Abstract
Background
Bupropion’s efficacy for smoking cessation in pregnant women is unknown.
Objectives
To determine if substance-dependent women prescribed bupropion smoked fewer cigarettes/day than those prescribed citalopram/escitalopram or no antidepressant medication.
Methods
Comparison of smoking in bupropion (n=11), citalopram/escitalopram (n=17), and no antidepressant (n=28) groups.
Results and Conclusions
Trend for greater decrease in smoking for the bupropion versus citalopram/escitalopram group [−6.4 versus −0.4 cigarettes/day (p=.276)], although the bupropion decrease was similar to that seen in the no antidepressant group [−5.3 cigarettes/day].
Scientific Significance
Data support continued study of bupropion in depressed pregnant substance-dependent smokers.
Introduction
Smoking during pregnancy
Smoking during pregnancy has been shown to cause adverse fetal and neonatal outcomes [e.g., spontaneous abortion, premature birth, low birth-weight, and sudden infant death syndrome (SIDS)]; and has been associated with childhood cognitive, emotional and behavioral problems.1 Rates of smoking among pregnant women vary from 12 percent in the general population,2 to 50 percent in depressed women,3 to 90 percent in women with substance use disorders (SUD).4 Women with SUD who become pregnant often have multiple risk factors for poor birth outcomes.5 Many women are motivated to quit smoking during pregnancy.6 Smoking interventions can be successfully employed during SA treatment.7 Additional risks associated with smoking during pregnancy underscore the importance of providing smoking cessation interventions in substance abuse (SA) treatment settings.
Major depressive disorder (MDD) during pregnancy
Like smoking, MDD is a significant problem with a high prevalence in reproductive age women 8. MDD occurs in up to 12% of pregnant women 9 and 40% of pregnant women with a SUD.10 Untreated depression is associated with risky maternal behaviors, and adverse maternal, fetal, and neonatal outcomes.11, 12
Pharmacologic treatment of MDD during pregnancy
Evidence regarding the relative benefits of antidepressant use during pregnancy is growing.13 The decision to prescribe an antidepressant medication to a pregnant woman is complex and requires an individualized appraisal of risk to the fetus of medication exposure, risks to the mother, fetus, and neonate associated with untreated depression, and available alternative therapies.14 During pregnancy, approximately 4–11% of women are exposed to at least one prescribed psychotropic medication.15–17 Many depressed women, in consultation with their physicians, proactively decide to take an antidepressant during pregnancy.18 Although the overall risk from antidepressant use during pregnancy is low, these medications should be given only if the potential benefits justify the potential risk to the fetus any medication use. The FDA has assigned most antidepressants, including bupropion, to pregnancy category C (human fetal risk cannot be ruled out), with the exception of paroxetine which is category D (positive evidence of human fetal risk exists).19 Available data regarding fetal exposure to atypical antidepressants, like bupropion, suggest no increased risk of adverse pregnancy events associated with medication use.20–25 Expert opinion and consensus generally recommends using the lowest effective dose of a single antidepressant, based on personal/family history of effectiveness, prior exposure during pregnancy, and available reproductive safety information.14
Nicotine replacement therapy (NRT) during pregnancy
Based primarily upon animal studies, it has been postulated that nicotine causes uteroplacental insufficiency and fetal neurotoxicity, and that it inhibits pulmonary cell maturation and increases the risk of SIDS.26 In pregnant women, three NRT trials have been published, yet evidence remains inadequate to fully evaluate NRT’s efficacy and safety during pregnancy.27
Pharmacologic treatment of both smoking and MDD during pregnancy
Given the prevalence of smoking in depressed pregnant women, and the desire to use a single medication to limit fetal exposure, a pharmacologic alternative to NRT that targets both smoking and depression would be ideal during pregnancy. This medication should have demonstrated efficacy for both smoking and MDD in non-pregnant individuals, and a relatively good safety profile for use during pregnancy. Sustained release bupropion (Zyban®), optimally dosed at 300 mg daily, was the first non-nicotine medication approved by the US FDA for smoking cessation in non-pregnant adults.28 Prior to its approval as an effective smoking treatment, bupropion (Wellbutrin®), was indicated only as an antidepressant. Its main mechanism of action is reputed to be via dopamine and noradrenalin reuptake inhibition.29 Given bupropion’s known efficacy for both smoking and MDD in general populations, and its relatively good pregnancy safety profile,20–25 bupropion is an ideal candidate medication to treat both smoking and MDD during pregnancy. To date, there are no published data available regarding the efficacy of bupropion for either smoking or depression treatment in controlled trials of pregnant women, 30 although one small observational study has suggested bupropion’s effectiveness for smoking cessation during pregnancy.31 Not surprisingly, bupropion is commonly used to treat both pregnant women with MDD 24 and, to a lesser extent, pregnant smokers. 32 The maternal and fetal effectiveness and safety of bupropion SR during pregnancy has been recommended as a research topic.28
