Abstract
Oligopeptide derivatives of metenkephalin were found to stimulate growth-hormone (GH) release directly by pituitary somatotrope cells in vitro in 1977. Members of this class of peptides and nonpeptidyl mimetics are referred to as GH secretagogues (GHSs). A specific guanosine triphosphatate-binding protein-associated heptahelical transmembrane receptor for GHS was cloned in 1996. An endogenous ligand for the GHS receptor, acylghrelin, was identified in 1999. Expression of ghrelin and homonymous receptor occurs in the brain, pituitary gland, stomach, endothelium/vascular smooth muscle, pancreas, placenta, intestine, heart, bone, and other tissues. Principal actions of this peptidergic system include stimulation of GH release via combined hypothalamopituitary mechanisms, orexigenesis (appetitive enhancement), insulinostasis (inhibition of insulin secretion), cardiovascular effects (decreased mean arterial pressure and vasodilation), stimulation of gastric motility and acid secretion, adipogenesis with repression of fat oxidation, and antiapoptosis (antagonism of endothelial, neuronal, and cardiomyocyte death). The array of known and proposed interactions of ghrelin with key metabolic signals makes ghrelin and its receptor prime targets for drug development.
1. Overview
Fundamental questions in peptide biology are the extent to which any given peptide operates in isolation versus interdependently, locally or systemically, and via a single pleiotropic or multiple distinct receptors. Identification of the ghrelin/GHS family initially disclosed GH-releasing properties [1]. Investigations subsequently unveiled multiorgan expression [2–4], multivariate actions [5], and complex modulation of and by collateral effectors [1, 4]: Table 1. The burgeoning repertoire of ghrelin actions mimics that of inhibin and activin [6, 7], which were originally isolated as regulators of follicle-stimulating hormone secretion, and thereafter recognized for hematopoietic and oncologic activity. Analogously, prominent clinical applications of ghrelin/GHS may involve not only GH-stimulating effects but also appetitive, metabolic, cardiovascular, locomotive, and gastrointestinal signaling: Figure 1. Recent development of transgenic mice expressing ghrelin-eGFP (enhanced green fluorescent protein) should permit more detailed mapping of ghrelin-expressing neurons in hypothalamic arcuate and ventromedial nuclei [8–10] and ghrelin-expressing cells in gastric oxyntic glands, pancreatic islets (epsilon cells), the anterior pituitary gland, bone marrow, and other less well-studied sites [4, 11, 12].
Table 1.
Interactions with ghrelin.
| (a) Regulation of ghrelin gene | |
|---|---|
| Stimulation | Repression |
| (1) GHRH (pituitary) | (1) Leptin (hypothalamus) |
| (2) Octanoate (stomach) | (2) Glucagon-like peptide (hypothalamus) |
| (3) Estradiol (stomach) | (3) Peptide YY (3-36) (hypothalamus) |
| (4) Glucagon (rat stomach) | (4) Insulin (stomach) |
| (5) Cholecystokinin (stomach) | (5) Somatostatin (pituitary, stomach) |
| (6) Hypoglycemia (stomach) | (6) Histamine (stomach) |
| (7) Acetylcholine (stomach) | (7) Hypoglycemia (brain) |
| (8) Leptin (stomach) | (8) Glucagon* |
|
| |
| (b) Regulation of ghrelin receptor | |
|
| |
| Activation | Suppression |
| (1) Constitutive expression | (1) Estradiol (appetitive effects) |
| (2) Acylghrelin | (2) GH (hypothalamus and pituitary) |
| (3) Estradiol (in vitro) | (3) IGF-I (pituitary) |
|
| |
| (c) Modulation of ghrelin action | |
|
| |
| Potentiation | Inhibition |
| (1) GHRH (GH release) | (1) Testosterone (dog and rat) |
| (2) Estradiol (GH release) | (2) Free Fatty acids (pituitary) |
| (3) L-arginine (GH release) | (3) Leptin (neurons) |
| (3) Desacyl-ghrelin (hunger, insulinostasis) | |
|
| |
| (d) Mediation of ghrelin actions | |
|
| |
| Appetitive | Cardiovascular |
| (1) NPY (↑) | (1) Nitric oxide (↑) |
| (2) Orexin A (↑) | (2) Extracellular-receptor activated kinases (↑) |
| (3) Leptin (↓) | (3) Unknown desacyl-ghrelin receptor |
| (4) Insulin (↓) | (4) CD36 (type B scavenger receptor) |
| Stomach | Pituitary |
| (1) GHS receptor-1a | (1) Phospholipase C |
| (2) ? CRH receptor-2 | (2) Diacylglycerol |
| (3) Protein-kinase C | |
| (4) Ca2+ signals | |
| Pancreas | |
| (1) Inward Ca2+ and outward K+ channels | |
|
| |
| (e) Regulation of ghrelin octanoyl-acyl transferase (GOAT) | |
|
| |
| Stimulation | Inhibition |
| (1) Long-term fasting (stomach) | (1) By acylghrelin (stomach) |
| (2) Acetylcholine | |
| (3) Leptin | |
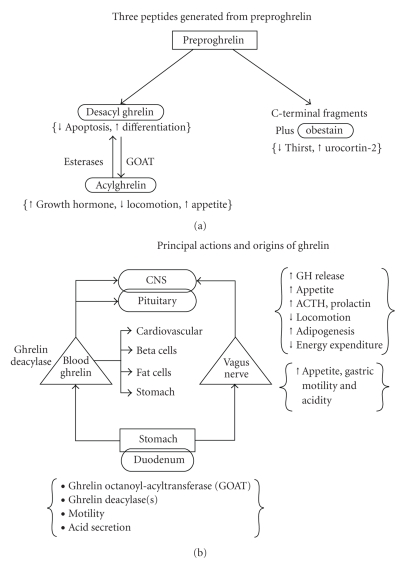
Figure 1.
Principal peptide products of preproghrelin (a) and primary actions of ghrelin recognized to date (b) (unpublished line drawing).
2. Unique Facets of Ghrelin
2.1. Multiplicity of Roles
The etymology of ghrelin is “ghre” for “growth”, consonant with the report by Bowers et al. of direct pituitary GH-releasing effects of metenkephalin-derived synthetic oligopeptides three decades ago [1]. The peptides were initially termed GH-releasing peptides (GHRPs), and more recently GH secretagogues (GHSs) to include congeneric molecules [2]. Multiple peptidyl and several nonpeptidyl analogs of ghrelin (GHS) have been developed and assessed functionally over the last three decades. The ghrelin receptor was cloned in 1996 by Howard et al. based upon phospholipase C-mediated intracellular inositol triphosphatate-dependent Ca2+ signal generation in transfected cells [5]. In certain systems, such as pituitary somatotropes, gastric oxyntic cells, appetitive neurons, pancreatic islets, and endothelial cells, additional messengers like phosphoinositidyl-3-kinase, Akt/protein kinase B, adenosine monophosphate protein kinase (AMP kinase), and nitric oxide may modulate ghrelin's actions [13–20]: Table 1. Mitogen-activated protein kinases (MAPKs) and extracellularly regulated kinases (ERKs 1/2) may mediate certain of ghrelin's proliferative and antiapoptic effects [15, 21, 22].
2.2. GH-Releasing Mechanisms
Ghrelin and cognate receptor, GHS-R1a, are distinct entirely from GH-releasing hormone, GHRH, and its receptor isolated in 1982 by the Guillemain and Vale laboratories from human pancreatic neoplasms [23, 24]. The homotypic GHRH receptor is an adenylyl cyclase-activating seven transmembrane-spanning GTP-binding protein [25]. GHRH neurons in the arcuate nucleus express GHS-R1a, but hypothalamic ghrelin neurons do not manifest GHRH receptors [4]. Both neuronal ensembles are extensive and project to the external layer of the pituitary stalk [11]. Nonetheless, to date, any direct role for hypothalamic release of ghrelin to the pituitary gland remains undocumented [26]. Ghrelin is unusual in that existence of the receptor was predicted three decades and cloned three years, before the natural ligand was identified by Kojima et al. in 1999 [3]. The ghrelin receptor exhibits high basal activity even unstimulated. Mutational disruption of constitutive GHS-R1a activity is associated with short stature in humans [27], whereas overexpression of GHS-R1a on GHRH neurons augments postweaning growth, reduces fat mass, and augments GHRH and GH gene expression in mice [28]. Thus, GHS-R1a is an amplifier of GHRH outflow as well as a direct effector of pituitary GH secretion. Exogenous GHS partially overcomes GH inhibition by glucose and infused somatostatin [29, 30]. Neurophysiological data and immediate-early gene responses have revealed two additional mechanisms of ghrelin action, namely, partial antagonism of noncompetitive hypothalamic and pituitary inhibition by somatostatin and of hypothalamic melanocortin and leptin pathways [31–40]. Figure 2 depicts a model, which incorporates several major actions of ghrelin/GHS within the GH axis [41]. This model does not include pituitary ghrelin, which is downregulated by excessive thyroxine, glucocorticoid, or brain-GH feedback and upregulated by GHRH [42]. Selective silencing of the pituitary ghrelin gene will be required to discern its physiological role.
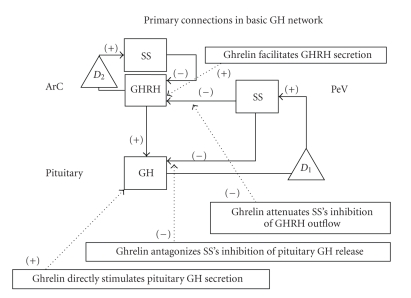
Figure 2.
Model-based functional networks subserving GH secretion, showing major effects of GH-releasing hormone (GHRH) and somatostatin (SS) as modified by GHS (ghrelin). D 1 and D 2 denote time delays. ArC and PeV define arcuate and periventricular nuclei (adapted from [41]).
2.3. Structural Features
Ghrelin is unique as the first acylated peptide recognized in mammals requiring a short-chain fatty-acid (octanoyl, decanoyl, or decenoyl) moiety linked to the third N-terminal amino acid (usually serine) for primary biological activity, namely. GHRH and GH release, locomotor suppression and appetite stimulation [26, 43–45]. Decanoyl and octanoyl moieties seem equipotent. The enzyme mediating Ser3 acylation was cloned by the Brown and Goldstein laboratory in 2008 and named ghrelin octanoyl-acyltransferase (GOAT) [46–48]. GOAT is one of 16 members of membrane-bound O-acyltransferases [49]. It is expressed in pancreas, stomach, skeletal muscle, heart, intestine, bone, and other organs and is endproduct inhibited and fatty-acyl substrate and fasting induced [50–52]. GOAT protein and transcripts exist in individual ghrelin-containing gastric mucosal oxyntic cells [53]. Regulation may exist pretranslationally also, since natural antisense ghrelin RNA's can be demonstrated by analysis of human genomic DNA [54, 55]. A second acyltransferase, termed microsomal zinc-stimulated serine (Ser2 ghrelin) octanoyltransferase, distinct from GOAT, was cloned by Ozawa et al. in 2009 [56]. This enzyme is concentrated in the endoplasmic reticulum of human erythroleukemia cells. Its in vivo role is not yet known but might include negative regulation of GOAT via the octanoylated products generated.
Unacylated ghrelin competes with acylghrelin for GHS-R1a to a negligible degree, namely, with a k d of 13 μM, which is four orders of magnitude greater than that for bioactive peptide [57]. Deacylases (esterases) of ghrelin exist in the blood and stomach, which may be nonselective [58, 59]. GOAT can utilize both ghrelin (1–4) and ghrelin (1–5) as substrates for serine acylation, consistent with their core structure [46, 60]. Des-Gln acylghrelin (1–27), an alternative transcript lacking glutamine in N-terminal position 14, is also fully active on the GHS-R1a receptor [61]. Ghrelin's amino-acid sequence is significantly conserved in fish, reptiles, amphibia, birds, and mammals [62]. Congeneric molecules include GHRP-2, GHRP-6, hexarelin, ipamorelin, ibutamorelin, and the nonpeptide MK-0677, which like ghrelin rapidly induce inositol triphosphate, diacylglycerol, and Ca2+ release via the GHS-R1a [51, 63–67].
2.4. Blood-Borne Ghrelin
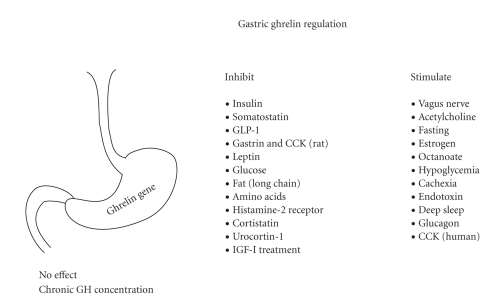
Ghrelin concentrations in blood comprise principally desacyl-ghrelin (85%–90%) and in lesser amounts acylghrelin (10%–15%) and C-terminal proghrelin peptides [68, 69]. In large cohorts, ghrelin is higher in women than men [70] and declines with age, body mass index (BMI), hypertension, and other markers of the metabolic syndrome [70]. The inverse relationships between ghrelin and both BMI and insulin concentrations appear to explain much of the age effect [71]. Appropriate sample collection and storage are necessary to limit ghrelin deacylation before assay [72, 73]. Although its exact role is not known, desacyl-ghrelin can exert a variety of agonistic and antagonistic actions [74–76], as discussed further in the relation to the ghrelin receptor. Octanoylated and total ghrelin levels in the stomach and blood rise between meals, during fasting, in cachexia, anorexia, or malnutrition, type I (insulinopenic) diabetes mellitus, after acute endotoxin exposure, overnight during initial deep sleep and in response to estradiol (E2), acute hypoglycemia, glucagon infusion, vagal stimulation, and chronic octanoate ingestion [77–89]: Figure 3. In mice, plasma bioactive decanoylated ghrelin increases and octanoylated ghrelin paradoxically decreases during fasting [90], suggesting precise posttranslational control [72]. Unlike gastric ghrelin, hypothalamic ghrelin gene transcript and peptide levels fall during short-term fasting [9].
Figure 3.
Key hormonal, gastrointestinal, nutritional, stress-related, infectious, and physiological regulators of gastric ghrelin secretion inferred in mammalian species.
In various studies, serum total ghrelin concentrations correlate positively with E2, IGFBP-1, and creatinine concentrations, and negatively with somatostatin, insulin, thyroxine, leptin, and testosterone (T) concentrations and arterial blood pressure [70, 82, 86, 91–98]. Thus, ghrelin levels rise not only in fasting individuals but also in estrogen/progestin-treated women [99–101], combined antiandrogen and progestin-treated men [82], and the estrogen-rich milieu of the late follicular phase of the menstrual cycle in one but not another study [92, 102]. Conversely, ghrelin levels fall in the high-testosterone milieu of male puberty [93]. Gastric ghrelin-secreting cells express estrogen receptor-alpha [103], thus potentially mediating some sex-steroid effects. Indeed, gonadal downregulation in girls and parenteral (but not transdermal) testosterone administration in boys diminish plasma ghrelin concentrations [104, 105]. In addition, clinical investigations indicate that E2 or T supplementation can potentiate GH secretion stimulated by a fixed submaximal dose of GHS/ghrelin [106–112]. On the other hand, low-dose transdermal E2 administration did not augment maximal GH stimulation by ghrelin in postmenopausal women [113], and the potentiating action of T administration on GHS was not evident in the dog, rat, or older men [114, 115]. Thus, more data are needed on the developmental dependence of sex-steroidal facilitation of GHS action. The differences before and after puberty do not seem to be due to feedback by differing GH levels, since acute infusion of GH or a GH-receptor antagonist does not feed back onto ghrelin secretion [116, 117]. Chronic GH excess or deficiency and exercise also do not consistently modify ghrelin production in humans or animals [118–120].
Food intake, especially amino acids, glucose but not fructose, dodecanoate, and other long-chain fatty acids, T administration, euglycemic hyperinsulinemia, impaired glucose tolerance, infusion of free fatty acids, somatostatin, cortistatin or urocortin, obesity (elevated body-mass index and increased total, subcutaneous or visceral fat), weight gain, hyperthyroidism, and leptin injection suppress ghrelin concentrations [86, 97, 116, 117, 128–144]. A possible intracellular mediator of fasting and feeding's reciprocal control of ghrelin secretion is the mammalian target of rapamycin [145], which is suppressed by fasting [145]. In general, total ghrelin concentrations nearly double before meals and fall to a nadir about one hour thereafter [146]. Protein (more than lipid) ingestion strongly suppresses plasma acylghrelin, whereas carbohydrate initially suppresses and then elevates circulating ghrelin in humans [147]. Gastrectomy reduces total ghrelin concentrations by 65%–80% (to 20%–35% of baseline) [148], thereby verifying that the stomach is the major source of this hormone.
Acylated ghrelin typically changes in parallel with total ghrelin availability and in the fed state may rise recurrently before GH pulses [149]. Dissociations between acyl and desacyl-ghrelin levels occur after fiber versus total energy intake [150], intravenous glucose infusion [142], in renal failure [86], and in the portal vis-à-vis hepatic veins because of preferential liver extraction of the acylated moiety [142]. In clinical studies, meal-induced depression of ghrelin levels may be attenuated in children compared with adults, and in women with polycystic ovarian syndrome (PCOS) compared with healthy women, but accentuated in African-American compared with Caucasian women [151–154]. The antiandrogen, flutamide, increased ghrelin levels in patients with PCOS, suggesting suppression of ghrelin production via the androgen receptor in this hyperandrogenemic syndrome [152].
2.5. Metabolism of Ghrelin
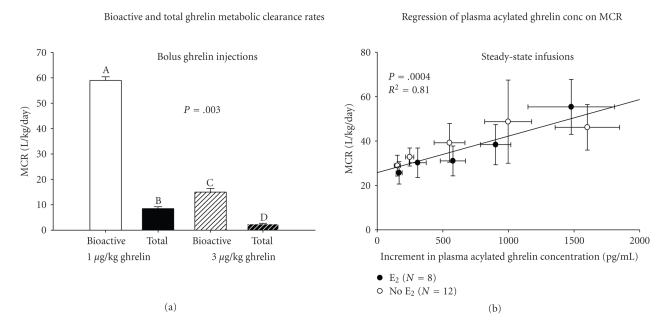
The metabolic clearance rates of acyl and desacyl-ghrelin injected by bolus in humans differ by several-fold [91]: Figure 4(a). Active ghrelin (1 μg/kg) is removed more rapidly (half-life 21 minutes) than unmodified ghrelin peptide (36 minutes) and partitions into a larger but finite distribution volume consistent with greater lipophilicity. At a higher ghrelin dose (3 μg/kg), half-lives are 47 and 64 minutes for acylated and unacylated peptide. This concentration-dependence suggests interconversion of ghrelin isoforms and/or 2-compartment kinetics of acylated peptide [155]. Figure 4(b) illustrates that steady-state bioactive-ghrelin concentrations are not saturable at metabolic clearance rates of up to 60 L/kg/day. Acylated peptide binds to plasma high-density lipoproteins containing paraoxonase (an esterase) [156], but the size and fate of this reservoir are not known. An additional gastric ghrelin deacylation enzyme has been identified, lysophospholipase I [58]. The exact degree to which this enzyme regulates ghrelin biosynthesis secretion is still unknown. Desacyl-ghrelin appears to undergo significant renal clearance [86], whereas acylghrelin is extracted substantially by the liver [142, 157].
Figure 4.
Strong impact of bolus ghrelin dose and ghrelin isotype (acylated [bioactive] or total ghrelin) on the metabolic clearance rate (MCR) of ghrelin in postmenopausal women. Means with different superscripts differ significantly by post hoc analysis after ANOVA (P = .003) (a). Linear relationship of steady-state MCR of acylated ghrelin to plasma acylghrelin concentration during constant ghrelin infusion (b). Adapted from [91] with permission.
3. Ghrelin (GHS) Receptor-1a
The ghrelin receptor exhibits high (about 50%) basal constitutive activity [158, 159] and responds to inverse agonists, partial agonists, and allosteric antagonists [160, 161]. In particular, inverse agonists repress basal receptor activity, as defined by inositol-triphosphate, Ca2+, or diacylglycerol signal generation [159, 162]. Since blood ghrelin levels rise between meals and overnight, a ghrelin-receptor inverse agonist might be used to minimize hunger at these times and overnight [163]. In two families, short stature accompanied GHS-R1a mutations that reduced constitutive GHS-R1a activity [27], thereby implying biological relevance of basal receptor activity.
Multiple experimental strategies have been employed to test the biological impact of silencing ghrelin or GHS-R1a activity: Table 2. Consistent outcomes in animal models comprise loss of appetitive, locomotor, and somatotropic regulation by exogenous ghrelin; modest reduction of body weight, IGF-I concentrations, and GH pulses in the female animal; increased fat oxidation; a rise in mean arterial blood pressure; reduced obesity and improved glucose tolerance, but with a potentially higher risk of hypoglycemia during prolonged fasting; and decreased development of fatty diet-induced diabetes mellitus [164–168, 174–185]. Double transgenic knockout of ghrelin and cognate receptor is marked by diminished adult body weight, greater energy expenditure, and higher locomotor activity [169, 186]. Thus, GHS-R1a is a physiological mediator of ghrelin's stimulation of GH secretion, repression of oxygen consumption and locomotor activity, and enhancement of appetite. GHS receptor type 1b arises from a nonspliced transcript, whose product does not bind acylghrelin or confer known bioactivity [5, 187].
Table 2.
Experimental strategies for verifying ghrelin action.
| Genetic |
| (1) transgenic silencing of ghrelin gene |
| (2) transgenic silencing of ghrelin receptor |
| (3) double knockout |
| (4) antisense transgene to neuronal ghrelin receptor |
| (5) overexpression of ghrelin |
| Immunologic |
| (1) immunoneutralization |
| (2) catalytic antibodies |
| Antagonists |
| (1) peptides |
| (2) nonpeptides |
| (3) RNA Spiegelmer |
| Infusion of ghrelin or desacyl-ghrelin |
| (1) agonism and antagonism |
| Anatomic definition of ghrelin-neural network |
| (1) transgenic green fluorescent protein-linked ghrelin |
Unlike ghrelin, synthetic GHS's acting via GHS-R1a typically do not require acylation and are not known GOAT substrates. Moreover, multiple biological effects have been reported for desacyl-ghrelin, which essentially does not bind GHS-R1a. A partial registry of effects comprises differentiation of skeletal muscle; relaxation of vascular smooth muscle; suppression of proinflammatory cytokines; inhibition of apoptosis of cardiomyocytes, pancreatic beta cells, and endothelial cells; antagonism of ghrelin's stimulation of somatic growth, hepatic gluconeogenesis, and appetite; hypotensive effects; repression of fatty-acid oxidation; and stimulation of adipogenesis [22, 75, 188–201]: Table 3. Although local acylation could explain certain actions of unacylated ghrelin, other effects that oppose or differ from those of acylated peptide raise the possibility of non-GHS-R1a mediation. Synthetic analogs of ghrelin likewise may exhibit partial agonism (e.g., stimulation of appetite but not GH release, and vice versa), antagonism, and inverse agonism despite similar GHS-R1a binding affinities [160, 202–207]. To date, no desacyl-ghrelin and no other GHS receptors have been cloned to explain such data [175].
Table 3.
Reported actions of desacyl-ghrelin.
| Effect of desacyl-ghrelin | Compared with acylghrelin | Reference |
|---|---|---|
| shared or opposite | ||
| Adipocytes | ||
| (1) ↓ fat oxidation | ||
| (2) ↑ glucose uptake | ||
| (3) ↑ differentiation | shared | [195, 199] |
| (4) ↑ hypertrophy | ||
| (5) ↓ lipolysis | ||
| anorexigenic | opposite | [190, 196] |
| Antiapoptotic | ||
| (1) islet beta cells | shared | [22, 194] |
| (2) cardiomyocytes | ||
| antiinflammatory | shared and unshared | [188, 200] |
| body weight | opposite | [75] |
| cardioprotection | shared | [194, 201] |
| gastric motility | opposite | [196, 208] |
| ↓ hepatic gluconeogenesis | opposite | [198] |
| hypotension | shared | [197] |
| locomotion | unknown | [209] |
| ↑ insulin sensitivity | opposite | [210] |
| neurogenesis | shared | [211–214] |
| skeletal-muscle differentiation | shared | [193] |
| ↓ somatic growth | opposite | [75] |
| vascular smooth-muscle relaxation | similar | [197] |
Acylghrelin, unlike the naked peptide, is a consistently effective agonist of GH secretion in multiple species from fish to mammals [34, 65, 215–221]. Nonacylated synthetic GHS can stimulate GH secretion in occasional models [222–224]. In fish, desacyl-ghrelin may function as an inhibitor of ghrelin's stimulation of appetite and locomotion [225]. In mice, transgenic overexpression of desacyl-ghrelin decreases food intake, gastric emptying, GH (female animal) and IGF-I (both sexes) concentrations, body weight and length, and GH release induced by exogenous ghrelin [75]. CNS delivery of desacyl-ghrelin in the rat likewise impedes food intake and gastric emptying [190, 196]. In humans, exogenous desacyl-ghrelin does not restrict ghrelin-induced GH secretion but disinhibits ghrelin's repression of insulin secretion [210, 221, 226]. Acylated ghrelin, unlike GHRH, does not induce GH-gene transcription. Exceptions include the pituitary glands of embryonic fish and prepubertal rats in vivo and ovine somatotropes in vitro [227–229]. Both GH and IGF-I can feed back to inhibit hypothalamopituitary stimulation by ghrelin/GHS, but feedback inhibition is less marked on GHS than GHRH stimulation [111]. Feedback appears to involve induction of periventricular hypothalamic somatostatin outflow [230], which quenches both GHRH and GH secretion [4].
4. Ghrelin and GHRH Synergy
Active ghrelin acts as an amplifier of GHRH, the primary agonist of GH secretion [4]. Human and murine hypothalami contain a wide network of ghrelin-expressing neurons, which extends across the arcuate, ventromedial, paraventricular and multiple other nuclei [8–11, 231–235]. Antisense RNA-mediated silencing of the murine GHS-1a receptor localized to GHRH neurons resulted in reduced adult weight, fat mass, pulsatile GH secretion, and IGF-I production in the adult female only [165]. Arcuate-nucleus GHRH-gene expression also declined in these animals [236], indicating that GHS-R1a not only transduces GHRH release but also maintains GHRH gene transcription [237, 238]. Consistent with this inference, GHS' stimulation of maximal (5-35-fold) GH release requires an intact hypothalamopituitary unit allowing GHRH outflow to the pituitary [239–242]. Accordingly, GHRH (−/−) mice, GHRH-receptor (−/−) mice, and rare patients with inactivating mutations of the GHRH receptor or congenital aplasia of the pituitary stalk are hyposomatotropic, and respond sparingly (<3-fold) to ghrelin/GHS [241, 243–246]. Immunoneutralization of GHRH and antagonists of GHRH also restrict GHS's effects markedly, resulting in responses of somatotrope cells to GHS similar in magnitude to those in inferred directly in vitro [1, 247].
Ghrelin or synthetic GHS achieves synergy with GHRH (supraadditive stimulation of GH secretion) when a near-threshold dose of GHS is combined with a maximally effective dose of GHRH in the human, rat, pig, cow, and dog in vivo [248–255]. Synergism is not observed after combined stimulation with either maximal GHRH and maximal GHS or submaximal GHRH and maximal GHS stimulation [65, 254]. Synergistic stimulation of GH release is absent in pituitary cells cultured in vitro and in patients with destructive lesions that separate the hypothalamus and pituitary gland [65, 216, 219, 242, 250, 256, 257], thus defining a critical role for joint hypothalamopituitary effects. Nonetheless, precise mechanisms subserving synergy are not established. Proposed mechanisms include ghrelin-mediated (a) opposition to hypothalamopituitary actions of SS and/or (b) stimulation of an unknown (“U”) synergy factor [4, 29, 65, 151, 255, 258–260]. A substance like galanin or an endogenous opiatergic peptide might represent such a factor, since both peptides release and synergize with GHRH and their neurons innervate periventricular SS neurons [261–267]. Reduced pituitary action of SS is unlikely to be the sole potentiating mechanism subserving GHS action, since GHS and GHRH synergize even after immunoneutralization of SS [268]. Although GHRH can induce the pituitary ghrelin and GHS-R1a genes [42], the physiological impact of this potentially amplifying mechanism is not known.
Since highly selective GHS-R1a antagonists are not available for clinical investigation, determining the exact extent to which endogenous ghrelin participates in amplifying GH secretion in human physiology, including in utero, in infancy, childhood, puberty, adulthood, and senescence remains difficult [4, 65, 250]. Nonetheless, mutational reduction of constitutive ghrelin-receptor activity in humans is associated with short stature [27]. Conversely, overexpression of neuronal GHS-R1a in female mice elevates GH and GHRH gene expression [28]. In addition, prolonged administration of ghrelin or synthetic GHS in humans augments GH, IGF-I and IGFBP-3 concentrations and lean-body mass, and diminishes total-body fat, while eliciting transient secretion of adenocorticotropin hormone (ACTH), cortisol, and prolactin [64, 269–273]. The last observation is significant, because higher doses of ghrelin/GHS evoke ACTH, cortisol, and prolactin secretion acutely in humans and animals, putatively by inducing hypothalamic secretion of one or both primary ACTH-releasing peptides, corticotrophin-releasing hormone (CRH), and arginine vasopressin (AVP) [272, 274–278]. How GHS induces prolactin release is not clear. Doubling or tripling plasma ghrelin concentrations evokes GH secretion in humans without measurably altering ACTH, prolactin, insulin, or free fatty-acid concentrations [279]. The basis for the dose-response difference and tachyphylaxis of the corticotropic, but not the GH-releasing, effect of GHS is not yet evident [280]. Likewise, why age and obesity impair GHS-induced secretion of GH but not of ACTH and prolactin is not known [113, 281].
Depending upon chemical structure and dose, synthetic GHS can stimulate GH secretion after intranasal, oral, s.c., or i.v. administration [4, 65, 250, 272, 282, 283]. Entry of acylghrelin (but not desacyl-ghrelin) into and exit of the same from the brain is via structurally selective saturable transport [284–286]. Since hypothalamic GHRH release is prerequisite to maximal GH stimulation, GHSs have clinical therapeutic potential in treating: (i) idiopathic short stature in patients with preserved CNS outflow of GHRH [64, 287–290]; (ii) hyposomatotropism in aging, wherein GHRH release may be diminished but is not absent [271, 291, 292]; (iii) hyposomatotropism associated with visceral adiposity, including the HIV lipodystrophy syndrome [167, 179, 190, 293–295]; and (iv) conditions of partial GH resistance accompanying anorexia, cachexia, or heightened catabolism, such as metastatic cancer, hepatic or renal failure, chronic obstructive pulmonary disease, systemic inflammation, and advanced heart failure [133, 250, 296–298]. Preclinical data in these areas and clinical data in healthy subjects suggest the merit and feasibility of more comprehensive investigations [270, 271, 299–301], since prospective, double-blind, placebo-controlled trials are lacking. Critical issues to be resolved include long-term safety, duration, efficacy, and indications for GHS administration in selected GH-deficient states.
