Abstract
Golgi cells are important players in the function of the Cerebellar cortex, controlling the flow of incoming information from mossy fibres to the granule cells which excite other cortical neurons. We recently showed that in anaesthetised rats most Golgi cells respond to stimulation of afferents from a very wide peripheral receptive field with a long lasting depression of firing. These responses are mediated via a crossed ascending afferent pathway, but the supraspinal part of this pathway is unknown. Here we have examined the hypothesis that the lateral reticular nucleus, a brainstem nucleus with known broad afferent convergence and which projects mossy fibres to much of the cerebellum, is involved. First, we show that single pulse electrical microstimulation within the lateral reticular nucleus can elicit long lasting depressions in Golgi cells which are qualitatively similar to those evoked by peripheral afferent stimulation. Second, we show that the amplitude of the depressions of Golgi cell firing evoked by peripheral stimulation can be reduced by pharmacological manipulation of the lateral reticular nucleus, either ipsilateral or contralateral to the stimulus site, with local injections of either the GABAA receptor agonist muscimol or the AMPA receptor blocker CNQX. This evidence suggests that the lateral reticular nucleus is a relay nucleus in the brainstem for peripheral afferent information in a pathway that generates Golgi cell long lasting depression responses.
Keywords: Cerebellum, Golgi cell, Mossy Fibre, Lateral Reticular Nucleus, Rat
INTRODUCTION
Cerebellar Golgi cells are one of only 5 major neuron types in the cerebellar cortex, and are critical to its function (Watanabe et al 1998). They play an important role in modulating the transmission of mossy fibre input into the cerebellar cortex by both direct and spillover inhibition of granule cells (Hamann et al., 2002; Mitchell & Silver, 2003). Golgi cells have excitatory inputs from mossy fibres and parallel fibres which can generate short lasting excitations (Vos et al., 1999; Holtzman et al., 2006a), but we recently reported that in vivo the most frequently encountered response to peripheral afferent stimulation is a depression of firing which may last hundreds of milliseconds (Holtzman et al., 2006a; Holtzman et al., 2006b; Xu & Edgley, 2008). The long duration of these responses may be significant because the spillover effects of GABA released by Golgi cells in the glomerulus have a long duration ‘tonic’ component that would only abate if the Golgi cells changed firing for hundreds of milliseconds. In behaving animals depressions of Golgi cell firing to rates below an otherwise continual, spontaneous firing of 10-30 Hz also occur during behaviour (Edgley & Lidierth, 1987; Barmack & Yakhnitsa, 2008; Prsa et al., 2009). These depressions are intriguing as there are few inhibitory pathways to Golgi cells (see Holtzman et al. 2006a). Importantly, these responses can be evoked from very wide receptive fields, including most of the body. This, combined with the demonstration that they involve a crossed anterolateral funiculus pathway (Holtzman et al 2006b) led us to speculate that they may be mediated by spinoreticulocerebellar mossy fibre pathways, such as those relayed in the lateral reticular nucleus (LRN). The LRN has spinal afferent inputs that are multisensory, bilateral and widely convergent (Rosen & Scheid, 1973; Clendenin et al., 1974c; Menetrey et al., 1983; Ness et al., 1998; Robbins et al., 2005), and many afferents to the LRN ascend to the spinal cord in the anterolateral funiculus (Rosen & Scheid, 1973). In turn the LRN projects mossy fibres bilaterally to much of the cerebellum (Clendenin et al., 1974d; Wu et al., 1999). Studies of the integration of information in the LRN have shown a mixture of excitation and inhibition evoked by stimulation of peripheral afferents (Clendenin et al., 1974a,, 1975; Ekerot, 1990a, 1990b). In the experiments described here, we tested the hypothesis that the LRN is involved in the transmission of the signals that generate long lasting depressions of Golgi cell firing evoked by peripheral afferent stimulation, by both electrically stimulating and by pharmacologically blocking the LRN.
METHODS
Experiments were performed on 36 adult Wistar rats (300–350 g) that were anaesthetized using urethane (1–1.2 g kg−1 i.p. initially, supplemented with small doses of a fentanyl fluanisone mixture (Hypnorm™ Vetpharma, UK), i.p. as required to maintain anaesthesia). All procedures were approved by the UK Home Office regulations and the Local Ethical Committee of the University of Cambridge.
Surgery
Anaesthetized animals were fixed in a stereotaxic headholder. A heating blanket regulated by feedback from a rectal thermometer was used to maintain core body temperature at 37-38°C. The obex was exposed by removal of the overlying muscle and dura at the foramen magna, and a small craniotomy exposed the cerebellar cortex over the vermis.
Recording
Extracellular single-unit recordings were made using metal (stainless steel or tungsten electrodes insulated with parylene C with impedances ranging from 2 to 5 MΩ) or glass electrodes filled with 1M NaCl with impedance ranging from 6 to 15 MΩ. Signals from the microelectrodes were amplified (gain, x10 000), filtered (band-pass, 0.3–10 kHz) and digitized at 25 kHz (1401 and Spike 2 software from Cambridge Electronic Design, UK).
Stimulation
In the stimulation experiments an insulated stainless steel stimulating electrode (impedance about 150 kΩ) was inserted into the LRN through the dorsal aspect of the brain stem at a 30° angle, tip rostral, aimed at stereotaxic coordinates: −4.2mm posterior and 0.3 mm below the interaural line and 1.9mm lateral to the midline. Biphasic stimulus pulses of 0.2ms duration were delivered at a rate of approximately 0.6 Hz. Accurate placement of the stimulating electrode was verified using post mortem histology (e.g. figure 1A). Stimulation of peripheral afferents was achieved as described by Holtzman et al. (2006a) using pairs of percutaneous pins inserted into the skin of the vibrissae and the paws of the fore- and hindlimbs.
