Abstract
Glutathione S-Transferases (GST) play an important role in multidrug resistance and are upregulated in multiple cancers. We have designed a prodrug class that releases NO on metabolism by GST. O2-(2,4-Dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (JS-K, a member of this class) has potent anti-neoplastic activity. We studied the effect of JS-K on angiogenesis. JS-K inhibited the proliferation of HUVEC’s with a 50% inhibitory concentration (IC50) of 0.432, 0.466, and 0.505 µM at 24, 48, and 72 hours, respectively. In the cord formation assay, JS-K led to a decrease in the number of cord junctions and cord length with an IC50 of 0.637 and 0.696 µM, respectively. JS-K inhibited cell migration at 5 hours using VEGF as a chemoattractant. Migration inhibition occurred with an IC50 of 0.493 µM. In the chick aortic ring assay using VEGF or FGF-b for vessel growth stimulation, 0.5 µM JS-K completely inhibited vessel growth. JS-K inhibited tumor angiogenesis in vivo in NIH III mice implanted subcutaneously with OPM1 multiple myeloma cells. JS-K is a potent inhibitor of angiogenesis in vitro and tumor vessel growth in vivo. As such, it establishes a new class of anti-neoplastic agents that target the malignant cells directly as well as their microenvironment.
Keywords: JS-K, nitric oxide, angiogenesis, myeloma, HUVEC
Introduction
Nitric oxide (NO) has significant growth-inhibitory activity against acute myeloid leukemia (AML) cells (1–3). The use of compounds that generate NO spontaneously for the treatment of malignancies is precluded by the pleiotropic and potentially toxic effects of NO (4). A strategy that targets NO to malignant cells is therefore likely to be more clinically applicable. In an effort to develop NO-based therapies for the treatment of malignant diseases, we have developed a class of arylated diazeniumdiolate NO-generating agents. These compounds are pro-drugs that do not release high levels of NO spontaneously (5). However, they are activated to release NO upon nucleophilic attack by reduced thiols, especially those of the abundant peptide glutathione (GSH). The activation reaction is catalyzed by the glutathione S-transferases (GST) (5). This drug design strategy seeks to exploit the overexpression of GST in malignant cells as compared to normal tissues (6,7).
Upon screening a library of arylated diazeniumdiolates for their in vitro anti-leukemic activity, we identified O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (JS-K) as a lead compound. JS-K inhibits the growth of HL-60 (human myeloid leukemia) cells with an in vitro 50% growth inhibitory concentration (IC50) of 0.25 – 0.5 µM (5). JS-K also inhibited the growth of HL-60 cells in a subcutaneous xenograft model in NOD/SCID mice (5). In separate experiments, JS-K was found to be a potent inhibitor of multiple myeloma (MM) cell growth, including multi-drug resistant clones (8). In that study JS-K treatment led to significant survival prolongation of NIH III mice subcutaneously implanted with OPM1 MM cells (8). The purpose of the experiments presented in this paper was to determine the effect of JS-K on angiogenesis. We show that JS-K is a potent inhibitor of angiogenesis in vitro and of tumor vessel growth in vivo.
Methods
Chemicals
JS-K was synthesized as previously described (9). All other chemicals were from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Cells
In vitro angiogenesis experiments were conducted using Human Umbilical Vein Endothelial Cells (HUVEC, VEC Technologies, New York, NY). For in vivo experiments OPM1 MM cells were obtained from Dr. Lief Bergsagel (Mayo Clinic, Scottsdale, AZ).
Growth Inhibition Assay
The growth inhibition assay was conducted as previously described (10). In brief, HUVECs (1.5×103) were plated in 100 µl of EBM-2 medium (Clonetics, Walkersville, MD). After 24 hours (day 0), JS-K was added at the indicated concentrations. On day 0, one plate was stained with 0.5% crystal violet in 20% methanol, rinsed with water, and air-dried. The remaining plates were incubated at 37°C. After 24, 48 and 72 hours, plates were stained with crystal violet. The stain was eluted with a solution of 0.1 M sodium citrate and ethanol (1:1) and absorbance was measured at 540 nm with a microplate reader. Day 0 absorbance was subtracted from the test plates and data were plotted as percentage of control proliferation (vehicle-treated cells).
Cord formation on Matrigel
Cord formation was evaluated as previously described by plating HUVEC on basement membrane matrix preparations (Matrigel, Becton Dickinson, Franklin Lakes, NJ) distributed in 96-well plate (10). HUVEC were treated with the indicated concentrations of JS-K for 24 hours. Cells were then harvested, washed, and resuspended in growth factor-supplemented EBM-2 medium before distributing in 96-well plates (2 X 105/100 µL). After 24 hours, tube formation was observed using an inverted phase contrast microscope (DM-IRB; Leica Inc.) at 5 X magnification and images were captured with a CCD camera. Quantitation of tube formation was determined by measuring the length of tubes and counting junctions in three random fields from each well (2 wells per data point) using the Bioquant Image Analysis System (Nashville, TN). Data were plotted and the IC50 was derived from the resultant curves.