5. Multifaceted Actions
Short-term ghrelin/GHS administration stimulates GH secretion, locomotor activity, and appetite, increases plasma free fatty acids, imposes mild peripheral (muscle) insulin resistance, suppresses insulin secretion, inhibits fat oxidation, and promotes adipocyte glucose metabolism [4, 65, 170, 209, 210, 302–307]. Moreover, GHS is both antiproliferative and proliferative depending upon cell type [308–310]. These and other multifaceted actions of ghrelin are discussed next.
5.1. Appetitive Effects of Ghrelin
Ghrelin consistently enhances appetite by 25%–30% in fasting humans and animals with the possible exception of chicken, quail, and sheep [37, 39, 295, 311–315]. Endogenous ghrelin may enhance anticipatory motor activity, before scheduled meals [316]. Active ghrelin stimulates and suppresses glucose-sensitive neurons in the lateral and ventromedial hypothalamus, respectively, stimulating hunger and inhibiting satiety [317]. The orexigenic effect arises by combined activation of neuropeptide Y/agouti-related peptide (NPY/AGRP) and orexin A neurons [314, 318–322]. Concomitantly, acylghrelin antagonizes satiety-promoting and anorexigenic signals, such as leptin [38, 323], corticotropin-releasing hormone (CRH), cocaine and amphetamine-regulated transcript (CART), proopiomelanocortin (POMC), and alpha-melanocyte-stimulating hormone (alpha-MSH) [324–326]: Figure 5. Obestatin is not pictured because of its controversial role in appetite. AGRP is an endogenous inhibitor of alpha-MSH, thereby promoting positive energy balance. Ghrelin may act in part by inducing the intracellular signal mammalian target of rapamycin in AGRP neurons, a messenger which promotes protein synthesis and limits apoptosis [327]. Both NPY and AGRP participate in the orexigenic action of ghrelin, since transgenic disruption of both mediators is needed to quench appetite [303, 321]. The potent orexigen, orexin (hypocretin), which is a key peptide in maintaining wakefulness [328], is also involved in the appetitive action of ghrelin, in that orexin-A immunoneutralization or receptor silencing attenuates ghrelin's orexigenic action [329]. A key neuronal biochemical mediator of energy-depletion mediated appetitive drive may be the low-ATP sensing protein kinase, AMP kinase, which is stimulated by ghrelin and cannabinoids [330]. Desacyl-ghrelin exerts opposite effects, namely. decreases food intake, fat mass, and gastric emptying [190, 293]. Ghrelin also suppresses pancreatic beta-cell insulin secretion, which may contribute to orexigenesis, given that insulin can function as a CNS satiety signal [331].
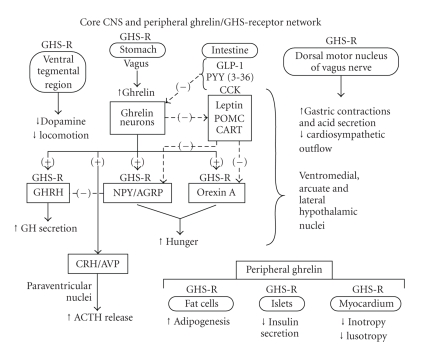
Figure 5.
Basic ghrelin network influencing locomotion (left upper quadrant), gastrointestinal signals to appetitive and anorexigenic centers (middle section), gastric motility (right upper quadrant) or peripheral target tissues (right lower segment). Unpublished schema.
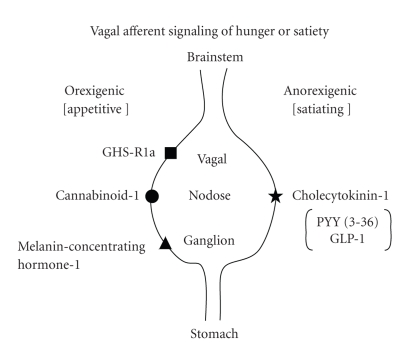
The peripheral vagus nerve and its dorsal motor nucleus in the brainstem and ventral tegmental neurons mediate some of systemic ghrelin's appetitive effects [332–336]. Hunger-suppressing vagal afferent impulses from the stomach are subdued by ghrelin and by other orexigenic hormones, acting via the GHS-R1a, cannabinoid-1 (CB-1), and melanin-concentrating hormone-1 (MCH-1) and activated by anorexigenic receptors, like cholecystokinin-A (CCK-A), peptide YY (3–36), and glucagon-like peptide-1 [334, 337]: Figure 6. In fact, a CB-1 antagonist impedes ghrelin's appetitive effect, illustrating key facilitative interactions [338]. In addition, a CCK antagonist abolishes the capability of intraduodenal fat to suppress gastric ghrelin secretion [339], consistent with gastroduodenal feedback [340]. The dorsal motor nucleus of the vagus expresses GHS-R1a, which modulates limbic-system dopamine- and brainstem gaba, glutamine, and noradrenergic transmission to hypothalamic appetitive and satiety centers [335, 341–343]. Dorsal vagal neuronal GHS-R1a levels may decline with aging in the rat [344]. Ghrelin crosses the blood-brain barrier and directly inhibits leptin neurons and stimulates NPY/AGRP and orexin neurons [333], which are located, respectively, in the arcuate nucleu and lateral hypothalamus. MCH inhibits lateral hypothalamic neurons, providing additional feedback control [345]. Inhibition of brain (rather than exclusively peripheral vagal) GHS-R1a seems to explain the anorexigenic properties of ghrelin antagonists [346, 347].
Figure 6.
Complementarity of ghrelin's vagal-nerve signaling via GHS-R1a with that of other orexigenic (left) or anorexigenic (right) peptides. PYY: polypeptide YY; GLP-1: glucagon-like peptide. Unpublished sketch.
Although genetic disruption of GHS-R1a abrogates orexigenic stimulation by ghrelin, cachexia does not develop in the transgenic animals, including those with combined ghrelin and GHS-R1a knockout, putatively reflecting signal redundancy within nutritional networks [167, 353–355]. In particular, pathways mediating orexigenesis and satiety are convergent, oppositional, adaptive, and complex [186, 295, 319, 356–358], involving hormones from endocrine glands as well as gut mucosa and vagal afferent signals [35, 38, 359–362]. For example, intestinal mucosal L-cell-derived oxyntomodulin, a satiety signal, acts via the hypothalamus to inhibit vagally driven gastric secretion of acylghrelin, thus quelling hunger [363]. Plasma leptin and ghrelin concentrations tend to vary inversely in various clinical settings [364], and CNS leptin is a strong ghrelin antagonist [35], possibly by modulating NPY-Y1 or Y5 signaling [8, 38]. GHS-R1a is subject to systemic modulation, including upregulation by thyroxine and E2 and downregulation by glucocorticoids and GH [122]. In addition, E2 inhibits the acute orexigenic effect of exogenous ghrelin in the male and female rat [123]. Accordingly, the ghrelin system is a pivotal but nonexclusive member of a robust nutritional network in mammals [10, 294, 314, 319, 365–368]. However, ghrelin reduces food intake in neonatal chickens, possibly by elevating brain fatty-acid synthesis [369].
GHS receptors are located in the stomach, gastrointestinal myenteric plexus, vagal nodose ganglion, dorsal motor nucleus of the vagus, arcuate-nucleus orexigenic neurons, such as NPY/AGRP and orexin A-expressing cells, anorexigenic neurons that are either leptin-sensing or leptin-producing, and multiple other unidentified neurons [314, 370, 371]. Silencing any one of NPY, AGRP or orexin-A-receptor genes limits but does not abolish ghrelin's orexigenic effect [329, 372–374]. Triple negation of NPY, AGRP, and orexin is necessary to eliminate ghrelin's appetitive effect [375]. Small interfering DNA-mediated neutralization of paraventricular neuronal GHS-R1a in rats imposes weight loss and reduces serum ghrelin without altering food intake, suggesting effects on energy expenditure as well [376]. Network robustness is conferred by the facts that ghrelin activates whereas leptin represses both NPY/AGRP, and orexin neurons; orexin A potentiates (feeds forward onto) NPY release via the orexin-1 receptor; NPY represses orexin release via NPY-Y1 receptors; neuropeptide W and galanin-like peptide further modulate orexin neurons [377, 378] NPY and orexin, respectively, suppress (feed back) and induce (feed forward onto) the potent anorexigens, leptin, and proopiomelanocortin, which in turn feed back on ghrelinergic cells [186, 235, 379–387]. Moreover, NPY and ghrelin neurons synapse on themselves, suggesting autofeedback-dependent regulation [235]. Collective feedforward/feedback circuits presumably subserve enhancement and suppression of food intake in a manner defined by sex, species, age, physical activity, ultradian rhythmicity, and sleep-wake cycles [35, 231, 295, 328, 388]. For example, sleep curtailment may augment appetite by simultaneously lowering circulating leptin and elevating ghrelin levels. The latter correlates with meal initiation [389]. These and other experiments illustrate the complex adaptability of nutritionally regulated neural networks [234, 278, 353, 362, 390].
A 23-amino-acid C-peptidyl fragment of preproghrelin, named obestatin, has not fulfilled its original nomenclature as an antagonist of ghrelin's orexigenic effects [394–399]: Figure 7. In addition, transgenic knockout of the proposed obestatin receptor, GPR39, does not affect food intake [394, 396, 400, 401]. Whereas obestatin (probably better termed, ghrelin-associated peptide) does not alter pituitary hormone secretion [402], this peptide may suppress thirst, promote beta-cell survival and regeneration, activate urocortin-2 pathways and thereby inhibit gastroduodenal peristalsis, and induce early-response genes in gastric mucosa and preadipocytes [403–410]. A physiological distinction pertinent to CNS actions of ghrelin, but not obestatin, is that only ghrelin exhibits specific saturable binding to and endocytosis by cerebral microvessel endothelial cells, thereby allowing measurable permeation of the blood-brain barrier [284, 286]. However, obestatin attenuates the hypothermic effect of preproghrelin gene deletion [411].
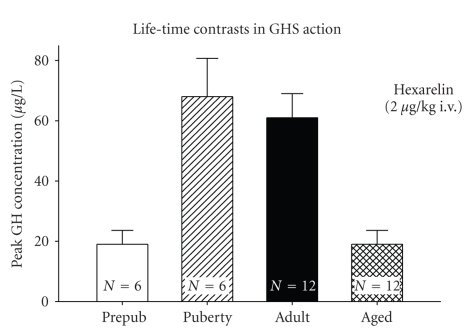
Figure 7.
Lifetime variations in GHS (hexarelin) action to induce GH secretion in prepubertal, pubertal, adult, and aged humans (redrawn with permission from [488]).
Transgenic overexpression of CNS and gastric ghrelin causes hyperphagia, increases energy expenditure, and decreases sensitivity to insulin and leptin in mice [170]. The orexigenic action of ghrelin does not seem to wane in this model, since adult transgenic animals continue to eat excessively after fat depots reach a maximum [412]. In a second model, 8 weeks of GHS treatment maintained orexigenesis in the rat [413]. In a third model, overexpression of GHS-R1a in GHRH neurons increased organ and muscle weight, while decreasing body fat in female animals [28]. Conversely, in a fourth model, transgenic reduction of GHS-R1a expression on GHRH neurons suppressed both GHRH and NPY gene expression, thereby diminishing GH and fat mass in the female [236]. In patients with renal or cardiac failure or chronic lung disease and cachexia, short-term (up to 3 weeks) administration of ghrelin once or twice daily may stimulate appetite and weight gain as assessed in uncontrolled studies [298–300, 414, 415]. However, in the perioperative orthopedic setting, ghrelin infusions for 3 weeks did not improve overall functional physical performance [416]. Nonetheless, the precise extent to which GHS agonists and antagonists will alter hunger or satiety over the longterm remains difficult to forecast [74, 232]. This issue is important since studies with leptin, an anorexigenic adipokine that antagonizes ghrelin, revealed tachyphylaxis to satiety-promoting effects [186, 417].
Ghrelin itself undergoes tissue-specific regulation by short-term fasting, hypoglycemia, and nutrient deprivation. These factors depress brain ghrelin expression, while promoting gastric ghrelin secretion and amplifying CNS responses to ghrelin [9, 418, 419]. In species in which desacyl-ghrelin can antagonize the orexigenic effects of acylghrelin [293], regulation of GOAT activity in both the stomach and brain may be important to appetitive homeostasis [191, 196]. GHS analogs that do not require acylation have the merit of bypassing GOAT [420].
Gastric-bypass procedures, especially when combined with truncal vagotomy, often reduce serum total ghrelin concentrations initially [426, 427]. In principle, reduced ghrelin availability would further attenuate adipogenesis [191, 195, 199]. However, in some studies prolonged weight loss after gastric bypass had no effect on fasting ghrelin levels [250, 428, 429] or reduced only acylated ghrelin [430]. In rodents, vagotomy impedes both appetitive and GH-stimulating effects of ghrelin [334, 336, 431, 432]. In humans, pharmacologic or surgical interruption of vagal cholinergic traffic lowers ghrelin concentrations and blunts appetitive stimulation but does not block GHS-induced GH release [336, 431, 433].
5.2. Insulinostasis
Ghrelin inhibits insulin (beta cells) and somatostatin (delta cells) but stimulates glucagon (alpha cells), secretion in vivo and in isolated pancreatic islets in vitro [304, 434–439]. Some early studies reported that GHS induces insulin secretion [440]. Inconsistencies may reflect the capabilities of desacyl-ghrelin to suppress hepatic glucose production and/or block acylghrelin's inhibition of insulin secretion [198, 221, 434, 441]. Other studies did not control for the fact that acutely elevated GH and glucose concentrations (induced by GHS) stimulate insulin secretion acutely. GHS receptors are expressed by both beta and alpha cells [442]. Ghrelin's inhibition requires Gi, which activates outward K+ currents and disables inward Ca2+ flux [443, 444]. These effects particularly impede rapid first-phase insulin release [444]. Moreover, acylghrelin antagonizes peripheral insulin action (muscle > liver), which effect is reversed by desacyl-ghrelin in GH-deficient adults [210]. However, desacyl-ghrelin has little if any effect on glucose, insulin, free fatty acid, or GH releases in (obese) humans [445].
From a reciprocal perspective, insulin and somatostatin repress, whereas glucagon induces the gastric ghrelin gene in the rat [88, 124, 130]. In humans, glucagon injection inhibits ghrelin secretion [121], but via uncertain pathways. In rodents, insulin represses not only gastric but also CNS preproghrelin expression [446]. In accordance with insulin's negative effects, hyperinsulinemia associated with hepatic or skeletal-muscle insulin resistance and/or obesity correlates inversely with fasting ghrelin concentrations [71, 447, 448]. An exception is the genetic Prader-Willi syndrome marked by obesity, mild insulin resistance, and unexplained hyperghrelinemia with increased numbers of gastric ghrelin-expressing cells [449–451].
Adult pancreatic islets express relatively little ghrelin protein and few GHS receptors, but fetal and neonatal islets exhibit abundant ghrelin gene transcripts and peptide in epsilon cells [452–454]. Although both acyl and desacyl-ghrelin promote beta-cell regeneration [408, 455], endogenous pancreatic ghrelin inhibits beta-cell function, since perfusion of the pancreas with ghrelin antiserum augments insulin secretion [408, 438, 444, 456]. In addition, genetic knockout of ghrelin and administration of GHS-receptor antagonists diminish fasting hyperinsulinemia and enhance glucose tolerance in rodent models [205, 439, 443, 444]. Thus, in knockout ghrelin mice, increased constitutive ghrelin-receptor activity may result in increased insulin secretion, which would be susceptible to inhibition by a GHS receptor antagonist with inverse agonist activity. Further investigations are required to appraise the influence of diet, age, gender, species, and obesity on pancreatic actions of ghrelin, given the high importance of drug development in this area. A desirable ghrelin antagonist would limit appetite, disinhibit insulin secretion, and minimize adiposity (by favoring fat oxidation) in patients at risk for the metabolic syndrome or type II diabetes mellitus [84, 205, 223, 457, 458]. Table 4 highlights some of these issues and identifies potential adverse consequences of prolonged GHS-receptor blockade.
Table 4.
Cardiovascular actions of GHS.
| ↓ mean arterial pressure |
| ↑ inotropy (myocardial tension generation) |
| ↓ lusitrophy (tension relaxation) |
| ↓ ventricular end-systolic pressure |
| ↓ cardiomyocyte apoptosis |
| ↓ endothelial apoptosis |
| ↓ pulmonary hypertension |
| ↑ renal perfusion |
| ↑ coronary perfusion pressure |
| ↑ left-ventricular ejection fraction |
| ↓ oxygen consumption |
| ↓ cardiac sympathetic drive |
5.3. Cardiovascular Effects
At doses that induce maximal GH secretion, ghrelin and GHS can exert direct vasodilatory, cardiotropic, and brainstem-mediated hypotensive effects, typified by a 10%–20% decrease in mean arterial blood pressure and an increase in left-ventricular ejection fraction [14, 183, 201, 459–464]. Desacyl-ghrelin also causes vasodilation and opposes cardiomyocyte and endothelial apoptosis [194, 465]. Conversely, a ghrelin antagonist elevates heart rate and arterial pressure in the conscious rat [183]. The mechanisms of ghrelin's effects include central suppression of sympathetic cardiac drive, systemic vasodilation, antagonism of angiotensin II and endothelin-induced vasoconstriction including of the pulmonary arteries, and potentiation of nitric oxide-mediated relaxation of vascular smooth muscle [14, 20, 197, 465–471]: Table 4. GHS receptors exist on endothelium, vascular smooth-muscle cells, and cardiomyocytes [13, 201, 472, 473]. Furthermore, ghrelin transcripts and protein are expressed in endothelial cells and cardiac muscle [466, 472]. Both ghrelin and desacyl-ghrelin reduce the rate of myocardial tension generation (negative inotropic effect) and the rate of tension relaxation (negative lusitropic effect), possibly in part independently of the GHS-1a receptor [13, 201, 330, 474]. Obestatin does not initiate these responses [475]. The membrane glycoprotein, CD36 initially recognized as a macrophage type B LDL-scavenger receptor and recently as a fatty-acid binding protein, may transduce or modulate certain cardiovascular and anti-inflammatory effects of ghrelin [188, 207, 474].
The capability of ghrelin to limit apoptosis of endothelial cells and cardiomyocytes in vitro introduces the possibility of reducing endothelial dysfunction associated with atherosclerotic risk [476–478] and salvaging myocardium in zones of marginal chemotoxicity [479] or ischemia [22, 194, 461, 480]. Desacyl-ghrelin shares cardioprotective effects, but the mechanism is not known [194]. Increased coronary-artery perfusion pressure, reduced cardiac sympathetic activity, antagonism of L-type Ca2+ channel-mediated contractility, and activation of low-energy AMP-kinase may contribute to cardiotropism [461, 481, 482]. Resultant effects are reduced left-ventricular endsystolic volume and increased cardiac output [414, 460]: Table 5. How much of this benefit is due to stimulated secretion of GH, which exerts cardiotropic effects, is not known [483].
Table 5.
Reported modulatory messengers of ghrelin.
| Messenger/mediator | Site/mechanism |
|---|---|
| (1) phospholipase C | GHS receptor-transfected cells |
| (2) protein kinase C | neurons |
| (3) cAMP potentiation | pituitary |
| (4) nitric oxide | vasculature |
| (5) urocortin-2 receptor | pancreas, stomach |
| (6) mitogen-activated protein kinase | cardiomyocytes |
| (7) extracellular-regulated kinase | cardiomyocytes |
| (8) potassium and calcium channels | islets, somatotropes |
| (9) AMP kinase | neurons, gastric mucosa |
Elevated ghrelin concentrations in patients with chronic heart failure may indicate a compensatory mechanism in particular [484] or reflect partial tissue resistance to ghrelin in cachetic states more generally [83, 485]. Three- and four-week pilot studies of exogenous ghrelin's anabolic and anticatabolic effects in chronic cardiac cachexia in the rat and human seem favorable [194, 301, 461, 486], although parallel-cohort prospectively randomized double-blind controlled clinical studies are not available [414]. Collective data invite more rigorous interventional studies in experimental models of both early and advanced myocardial injury and failure. In addition, further investigations are necessary to assess how GHS affects perfusion of the brain, skeletal muscle, kidney, liver, and intestine [296, 487].
5.4. Gastric Motility
Ghrelin is expressed in granules in gastric X/A-like oxyntic cells [489, 490]. The same granules contain immunoreactive motilin, a comparably strong but distinct prokinetic peptide [491, 492]. Obestatin is additionally present in many of the same cells, but its gastrointestinal function is not known [189, 404]. In one report, obestatin was able to elicit pancreatic zymogen secretion [493]. In other contexts, desacyl-ghrelin and obestatin, unlike ghrelin, inhibit gastric antral contractions [189]. Ingestion of medium-chain triglycerides in the infant or adult enhances gastric acylghrelin concentrations [494, 495]. Inactivation of prohormone convertase 1/3 (a preproghrelin endopeptidase) also increases ghrelin gene transcripts, suggesting negative feedback by ghrelin on its own transcription [496]. Fasting induces the preproghrelin gene in fish and mammals via efferent vagal cholinergic signals [497–499]. The cephalic (meal-visualization) phase of digestion in humans clearly depends on vagal efferents, which transduce prandial inhibition of ghrelin secretion [500]. In sheep, cholinergic blockade stimulates stomach ghrelin output [311], suggesting baseline cephalic phase-like inhibition in this species.
Motilin and ghrelin receptors are 36% homologous in peptide sequences; yet each is activated principally by the homologous agonist at physiological concentrations [492, 501, 502]. Further unlike GHS-R1a, the motilin receptor exhibits little constitutive activity. The GHS-R1a gene is conserved with 58% nucleotide homology in puffer fish and humans [63]. The receptor is present in the myenteric (neuronal) plexus, gastroduodenal mucosa, smooth muscle, and vagal nodose ganglia [503, 504]. Ghrelin stimulates smooth-muscle contractions and type III primary migrating-motor complexes in the stomach and proximal duodenum of the eel, rat, rabbit, mouse, guinea pig, and human; inhibits postprandial contractions (dog) and gastric accommodation (decreases residual stomach volume); and promotes gastric-acid secretion, which facilitates protein hydrolysis and Ca2+ absorption [505–511]. Current promotility drugs for the treatment of idiopathic, postvagotomy and diabetic gastric atony, and morphine-induced postoperative ileus include serotoninergic-3 agonists, dopamine antagonists, and motilin-receptor agonists, like erythromycin [492, 512–514]. Whereas desacyl-ghrelin may block certain promotility effects of ghrelin [208], ghrelin is unique in acting on all four of gastroduodenal myenteric neurons, smooth-muscle cells, the vagus nerve, and brain GHS-1a receptors to augment gastric emptying and lower pH [333, 512]. An additional effect is gastroprotection against alcohol and ischemic mucosal stress [515]. Histamine-2 agonists and gastrin synergize in promoting preprandial acid secretion [516, 517], thus illustrating intragastric interactions with ghrelin.
Preclinical and clinical data indicate that GHS-receptor agonists can accelerate gastric emptying even in the presence of autonomic denervation, and reduce postoperative gastrointestinal ileus [492, 506, 509, 511, 512, 518–522]. Patients with diabetic gastroparesis may have low plasma total ghrelin levels, possibly due to vagal denervation and/or chronic glucagon excess [121, 523, 524]. What is unclear is the risk/benefit ratio of systemic GHS treatment in patients with GI bleeding, autonomic neuropathy, and/or concurrent postoperative needs for narcotics, anticholinergics, and other drugs. Concerns would include possible hypotension due to GHS-mediated relaxation of vascular smooth muscle and negative cardiac inotropy and possible deterioration of glucose tolerance due to suppression of insulin secretion and augmentation of hepatic glucose output [198]. Thus, high agonist selectivity will be necessary to ensure clinical safety.
5.5. Sleep-Wake Regulation
Ghrelin concentrations rise during the first four hours of normal sleep in humans [525]. The mechanism is not known. Viewed conversely, ghrelin administration enhances slow-wave (non-REM) sleep including in young and older men but not women [526–530]. In one clinical study, the GHS peptide, hexarelin, reduced non-REM sleep whereas GHRP-6 increased the same, suggesting GHS-receptor pleiotropy or multiplicity [531]. How ghrelin can enhance deep sleep and yet activate orexin-A neurons [328], which are coupled to arousal-wakefulness and appetite, is not yet clear [328, 532]. In addition, why patients with obstructive sleep apnea, albeit obese, have elevated acylghrelin concentrations has not been elucidated [533]. However, reduction of hypoxic episodes decreased ghrelin, suggesting that ghrelin is a stress-responsive hormone.
5.6. Cell Survival and Cachexia
Ghrelin and GHS analogs exert differentiative as well as proliferative and antiproliferative effects in vitro and in vivo [193, 211, 455, 534]. Examples include mitogenic and antiapoptic actions on pancreatic islets, spinal-cord, cortical and brainstem neurons, osteoblasts, fetal lung branches, endothelium, cardiomyocytes, and adipocytes on the one hand, and apoptotic effects in certain adrenal, lung, and prostate cancer cell lines on the other hand [15, 21, 22, 192, 211–213, 309, 391, 480, 535–541]. Several cell-cycle effects can be induced by incubation with either acyl- or desacyl-ghrelin, raising the possibility of involvement of both GHS-R1a and non-GHS-R1a receptor pathways [58, 537]. This reflects the fact that GHS-1a is essentially unresponsive to desacyl-ghrelin [22, 177]. Short-term ghrelin/GHS administration to enhance neuronal regeneration after ischemic or toxic insults thus represents another major point of potential therapeutic focus [214, 542, 543]. Because ghrelin can also stimulate proliferation of certain carcinoma cell lines in vitro [308, 309, 544], studies to exclude longterm oncogenic effects are needed.
Inasmuch as appetite wanes in the cachectic stage of carcinomatosis, ghrelin is being evaluated as an anticachectic agent. Studies in the rat, mouse, and human indicate that ghrelin/GHS administration can dose-dependently enhance appetite, body weight, and fat mass in several short-term models of disseminated neoplasia [545–549]. Caveats are that rigorous controls and blinding are often lacking, and evaluations are necessarily of short duration initially. In addition, some studies do not show orexigenic benefits [550, 551]. A preliminary study in 12 cachectic patients with dialysis-dependent kidney failure showed appetitive enhancement by ghrelin compared with randomized double-blind placebo injection over a 1-week interval [415].
5.7. Adipogenesis
Prominent effects of ghrelin/GHS include direct promotion of preadipocyte proliferation and adipocytic differentiation and hypertrophy [76, 552]: Table 6. GH-independent CNS actions may participate in lipogenesis [553]. Desacyl-ghrelin shares several of these actions [191, 199]. Relevant mechanisms embrace repression of insulin-sensitizing genes, such as adiponectin, and induction of adipocyte leptin and peroxisome-proliferating activator receptor- (PPAR-) gamma genes; stimulation of endothelial lipoprotein lipase, adipocyte fatty acid synthase, acetyl CoA carboxylase, and fat-cell glucose uptake; and inhibition of fat oxidation via rate-limiting carnitine-palmitoyl transferase [167, 171, 172, 191, 554, 555]. As a more general metabolic mechanism, ghrelin mediates inhibition of sympathetic outflow to thermogenic fat depots, reduction of uncoupling protein-1 expression, and thereby decreased resting energy expenditure [556, 557]. Species appears to influence some mechanisms. For example, in sheep, ghrelin potentiates glucose-induced insulin secretion, which is antilipoytic [558]. In rodents, unacylated ghrelin can elevate insulin concentrations, which is adipogenic [441]. Neither is the case in humans [210].
Table 6.
Adipogenic effects of ghrelin.
| (1) decrease fat-cell lipid export |
| (2) enhance lipoprotein lipase |
| (3) reduce insulin sensitivity |
| (4) stimulate preadipocyte proliferation |
| (5) promote adipocyte differentiation |
| (6) inhibit 5′-adenosine monophosphate protein kinase (AMP-kinase) |
| (7) augment hepatic glucose output and triacylglyceride content |
| (8) activate acetyl CoA carboxylase |
| (9) inhibit fatty acid oxidation |
| (10) induce leptin, sterol-response element binding protein-1c and |
| (11) PPAR-gamma |
| (12) suppress adiponectin |
| (13) increase appetite |
CNS pathways may participate in stimulating adipocyte hypertrophy [171] and increasing the respiratory coefficient (increased ratio of glucose/fatty-acid oxidation) [234, 392, 555]. In particular, ghrelin acts in part by repressing leptin, CRH, and histamine (anorexigenic) and activating orexin and NPY (appetitive) signaling [184, 559–561]. Orexin A in turn inhibits GHRH gene expression, thus limiting GH-dependent lipolysis [562]. Thus, orexin contributes to fat accumulation by augmenting appetite, attenuating satiation, and restricting GH secretion [38, 171, 443].
Despite ghrelin's strong adipogenic effects, subcutaneous, visceral, and total adiposity correlate negatively with blood ghrelin concentrations [116, 563]. This may be because a significant longterm effect of ghrelin is to drive pulsatile GH secretion, which is strongly lipolytic [4]. Animal models indicate that ghrelin antagonists are able to reduce hyperglycemia and adipogenesis, enhance energy expenditure and fat oxidation, stimulate insulin secretion and action, and heighten resistance to diet-induced obesity [167, 168, 179, 182, 184, 205, 564]. A potential risk of prolonged antagonist administration in the fasting state could be endogenous insulin-induced hypoglycemia [166], although this adverse event has not been observed in humans.
5.8. Bone Formation
GHS-R1a and ghrelin peptide are synthesized in bone cells, such as chondrocytes and osteoblasts [12, 565]. Albeit moderately well understood in other tissues, mechanisms mediating regulation of GHS-R1a in bone remain unclear: Table 7. Acylated and unacylated ghrelin stimulate osteoblast proliferation and differentiation, increase osteoblastic markers like osteocalcin and bone alkaline phosphatase, and repress osteoblast apoptosis putatively via phosphatidylinositol 3-kinase and mitogen-activated protein kinase signaling [566]. Moreover, in GH-deficient rodents, ghrelin administration augments Ca2+ retention in the skeleton, thereby elevating bone-mineral density [565]. The clinical impact of these effects is not yet established.
Table 7.
Key regulators of GHS-1a receptor.
| Downregulation | Stimulation |
|---|---|
| glucocorticoids (rodent) | estrogen (VMN, stomach) |
| ghrelin (fish) | thyroxine (rat) |
| certain promoter hyplotypes | lactation (rat hypothalamus/pituitary) |
| age (human brain) | puberty (rat, pituitary) |
| GH (arcuate nucleus) | GHRH (pituitary) |
| atherosclerosis (human) | |
| ghrelin-responsive corticotropinoma (human) |
5.9. Stress Adaptations
Recent studies point to a role for ghrelin in modifying pathophysiological adaptations to stress [275, 567, 568]. For example, ghrelin concentrations rise acutely after major surgery along with inflammatory cytokines like tumor-necrosis factor-alpha and interleukin (IL)-6 [569]. In mice, transgenic knockdown of the ghrelin receptor accentuates adverse effects of chronic social-defeat stress [570]. Analogously, ghrelin may permit adaptation to pain, since it blocks spinal-cord nociceptive signals [571]. Lipopolysaccharide endotoxin stress lowers blood ghrelin levels, causing an associated reduction in gastric emptying [572]. The latter is substantially relieved by ghrelin infusion. Under some conditions, desacyl-ghrelin inhibits gastric contractions by activating stress-adaptive CRH receptor-2, for which urocortin is the natural ligand [208, 348]. Ghrelin also induces the anorexigenic hypothalamic CRH gene [567], which normally stimulates ACTH release. Whether CNS-mediated inhibition of food intake by desacyl-ghrelin proceeds by activating the CRH pathway is not known [196].
