of nicotine on chemotaxis of hMSCs in vitro was attenuated in the presence of α-bungarotoxin suggesting a role for α7 subunits of nAChR in mediating these effects of nicotine. Unfortunately it was not feasible to block the effects of nicotine on hMSCs migration in vivo due to toxic side effects of α-bungarotoxin (not shown).
There are contradictory reports regarding the effects of nicotine on cell migration. While some groups reported that nicotine stimulates cell migration (Totti, McCusker et al. 1984; Nowak, Ruta et al. 1990; Li, Zhao et al. 2004; Di Luozzo, Pradhan et al. 2005), others demonstrated that nicotine is an inhibitory factor (Bridges, Kraal et al. 1977; Giannopoulou, Geinoz et al. 1999; Serobyan, Schraufstatter et al. 2005). We and others have previously published that nicotine induces signal transduction in non-neural cells (Kihara, Shimohama et al. 2001; Serobyan, Schraufstatter et al. 2005; Hoogduijn, Cheng et al. 2008), activation of which can be associated with increased cell motility. However, the signaling mechanisms activated might depend on the specific cell types and the diverse experimental conditions that were used in these different studies. For example, we detected Erk1/2 phosphorylation in endothelial cells (Serobyan, Schraufstatter et al. 2005), but not in hMSC (Supplementary Figure 1).
Furthermore, the effect of nicotine on cells exposed to chemotactic factors might be different from that on serum-starved cells usually used for studying signal transduction. In addition, the effects of nicotine on cell migration may not necessarily be associated with signaling, but could be the result of a detrimental effect of nicotine on the expression of receptors for chemotactic factors, which was previously shown for CXCR4 (Serobyan, Jagannathan et al. 2007). Thus, further studies on the molecular mechanisms that mediate the effect of nicotine on cell migration are needed since nAChRs may represent an attractive therapeutic target for regulating the migration of non-neural cells.
Supplementary Material
Acknowledgments
We thank Dr. Dr. Marina Skok for providing the nAchR specific antibodies used in this study. We thank Valentina Goncharova for excellent technical support. Human MSCs employed in this work were provided by the Tulane Center for Gene Therapy through a grant from NCRR of the NIH, Grant # P40RR017447. This project was funded by the University of California Tobacco Related Disease Research Program (TRDRP-16RT-0134) and NIH (R21NS062428) to SKK.
References
- Beckel JM, Kanai A, et al. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2006;290(1):F103–10. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, et al. Calcium--a life and death signal. Nature. 1998;395(6703):645–8. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- Bridges RB, Kraal JH, et al. Effects of cigarette smoke components on in vitro chemotaxis of human polymorphonuclear leukocytes. Infect Immun. 1977;16(1):240–8. doi: 10.1128/iai.16.1.240-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty CS, Soloway PD, et al. Nicotine and cotinine stimulate secretion of basic fibroblast growth factor and affect expression of matrix metalloproteinases in cultured human smooth muscle cells. J Vasc Surg. 1996;24(6):927–34. doi: 10.1016/s0741-5214(96)70038-1. discussion 934–5. [DOI] [PubMed] [Google Scholar]
- Chamberlain G, Fox J, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Chen D, Patrick JW. The alpha-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the alpha7 subunit. J Biol Chem. 1997;272(38):24024–9. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151(7):915–29. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch VR, Pick M, et al. The stress-associated acetylcholinesterase variant AChE-R is expressed in human CD34(+) hematopoietic progenitors and its C-terminal peptide ARP promotes their proliferation. Exp Hematol. 2002;30(10):1153–61. doi: 10.1016/s0301-472x(02)00900-1. [DOI] [PubMed] [Google Scholar]
- Di Luozzo G, Pradhan S, et al. Nicotine induces mitogen-activated protein kinase dependent vascular smooth muscle cell migration. Atherosclerosis. 2005;178(2):271–7. doi: 10.1016/j.atherosclerosis.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Fujii T, Takada-Takatori Y, et al. Basic and clinical aspects of non-neuronal acetylcholine: expression of an independent, non-neuronal cholinergic system in lymphocytes and its clinical significance in immunotherapy. J Pharmacol Sci. 2008;106(2):186–92. doi: 10.1254/jphs.fm0070109. [DOI] [PubMed] [Google Scholar]
- Fujii YX, Fujigaya H, et al. Enhanced serum antigen-specific IgG1 and proinflammatory cytokine production in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. J Neuroimmunol. 2007;189(1–2):69–74. doi: 10.1016/j.jneuroim.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. Aaps J. 2005;7(4):E885–94. doi: 10.1208/aapsj070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallowitsch-Puerta M, Tracey KJ. Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine alpha 7 receptor. Ann NY Acad Sci. 2005;1062:209–19. doi: 10.1196/annals.1358.024. [DOI] [PubMed] [Google Scholar]