Cell migration assay
Cell migration was evaluated as previously described (10). Migration assay was performed in a 96-well disposable chamber (ChemoTx® 101-8, Neuroprobe, Gaithersburg, MD). Both sides of the framed filter were coated with 25 µl/well of rat tail collagen Type I (BD Biosciences, Bedford, MA) for 30 minutes and dried for 1 hour in a laminar flow hood. Assay buffer (27–29 µl) for normal negative control or 10 ng/ml vascular endothelial growth factor (VEGF, R&D Systems, Minneapolis, MN) as a chemoattractant was added to the wells of the bottom plate to form a small positive meniscus over the wells. The collagen-coated framed filter was then placed over the microplate. HUVEC were harvested and a cell suspension was prepared at 2 X 106 /ml. Prior to addition to the migration plate, cells were mixed with JS-K prepared in media containing 1% bovine serum albumin. Untreated control or JS-K-treated cell suspensions (30 µl) were placed on top of each well of the filter in quadruplets. Plates were incubated at 37°C for 5 hours. At the end of the incubation period, the filter was fixed and stained using the Hema 3 staining kit (Fisher Diagnostics, Middletown, VA). The filter was rinsed with distilled water and cells that had not migrated from the top of the filter were removed using a wet Kimwipe. Cells that had migrated were counted from five fields from each well under high power. The number of cells that had spontaneously migrated (negative control wells) was subtracted from the number of cells that had migrated in untreated control or the JS-K treatment variable. Data are expressed as the percent of control migration (vehicle-treated cells). Data were plotted and the IC50 was derived from the resultant curves.
Chick aortic arch assay
Aortic arches were dissected from day 12–14 chick embryos. Periaortic fibroadipose tissue was removed from the aortic arches which were then cut into 1-mm-long rings. Aortic rings were placed on growth factor-depleted Matrigel (Becton Dickinson, Franklin Lakes, NJ). Rings were cultured at 37°C in a humidified 5% CO2 atmosphere. VEGF or Fibroblast Growth Factor-2 (FGF-2) at a concentration of 50 ng/ml was used to stimulate vessel growth. JS-K was added at concentrations ranging from 0.1 to 0.5 µM. After 48 hours in culture, rings were stained with Calcein AM (Molecular Probes, Eugene, OR) and evaluated for vessel growth using an inverted microscope (DM-IRB, Leica Inc.). As shown, the effects of JS-K on vessel growth were so significant (total inhibition) that no delineation of the outgrowth area for quantitation purposes was conducted.
Animal studies
NIH III mice (5 to 6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). All animal studies were conducted according to protocols approved by the Animal Ethics Committee of the Dana-Farber Cancer Institute according to accepted standards. The mice were inoculated subcutaneously in the right flank with 3×107 OPM1 MM cells in 100 µl of RPMI 1640 and 100 µl of Matrigel basement membrane matrix (Becton Dickinson, Franklin Lakes, NJ). When tumors were palpable, 9 mice were assigned to the treatment group receiving 4 µmol/kg of JS-K intravenously three times per week and 8 mice to the control group receiving an equivalent volume of vehicle alone according to the same schedule. JS-K stocks in dimethyl sulfoxide (DMSO) were serially diluted in phosphate buffered saline (PBS) before injection. The final concentration of DMSO was less than 10%. Vehicle control consisted of 10% DMSO in PBS. Caliper measurements of the longest perpendicular tumor diameters were performed every other day to estimate the tumor volume. Animals were sacrificed when tumors reached 2 cm3 or if the mice appeared moribund. The first day of sacrifice was 21 and 43 days after start of treatment for the vehicle control and JS-K-treated groups, respectively. Upon sacrifice, tumors were dissected out of the animals and stained with a murine specific anti-CD34 rat monoclonal antibody (Abcam Clone MEC14.7) to mark vessels. Vessel density was evaluated visually using light microscopy. Photomicrographs shown are representative of all the sections evaluated. Again here, inhibition of tumor vessel growth by JS-K was so significant that no attempt at quantitation was undertaken using hot spot analysis or vessel scoring.
Statistics
The Student t test was used to compare results between treated cells and controls. The Bonferroni procedure was used to adjust p-values for multiple comparisons within each experiment. Bonferroni adjusted p-values < 0.05 were considered statistically significant.