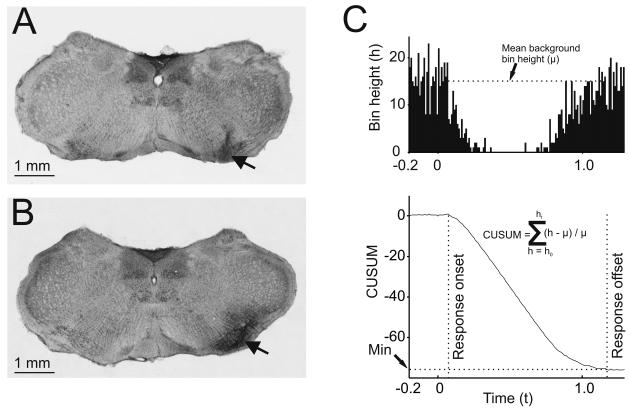
Figure 1.
Site of brainstem stimulation and injection and derivation of the size of Golgi cell inhibitory responses. A and B: coronal sections taken from 2 different experiments (approximately 4.5 mm caudal to the interaural plane). A shows an electrolytic lesion (arrow) made at the site of the electrode in the LRN from an electrical stimulation experiment. B shows the spread of dye after 0.2 μl drug injection into the LRN (arrow) in a pharmacology experiment. C. The upper panel shows a PSTH from a typical Golgi cell showing a depression of firing evoked by peripheral afferent stimulation. The lower graph shows the CUSUM derived from the PSTH, from which response onset and offset latencies were determined (vertical lines) and the integral of mean background bin height (μ) minus actual bin height (h) and then divided by mean background bin height (μ) used to quantify the response.
Drug injection
In separate experiments pharmacological blockade in the region of the LRN was achieved with microinjection of either the GABAA receptor agonist muscimol or the competitive AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), (dissolved in 0.9% saline to concentrations of respectively 8.24mM and 3mM, both substances obtained from Tocris Bioscience UK). Injections of 0.2 μl of either solution were made via a glass pipette (tip diameter 10-20 μm) attached to a Hamilton microsyringe positioned with the tip at the same stereotaxic co-ordinates as the stimulating electrode used in the experiments described above (figure 1B). The injection solutions also contained 0.2% Pontamine blue dye, which allowed the sites of injections and extent of spread to be assessed post mortem (e.g. figure 1B).
Analysis
Single unit spikes were discriminated off-line using a custom-made program (LabSpike – Bhumbra, http://www.pdn.cam.ac.uk/staff/dyball/labspike.html, Department of Physiology Development and Neuroscience, University of Cambridge). Responses were evaluated as poststimulus time histograms (PSTH) made in MATLAB (The MathWorks Inc., Natick, MA, USA) and incorporated a cumulative sum (CUSUM) derivative analysis (Davey et al., 1986), which facilitates the detection of persistent trends in the PSTH. As standard, PSTHs were made with 10ms bins and included at least 100 stimulus deliveries. A period of 200 ms immediately preceding the stimulus was used as an estimate of background spontaneous firing to establish the CUSUM baseline (the mean height of 20 10ms-wide bins). Responses were measured relative to this baseline.
Depressions of cell firing were quantified from the PSTHs. Mean background bin height was derived from a 0.2s-long prestimulus period. Responses were quantified as the sum of the normalized differences between this mean and the actual bin counts during the poststimulus period of the response. The dashed horizontal line on the PSTH in figure 1C shows the mean background bin height. The amplitude of the response is the area below this line and above the bins of the PSTH. The lower graph plots the sum of the normalized (to background) size of the difference between binheights in the PSTH and the background mean binheight value with respect to time, i.e. the CUSUM (Davey et al., 1986). The modulus of the minimum value of this function (Min) is termed the cumulative response, and is an index of the size of the depression of Golgi cell firing. The time after stimulus at which this minimum is reached indicates the offset of the inhibitory response. Statistical comparisons were made using SigmaStat software (version 3.1).
RESULTS
Responses of cerebellar Golgi and Purkinje cells to LRN stimulation
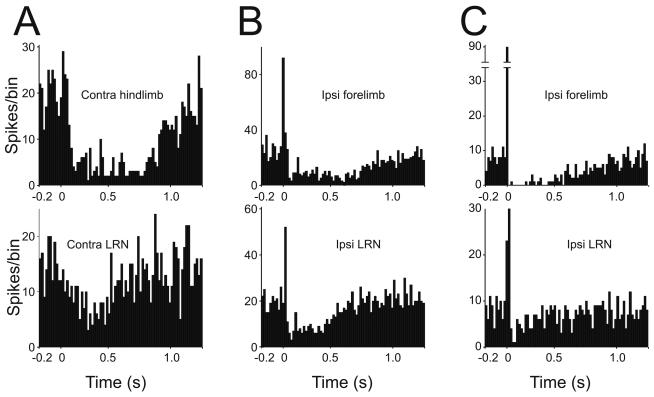
Single unit spike recordings were made from cerebellar Golgi and Purkinje cells in the vermis in lobules V/VI. Golgi cells were identified by their characteristic spontaneous spike firing and by their characteristic depressing responses to electrical stimulation of peripheral receptive fields, which were identical to those described previously (Holtzman et al., 2006a; see figure 2 top row of PSTHs). Purkinje cells were identified by the presence of both simple and complex spikes. In 27 cases Golgi cells were recorded whilst single pulse electrical stimuli were delivered in either the ipsilateral or the contralateral LRN. These stimuli evoked responses that were qualitatively very similar to those evoked by peripheral receptive field stimulation as described previously (Holtzman et al., 2006a; Holtzman et al., 2006b; Xu & Edgley, 2008). Examples of the response patterns of Golgi cells to electrical stimulation in the LRN in are shown in figure 2, (bottom row).
