Abstract
Background
Information is currently limited on the long-term follow up of HIV-1 infected women who are on highly active antiretroviral therapy (HAART) that contains nevirapine and lamivudine and who were previously exposed to antiretroviral drugs for the prevention of mother to child transmission (PMTCT) of HIV.
Methods
We studied the 36-month immunological response to HAART in HIV-1 infected women in Côte d'Ivoire. The women were previously exposed to antiretroviral drug regimens for PMTCT, including single-dose nevirapine and/or short-course zidovudine with or without lamivudine. All HAART regimens included a non-nucleoside reverse transcriptase inhibitor.
Results
At 36 months: the median absolute increase in CD4+ T cell count was +359 cells/mm3 (IQR: 210-466) in 200 women who had undergone 36-month follow-up visits; +359 cells/mm3 (IQR: 222-491) in 88 women not exposed to PMTCT antiretrovirals; and +363 cells/mm3 (IQR: 200-464) in 112 women exposed to at least one antiretroviral PMTCT regimen. Overall, 49 (19.8%) of the 247 women who initiated HAART met the immunological failure criteria at least once during follow up. The overall probability of immunological failure was 0.08 (95% CI: 0.12-0.15) at 12 months, and 0.21 (95% CI: 0.16-0.27) at 36 months. No difference was observed according to the presence or absence of resistance mutations to nevirapine or lamivudine in women tested at four weeks postpartum. In addition, at 36 months, 23% of women were lost to follow up, dead or had stopped their treatment.
Conclusions
A non-nucleoside reverse transcriptase inhibitor-based antiretroviral regimen, initiated a year or more after PMTCT exposure and that includes nevirapine, remains a good option for at least the first 36 months of treatment.
Background
Information is currently limited on the long-term follow-up of HIV-1 infected women who are on highly active antiretroviral therapy (HAART) containing nevirapine (NVP) and lamivudine (3TC) and who were previously exposed to antiretroviral (ARV) drugs for the prevention of mother to child transmission (PMTCT) of HIV [1-4].
A 12-month study showed a good immunological response in women previously exposed to ARV drugs for PMTCT [2]. However, in this study we found a higher risk of virological failure in women who had 3TC-acquired resistance mutation four weeks postpartum [2]. We now report the immunological response to HAART at 36 months in women previously exposed to short-course ARV prophylaxis and study factors associated with immunological failure or with immunological failure and death according to the history of PMTCT exposure.
Methods
A prospective cohort study was conducted in Abidjan, Côte d'Ivoire, between August 2003 and June 2009 among all HIV-infected women who initiated HAART in the MTCT-Plus initiative. The study population and study design have previously been described [2].
Very briefly, our population consisted of: (1) women never exposed to any treatment for PMTCT; (2) women exposed to single-dose NVP (sdNVP) and zidovudine (ZDV) for PMTCT; and (3) women exposed to short-course zidovudine (scZDV) and 3TC for PMTCT. The primary variable of interest was the presence of viral resistance mutation to NVP or 3TC measured at Week 4 postpartum.
Two outcomes were considered after 36 months on HAART: (1) immunological failure, defined as a 50% fall from absolute CD4+ T cell count peak level [5]; and (2) a combined criteria, defined as either immunological failure or the occurrence of death during the first 36 months of follow up. The virological analyses were done retrospectively, and results were not available for clinical use. Decisions to switch antiretroviral regimens were thus made by local clinicians based on routinely collected immunological and clinical data.
Other study variables measured at time of HAART initiation were included in the analysis: age, WHO clinical stage, body mass index, hemoglobinemia at HAART initiation, and self-reported adherence (seven-day self-report at six, 12, 18, 24, 30 and 36 months) [5]. Cox regression was used to identify factors associated in univariable analysis (p < 0.20) with immunological failure or the combined criteria. We censored the follow up of each patient at the date of last visit, or death, or date of switch to a protease inhibitor (PI) to evaluate the response to non-nucleoside reverse transcriptase inhibitor (NNRTI)-based treatment.
Results
From August 2003 to September 2005, 247 women initiated 3TC-containing HAART with either NVP or efavirenz (EFV). At HAART initiation, their median age was 28 years (interquartile range: 25-32) and their median CD4+ count was 188 cells/mm3 (IQR: 126-264). Overall, 28 women (11.3%) were classified at WHO clinical Stage 1; 110 (44.5%) at Stage 2; 96 (38.9%) at Stage 3; and 13 (5.3%) at Stage 4. A total of 109 women (44.1%) had never been exposed to a PMTCT ARV regimen during a previous pregnancy, and 138 (55.9%) had previously received a PMTCT regimen: 50 had received scZDV + sdNVP and two sdNVP only; 81 had received scZDV+sc3TC+sdNVP and five scZDV+3TC only. Among 73 of the 86 3TC-exposed women tested for resistance mutations at Week 4 postpartum, 11 (15.1%) had detectable 3TC resistance mutations. Among 111 of the 133 sdNVP-exposed women tested at Week 4 postpartum, 19 (17.1%) had detectable NVP resistance mutations.