Results
Effect of JS-K on HUVEC proliferation
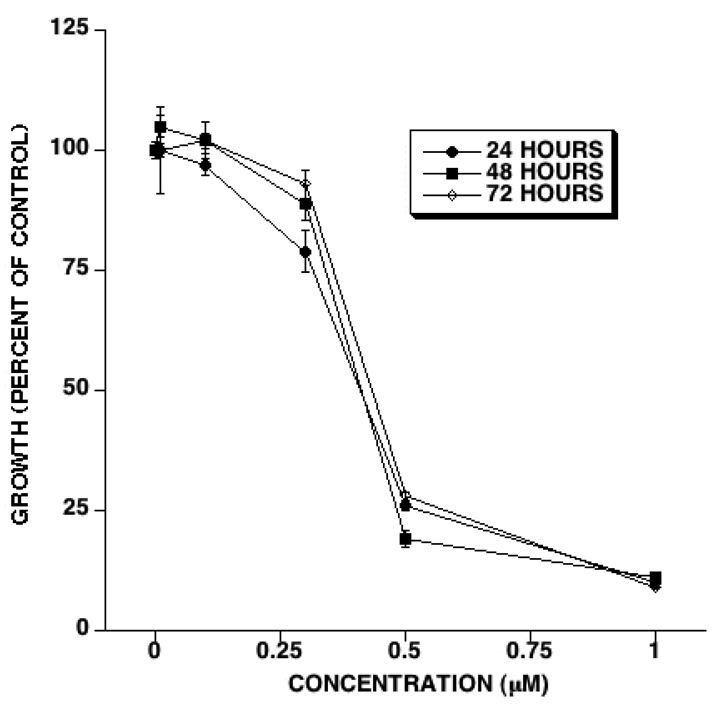
We first determined the effect of JS-K on HUVEC proliferation in vitro. HUVEC growth was evaluated 24, 48, and 72 hours after adding JS-K. JS-K inhibited HUVEC growth in a dose-dependent fashion (Figure 1). HUVEC growth inhibition by JS-K as a percent of control occurred to the same extent at every time point (Figure 1). The IC50 for HUVEC growth inhibition by JS-K was 0.432, 0.466, and 0.505 µM after 24, 48, and 72 hours of exposure, respectively. A JS-K concentration of 1 µM almost completely inhibited HUVEC proliferation (Figure 1).
Figure 1. JS-K inhibits HUVEC growth.
HUVEC were cultured with the indicated concentrations of JS-K. At the indicated time points cell growth was scored as outlined in the Methods section. JS-K treatment led to a dose-dependent inhibition of HUVEC growth. The IC50 at 24, 48, and 72 hours was 0.432, 0.466, and 0.505 µM, respectively. (Averages and SEM of 3 separate experiments).
Effect of JS-K on cord formation
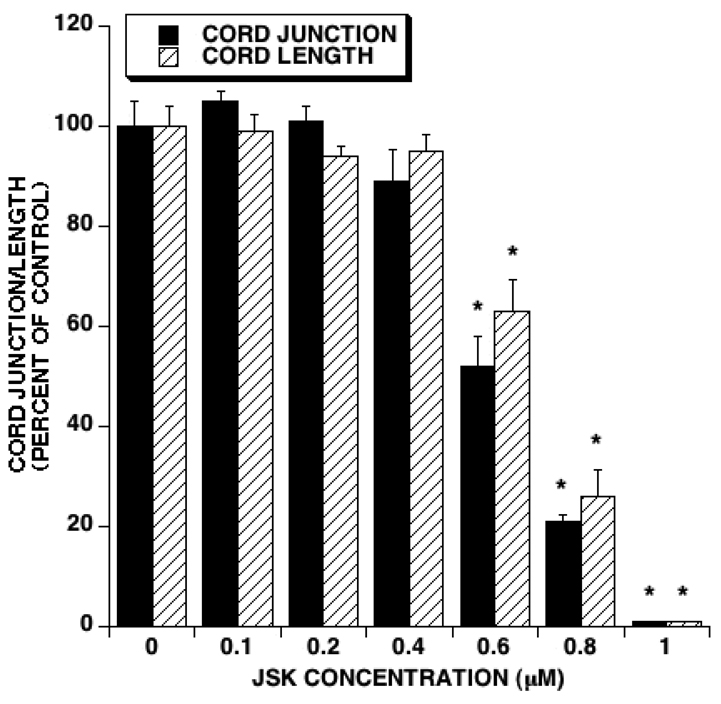
The cord formation assay is indicative of new vessel formation. We therefore determined the effect of JS-K on cord formation in vitro. After a 24 hour exposure to JS-K, a dose dependent decrease in the number of cord junctions was observed (Figure 2). At the same time points, cord length was significantly diminished as well (Figure 2). The IC50 for JS-K-induced decrease in the number of cord junctions and cord length was 0.637 and 0.696 µM, respectively.
Figure 2. JS-K inhibits cord formation.
HUVECs were plated in Matrigel after treatment with the indicated concentrations of JS-K. Twenty four hours later cord formation was evaluated by scoring cord length and cord junctions. JS-K treatment led to a dose-dependent inhibition of cord formation. The IC50s for cord junction and cord length were 0.637 and 0.696 µM, respectively. (Averages and SEM of 3 separate experiments. Asterisks indicate statistically significant differences between treatments and controls).