Figure 2.
Comparison of Golgi cell responses to LRN and periphery stimulation. Responses of 3 different Golgi cells to single-pulse electrical stimulation of peripheral afferents (top row – source indicated on the PSTHs) and to a single electrical stimulus delivered in the LRN (bottom row, laterality indicated). Each PSTH is constructed from 100 stimulus deliveries. Stimulus occurs at time = 0. Binwidth = 10ms. LRN stimulus intensities are 70, 80 and 50μA for respectively A, B and C).
Single pulse stimulation in the LRN evoked two different types of responses, each of which paralleled the responses elicited by peripheral afferent stimulation, as illustrated in figure 2. The most common response was a long lasting (minimally 370ms) depression of firing that was qualitatively similar to their responses evoked by peripheral stimulation: this was the only response to LRN stimulation seen in the majority (18 of 27, 67%) of the Golgi cells tested. An example of this type of response is shown in figure 2A. Initial excitations were also seen, albeit more rarely (9/27, 33% of neurons). Of these, 6 (22% of the total) also showed a subsequent depression of firing, the shortest of which lasted for 440 ms. (an example is illustrated in figure 2B); the remaining 3 cells responded with only a short latency excitation (SLE) (figure 2C). Since Golgi cells rarely discharge short intervals, the absence of firing immediately following the SLE is interpreted to reflect the refractoriness of the Golgi cell. All of the 9 Golgi cells that showed an SLE to LRN stimulation also showed an SLE response to at least one of the sources of peripheral afferents tested (figure 2 B & C, upper PSTHs).
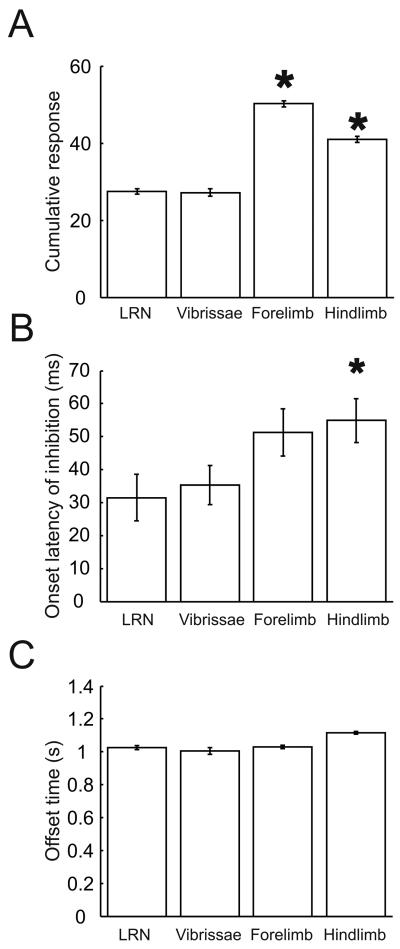
In order to quantitatively compare the depressions of Golgi cells evoked by LRN and peripheral stimulation the approach described in methods was used. The amplitude of the firing depression normalised to spontaneous background firing (defined as the cumulative response) was calculated for each Golgi cell's responses to both LRN and peripheral stimulation (all receptive fields tested). For the 6 cells where short latency excitation preceded the depression, the bins of the excitatory response were substituted with bin heights equal to the mean background, calculated from the 0.2s pre-stimulus time. The amplitudes of depressions evoked by LRN and peripheral stimulation are compared in figure 3A. The cumulative response values for LRN stimulation are significantly smaller those for forelimb and hindlimb afferent stimulation (P<0.05, unpaired Student's t-tests), but not significantly different to the response to vibrissal stimulation (P=0.95, unpaired Student's t-test). Since LRN-derived mossy fibres project to the cerebellar cortex bilaterally (Wu et al., 1999) it is likely that Golgi cells receive inputs from the LRN on both sides, but in this experiment the LRN was activated unilaterally, and with a single stimulus of less than 100 μA.
Figure 3.
Comparison of Golgi cell responses elicited from peripheral stimulation and LRN stimulation. Comparison of the size (A), mean onset latencies of pure inhibitory responses (B), and mean offset latencies (C) of Golgi cell responses to peripheral and LRN stimulation. * indicates statistical significance at 5% confidence interval with LRN stimulation (P<0,05, upaired Student's t-tests).
The onset times of the depressions evoked from LRN and peripheral stimulation were measured by eye from deviations in the CUSUM (figure 1C) and are compared in figure 3B. The depressions evoked by LRN stimulation (single pulse) had a mean onset latency of 32 ± 6.7 ms (mean ± SEM). These onset latencies were significantly shorter than those evoked from the hindlimbs (P= 0.013, unpaired Student's t-test), but not significantly different to those evoked from forelimb or trigeminal afferents (P>0.05, unpaired Student's t-tests). Although single stimuli were delivered, the depressions of firing had durations averaging almost a second, similar to the peripheral afferent evoked depressions. The offset times of the Golgi cell inhibitory response (measured from the time of the stimulus) were measured by taking the time at which the CUSUM of the PSTH reached its minimum (see methods and Figure 1C) and are compared in figure 3C; the mean time of termination of the response was 969±78ms (mean ± SEM) after the time of the stimulus and was not significantly different to values elicited from any of the peripheral receptive fields (P>0.05, unpaired Student's t-tests).