Purpose of the present study
The present study represents an initial evaluation of whether changes in cigarette use would differ between depressed pregnant substance-dependent smokers prescribed bupropion vs. citalopram/escitalopram. The primary hypothesis is that bupropion is superior compared to citalopram/escitalopram in reducing cigarette use, with comparable effectiveness in improving depression symptoms. The comparison medications were chosen for several reasons: 1) they are selective serotonin reuptake inhibitor (SSRI) antidepressants with no known cigarette smoking cessation effect, like all other SSRIs; 2) they are antidepressants that are well accepted, effective, and tolerated by many pregnant women, like all other SSRIs; 3) at the time of the study, they were both antidepressants with a relatively good safety profile in pregnancy and lactation, unlike all other SSRIs; 4) they are stereo-isomers of one another and together were used by a greater number of patients than any individual antidepressant during the study period. Other SSRIs were not included in the study cohort due to data collection constraints. This study also compared smoking in women using bupropion or citalopram/escitalopram for depression to smoking among non-depressed women (i.e., those using no antidepressant).
Materials and Methods
Setting
The Center for Addiction and Pregnancy (CAP) is a comprehensive care program for substance-dependent pregnant women. CAP patients typically complete an initial 7-day residential stay, followed by outpatient treatment.33
Participants and sampling
The Johns Hopkins University Institutional Review Board approved the study and waived the requirement for informed consent. Medical records for 503 CAP patients admitted between January 1, 2007 and June 30, 2008 were evaluated for possible study inclusion. Potential participants were identified by self-reported bupropion, citalopram/escitalopram or no antidepressant use and self-reported smoking at least one cigarette per day (CPD), and were included in the final analyses if they were living in a non-restricted smoking environment, not using NRT, and not enrolled in a behavioral smoking cessation research study. Participants were 56 pregnant substance-dependent smokers: 28 depressed smokers prescribed medication for depression and 28 non-depressed smokers prescribed no antidepressant (matched for interval of time between initial and final treatment visits). All participants met criteria for moderate or severe major depressive disorder on clinical psychiatric interview using Diagnostic and Statistical Manual (DSM) criteria. No data were collected on co-morbid diagnoses. In addition to nicotine, participants reported use of opioids, cocaine, alcohol and/or marijuana on admission to treatment Participants were classified into one of three groups according to the medical record: (1) bupropion (n=11), (2) medication control (citalopram or escitalopram) (n=17), or (3) non-depressed control (n=28).
Data sources and measures
All data were collected from medical records. To evaluate smoking, self-report was compared from two points during treatment: (1) initial visit upon admission and (2) final treatment visit. For patients prescribed antidepressants, the final visit was the final psychiatric visit on study medication. Psychiatric visits were scheduled on an individual basis and were not scheduled at the same or regular intervals for all patients. For antidepressant non-users, the final visit was the obstetric visit matched for length of time between initial and final visits similar to that of the antidepressant patients. Data were not available from the data set regarding history of exposure prior to pregnancy to study medications. Adherence to medication relied on self-report.
To evaluate smoking, patients reported the total number of cigarettes smoked in the previous 24 hours. To evaluate mood, patients reported overall mood in the previous 24 hours on a 0–10 scale, with 0=worst mood and 10=best mood. Smoking reports were recorded on admission and at all subsequent psychiatric or obstetric visits. Mood reports were recorded at psychiatric visits. Data regarding participant use of non-nicotine substances at visits were not available from the data set.