Effect of JS-K on endothelial cell migration
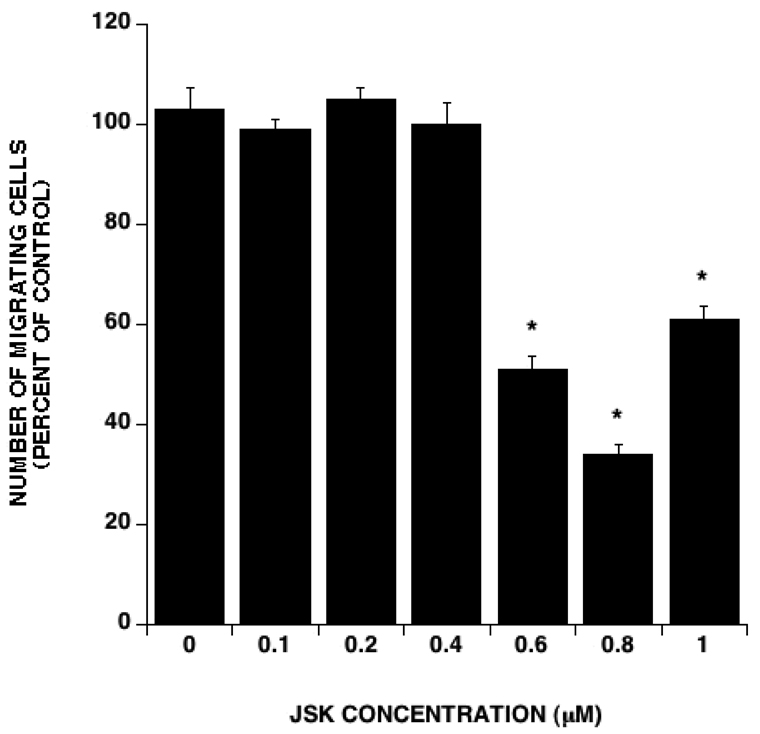
Endothelial cell migration is critical to angiogenesis. We therefore sought to determine the effect of JS-K on HUVEC migration in vitro. In this assay, HUVEC were subjected to chemotaxis by VEGF stimulation for 5 hours with or without JS-K. At concentrations above 0.4 µM, JS-K significantly inhibited HUVEC migration (Figure 4). At that time point, visual inspection revealed no evidence of cell death at any JS-K concentration, indicating that the observed effect was due to migration inhibition rather than cytotoxicity of the drug towards HUVECs. Cytotoxicity by JS-K at these concentrations was not observed until later time points (24 hours or more). The IC50 for migration inhibition was 0.493 µM.
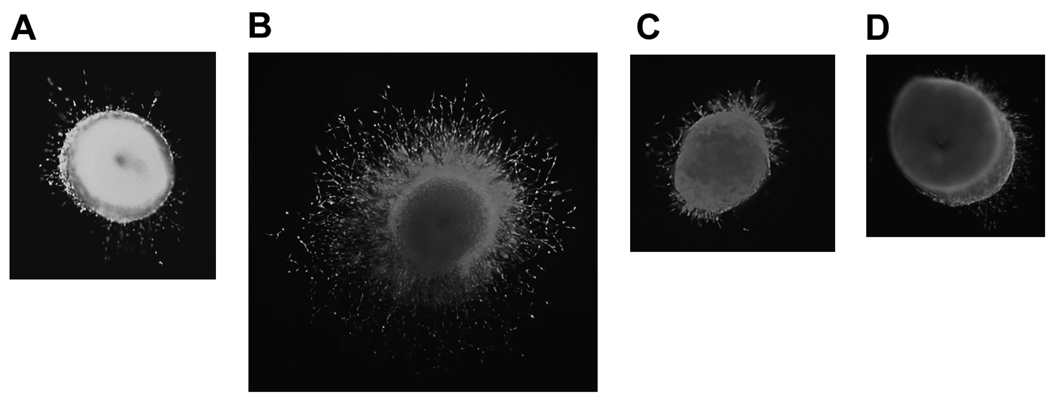
Figure 4. JS-K inhibits vessel growth in the chick aortic ring assay.
Chick aortic rings were cultured in vitro with different additives as detailed in the Methods section. After 2 days in culture, rings were evaluated for vessel growth. A- No additive control; B- FGF-b 50 ng/ml; C- FGF-b 50 ng/ml + JS-K 0.1 µM; D- FGF-b 50 ng/ml + JS-K 0.5 µM. Pictures are representative of 2 separate experiments. Similar results were observed when 50 ng/ml VEGF was used to stimulate angiogenesis (not shown).
Effect of JS-K in the chick aortic ring assay
In addition to endothelial cells, the chick aortic ring assay includes the surrounding non-endothelial cells such as fibroblasts. Furthermore, in this assay, endothelial cells have not been modified by repeated in vitro passages. Consequently, the chick aortic ring assay is more reflective of in vivo angiogenesis. We therefore determined the effect of JS-K on vessel growth in this assay. Two sets of experiments were conducted using FGF-2 or VEGF to stimulate angiogenesis as detailed in the Methods section. Culture of the aortic rings without stimulation led to minimal vessel growth (Figure 4A) while stimulation with 50 ng/ml FGF-2 led to extensive angiogenesis (Figure 4B). FGF-2-induced angiogenesis was diminished by the addition of 0.1 µM JS-K and almost completely abolished when the latter was added at a concentration of 0.5 µM (Figures 4C and 4D, respectively). Similar observations were made when 50 ng/ml VEGF was used to stimulate angiogenesis (not shown).