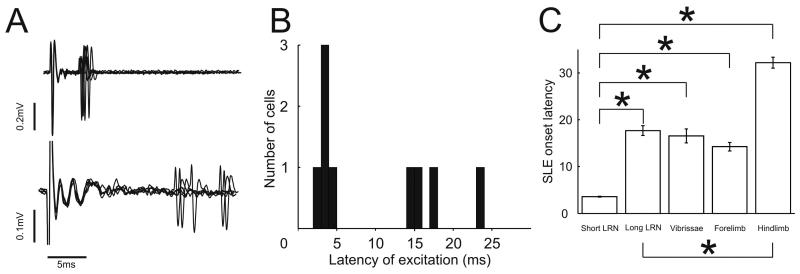
Excitatory responses evoked by LRN stimulation were rarer than depressions, as they were for peripheral stimulation. In total 9/27 cells showed excitatory responses. Their onset latencies fell into two distinct groups. In one group (5 cells), the latencies of the first spike of the SLE were brief, with little variance, ranging from 2.9ms to 4.3 ms (mean ±SEM = 3.6 ± 0.22 ms), in the other group (4 cells) the excitatory response onsets had longer and more variable latencies, ranging from 14.1ms to 23.6ms (mean latency ±SEM = 17.7 ± 4.2 ms). Bimodality of the population was verified by performing a Kolmogorov-Smirnov normality test which showed that although each subgroup was normally distributed (P>0.2) the combined population was not (P=0.01). Example recordings from one neuron of each group are shown in figure 4A and the histogram of all SLE onset latencies is shown in figure 4B. Figure 4C plots the mean and standard errors of the onset latencies of the shorter and longer latency response groups compared with latencies of SLE responses evoked from peripheral afferents. The various inter-group statistical relationships are indicated on the graph: the short latency LRN-evoked responses had latencies significantly shorter than those evoked from any source of peripheral afferents (P<0.05, unpaired Student's t-tests). The coefficient of variation (standard deviation ÷ mean) for the shorter and longer latency groups are 0.139 and 0.239 respectively. This means that the group with shorter onset latencies had less variation in their onset latencies than the group with longer latencies (as can be seen from the raw data in figure 4A and the relative sizes of the error bar in figure 4C). All of the Golgi cells in which LRN stimulation evoked an SLE without a following depression of firing (such as in figure 2C) belonged in the longer latency group. The differences in the variance of the two groups could be because the SLE responses in the shorter group are due to direct mossy fibre to Golgi cell connections. (Antidromic latencies have been previously reported to be less than 3ms in the cat (Clendenin et al., 1974d)) whereas the SLE responses in the longer group may be via the mossy fibre – granule cell – Golgi cell pathway. The shorter and longer latencies of SLE from LRN stimulation are compared with the SLE latencies from peripheral stimulation and with each other in figure 4C. The small numbers of cells limit the conclusion that can be drawn from this comparison.
Figure 4.
Bimodal distribution of excitatory response onset latencies to LRN stimulation. A. Raw data overlays of spike recordings from two Golgi cells with different SLE onset latencies lined up by stimulus artifact. B. Histogram of mean SLE onset latencies showing two groups (binwidth = 1ms). C. Mean and standard error of SLE onset latency for the shorter and longer group. * indicates significant difference at 5% confidence interval (P<0,05, upaired Student's t-tests).
Stimulation of both the ipsilateral and the contralateral LRN could elicit similar responses in Golgi cells. The cumulative response values, onset and offset latencies elicited from two sides of the body were not statistically different (P< 0.05, unpaired Student's t-tests). This may well reflect the extensive crossing of LRN derived mossy fibres in the cerebellar cortex (Wu et al., 1999). Single pulse stimulation of the LRN at this intensity had no effect on the majority of cerebellar Purkinje cells recorded. Of 24 Purkinje cells recorded in the vicinity of the Golgi cells described above, only one showed a clear simple spike response following single pulse LRN stimulation: a brief excitation in simple spike firing followed by a depression of firing lasting <100 ms (most likely due to the action of molecular layer interneurons (Bengtsson & Jorntell, 2009)). This particular cell's response is in agreement with numerous previous studies describing the cerebellar projections of LRN neurons as mossy fibres (Sasaki & Strata, 1967; Clendenin et al., 1974d, 1974b; Wu et al., 1999). However, the fact that the rest of the Purkinje cells tested were indifferent to LRN stimulation suggests that either the LRN derived parallel fibre synapses on Purkinje cells of this region were insufficiently strong to influence Purkinje cell firing, or that Golgi cells receive a distinct and more potent input from LRN neurons (Arshavsky et al., 1986). In none of the cases did the electrical stimulation in the LRN evoke climbing fibre responses in local Purkinje cells at the intensities we used in this study, verifying that we were not activating climbing fibres
Changes in Golgi cell responses after pharmacological blockade of the LRN
The above results show that single electrical stimuli delivered within the LRN generate responses very similar to those generated by stimulation of peripheral afferents in Golgi cells, consistent with the LRN forming part of the afferent pathway by which Golgi cells in the rat receive their widely convergent peripheral inputs. In a separate series of experiments we tested whether interference with LRN function affects the Golgi cell responses to peripheral afferent stimulation. To achieve this we injected 0.2 μl of either the GABAA receptor agonist muscimol (8.24mM: 1mg/ml) or the AMPA/Kainate receptor antagonist CNQX (3mM) into the LRN unilaterally whilst afferents from the hindlimbs on both sides of the body were stimulated alternately (interstimulus interval = 2s). PSTHs of the Golgi cell responses were constructed from a control period of at least100 trials before and 100 trials following drug injection for both ipsi- and contralateral hindlimbs. To compare the PSTHs taken before and after the injection we defined the response duration to be the period of time in the pre-injection PSTH from the stimulus time to the end of the inhibitory response (measured by taking the time at which the CUSUM first reaches its minimum value). The PSTH bin values during the response (the grey bins in figure 5B and C) for each stimulus were normalised to the background mean bin height. Pre- and post-injection normalised binheight values were compared using 2-tailed paired Student's t-tests.