The first-line HAART regimen was ZDV+3TC+NVP in 234 (95.1%) women, and stavudine (d4T)+3TC+NVP in seven (2.9%) women; five women started HAART with ZDV+3TC+EFV and one began with d4T+3TC+EFV. The median time between exposure to sdNVP and initiation of HAART was 21 months (IQR: 13-26). During the 36 months of follow up, 30 women (12.1%) were lost to follow up, 10 (4.0%) stopped treatment at their own request, and 17 (6.9%) died. Furthermore, 19 out of 247 (7.7%) HIV-infected women switched to PI-based HAART. The reasons for switching were immunological failure in four patients and side effects related to NVP or EFV in 15 cases. There was no association between switching to PI and PMTCT-acquired resistance to NVP (p = 0.43) or to 3TC (p = 0.93).
At 36 months, the median absolute increase in CD4+ count was +359 cells/mm3 (IQR: 210-466) in 200 women who had undergone 36-month follow-up visits. The increase was +359 cells/mm3 (IQR: 222-491) in 88 women not exposed to PMTCT ARV, and +363 cells/mm3 (IQR: 200-464) in 112 women exposed to at least one ARV PMTCT regimen. The median absolute increase in CD4 count was +366 cells/mm3 (IQR: 174-461) in the 18 women with NVP resistance at Week 4 postpartum, and > 230 cells/mm3 (IQR: 130-403) in the eight women with 3TC resistance mutations detected at Week 4 postpartum (Figures 1A and 1B). When analyses of immunological response were restricted to the 184 women who did not switch to a PI during the follow up, the median absolute increase in CD4+ count was 361 cells/mm3 (IQR: 220-466) at 36 months.
Figure 1.
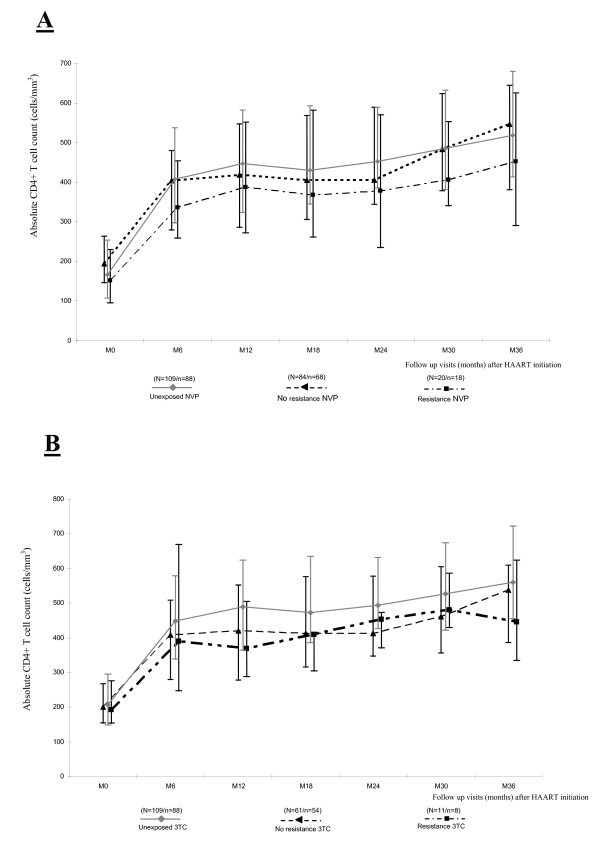
Immunological response in HIV-infected women. A. Immunological response in HIV-infected women exposed to nevirapine or acquired PMTCT resistance to nevirapine. B. Immunological response in HIV-infected women exposed to lamivudine or acquired PMTCT resistance to lamivudine. NVP = nevirapine. 3TC = lamivudine. N = number of patients with CD4 count available at baseline (M0). n = number of patients with CD4 count available at month 36 (M36).
Overall, 49 (19.8%), (95% CI 15.0-25.3%) of the 247 women who initiated HAART met the immunological failure criteria at least once during follow up. Regarding immunologic failure, no statistical difference was found between the women unexposed to sdNVP, those exposed to NVP without resistance mutations, and those with NVP resistance mutations at Week 4 postpartum (14.7% vs. 19.8% vs. 25.0%, respectively, p = 0.50). The same conclusion was drawn for 3TC exposure (14.7% in women unexposed to 3TC vs. 14.8% in women exposed without 3TC-resistance mutation vs. 18.2% in women exposed with 3TC-resistance mutations, p = 0.71).