Effect of JS-K on tumor angiogenesis in vivo
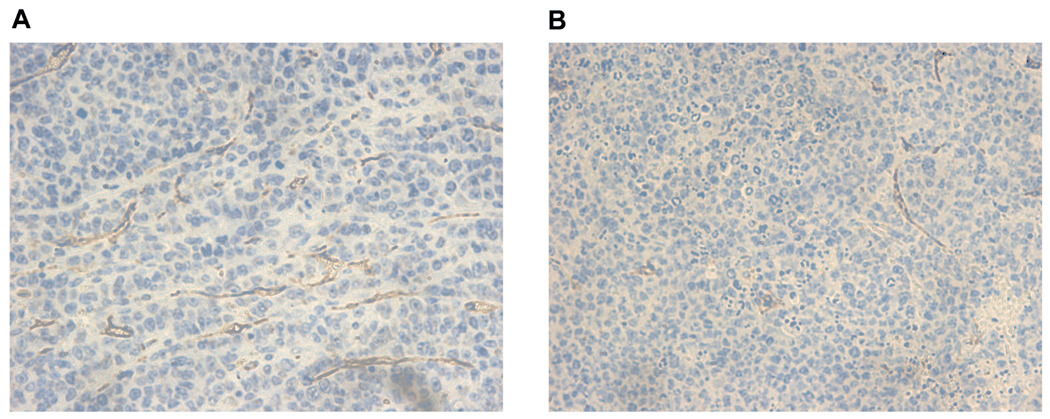
In order to determine whether JS-K affects tumor angiogenesis in vivo, we used a murine subcutaneous plasmacytoma model. In this experiment, NIH III mice were implanted subcutaneously with OPM 1 MM cells and treated with JS-K at a dose of 4 µmol/kg given intravenously three times a week. We have previously observed that this dose and schedule are safe and effective for the treatment of AML in NOD/SCID mouse xenografts (5). As previously reported, JS-K treatment of NIH III mice implanted with OPM 1 cells led to significant inhibition of tumor growth and prolongation of survival (8). Tumor explants obtained from control vehicle-treated animals at 21 days after start of treatment revealed substantial tumor angiogenesis as evidenced by CD34 staining (Figure 5A). JS-K treatment led to markedly decreased tumor angiogenesis at the 43-day time point (Figure 5B).
Figure 5. JS-K inhibits tumor angiogenesis in an in vivo plasmacytoma model.
NIH III mice were implanted with OPM1 MM cells and treated with vehicle control (A) or JS-K at a dose of 4 µmol/kg thrice weekly (B). Control and JS-K-treated mice were sacrificed starting 21 and 43 days after starting treatment, respectively. Tumor explants were stained with a CD34 antibody to mark vessels and slides were evaluated under 400X magnification. JS-K significantly inhibited tumor angiogenesis in vivo. Pictures are representative of slides from 8 control and 9 JS-K-treated animals, respectively.
Discussion
The experiments we present here show a previously unknown effect of JS-K, namely inhibition of angiogenesis. Using different in vitro assays, we show that JS-K inhibits 3 key aspects of angiogenesis, namely endothelial cell division, endothelial cell migration, and new vessel formation. Importantly, JS-K inhibited tumor angiogenesis in vivo. In the in vivo experiment described here and in our previously reported in vivo experiments, JS-K was administered to mice at a dose of 4 µmol/kg without observable toxicity (5,8). Such a dose would be expected to lead to peak blood levels of around 17 µM in a 20 g mouse. This concentration is considerably higher than the concentrations at which JS-K was inhibitory in the different angiogenesis assays described in this paper. Consequently, as shown in the in vivo model presented here, angiogenesis inhibition by JS-K is achievable in vivo and therefore is relevant to the clinical development of this drug.
Another point to make regarding the relevance of our observations is whether JS-K would get activated in endothelial cells in vivo. We have used HUVEC in our in vitro assays of angiogenesis. These cells have become a standard in the study of angiogenesis (11). However, data suggest that tumor endothelial cells are different from normal endothelial cells and therefore could respond differently to cytotoxic or anti-angiogenic agents (12). JS-K is designed to be activated to release NO upon interaction with GSH in a reaction catalyzed by GST (5). GST are expressed in normal endothelial cells (13). No data are available comparing levels of expression of GST between normal and tumor endothelial cells. However, since endothelial cells have been shown to express GST (13), one would expect that JS-K would get activated in the tumor vasculature to inhibit tumor angiogenesis in vivo. Furthermore, it is obvious that a single assay of angiogenesis is not enough to demonstrate whether a chemotherapeutic agent is truly an angiogenesis inhibitor (11). However, the combined analysis of the effect of JS-K in 4 different in vitro assays, clearly supports the fact that it is an angiogenesis inhibitor. Most importantly, our demonstration that JS-K inhibits tumor angiogenesis in vivo in a murine MM model certainly makes our observations relevant to clinical situations.