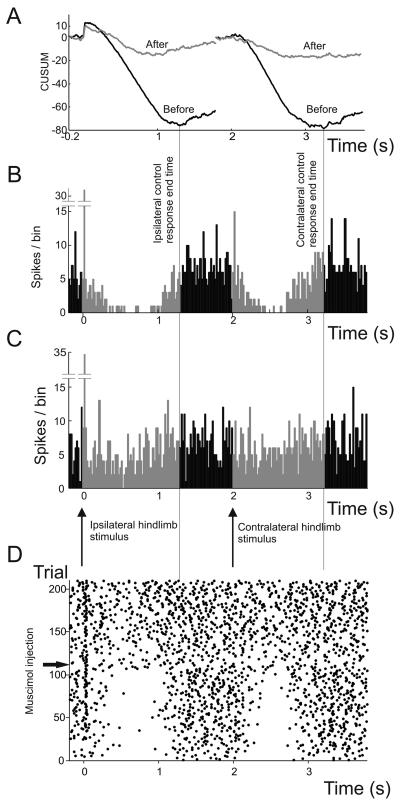
Figure 5.
Example of the effect of muscimol injection in the LRN on the response of a Golgi cell to ipsilateral and contralateral receptive field stimulation. CUSUMs (A) were constructed from PSTHs derived from 100 trials before (B) and after (C) muscimol injection (black and grey lines, respectively). D shows the same data in raster form. Responses to stimulation of both ipsilateral hindlimb afferents (time 0) and contralateral hindlimb afferents (time 2s) are shown. The offsets of the responses were taken as the times of the minimum of the CUSUM (black lines in A, derived from the PSTHs in B) before injection of muscimol. Injection of 0.2 μl muscimol reduced the responses as can be seen from the PSTHs in C and the CUSUM (grey line in A). For both the ipsilateral and contralateral responses, the PSTH bins between the stimulus and the control response end time (highlighted in grey) were selected and their binheight values normalised to mean pre-stimulus binheights. These normalised binheight values can then be compared before (B) and after (C) muscimol injection and tested for statistically significant differences. The onset of the effect of muscimol was rapid, as can be seen by the change in the raster of spikes after injection (D). Note that in addition to the depression evoked by stimulation of afferents from either hindlimb, those from the ipsilateral hindlimb also evoked a short latency excitation (the high bin after time 0 in the PSTHs in B & C), which was unaffected by the muscimol injection.
In 7 animals we injected muscimol into the LRN. For each animal only one Golgi cell was tested to guard against potential long-lasting effects of the drugs. In 5 of these, unilateral injection caused a significant decrease (P < 0.05, paired Student's t-tests) in the magnitude of the depressions of Golgi cell firing evoked by both ipsilateral and contralateral hindlimb stimulation. Figure 5 shows one such example. The remaining two cells showed a significant decrease in the depression evoked by stimulation of one hindlimb but not the other (one ipsilateral, the other contralateral). The pre-stimulus control firing rates were not significantly altered by the muscimol injections (P=0.43, paired Student's t-test).
We injected CNQX in 8 animals, again recording only one Golgi cell from each. In 2 of these there was a significant decrease in the depression evoked by stimulation of afferents from both hindlimbs; in 3 other cells the response to stimulation of contralateral, but not ipsilateral hindlimb afferents was significantly decreased. In one cell the response to stimulation of ipsilateral, but not contralateral hindlimb afferents was significantly decreased (In all cases significance was accepted at P<0.05, paired Student's t-tests). The remaining 2 cells did not show significant changes in their responses to either hindlimb. In none of the cases were there significant increases in inhibitory response amplitude after either muscimol or CNQX injection. The pre stimulus control firing rates were reduced after CNQX injection (from a mean of 5.6Hz to 5 Hz, P = 0.039; paired Student's t-test).