Acute vascular-endothelial biochemical stress responses are modified by ghrelin. This peptide induces nitric oxide synthase (NOS) and represses the generation of reactive oxygen species, thereby attenuating endothelial injury [20, 573]. The protective effects require GHS-R1a, phosphotidylinositol-3 kinase, and Akt/protein kinase B [20]. A long-term action of ghrelin may be to retard endothelial apoptosis [574]. Ghrelin can also directly stimulate human vascular endothelial-cell migration, but the impact of this effect is not so clear [575]. Substantial additional work is needed to extend understanding of these aspects of ghrelin pathophysiology.
5.10. Immune Modulation
Ghrelin, GHS receptors, GH, and GH receptors are expressed in human monocytes and B and T lymphocytes [576]. In general, ghrelin's effects are antiinflammatory and immune-enhancing, for example, diminution of monocytic, bacterial and endothelial inflammatory factors driven by endotoxin exposure, sepsis, arthritis, interleukin 1 and 6, tumor necrosis factor-alpha, and nuclear-factor kappa B [200, 577–579], and stimulation of proliferation of thymic epithelial cells and T lymphocytes [580, 581]. Ghrelin also suppresses neutrophil and macrophage migration, caspase activation, reactive oxygen-species generation, and endoplasmic-reticular stress activation [582–584]. The combined impact may be to restrict lethal hepatic and pulmonary microvascular injury in sepsis [515, 585–587]. Ghrelin concurrently induces antiinflammatory cytokines, like interleukin-10 [578], and augments organ perfusion pressure in sepsis [515, 588]. Sepsis-associated gastric mucosal injury, an additional cause of mortality due to hemorrhage, is prevented in some models [589]. Certain of these effects may require the vagus nerve [590]. In acute renal failure, ghrelin treatment can repress inflammatory cytokines in the blood and brain, limit protein catabolism, enhance food intake, and attenuate renal injury due to endotoxemia and ischemia [415, 591–594]. Total but not acylghrelin concentrations are elevated in endstage renal failure [595] and normalize following kidney transplantation.
Long-term surveillance of ghrelin and GHS receptor-deficient and ghrelin-overexpressing transgenic animals will be needed to assess whether prolonged changes in ghrelin availability influence immune function or inflammatory disease.
5.11. GHS Administration in Protracted Critical Illness
Although acute stress elevates GH secretion [596], extended critical illness suppresses all three of GH, IGF-I, and IGFBP-3 concentrations and inhibits tissue actions of GH. In unfed patients with multiorgan failure, total ghrelin levels rise [86, 131]. Infusions of GHS alone or combined with GHRH substantially reverse biochemical markers of hyposomatotropism in this setting [597, 598]. However, recovery from multiorgan failure and inflammation is necessary to alleviate tissue resistance to GH [4]. In animal models, ghrelin administration reduces sympathetic outflow, acute renal failure, inflammation in the lung, stomach and liver, and lethality of sepsis [200, 344, 515, 586–590, 599–601]. Contrastingly, glucocorticoid deficiency, and glucocorticoid excess alter the GHS axis by, respectively, diminishing blood ghrelin and brain GHS-R1a levels [421, 602, 603]. Further investigations are needed in this meritorious area.
5.12. Ghrelin in Pregnancy and Lactation
Ghrelin is expressed abundantly by the placenta [604], and ghrelin can stimulate GH release by human fetal pituitary cells in vitro [605]. Immunoneutralization of maternal ghrelin in the rat diminishes fetal body weight, raising the possibility of maternal-fetal exchange of ghrelin [606]. Maternal ghrelin and placentally derived variant-GH concentrations in the human peak at about 18 and 34 weeks gestation, respectively, fall thereafter, and reach a nadir approximately 3 days postpartum [607–609]. The decline in ghrelin in the third trimester of pregnancy is inversely related to blood pressure, resistin, and TNF-alpha levels [95, 610]. Although human umbilical-cord acylghrelin levels exceed those in the mother, the role of placental ghrelin is not well understood [611]. Placental insufficiency, low birth weight, and maternal fasting elevate fetal ghrelin levels [611, 612]. Postpartum acylghrelin concentrations increase by several-fold over midpregnancy values [613], but GH responses to GHS are reduced in breastfeeding women, especially in the hyperprolactinemic setting [614]. The basis may involve pregnancy-associated suppression of hypothalamic GHRH and GHS-R1a with reciprocal induction of the SS gene due to feedback by high placental somatomammotropin (GH isotype V) and maternal IGF-I concentrations [422].
In the rat, lactation induces both hypothalamic and pituitary GHS receptors [615]. Pituitary GHS-R1a is maximal in the newborn pup and pubertal animal [423]: Table 7. Estradiol, T4, and GHRH may contribute to these maxima, since the GHS-R1a promoter is responsive to estrogen and cyclic AMP [122, 187]. Ghrelin is secreted into colostrum and milk, but its effect on the suckling infant is not well delineated [616]. In one preclinical study, treating rat pups with ghrelin reduced pancreatic exocrine development before weaning and exerted the opposite effect after weaning [617].
5.13. Antireproductive Effects of GHS
Exogenous ghrelin reduces LH pulse frequency in the adult male rat, cyclic or gonadectomized female rat, and ovariectomized monkey [618–622]. Ghrelin may also inhibit embryo development, decrease litter size, and delay pubertal onset in the male rat [623, 624]. The mechanism may involve neuropeptide Y (Y1 or Y5) or CRH-dependent inhibition of kisspeptin, which together supervise gonadotropin-releasing hormone secretion, and thereby pulsatile LH secretion [622, 624–627]. Other sites of inhibition may include uterine epithelium (by inducing IGFBP-1 and lowering free IGF-I), Leydig cells in the testis, and granulosa-luteal cells in the ovary (by blocking steroidogenesis) [624, 628–633]. Although physiological effects are not clear, ghrelin is expressed in sheep oocytes [634], and GHS-R1a in Sertoli (nurse) cells in spermatogenic tubules [623]. Further study is required to verify these inferences and evaluate clinical relevance [623]. For example, ghrelin infusion had no demonstrable effects on LH secretion in the early follicular phase of the menstrual cycle [635].
5.14. Neuroendocrine Tumors
Both ghrelin and obestatin are detected in various neoplasms, such as enterochromaffin tumors (e.g., carcinoids), pituitary adenomas, and carcinoma of the pancreas, lung, and breast [406, 636, 637]. The amount of peptide secreted is usually insufficient to serve as a tumor marker or to elicit clinical symptoms or signs.
5.15. Body-Composition Effects
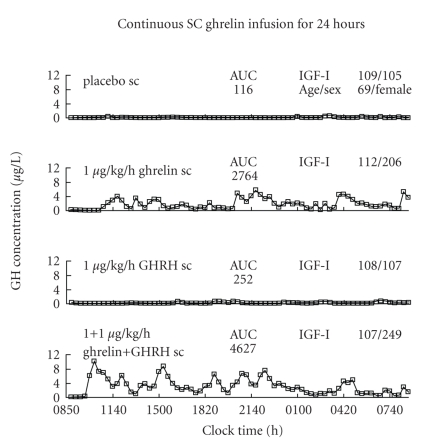
Clinical studies in older individuals indicate that prolonged (up to 1 year) administration of GHS orally can increase lean-body mass, decrease abdominal visceral fat, and possibly improve certain performance measures, such as stair climbing and timed walking [548, 638]. In analyses comprising 1-to-30 days of parenteral GHS delivery, IGF-I concentrations also rise, but cortisol and prolactin do not [270–272]: Figure 8. Adverse events included insomnia, fatigue, small increases in fasting glucose or glycated hemoglobin, and mild insulin resistance [638]. An important consideration is that acute diabetogenic effects of GHS may be attenuated by its longterm antidiabetic effects: Table 8. Insomnia could reflect GHS's stimulation of orexin pathways, whereas glucose intolerance could reflect insulinostasis and insulin resistance due to acute free fatty-acid release in humans [306]. Short-term use of ghrelin in severe cachexia enhanced appetite and/or weight gain in some studies [298, 300]. Whereas both glucocorticoid deficiency and excess in chronic diseases can reduce GH response to GHS [4, 421], combined GHRH and ghrelin infusion is more effective at driving GH secretion than ghrelin alone [270]: Figure 8. Viral vector delivery of ghrelin augmented weight in the rat [639], introducing an additional potential avenue of treatment beyond subcutaneous, intravenous, oral, or intranasal administration [282].
Figure 8.
Continuous subcutaneous (SC) infusion of saline, GHRH or ghrelin, or both (1 μg/kg/hour) for 24 hours in a normal 69-year-old woman. Data are 20-minutes GH concentrations (y-axis) plotted against time (x-axis). AUC: area under the GH versus time curve. IGF-I concentrations at the start and end of each infusion are stated in the upper-right corner of each panel in units of μg/L.
Table 8.
Diabetogenic and antidiabetogenic actions of ghrelin.
| Prodiabetic effects | Antidiabetic effects |
|---|---|
| stimulation of hepatic glucose output | chronic ↑ GH (lipolysis) |
| adipogenesis* | increase lean-body mass (chronic) |
| inhibition of insulin secretion | decrease oxygen consumption |
| appetite enhancement | increase uncoupling protein-1 |
| acute free-fatty acid release* (human) | |
| Antithermogenesis | |
| decreased sympathetic outflow |
A small percentage of GH-deficient adults (10%) also respond acutely to GHS, suggesting some preservation of somatotrope function and GHRH availability [640]. In other settings, injected GHS showed high specificity (95%) but low sensitivity (80%) in detecting GH deficiency [641]. Combining GHS with GHRH and/or L-arginine improves test sensitivity [4, 163].
5.16. Species Differences
Species differences in ghrelin structure, and to a lesser degree ghrelin action, have been articulated [2, 26, 62, 276, 315, 642]. In the eel, ghrelin-21 predominates instead of ghrelin-28 [643]. In fish, decanoyl rather than octanoyl ghrelin stimulates food consumption and increases liver and fat mass [644].
5.17. Genetic Considerations
Epidemiological studies have not identified common genetic haplotypes of GHS-R1a, which predispose to obesity or short stature [645]. Two-weak associations of GHS-R1a polymorphisms with metabolic syndrome or cardiovascular risk require confirmation [646, 647]. Similar preliminary data apply to polymorphisms of the ghrelin gene [393, 648].
6. Summary
In addition to increasing GH, as the term ghrelin implies, GHS regulates inflammation, cellular proliferation, apoptosis, differentiation, and hormone secretion via receptors located in the brain, stomach, intestine, heart, arterial wall, bone, fat cells, and pancreas (exocrine and endocrine). Acylated and unacylated ghrelin can exert both similar (antiapoptotic) and opposite (appetitive) effects. Promising clinical applications of ghrelin agonists and antagonists arise in relation to metabolic, gastric, GH-stimulating, anti-inflammatory, and cardiotropic effects. Nonetheless, there are both desirable and potentially undesirable aspects of chronic administration of ghrelin agonists or antagonists: Table 9. Accordingly, substantial further advances in ghrelin biology will be important.
Table 9.
Issues concerning longterm administration of ghrelin antagonists.
| Desirable |
| ↓ hyperglycemia |
| ↑ insulin secretion |
| ↓ appetite and food intake |
| ↑ fat oxidation |
| ↑ LH secretion? |
|
|
| Undesirable |
| ↓ gastric emptying |
| ↑ blood pressure (vasoconstrict) |
| ↑ cardiac oxygen consumption? |
| ↓ neoplastic apoptosis? |
| ↑ inflammatory mediators? |
| ↓ bone growth? |
| ↑ gastric alkalinity and mucosal permeability? |
| ↓ GH secretion (female)? |
| ↓ neurogenesis (brainstem, cortex)? |
| ↑ hypoglycemia during prolonged fast? |
7. Speculations
A saga is evolving within the ghrelin system due to the recent identification of the ghrelin O-acyl transferase (GOAT) enzyme [47, 48]. The octanoyl addition is of major biochemical and functional significance. Not only is ghrelin the first natural hormone with a fatty-acid addition, but also octanoylation is essential for binding and activating the receptor, GHS-R1a, probably by determining the active receptor-specific conformation of the ghrelin 1–28 molecule. Final isolation of the GOAT enzyme may not be so easy, since it is tightly bound by 12 transmembrane domains spanning the endoplasmic reticulum (ER) [47].
Important issues arise regarding the chemistry and biology of posttranslational octanoylation of ghrelin hormone. The current concept is that preproghrelin 1–117 or proghrelin 1–94 is octanoylated for fundamental biological reasons. In consonance with this postulate, production of octanoylated ghrelin 1–28 is restricted to the conjoint anatomical and intracellular sites of GOAT and preproghrelin 1–117 biosynthesis. Furthermore, if the desacylated ghrelin 1–28 is not directly octanoylated by GOAT, the specificity of biological regulation is made even more precise. Available evidence indirectly suggests that GOAT first octanoylates the preproghrelin 1–117 or the proghrelin 1–94 molecule. In the former case, octanoylation would occur cotranslationally rather than posttranslationally. This would require an integrated cleavage of the signal peptide. Subsequently, several prohormone convertases (PC1/3, PC2, and furin) permit sequential formation of octanoylated Ser3 proghrelin 1–94 and octanoylated Ser3 ghrelin 1–28 [496, 649, 650].
Before the identification of GOAT, Zhu, Cao, Voogd, and Steiner published the finding that 1–94 proghrelin was octanoylated, whereas ghrelin 1–28 remained unoctanoylated [496]. These results support the inference that octanoylated Ser3 ghrelin 1–28 is derived from octanoylated proghrelin 1–94. The specific actions of convertases are now being resolved. Zhu et al. demonstrated that PC1 must be the enzyme that cleaves proghrelin in vivo, because only proghrelin and not processed ghrelin is made in PC1-knockout mice [496]. In addition, furin can cleave proghrelin in vitro as described in the laboratory of Lindberg and, more recently, that of Kojima in transfection studies [72, 649]. Why furin does not suffice in the PC1 gene-deletion model is not clear.
In addition to GOAT, another human ER oxyesterase has been purified and characterized from a stable human erythroleukemia cell line by Ozawa, Speaker, and Lindberg [56]. The acronym of this additional oxyesterase is ERAT for endoplasmic reticulum O-acyl transferase. ERAT will esterify modified proghrelin having an N-terminal tripeptide extension, but not the proghrelin 1–94 molecule. Notably ERAT octanoylation is limited to the Ser2 amino-acid residue, while Ser3, Ser6, or Ser18 of ghrelin 1–28 are not octanoylated. In addition, ERAT may transfer an array of long-chain natural fatty acids, not just octanoic, to as yet unknown substrates as well as to ghrelin 1–28, when the latter contains an N-terminal tripeptide extension. This specificity is reminiscent of N-myristoyltransferase. Furthermore, ERAT is a soluble enzyme, but firmly bound to the ER membrane, whereas GOAT is an insoluble integral ER membrane-spanning enzyme. If ERAT and GOAT were colocalized in the human stomach, one could envision both independent and interdependent roles of ERAT and GOAT. Although mammalian GOAT was initially considered to only add octanoic or decanoic fatty acids to Ser3, the laboratory of Kojima reported that GOAT effectively acylates truncated ghrelin peptides with n-hexanoic acid in cultured cells [134]. ERAT might interact by limiting in situ availability of octanoic acid to GOAT. A significant analytical point is that Ser2 versus Ser3 octanoylated ghrelin would not be distinguishable by mass spectrometry, HPLC, or probably immunologically, requiring instead mutagenesis studies, amino-acid sequencing, binding, and biological activity studies.
Whether ERAT acylates proghrelin in vivo is not known. Albeit low in potency, Ser2,3 dioctanoylated ghrelin 1–28 can release GH in vivo in rats [125]. In addition, scientists at Merck laboratories demonstrated that Ser2 mono-octanoylated ghrelin 1–28 binds to GHS-R1a in vitro. Since octanoylated ghrelin 1–28 may exert endproduct repression of GOAT acyltransferase activity and since binding sites for GOAT, ERAT, and GHS-R1a may overlap, small amounts of Ser2 or Ser2,3 octanoylated ghrelin 1–28 might have GOAT inhibitory activity. The proposition would be that endogenous ERAT-derived ghrelin peptides may act on GOAT only at intracellular sites.
Notable are two ghrelin deacylation enzymes, plasma paraoxanase and gastric lysophospholipase I [156, 651]. Plasma paraoxanase is bound to high-density lipoproteins and may especially determine physiological plasma ghrelin levels. An interesting question is whether a major role of blood-borne paroxanase is to minimize the actions of octanoylated ghrelin or to maximize the actions of desacylated ghrelin. On the other hand, gastric lysophospholipase I may primarily regulate tissue ghrelin bioactivity and subsequent vagal-afferent activity as a function of the amount and type of oral fatty-acid intake. In this regard, oral octanoate increases, but oral dodecanoate decreases, plasma octanoylated ghrelin levels [134]. Accordingly, the activity of the two deacylases could be a function of amount and type of substrate as well as factors that modify synthesis of and catalysis by paroxanase and lysophospholipase I.
The multiple influences of the ghrelin system depend upon local and systemic posttranslational regulatory mechanisms and pleiotropic receptor-signaling in diverse target tissues [518]. Moreover, gastric ghrelin is subject to food-entrainable and circadian-clock inputs [652]. There is an additional possibility that partial overlap of ghrelin-binding properties of GHS-R1a, GOAT, and ERAT could contribute to differences and similarities between ghrelin and GHS mimetics as well as competition between full and partial GHS-R1a agonists [653]. Brown and Goldstein demonstrated that truncated ghrelin peptide analogs inhibit activity of GOAT in vitro [46]. They proposed that native octanoyl ghrelin 1–28 in higher concentrations may also inhibit GOAT. If so, one could envision that certain GHS mimetics might decrease or increase the activity of GOAT and ERAT as well as that of GHS-R1a. For example, at a low dosage Dap3 octanoylated ghrelin 1–28 inhibits octanoyl transferase activity in vitro indicating that GOAT is a significant site of action, whereas this analog stimulates both GH release and food intake in vivo, thus defining a GHS-R1a (ghrelin receptor) site of action [46, 654]. To assess the biochemistry, physiology, endocrinology, and therapeutic implications of peptide/non-peptide GHS mimetics and ghrelin will require combined in vitro and in vivo structure-function and dose-response analyses.
Acknowledgments
The authors thank Donna Scott for support of manuscript preparation and Ashley Bryant for graphics assistance. Exchanges with Iris Lindberg about the Speculations Section were most valuable. This review was supported in part via the Center for Translational Science Activities (CTSA) Grant no. 1 UL 1 RR024150 to Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD) and R01 NIA AG19695, and AG29362 from the National Institutes of Health (Bethesda, MD).
References
- 1.Bowers CY, Chang J, Momany F, Folkers K. Effect of the enkephalins and enkephalin analogs on release of pituitary hormones in vitro. In: MacIntyre I, Szelke M, editors. Molecular Endocrinology. Amsterdam, The Netherlands: Elsevier/North Holland; 1977. pp. 287–292. [Google Scholar]
- 2.Davenport AP, Bonner TI, Foord SM, et al. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacological Reviews. 2005;57(4):541–546. doi: 10.1124/pr.57.4.1. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 4.Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocrine Reviews. 2006;27(2):101–140. doi: 10.1210/er.2005-0006. [DOI] [PubMed] [Google Scholar]
- 5.Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 6.Robertson DM. Transforming growth factor β/inhibin family. Bailliere’s Clinical Endocrinology and Metabolism. 1991;5(4):615–634. doi: 10.1016/s0950-351x(10)80006-0. [DOI] [PubMed] [Google Scholar]
- 7.Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Experimental Biology and Medicine. 2002;227(9):724–752. doi: 10.1177/153537020222700905. [DOI] [PubMed] [Google Scholar]
- 8.Bagnasco M, Tulipano G, Melis MR, Argiolas A, Cocchi D, Muller EE. Endogenous ghrelin is an orexigenic peptide acting in the arcuate nucleus in response to fasting. Regulatory Peptides. 2003;111(1–3):161–167. doi: 10.1016/s0167-0115(02)00283-5. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Fukue Y, Teranishi H, Yoshida Y, Kojima M. Molecular forms of hypothalamic ghrelin and its regulation by fasting and 2-deoxy-D-glucose administration. Endocrinology. 2005;146(6):2510–2516. doi: 10.1210/en.2005-0174. [DOI] [PubMed] [Google Scholar]
- 10.Kageyama H, Kitamura Y, Hosono T, et al. Visualization of ghrelin-producing neurons in the hypothalamic arcuate nucleus using ghrelin-EGFP transgenic mice. Regulatory Peptides. 2008;145(1–3):116–121. doi: 10.1016/j.regpep.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Menyhért J, Wittmann G, Hrabovszky E, et al. Distribution of ghrelin-immunoreactive neuronal networks in the human hypothalamus. Brain Research. 2006;1125(1):31–36. doi: 10.1016/j.brainres.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 12.Caminos JE, Gualillo O, Lago F, et al. The endogenous growth hormone secretagogue (ghrelin) is synthesized and secreted by chondrocytes. Endocrinology. 2005;146(3):1285–1292. doi: 10.1210/en.2004-1379. [DOI] [PubMed] [Google Scholar]
- 13.Soares J-B, Rocha-Sousa A, Castro-Chaves P, Henriques-Coelho T, Leite-Moreira AF. Inotropic and lusitropic effects of ghrelin and their modulation by the endocardial endothelium, NO, prostaglandins, GHS-R1a and KCa channels. Peptides. 2006;27(7):1616–1623. doi: 10.1016/j.peptides.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Bong SJ, Chang HH, Jin Z-G. Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation. Endocrinology. 2008;149(8):4183–4192. doi: 10.1210/en.2008-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kui L, Weiwei Z, ling L, et al. Ghrelin inhibits apoptosis induced by high glucose and sodium palmitate in adult rat cardiomyocytes through the PI3K-Akt signaling pathway. Regulatory Peptides. 2009;155(1–3):62–69. doi: 10.1016/j.regpep.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Pacheco F, Luque RM, Tena-Sempere M, Malagón MM, Castaño JP. Ghrelin induces growth hormone secretion via a nitric oxide/cGMP signalling pathway. Journal of Neuroendocrinology. 2008;20(3):406–412. doi: 10.1111/j.1365-2826.2008.01645.x. [DOI] [PubMed] [Google Scholar]
- 17.Bilgin HM, Tumer C, Diken H, Kelle M, Sermet A. Role of ghrelin in the regulation of gastric acid secretion involving nitrergic mechanisms in rats. Physiological Research. 2008;57(4):563–568. doi: 10.33549/physiolres.931234. [DOI] [PubMed] [Google Scholar]
- 18.Gaskin FS, Farr SA, Banks WA, Kumar VB, Morley JE. Ghrelin-induced feeding is dependent on nitric oxide. Peptides. 2003;24(6):913–918. doi: 10.1016/s0196-9781(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 19.Konturek PC, Brzozowski T, Walter B, et al. Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. European Journal of Pharmacology. 2006;536(1-2):171–181. doi: 10.1016/j.ejphar.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Iantorno M, Chen H, Kim J-A, et al. Ghrelin has novel vascular actions that mimic PI 3-kinase-dependent actions of insulin to stimulate production of NO from endothelial cells. American Journal of Physiology. 2007;292(3):E756–E764. doi: 10.1152/ajpendo.00570.2006. [DOI] [PubMed] [Google Scholar]
- 21.Chung H, Seo S, Moon M, Park S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3β and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. Journal of Endocrinology. 2008;198(3):511–521. doi: 10.1677/JOE-08-0160. [DOI] [PubMed] [Google Scholar]
- 22.Baldanzi G, Filigheddu N, Cutrupi S, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. Journal of Cell Biology. 2002;159(6):1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivier J, Spiess J, Thorner M, Vale W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature. 1982;300(5889):276–278. doi: 10.1038/300276a0. [DOI] [PubMed] [Google Scholar]
- 24.Guillemin R, Brazeau P, Bohlen P, Esch F, Ling N, Wehrenberg WB. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982;218(4572):585–587. doi: 10.1126/science.6812220. [DOI] [PubMed] [Google Scholar]
- 25.Mayo KE, Godfrey PA, Suhr ST, Kulik DJ, Rahal JO. Growth hormone-releasing hormone: synthesis and signaling. Recent Progress in Hormone Research. 1995;50(1):35–73. doi: 10.1016/b978-0-12-571150-0.50007-x. [DOI] [PubMed] [Google Scholar]
- 26.Kojima M, Kangawa K. Ghrelin: structure and function. Physiological Reviews. 2005;85(2):495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 27.Pantel J, Legendre M, Cabrol S, et al. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. Journal of Clinical Investigation. 2006;116(3):760–768. doi: 10.1172/JCI25303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lall S, Balthasar N, Carmignac D, et al. Physiological studies of transgenic mice overexpressing growth hormone (GH) secretagogue receptor 1A in GH-releasing hormone neurons. Endocrinology. 2004;145(4):1602–1611. doi: 10.1210/en.2003-1509. [DOI] [PubMed] [Google Scholar]
- 29.Di Vito L, Broglio F, Benso A, et al. The GH-releasing effect of ghrelin, a natural GH secretagogue, is only blunted by the infusion of exogenous somatostatin in humans. Clinical Endocrinology. 2002;56(5):643–648. doi: 10.1046/j.1365-2265.2002.01530.x. [DOI] [PubMed] [Google Scholar]
- 30.McMahon CD, Chapin LT, Radcliff RP, Lookingland KJ, Tucker HA. GH-releasing peptide-6 overcomes refractoriness of somatotropes to GHRH after feeding. Journal of Endocrinology. 2001;170(1):235–241. doi: 10.1677/joe.0.1700235. [DOI] [PubMed] [Google Scholar]
- 31.Dickson SL, Viltart O, Bailey ART, Leng G. Attenuation of the growth hormone secretagogue induction of fos protein in the rat arcuate nucleus by central somatostatin action. Neuroendocrinology. 1997;66(3):188–194. doi: 10.1159/000127237. [DOI] [PubMed] [Google Scholar]
- 32.Tolle V, Zizzari P, Tomasetto C, Rio M-C, Epelbaum J, Bluet-Pajot M-T. In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat. Neuroendocrinology. 2001;73(1):54–61. doi: 10.1159/000054620. [DOI] [PubMed] [Google Scholar]
- 33.Herrington J, Hille B. Growth hormone-releasing hexapeptide elevates intracellular calcium in rat somatotropes by two mechanisms. Endocrinology. 1994;135(3):1100–1108. doi: 10.1210/endo.135.3.8070352. [DOI] [PubMed] [Google Scholar]
- 34.Hashizume T, Horiuchi M, Tate N, et al. Effects of ghrelin on growth hormone secretion from cultured adenohypophysial cells in cattle. Endocrine Journal. 2003;50(3):289–295. doi: 10.1507/endocrj.50.289. [DOI] [PubMed] [Google Scholar]
- 35.Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. Journal of Neuroendocrinology. 2002;14(7):580–586. doi: 10.1046/j.1365-2826.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- 36.Menyhert J, Wittmann G, Hrabovszky E, Keller E, Liposits Z, Fekete C. Interconnection between orexigenic neuropeptide Y- and anorexigenic α-melanocyte stimulating hormone-synthesizing neuronal systems of the human hypothalamus. Brain Research. 2006;1076(1):101–105. doi: 10.1016/j.brainres.2005.12.118. [DOI] [PubMed] [Google Scholar]
- 37.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. The Journal of Clinical Endocrinology & Metabolism. 2001;86(12):5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 38.Shintani M, Ogawa Y, Ebihara K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50(2):227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 39.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 40.Tung YCL, Hewson AK, Dickson SL. Actions of leptin on growth hormone secretagogue-responsive neurones in the rat hypothalamic arcuate nucleus recorded in vitro. Journal of Neuroendocrinology. 2001;13(2):209–215. doi: 10.1046/j.1365-2826.2001.00615.x. [DOI] [PubMed] [Google Scholar]
- 41.Farhy LS, Veldhuis JD. Deterministic construct of amplifying actions of ghrelin on pulsatile growth hormone secretion. American Journal of Physiology. 2005;288(6):R1649–R1663. doi: 10.1152/ajpregu.00451.2004. [DOI] [PubMed] [Google Scholar]
- 42.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Regulation of the ghrelin gene: growth hormone-releasing hormone upregulates ghrelin mRNA in the pituitary. Endocrinology. 2001;142(9):4154–4157. doi: 10.1210/endo.142.9.8492. [DOI] [PubMed] [Google Scholar]
- 43.Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nature Reviews Neuroscience. 2001;2(8):551–560. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- 44.Abizaid A, Liu Z-W, Andrews ZB, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. Journal of Clinical Investigation. 2006;116(12):3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang-Christensen M, Vrang N, Ortmann S, Bidlingmaier M, Horvath TL, Tschöp M. Central administration of ghrelin and agouti-related protein (83–132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology. 2004;145(10):4645–4652. doi: 10.1210/en.2004-0529. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Zhao T-J, Goldstein JL, Brown MS. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(31):10750–10755. doi: 10.1073/pnas.0805353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(17):6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang S-C, Magee AI. Acyltransferases for secreted signalling proteins (Review) Molecular Membrane Biology. 2009;26(1-2):104–113. doi: 10.1080/09687680802706432. [DOI] [PubMed] [Google Scholar]
- 50.Kirchner H, Gutierrez JA, Solenberg PJ, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nature Medicine. 2009;15(7):741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Root AW, Root MJ. Clinical pharmacology of human growth hormone and its secretagogues. Current Drug Targets. Immune, Endocrine and Metabolic Disorders. 2002;2(1):27–52. [PubMed] [Google Scholar]