Data Analyses
All analyses were performed using SPSS Version 16.0 for the Macintosh (SPSS, Inc). Chi-square, ANOVA and Tukey’s HSD tests were used to analyze data by medication status (bupropion, citalopram/escitalopram, and no antidepressant). Secondary analysis was performed on a subset of the cohort (bupropion n=8; citalopram/escitalopram n=11) for whom there were self-reports of mood ratings at both the initial and final visits.
Results
Treatment Admission Demographics and Substance Use (Table 1)
Table 1.
| Total (N=56) | Bupropion (n=11) | Citalopram/Escitalopram (n=17) | No Antidepressant (n=28) | P value | |
|---|---|---|---|---|---|
| Measure | |||||
| Age, mean yrs (SD) | 30.9 (5.8) | 28.5 (4.9) | 32.2 (5.7) | 31.0 (6.0) | 0.238 |
| White, % | 60.7 | 72.7 | 47.1 | 64.3 | 0.342 |
| EGA, mean weeks (SD) | 15.2 (8.0) | 14.6 (5.4) | 14.8 (8.2) | 15.6 (8.9) | 0.920 |
| Education, mean years (SD)** | 10.8 (1.8) | 10.7 (1.4) | 10.9 (1.5) | 10.6 (2.0) | 0.870 |
| Methadone-maintained, %^ | 85.2 | 90.9 | 75.0 | 88.9 | 0.388 |
| Substance of abuse/dependence reported on admission: | |||||
| Alcohol, % | 25.0 | 18.2 | 41.2 | 17.9 | 0.182 |
| Cocaine, % | 62.5 | 63.6 | 58.8 | 64.3 | 0.931 |
| Opioid, % | 92.9 | 90.9 | 88.2 | 96.4 | 0.563 |
| Marijuana, % | 12.5 | 9.1 | 11.8 | 14.3 | 0.902 |
| Cigarettes per day reported at initial visit (SD) | 14.7 (8.6) | 14.6 (10.0) | 14.5 (9.7) | 14.9 (7.6) | 0.992 |
EGA=estimated gestational age; SD=standard deviation
missing data for 1 subject from the citalopram/escitalopram group
missing data for 1 subject from the citalopram/escitalopram group and 1 from the no antidepressant group
Baseline demographic and substance use characteristics of the sample did not differ between groups.
Smoking (Primary Analysis) (Figure 1)
Figure 1.
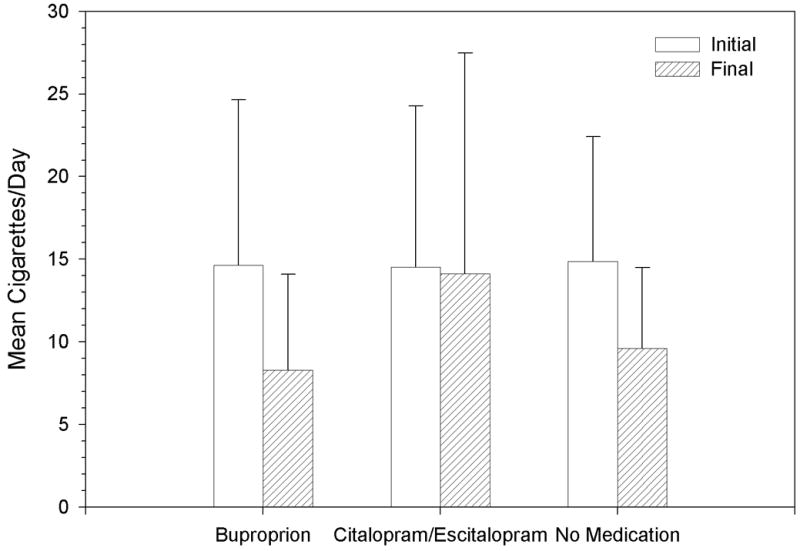
Mean time (weeks) between the initial and final visit for all patients was 16.1 (SD 10.2). Mean CPD at the initial visit for the total sample was 14.7 (SD 8.6). Mean CPD at initial visit for the bupropion, citalopram/escitalopram, and no medication groups was 14.6 (SD 10.0), 14.5 (SD 9.7), and 14.9 (SD 7.6), respectively. Mean daily medication dose (mg)/duration (weeks) for bupropion and citalopram/escitalopram groups was 282 (SD 147)/5 (SD 3) and 29 (SD 15)/7 (SD 6), respectively. Data regarding the mean daily medication dose for citalopram versus escitalopram doses are not available from the data set. No serious adverse events were noted with any medication. Data regarding other adverse events were not systematically recorded and were not available from the data set.