One obvious question arising in data interpretation is whether our observations are due to a simple cytotoxic effect of JS-K on endothelial cells rather than true inhibition of angiogenesis. Data presented in Figure 2 show that JS-K did not inhibit cord formation or cord junction at a concentration of 0.4 µM. As shown in Figure 1, at this concentration, JS-K would be expected to induce around 50% inhibition of HUVEC growth. If the effect of JS-K on cord formation/cord junction was due to a cytotoxic effect of HUVEC’s, then one would expect that at a concentration (0.4 µM) that induces 50% growth inhibition in HUVEC’s one would observe some cord formation/cord junction inhibition. Similarly, data shown in Figure 3 show that JS-K did not inhibit HUVEC migration at a concentration of 0.4 µM. Again, at that concentration JS-K induced almost 50% growth inhibition of HUVEC’s (Figure 1). If JS-K inhibited HUVEC migration solely through a cytotoxic effect, one would expect to observe some migration inhibition at a concentration of 0.4 µM. Furthermore, migration was evaluated at 5 hours. At that point, we did not see any difference in cell density between JS-K-treated HUVEC’s and controls. Consequently, we believe that the observed effects of JS-K are due to a genuine anti-angiogenic effect rather than simple cytotoxicity.
Figure 3. JS-K inhibits HUVEC migration.
HUVEC migration at 5 hours with the indicated concentrations of JS-K was assayed using 10 ng/ml VEGF as a chemoattractant. JS-K treatment led to a dose-dependent inhibition of HUVEC migration. The IC50 for migration inhibition was 0.496 µM. At the 5-hours time point there was no evidence of growth inhibition. (Averages and SEM of 3 different experiments. Asterisks indicate statistically significant differences between treatments and control).
The mechanism by which JS-K inhibits angiogenesis is yet to be elucidated. JS-K is a NO-generating compound. The effect of NO on angiogenesis has been the subject of much work as well as controversy. NO plays an important role in the initiation of angiogenesis by inducing vasodilation through the activation of the soluble guanylate cyclase and cGMP production (14). NO also upregulates the production of VEGF (15). On the other hand, Pipili-Synetos et al proposed that NO is an endogenous inhibitor of angiogenesis (16), and Jia et al (17) described the direct anti-angiogenic effect of S-nitrosocaptopril (an S-nitrosothiol type of NO donor compound). In the latter experiments, S-nitrosocaptopril inhibited angiogenesis in vitro (proliferation and tube formation of capillary endothelial cells), and in vivo (neovasculature formation induced by VEGF on the chick embryo chorioallantoic membrane) (17). In these experiments, the anti-angiogenic effects of S-nitrosocaptopril were ascribed to NO because captopril itself did not affect angiogenesis. If NO is involved in the effects of JS-K on angiogenesis, it is clearly inhibitory in the current context. On the other hand, microarray studies have shown that JS-K upregulates in HL-60 cells the expression of the endogenous angiogenesis inhibitor Thrombospondin-1 and its receptor CD36 (18). In the same cells, it also upregulated the expression of several tissue inhibitors of metalloproteinases (TIMP) (18). Consequently, it is possible that the anti-angiogenic effects of JS-K are mediated by modulating the expression of key factors involved in the angiogenesis process.
Arylation reactions (the reaction of an aryl ring with free protein thiols) have been shown to affect protein function (19). The structure of JS-K includes a diazeniumdiolate moiety as well as a dinitrophenyl ring. Consequently, arylation could contribute to JS-K’s mechanism of action. Indeed, we have previously shown that protein arylation plays a role in the anti-leukemic effects of JS-K (20). It is therefore likely that JS-K induces arylation of key proteins in endothelial cells with resultant inhibition of angiogenesis. On the other hand, the JS-K derivative PABA/NO has been shown to induce extensive protein glutathionylation in ovarian cancer cells (21). Similar to arylation, glutathionylation of thiols can have an inhibitory effect on protein function (22). Whether JS-K exerts its effects on endothelial cells (and therefore angiogenesis) through arylation or glutathionylation in addition to NO release remains to be proven. However, similar to leukemic cells, since JS-K has multiple biologic effects, it is likely to exert its action on endothelial cells through multiple mechanisms, including NO release.
Arylated diazeniumdiolates constitute a large group of NO-generating compounds that share a common backbone. Our lead optimization efforts have allowed the identification of JS-K as the most active anti-neoplastic agent of this family as determined by screening their ability to inhibit HL-60 cell proliferation (20). Even though they vary in their direct cytotoxic potency, arylated diazeniumdiolates share the common properties of NO generation and arylation of thiols. No data are currently available on the effect of other arylated diazeniumdiolates on angiogenesis. Consequently, it is not possible to tell if the anti-angiogenic properties of JS-K are unique to this compound or constitute a class effect. We have previously determined that any substitution on the dinitrophenyl ring of JS-K diminished its direct cytotoxic effects (20). Any conclusion as to whether a similar effect would be observed with JS-K’s anti-angiogenic properties is speculative at this point.