- 52.González CR, Vázquez MJ, López M, Diéguez C. Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosa. Journal of Molecular Endocrinology. 2008;41(5-6):415–421. doi: 10.1677/JME-08-0102. [DOI] [PubMed] [Google Scholar]
- 53.Sakata I, Yang J, Lee CE, et al. Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. American Journal of Physiology. 2009;297(1):E134–E141. doi: 10.1152/ajpendo.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seim I, Collet C, Herington AC, Chopin LK. Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genomics. 2007;8, article 298 doi: 10.1186/1471-2164-8-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seim I, Amorim L, Walpole C, Carter S, Chopin LK, Herington AC. Ghrelin gene-related peptides: multifunctional endocrine/autocrine modulators in health and disease. Clinical and Experimental Pharmacology and Physiology. 2010;37(1):125–131. doi: 10.1111/j.1440-1681.2009.05241.x. [DOI] [PubMed] [Google Scholar]
- 56.Ozawa A, Speaker RB, III, Lindberg I. Enzymatic characterization of a human acyltransferase activity. PLoS ONE. 2009;4(5, article e5426) doi: 10.1371/journal.pone.0005426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gauna C, van de Zande B, van Kerkwijk A, Themmen APN, van der Lely AJ, Delhanty PJD. Unacylated ghrelin is not a functional antagonist but a full agonist of the type 1a growth hormone secretagogue receptor (GHS-R) Molecular and Cellular Endocrinology. 2007;274(1-2):30–34. doi: 10.1016/j.mce.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Shanado Y, Kometani M, Uchiyama H, Koizumi S, Teno N. Lysophospholipase I identified as a ghrelin deacylation enzyme in rat stomach. Biochemical and Biophysical Research Communications. 2004;325(4):1487–1494. doi: 10.1016/j.bbrc.2004.10.193. [DOI] [PubMed] [Google Scholar]
- 59.Hosoda H, Doi K, Nagaya N, et al. Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clinical Chemistry. 2004;50(6):1077–1080. doi: 10.1373/clinchem.2003.025841. [DOI] [PubMed] [Google Scholar]
- 60.Ohgusu H, Shirouzu K, Nakamura Y, et al. Ghrelin O-acyltransferase (GOAT) has a preference for n-hexanoyl-CoA over n-octanoyl-CoA as an acyl donor. Biochemical and Biophysical Research Communications. 2009;386(1):153–158. doi: 10.1016/j.bbrc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Hosoda H, Kojima M, Matsuo H, Kangawa K. Purification and characterization of rat des-Gln14-ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. Journal of Biological Chemistry. 2000;275(29):21995–22000. doi: 10.1074/jbc.M002784200. [DOI] [PubMed] [Google Scholar]
- 62.Kaiya H, Miyazato M, Kangawa K, Peter RE, Unniappan S. Ghrelin: a multifunctional hormone in non-mammalian vertebrates. Comparative Biochemistry and Physiology Part A. 2008;149(2):109–128. doi: 10.1016/j.cbpa.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Smith RG, Leonard R, Bailey ART, et al. Growth hormone secretagogue receptor family members and ligands. Endocrine. 2001;14(1):9–14. doi: 10.1385/ENDO:14:1:009. [DOI] [PubMed] [Google Scholar]
- 64.Codner E, Cassorla F, Tiulpakov AN, et al. Effects of oral administration of ibutamoren mesylate, a nonpeptide growth hormone secretagogue, on the growth hormone-insulin-like growth factor I axis in growth hormone-deficient children. Clinical Pharmacology and Therapeutics. 2001;70(1):91–98. doi: 10.1067/mcp.2001.116514. [DOI] [PubMed] [Google Scholar]
- 65.Bowers CY, Chang J-K, Wu S, Linse KD, Hurley DL, Veldhuis JD. Biochemistry of the growth hormone-releasing peptides, secretagogues and ghrelin. In: Mantovani G, Anker SD, Inui A, et al., editors. Cachexia and Wasting: A Modern Approach. New York, NY, USA: Springer; 2006. pp. 219–234. [Google Scholar]
- 66.Aagaard NK, Grøfte T, Greisen J, et al. Growth hormone and growth hormone secretagogue effects on nitrogen balance and urea synthesis in steroid treated rats. Growth Hormone and IGF Research. 2009;19(5):426–431. doi: 10.1016/j.ghir.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Raun K, Hansen BS, Johansen NL, et al. Ipamorelin, the first selective growth hormone secretagogue. European Journal of Endocrinology. 1998;139(5):552–561. doi: 10.1530/eje.0.1390552. [DOI] [PubMed] [Google Scholar]
- 68.Pemberton CJ, Richards AM. Biochemistry of ghrelin precursor peptides. Vitamins & Hormones. 2007;77:13–30. doi: 10.1016/S0083-6729(06)77002-9. [DOI] [PubMed] [Google Scholar]
- 69.Pemberton C, Wimalasena P, Yandle T, Soule S, Richards M. C-terminal pro-ghrelin peptides are present in the human circulation. Biochemical and Biophysical Research Communications. 2003;310(2):567–573. doi: 10.1016/j.bbrc.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 70.Ingelsson E, Larson MG, Yin X, et al. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. The Journal of Clinical Endocrinology & Metabolism. 2008;93(8):3149–3157. doi: 10.1210/jc.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purnell JQ, Weigle DS, Breen P, Cummings DE. Ghrelin levels correlate with insulin levels, insulin resistance, and high-density lipoprotein cholesterol, but not with gender, menopausal status, or cortisol levels in humans. The Journal of Clinical Endocrinology & Metabolism. 2003;88(12):5747–5752. doi: 10.1210/jc.2003-030513. [DOI] [PubMed] [Google Scholar]
- 72.Hosoda H, Kojima M, Mizushima T, Shimizu S, Kangawa K. Structural divergence of human ghrelin: identification of multiple ghrelin-derived molecules produced by post-translational processing. Journal of Biological Chemistry. 2003;278(1):64–70. doi: 10.1074/jbc.M205366200. [DOI] [PubMed] [Google Scholar]
- 73.Chandarana K, Drew ME, Emmanuel J, et al. Subject standardization, acclimatization, and sample processing affect gut hormone levels and appetite in humans. Gastroenterology. 2009;136(7):2115–2126. doi: 10.1053/j.gastro.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 74.Toshinai K, Yamaguchi H, Sun Y, et al. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147(5):2306–2314. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- 75.Ariyasu H, Takaya K, Iwakura H, et al. Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology. 2005;146(1):355–364. doi: 10.1210/en.2004-0629. [DOI] [PubMed] [Google Scholar]
- 76.Thompson NM, Gill DAS, Davies R, et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directlyin vivo by a mechanism independent of GHS-R1a. Endocrinology. 2004;145(1):234–242. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- 77.Broglio F, Gianotti L, Destefanis S, et al. The endocrine response to acute ghrelin administration is blunted in patients with anorexia nervosa, a ghrelin hypersecretory state. Clinical Endocrinology. 2004;60(5):592–599. doi: 10.1111/j.1365-2265.2004.02011.x. [DOI] [PubMed] [Google Scholar]
- 78.Dong J, Peeters TL, De Smet B, et al. Role of endogenous ghrelin in the hyperphagia of mice with streptozotocin-induced diabetes. Endocrinology. 2006;147(6):2634–2642. doi: 10.1210/en.2005-1335. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka M, Nakahara T, Kojima S, et al. Effect of nutritional rehabilitation on circulating ghrelin and growth hormone levels in patients with anorexia nervosa. Regulatory Peptides. 2004;122(3):163–168. doi: 10.1016/j.regpep.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 80.Troisi A, Di Lorenzo G, Lega I, et al. Plasma ghrelin in anorexia, bulimia, and binge-eating disorder: relations with eating patterns and circulating concentrations of cortisol and thyroid hormones. Neuroendocrinology. 2005;81(4):259–266. doi: 10.1159/000087923. [DOI] [PubMed] [Google Scholar]
- 81.Toshinai K, Mondal MS, Nakazato M, et al. Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochemical and Biophysical Research Communications. 2001;281(5):1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 82.Gambineri A, Pagotto U, De Iasio R, et al. Short-term modification of sex hormones is associated with changes in ghrelin circulating levels in healthy normal-weight men. Journal of Endocrinological Investigation. 2005;28(3):241–246. doi: 10.1007/BF03345380. [DOI] [PubMed] [Google Scholar]
- 83.Itoh T, Nagaya N, Yoshikawa M, et al. Elevated plasma ghrelin level in underweight patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2004;170(8):879–882. doi: 10.1164/rccm.200310-1404OC. [DOI] [PubMed] [Google Scholar]
- 84.Gottero C, Broglio F, Prodam F, et al. Ghrelin: a link between eating disorders, obesity and reproduction. Nutritional Neuroscience. 2004;7(5-6):255–270. doi: 10.1080/10284150400017363. [DOI] [PubMed] [Google Scholar]
- 85.Vila G, Maier C, Riedl M, et al. Bacterial endotoxin induces biphasic changes in plasma ghrelin in healthy humans. The Journal of Clinical Endocrinology & Metabolism. 2007;92(10):3930–3934. doi: 10.1210/jc.2007-1194. [DOI] [PubMed] [Google Scholar]
- 86.Yoshimoto A, Mori K, Sugawara A, et al. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. Journal of the American Society of Nephrology. 2002;13(11):2748–2752. doi: 10.1097/01.asn.0000032420.12455.74. [DOI] [PubMed] [Google Scholar]
- 87.Ryber L, Obrink K, Houe N, Frystyk J, Jorgensen JOL. Serum ghrelin levels are suppressed in hypopituitary patients following insulin-induced hypoglycaemia irrespective of GH status. Clinical Endocrinology. 2006;65(2):210–214. doi: 10.1111/j.1365-2265.2006.02575.x. [DOI] [PubMed] [Google Scholar]
- 88.Wei W, Wang G, Qi X, Englander EW, Greeley GH., Jr. Characterization and regulation of the rat and human ghrelin promoters. Endocrinology. 2005;146(3):1611–1625. doi: 10.1210/en.2004-1306. [DOI] [PubMed] [Google Scholar]
- 89.Matsuoka H, Hosoda H, Sugawara H, et al. Short-term secretory regulation of ghrelin during growth hormone provocative tests in prepubertal children with various growth hormone secretory capacities. Hormone Research. 2005;64(6):274–279. doi: 10.1159/000089294. [DOI] [PubMed] [Google Scholar]
- 90.Hiejima H, Nishi Y, Hosoda H, et al. Regional distribution and the dynamics of n-decanoyl ghrelin, another acyl-form of ghrelin, upon fasting in rodents. Regulatory Peptides. 2009;156(1–3):47–56. doi: 10.1016/j.regpep.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Paulo RC, Brundage R, Cosma M, Mielke KL, Bowers CY, Veldhuis JD. Estrogen elevates the peak overnight production rate of acylated ghrelin. The Journal of Clinical Endocrinology & Metabolism. 2008;93(11):4440–4447. doi: 10.1210/jc.2008-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barkan AL, Dimaraki EV, Jessup SK, Symons KV, Ermolenko M, Jaffe CA. Ghrelin secretion in humans is sexually dimorphic, suppressed by somatostatin, and not affected by the ambient growth hormone levels. The Journal of Clinical Endocrinology & Metabolism. 2003;88(5):2180–2184. doi: 10.1210/jc.2002-021169. [DOI] [PubMed] [Google Scholar]
- 93.Whatmore AJ, Hall CM, Jones J, Westwood M, Clayton PE. Ghrelin concentrations in healthy children and adolescents. Clinical Endocrinology. 2003;59(5):649–654. doi: 10.1046/j.1365-2265.2003.01903.x. [DOI] [PubMed] [Google Scholar]
- 94.Riis ALD, Hansen TK, Møller N, Weeke J, Jorgensen JOL. Hyperthyroidism is associated with suppressed circulating ghrelin levels. The Journal of Clinical Endocrinology & Metabolism. 2003;88(2):853–857. doi: 10.1210/jc.2002-021302. [DOI] [PubMed] [Google Scholar]
- 95.Makino Y, Hosoda H, Shibata K, et al. Alteration of plasma ghrelin levels associated with the blood pressure in pregnancy. Hypertension. 2002;39(3):781–784. doi: 10.1161/hy0302.105221. [DOI] [PubMed] [Google Scholar]
- 96.Pöykkö SM, Kellokoski E, Hörkkö S, Kauma H, Kesäniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003;52(10):2546–2553. doi: 10.2337/diabetes.52.10.2546. [DOI] [PubMed] [Google Scholar]
- 97.Norrelund H, Hansen TK, Orskov H, et al. Ghrelin immunoreactivity in human plasma is suppressed by somatostatin. Clinical Endocrinology. 2002;57(4):539–546. doi: 10.1046/j.1365-2265.2002.01649.x. [DOI] [PubMed] [Google Scholar]
- 98.Akamizu T, Murayama T, Teramukai S, et al. Plasma ghrelin levels in healthy elderly volunteers: the levels acylated ghrelin in elderly females correlate positively with serum IGF-I levels and bowel movement frequency and negatively with systolic blood pressure. Journal of Endocrinology. 2006;188(2):333–344. doi: 10.1677/joe.1.06442. [DOI] [PubMed] [Google Scholar]
- 99.Grinspoon S, Miller KK, Herzog DB, Grieco KA, Klibanski A. Effects of estrogen and recombinant human insulin-like growth factor-I on ghrelin secretion in severe undernutrition. The Journal of Clinical Endocrinology & Metabolism. 2004;89(8):3988–3993. doi: 10.1210/jc.2004-0287. [DOI] [PubMed] [Google Scholar]
- 100.Kellokoski E, Pöykkö SM, Karjalainen AH, et al. Estrogen replacement therapy increases plasma ghrelin levels. The Journal of Clinical Endocrinology & Metabolism. 2005;90(5):2954–2963. doi: 10.1210/jc.2004-2016. [DOI] [PubMed] [Google Scholar]
- 101.Di Carlo C, Tommaselli GA, Gargano V, et al. Effects of estrogen-progestin therapy on serum levels of RANKL, osteoprotegerin, osteocalcin, leptin, and ghrelin in postmenopausal women. Menopause. 2007;14(1):38–44. doi: 10.1097/01.gme.0000227855.04732.7b. [DOI] [PubMed] [Google Scholar]
- 102.Messini CI, Dafopoulos K, Chalvatzas N, Georgoulias P, Messinis IE. Growth hormone and prolactin response to ghrelin during the normal menstrual cycle. Clinical Endocrinology. 2009;71(3):383–387. doi: 10.1111/j.1365-2265.2008.03505.x. [DOI] [PubMed] [Google Scholar]
- 103.Matsubara M, Sakata I, Wada R, Yamazaki M, Inoue K, Sakai T. Estrogen modulates ghrelin expression in the female rat stomach. Peptides. 2004;25(2):289–297. doi: 10.1016/j.peptides.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 104.Maffeis C, Franceschi R, Moghetti P, Camilot M, Lauriola S, Tato L. Circulating ghrelin levels in girls with central precocious puberty are reduced during treatment with LHRH analog. European Journal of Endocrinology. 2007;156(1):99–103. doi: 10.1530/eje.1.02320. [DOI] [PubMed] [Google Scholar]
- 105.Lebenthal Y, Gat-Yablonski G, Shtaif B, Padoa A, Phillip M, Lazar L. Effect of sex hormone administration on circulating ghrelin levels in peripubertal children. The Journal of Clinical Endocrinology & Metabolism. 2006;91(1):328–331. doi: 10.1210/jc.2005-0204. [DOI] [PubMed] [Google Scholar]
- 106.Villa P, Costantini B, Perri C, Suriano R, Ricciardi L, Lanzone A. Estro-progestin supplementation enhances the growth hormone secretory responsiveness to ghrelin infusion in postmenopausal women. Fertility and Sterility. 2008;89(2):398–403. doi: 10.1016/j.fertnstert.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 107.Kok P, Paulo RC, Cosma M, et al. Estrogen supplementation selectively enhances hypothalamo-pituitary sensitivity to ghrelin in postmenopausal women. The Journal of Clinical Endocrinology & Metabolism. 2008;93(10):4020–4026. doi: 10.1210/jc.2008-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cappa M, Setzu S, Bernardini S, et al. Exogenous growth hormone administration does not inhibit the growth hormone response to hexarelin in normal men. Journal of Endocrinological Investigation. 1995;18(10):762–766. doi: 10.1007/BF03349808. [DOI] [PubMed] [Google Scholar]
- 109.Loche S, Colao A, Cappa M, et al. The growth hormone response to hexarelin in children: reproducibility and effect of sex steroids. The Journal of Clinical Endocrinology & Metabolism. 1997;82(3):861–864. doi: 10.1210/jcem.82.3.3795. [DOI] [PubMed] [Google Scholar]
- 110.Veldhuis JD, Keenan DM, Iranmanesh A, Mielke K, Miles JM, Bowers CY. Estradiol potentiates ghrelin-stimulated pulsatile growth hormone secretion in postmenopausal women. The Journal of Clinical Endocrinology & Metabolism. 2006;91(9):3559–3565. doi: 10.1210/jc.2006-0948. [DOI] [PubMed] [Google Scholar]
- 111.Anderson SM, Wideman L, Patrie JT, Weltman A, Bowers CY, Veldhuis JD. E2 supplementation selectively relieves GH’s autonegative feedback on GH-releasing peptide-2-stimulated GH secretion. The Journal of Clinical Endocrinology & Metabolism. 2001;86(12):5904–5911. doi: 10.1210/jcem.86.12.8076. [DOI] [PubMed] [Google Scholar]
- 112.Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD. Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. The Journal of Clinical Endocrinology & Metabolism. 2001;86(2):551–560. doi: 10.1210/jcem.86.2.7240. [DOI] [PubMed] [Google Scholar]
- 113.Broglio F, Benso A, Castiglioni C, et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. The Journal of Clinical Endocrinology & Metabolism. 2003;88(4):1537–1542. doi: 10.1210/jc.2002-021504. [DOI] [PubMed] [Google Scholar]
- 114.Veldhuis JD, Keenan DM, Mielke K, Miles JM, Bowers CY. Testosterone supplementation in healthy older men drives GH and IGF-I secretion without potentiating peptidyl secretagogue efficacy. European Journal of Endocrinology. 2005;153(4):577–586. doi: 10.1530/eje.1.02001. [DOI] [PubMed] [Google Scholar]
- 115.Rigamonti AE, Cella SG, Giordani C, et al. Testosterone inhibition of growth hormone release stimulated by a growth hormone secretagogue: studies in the rat and dog. Neuroendocrinology. 2007;84(2):115–122. doi: 10.1159/000096998. [DOI] [PubMed] [Google Scholar]
- 116.Hansen TK, Dall R, Hosoda H, et al. Weight loss increases circulating levels of ghrelin in human obesity. Clinical Endocrinology. 2002;56(2):203–206. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 117.Gormsen LC, Nielsen C, Gjedsted J, et al. Effects of free fatty acids, growth hormone and growth hormone receptor blockade on serum ghrelin levels in humans. Clinical Endocrinology. 2007;66(5):641–645. doi: 10.1111/j.1365-2265.2007.02786.x. [DOI] [PubMed] [Google Scholar]
- 118.Nass R, Liu J, Hellmann P, et al. Chronic changes in peripheral growth hormone levels do not affect ghrelin stomach mRNA expression and serum ghrelin levels in three transgenic mouse models. Journal of Neuroendocrinology. 2004;16(8):669–675. doi: 10.1111/j.1365-2826.2004.01220.x. [DOI] [PubMed] [Google Scholar]
- 119.Janssen JA, van der Toorn FM, Hofland LJ, et al. Systemic ghrelin levels in subjects with growth hormone deficiency are not modified by one year of growth hormone replacement therapy. European Journal of Endocrinology. 2001;145(6):711–716. doi: 10.1530/eje.0.1450711. [DOI] [PubMed] [Google Scholar]
- 120.Dall R, Kanaley J, Hansen TK, et al. Plasma ghrelin levels during exercise in healthy subjects and in growth hormone-deficient patients. European Journal of Endocrinology. 2002;147(1):65–70. doi: 10.1530/eje.0.1470065. [DOI] [PubMed] [Google Scholar]
- 121.Arafat AM, Perschel FH, Otto B, et al. Glucagon suppression of ghrelin secretion is exerted at hypothalamus-pituitary level. The Journal of Clinical Endocrinology & Metabolism. 2006;91(9):3528–3533. doi: 10.1210/jc.2006-0225. [DOI] [PubMed] [Google Scholar]
- 122.Kaji H, Kishimoto M, Kirimura T, et al. Hormonal regulation of the human ghrelin receptor gene transcription. Biochemical and Biophysical Research Communications. 2001;284(3):660–666. doi: 10.1006/bbrc.2001.5035. [DOI] [PubMed] [Google Scholar]
- 123.Clegg DJ, Brown LM, Zigman JM, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56(4):1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 124.Katayama T, Shimamoto S, Oda H, Nakahara K, Kangawa K, Murakami N. Glucagon receptor expression and glucagon stimulation of ghrelin secretion in rat stomach. Biochemical and Biophysical Research Communications. 2007;357(4):865–870. doi: 10.1016/j.bbrc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 125.Chen C-Y, Fujimiya M, Asakawa A, et al. At the cutting edge: ghrelin gene products in food intake and gut motility. Neuroendocrinology. 2009;89(1):9–17. doi: 10.1159/000165004. [DOI] [PubMed] [Google Scholar]
- 126.Friis-Hansen L, Wierup N, Rehfeld JF, Sundler F. Reduced ghrelin, islet amyloid polypeptide, and peptide YY expression in the stomach of gastrin-cholecystokinin knockout mice. Endocrinology. 2005;146(10):4464–4471. doi: 10.1210/en.2004-0938. [DOI] [PubMed] [Google Scholar]
- 127.Arakawa M, Suzuki H, Minegishi Y, et al. Enhanced ghrelin expression and subsequent acid secretion in mice with genetic H2-receptor knockout. Journal of Gastroenterology. 2007;42(9):711–718. doi: 10.1007/s00535-007-2084-2. [DOI] [PubMed] [Google Scholar]
- 128.Broglio F, Van Koetsveld P, Benso A, et al. Ghrelin secretion is inhibited by either somatostatin or cortistatin in humans. The Journal of Clinical Endocrinology & Metabolism. 2002;87(10):4829–4832. doi: 10.1210/jc.2002-020956. [DOI] [PubMed] [Google Scholar]
- 129.Teff KL, Elliott SS, Tschöp M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. The Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 130.Flanagan DE, Evans ML, Monsod TP, et al. The influence of insulin on circulating ghrelin. American Journal of Physiology. 2003;284(2):E313–E316. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- 131.Takahashi H, Kato A, Onodera K, Suzuki K. Fasting plasma ghrelin levels reflect malnutrition state in patients with liver cirrhosis. Hepatology Research. 2006;34(2):117–123. doi: 10.1016/j.hepres.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 132.Jarkovská Z, Hodková M, Sazamová M, et al. Plasma levels of active and total ghrelin in renal failure: a relationship with GH/IGF-I axis. Growth Hormone and IGF Research. 2005;15(6):369–376. doi: 10.1016/j.ghir.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 133.Kojima M, Kangawa K. Drug insight: the functions of ghrelin and its potential as a multitherapeutic hormone. Nature Clinical Practice Endocrinology and Metabolism. 2006;2(2):80–88. doi: 10.1038/ncpendmet0080. [DOI] [PubMed] [Google Scholar]
- 134.Feltrin KL, Patterson M, Ghatei MA, et al. Effect of fatty acid chain length on suppression of ghrelin and stimulation of PYY, GLP-2 and PP secretion in healthy men. Peptides. 2006;27(7):1638–1643. doi: 10.1016/j.peptides.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 135.Blom WAM, Lluch A, Stafleu A, et al. Effect of a high-protein breakfast on the postprandial ghrelin response. American Journal of Clinical Nutrition. 2006;83(2):211–220. doi: 10.1093/ajcn/83.2.211. [DOI] [PubMed] [Google Scholar]
- 136.Otto B, Cuntz U, Fruehauf E, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. European Journal of Endocrinology. 2001;145(5):669–673. [PubMed] [Google Scholar]
- 137.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 138.Katsuki A, Urakawa H, Gabazza EC, et al. Circulating levels of active ghrelin is associated with abdominal adiposity, hyperinsulinemia and insulin resistance in patients with type 2 diabetes mellitus. European Journal of Endocrinology. 2004;151(5):573–577. doi: 10.1530/eje.0.1510573. [DOI] [PubMed] [Google Scholar]
- 139.Kelishadi R, Hashemipour M, Mohammadifard N, Alikhassy H, Adeli K. Short- and long-term relationships of serum ghrelin with changes in body composition and the metabolic syndrome in prepubescent obese children following two different weight loss programmes. Clinical Endocrinology. 2008;69(5):721–729. doi: 10.1111/j.1365-2265.2008.03220.x. [DOI] [PubMed] [Google Scholar]
- 140.Lee C-C, Lee R-P, Subeq Y-M, Wang C-H, Fang T-C, Hsu B-G. Fasting serum total ghrelin level inversely correlates with metabolic syndrome in hemodialysis patients. Archives of Medical Research. 2008;39(8):785–790. doi: 10.1016/j.arcmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 141.Nakagawa E, Nagaya N, Okumura H, et al. Hyperglycaemia suppresses the secretion of ghrelin, a novel growth-hormone-releasing peptide: responses to the intravenous and oral administration of glucose. Clinical Science. 2002;103(3):325–328. doi: 10.1042/cs1030325. [DOI] [PubMed] [Google Scholar]
- 142.Gauna C, Uitterlinden P, Kramer P, et al. Intravenous glucose administration in fasting rats has differential effects on acylated and unacylated ghrelin in the portal and systemic circulation: a comparison between portal and peripheral concentrations in anesthetized rats. Endocrinology. 2007;148(11):5278–5287. doi: 10.1210/en.2007-0225. [DOI] [PubMed] [Google Scholar]
- 143.Lucidi P, Murdolo G, Di Loreto C, et al. Ghrelin is not necessary for adequate hormonal counterregulation of insulin-induced hypoglycemia. Diabetes. 2002;51(10):2911–2914. doi: 10.2337/diabetes.51.10.2911. [DOI] [PubMed] [Google Scholar]
- 144.Genís BB, Granada ML, Alonso N, et al. Ghrelin, glucose homeostasis, and carotid intima media thickness in kidney transplantation. Transplantation. 2007;84(10):1248–1254. doi: 10.1097/01.tp.0000287456.82676.01. [DOI] [PubMed] [Google Scholar]
- 145.Xu G, Li Y, An W, et al. Gastric mammalian target of rapamycin signaling regulates ghrelin production and food intake. Endocrinology. 2009;150(8):3637–3644. doi: 10.1210/en.2009-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 147.Foster-Schubert KE, Overduin J, Prudom CE, et al. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. The Journal of Clinical Endocrinology & Metabolism. 2008;93(5):1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takachi K, Doki Y, Ishikawa O, et al. Postoperative ghrelin levels and delayed recovery from body weight loss after distal or total gastrectomy. Journal of Surgical Research. 2006;130(1):1–7. doi: 10.1016/j.jss.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 149.Nass R, Farhy LS, Liu J, et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. The Journal of Clinical Endocrinology & Metabolism. 2008;93(5):1988–1994. doi: 10.1210/jc.2007-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.St-Pierre DH, Rabasa-Lhoret R, Lavoie M-E, et al. Fiber intake predicts ghrelin levels in overweight and obese postmenopausal women. European Journal of Endocrinology. 2009;161(1):65–72. doi: 10.1530/EJE-09-0018. [DOI] [PubMed] [Google Scholar]
- 151.Bellone S, Castellino N, Broglio F, et al. Ghrelin secretion in childhood is refractory to the inhibitory effect of feeding. The Journal of Clinical Endocrinology & Metabolism. 2004;89(4):1662–1665. doi: 10.1210/jc.2003-031207. [DOI] [PubMed] [Google Scholar]
- 152.Gambineri A, Pagotto U, Tschöp M, et al. Anti-androgen treatment increases circulating ghrelin levels in obese women with polycystic ovary syndrome. Journal of Endocrinological Investigation. 2003;26(7):629–634. doi: 10.1007/BF03347020. [DOI] [PubMed] [Google Scholar]
- 153.Barber TM, Casanueva FF, Karpe F, et al. Ghrelin levels are suppressed and show a blunted response to oral glucose in women with polycystic ovary syndrome. European Journal of Endocrinology. 2008;158(4):511–516. doi: 10.1530/EJE-07-0683. [DOI] [PubMed] [Google Scholar]
- 154.Brownley KA, Light KC, Grewen KM, Bragdon EE, Hinderliter AL, West SG. Postprandial ghrelin is elevated in black compared with white women. The Journal of Clinical Endocrinology & Metabolism. 2004;89(9):4457–4463. doi: 10.1210/jc.2004-0607. [DOI] [PubMed] [Google Scholar]
- 155.Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocrine Reviews. 2008;29(7):823–864. doi: 10.1210/er.2008-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Beaumont NJ, Skinner VO, Tan TM, et al. Ghrelin can bind to a species of high density lipoprotein associated with paraoxonase. Journal of Biological Chemistry. 2003;278(11):8877–8880. doi: 10.1074/jbc.C200575200. [DOI] [PubMed] [Google Scholar]
- 157.Banks WA, Burney BO, Robinson SM. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides. 2008;29(11):2061–2065. doi: 10.1016/j.peptides.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor—identification of a potent inverse agonist. Molecular Endocrinology. 2003;17(11):2201–2210. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- 159.Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. Common structural basis for constitutive activity of the ghrelin receptor family. Journal of Biological Chemistry. 2004;279(51):53806–53817. doi: 10.1074/jbc.M407676200. [DOI] [PubMed] [Google Scholar]
- 160.Holst B, Brandt E, Bach A, Heding A, Schwartz TW. Nonpeptide and peptide growth hormone secretagogues act both as ghrelin receptor agonist and as positive or negative allosteric modulators of ghrelin signaling. Molecular Endocrinology. 2005;19(9):2400–2411. doi: 10.1210/me.2005-0059. [DOI] [PubMed] [Google Scholar]
- 161.Fraser GL, Hoveyda HR, Tannenbaum GS. Pharmacological demarcation of the growth hormone, gut motility and feeding effects of ghrelin using a novel ghrelin receptor agonist. Endocrinology. 2008;149(12):6280–6288. doi: 10.1210/en.2008-0804. [DOI] [PubMed] [Google Scholar]
- 162.Holst B, Mokrosinski J, Lang M, et al. Identification of an efficacy switch region in the ghrelin receptor responsible for interchange between agonism and inverse agonism. Journal of Biological Chemistry. 2007;282(21):15799–15811. doi: 10.1074/jbc.M609796200. [DOI] [PubMed] [Google Scholar]
- 163.Cordido F, Isidro ML, Nemiña R, Sangiao-Alvarellos S. Ghrelin and growth hormone secretagogues, physiological and pharmacological aspect. Current Drug Discovery Technologies. 2009;6(1):34–42. doi: 10.2174/157016309787581048. [DOI] [PubMed] [Google Scholar]
- 164.Mayorov AV, Amara N, Chang JY, et al. Catalytic antibody degradation of ghrelin increases whole-body metabolic rate and reduces refeeding in fasting mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17487–17492. doi: 10.1073/pnas.0711808105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shuto Y, Shibasaki T, Otagiri A, et al. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. Journal of Clinical Investigation. 2002;109(11):1429–1436. doi: 10.1172/JCI13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149(2):843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zigman JM, Nakano Y, Coppari R, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. Journal of Clinical Investigation. 2005;115(12):3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shearman LP, Wang S-P, Helmling S, et al. Ghrelin neutralization by a ribonucleic acid-SPM ameliorates obesity in diet-induced obese mice. Endocrinology. 2006;147(3):1517–1526. doi: 10.1210/en.2005-0993. [DOI] [PubMed] [Google Scholar]