An ANOVA showed no statistically significant between-group difference in decrease in CPD (F2,53=1.64, p=0.202) (Figure 1). Subsequent pair-wise comparisons were performed as part of the original analysis plan. These comparisons showed no differences in the decrease of CPD between users of bupropion and citalopram/escitalopram (6.4 vs. 0.4, p=0.276), between users of bupropion and nonusers of antidepressants (6.4 vs 5.3, p=0.947), or between users of citalopram/escitalopram and nonusers of antidepressants (0.4 vs 5.3, p=0.260).
It was not possible to match the interval between the initial and final visits across the three groups. Matching was performed between medication and no medication groups only. Thus, CPD/weeks of treatment was calculated in order to ensure comparability across the three groups. An ANOVA of decrease in CPD/weeks of treatment showed a non-statistically significant between-group difference (F 2,53=2.75, p=0.073), Subsequent pair-wise comparisons showed no differences between nonusers and either buproprion or citalopram/escitalopram (p=0.161 and 0.736, respectively) but a trend toward a greater rate of decrease in CPD/week of treatment between users of bupropion and users of citalopram/escitalopram (p=0.064)
Mood (Secondary Analysis) (Figure 2)
Figure 2.
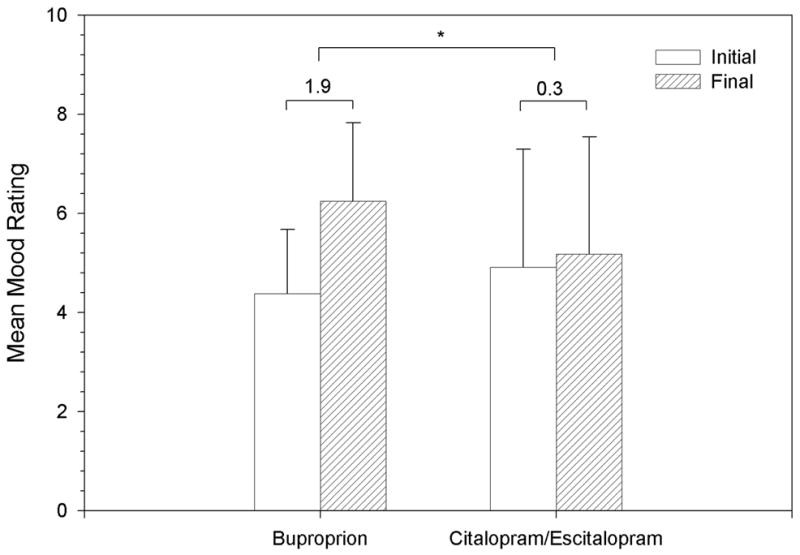
The subset of the cohort used for this analysis did not differ significantly from the total sample on baseline demographics and substance use. Mean time (weeks) between initial and final visit for all patients remained 16.1 (SD 10.2).
The mean daily dose (mg)/duration (weeks) for bupropion and citalopram/escitalopram users was 256 (SD 161)/5 (SD 3) and 31 (SD 12)/4 (SD 3.5), respectively. The mean mood rating at the initial visit for the bupropion and citalopram/escitalopram groups was 4.4 (SD 1.3) and 4.9 (SD 2.4), respectively. Changes in mood ratings were significantly different (p=.028) between bupropion and citalopram/escitalopram groups, with greater improvement in mood rating for the bupropion group [1.9 (SD 1.6) vs. 0.3 (SD 1.3)] (Figure 2).