Using a murine subcutaneous plasmacytoma model, we show here that JS-K inhibits tumor angiogenesis in vivo. We have previously demonstrated that JS-K is directly cytotoxic to MM cells by activating both the intrinsic and extrinsic apoptosis pathways (8). Furthermore, JS-K overcomes the survival and growth advantages imparted to MM cells by exogenous Interleukin-6 and Insulin Growth Factor-1 (IGF-1), or by adherence of MM cells to bone marrow stromal cells (8). It is established that MM cells thrive on the normal microenviroment of the tumor cell (23). Part of this is due to recruitment of new vessels. Bone marrow angiogenesis has also been shown to correlate negatively with survival of MM patients (24,25). Our previous work and results presented here show that JS-K has anti-myeloma activity by a direct cytotoxic effect as well as by affecting the interaction of the malignant cells with their microenvironment. This dual effect makes JS-K a very potent new agent for the treatment of MM. Besides MM, we have shown that JS-K has significant anti-neoplastic properties in vivo against AML, prostate cancer and hepatoma cells (5,20). In these models, JS-K was directly cytotoxic to the malignant cells. The in vitro angiogenesis assays we used here are not specific to a particular malignancy. It is therefore likely that JS-K would inhibit angiogenesis in different tumor models, including the ones we have studied in vivo so far.
Conclusion
We show here that JS-K potently inhibits several key elements of the angiogenesis process. Furthermore, it inhibits tumor angiogenesis in vivo. Consequently, JS-K establishes a new class of cancer chemotherapeutic agents acting by a direct cytotoxic effect as well as inhibition of tumor angiogenesis. As such, JS-K shows great promise for medicinal development for the treatment of different malignant diseases.
Acknowledgments
We acknowledge Jagadambal Thillainathan (SAIC-Frederick, Frederick, MD) for technical assistance.
-
Financial support:
Translational Research Award from the Leukemia and Lymphoma Society, NCI RAID Grant, NIH Grants R01 CA84496 and RO1 CA129611 (P.J.S). National Cancer Institute contract No. N01-CO-12400. Supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
-
Author contributions
Gurmeet Kaur: Performed research, reviewed and edited manuscript.
Tanyel Kiziltepe: Performed research, reviewed and edited manuscript.
Kenneth C. Anderson: Oversaw research, reviewed and edited manuscript.
Jeffery Kutok: Performed research, reviewed and edited manuscript.
Lee Jia: Oversaw research, reviewed and edited manuscript.
Kenneth M. Boucher: Performed statistical analysis, reviewed and edited manuscript.
Joseph E. Saavedra: Designed, synthesized and provided JS-K. Reviewed and edited manuscript.
Larry K. Keefer: Designed JS-K. Reviewed and edited manuscript.
Paul J. Shami: Analyzed and interpreted data, wrote manuscript.
Conflict of interest: Paul J. Shami is a founder, member of the Board of Directors, Chief Medical Officer, and holds stock in JSK Therapeutics Inc.
Abbreviations List
- AML
acute myeloid leukemia
- DMSO
dimethyl sulfoxide
- GSH
glutathione
- GST
Glutathione S-Transferases
- NO
nitric oxide
- HUVEC
human umbilical vein endothelial cells
- IC50
50% growth inhibitory concentration
- JS-K
O2-(2,4-Dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate
- MM
multiple myeloma
- PBS
phosphate buffered saline
- VEGF
vascular endothelial growth factor
- TIMP
tissue inhibitors of metalloproeinases
References
- 1.Magrinat G, Mason SN, Shami PJ, Weinberg JB. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992;80:1880–1884. [PubMed] [Google Scholar]
- 2.Shami PJ, Moore JO, Gockerman JP, Hathorn JW, Misukonis MA, Weinberg JB. Nitric oxide modulation of the growth and differentiation of freshly isolated acute non-lymphocytic leukemia cells. Leuk Res. 1995;19:527–533. doi: 10.1016/0145-2126(95)00013-e. [DOI] [PubMed] [Google Scholar]
- 3.Shami PJ, Sauls DL, Weinberg JB. Schedule and concentration-dependent induction of apoptosis in leukemia cells by nitric oxide. Leukemia. 1998;12:1461–1466. doi: 10.1038/sj.leu.2401131. [DOI] [PubMed] [Google Scholar]
- 4.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 5.Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV, Gu Y, Fox SD, Buzard GS, Citro ML, Waterhouse DJ, Davies KM, Ji X, Keefer LK. JS-K, a Glutathione S-transferase-activated nitric oxide donor with potent anti-neoplastic activity. Mol Cancer Ther. 2003;2:409–417. [PubMed] [Google Scholar]
- 6.Baines P, Cumber P, Padua RA. Multidrug resistance in leukaemia. Baillieres in Clin Hematol. 1992;5:943–960. doi: 10.1016/s0950-3536(11)80053-3. [DOI] [PubMed] [Google Scholar]