- 169.Pfluger PT, Kirchner H, Günnel S, et al. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. American Journal of Physiology. 2008;294(3):G610–G618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- 170.Bewick GA, Kent A, Campbell D, et al. Mice with hyperghrelinemia are hyperphagic and glucose intolerant and have reduced leptin sensitivity. Diabetes. 2009;58(4):840–846. doi: 10.2337/db08-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Theander-Carrillo C, Wiedmer P, Cettour-Rose P, et al. Ghrelin action in the brain controls adipocyte metabolism. Journal of Clinical Investigation. 2006;116(7):1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zorrilla EP, Iwasaki S, Moss JA, et al. Vaccination against weight gain. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):13226–13231. [Google Scholar]
- 173.Wortley KE, Del Rincon J-P, Murray JD, et al. Absence of ghrelin protects against early-onset obesity. Journal of Clinical Investigation. 2005;115(12):3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Molecular and Cellular Biology. 2003;23(22):7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cruz CR, Smith RG. The growth hormone secretagogue receptor. Vitamins & Hormones. 2007;77:47–88. doi: 10.1016/S0083-6729(06)77004-2. [DOI] [PubMed] [Google Scholar]
- 176.Vizcarra JA, Kirby JD, Kim SK, Galyean ML. Active immunization against ghrelin decreases weight gain and alters plasma concentrations of growth hormone in growing pigs. Domestic Animal Endocrinology. 2007;33(2):176–189. doi: 10.1016/j.domaniend.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 177.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wellman PJ, Hollas CN, Elliott AE. Systemic ghrelin sensitizes cocaine-induced hyperlocomotion in rats. Regulatory Peptides. 2008;146(1–3):33–37. doi: 10.1016/j.regpep.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wortley KE, Anderson KD, Garcia K, et al. Genetic deletion of ghrelin does decrease food intake but influences metabolic fuel preference. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8227–8232. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Helmling S, Maasch C, Eulberg D, et al. Inhibition of ghrelin action in vitro and in vivo by an RNA-Spiegelmer. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13174–13179. doi: 10.1073/pnas.0404175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zizzari P, Halem H, Taylor J, et al. Endogenous ghrelin regulates episodic GH secretion by amplifying GH pulse amplitude: evidence from antagonism of the GHS-R1a receptor. Endocrinology. 2005;146(9):3836–3842. doi: 10.1210/en.2005-0212. [DOI] [PubMed] [Google Scholar]
- 182.Salomé N, Hansson C, Taube M, et al. On the central mechanism underlying ghrelin’s chronic pro-obesity effects in rats: new insights from studies exploiting a potent ghrelin receptor antagonist. Journal of Neuroendocrinology. 2009;21(9):777–785. doi: 10.1111/j.1365-2826.2009.01895.x. [DOI] [PubMed] [Google Scholar]
- 183.Vlasova MA, Järvinen K, Herzig K-H. Cardiovascular effects of ghrelin antagonist in conscious rats. Regulatory Peptides. 2009;156(1–3):72–76. doi: 10.1016/j.regpep.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 184.Asakawa A, Inui A, Kaga T, et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52(7):947–952. doi: 10.1136/gut.52.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Esler WP, Rudolph J, Claus TH, et al. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148(11):5175–5185. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- 186.Nogueiras R, López M, Lage R, et al. Bsx, a novel hypothalamic factor linking feeding with locomotor activity, is regulated by energy availability. Endocrinology. 2008;149(6):3009–3015. doi: 10.1210/en.2007-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Petersenn S, Rasch AC, Penshorn M, Beil FU, Schulte HM. Genomic structure and transcriptional regulation of the human growth hormone secretagogue receptor. Endocrinology. 2001;142(6):2649–2659. doi: 10.1210/endo.142.6.8184. [DOI] [PubMed] [Google Scholar]
- 188.Bulgarelli I, Tamiazzo L, Bresciani E, et al. Desacyl-ghrelin and synthetic GH-secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by β-amyloid fibrils. Journal of Neuroscience Research. 2009;87(12):2718–2727. doi: 10.1002/jnr.22088. [DOI] [PubMed] [Google Scholar]
- 189.Fujimiya M, Asakawa A, Ataka K, Kato I, Inui A. Different effects of ghrelin, des-acyl ghrelin and obestatin on gastroduodenal motility in conscious rats. World Journal of Gastroenterology. 2008;14(41):6318–6326. doi: 10.3748/wjg.14.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Inhoff T, Mönnikes H, Noetzel S, et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29(12):2159–2168. doi: 10.1016/j.peptides.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Giovambattista A, Gaillard RC, Spinedi E. Ghrelin gene-related peptides modulate rat white adiposity. Vitamins & Hormones. 2007;77:171–205. doi: 10.1016/S0083-6729(06)77008-X. [DOI] [PubMed] [Google Scholar]
- 192.Muscaritoli M, Molfino A, Chiappini MG, et al. Anorexia in hemodialysis patients: the possible role of des-acyl ghrelin. American Journal of Nephrology. 2007;27(4):360–365. doi: 10.1159/000103798. [DOI] [PubMed] [Google Scholar]
- 193.Filigheddu N, Gnocchi VF, Coscia M, et al. Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Molecular Biology of the Cell. 2007;18(3):986–994. doi: 10.1091/mbc.E06-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li L, Zhang L-K, Pang Y-Z, et al. Cardioprotective effects of ghrelin and des-octanoyl ghrelin on myocardial injury induced by isoproterenol in rats. Acta Pharmacologica Sinica. 2006;27(5):527–535. doi: 10.1111/j.1745-7254.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 195.Patel AD, Stanley SA, Murphy KG, et al. Ghrelin stimulates insulin-induced glucose uptake in adipocytes. Regulatory Peptides. 2006;134(1):17–22. doi: 10.1016/j.regpep.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 196.Chen CY, Chao Y, Chang FY, Chien EJ, Lee SD, Doong ML. Intracisternal des-acyl ghrelin inhibits food intake and non-nutrient gastric emptying in conscious rats. International Journal of Molecular Medicine. 2005;16(4):695–699. [PubMed] [Google Scholar]
- 197.Tsubota Y, Owada-Makabe K, Yukawa K, Maeda M. Hypotensive effect of des-acyl ghrelin at nucleus tractus solitarii of rat. NeuroReport. 2005;16(2):163–166. doi: 10.1097/00001756-200502080-00019. [DOI] [PubMed] [Google Scholar]
- 198.Gauna C, Delhanty PJD, Hofland LJ, et al. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. The Journal of Clinical Endocrinology & Metabolism. 2005;90(2):1055–1060. doi: 10.1210/jc.2004-1069. [DOI] [PubMed] [Google Scholar]
- 199.Muccioli G, Pons N, Ghe C, Catapano F, Granata R, Ghigo E. Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type 1a growth hormone secretagogue receptor. European Journal of Pharmacology. 2004;498(1–3):27–35. doi: 10.1016/j.ejphar.2004.07.066. [DOI] [PubMed] [Google Scholar]
- 200.Li WG, Gavrila D, Liu X, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-κB activation in human endothelial cells. Circulation. 2004;109(18):2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 201.Bedendi I, Alloatti G, Marcantoni A, et al. Cardiac effects of ghrelin and its endogenous derivatives des-octanoyl ghrelin and des-Gln14-ghrelin. European Journal of Pharmacology. 2003;476(1-2):87–95. doi: 10.1016/s0014-2999(03)02083-1. [DOI] [PubMed] [Google Scholar]
- 202.Halem HA, Taylor JE, Dong JZ, et al. A novel growth hormone secretagogue-1a receptor antagonist that blocks ghrelin-induced growth hormone secretion but induces increased body weight gain. Neuroendocrinology. 2005;81(5):339–349. doi: 10.1159/000088796. [DOI] [PubMed] [Google Scholar]
- 203.Holst B, Lang M, Brandt E, et al. Ghrelin receptor inverse agonists: identification of an active peptide core and its interaction epitopes on the receptor. Molecular Pharmacology. 2006;70(3):936–946. doi: 10.1124/mol.106.024422. [DOI] [PubMed] [Google Scholar]
- 204.Moulin A, Demange L, Bergé G, et al. Toward potent ghrelin receptor ligands based on trisubstituted 1,2,4-triazole structure. 2. Synthesis and pharmacological in vitro and in vivo evaluations. Journal of Medicinal Chemistry. 2007;50(23):5790–5806. doi: 10.1021/jm0704550. [DOI] [PubMed] [Google Scholar]
- 205.Rudolph J, Esler WP, O’Connor S, et al. Quinazolinone derivatives as orally available ghrelin receptor antagonists for the treatment of diabetes and obesity. Journal of Medicinal Chemistry. 2007;50(21):5202–5216. doi: 10.1021/jm070071+. [DOI] [PubMed] [Google Scholar]
- 206.Demange L, Boeglin D, Moulin A, et al. Synthesis and pharmacological in vitro and in vivo evaluations of novel triazole derivatives as ligands of the ghrelin receptor. 1. Journal of Medicinal Chemistry. 2007;50(8):1939–1957. doi: 10.1021/jm070024h. [DOI] [PubMed] [Google Scholar]
- 207.Muccioli G, Baragli A, Granata R, Papotti M, Ghigo E. Heterogeneity of ghrelin/growth hormone secretagogue receptors: toward the understanding of the molecular identity of novel ghrelin/GHS receptors. Neuroendocrinology. 2007;86(3):147–164. doi: 10.1159/000105141. [DOI] [PubMed] [Google Scholar]
- 208.Chen C-Y, Inui A, Asakawa A, et al. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129(1):8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 209.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addiction Biology. 2007;12(1):6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 210.Gauna C, Meyler FM, Janssen JA, et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. The Journal of Clinical Endocrinology & Metabolism. 2004;89(10):5035–5042. doi: 10.1210/jc.2004-0363. [DOI] [PubMed] [Google Scholar]
- 211.Johansson I, Destefanis S, Aberg ND, et al. Proliferative and protective effects of growth hormone secretagogues on adult rat hippocampal progenitor cells. Endocrinology. 2008;149(5):2191–2199. doi: 10.1210/en.2007-0733. [DOI] [PubMed] [Google Scholar]
- 212.Sato M, Nakahara K, Goto S, et al. Effects of ghrelin and des-acyl ghrelin on neurogenesis of the rat fetal spinal cord. Biochemical and Biophysical Research Communications. 2006;350(3):598–603. doi: 10.1016/j.bbrc.2006.09.088. [DOI] [PubMed] [Google Scholar]
- 213.Zhang W, Hu Y, Lin TR, Fan Y, Mulholland MW. Stimulation of neurogenesis in rat nucleus of the solitary tract by ghrelin. Peptides. 2005;26(11):2280–2288. doi: 10.1016/j.peptides.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 214.Zhang W, Lin TR, Hu Y, et al. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. Journal of Physiology. 2004;559(3):729–737. doi: 10.1113/jphysiol.2004.064121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hayashida T, Murakami K, Mogi K, et al. Ghrelin in domestic animals: distribution in stomach and its possible role. Domestic Animal Endocrinology. 2001;21(1):17–24. doi: 10.1016/s0739-7240(01)00104-7. [DOI] [PubMed] [Google Scholar]
- 216.Kineman RD, Luque RM. Evidence that ghrelin is as potent as growth hormone (GH)-releasing hormone (GHRH) in releasing GH from primary pituitary cell cultures of a nonhuman primate (Papio anubis), acting through intracellular signaling pathways distinct from GHRH. Endocrinology. 2007;148(9):4440–4449. doi: 10.1210/en.2007-0441. [DOI] [PubMed] [Google Scholar]
- 217.Glavaski-Joksimovic A, Jeftinija K, Scanes CG, Anderson LL, Jeftinija S. Stimulatory effect of ghrelin on isolated porcine somatotropes. Neuroendocrinology. 2003;77(6):367–379. doi: 10.1159/000071309. [DOI] [PubMed] [Google Scholar]
- 218.Ahmed S, Harvey S. Ghrelin: a hypothalamic GH-releasing factor in domestic fowl (Gallus domesticus) Journal of Endocrinology. 2002;172(1):117–125. doi: 10.1677/joe.0.1720117. [DOI] [PubMed] [Google Scholar]
- 219.Shupnik MA. Regulational effect of ghrelin on growth hormone secretion from perifused rat anterior pituitary cells. Journal of Neuroendocrinology. 2002;14(2):156–162. doi: 10.1046/j.0007-1331.2001.00757.x. [DOI] [PubMed] [Google Scholar]
- 220.Broglio F, Benso A, Gottero C, et al. Non-acylated ghrelin does not possess the pituitaric and pancreatic endocrine activity of acylated ghrelin in humans. Journal of Endocrinological Investigation. 2003;26(3):192–196. doi: 10.1007/BF03345156. [DOI] [PubMed] [Google Scholar]
- 221.Broglio F, Gottero C, Prodam F, et al. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. The Journal of Clinical Endocrinology & Metabolism. 2004;89(6):3062–3065. doi: 10.1210/jc.2003-031964. [DOI] [PubMed] [Google Scholar]
- 222.Nagamine J, Nagata R, Seki H, et al. Pharmacological profile of a new orally active growth hormone secretagogue, SM-130686. Journal of Endocrinology. 2001;171(3):481–489. doi: 10.1677/joe.0.1710481. [DOI] [PubMed] [Google Scholar]
- 223.Hansen BS, Raun K, Nielsen KK, et al. Pharmacological characterisation of a new oral GH secretagogue, NN703. European Journal of Endocrinology. 1999;141(2):180–189. doi: 10.1530/eje.0.1410180. [DOI] [PubMed] [Google Scholar]
- 224.Garcia JM, Polvino WJ. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Hormone and IGF Research. 2009;19(3):267–273. doi: 10.1016/j.ghir.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 225.Matsuda K, Miura T, Kaiya H, et al. Regulation of food intake by acyl and des-acyl ghrelins in the goldfish. Peptides. 2006;27(9):2321–2325. doi: 10.1016/j.peptides.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 226.Kaplan SL, Grumbach MM, Aubert ML. The ontogenesis of pituitary hormones and hypothalamic factors in the human fetus: maturation of central nervous system regulation of anterior pituitary function. Recent Progress in Hormone Research. 1976;32:161–243. doi: 10.1016/b978-0-12-571132-6.50015-4. [DOI] [PubMed] [Google Scholar]
- 227.Yan M, Hernandez M, Xu R, Chen C. Effect of GHRH and GHRP-2 treatment in vitro on GH secretion and levels of GH, pituitary transcription factor-1, GHRH-receptor, GH-secretagogue-receptor and somatostatin receptor mRNAs in ovine pituitary cells. European Journal of Endocrinology. 2004;150(2):235–242. doi: 10.1530/eje.0.1500235. [DOI] [PubMed] [Google Scholar]
- 228.Li X, He J, Hu W, Yin Z. The essential role of endogenous ghrelin in growth hormone expression during zebrafish adenohypophysis development. Endocrinology. 2009;150(6):2767–2774. doi: 10.1210/en.2008-1398. [DOI] [PubMed] [Google Scholar]
- 229.Torsello A, Luoni M, Grilli R, et al. Hexarelin stimulation of growth hormone release and mRNA levels in an infant and adult rat model of impaired GHRH function. Neuroendocrinology. 1997;65(2):91–97. doi: 10.1159/000127168. [DOI] [PubMed] [Google Scholar]
- 230.Bailey ART, Giles ME, Brown CH, et al. Chronic central infusion of growth hormone secretagogues: effects on Fos expression and peptide gene expression in the rat arcuate nucleus. Neuroendocrinology. 1999;70(2):83–92. doi: 10.1159/000054462. [DOI] [PubMed] [Google Scholar]
- 231.Mondal MS, Date Y, Yamaguchi H, et al. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regulatory Peptides. 2005;126(1-2):55–59. doi: 10.1016/j.regpep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 232.Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 233.Lu S, Guan J-L, Wang Q-P, et al. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neuroscience Letters. 2002;321(3):157–160. doi: 10.1016/s0304-3940(01)02544-7. [DOI] [PubMed] [Google Scholar]
- 234.Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. American Journal of Physiology. 2005;289(2):R353–R358. doi: 10.1152/ajpregu.00756.2004. [DOI] [PubMed] [Google Scholar]
- 235.Hori Y, Kageyama H, Guan J-L, et al. Synaptic interaction between ghrelin- and ghrelin-containing neurons in the rat hypothalamus. Regulatory Peptides. 2008;145(1–3):122–127. doi: 10.1016/j.regpep.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 236.Mano-Otagiri A, Nemoto T, Sekino A, et al. Growth hormone-releasing hormone (GHRH) neurons in the arcuate nucleus (Arc) of the hypothalamus are decreased in transgenic rats whose expression of ghrelin receptor is attenuated: evidence that ghrelin receptor is involved in the up-regulation of GHRH expression in the ARC. Endocrinology. 2006;147(9):4093–4103. doi: 10.1210/en.2005-1619. [DOI] [PubMed] [Google Scholar]
- 237.Fletcher TP, Thomas GB, Clarke IJ. Growth hormone-releasing hormone and somatostatin concentrations in the hypophysial portal blood of conscious sheep during the infusion of growth hormone-releasing peptide-6. Domestic Animal Endocrinology. 1996;13(3):251–258. doi: 10.1016/0739-7240(96)00017-3. [DOI] [PubMed] [Google Scholar]
- 238.Wren AM, Small CJ, Fribbens CV, et al. The hypothalamic mechanisms of the hypophysiotropic action of ghrelin. Neuroendocrinology. 2002;76(5):316–324. doi: 10.1159/000066629. [DOI] [PubMed] [Google Scholar]
- 239.Alba M, Fintini D, Bowers CY, Parlow AF, Salvatori R. Effects of long-term treatment with growth hormone-releasing peptide-2 in the GHRH knockout mouse. American Journal of Physiology. 2005;289(5):E762–E767. doi: 10.1152/ajpendo.00203.2005. [DOI] [PubMed] [Google Scholar]
- 240.Roh S-G, Chen C, Choi K-C, Shrestha Y, Sasaki S-I. Is GHRH receptor essential to GHRP-2-induced GH secretion in primary cultured rat pituitary cells? Endocrinology. 2002;143(5):1964–1967. doi: 10.1210/endo.143.5.8893. [DOI] [PubMed] [Google Scholar]
- 241.Roelfsema F, Biermasz NR, Veldman RG, et al. Growth hormone (GH) secretion in patients with an inactivating defect of the GH-releasing hormone (GHRH) receptor is pulsatile: evidence for a role for non-GHRH inputs into the generation of GH pulses. The Journal of Clinical Endocrinology & Metabolism. 2001;86(6):2459–2464. doi: 10.1210/jcem.86.6.7536. [DOI] [PubMed] [Google Scholar]
- 242.Popovic V, Damjanovic S, Micic D, Djurovic M, Dieguez C, Casanueva FF. Blocked growth hormone-releasing peptide (GHRP-6)-induced GH secretion and absence of the synergic action of GHRP-6 plus GH-releasing hormone in patients with hypothalamopituitary disconnection: evidence that GHRP-6 main action is exerted at the hypothalamic level. The Journal of Clinical Endocrinology & Metabolism. 1995;80(3):942–947. doi: 10.1210/jcem.80.3.7883854. [DOI] [PubMed] [Google Scholar]
- 243.Fintini D, Alba M, Schally AV, Bowers CY, Parlow AF, Salvatori R. Effects of combined long-term treatment with a growth hormone-releasing hormone analogue and a growth hormone secretagogue in the growth hormone-releasing hormone knock out mouse. Neuroendocrinology. 2005;82(3-4):198–207. doi: 10.1159/000092520. [DOI] [PubMed] [Google Scholar]
- 244.Dickson SL, Doutrelant-Viltart O, Leng G. GH-deficient dw/dw rats and lit/lit mice show increased Fos expression in the hypothalamic arcuate nucleus following systemic injection of GH-releasing peptide-6. Journal of Endocrinology. 1995;146(3):519–526. doi: 10.1677/joe.0.1460519. [DOI] [PubMed] [Google Scholar]
- 245.Maghnie M, Pennati MC, Civardi E, et al. GH response to ghrelin in subjects with congenital GH deficiency: evidence that ghrelin action requires hypothalamic-pituitary connections. European Journal of Endocrinology. 2007;156(4):449–454. doi: 10.1530/EJE-06-0642. [DOI] [PubMed] [Google Scholar]
- 246.Maheshwari HG, Rahim A, Shalet SM, Baumann G. Selective lack of growth hormone (GH) response to the GH-releasing peptide hexarelin in patients with GH-releasing hormone receptor deficiency. The Journal of Clinical Endocrinology & Metabolism. 1999;84(3):956–959. doi: 10.1210/jcem.84.3.5523. [DOI] [PubMed] [Google Scholar]
- 247.Pandya N, DeMott-Friberg R, Bowers CY, Barkan AL, Jaffe CA. Growth hormone (GH)-releasing peptide-6 requires endogenous hypothalamic GH-releasing hormone for maximal GH stimulation. The Journal of Clinical Endocrinology & Metabolism. 1998;83(4):1186–1189. doi: 10.1210/jcem.83.4.4711. [DOI] [PubMed] [Google Scholar]
- 248.Hickey GJ, Drisko J, Faidley T, et al. Mediation by the central nervous system is critical to the in vivo activity of the GH secretagogue L-692,585. Journal of Endocrinology. 1996;148(2):371–380. doi: 10.1677/joe.0.1480371. [DOI] [PubMed] [Google Scholar]
- 249.ThidarMyint H, Yoshida H, Ito T, He M, Inoue H, Kuwayama H. Combined administration of ghrelin and GHRH synergistically stimulates GH release in Holstein preweaning calves. Domestic Animal Endocrinology. 2008;34(1):118–123. doi: 10.1016/j.domaniend.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 250.Bowers CY, Laferrere B, Hurley DL, Veldhuis JD. The role of growth hormone and ghrelin in feeding and body composition. In: Donahoe PA, editor. Energy Metabolism and Obesity: Research and Clinical Applications. Totowa, NJ, USA: The Humana Press; 2008. pp. 125–154. [Google Scholar]
- 251.Arvat E, Maccario M, Di Vito L, et al. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. The Journal of Clinical Endocrinology & Metabolism. 2001;86(3):1169–1174. doi: 10.1210/jcem.86.3.7314. [DOI] [PubMed] [Google Scholar]
- 252.Bowers CY, Reynolds GA, Durham D, Barrera CM, Pezzoli SS, Thorner MO. Growth hormone (GH)-releasing peptide stimulates GH release in normal men and acts synergistically with GH-releasing hormone. The Journal of Clinical Endocrinology & Metabolism. 1990;70(4):975–982. doi: 10.1210/jcem-70-4-975. [DOI] [PubMed] [Google Scholar]
- 253.Bowers CY, Granda-Ayala R. Growth hormone/insulin-like growth factor-1 response to acute and chronic growth hormone-releasing peptide-2, growth hormone-releasing hormone 1-44NH2 and in combination in older men and women with decreased growth hormone secretion. Endocrine. 2001;14(1):79–86. doi: 10.1385/ENDO:14:1:079. [DOI] [PubMed] [Google Scholar]
- 254.Hataya Y, Akamizu T, Takaya K, et al. A low dose of ghrelin stimulates growth hormone (GH) release synergistically with GH-releasing hormone in humans. The Journal of Clinical Endocrinology & Metabolism. 2001;86(9):4552–4555. doi: 10.1210/jcem.86.9.8002. [DOI] [PubMed] [Google Scholar]
- 255.Bowers CY, Momany FA, Reynolds GA, Hong A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology. 1984;114(5):1537–1545. doi: 10.1210/endo-114-5-1537. [DOI] [PubMed] [Google Scholar]
- 256.Rico M, Rueda V, Lorenzo MT, Núñez A, De la Cruz LF. Effect of growth hormone-releasing peptide 1–6 on GH secretion-stimulated by GHRH and pyridostigmine in lambs. Journal of Physiology and Biochemistry. 1998;54(2):67–76. [PubMed] [Google Scholar]
- 257.Bowers CY, Veeraragavan K, Sethumadhavan K. Atypical growth hormone releasing peptides. In: Bercu BB, Walker RF, editors. Growth Hormone. II. Basic and Clinical Aspects. New York, NY, USA: Springer; 1994. pp. 203–222. [Google Scholar]
- 258.Wells T, Flavell DM, Wells SE, Carmignac DF, Robinson ICAF. Effects of growth hormone secretagogues in the transgenic growth-retarded (Tgr) rat. Endocrinology. 1997;138(2):580–587. doi: 10.1210/endo.138.2.4917. [DOI] [PubMed] [Google Scholar]
- 259.Jimenez-Reina L, Canete R, de la Torre MJ, Bernal G. Influence of chronic treatment with the growth hormone secretagogue Ipamorelin, in young female rats: somatotroph response in vitro. Histology and Histopathology. 2002;17(3):707–714. doi: 10.14670/HH-17.707. [DOI] [PubMed] [Google Scholar]
- 260.Russell-Jones DL, Umpleby M. Protein anabolic action of insulin, growth hormone and insulin-like growth factor I. European Journal of Endocrinology. 1996;135(6):631–642. doi: 10.1530/eje.0.1350631. [DOI] [PubMed] [Google Scholar]
- 261.Arvat E, Maccagno B, Ramunni J, et al. Influence of galanin and serotonin on the endocrine response to Hexarelin, a synthetic peptidyl GH-secretagogue, in normal women. Journal of Endocrinological Investigation. 1998;21(10):673–679. doi: 10.1007/BF03350797. [DOI] [PubMed] [Google Scholar]
- 262.Davis TME, Burrin JM, Bloom SR. Growth hormone (GH) release in response to GH-releasing hormone in man in 3-fold enhanced by galanin. The Journal of Clinical Endocrinology & Metabolism. 1987;65(6):1248–1252. doi: 10.1210/jcem-65-6-1248. [DOI] [PubMed] [Google Scholar]
- 263.Kitajima N, Chihara K, Abe H, Okimura Y, Shakutsui S. Galanin stimulates immunoreactive growth hormone-releasing factor secretion from rat hypothalamic slices perifused in vitro. Life Sciences. 1990;47(25):2371–2376. doi: 10.1016/0024-3205(90)90277-x. [DOI] [PubMed] [Google Scholar]
- 264.Liposits Z, Merchenthaler I, Reid JJ, Negro-Vilar A. Galanin-immunoreactive axons innervate somatostatin-synthesizing neurons in the anterior periventricular nucleus of the rat. Endocrinology. 1993;132(2):917–923. doi: 10.1210/endo.132.2.7678803. [DOI] [PubMed] [Google Scholar]
- 265.Delemarre-van De Waal HA, Burton KA, Kabigting EB, Steiner RA, Clifton DK. Expression and sexual dimorphism of galanin messenger ribonucleic acid in growth hormone-releasing hormone neurons of the rat during development. Endocrinology. 1994;134(2):665–671. doi: 10.1210/endo.134.2.7507832. [DOI] [PubMed] [Google Scholar]
- 266.Maiter DM, Hooi SC, Koenig JI, Martin JB. Galanin is a physiological regulator of spontaneous pulsatile secretion of growth hormone in the male rat. Endocrinology. 1990;126(2):1216–1222. doi: 10.1210/endo-126-2-1216. [DOI] [PubMed] [Google Scholar]
- 267.Cremagnani L, Vaccari M, Maronati E, et al. Potentiating effect of galanin on GHRH-induced GH release. Comparison between old and young subjects. Hormone and Metabolic Research. 1996;28(2):101–104. doi: 10.1055/s-2007-979137. [DOI] [PubMed] [Google Scholar]
- 268.Yagi H, Kaji H, Sato M, Okimura Y, Chihara K. Effect of intravenous or intracerebroventricular injections of His-D-Trp-Ala-Trp-D-Phe-Lys-NH2 on GH release in conscious, freely moving male rats. Neuroendocrinology. 1996;63(2):198–206. doi: 10.1159/000126958. [DOI] [PubMed] [Google Scholar]
- 269.Chapman IM, Bach MA, Van Cauter E, et al. Stimulation of the growth hormone (GH)-insulin-like growth factor I axis by daily oral administration of a GH secretogogue (MK-677) in healthy elderly subjects. The Journal of Clinical Endocrinology & Metabolism. 1996;81(12):4249–4257. doi: 10.1210/jcem.81.12.8954023. [DOI] [PubMed] [Google Scholar]
- 270.Veldhuis JD, Reynolds GA, Iranmanesh A, Bowers CY. Twenty-four hour continuous ghrelin infusion augments physiologically pulsatile, nycthemeral, and entropic (feedback-regulated) modes of growth hormone secretion. The Journal of Clinical Endocrinology & Metabolism. 2008;93(9):3597–3603. doi: 10.1210/jc.2008-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bowers CY, Granda R, Mohan S, Kuipers J, Baylink D, Veldhuis JD. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. The Journal of Clinical Endocrinology & Metabolism. 2004;89(5):2290–2300. doi: 10.1210/jc.2003-031799. [DOI] [PubMed] [Google Scholar]
- 272.Rahim A, O’Neill PA, Shalet SM. The effect of chronic hexarelin administration on the pituitary-adrenal axis and prolactin. Clinical Endocrinology. 1999;50(1):77–84. doi: 10.1046/j.1365-2265.1999.00609.x. [DOI] [PubMed] [Google Scholar]
- 273.Frieboes R-M, Murck H, Antonijevic IA, Steiger A. Effects of growth hormone-releasing peptide-6 on the nocturnal secretion of GH, ACTH and cortisol and on the sleep EEG in man: role of routes of administration. Journal of Neuroendocrinology. 1999;11(6):473–478. doi: 10.1046/j.1365-2826.1999.00364.x. [DOI] [PubMed] [Google Scholar]
- 274.Arvat E, Maccagno B, Ramunni J, et al. Hexarelin, a synthetic growth-hormone releasing peptide, shows no interaction with corticotropin-releasing hormone and vasopressin on adrenocorticotropin and cortisol secretion in humans. Neuroendocrinology. 1997;66(6):432–438. doi: 10.1159/000127269. [DOI] [PubMed] [Google Scholar]
- 275.Arvat E, Di Vito L, Maccagno B, et al. Effects of GHRP-2 and hexarelin, two synthetic GH-releasing peptides, on GH, prolactin, ACTH and cortisol levels in man. Comparison with the effects of GHRH, TRH and hCRH. Peptides. 1997;18(6):885–891. doi: 10.1016/s0196-9781(97)00016-8. [DOI] [PubMed] [Google Scholar]
- 276.Anderson LL, Jeftinija S, Scanes CG, et al. Physiology of ghrelin and related peptides. Domestic Animal Endocrinology. 2005;29(1):111–144. doi: 10.1016/j.domaniend.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 277.Frieboes R-M, Murck H, Maier P, Schier T, Holsboer F, Steiger A. Growth hormone-releasing peptide-6 stimulates sleep, growth hormone, ACTH and cortisol release in normal man. Neuroendocrinology. 1995;61(5):584–589. doi: 10.1159/000126883. [DOI] [PubMed] [Google Scholar]
- 278.Johnstone LE, Srisawat R, Kumarnsit E, Leng G. Hypothalamic expression of NPY mRNA, vasopressin mRNA and CRF mRNA in response to food restriction and central administration of the orexigenic peptide GHRP-6. Stress. 2005;8(1):59–67. doi: 10.1080/10253890500095283. [DOI] [PubMed] [Google Scholar]
- 279.Lucidi P, Murdolo G, Di Loreto C, et al. Metabolic and endocrine effects of physiological increments in plasma ghrelin concentrations. Nutrition, Metabolism and Cardiovascular Diseases. 2005;15(6):410–417. doi: 10.1016/j.numecd.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 280.Maccario M, Veldhuis JD, Broglio F, et al. Impact of two or three daily subcutaneous injections of hexarelin, a synthetic growth hormone (GH) secretagogue, on 24-h GH, prolactin, adrenocorticotropin and cortisol secretion in humans. European Journal of Endocrinology. 2002;146(3):310–318. doi: 10.1530/eje.0.1460310. [DOI] [PubMed] [Google Scholar]
- 281.Tassone F, Broglio F, Destefanis S, et al. Neuroendocrine and metabolic effects of acute ghrelin administration in human obesity. The Journal of Clinical Endocrinology & Metabolism. 2003;88(11):5478–5483. doi: 10.1210/jc.2003-030564. [DOI] [PubMed] [Google Scholar]
- 282.Yu H, Kim K. Direct nose-to-brain transfer of a growth hormone releasing neuropeptide, hexarelin after intranasal administration to rabbits. International Journal of Pharmaceutics. 2009;378(1-2):73–79. doi: 10.1016/j.ijpharm.2009.05.057. [DOI] [PubMed] [Google Scholar]
- 283.Moulin A, Ryan J, Martinez J, Fehrentz J-A. Recent developments in ghrelin receptor ligands. ChemMedChem. 2007;2(9):1242–1259. doi: 10.1002/cmdc.200700015. [DOI] [PubMed] [Google Scholar]