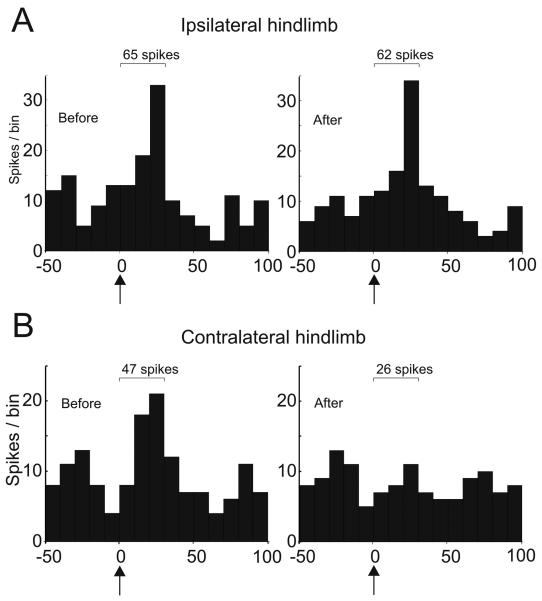
A small proportion of Golgi cells show SLE responses (Holtzman et al., 2006a); 2 of the Golgi cells tested with muscimol showed SLE responses evoked from both ipsilateral and contralateral hindlimbs that preceded the long-lasting depressions. In both cases, the contralaterally evoked SLE responses were abolished following the muscimol injections abolished, but the ipsilateral SLE responses were unaffected. Example PSTHs illustrating the responses of one of these neurons is shown in figure 6. A weak excitatory response evoked by stimulation of ipsilateral hindlimb afferents is unaffected by muscimol injection, while a similar response evoked from the contralateral hindlimb is abolished. Two other cells showed an SLE response to only the ipsilateral hindlimb stimulation, and these were not visibly affected by muscimol injection (the cell illustrated in figure 5 is one). None of the cells tested with CNQX injection had SLE responses.
Figure 6.
Muscimol injections abolished contralaterally but not ipsilaterally evoked Golgi cell SLE responses. A. PSTHs showing 50 ms before and 100ms after stimulation of ipsilateral and contralateral hindlimb afferents, respectively. B PSTHs for the same stimuli in the same cell following injection of muscimol into the LRN. Numbers on each PSTH show the total spike count for the 3 bins following the stimulus in each case.
Judging from the raster plots (e.g. figure 5D) the drug injections had relatively rapid onsets (a few seconds). By assessing the responses in exactly 100 trials after drug injection we tried to control for the possibility that the effects of the drugs could wane over time. However recovery was not seen for up to 45 minutes after injection.
In control experiments we injected 0.2μl of 0.8% saline into the LRN we did not detect any statistically significant effects on Golgi cell responses (n = 5 cells, P>0.05, paired Student's t-tests).
DISCUSSION
The objective of this work was to test whether the LRN is a brainstem relay for the long lasting depressions of Golgi cell activity that are reliably evoked by stimulation of peripheral afferents. Both the approaches we have used provide evidence to further implicate the LRN as a relay that mediates these responses. Single pulse stimulation within the LRN has very similar effects to stimulating peripheral afferents as far as Golgi cell responses are concerned. Furthermore, pharmacological blockade of the LRN attenuates the Golgi cell responses. Therefore, these results confirm the proposal that the LRN is involved in Golgi cell responses (Holtzman et al. 2006b). Prior anatomical studies showed that LRN-derived mossy fibres project to the cerebellar cortex (Clendenin et al., 1974b; Wu et al., 1999) and therefore can act to directly influence Granule and Golgi cell firing.
Both approaches that we used will have manipulated LRN output. The electrical stimuli we used were of relatively low intensities because LRN projection axons are relatively large should be easily activated. Structures other than the LRN may potentially have been activated, one that we need to consider is the inferior olive or olivocerebellar climbing fibre axons, since stimulation of climbing fibres generates depressions of Golgi cell firing (Schulman & Bloom, 1981; Xu & Edgley, 2008). However the stimuli we used did not evoke complex spikes in Purkinje cells recorded close to the Golgi cells we sampled.
A second issue arising from the use of electrical stimulation is the potential activation of other fibres of passage in the region of the LRN. This was the impetus behind the pharmacological experiments. The agents we used selectively depress transmission by neurons rather than axons and reduced Golgi cell responses. The results strongly implicate the LRN and the region around it in the long lasting depressions of Golgi cell firing.
Laterality of responses
The present results, when interpreted in isolation, fits with a scheme where the inhibitory component of the Golgi cell response is carried by a pathway activated by peripheral afferents that projects bilaterally to both LRNs but the early excitatory component that was identified in some Golgi cells projects via the contralateral LRN only. The output mossy fibres of the LRN are then responsible for the response of the cerebellar Golgi cells (Holtzman et al., 2006a).
However previous work (Holtzman et al., 2006b) showed that lateral hemisection of the lumbar spinal cord strongly reduced or abolished the Golgi cell responses to stimulation of afferents below and contralateral to the lesion, but not responses to stimulation of ipsilateral afferents. Their conclusion was that the ascending spinal pathway was crossed and unilateral. These two seemingly contradictory conclusions could be reconciled if convergence of information from both sides of the body occurs supraspinally, in structure(s) rostral to the lumbar spinal cord, but before or at the LRN. Convergence could occur at the spinal cord level via structures similar to the bilateral ventral flexor reflex tract (bVFRT) described by Clendinin et al (Clendenin et al., 1974e) or on neurons within the LRN itself. However many intermediate relays this pathway may contain, it is likely that the LRN is the final relay before the signal reaches the cerebellum since LRN neurons project mossy fibres directly to the cerebellum (Wu et al., 1999)
The question remains as to how excitatory mossy fibres from the LRN can induce depressions of Golgi cell firing Mossy fibres make excitatory synapses onto cerebellar granule cells (Eccles et al., 1966) as well as direct synapses onto Golgi cells (Palay & Chan-Palay, 1974). Indeed Eccles et al (1966) recorded excitations in Golgi cells upon direct mossy fibre stimulation. This was postulated to be mediated via the mossy fibre – granule cell pathway. However subsequently the efficacy of the parallel fibre-Golgi cell synapses was found to be rather low (Dieudonne, 1998). Furthermore in vitro direct parallel fibre stimulation induces IPSCs in Golgi cells that are mediated by mGluR2 receptors (Watanabe & Nakanishi, 2003). Therefore it is possible that the dominant response (in terms of number of spikes lost or gained) of Golgi cells to glutamatergic mossy fibre input (direct and/or via parallel fibres) can be that of an inhibition in its firing. Hence it may be possible for activation of the LRN (a major source of mossy fibres) to produce a net inhibition of Golgi cells.