- 7.Sargent JM, Williamson C, Hall AG, Elgie AW, Taylor CG. Evidence for the involvement of the glutathione pathway in drug resistance in AML. Adv Exp Med Biol. 1999;457:205–209. doi: 10.1007/978-1-4615-4811-9_22. [DOI] [PubMed] [Google Scholar]
- 8.Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, Li C-Q, Trudel LJ, Yasui H, Vallet S, Kutok JL, Chauhan D, Mitsiades CS, Saavedra JE, Wogan GN, Keefer LK, Shami PJ, Anderson KC. JS-K, a GST-activated nitric oxide generator, induces DNA double strand breaks, activates DNA damage response pathways, and induces apoptosis in human multiple myeloma cells. Blood. 2007;110:709–718. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saavedra JE, Srinivasan A, Bonifant CL, Chu J, Shanklin AP, Flippen-Anderson JL, Rice WG, Turpin JA, Davies KM, Keefer LK. The secondary amine/nitric oxide complex ion R2N[N(O)NO]− as nucleophile and leaving group in SNAr reactions. J Org Chem. 2001;66:3090–3098. doi: 10.1021/jo0016529. [DOI] [PubMed] [Google Scholar]
- 10.Kaur G, Belotti D, Burger AM, Fisher-Nielson K, Borsotti P, Riccardi E, Thillainathan J, Hollingshead M, Sausville EA, Giavazzi R. Antiangiogenic properties of 17-(dimethylaminoethylamino)-17-demethoxy-geldanamycin: an orally bioavailable heat shock protein 90 modulator. Clin Cancer Res. 2004;10:4813–4821. doi: 10.1158/1078-0432.CCR-03-0795. [DOI] [PubMed] [Google Scholar]
- 11.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis Assays: A Critical Overview. Clinical Chemistry. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 12.de Vos FY, Wllemse PH, de Vries EG, Gitema JA. Endothelial cell effects of cytotoxics: balance between desired and unwanted effects. Cancer Treat Rev. 2004;30(6):495–513. doi: 10.1016/j.ctrv.2004.05.003. [DOI] [PubMed] [Google Scholar]; 12 Bruneel A, Labas V, Mailloux A, Sharma S, Vinh J, Vaubourdolle M, Baudin B. Proteomic study of human umbilical vein endothelial cells in culture. Proteomics. 2003;3:714–723. doi: 10.1002/pmic.200300409. [DOI] [PubMed] [Google Scholar]
- 13.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 14.Kimura H, Weisz A, Kurashima Y, et al. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–197. [PubMed] [Google Scholar]
- 15.Pipili-Synetos E, Sakkoula E, Haralabopoulos G, et al. Evidence that nitric oxide is an endogenous antiangiogenic mediator. Br J Pharmacol. 1994;111:894–902. doi: 10.1111/j.1476-5381.1994.tb14822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia L, Wu C, Young X. Anti-angiogenetic effects of S-nitrosocaptopril crystals as a nitric oxide donor. Eur J Pharmacol. 2000;391:137–144. doi: 10.1016/s0014-2999(99)00794-3. [DOI] [PubMed] [Google Scholar]
- 17.Shami PJ, Malaviya S, Tari A, Saavedra JE, Keefer LK, Tokar E, Sun Y, Waalkes MP, Liu J. Gene expression profiling for nitric oxide prodrug JS-K to kill HL-60 myeloid leukemia cells. Genomics. 2009;94:32–38. doi: 10.1016/j.ygeno.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen SD, Khairallah EA. Selective protein arylation and acetaminophen-induced hepatotoxicity. Drug Metabolism Reviews. 1997;29:59–77. doi: 10.3109/03602539709037573. [DOI] [PubMed] [Google Scholar]
- 19.Shami PJ, Saavedra JE, Bonifant CL, Chu J, Udupi V, Malaviya S, Carr BI, Kar S, Wang M, Jia L, Ji X, Keefer LK. Antitumor activity of JS-K [O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] and related O2-aryl diazeniumdiolates in vitro and in vivo. J Med Chem. 2006;49:4356–4366. doi: 10.1021/jm060022h. [DOI] [PubMed] [Google Scholar]
- 20.Townsend DM, Findlay VJ, Fazilev F, Ogle M, Fraser J, Saavedra JE, Ji X, Keefer LK, Tew KD. A glutathione S-transferase p-activated prodrug causes kinase activation concurrent with S-glutathionylation of proteins. Mol Pharmacol. 2006;69:501–508. doi: 10.1124/mol.105.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxidants and Redox Signaling. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podar K, Richardson PG, Hideshima T, Chauhan D, Anderson KC. Best Practice and Research. The malignant clone and the bone-marrow environment. Clinical Haematology. 2007;20:597–612. doi: 10.1016/j.beha.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Rajkumar SV, Leong T, Roche PC, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, Kyle RA, Gertz MA. Greipp PR. Prognostic value of bone marrow angiogenesis in multiple myeloma. Clin Cancer Res. 2000;6:3111–3116. [PubMed] [Google Scholar]
- 24.Kumar S, Gertz MA, Dispenzieri A, Lacy MQ, Wellik LA, Fonseca R, Lust JA, Witzig TE, Kyle RA, Greipp PR, Rajkumar SV. Prognostic value of bone marrow angiogenesis in patients with multiple myeloma undergoing high-dose therapy. Bone Marrow Transplant. 2004;34:235–239. doi: 10.1038/sj.bmt.1704555. [DOI] [PubMed] [Google Scholar]