- 284.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. Journal of Pharmacology and Experimental Therapeutics. 2002;302(2):822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 285.Muccioli G, Tschop M, Papotti M, Deghenghi R, Heiman M, Ghigo E. Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. European Journal of Pharmacology. 2002;440(2-3):235–254. doi: 10.1016/s0014-2999(02)01432-2. [DOI] [PubMed] [Google Scholar]
- 286.Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27(4):911–916. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 287.Klinger B, Silbergeld A, Deghenghi R, Frenkel J, Laron Z. Desensitization from long-term intranasal treatment with hexarelin does not interfere with the biological effects of this growth hormone-releasing peptide in short children. European Journal of Endocrinology. 1996;134(6):716–719. doi: 10.1530/eje.0.1340716. [DOI] [PubMed] [Google Scholar]
- 288.Mericq V, Cassorla F, Salazar T, et al. Effects of eight months treatment with graded doses of a growth hormone (GH)-releasing peptide in GH-deficient children. The Journal of Clinical Endocrinology & Metabolism. 1998;83(7):2355–2360. doi: 10.1210/jcem.83.7.4969. [DOI] [PubMed] [Google Scholar]
- 289.Pihoker C, Badger TM, Reynolds GA, Bowers CY. Treatment effects of intranasal growth hormone releasing peptide-2 in children with short stature. Journal of Endocrinology. 1997;155(1):79–86. doi: 10.1677/joe.0.1550079. [DOI] [PubMed] [Google Scholar]
- 290.Laron Z, Frenkel J, Deghenghi R, Anin S, Klinger B, Silbergeld A. Intranasal administration of the GHRP hexarelin accelerates growth in short children. Clinical Endocrinology. 1995;43(5):631–635. doi: 10.1111/j.1365-2265.1995.tb02929.x. [DOI] [PubMed] [Google Scholar]
- 291.Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Annals of Internal Medicine. 2008;149(9):601–611. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ariyasu H, Iwakura H, Yamada G, Nakao K, Kangawa K, Akamizu T. Efficacy of ghrelin as a therapeutic approach for age-related physiological changes. Endocrinology. 2008;149(7):3722–3728. doi: 10.1210/en.2007-1650. [DOI] [PubMed] [Google Scholar]
- 293.Asakawa A, Inui A, Fujimiya M, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54(1):18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kohno D, Sone H, Minokoshi Y, Yada T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochemical and Biophysical Research Communications. 2008;366(2):388–392. doi: 10.1016/j.bbrc.2007.11.166. [DOI] [PubMed] [Google Scholar]
- 295.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26(11):2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 296.Nagaya N, Miyatake K, Uematsu M, et al. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. The Journal of Clinical Endocrinology & Metabolism. 2001;86(12):5854–5859. doi: 10.1210/jcem.86.12.8115. [DOI] [PubMed] [Google Scholar]
- 297.Nagaya N, Kojima M, Kangawa K. Ghrelin, a novel growth hormone-releasing peptide, in the treatment of cardiopulmonary-associated cachexia. Internal Medicine. 2006;45(3):127–134. doi: 10.2169/internalmedicine.45.1402. [DOI] [PubMed] [Google Scholar]
- 298.Neary NM, Small CJ, Wren AM, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. The Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2832–2836. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 299.Nagaya N, Kangawa K. Ghrelin improves left ventricular dysfunction and cardiac cachexia in heart failure. Current Opinion in Pharmacology. 2003;3(2):146–151. doi: 10.1016/s1471-4892(03)00013-4. [DOI] [PubMed] [Google Scholar]
- 300.Nagaya N, Itoh T, Murakami S, et al. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128(3):1187–1193. doi: 10.1378/chest.128.3.1187. [DOI] [PubMed] [Google Scholar]
- 301.Nagaya N, Uematsu M, Kojima M, et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104(12):1430–1435. doi: 10.1161/hc3601.095575. [DOI] [PubMed] [Google Scholar]
- 302.Vestergaard ET, Hansen TK, Gormsen LC, et al. Constant intravenous ghrelin infusion in healthy young men: clinical pharmacokinetics and metabolic effects. American Journal of Physiology. 2007;292(6):E1829–E1836. doi: 10.1152/ajpendo.00682.2006. [DOI] [PubMed] [Google Scholar]
- 303.Tschop M, Statnick MA, Suter TM, Heiman ML. GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology. 2002;143(2):558–568. doi: 10.1210/endo.143.2.8633. [DOI] [PubMed] [Google Scholar]
- 304.Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural gh secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. The Journal of Clinical Endocrinology & Metabolism. 2001;86(10):5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 305.Zhang W, Chai B, Li J-Y, Wang H, Mulholland MW. Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology. 2008;149(9):4710–4716. doi: 10.1210/en.2008-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Vestergaard ET, Gormsen LC, Jessen N, et al. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes. 2008;57(12):3205–3210. doi: 10.2337/db08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Broglio F, Prodam F, Riganti F, et al. The continuous infusion of acylated ghrelin enhances growth hormone secretion and worsens glucose metabolism in humans. Journal of Endocrinological Investigation. 2008;31(9):788–794. doi: 10.1007/BF03349259. [DOI] [PubMed] [Google Scholar]
- 308.Duxbury MS, Waseem T, Ito H, et al. Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochemical and Biophysical Research Communications. 2003;309(2):464–468. doi: 10.1016/j.bbrc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 309.Delhanty PJD, Van Koetsveld PM, Gauna C, et al. Ghrelin and its unacylated isoform stimulate the growth of adrenocortical tumor cells via an anti-apoptotic pathway. American Journal of Physiology. 2007;293(1):E302–E309. doi: 10.1152/ajpendo.00377.2006. [DOI] [PubMed] [Google Scholar]
- 310.Kheradmand A, Roshangar L, Taati M. The role of ghrelin on the morphometry and intracellular changes in the rat testis. Tissue and Cell. 2009;41(2):105–111. doi: 10.1016/j.tice.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 311.Sugino T, Yamaura J, Yamagishi M, et al. Involvement of cholinergic neurons in the regulation of the ghrelin secretory response to feeding in sheep. Biochemical and Biophysical Research Communications. 2003;304(2):308–312. doi: 10.1016/s0006-291x(03)00593-x. [DOI] [PubMed] [Google Scholar]
- 312.Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50(7–12):2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 313.Wren AM, Small CJ, Ward HL, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141(11):4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 314.Lawrence CB, Snape AC, Baudoin FM-H, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143(1):155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 315.Kaiya H, Furuse M, Miyazato M, Kangawa K. Current knowledge of the roles of ghrelin in regulating food intake and energy balance in birds. General and Comparative Endocrinology. 2009;163(1-2):33–38. doi: 10.1016/j.ygcen.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 316.Blum ID, Patterson Z, Khazall R, et al. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164(2):351–359. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Chen X, Ge Y-L, Jiang Z-Y, Liu C-Q, Depoortere I, Peeters TL. Effects of ghrelin on hypothalamic glucose responding neurons in rats. Brain Research. 2005;1055(1-2):131–136. doi: 10.1016/j.brainres.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 318.Dickson SL, Luckman SM. Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology. 1997;138(2):771–777. doi: 10.1210/endo.138.2.4907. [DOI] [PubMed] [Google Scholar]
- 319.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50(7–12):2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 320.Kohno D, Gao H-Z, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52(4):948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- 321.Chen HY, Trumbauer ME, Chen AS, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145(6):2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 322.Keen-Rhinehart E, Bartness TJ. NPY Y1 receptor is involved in ghrelin- and fasting-induced increases in foraging, food hoarding, and food intake. American Journal of Physiology. 2007;292(4):R1728–R1737. doi: 10.1152/ajpregu.00597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Nogueiras R, Tovar S, Mitchell SE, et al. Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes. 2004;53(10):2552–2558. doi: 10.2337/diabetes.53.10.2552. [DOI] [PubMed] [Google Scholar]
- 324.Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Annals of the New York Academy of Sciences. 2003;994:175–186. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- 325.Bray GA. Afferent signals regulating food intake. Proceedings of the Nutrition Society. 2000;59(3):373–384. doi: 10.1017/s0029665100000422. [DOI] [PubMed] [Google Scholar]
- 326.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutrition, Metabolism and Cardiovascular Diseases. 2008;18(2):158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 327.Villanueva EC, Munzberg H, Cota D, et al. Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology. 2009;150(10):4541–4551. doi: 10.1210/en.2009-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Frontiers in Neuroendocrinology. 2008;29(1):70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 329.Toshinai K, Date Y, Murakami N, et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144(4):1506–1512. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- 330.Kola B, Hubina E, Tucci SA, et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. Journal of Biological Chemistry. 2005;280(26):25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- 331.Osto M, Wielinga PY, Alder B, Walser N, Lutz TA. Modulation of the satiating effect of amylin by central ghrelin, leptin and insulin. Physiology and Behavior. 2007;91(5):566–572. doi: 10.1016/j.physbeh.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 332.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. Journal of Neuroscience. 2006;26(43):11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochemical and Biophysical Research Communications. 2001;280(3):904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- 334.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123(4):1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 335.Kawahara Y, Kawahara H, Kaneko F, et al. Peripherally administered ghrelin induces bimodal effects on the mesolimbic dopamine system depending on food-consumptive states. Neuroscience. 2009;161(3):855–864. doi: 10.1016/j.neuroscience.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 336.Le Roux CW, Neary NM, Halsey TJ, et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. The Journal of Clinical Endocrinology & Metabolism. 2005;90(8):4521–4524. doi: 10.1210/jc.2004-2537. [DOI] [PubMed] [Google Scholar]
- 337.Malcher-Lopes R, Di S, Marcheselli VS, et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. Journal of Neuroscience. 2006;26(24):6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Tucci SA, Rogers EK, Korbonits M, Kirkham TC. The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. British Journal of Pharmacology. 2004;143(5):520–523. doi: 10.1038/sj.bjp.0705968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Degen L, Drewe J, Piccoli F, et al. Effect of CCK-1 receptor blockade on ghrelin and PYY secretion in men. American Journal of Physiology. 2007;292(4):R1391–R1399. doi: 10.1152/ajpregu.00734.2006. [DOI] [PubMed] [Google Scholar]
- 340.Lippl F, Kircher F, Erdmann J, Allescher H-D, Schusdziarra V. Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regulatory Peptides. 2004;119(1-2):93–98. doi: 10.1016/j.regpep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 341.Dixit VD, Yang H, Cooper-Jenkins A, Giri BB, Patel K, Taub DD. Reduction of T cell-derived ghrelin enhances proinflammatory cytokine expression: implications for age-associated increases in inflammation. Blood. 2009;113(21):5202–5205. doi: 10.1182/blood-2008-09-181255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Koda S, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146(5):2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 343.Date Y, Toshinai K, Koda S, et al. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146(8):3518–3525. doi: 10.1210/en.2004-1240. [DOI] [PubMed] [Google Scholar]
- 344.Wu R, Zhou M, Dong W, et al. Ghrelin hyporesponsiveness contributes to age-related hyperinflammation in septic shock. Annals of Surgery. 2009;250(1):126–133. doi: 10.1097/SLA.0b013e3181ad85d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Gao X-B. Electrophysiological effects of MCH on neurons in the hypothalamus. Peptides. 2009;30(11):2025–2030. doi: 10.1016/j.peptides.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Xin Z, Serby MD, Zhao H, et al. Discovery and pharmacological evaluation of growth hormone secretagogue receptor antagonists. Journal of Medicinal Chemistry. 2006;49(15):4459–4469. doi: 10.1021/jm060461g. [DOI] [PubMed] [Google Scholar]
- 347.Salome N, Haage D, Perrissoud D, et al. Anorexigenic and electrophysiological actions of novel ghrelin receptor (GHS-R1A) antagonists in rats. European Journal of Pharmacology. 2009;612(1–3):167–173. doi: 10.1016/j.ejphar.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 348.Wang L, Martinez V, Rivier JE, Taché Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. American Journal of Physiology. 2001;281(5):R1401–R1410. doi: 10.1152/ajpregu.2001.281.5.R1401. [DOI] [PubMed] [Google Scholar]
- 349.Cunha SR, Mayo KE. Ghrelin and growth hormone (GH) secretagogues potentiate GH-releasing hormone (GHRH)-induced cyclic adenosine 3′,5′-monophosphate production in cells expressing transfected GHRH and GH secretagogue receptors. Endocrinology. 2002;143(12):4570–4582. doi: 10.1210/en.2002-220670. [DOI] [PubMed] [Google Scholar]
- 350.Xu R, Zhao Y, Chen C. Growth hormone-releasing peptide-2 reduces inward rectifying K+ currents via a PKA-cAMP-mediated signalling pathway in ovine somatoropes. Journal of Physiology. 2002;545(2):421–433. doi: 10.1113/jphysiol.2002.030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Chen C, Clarke IJ. Effects of growth hormone-releasing peptide-2 (GHRP-2) on membrane Ca2+ permeability in cultured ovine somatotrophs. Journal of Neuroendocrinology. 1995;7(3):179–186. doi: 10.1111/j.1365-2826.1995.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 352.Kuramochi M, Kohno D, Onaka T, Kato S, Yada T. Galanin-like peptide and ghrelin increase cytosolic Ca2+ in neurons containing growth hormone-releasing hormone in the arcuate nucleus. Regulatory Peptides. 2005;126(1-2):85–89. doi: 10.1016/j.regpep.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 353.Shioda S, Takenoya F, Yagi M, Wang L, Hori Y, Kageyama H. Neural networks of several novel neuropeptides involved in feeding regulation. Nutrition. 2008;24(9):848–853. doi: 10.1016/j.nut.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 354.Johansson A, Fredriksson R, Winnergren S, Hulting A-L, Schioth HB, Lindblom J. The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides. 2008;29(9):1588–1595. doi: 10.1016/j.peptides.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 355.Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell. 2007;129(2):251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Hu Z, Seung HC, van Haasteren G, Wang J, Lane MD. Effect of centrally administered C75, a fatty acid synthase inhibitor, on ghrelin secretion and its downstream effects. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(11):3972–3977. doi: 10.1073/pnas.0500619102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Tung YCL, Hewson AK, Carter RN, Dickson SL. Central responsiveness to a grelin mimetic (GHRP-6) is rapidly altered by acute changes in nutritional status in rats. Journal of Neuroendocrinology. 2005;17(6):387–393. doi: 10.1111/j.1365-2826.2005.01316.x. [DOI] [PubMed] [Google Scholar]
- 358.Tups A, Helwig M, Khorooshi RMH, Archer ZA, Klingensport M, Mercer JG. Circulating ghrelin levels and central ghrelin receptor expression are elevated in response to food deprivation in a seasonal mammal (Phodopus sungorus) Journal of Neuroendocrinology. 2004;16(11):922–928. doi: 10.1111/j.1365-2826.2004.01251.x. [DOI] [PubMed] [Google Scholar]
- 359.Yi C-X, van der Vliet J, Dai J, Yin G, Ru L, Buijs RM. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology. 2006;147(1):283–294. doi: 10.1210/en.2005-1051. [DOI] [PubMed] [Google Scholar]
- 360.Tamura H, Kamegai J, Shimizu T, Ishii S, Sugihara H, Oikawa S. Ghrelin stimulates GH but not food intake in arcuate nucleus ablated rats. Endocrinology. 2002;143(9):3268–3275. doi: 10.1210/en.2002-220268. [DOI] [PubMed] [Google Scholar]
- 361.Seoane LM, Lopez M, Tovar S, Casanueva FF, Senaris R, Dieguez C. Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology. 2003;144(2):544–551. doi: 10.1210/en.2002-220795. [DOI] [PubMed] [Google Scholar]
- 362.Riediger T, Bothe C, Becskei C, Lutz TA. Peptide YY directly inhibits ghrelin-activated neurons of the arcuate nucleus and reverses fasting-induced c-Fos expression. Neuroendocrinology. 2004;79(6):317–326. doi: 10.1159/000079842. [DOI] [PubMed] [Google Scholar]
- 363.Patterson M, Murphy KG, Patel SR, et al. Hypothalamic injection of oxyntomodulin suppresses circulating ghrelin-like immunoreactivity. Endocrinology. 2009;150(8):3513–3520. doi: 10.1210/en.2008-0796. [DOI] [PubMed] [Google Scholar]
- 364.Monti V, Carlson JJ, Hunt SC, Adams TD. Relationship of ghrelin and leptin hormones with body mass index and waist circumference in a random sample of adults. Journal of the American Dietetic Association. 2006;106(6):822–828. doi: 10.1016/j.jada.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 365.Ida T, Mori K, Miyazato M, et al. Neuromedin S is a novel anorexigenic hormone. Endocrinology. 2005;146(10):4217–4223. doi: 10.1210/en.2005-0107. [DOI] [PubMed] [Google Scholar]
- 366.Kurose Y, Iqbal J, Rao A, et al. Changes in expression of the genes for the leptin receptor and the growth hormone-releasing peptide/ghrelin receptor in the hypothalamic arcuate nucleus with long-term manipulation of adiposity by dietary means. Journal of Neuroendocrinology. 2005;17(6):331–340. doi: 10.1111/j.1365-2826.2005.01318.x. [DOI] [PubMed] [Google Scholar]
- 367.Kobelt P, Tebbe JJ, Tjandra I, et al. CCK inhibits the orexigenic effect of peripheral ghrelin. American Journal of Physiology. 2005;288(3):R751–R758. doi: 10.1152/ajpregu.00094.2004. [DOI] [PubMed] [Google Scholar]
- 368.Kobelt P, Goebel M, Stengel A, et al. Bombesin, but not amylin, blocks the orexigenic effect of peripheral ghrelin. American Journal of Physiology. 2006;291(4):R903–R913. doi: 10.1152/ajpregu.00681.2005. [DOI] [PubMed] [Google Scholar]
- 369.Buyse J, Janssen S, Geelissen S, et al. Ghrelin modulates fatty acid synthase and related transcription factor mRNA levels in a tissue-specific manner in neonatal broiler chicks. Peptides. 2009;30(7):1342–1347. doi: 10.1016/j.peptides.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 370.De Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. Journal of Neuroscience. 2007;27(11):2876–2882. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Shimizu H, Oh-I S, Hashimoto K, et al. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150(2):662–671. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 372.Toshinai K, Mondal MS, Shimbara T, et al. Ghrelin stimulates growth hormone secretion and food intake in aged rats. Mechanisms of Ageing and Development. 2007;128(2):182–186. doi: 10.1016/j.mad.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 373.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essentials for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 374.Solomon A, De Fanti BA, Martínez JA. Peripheral ghrelin interacts with orexin neurons in glucostatic signalling. Regulatory Peptides. 2007;144(1–3):17–24. doi: 10.1016/j.regpep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 375.Akimoto-Takano S, Sakurai C, Kanai S, Hosoya H, Ohta M, Miyasaka K. Differences in the appetite-stimulating effect of orexin, neuropeptide Y and ghrelin among young, adult and old rats. Neuroendocrinology. 2006;82(5-6):256–263. doi: 10.1159/000092754. [DOI] [PubMed] [Google Scholar]
- 376.Shrestha YB, Wickwire K, Giraudo S. Effect of reducing hypothalamic ghrelin receptor gene expression on energy balance. Peptides. 2009;30(7):1336–1341. doi: 10.1016/j.peptides.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Shimomura Y, Harada M, Goto M, et al. Identification of neuropeptide W as the endogenous ligand for orphan G-protein-coupled receptors GPR7 and GPR8. Journal of Biological Chemistry. 2002;277(39):35826–35832. doi: 10.1074/jbc.M205337200. [DOI] [PubMed] [Google Scholar]
- 378.Takenoya F, Kitamura S, Kageyama H, et al. Neuronal interactions between neuropeptide W- and orexin- or melanin-concentrating hormone-containing neurons in the rat hypothalamus. Regulatory Peptides. 2008;145(1–3):159–164. doi: 10.1016/j.regpep.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 379.Acuna-Goycolea C, van den Pol AN. Neuroendocrine proopiomelanocortin neurons are excited by hypocretin/orexin. Journal of Neuroscience. 2009;29(5):1503–1513. doi: 10.1523/JNEUROSCI.5147-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron. 2004;41(5):711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- 381.Broberger C, Landry M, Wong H, Walsh JN, Hokfelt T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66(6):393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- 382.Wang J-H, Wang F, Yang M-J, et al. Leptin regulated calcium channels of neuropeptide Y and proopiomelanocortin neurons by activation of different signal pathways. Neuroscience. 2008;156(1):89–98. doi: 10.1016/j.neuroscience.2008.04.079. [DOI] [PubMed] [Google Scholar]
- 383.Guan J-L, Wang Q-P, Kageyama H, et al. Synaptic interactions between ghrelin- and neuropeptide Y-containing neurons in the rat arcuate nucleus. Peptides. 2003;24(12):1921–1928. doi: 10.1016/j.peptides.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 384.Guan J-L, Saotome T, Wang Q-P, et al. Orexinegic innervation of POMC-containing neurons in the rat arcuate nucleus. NeuroReport. 2001;12(3):547–551. doi: 10.1097/00001756-200103050-00023. [DOI] [PubMed] [Google Scholar]
- 385.Funahashi H, Takenoya F, Guan J-L, Kageyama H, Yada T, Shioda S. Hypothalamic neuronal networks and feeding-related peptides involved in the regulation of feeding. Anatomical Science International. 2003;78(3):123–138. doi: 10.1046/j.0022-7722.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 386.Jureus A, Cunningham MJ, McClain ME, Clifton DK, Steiner RA. Galanin-like peptide (GALP) is a target for regulation by leptin in the hypothalamus of the rat. Endocrinology. 2000;141(7):2703–2706. doi: 10.1210/endo.141.7.7669. [DOI] [PubMed] [Google Scholar]
- 387.Baskin DG, Hahn TM, Schwartz MW. Leptin sensitive neurons in the hypothalamus. Hormone and Metabolic Research. 1999;31(5):345–350. doi: 10.1055/s-2007-978751. [DOI] [PubMed] [Google Scholar]
- 388.Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. The Journal of Clinical Endocrinology & Metabolism. 2001;86(10):4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 389.Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. American Journal of Physiology. 2004;287(2):E297–E304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- 390.van den Pol AN, Yao Y, Fu L-Y, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong renilla green fluorescent protein in NPY neurons. Journal of Neuroscience. 2009;29(14):4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Akamizu T, Takaya K, Irako T, et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. European Journal of Endocrinology. 2004;150(4):447–455. doi: 10.1530/eje.0.1500447. [DOI] [PubMed] [Google Scholar]
- 392.Davies JS, Kotokorpi P, Eccles SR, et al. Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Molecular Endocrinology. 2009;23(6):914–924. doi: 10.1210/me.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Garcia EA, King P, Sidhu K, et al. The role of ghrelin and ghrelin-receptor gene variants and promoter activity in type 2 diabetes. European Journal of Endocrinology. 2009;161(2):307–315. doi: 10.1530/EJE-09-0122. [DOI] [PubMed] [Google Scholar]
- 394.Tremblay F, Perreault M, Klaman LD, Tobin JF, Smith E, Gimeno RE. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology. 2007;148(2):501–506. doi: 10.1210/en.2006-1275. [DOI] [PubMed] [Google Scholar]
- 395.De Smet B, Thijs T, Peeters TL, Depoortere I. Effect of peripheral obestatin on gastric emptying and intestinal contractility in rodents. Neurogastroenterology and Motility. 2007;19(3):211–217. doi: 10.1111/j.1365-2982.2006.00883.x. [DOI] [PubMed] [Google Scholar]
- 396.Moechars D, Depoortere I, Moreaux B, et al. Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology. 2006;131(4):1131–1141. doi: 10.1053/j.gastro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 397.Gourcerol G, Coskun T, Craft LS, et al. Preproghrelin-derived peptide, obestatin, fails to influence food intake in lean or obese rodents. Obesity. 2007;15(11):2643–2652. doi: 10.1038/oby.2007.316. [DOI] [PubMed] [Google Scholar]
- 398.Gourcerol G, Million M, Adelson DW, et al. Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides. 2006;27(11):2811–2819. doi: 10.1016/j.peptides.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 399.Kobelt P, Wisser A-S, Stengel A, et al. Peripheral obestatin has no effect on feeding behavior and brain Fos expression in rodents. Peptides. 2008;29(6):1018–1027. doi: 10.1016/j.peptides.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Frutos MG-S, Cacicedo L, Fernández C, et al. Insights into a role of GH secretagogues in reversing the age-related decline in the GH/IGF-I axis. American Journal of Physiology. 2007;293(5):E1140–E1152. doi: 10.1152/ajpendo.00236.2007. [DOI] [PubMed] [Google Scholar]
- 401.Nogueiras R, Pfluger P, Tovar S, et al. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology. 2007;148(1):21–26. doi: 10.1210/en.2006-0915. [DOI] [PubMed] [Google Scholar]
- 402.Yamamoto D, Ikeshita N, Daito R, et al. Neither intravenous nor intracerebroventricular administration of obestatin affects the secretion of GH, PRL, TSH and ACTH in rats. Regulatory Peptides. 2007;138(2-3):141–144. doi: 10.1016/j.regpep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 403.Samson WK, White MM, Price C, Ferguson AV. Obestatin acts in brain to inhibit thirst. American Journal of Physiology. 2007;292(1):R637–R643. doi: 10.1152/ajpregu.00395.2006. [DOI] [PubMed] [Google Scholar]
- 404.Ataka K, Inui A, Asakawa A, Kato I, Fujimiya M. Obestatin inhibits motor activity in the antrum and duodenum in the fed state of conscious rats. American Journal of Physiology. 2008;294(5):G1210–G1218. doi: 10.1152/ajpgi.00549.2007. [DOI] [PubMed] [Google Scholar]
- 405.Volante M, Rosas R, Ceppi P, et al. Obestatin in human neuroendocrine tissues and tumours: expression and effect on tumour growth. Journal of Pathology. 2009;218(4):458–466. doi: 10.1002/path.2551. [DOI] [PubMed] [Google Scholar]
- 406.Tsolakis AV, Grimelius L, Stridsberg M, et al. Obestatin/ghrelin cells in normal mucosa and endocrine tumours of the stomach. European Journal of Endocrinology. 2009;160(6):941–949. doi: 10.1530/EJE-09-0001. [DOI] [PubMed] [Google Scholar]
- 407.Zhao C-M, Furnes MW, Stenstrom B, Kulseng B, Chen D. Characterization of obestatin- and ghrelin-producing cells in the gastrointestinal tract and pancreas of rats: an immunohistochemical and electron-microscopic study. Cell and Tissue Research. 2008;331(3):575–587. doi: 10.1007/s00441-007-0514-3. [DOI] [PubMed] [Google Scholar]
- 408.Kerem M, Salman B, Ozsoy S, et al. Exogenous ghrelin enhances endocrine and exocrine regeneration in pancreatectomized rats. Journal of Gastrointestinal Surgery. 2009;13(4):775–783. doi: 10.1007/s11605-008-0778-2. [DOI] [PubMed] [Google Scholar]
- 409.Granata R, Settanni F, Gallo D, et al. Obestatin promotes survival of pancreatic β-cells and human islets and induces expression of genes involved in the regulation of β-cell mass and function. Diabetes. 2008;57(4):967–979. doi: 10.2337/db07-1104. [DOI] [PubMed] [Google Scholar]
- 410.Zhang JV, Jahr H, Luo C-W, et al. Obestatin induction of early-response gene expression in gastrointestinal and adipose tissues and the mediatory role of G protein-coupled receptor, GPR39. Molecular Endocrinology. 2008;22(6):1464–1475. doi: 10.1210/me.2007-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Szentirmai É, Kapás L, Sun Y, Smith RG, Krueger JM. The preproghrelin gene is required for the normal integration of thermoregulation and sleep in mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(33):14069–14074. doi: 10.1073/pnas.0903090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Wei W, Qi X, Reed J, et al. Effect of chronic hyperghrelinemia on ingestive action of ghrelin. American Journal of Physiology. 2006;290(3):R803–R808. doi: 10.1152/ajpregu.00331.2005. [DOI] [PubMed] [Google Scholar]
- 413.Bresciani E, Pitsikas N, Tamiazzo L, et al. Feeding behavior during long-term hexarelin administration in young and old rats. Journal of Endocrinological Investigation. 2008;31(7):647–652. doi: 10.1007/BF03345618. [DOI] [PubMed] [Google Scholar]
- 414.Nagaya N, Moriya J, Yasumura Y, et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110(24):3674–3679. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- 415.Ashby DR, Ford HE, Wynne KJ, et al. Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney International. 2009;76(2):199–206. doi: 10.1038/ki.2009.114. [DOI] [PubMed] [Google Scholar]