- Giannopoulou C, Geinoz A, et al. Effects of nicotine on periodontal ligament fibroblasts in vitro. J Clin Periodontol. 1999;26(1):49–55. doi: 10.1034/j.1600-051x.1999.260109.x. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74(6):363–96. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, et al. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74(8):1102–11. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Grando SA. Biological functions of keratinocyte cholinergic receptors. J Investig Dermatol Symp Proc. 1997;2(1):41–8. doi: 10.1038/jidsymp.1997.10. [DOI] [PubMed] [Google Scholar]
- Grando SA, Kawashima K, et al. Recent progress in understanding the non-neuronal cholinergic system in humans. Life Sci. 2007;80(24–25):2181–5. doi: 10.1016/j.lfs.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Jang JJ, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7(7):833–9. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, et al. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Hoogduijn MJ, Cheng A, et al. Functional Nicotinic and Muscarinic Receptors on Mesenchymal Stem Cells. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0032. [DOI] [PubMed] [Google Scholar]
- Hukkanen JP, Jacob, et al. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Kalamida D, Poulas K, et al. Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. Febs J. 2007;274(15):3799–845. doi: 10.1111/j.1742-4658.2007.05935.x. [DOI] [PubMed] [Google Scholar]
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–16. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 2004;9:2063–85. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- Khaldoyanidi S. Directing stem cell homing. Cell Stem Cell. 2008;2(3):198–200. doi: 10.1016/j.stem.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Khaldoyanidi S, Sikora L, et al. Correlation between nicotine-induced inhibition of hematopoiesis and decreased CD44 expression on bone marrow stromal cells. Blood. 2001;98(2):303–12. doi: 10.1182/blood.v98.2.303. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, et al. alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J Biol Chem. 2001;276(17):13541–6. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- Li S, Zhao T, et al. Nicotinic acetylcholine receptor alpha7 subunit mediates migration of vascular smooth muscle cells toward nicotine. J Pharmacol Sci. 2004;94(3):334–8. doi: 10.1254/jphs.94.334. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2006 doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Millar NS, Harkness PC. Assembly and trafficking of nicotinic acetylcholine receptors (Review) Mol Membr Biol. 2008;25(4):279–92. doi: 10.1080/09687680802035675. [DOI] [PubMed] [Google Scholar]
- Nowak D, Ruta U, et al. Nicotine increases human polymorphonuclear leukocytes chemotactic response--a possible additional mechanism of lung injury in cigarette smokers. Exp Pathol. 1990;39(1):37–43. doi: 10.1016/s0232-1513(11)80218-5. [DOI] [PubMed] [Google Scholar]
- Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind alpha-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994;14(2):889–96. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothem DE, Rothem L, et al. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metab. 2009 doi: 10.1007/s00774-009-0075-5. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Discipio RG, et al. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol. 2009;182(6):3827–36. doi: 10.4049/jimmunol.0803055. [DOI] [PubMed] [Google Scholar]
- Serobyan N, Jagannathan S, et al. The cholinergic system is involved in regulation of the development of the hematopoietic system. Life Sci. 2007;80(24–25):2352–60. doi: 10.1016/j.lfs.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serobyan N, Orlovskaya I, et al. Exposure to nicotine during gestation interferes with the colonization of fetal bone marrow by hematopoietic stem/progenitor cells. Stem Cells Dev. 2005;14(1):81–91. doi: 10.1089/scd.2005.14.81. [DOI] [PubMed] [Google Scholar]
- Serobyan N, I, Schraufstatter U, et al. Nicotinic acetylcholine receptor-mediated stimulation of endothelial cells results in the arrest of haematopoietic progenitor cells on endothelium. Br J Haematol. 2005;129(2):257–65. doi: 10.1111/j.1365-2141.2005.05446.x. [DOI] [PubMed] [Google Scholar]
- Tipton DA, Dabbous MK. Effects of nicotine on proliferation and extracellular matrix production of human gingival fibroblasts in vitro. J Periodontol. 1995;66(12):1056–64. doi: 10.1902/jop.1995.66.12.1056. [DOI] [PubMed] [Google Scholar]
- Tomek RJ, Rimar S, et al. Nicotine regulates collagen gene expression, collagenase activity, and DNA synthesis in cultured cardiac fibroblasts. Mol Cell Biochem. 1994;136(2):97–103. doi: 10.1007/BF00926068. [DOI] [PubMed] [Google Scholar]
- Totti N, 3rd, McCusker KT, et al. Nicotine is chemotactic for neutrophils and enhances neutrophil responsiveness to chemotactic peptides. Science. 1984;223(4632):169–71. doi: 10.1126/science.6318317. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetlin V, Shelukhina I, et al. Detection of alpha7 nicotinic acetylcholine receptors with the aid of antibodies and toxins. Life Sci. 2007;80(24–25):2202–5. doi: 10.1016/j.lfs.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Walker LM, Preston MR, et al. Nicotinic regulation of c-fos and osteopontin expression in human-derived osteoblast-like cells and human trabecular bone organ culture. Bone. 2001;28(6):603–8. doi: 10.1016/s8756-3282(01)00427-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ, et al. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther. 1998;77(1):59–79. doi: 10.1016/s0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.