Functional role of the Golgi cell responses
Two major issues arise from this work. The first is that in the urethane anaesthetised rat responses are readily elicited in Golgi cells by stimuli that do not substantially affect the firing of the local Purkinje cells. The substantial impact that LRN stimulation has on Golgi cell firing suggests that, although carried by mossy fibres, the LRN afferents have a much more potent influence on Golgi cell firing than on Purkinje cell firing. The demonstration by Bengtsson & Jorntell that the limited convergence on granule cells can include mossy fibres of the same response type (including LRN mossy fibre response types) suggests that the activity of LRN afferents could be preserved in the granule cell pathways (Bengtsson & Jorntell, 2009). This might explain the different responses in Purkinje and Golgi cells. The second issue is that LRN neurons relay to Golgi cells signals generated by widely convergent inputs that are unlikely to be parametrically specific. It is more likely that they play a role in modulating the gain of mossy fibre – granule cell transmission associated with overall levels of sensory arousal (as postulated by Holtzman et al 2006a/b). It is possible that the LRN – Golgi cell system controls the signal-to-noise ratio of signals being passed onto Purkinje cells via the parallel fibres.
What might be the impact of the unusual convergence and response properties of Golgi cells on the processing in the granule cell layer? One possibility is that this LRN-Golgi cell sensory system functions to optimise the signal and noise ratio in parallel fibre inputs to Purkinje cells in the face of differing levels of sensory arousal. The way this operates may be explained by the chain performance theorem (Printz & Servan-Schreiber, 1990; Servan-Schreiber et al., 1990): a mechanism proposed to explain increases in signal to noise performance mediated by global catecholamine signalling (Servan-Schreiber et al., 1990). The theorem demonstrates that in a chain of two neurons where the output of the first neuron (termed the input unit) in the chain, convolved with gain-independent noise, forms the input to the second neuron (termed the response unit) in the chain, increases in the gain of inputs into the first neuron will increase the performance of signal detection by the second neuron. However, inappropriately high gain in a low noise situation will generate false positive signals, so gain needs to be controlled. Within the context of the cerebellar cortex, granule cells correspond to the input units and Purkinje cells correspond to the output units in the model (Servan-Schreiber et al., 1990). Golgi cells are ideally placed to alter the gain of mossy fibre inputs to granule cells: in a high arousal (high noise) situation the depression of Golgi cell firing would increase the gain of mossy fibre to granule cell transmission. Thus Golgi cells function to optimise signal detection by Purkinje cells according to arousal levels. Furthermore, our previous work (Xu & Edgley 2008) suggests that the strength of this proposed LRN - Golgi cell gain control system is subject to climbing fibre dependent reduction, thereby providing a potential mechanism for tuning out false positives in the presence of persistent increases in sensory arousal. Systems level modelling will be required to investigate this further.
ACKNOWLEDGEMENTS
WX was supported by the Elmore Fund of the Clinical School of Medicine, University of Cambridge. Part of this work was supported by NIH R01 NS040863-06.
ABBREVIATIONS
- bVFRT
bilateral ventral flexor reflex tract
- CUSUM
cumulative sum
- LRN
lateral reticular nucleus
- PSTH
Post stimulus time histogram
- SLE
short latency excitation
REFERENCES
- Arshavsky Y, Gelfand I, Orlovsky G. Cerebellum and rhythmical movements. Studies in brain function. 1986;13 [Google Scholar]
- Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J Neurosci. 2008;28:1140–1152. doi: 10.1523/JNEUROSCI.3942-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson F, Jorntell H. Sensory transmission in cerebellar granule cells relies on similarly coded mossy fiber inputs. Proc Natl Acad Sci U S A. 2009;106:2389–2394. doi: 10.1073/pnas.0808428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clendenin M, Ekerot CF, Oscarsson O. The Lateral Reticular Nucleus in the Cat III. Organization of Component Activated from Ipsilateral Forelimb Tract. Exp Brain Res. 1974a;21:501–513. doi: 10.1007/BF00237168. [DOI] [PubMed] [Google Scholar]
- Clendenin M, Ekerot CF, Oscarsson O. The Lateral Reticular Nucleus in the Cat IV. Activation from Dorsal Funiculus and Trigeminal Afferents. Exp Brain Res. 1975;24:131–144. doi: 10.1007/BF00234059. [DOI] [PubMed] [Google Scholar]
- Clendenin M, Ekerot CF, Oscarsson O, Rosen I. Distribution in cerebellar cortex of mossy fibre afferents from the lateral reticular nucleus in the cat. Brain Res. 1974b;69:136–139. doi: 10.1016/0006-8993(74)90378-3. [DOI] [PubMed] [Google Scholar]
- Clendenin M, Ekerot CF, Oscarsson O, Rosen I. The Lateral Reticular Nucleus in the Cat II. Orgnization of Component Activated from Bilateral Ventral Flexor Reflex Tract (bVFRT) Exp Brain Res. 1974c;21:487–500. doi: 10.1007/BF00237167. [DOI] [PubMed] [Google Scholar]