- 416.Akamizu T, Iwakura H, Ariyasu H, et al. Effects of ghrelin treatment on patients undergoing total hip replacement for osteoarthritis: different outcomes from studies in patients with cardiac and pulmonary cachexia. Journal of the American Geriatrics Society. 2008;56(12):2363–2365. doi: 10.1111/j.1532-5415.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 417.Blüher S, Mantzoros CS. Leptin in humans: lessons from translational research. American Journal of Clinical Nutrition. 2009;89(3):991S–997S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Scott V, McDade DM, Luckman SM. Rapid changes in the sensitivity of arcuate nucleus neurons to central ghrelin in relation to feeding status. Physiology and Behavior. 2007;90(1):180–185. doi: 10.1016/j.physbeh.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 419.Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003;24(4):597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 420.Strassburg S, Anker SD, Castaneda TR, et al. Long-term effects of ghrelin and ghrelin receptor agonists on energy balance in rats. American Journal of Physiology. 2008;295(1):E78–E84. doi: 10.1152/ajpendo.00040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Thomas GB, Bennett PA, Carmignac DF, Robinson IC. Glucocorticoid regulation of growth hormone (GH) secretagogue-induced growth responses and GH secretagogue receptor expression in the rat. Growth Hormone and IGF Research. 2000;10(1):45–52. doi: 10.1054/ghir.1999.0138. [DOI] [PubMed] [Google Scholar]
- 422.Bennett PA, Thomas GB, Howard AD, et al. Hypothalamic growth hormone secretagogue-receptor (GHS-R) expression is regulated by growth hormone in the rat. Endocrinology. 1997;138(11):4552–4557. doi: 10.1210/endo.138.11.5476. [DOI] [PubMed] [Google Scholar]
- 423.Kamegai J, Wakabayashi I, Kineman RD, Frohman LA. Growth hormone-releasing hormone receptor (GHRH-R) and growth hormone secretagogue receptor (GHS-R) mRNA levels during postnatal development in male and female rats. Journal of Neuroendocrinology. 1999;11(4):299–306. doi: 10.1046/j.1365-2826.1999.00330.x. [DOI] [PubMed] [Google Scholar]
- 424.Machado MC, Valeria de Sa S, Correa-Giannella ML, et al. Association between tumoral GH-releasing peptide receptor type 1a mRNA expression and in vivo response to GH-releasing peptide-6 in ACTH-dependent Cushing’s syndrome patients. European Journal of Endocrinology. 2008;158(5):605–613. doi: 10.1530/EJE-07-0659. [DOI] [PubMed] [Google Scholar]
- 425.Katugampola SD, Pallikaros Z, Davenport AP. [125I-his9]-ghrelin, a novel radioligand for localizing GHS orphan receptors in human and rat tissue; up-regulation of receptors with atherosclerosis. British Journal of Pharmacology. 2001;134(1):143–149. doi: 10.1038/sj.bjp.0704228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. The New England Journal of Medicine. 2002;346(21):1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 427.Roth CL, Reinehr T, Schernthaner G-H, Kopp H-P, Kriwanek S, Schernthaner G. Ghrelin and obestatin levels in severely obese women before and after weight loss after Roux-en-Y gastric bypass surgery. Obesity Surgery. 2009;19(1):29–35. doi: 10.1007/s11695-008-9568-x. [DOI] [PubMed] [Google Scholar]
- 428.Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. The Journal of Clinical Endocrinology & Metabolism. 2003;88(4):1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 429.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. International Journal of Obesity. 2009;33(7):786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Foschi D, Corsi F, Colombo F, et al. Different effects of vertical banded gastroplasty and Roux-en-Y gastric bypass on meal inhibition of ghrelin secretion in morbidly obese patients. Journal of Investigative Surgery. 2008;21(2):77–81. doi: 10.1080/08941930701883624. [DOI] [PubMed] [Google Scholar]
- 431.Takeno R, Okimura Y, Iguchi G, et al. Intravenous administration of ghrelin stimulates growth hormone secretion in vagotomized patients as well as normal subjects. European Journal of Endocrinology. 2004;151(4):447–450. doi: 10.1530/eje.0.1510447. [DOI] [PubMed] [Google Scholar]
- 432.Saliba J, Wattacheril J, Abumrad NN. Endocrine and metabolic response to gastric bypass. Current Opinion in Clinical Nutrition and Metabolic Care. 2009;12(5):515–521. doi: 10.1097/MCO.0b013e32832e1b14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Maier C, Riedl M, Vila G, et al. Cholinergic regulation of ghrelin and peptide YY release may be impaired in obesity. Diabetes. 2008;57(9):2332–2340. doi: 10.2337/db07-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Qader SS, Hakanson R, Rehfeld JF, Lundquist I, Salehi A. Proghrelin-derived peptides influence the secretion of insulin, glucagon, pancreatic polypeptide and somatostatin: a study on isolated islets from mouse and rat pancreas. Regulatory Peptides. 2008;146(1–3):230–237. doi: 10.1016/j.regpep.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 435.Arosio M, Ronchi CL, Gebbia C, Cappiello V, Beck-Peccoz P, Peracchi M. Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels. The Journal of Clinical Endocrinology & Metabolism. 2003;88(2):701–704. doi: 10.1210/jc.2002-021161. [DOI] [PubMed] [Google Scholar]
- 436.Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. European Journal of Endocrinology. 2002;146(2):241–244. doi: 10.1530/eje.0.1460241. [DOI] [PubMed] [Google Scholar]
- 437.Cui C, Ohnuma H, Daimon M, et al. Ghrelin infused into the portal vein inhibits glucose-stimulated insulin secretion in Wistar rats. Peptides. 2008;29(7):1241–1246. doi: 10.1016/j.peptides.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 438.Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacology and Therapeutics. 2008;118(2):239–249. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 439.Dezaki K, Kakei M, Yada T. Ghrelin uses Gα i2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes. 2007;56(9):2319–2327. doi: 10.2337/db07-0345. [DOI] [PubMed] [Google Scholar]
- 440.Lee HM, Wang G, Englander EW, Kojima M, Greeley GH., Jr. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143(1):185–190. doi: 10.1210/endo.143.1.8602. [DOI] [PubMed] [Google Scholar]
- 441.Gauna C, Kiewiet RM, Janssen JAMJL, et al. Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditions. American Journal of Physiology. 2007;293(3):E697–E704. doi: 10.1152/ajpendo.00219.2007. [DOI] [PubMed] [Google Scholar]
- 442.Kageyama H, Funahashi H, Hirayama M, et al. Morphological analysis of ghrelin and its receptor distribution in the rat pancreas. Regulatory Peptides. 2005;126(1-2):67–71. doi: 10.1016/j.regpep.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 443.Yada T, Dezaki K, Sone H, et al. Ghrelin regulates insulin release and glycemia: physiological role and therapeutic potential. Current Diabetes Reviews. 2008;4(1):18–23. doi: 10.2174/157339908783502352. [DOI] [PubMed] [Google Scholar]
- 444.Dezaki K, Hosoda H, Kakei M, et al. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: implication in the glycemic control in rodents. Diabetes. 2004;53(12):3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 445.Kiewiet RM, van Aken MO, van der Weerd K, et al. Effects of acute administration of acylated and unacylated ghrelin on glucose and insulin concentrations in morbidly obese subjects without overt diabetes. European Journal of Endocrinology. 2009;161(4):567–573. doi: 10.1530/EJE-09-0339. [DOI] [PubMed] [Google Scholar]
- 446.Fick LJ, Cai F, Belsham DD. Hypothalamic preproghrelin gene expression is repressed by insulin via both PI3-K/Akt and ERK1/2 MAPK pathways in immortalized, hypothalamic neurons. Neuroendocrinology. 2009;89(3):267–275. doi: 10.1159/000167698. [DOI] [PubMed] [Google Scholar]
- 447.Ikezaki A, Hosoda H, Ito K, et al. Fasting plasma ghrelin levels are negatively correlated with insulin resistance and PAI-1, but not with leptin, in obese children and adolescents. Diabetes. 2002;51(12):3408–3411. doi: 10.2337/diabetes.51.12.3408. [DOI] [PubMed] [Google Scholar]
- 448.Pagotto U, Gambineri A, Vicennati V, Heiman ML, Tschop M, Pasquali R. Plasma ghrelin, obesity, and the polycystic ovary syndrome: correlation with insulin resistance and androgen levels. The Journal of Clinical Endocrinology & Metabolism. 2002;87(12):5625–5629. doi: 10.1210/jc.2002-020776. [DOI] [PubMed] [Google Scholar]
- 449.Goldstone AP, Patterson M, Kalingag N, et al. Fasting and postprandial hyperghrelinemia in Prader-Willi syndrome is partially explained by hypoinsulinemia, and is not due to peptide YY 3-36 deficiency or seen in hypothalamic obesity due to craniopharyngioma. The Journal of Clinical Endocrinology & Metabolism. 2005;90(5):2681–2690. doi: 10.1210/jc.2003-032209. [DOI] [PubMed] [Google Scholar]
- 450.Tauber M, Auriol FC, Moulin P, Molinas C, Delagnes V, Salles JP. Hyperghrelinemia is a common feature of Prader-Willi syndrome and pituitary stalk interruption: a pathophysiological hypothesis. Hormone Research. 2004;62(1):49–54. doi: 10.1159/000078862. [DOI] [PubMed] [Google Scholar]
- 451.Choe YH, Sang YS, Paik K-H, et al. Increased density of ghrelin-expressing cells in the gastric fundus and body in Prader-Willi syndrome. The Journal of Clinical Endocrinology & Metabolism. 2005;90(9):5441–5445. doi: 10.1210/jc.2004-1935. [DOI] [PubMed] [Google Scholar]
- 452.Andralojc KM, Mercalli A, Nowak KW, et al. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia. 2009;52(3):486–493. doi: 10.1007/s00125-008-1238-y. [DOI] [PubMed] [Google Scholar]
- 453.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regulatory Peptides. 2002;107(1–3):63–69. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 454.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing β cells in two mouse models of pancreas development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):2924–2929. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Granata R, Settanni F, Biancone L, et al. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic β-cells and human islets: involvement of 3′, 5′-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-kinase/Akt signaling. Endocrinology. 2007;148(2):512–529. doi: 10.1210/en.2006-0266. [DOI] [PubMed] [Google Scholar]
- 456.Dezaki K, Sone H, Koizumi M, et al. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006;55(12):3486–3493. doi: 10.2337/db06-0878. [DOI] [PubMed] [Google Scholar]
- 457.Halem HA, Taylor JE, Dong JZ, et al. Novel analogs of ghrelin: physiological and clinical implications. European Journal of Endocrinology. 2004;151(supplement 1):S71–S75. doi: 10.1530/eje.0.151s071. [DOI] [PubMed] [Google Scholar]
- 458.Nogueiras R, Perez-Tilve D, Wortley KE, Tschop M. Growth hormone secretagogue (ghrelin-) receptors—a complex drug target for the regulation of body weight. CNS and Neurological Disorders: Drug Targets. 2006;5(3):335–343. doi: 10.2174/187152706777452227. [DOI] [PubMed] [Google Scholar]
- 459.Lin Y, Matsumura K, Fukuhara M, Kagiyama S, Fujii K, Iida M. Ghrelin acts at the nucleus of the solitary tract to decrease arterial pressure in rats. Hypertension. 2004;43(5):977–982. doi: 10.1161/01.HYP.0000122803.91559.55. [DOI] [PubMed] [Google Scholar]
- 460.Nagaya N, Kojima M, Uematsu M, et al. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. American Journal of Physiology. 2001;280(5):R1483–R1487. doi: 10.1152/ajpregu.2001.280.5.R1483. [DOI] [PubMed] [Google Scholar]
- 461.Xu X-B, Pang J-J, Cao J-M, et al. GH-releasing peptides improve cardiac dysfunction and cachexia and suppress stress-related hormones and cardiomyocyte apoptosis in rats with heart failure. American Journal of Physiology. 2005;289(4):H1643–H1651. doi: 10.1152/ajpheart.01042.2004. [DOI] [PubMed] [Google Scholar]
- 462.Matsumura K, Tsuchihashi T, Fujii K, Abe I, Iida M. Central ghrelin modulates sympathetic activity in conscious rabbits. Hypertension. 2002;40(5):694–699. doi: 10.1161/01.hyp.0000035395.51441.10. [DOI] [PubMed] [Google Scholar]
- 463.Shinde UA, Desai KM, Yu C, Gopalakrishnan V. Nitric oxide synthase inhibition exaggerates the hypotensive response to ghrelin: role of calcium-activated potassium channels. Journal of Hypertension. 2005;23(4):779–784. doi: 10.1097/01.hjh.0000163146.20330.bc. [DOI] [PubMed] [Google Scholar]
- 464.Enomoto M, Nagaya N, Uematsu M, et al. Cardiovascular and hormonal effects of subcutaneous administration of ghrelin, a novel growth hormone-releasing peptide, in healthy humans. Clinical Science. 2003;105(4):431–435. doi: 10.1042/CS20030184. [DOI] [PubMed] [Google Scholar]
- 465.Moazed B, Quest D, Gopalakrishnan V. Des-acyl ghrelin fragments evoke endothelium-dependent vasodilatation of rat mesenteric vascular bed via activation of potassium channels. European Journal of Pharmacology. 2009;604(1–3):79–86. doi: 10.1016/j.ejphar.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 466.Kleinz MJ, Maguire JJ, Skepper JN, Davenport AP. Functional and immunocytochemical evidence for a role of ghrelin and des-octanoyl ghrelin in the regulation of vascular tone in man. Cardiovascular Research. 2006;69(1):227–235. doi: 10.1016/j.cardiores.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 467.Yuan M-J, Huang C-X, Tang Y-H, et al. A novel peptide ghrelin inhibits neural remodeling after myocardial infarction in rats. European Journal of Pharmacology. 2009;618(1–3):52–57. doi: 10.1016/j.ejphar.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 468.Schwenke DO, Tokudome T, Shirai M, et al. Exogenous ghrelin attenuates the progression of chronic hypoxia-induced pulmonary hypertension in conscious rats. Endocrinology. 2008;149(1):237–244. doi: 10.1210/en.2007-0833. [DOI] [PubMed] [Google Scholar]
- 469.Marleau S, Mulumba M, Lamontagne D, Ong H. Cardiac and peripheral actions of growth hormone and its releasing peptides: relevance for the treatment of cardiomyopathies. Cardiovascular Research. 2006;69(1):26–35. doi: 10.1016/j.cardiores.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 470.Okumura H, Nagaya N, Enomoto M, Nakagawa E, Oya H, Kangawa K. Vasodilatory effect of ghrelin, an endogenous peptide from the stomach. Journal of Cardiovascular Pharmacology. 2002;39(6):779–783. doi: 10.1097/00005344-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 471.Wiley KE, Davenport AP. Comparison of vasodilators in human internal mammary artery: ghrelin is a potent physiological antagonist of endothelin-1. British Journal of Pharmacology. 2002;136(8):1146–1152. doi: 10.1038/sj.bjp.0704815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Iglesias MJ, Pineiro R, Blanco M, et al. Growth hormone releasing peptide (ghrelin) is synthesized and secreted by cardiomyocytes. Cardiovascular Research. 2004;62(3):481–488. doi: 10.1016/j.cardiores.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 473.Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. Journal of Clinical Endocrinology and Metabolism. 2002;87(6):2988–2991. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 474.Bodart V, Febbraio M, Demers A, et al. CD36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart. Circulation Research. 2002;90(8):844–849. doi: 10.1161/01.res.0000016164.02525.b4. [DOI] [PubMed] [Google Scholar]
- 475.Iglesias MJ, Salgado A, Pineiro R, et al. Lack of effect of the ghrelin gene-derived peptide obestatin on cardiomyocyte viability and metabolism. Journal of Endocrinological Investigation. 2007;30(6):470–476. doi: 10.1007/BF03346330. [DOI] [PubMed] [Google Scholar]
- 476.Tesauro M, Schinzari F, Iantorno M, et al. Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation. 2005;112(19):2986–2992. doi: 10.1161/CIRCULATIONAHA.105.553883. [DOI] [PubMed] [Google Scholar]
- 477.Rossi F, Bertone C, Petricca S, Santiemma V. Ghrelin inhibits angiotensin II-induced migration of human aortic endothelial cells. Atherosclerosis. 2007;192(2):291–297. doi: 10.1016/j.atherosclerosis.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 478.Conconi MT, Nico B, Guidolin D, et al. Ghrelin inhibits FGF-2-mediated angiogenesis in vitro and in vivo. Peptides. 2004;25(12):2179–2185. doi: 10.1016/j.peptides.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 479.Xu Z, Lin S, Wu W, et al. Ghrelin prevents doxorubicin-induced cardiotoxicity through TNF-alpha/NF-κB pathways and mitochondrial protective mechanisms. Toxicology. 2008;247(2-3):133–138. doi: 10.1016/j.tox.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 480.Zhang M, Yuan F, Liu H, Chen H, Qiu X, Fang W. Inhibition of proliferation and apoptosis of vascular smooth muscle cells by ghrelin. Acta Biochimica et Biophysica Sinica. 2008;40(9):769–776. [PubMed] [Google Scholar]
- 481.Pemberton CJ, Tokola H, Bagi Z, et al. Ghrelin induces vasoconstriction in the rat coronary vasculature without altering cardiac peptide secretion. American Journal of Physiology. 2004;287(4):H1522–H1529. doi: 10.1152/ajpheart.00193.2004. [DOI] [PubMed] [Google Scholar]
- 482.Chang L, Zhao J, Li G-Z, et al. Ghrelin protects myocardium from isoproterenol-induced injury in rats. Acta Pharmacologica Sinica. 2004;25(9):1131–1137. [PubMed] [Google Scholar]
- 483.Cittadini A, Saldamarco L, Marra AM, et al. Growth hormone deficiency in patients with chronic heart failure and beneficial effects of its correction. Journal of Clinical Endocrinology and Metabolism. 2009;94(9):3329–3336. doi: 10.1210/jc.2009-0533. [DOI] [PubMed] [Google Scholar]
- 484.Nagaya N, Uematsu M, Kojima M, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104(17):2034–2038. doi: 10.1161/hc4201.097836. [DOI] [PubMed] [Google Scholar]
- 485.Shimizu Y, Nagaya N, Isobe T, et al. Increased plasma ghrelin level in lung cancer cachexia. Clinical Cancer Research. 2003;9(2):774–778. [PubMed] [Google Scholar]
- 486.Akashi YJ, Palus S, Datta R, et al. No effects of human ghrelin on cardiac function despite profound effects on body composition in a rat model of heart failure. International Journal of Cardiology. 2009;137(3):267–275. doi: 10.1016/j.ijcard.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 487.Dimitrova DZ, Mihov DN, Wang R, et al. Contractile effect of ghrelin on isolated guinea-pig renal arteries. Vascular Pharmacology. 2007;47(1):31–40. doi: 10.1016/j.vph.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 488.Bellone J, Bartolotta E, Sgattoni C, et al. Hexarelin, a synthetic GH-releasing peptide, is a powerful stimulus of GH secretion in pubertal children and in adults but not in prepubertal children and in elderly subjects. Journal of Endocrinological Investigation. 1998;21(8):494–500. doi: 10.1007/BF03347334. [DOI] [PubMed] [Google Scholar]
- 489.Dornonville de la Cour C, Bjorkqvist M, Sandvik AK, et al. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regulatory Peptides. 2001;99(2-3):141–150. doi: 10.1016/s0167-0115(01)00243-9. [DOI] [PubMed] [Google Scholar]
- 490.Zhao Z, Sakai T. Characteristic features of ghrelin cells in the gastrointestinal tract and the regulation of stomach ghrelin expression and production. World Journal of Gastroenterology. 2008;14(41):6306–6311. doi: 10.3748/wjg.14.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Wierup N, Bjorkqvist M, Westrom B, Pierzynowski S, Sundler F, Sjolund K. Ghrelin and motilin are cosecreted from a prominent endocrine cell population in the small intestine. Journal of Clinical Endocrinology and Metabolism. 2007;92(9):3573–3581. doi: 10.1210/jc.2006-2756. [DOI] [PubMed] [Google Scholar]
- 492.Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nature Reviews Gastroenterology and Hepatology. 2009;6(6):343–352. doi: 10.1038/nrgastro.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Kapica M, Zabielska M, Puzio I, et al. Obestatin stimulates the secretion of pancreatic juice enzymes through a vagal pathway in anaesthetized rats—preliminary results. Journal of Physiology and Pharmacology. 2007;58(supplement 3):123–130. [PubMed] [Google Scholar]
- 494.Nishi Y, Hiejima H, Hosoda H, et al. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146(5):2255–2264. doi: 10.1210/en.2004-0695. [DOI] [PubMed] [Google Scholar]
- 495.Nishi Y, Hiejima H, Mifune H, Sato T, Kangawa K, Kojima M. Developmental changes in the pattern of ghrelin’s acyl modification and the levels of acyl-modified ghrelins in murine stomach. Endocrinology. 2005;146(6):2709–2715. doi: 10.1210/en.2004-0645. [DOI] [PubMed] [Google Scholar]
- 496.Zhu X, Cao Y, Voodg K, Steiner DF. On the processing of proghrelin to ghrelin. Journal of Biological Chemistry. 2006;281(50):38867–38870. doi: 10.1074/jbc.M607955200. [DOI] [PubMed] [Google Scholar]
- 497.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochemical and Biophysical Research Communications. 2000;279(3):909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 498.Erlanson-Albertsson C, Lindqvist A. Vagotomy and accompanying pyloroplasty down-regulates ghrelin mRNA but does not affect ghrelin secretion. Regulatory Peptides. 2008;151(1–3):14–18. doi: 10.1016/j.regpep.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 499.Amole N, Unniappan S. Fasting induces preproghrelin mRNA expression in the brain and gut of zebrafish, Danio rerio. General and Comparative Endocrinology. 2009;161(1):133–137. doi: 10.1016/j.ygcen.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 500.Arosio M, Ronchi CL, Beck-Peccoz P, et al. Effects of modified sham feeding on ghrelin levels in healthy human subjects. Journal of Clinical Endocrinology and Metabolism. 2004;89(10):5101–5104. doi: 10.1210/jc.2003-032222. [DOI] [PubMed] [Google Scholar]
- 501.Depoortere I, Thijs T, Thielemans L, Robberecht P, Peeters TL. Interaction of the growth hormone-releasing peptides ghrelin and growth hormone-releasing peptide-6 with the motilin receptor in the rabbit gastric antrum. Journal of Pharmacology and Experimental Therapeutics. 2003;305(2):660–667. doi: 10.1124/jpet.102.047563. [DOI] [PubMed] [Google Scholar]
- 502.Depoortere I, De Winter B, Thijs T, De Man J, Pelckmans P, Peeters T. Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitro. European Journal of Pharmacology. 2005;515(1–3):160–168. doi: 10.1016/j.ejphar.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 503.Xu L, Depoortere I, Tomasetto C, et al. Evidence for the presence of motilin, ghrelin, and the motilin and ghrelin receptor in neurons of the myenteric plexus. Regulatory Peptides. 2005;124(1–3):119–125. doi: 10.1016/j.regpep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 504.Olsson C, Holbrook JD, Bompadre G, et al. Identification of genes for the ghrelin and motilin receptors and a novel related gene in fish, and stimulation of intestinal motility in zebrafish (Danio rerio) by ghrelin and motilin. General and Comparative Endocrinology. 2008;155(1):217–226. doi: 10.1016/j.ygcen.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 505.Kudoh K, Shibata C, Funayama Y, et al. The effect of growth hormone releasing peptide-2 on upper gastrointestinal contractile activity and food intake in conscious dogs. Journal of Gastroenterology. 2009;44(4):297–304. doi: 10.1007/s00535-009-0025-y. [DOI] [PubMed] [Google Scholar]
- 506.Qiu W-C, Wang Z-G, Wang W-G, Yan J, Zheng Q. Gastric motor effects of ghrelin and growth hormone releasing peptide 6 in diabetic mice with gastroparesis. World Journal of Gastroenterology. 2008;14(9):1419–1424. doi: 10.3748/wjg.14.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Trudel L, Tomasetto C, Rio MC, et al. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. American Journal of Physiology. 2002;282(6):G948–G952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- 508.Levin F, Edholm T, Schmidt PT, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. Journal of Clinical Endocrinology and Metabolism. 2006;91(9):3296–3302. doi: 10.1210/jc.2005-2638. [DOI] [PubMed] [Google Scholar]
- 509.Sanger GJ, Westaway SM, Barnes AA, et al. GSK962040: a small molecule, selective motilin receptor agonist, effective as a stimulant of human and rabbit gastrointestinal motility. Neurogastroenterology and Motility. 2009;21(6):p. 657. doi: 10.1111/j.1365-2982.2008.01270.x. [DOI] [PubMed] [Google Scholar]
- 510.Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterology and Motility. 2007;19(8):675–680. doi: 10.1111/j.1365-2982.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 511.Ang D, Nicolai H, Vos R, et al. Influence of ghrelin on the gastric accommodation reflex and on meal-induced satiety in man. Neurogastroenterology and Motility. 2009;21(5):528–533. doi: 10.1111/j.1365-2982.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- 512.Murray CDR, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54(12):1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Katagiri F, Itoh H, Takeyama M. Effects of erythromycin on plasma gastrin, somatostatin, and motilin levels in healthy volunteers and postoperative cancer patients. Biological and Pharmaceutical Bulletin. 2005;28(7):1307–1310. doi: 10.1248/bpb.28.1307. [DOI] [PubMed] [Google Scholar]
- 514.Tack J. Prokinetics and fundic relaxants in upper functional GI disorders. Current Opinion in Pharmacology. 2008;8(6):690–696. doi: 10.1016/j.coph.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 515.Chang L, Zhao J, Yang J, Zhang Z, Du J, Tang C. Therapeutic effects of ghrelin on endotoxic shock in rats. European Journal of Pharmacology. 2003;473(2-3):171–176. doi: 10.1016/s0014-2999(03)01972-1. [DOI] [PubMed] [Google Scholar]
- 516.Yakabi K, Kawashima J, Kato S. Ghrelin and gastric acid secretion. World Journal of Gastroenterology. 2008;14(41):6334–6338. doi: 10.3748/wjg.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Andrews PLR, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Current Opinion in Pharmacology. 2002;2(6):650–656. doi: 10.1016/s1471-4892(02)00227-8. [DOI] [PubMed] [Google Scholar]
- 518.Inui A, Asakawa A, Bowers CY, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. The FASEB Journal. 2004;18(3):439–456. doi: 10.1096/fj.03-0641rev. [DOI] [PubMed] [Google Scholar]
- 519.Wargin W, Thomas H, Clohs L, et al. Contribution of protein binding to the pharmacokinetics of the ghrelin receptor agonist TZP-101 in healthy volunteers and adults with symptomatic gastroparesis: two randomized, double-blind studies and a binding profile study. Clinical Drug Investigation. 2009;29(6):409–418. doi: 10.2165/00044011-200929060-00004. [DOI] [PubMed] [Google Scholar]
- 520.Ejskjaer N, Vestergaard ET, Hellstrom PM, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Alimentary Pharmacology and Therapeutics. 2009;29(11):1179–1187. doi: 10.1111/j.1365-2036.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 521.Binn M, Albert C, Gougeon A, et al. Ghrelin gastrokinetic action in patients with neurogenic gastroparesis. Peptides. 2006;27(7):1603–1606. doi: 10.1016/j.peptides.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 522.Poitras P, Polvino WJ, Rocheleau B. Gastrokinetic effect of ghrelin analog RC-1139 in the rat: effect on post-operative and on morphine induced ileus. Peptides. 2005;26(9):1598–1601. doi: 10.1016/j.peptides.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 523.Asai S, Katabami T, Obi N, et al. No ghrelin response to oral glucose in diabetes mellitus with gastroparesis. Endocrine Journal. 2009;56(1):79–87. doi: 10.1507/endocrj.k08e-169. [DOI] [PubMed] [Google Scholar]
- 524.Harsch IA, Koebnick C, Tasi AM, Hahn EG, Konturek PC. Ghrelin and obestatin levels in type 2 diabetic patients with and without delayed gastric emptying. Digestive Diseases and Sciences. 2009;54(10):2161–2166. doi: 10.1007/s10620-008-0622-2. [DOI] [PubMed] [Google Scholar]
- 525.Dzaja A, Dalal MA, Himmerich H, Uhr M, Pollmacher T, Schuld A. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. American Journal of Physiology. 2004;286(6):E963–E967. doi: 10.1152/ajpendo.00527.2003. [DOI] [PubMed] [Google Scholar]
- 526.Kluge M, Gazea M, Schussler P, et al. Ghrelin increases slow wave sleep and stage 2 sleep and decreases stage 1 sleep and REM sleep in elderly men but does not affect sleep in elderly women. Psychoneuroendocrinology. 2010;35(2):297–304. doi: 10.1016/j.psyneuen.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 527.Steiger A. Ghrelin and sleep-wake regulation. American Journal of Physiology. 2007;292(1):R573–R574. doi: 10.1152/ajpregu.00618.2006. [DOI] [PubMed] [Google Scholar]
- 528.Kluge M, Schussler P, Bleninger P, et al. Ghrelin alone or co-administered with GHRH or CRH increases non-REM sleep and decreases REM sleep in young males. Psychoneuroendocrinology. 2008;33(4):497–506. doi: 10.1016/j.psyneuen.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 529.Weikel JC, Wichniak A, Ising M, et al. Ghrelin promotes slow-wave sleep in humans. American Journal of Physiology. 2003;284(2):E407–E415. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]
- 530.Schuessler P, Uhr M, Ising M, Schmid D, Weikel J, Steiger A. Nocturnal ghrelin levels—relationship to sleep EEG, the levels of growth hormone, ACTH and cortisol—and gender differences. Journal of Sleep Research. 2005;14(4):329–336. doi: 10.1111/j.1365-2869.2005.00486.x. [DOI] [PubMed] [Google Scholar]
- 531.Frieboes R-M, Antonijevic IA, Held K, et al. Hexarelin decreases slow-wave sleep and stimulates the secretion of GH, ACTH, cortisol and prolactin during sleep in healthy volunteers. Psychoneuroendocrinology. 2004;29(7):851–860. doi: 10.1016/S0306-4530(03)00152-5. [DOI] [PubMed] [Google Scholar]
- 532.Seoane LM, Tovar SA, Perez D, et al. Orexin A suppresses in vivo GH secretion. European Journal of Endocrinology. 2004;150(5):731–736. doi: 10.1530/eje.0.1500731. [DOI] [PubMed] [Google Scholar]
- 533.Takahashi K-I, Chin K, Akamizu T, et al. Acylated ghrelin level in patients with OSA before and after nasal CPAP treatment. Respirology. 2008;13(6):810–816. doi: 10.1111/j.1440-1843.2008.01357.x. [DOI] [PubMed] [Google Scholar]
- 534.Leite-Moreira AF, Rocha-Sousa A, Henriques-Coelho T. Cardiac, skeletal, and smooth muscle regulation by ghrelin. Vitamins and Hormones. 2007;77:207–238. doi: 10.1016/S0083-6729(06)77009-1. [DOI] [PubMed] [Google Scholar]