The overall probability of immunological failure was 0.08 (95% CI: 0.12-0.15) at 12 months, 0.14 (95% CI: 0.10-0.19) at 24 months and 0.21 (95% CI: 0.16-0.27) at 36 months. At 36 months, the probability of immunological failure was 0.25 (95% CI: 0.18-0.28) in women who selected a 3TC-resistant virus after PMTCT and 0.21 (95% CI: 0.14-0.31) in women who selected a NVP-resistant virus.
For the second outcome (death or immunological failure), the probability at 36 months was 0.26 (95% CI: 0.20-0.31) in the overall population. It was 0.32 (95% CI: 0.16-0.58) in women who had NVP resistance mutations at Week 4, and 0.30 (95% CI: 0.11-0.68) in women with 3TC resistance mutation at Week 4.
In multivariate analysis (n = 247), the only factor associated with immunological failure was poor self-reported adherence (adjusted Hazard Ratio, aHR, 2.61; 95% CI 1.43-4.74, p = 0.002), controlling for resistance mutations and exposure to NVP or 3TC, CD4 count, maternal age, WHO clinical stage, and hemoglobinemia at HAART initiation. Self-reported adherence was also associated with the combined criteria (aHR 4.14; 95% CI 2.39-7.19, p < 0.001).
Discussion
During the 36 months of follow up, we did not find any differences for immunological failure related to the presence or absence of NVP- or 3TC-resistance mutations at Week 4 postpartum. Our findings are consistent with those reported by others for shorter periods of follow up [1-4,6], and support the recommendation to use NNRTI-based regimens in women previously exposed to NVP when the delay between the exposure to NVP and HAART initiation is longer than 12 months [6]. The long delay between PMTCT exposure and HAART initiation in our study likely resulted in the fading of detectable resistance mutations acquired with PMTCT ARV exposure [7,8].
We also reported that 7.7% of the women switched from NNRTI-based to PI-based HAART during follow up. Among the 19 women who switched to PI-based HAART, only four switches were related to treatment failure; the other 15 were done to manage NNRTI-related drug toxicity. While 49 (19.8%) women met immunologic criteria for failure at least once during follow up, very few were changed to second-line therapy. Similar findings have been reported in the Médecins Sans Frontières multi-country cohort, with an incidence of switch of 4.8 per 1000 person -years [9]. Reasons that patients did not switch were not recorded, but we hypothesize that this is a common practice in settings with limited availability of ARV drugs, and where physicians often choose to reinforce adherence and postpone regimen changes in clinically stable patients.
Two limitations are noted. First, there is a lack of viral load data, which is not routinely monitored and recorded in our study area. Such data is important to fully understand the dynamics and rate of treatment failure in our population. Switching treatments without using viral load data for making these decisions is indeed of utmost concern [10,11]. Second, the limited sample size implies limited statistical power to detect any immunologic difference between the groups of interest variable studied. However, our results were consistent with previous reports [3,4,6].
In addition, 23% of women were lost to follow up, dead or had stopped treatment at 36 months, and were no longer on treatment despite the establishment of a well-funded programme with excellent resources [12]. However, very few data are available on long-term follow up of ART treatment in low income-countries to make any comparison between studies. In sub-Saharan Africa, 38% of patients were lost to care and therefore no longer on treatment after two years of follow up [13].
Conclusions
In conclusion, an NNRTI-based antiretroviral regimen, which includes NVP, initiated at least one year after PMTCT exposure remains a good option for at least the first 36 months of treatment. Larger studies, preferably with virological and genotypic test data, are needed to confirm our findings and to better decide when to switch HAART regimens.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DKE, PAC and FD designed the study. PAC and CAB collected the data. DKE and PC analyzed the data. DKE and PAC interpreted the data. All authors contributed to the writing of the manuscript, and all authors approved the manuscript for publication.
Contributor Information
Didier K Ekouevi, Email: ekouevi@aviso.ci.
Patrick A Coffie, Email: ahuatchi@gmail.com.
Marie-Laure Chaix, Email: marie-laure.chaix@nck.ap-hop-paris.fr.
Besigin Tonwe-Gold, Email: btonwe@gmail.com.
Clarisse Amani-Bosse , Email: abclarisse@yahoo.fr.
Valériane Leroy, Email: valeriane.leroy@isped.u-bordeaux2.fr.
Elaine J Abrams, Email: eja1@mail.cumc.columbia.edu.
François Dabis, Email: francois.dabis@gmail.com.
Acknowledgements
This study was presented at the 15th International Conference on AIDS and STIs in Africa (ICASA), Dakar, Senegal, 3-7 December 2009 [abstract 1842].
The MTCT-Plus care and treatment programme in Abidjan is supported by the MTCT-Plus Initiative through the International Center for AIDS Care and Treatment Programs at the Columbia University Mailman School of Public Health, New York, NY, USA. The MTCT-Plus Initiative is funded by several private US foundations http://www.mtctplus.org.