- Clendenin M, Ekerot CF, Oscarsson O, Rosen I. The lateral reticular nucleus in the cat. I. Mossy fibre distribution in cerebellar cortex. Exp Brain Res. 1974d;21:473–486. doi: 10.1007/BF00237166. [DOI] [PubMed] [Google Scholar]
- Clendenin M, Ekerot CF, Oscarsson O, Rosen I. The lateral reticular nucleus in the cat. II. Organization of component activated from bilateral ventral flexor reflex tract (bVFRT) Exp Brain Res. 1974e;21:487–500. doi: 10.1007/BF00237167. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Ellaway PH, Stein RB. Statistical limits for detecting change in the cumulative sum derivative of the peristimulus time histogram. J Neurosci Methods. 1986;17:153–166. doi: 10.1016/0165-0270(86)90068-3. [DOI] [PubMed] [Google Scholar]
- Dieudonne S. Submillisecond kinetics and low efficacy of parallel fibre-Golgi cell synaptic currents in the rat cerebellum. J Physiol. 1998;510(Pt 3):845–866. doi: 10.1111/j.1469-7793.1998.845bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. The mossy fibre-granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Exp Brain Res. 1966;1:82–101. doi: 10.1007/BF00235211. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Lidierth M. The discharges of cerebellar Golgi cells during locomotion in the cat. J Physiol. 1987;392:315–332. doi: 10.1113/jphysiol.1987.sp016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF. The lateral reticular nucleus in the cat. VI. Excitatory and inhibitory afferent paths. Exp Brain Res. 1990a;79(1):109–119. doi: 10.1007/BF00228879. [DOI] [PubMed] [Google Scholar]
- Ekerot CF. The lateral reticular nucleus in the cat. VII. Excitatory and inhibitory projection from the ipsilateral forelimb tract (iF tract) Exp Brain Res. 1990b;79(1):120–128. doi: 10.1007/BF00228880. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Holtzman T, Mostofi A, Phuah CL, Edgley SA. Cerebellar Golgi cells in the rat receive multimodal convergent peripheral inputs via the lateral funiculus of the spinal cord. J Physiol. 2006b;577:69–80. doi: 10.1113/jphysiol.2006.117218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman T, Rajapaksa T, Mostofi A, Edgley SA. Different responses of rat cerebellar Purkinje cells and Golgi cells evoked by widespread convergent sensory inputs. J Physiol. 2006a;574:491–507. doi: 10.1113/jphysiol.2006.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetrey D, Roudier F, Besson JM. Spinal neurons reaching the lateral reticular nucleus as studied in the rat by retrograde transport of horseradish peroxidase. J Comp Neurol. 1983;220:439–452. doi: 10.1002/cne.902200406. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Follett KA, Piper J, Dirks BA. Characterization of neurons in the area of the medullary lateral reticular nucleus responsive to noxious visceral and cutaneous stimuli. Brain Res. 1998;802:163–174. doi: 10.1016/s0006-8993(98)00608-8. [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex - Cytology and Organization. 1974 [Google Scholar]
- Printz H, Servan-Schreiber D. Foundations of a computational theory of catecholamine effects. Technical Report CMU-CS-90-105. 1990 [Google Scholar]
- Prsa M, Dash S, Catz N, Dicke PW, Thier P. Characteristics of responses of Golgi cells and mossy fibers to eye saccades and saccadic adaptation recorded from the posterior vermis of the cerebellum. J Neurosci. 2009;29:250–262. doi: 10.1523/JNEUROSCI.4791-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MT, Uzzell TW, Aly S, Ness TJ. Visceral nociceptive input to the area of the medullary lateral reticular nucleus ascends in the lateral spinal cord. Neurosci Lett. 2005;381:329–333. doi: 10.1016/j.neulet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Rosen I, Scheid P. Patterns of afferent input to the lateral reticular nucleus of the cat. Exp Brain Res. 1973;18:242–255. doi: 10.1007/BF00234595. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Strata P. Responses evoked in the cerebellar cortex by stimulating mossy fibre pathways to the cerebellum. Exp Brain Res. 1967;3:95–110. doi: 10.1007/BF00233255. [DOI] [PubMed] [Google Scholar]
- Schulman JA, Bloom FE. Golgi cells of the cerebellum are inhibited by inferior olive activity. Brain Res. 1981;210:350–355. doi: 10.1016/0006-8993(81)90908-2. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Vos BP, Volny-Luraghi A, De Schutter E. Cerebellar Golgi cells in the rat: receptive fields and timing of responses to facial stimulation. Eur J Neurosci. 1999;11:2621–2634. doi: 10.1046/j.1460-9568.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Nakanishi S. mGluR2 postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron. 2003;39:821–829. doi: 10.1016/s0896-6273(03)00530-0. [DOI] [PubMed] [Google Scholar]
- Wu HS, Sugihara I, Shinoda Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol. 1999;411:97–118. doi: 10.1002/(sici)1096-9861(19990816)411:1<97::aid-cne8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Xu W, Edgley SA. Climbing fibre-dependent changes in Golgi cell responses to peripheral stimulation. J Physiol. 2008;586:4951–4959. doi: 10.1113/jphysiol.2008.160879. [DOI] [PMC free article] [PubMed] [Google Scholar]