- 535.Nunes S, Nogueira-Silva C, Dias E, Moura RS, Correia-Pinto J. Ghrelin and obestatin: different role in fetal lung development? Peptides. 2008;29(12):2150–2158. doi: 10.1016/j.peptides.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 536.Kim SW, Her SJ, Park SJ, et al. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone. 2005;37(3):359–369. doi: 10.1016/j.bone.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 537.Hwang S, Moon M, Kim S, Hwang L, Ahn KJ, Park S. Neuroprotective effect of ghrelin is associated with decreased expression of prostate apoptosis response-4. Endocrine Journal. 2009;56(4):609–617. doi: 10.1507/endocrj.k09e-072. [DOI] [PubMed] [Google Scholar]
- 538.Delgado-Rubin de Celix A, Chowen JA, Argente J, Frago LM. Growth hormone releasing peptide-6 acts as a survival factor in glutamate-induced excitotoxicity. Journal of Neurochemistry. 2006;99(3):839–849. doi: 10.1111/j.1471-4159.2006.04122.x. [DOI] [PubMed] [Google Scholar]
- 539.Kim MS, Yoon CY, Jang PG, et al. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Molecular Endocrinology. 2004;18(9):2291–2301. doi: 10.1210/me.2003-0459. [DOI] [PubMed] [Google Scholar]
- 540.Cassoni P, Allia E, Marrocco T, et al. Ghrelin and cortistatin in lung cancer: expression of peptides and relaed receptors in human primary tumors and in vitro effect on the H345 small cell carcinoma cell line. Journal of Endocrinological Investigation. 2006;29(9):781–790. doi: 10.1007/BF03347371. [DOI] [PubMed] [Google Scholar]
- 541.Lau PN, Chow KBS, Chan C-B, Cheng CHK, Wise H. The constitutive activity of the ghrelin receptor attenuates apoptosis via a protein kinase C-dependent pathway. Molecular and Cellular Endocrinology. 2009;299(2):232–239. doi: 10.1016/j.mce.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 542.Chung H, Kim E, Lee DH, et al. Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology. 2007;148(1):148–159. doi: 10.1210/en.2006-0991. [DOI] [PubMed] [Google Scholar]
- 543.Ammori JB, Zhang W-Z, Li J-Y, Chai B-X, Mulholland MW. Effects of ghrelin on neuronal survival in cells derived from dorsal motor nucleus of the vagus. Surgery. 2008;144(2):159–167. doi: 10.1016/j.surg.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Cassoni P, Ghe C, Marrocco T, et al. Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. European Journal of Endocrinology. 2004;150(2):173–184. doi: 10.1530/eje.0.1500173. [DOI] [PubMed] [Google Scholar]
- 545.Wang W, Andersson M, Iresjo BM, Lonnroth C, Lundholm K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. International Journal of Oncology. 2006;28(6):1393–1400. [PubMed] [Google Scholar]
- 546.Hanada T, Toshinai K, Kajimura N, et al. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochemical and Biophysical Research Communications. 2003;301(2):275–279. doi: 10.1016/s0006-291x(02)03028-0. [DOI] [PubMed] [Google Scholar]
- 547.DeBoer MD, Zhu XX, Levasseur P, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148(6):3004–3012. doi: 10.1210/en.2007-0016. [DOI] [PubMed] [Google Scholar]
- 548.Garcia JM, Polvino WJ. Effect on body weight and safety of RC-1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo-controlled, multiple-dose study in healthy volunteers. Oncologist. 2007;12(5):594–600. doi: 10.1634/theoncologist.12-5-594. [DOI] [PubMed] [Google Scholar]
- 549.Perboni S, Bowers C, Kojima S, Asakawa A, Inui A. Growth hormone releasing peptide 2 reverses anorexia associated with chemotherapy with 5-fluoruracil in colon cancer cell-bearing mice. World Journal of Gastroenterology. 2008;14(41):6303–6305. doi: 10.3748/wjg.14.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Chance WT, Dayal R, Friend LA, Thomas I, Sheriff S. Continuous intravenous infusion of ghrelin does not stimulate feeding in tumor-bearing rats. Nutrition and Cancer. 2008;60(1):75–90. doi: 10.1080/01635580701753016. [DOI] [PubMed] [Google Scholar]
- 551.Strasser F, Lutz TA, Maeder MT, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. British Journal of Cancer. 2008;98(2):300–308. doi: 10.1038/sj.bjc.6604148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Zwirska-Korczala K, Adamczyk-Sowa M, Sowa P, et al. Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. Journal of Physiology and Pharmacology. 2007;58(supplement 1):53–64. [PubMed] [Google Scholar]
- 553.Sangiao-Alvarellos S, Vazquez MJ, Varela L, et al. Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology. 2009;150(10):4562–4574. doi: 10.1210/en.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Ott V, Fasshauer M, Dalski A, et al. Direct peripheral effects of ghrelin include suppression of adiponectin expression. Hormone and Metabolic Research. 2002;34(11-12):640–645. doi: 10.1055/s-2002-38261. [DOI] [PubMed] [Google Scholar]
- 555.Choi K, Roh S-G, Hong Y-H, et al. The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology. 2003;144(3):754–759. doi: 10.1210/en.2002-220783. [DOI] [PubMed] [Google Scholar]
- 556.Yasuda T, Masaki T, Kakuma T, Yoshimatsu H. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neuroscience Letters. 2003;349(2):75–78. doi: 10.1016/s0304-3940(03)00789-4. [DOI] [PubMed] [Google Scholar]
- 557.Tsubone T, Masaki T, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H. Ghrelin regulates adiposity in white adipose tissue and UCP1 mRNA expression in brown adipose tissue in mice. Regulatory Peptides. 2005;130(1-2):97–103. doi: 10.1016/j.regpep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 558.Takahashi H, Kurose Y, Suzuki Y, et al. Ghrelin differentially modulates the GH secretory response to GHRH between the fed and fasted states in sheep. Domestic Animal Endocrinology. 2009;37(1):55–60. doi: 10.1016/j.domaniend.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 559.Yasuda T, Masaki T, Kakuma T, Yoshimatsu H. Hypothalamic melanocortin system regulates sympathetic nerve activity in brown adipose tissue. Experimental Biology and Medicine. 2004;229(3):235–239. doi: 10.1177/153537020422900303. [DOI] [PubMed] [Google Scholar]
- 560.Yasuda T, Masaki T, Kakuma T, et al. Dual regulatory effects of orexins on sympathetic nerve activity innervating brown adipose tissue in rats. Endocrinology. 2005;146(6):2744–2748. doi: 10.1210/en.2004-1226. [DOI] [PubMed] [Google Scholar]
- 561.Yasuda T, Masaki T, Sakata T, Yoshimatsu H. Hypothalamic neuronal histamine regulates sympathetic nerve activity and expression of uncoupling protein 1 mRNA in brown adipose tissue in rats. Neuroscience. 2004;125(3):535–540. doi: 10.1016/j.neuroscience.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 562.Lopez M, Seoane LM, Tovar S, Nogueiras R, Dieguez C, Senaris R. Orexin-A regulates growth hormone-releasing hormone mRNA content in a nucleus-specific manner and somatostatin mRNA content in a growth hormone-dependent fashion in the rat hypothalamus. European Journal of Neuroscience. 2004;19(8):2080–2088. doi: 10.1111/j.0953-816X.2004.03318.x. [DOI] [PubMed] [Google Scholar]
- 563.Sondergaard E, Gormsem LC, Nellemann B, Vestergaard ET, Christiansen JS, Nielsen S. Visceral fat mass is a strong predictor of circulating ghrelin levels in premenopausal women. European Journal of Endocrinology. 2009;160(3):375–379. doi: 10.1530/EJE-08-0735. [DOI] [PubMed] [Google Scholar]
- 564.Soares J-B, Roncon-Albuquerque R, Jr., Leite-Moreira A. Ghrelin and ghrelin receptor inhibitors: agents in the treatment of obesity. Expert Opinion on Therapeutic Targets. 2008;12(9):1177–1189. doi: 10.1517/14728222.12.9.1177. [DOI] [PubMed] [Google Scholar]
- 565.Fukushima N, Hanada R, Teranishi H, et al. Ghrelin directly regulates bone formation. Journal of Bone and Mineral Research. 2005;20(5):790–798. doi: 10.1359/JBMR.041237. [DOI] [PubMed] [Google Scholar]
- 566.Delhanty PJD, van der Eerden BCJ, van der Velde M, et al. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. Journal of Endocrinology. 2006;188(1):37–47. doi: 10.1677/joe.1.06404. [DOI] [PubMed] [Google Scholar]
- 567.Asakawa A, Inui A, Kaga T, et al. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74(3):143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- 568.Kawakami A, Okada N, Rokkaku K, Honda K, Ishibashi S, Onaka T. Leptin inhibits and ghrelin augments hypothalamic noradrenaline release after stress. Stress. 2008;11(5):363–369. doi: 10.1080/10253890701820257. [DOI] [PubMed] [Google Scholar]
- 569.Maruna P, Gurlich R, Rosicka M. Ghrelin as an acute-phase reactant during postoperative stress response. Hormone and Metabolic Research. 2008;40(6):404–409. doi: 10.1055/s-2008-1065329. [DOI] [PubMed] [Google Scholar]
- 570.Lutter M, Sakata I, Osborne-Lawrence S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nature Neuroscience. 2008;11(7):752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Vergnano AM, Ferrini F, Salio C, Lossi L, Baratta M, Merighi A. The gastrointestinal hormone ghrelin modulates inhibitory neurotransmission in deep laminae of mouse spinal cord dorsal horn. Endocrinology. 2008;149(5):2306–2312. doi: 10.1210/en.2007-1164. [DOI] [PubMed] [Google Scholar]
- 572.Wang L, Basa NR, Shaikh A, et al. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. American Journal of Physiology. 2006;291(4):G611–G620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 573.Hedayati N, Annambhotla S, Jiang J, et al. Growth hormone-releasing peptide ghrelin inhibits homocysteine-induced endothelial dysfunction in porcine coronary arteries and human endothelial cells. Journal of Vascular Surgery. 2009;49(1):199–207. doi: 10.1016/j.jvs.2008.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Zhao H, Liu G, Wang Q, et al. Effect of ghrelin on human endothelial cells apoptosis induced by high glucose. Biochemical and Biophysical Research Communications. 2007;362(3):677–681. doi: 10.1016/j.bbrc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 575.Li A, Cheng G, Zhu G, Tarnawski AS. Ghrelin stimulates angiogenesis in human microvascular endothelial cells: implications beyond GH release. Biochemical and Biophysical Research Communications. 2007;353(2):238–243. doi: 10.1016/j.bbrc.2006.11.144. [DOI] [PubMed] [Google Scholar]
- 576.Hattori N, Saito T, Yagyu T, Jiang B-H, Kitagawa K, Inagaki C. GH, GH receptor, GH secretagogue receptor, and Ghrelin expression in human T cells, B cells, and neutrophils. Journal of Clinical Endocrinology and Metabolism. 2001;86(9):4284–4291. doi: 10.1210/jcem.86.9.7866. [DOI] [PubMed] [Google Scholar]
- 577.Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. American Journal of Physiology. 2005;288(3):E486–E492. doi: 10.1152/ajpendo.00196.2004. [DOI] [PubMed] [Google Scholar]
- 578.Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery. 2008;143(3):334–342. doi: 10.1016/j.surg.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Himmerich H, Sheldrick AJ. TNF-alpha and ghrelin: opposite effects on immune system, metabolism and mental health. doi: 10.2174/092986610790225941. Protein & Peptide Letters,2010, In press. [DOI] [PubMed] [Google Scholar]
- 580.Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Hormone and IGF Research. 2009;19(3):187–197. doi: 10.1016/j.ghir.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 581.Youm Y-H, Yang H, Sun Y, et al. Deficient ghrelin receptor-mediated signaling compromises thymic stromal cell microenvironment by acceleratingthymic adiposity. Journal of Biological Chemistry. 2009;284(11):7068–7077. doi: 10.1074/jbc.M808302200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Zhang G-G, Teng X, Liu Y, et al. Inhibition of endoplasm reticulum stress by ghrelin protects against ischemia/reperfusion injury in rat heart. Peptides. 2009;30(6):1109–1116. doi: 10.1016/j.peptides.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 583.El Eter E, Al Tuwaijiri A, Hagar H, Arafa M. In vivo and in vitro antioxidant activity of ghrelin: attenuation of gastric ischemic injury in the rat. Journal of Gastroenterology and Hepatology. 2007;22(11):1791–1799. doi: 10.1111/j.1440-1746.2006.04696.x. [DOI] [PubMed] [Google Scholar]
- 584.Kawczynska-Drozdz A, Olszanecki R, Jawien J, et al. Ghrelin inhibits vascular superoxide production in spontaneously hypertensive rats. American Journal of Hypertension. 2006;19(7):764–767. doi: 10.1016/j.amjhyper.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 585.Liu Z, Yu Y, Jiang Y, Li J. Growth hormone increases circulating neutrophil activation and provokes lung microvascular injury in septic peritonitis rats. Journal of Surgical Research. 2002;105(2):195–199. doi: 10.1006/jsre.2002.6374. [DOI] [PubMed] [Google Scholar]
- 586.Granado M, Martin AI, Lopez-Menduina M, Lopez-Calderon A, Villanua MA. GH-releasing peptide-2 administration prevents liver inflammatory response in endotoxemia. American Journal of Physiology. 2008;294(1):E131–E141. doi: 10.1152/ajpendo.00308.2007. [DOI] [PubMed] [Google Scholar]
- 587.Wu R, Dong W, Zhou M, et al. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. American Journal of Respiratory and Critical Care Medicine. 2007;176(8):805–813. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Wu R, Dong W, Zhou M, Cui X, Hank SH, Wang P. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovascular Research. 2005;68(2):318–326. doi: 10.1016/j.cardiores.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 589.Wu R, Dong W, Qiang X, et al. Orexigenic hormone ghrelin ameliorates gut barrier dysfunction in sepsis in rats. Critical Care Medicine. 2009;37(8):2421–2426. doi: 10.1097/CCM.0b013e3181a557a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. Journal of Immunology. 2008;180(12):8369–8377. doi: 10.4049/jimmunol.180.12.8369. [DOI] [PubMed] [Google Scholar]
- 591.Takeda R, Nishimatsu H, Suzuki E, et al. Ghrelin improves renal function in mice with ischemic acute renal failure. Journal of the American Society of Nephrology. 2006;17(1):113–121. doi: 10.1681/ASN.2004080626. [DOI] [PubMed] [Google Scholar]
- 592.DeBoer MD, Zhu X, Levasseur PR, et al. Ghrelin treatment of chronic kidney disease: improvements in lean body mass and cytokine profile. Endocrinology. 2008;149(2):827–835. doi: 10.1210/en.2007-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Wynne K, Giannitsopoulou K, Small CJ, et al. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. Journal of the American Society of Nephrology. 2005;16(7):2111–2118. doi: 10.1681/ASN.2005010039. [DOI] [PubMed] [Google Scholar]
- 594.Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30(8):771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 595.Arbeiter AK, Buscher R, Petersenn S, Hauffa BP, Mann K, Hoyer PF. Ghrelin and other appetite-regulating hormones in paediatric patients with chronic renal failure during dialysis and following kidney transplantation. Nephrology Dialysis Transplantation. 2009;24(2):643–646. doi: 10.1093/ndt/gfn529. [DOI] [PubMed] [Google Scholar]
- 596.Kola B, Farkas I, Christ-Crain M, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE. 2008;3(3, article e1797) doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Van den Berghe G, de Zegher F, Bowers CY, et al. Pituitary responsiveness to GH-releasing hormone, GH-releasing peptide-2 and thyrotrophin-releasing hormone in critical illness. Clinical Endocrinology. 1996;45(3):341–351. doi: 10.1046/j.1365-2265.1996.00805.x. [DOI] [PubMed] [Google Scholar]
- 598.Van den Berghe G, Baxter RC, Weekers F, et al. The combined administration of GH-releasing peptide-2 (GHRP-2), TRH and GnRH to men with prolonged critical illness evokes superior endocrine and metabolic effects compared to treatment with GHRP-2 alone. Clinical Endocrinology. 2002;56(5):655–669. doi: 10.1046/j.1365-2265.2002.01255.x. [DOI] [PubMed] [Google Scholar]
- 599.Wu R, Zhou M, Das P, et al. Ghrelin inhibits sympathetic nervous activity in sepsis. American Journal of Physiology. 2007;293(6):E1697–E1702. doi: 10.1152/ajpendo.00098.2007. [DOI] [PubMed] [Google Scholar]
- 600.Wu R, Zhou M, Cui X, Simms HH, Wang P. Upregulation of cardiovascular ghrelin receptor occurs in the hyperdynamic phase of sepsis. American Journal of Physiology. 2004;287(3):H1296–H1302. doi: 10.1152/ajpheart.00852.2003. [DOI] [PubMed] [Google Scholar]
- 601.Wu R, Zhou M, Cui X, Simms HH, Wang P. Ghrelin clearance is reduced at the late stage of polymicrobial sepsis. International Journal of Molecular Medicine. 2003;12(5):777–781. [PubMed] [Google Scholar]
- 602.Proulx K, Vahl TP, Drazen DL, Woods SC, Seeley RJ. The effect of adrenalectomy on ghrelin secretion and orexigenic action. Journal of Neuroendocrinology. 2005;17(7):445–451. doi: 10.1111/j.1365-2826.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- 603.Otto B, Tschop M, Heldwein W, Pfeiffer AFH, Diederich S. Endogenous and exogenous glucocorticoids decrease plasma ghrelin in humans. European Journal of Endocrinology. 2004;151(1):113–117. doi: 10.1530/eje.0.1510113. [DOI] [PubMed] [Google Scholar]
- 604.Gualillo O, Caminos JE, Blanco M, et al. Ghrelin, a novel placental-derived hormone. Endocrinology. 2001;142(2):788–794. doi: 10.1210/endo.142.2.7987. [DOI] [PubMed] [Google Scholar]
- 605.Rubinfeld H, Hadani M, Taylor JE, et al. Novel ghrelin analogs with improved affinity for the GH secretagogue receptor stimulate GH and prolactin release from human pituitary cells. European Journal of Endocrinology. 2004;151(6):787–795. doi: 10.1530/eje.0.1510787. [DOI] [PubMed] [Google Scholar]
- 606.Nakahara K, Nakagawa M, Baba Y, et al. Maternal ghrelin plays an important role in rat fetal development during pregnancy. Endocrinology. 2006;147(3):1333–1342. doi: 10.1210/en.2005-0708. [DOI] [PubMed] [Google Scholar]
- 607.Fuglsang J, Sandager P, Moller N, Fisker S, Frystyk J, Ovesen P. Peripartum maternal and foetal ghrelin, growth hormones, IGFs and insulin interrelations. Clinical Endocrinology. 2006;64(5):502–509. doi: 10.1111/j.1365-2265.2006.02498.x. [DOI] [PubMed] [Google Scholar]
- 608.Fuglsang J, Skjaerbaek C, Espelund U, et al. Ghrelin and its relationship to growth hormones during normal pregnancy. Clinical Endocrinology. 2005;62(5):554–559. doi: 10.1111/j.1365-2265.2005.02257.x. [DOI] [PubMed] [Google Scholar]
- 609.Fuglsang J, Lauszus FF, Fisker S, Flyvbjerg A, Ovesen P. Growth hormone binding protein and maternal body mass index in relation to placental growth hormone and insulin requirements during pregnancy in type 1 diabetic women. Growth Hormone and IGF Research. 2005;15(3):223–230. doi: 10.1016/j.ghir.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 610.Palik E, Baranyi E, Melczer Z, et al. Elevated serum acylated (biologically active) ghrelin and resistin levels associate with pregnancy-induced weight gain and insulin resistance. Diabetes Research and Clinical Practice. 2007;76(3):351–357. doi: 10.1016/j.diabres.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 611.Yokota I, Kitamura S, Hosoda H, Kotani Y, Kangawa K. Concentration of the n-octanoylated active form of ghrelin in fetal and neonatal circulation. Endocrine Journal. 2005;52(2):271–276. doi: 10.1507/endocrj.52.271. [DOI] [PubMed] [Google Scholar]
- 612.Bouhours-Nouet N, Boux de Casson F, Rouleau S, et al. Maternal and cord blood ghrelin in the pregnancies of smoking mothers: possible markers of nutrient availability for the fetus. Hormone Research. 2006;66(1):6–12. doi: 10.1159/000092807. [DOI] [PubMed] [Google Scholar]
- 613.Tham E, Liu J, Innis S, et al. Acylated ghrelin concentrations are markedly decreased during pregnancy in mothers with and without gestational diabetes: relationship with cholinesterase. American Journal of Physiology. 2009;296(5):E1093–E1100. doi: 10.1152/ajpendo.90866.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.de Zegher F, Spitz B, Van den Berghe G, et al. Postpartum hyperprolactinemia and hyporesponsiveness of growth hormone (GH) to GH-releasing peptide. Journal of Clinical Endocrinology and Metabolism. 1998;83(1):103–106. doi: 10.1210/jcem.83.1.4483. [DOI] [PubMed] [Google Scholar]
- 615.Abizaid A, Schiavo L, Diano S. Hypothalamic and pituitary expression of ghrelin receptor message is increased during lactation. Neuroscience Letters. 2008;440(3):206–210. doi: 10.1016/j.neulet.2008.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Kotunia A, Zabielski R. Ghrelin in the postnatal development of the gastrointestinal tract. Journal of Physiology and Pharmacology. 2006;57(supplement 5):97–111. [PubMed] [Google Scholar]
- 617.Dembinski A, Warzecha Z, Ceranowicz P, et al. Variable effect of ghrelin administration on pancreatic development in young rats. role of insulin-like growth factor-1. Journal of Physiology and Pharmacology. 2005;56(4):555–570. [PubMed] [Google Scholar]
- 618.Martini AC, Fernandez-Fernandez R, Tovar S, et al. Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology. 2006;147(5):2374–2382. doi: 10.1210/en.2005-1422. [DOI] [PubMed] [Google Scholar]
- 619.Fernandez-Fernandez R, Navarro VM, Barreiro ML, et al. Effects of chronic hyperghrelinemia on puberty onset and pregnancy outcome in the rat. Endocrinology. 2005;146(7):3018–3025. doi: 10.1210/en.2004-1622. [DOI] [PubMed] [Google Scholar]
- 620.Barreiro ML, Gaytan F, Castellano JM, et al. Ghrelin inhibits the proliferative activity of immature Leydig cells in vivo and regulates stem cell factor messenger ribonucleic acid expression in rat testis. Endocrinology. 2004;145(11):4825–4834. doi: 10.1210/en.2004-0732. [DOI] [PubMed] [Google Scholar]
- 621.Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochemical and Biophysical Research Communications. 2001;288(4):780–785. doi: 10.1006/bbrc.2001.5854. [DOI] [PubMed] [Google Scholar]
- 622.Fernandez-Fernandez R, Tena-Sempere M, Navarro VM, et al. Effects of ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology. 2005;82(5-6):245–255. doi: 10.1159/000092753. [DOI] [PubMed] [Google Scholar]
- 623.Tena-Sempere M. Ghrelin and reproduction: ghrelin as novel regulator of the gonadotropic axis. Vitamins and Hormones. 2007;77:285–300. doi: 10.1016/S0083-6729(06)77012-1. [DOI] [PubMed] [Google Scholar]
- 624.Barreiro ML, Tena-Sempere M. Ghrelin and reproduction: a novel signal linking energy status and fertility? Molecular and Cellular Endocrinology. 2004;226(1-2):1–9. doi: 10.1016/j.mce.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 625.Vulliemoz NR, Xiao E, Xia-Zhang L, Rivier J, Ferin M. Astressin B, a nonselective corticotropin-releasing hormone receptor antagonist, prevents the inhibitory effect of ghrelin on luteinizing hormone pulse frequency in the ovariectomized rhesus monkey. Endocrinology. 2008;149(3):869–874. doi: 10.1210/en.2007-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Lebrethon M-C, Aganina A, Fournier M, Gerard A, Parent AS, Bourguignon JP. Effects of in vivo and in vitro administration of ghrelin, leptin and neuropeptide mediators on pulsatile gonadotrophin-releasing hormone secretion from male rat hypothalamus before and after puberty. Journal of Neuroendocrinology. 2007;19(3):181–188. doi: 10.1111/j.1365-2826.2006.01518.x. [DOI] [PubMed] [Google Scholar]
- 627.Forbes S, Li XF, Kinsey-Jones J, O’Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neuroscience Letters. 2009;460(2):143–147. doi: 10.1016/j.neulet.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 628.Gaytan F, Barreiro ML, Caminos JE, et al. Expression of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in normal human testis and testicular tumors. Journal of Clinical Endocrinology and Metabolism. 2004;89(1):400–409. doi: 10.1210/jc.2003-031375. [DOI] [PubMed] [Google Scholar]
- 629.Viani I, Vottero A, Tassi F, et al. Ghrelin inhibits steroid biosynthesis by cultured granulosa-lutein cells. Journal of Clinical Endocrinology and Metabolism. 2008;93(4):1476–1481. doi: 10.1210/jc.2007-2063. [DOI] [PubMed] [Google Scholar]
- 630.Iqbal J, Kurose Y, Canny B, Clarke IJ. Effects of central infusion of ghrelin on food intake and plasma levels of growth hormone, luteinizing hormone, prolactin, and cortisol secretion in sheep. Endocrinology. 2006;147(1):510–519. doi: 10.1210/en.2005-1048. [DOI] [PubMed] [Google Scholar]
- 631.Caminos JE, Tena-Sempere M, Gaytan F, et al. Expression of ghrelin in the cyclic and pregnant rat ovary. Endocrinology. 2003;144(4):1594–1602. doi: 10.1210/en.2002-221058. [DOI] [PubMed] [Google Scholar]
- 632.Gaytan F, Barreiro ML, Chopin LK, et al. Immunolocalization of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in the cyclic human ovary. Journal of Clinical Endocrinology and Metabolism. 2003;88(2):879–887. doi: 10.1210/jc.2002-021196. [DOI] [PubMed] [Google Scholar]
- 633.Tawadros N, Salamonsen LA, Dimitriadis E, Chen C. Facilitation of decidualization by locally produced ghrelin in the human endometrium. Molecular Human Reproduction. 2007;13(7):483–489. doi: 10.1093/molehr/gam029. [DOI] [PubMed] [Google Scholar]
- 634.Du C, Xilingaowa, Cao G, et al. Expression of the orexigenic peptide ghrelin in the sheep ovary. Domestic Animal Endocrinology. 2009;36(2):89–98. doi: 10.1016/j.domaniend.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 635.Messini CI, Dafopoulos K, Chalvatzas N, Georgoulias P, Messinis IE. Effect of ghrelin on gonadotrophin secretion in women during the menstrual cycle. Human Reproduction. 2009;24(4):976–981. doi: 10.1093/humrep/den458. [DOI] [PubMed] [Google Scholar]
- 636.Tsolakis AV, Stridsberg M, Grimelius L, et al. Ghrelin immunoreactive cells in gastric endocrine tumors and their relation to plasma ghrelin concentration. Journal of Clinical Gastroenterology. 2008;42(4):381–388. doi: 10.1097/MCG.0b013e318032338c. [DOI] [PubMed] [Google Scholar]
- 637.Wasko R, Jaskula M, Kotwicka M, et al. The expression of ghrelin in somatotroph and other types of pituitary adenomas. Neuroendocrinology Letters. 2008;29(6):929–938. [PubMed] [Google Scholar]
- 638.White HK, Petrie CD, Landschulz W, et al. Effects of an oral growth hormone secretagogue in older adults. Journal of Clinical Endocrinology and Metabolism. 2009;94(4):1198–1206. doi: 10.1210/jc.2008-0632. [DOI] [PubMed] [Google Scholar]
- 639.Xie QF, Wu CX, Meng QY, Li N. Ghrelin and truncated ghrelin variant plasmid vectors administration into skeletal muscle augments long-term growth in rats. Domestic Animal Endocrinology. 2004;27(2):155–164. doi: 10.1016/j.domaniend.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 640.Svensson J, Monson JP, Vetter T, et al. Oral administration of the growth hormone secretagogue NN703 in adult patients with growth hormone deficiency. Clinical Endocrinology. 2003;58(5):572–580. doi: 10.1046/j.1365-2265.2003.01754.x. [DOI] [PubMed] [Google Scholar]
- 641.Alaioubi B, Mann K, Petersenn S. Diagnosis of growth hormone deficiency in adults: provocative testing with GHRP6 in comparison to the insulin tolerance test. Hormone and Metabolic Research. 2009;41(3):238–243. doi: 10.1055/s-0028-1093350. [DOI] [PubMed] [Google Scholar]
- 642.Pazos Y, Casanueva FF, Camina JP. Basic aspects of ghrelin action. Vitamins and Hormones. 2007;77:89–119. doi: 10.1016/S0083-6729(06)77005-4. [DOI] [PubMed] [Google Scholar]
- 643.Kaiya H, Kojima M, Hosoda H, et al. Amidated fish ghrelin: purification, cDNA cloning in the Japanese eel and its biological activity. Journal of Endocrinology. 2003;176(3):415–423. doi: 10.1677/joe.0.1760415. [DOI] [PubMed] [Google Scholar]
- 644.Riley LG, Fox BK, Kaiya H, Hirano T, Grau EG. Long-term treatment of ghrelin stimulates feeding, fat deposition, and alters the GH/IGF-I axis in the tilapia, Oreochromis mossambicus . General and Comparative Endocrinology. 2005;142(1-2):234–240. doi: 10.1016/j.ygcen.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 645.Garcia EA, Heude B, Petry CJ, et al. Ghrelin receptor gene polymorphisms and body size in children and adults. Journal of Clinical Endocrinology and Metabolism. 2008;93(10):4158–4161. doi: 10.1210/jc.2008-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Baessler A, Fischer M, Mayer B, et al. Epistatic interaction between haplotypes of the ghrelin ligand and receptor genes influence susceptibility to myocardial infarction and coronary artery disease. Human Molecular Genetics. 2007;16(8):887–899. doi: 10.1093/hmg/ddm033. [DOI] [PubMed] [Google Scholar]
- 647.Li W-J, Zhen Y-S, Sun K, et al. Ghrelin receptor gene polymorphisms are associated with female metabolic syndrome in Chinese population. Chinese Medical Journal. 2008;121(17):1666–1669. [PubMed] [Google Scholar]
- 648.Korbonits M, Gueorguiev M, O’Grady E, et al. A variation in the ghrelin gene increases weight and decreases insulin secretion in tall, obese children. Journal of Clinical Endocrinology and Metabolism. 2002;87(8):4005–4008. doi: 10.1210/jcem.87.8.8881. [DOI] [PubMed] [Google Scholar]
- 649.Ozawa A, Cai Y, Lindberg I. Production of bioactive peptides in an in vitro system. Analytical Biochemistry. 2007;366(2):182–189. doi: 10.1016/j.ab.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Takahashi T, Ida T, Sato T, et al. Production of n-octanoyl-modified ghrelin in cultured cells requires prohormone processing protease and ghrelin o-acyltransferase, as well as n-octanoic acid. Journal of Biochemistry. 2009;146(5):675–682. doi: 10.1093/jb/mvp112. [DOI] [PubMed] [Google Scholar]
- 651.Yang Y, Cao J, Shi Y. Identification and characterization of a gene encoding human LPGAT1, an endoplasmic reticulum-associated lysophosphatidylglycerol acyltransferase. Journal of Biological Chemistry. 2004;279(53):55866–55874. doi: 10.1074/jbc.M406710200. [DOI] [PubMed] [Google Scholar]
- 652.LeSautera J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(32):13582–13587. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Bennett KA, Langmead CJ, Wise A, Milligan G. Growth hormone secretagogues and growth hormone releasing peptides act as orthosteric super-agonists but not allosteric regulators for activation of the G protein Gαo1 by the ghrelin receptor. Molecular Pharmacology. 2009;76(4):802–811. doi: 10.1124/mol.109.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Tannenbaum GS, Khoja ZJ, Chang J-K, Veldhuis JD, Bowers CY. In vivo activity of in vitro ghrelin O-acyltransferase (GOAT) inhibitors on food intake and GH release in rats. In: Proceedings of the 91st Annual Meeting of the Endocrine Society; 2009; Washington, DC, USA. [Google Scholar]