The ANRS 1201/1202 Ditrame Plus trial cohort, on which the MTCT-Plus Abidjan programme was built, was funded by the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS, Paris, France), with additional support from the French Charity Sidaction (Paris, France).
Didier Ekouevi was a fellow of the French Charity Sidaction (2002-2004), and then of the European and Developing Countries Clinical Trials Partnership. Patrick Coffie is now a fellow of the French Charity Sidaction. This project received additional unrestricted financial support from GlaxoSmithKline.
References
- Chi BH, Sinkala M, Stringer EM, Cantrell RA, Mtonga V, Bulterys M, Zulu I, Kankasa C, Wilfert C, Weidle PJ, Vermund SH, Stringer JS. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;13(8):957–964. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffie PA, Ekouevi DK, Chaix ML, Tonwe-Gold B, Clarisse AB, Becquet R, Viho I, N'dri-Yoman T, Leroy V, Abrams EJ, Rouzioux C, Dabis F. Maternal 12-month response to antiretroviral therapy following prevention of mother-to-child transmission of HIV type 1, Ivory Coast, 2003-2006. Clin Infect Dis. 2008;13(4):611–621. doi: 10.1086/526780. [DOI] [PubMed] [Google Scholar]
- Jourdain G, Ngo-Giang-Huong N, Le Coeur S, Bowonwatanuwong C, Kantipong P, Leechanachai P, Ariyadej S, Leenasirimakul P, Hammer S, Lallemant M. Perinatal HIV Prevention Trial Group. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;13(3):229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, Chand F, Makhema J, Moffat C, Asmelash A, Ndase P, Arimi P, van Widenfelt E, Mazhani L, Novitsky V, Lagakos S, Essex M. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;13(2):135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants in resource-limited settings: towards universal access. Recommendations for a public health approach. 2006. http://www.who.int/hiv/pub/mtct/arv_guidelines_mtct.pdf (accessed 26 March 2010)
- Stringer JS, McConnell MS, Kiarie J, Bolu O, Anekthananon T, Jariyasethpong T, Potter D, Mutsotso W, Borkowf CB, Mbori-Ngacha D, Muiruri P, Ong'ech JO, Zulu I, Njobvu L, Jetsawang B, Pathak S, Bulterys M, Shaffer N, Weidle PJ. Effectiveness of non-nucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi-country, prospective cohort study. PLoS Med. 2010;13(2):e1000233.. doi: 10.1371/journal.pmed.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix ML, Ekouevi DK, Peytavin G, Rouet F, Tonwe-Gold B, Viho I, Bequet L, Amani-Bosse C, Menan H, Leroy V, Rouzioux C, Dabis F. Impact of nevirapine (NVP) plasma concentration on selection of resistant virus in mothers who received single-dose NVP to prevent perinatal human immunodeficiency virus type 1 transmission and persistence of resistant virus in their infected children. Antimicrob Agents Chemother. 2007;13(3):896–901. doi: 10.1128/AAC.00910-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman SH, Mracna M, Guay LA, Deseyve M, Cunningham S, Mirochnick M, Musoke P, Fleming T, Glenn Fowler M, Mofenson LM, Mmiro F, Jackson JB. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;13(15):1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- Pujades-Rodriguez M, O'Brien D, Humblet P, Calmy A. Second-line antiretroviral therapy in resource-limited settings: the experience of Medecins Sans Frontieres. AIDS. 2008;13(11):1305–1312. doi: 10.1097/QAD.0b013e3282fa75b9. [DOI] [PubMed] [Google Scholar]
- Calmy A, Ford N, Hirschel B, Reynolds SJ, Lynen L, Goemaere E, Garcia de la Vega F, Perrin L, Rodriguez W. HIV viral load monitoring in resource-limited regions: optional or necessary? Clin Infect Dis. 2007;13(1):128–134. doi: 10.1086/510073. [DOI] [PubMed] [Google Scholar]
- Reynolds SJ, Nakigozi G, Newell K, Ndyanabo A, Galiwongo R, Boaz I, Quinn TC, Gray R, Wawer M, Serwadda D. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. AIDS. 2009;13(6):697–700. doi: 10.1097/QAD.0b013e3283262a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonwe-Gold B, Ekouevi DK, Bosse CA, Toure S, Kone M, Becquet R, Leroy V, Toro P, Dabis F, El Sadr WM, Abrams EJ. Implementing family-focused HIV care and treatment: the first 2 years' experience of the mother-to-child transmission-plus program in Abidjan, Cote d'Ivoire. Trop Med Int Health. 2009;13(2):204–212. doi: 10.1111/j.1365-3156.2008.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;13(10):e298.. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]