Discussion
The frequent co-occurrence of smoking and MDD in pregnant women with SUD, coupled with the desire to limit fetal exposure to multiple toxins, provides the rationale for identifying a single drug to treat both smoking and MDD in this population. Data from this study show a trend towards a greater reduction in smoking in depressed pregnant smokers using bupropion compared to citalopram/escitalopram, with decreases for bupropion similar to that seen in patients who are not depressed. Bupropion users in this study also had a greater improvement in mood rating compared to citalopram/escitalopram users. These differences occurred despite similarly prescribed effective doses of each antidepressant.
One interpretation of these findings is that bupropion is just as effective as no medication treatment for smoking, and that citalopram/escitalopram is worse than no treatment as a smoking cessation aid (i.e., that citalopram/escitalopram might actually worsen the likelihood that a patient would stop smoking). However, the no antidepressant group differed in two important ways from the antidepressant groups – first, in the use of an antidepressant medication, and second, in the presence of a depressive disorder. Ideally, the bupropion and citalopram/escitalopram groups would be compared to a group of depressed smokers who received no medication treatment (or placebo). However, in the absence of such a group, the findings for the citalopram/escitalopram patients should not be over interpreted as suggesting citalopram/escitalopram worsens outcome.
The fact that bupropion showed a trend toward some efficacy is promising, especially given that bupropion was not used specifically as a smoking cessation aid in these patients. Thus, target quit dates designed relative to the start of medication were not set, and other behavioral and counseling interventions for smoking cessation were not specified with these patients. Such interventions would be expected to produce better outcomes for patients treated with bupropion.
This study has several limitations. It used broadly defined measures of smoking and mood. A prospective trial examining smoking and mood could include more comprehensive and sensitive measures and yield results supporting stronger conclusions. The short duration of time on bupropion or citalopram/escitalopram required for study inclusion, non-observed medication use, and differences in duration of time on drug between the two groups may minimize smoking and mood findings. Although the mean bupropion dose prescribed is adequate in non-pregnant substance-dependent populations for smoking cessation response, it has not been studied in pregnant patients and may minimize smoking findings in the present study. The lack of observed medication ingestion may also minimize smoking and mood findings. In addition, heterogeneity of women’s trimester status, co-occurring psychiatric disorders (which were not systematically assesed), ongoing substance abuse, and control medications may have played a role in the outcomes. Sample sizes are modest and may limit the power to detect differences. Also noteworthy is the recent FDA-required new Boxed Warnings, highlighting the risk of serious neuropsychiatric symptoms in patients using smoking cessation aids varenicline and bupropion. These symptoms include changes in behavior, agitation, depressed mood, suicidal thoughts, and attempted suicide.34 (The same changes will also be required for bupropion’s indication for the treatment of depression and seasonal affective disorder.) Despite these limitations, the present study provides some of the first data examining the relationship between bupropion and smoking in pregnant women enrolled in comprehensive drug treatment.
These results comparing bupropion and citalopram/escitalopram demonstrate a trend towards a greater reduction in cigarettes smoked and greater improvement in mood for women treated with bupropion. This suggests the value in further studying the smoking cessation efficacy and safety of bupropion in populations of pregnant substance-dependent smokers.
Contributor Information
Margaret Chisolm, Email: mchisol1@jhmi.edu, 5300 Alpha Commons Drive, 4th floor, Baltimore, MD, 21224, USA.
Ms Michelle Tuten, Email: mtuten@jhmi.edu, Johns Hopkins University, Psychiatry and Behavioral Sciences, Baltimore, USA.
Dr Emily P Brigham, Email: epfeil1@jhmi.edu, Johns Hopkins University, General Internal Medicine, Baltimore, USA.
Dr Eric C Strain, Email: ECSGSS@aol.com, Johns Hopkins University, Psychiatry and Behavioral Sciences, Baltimore, USA.
References
- 1.U.S. Department of Health and Human Services. The health consequences of smoking: A report of the surgeon general. 2004. [Google Scholar]
- 2.U.S. Department of Health and Human Services. Women and smoking: A report of the surgeon general. Rockville (MD): U.S. department of health and human services, public health service, office of the surgeon general; 2001. [Google Scholar]
- 3.Hoffman S, Hatch MC. Depressive symptomatology during pregnancy: Evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol. 2000;19:535–543. [PubMed] [Google Scholar]
- 4.Haug NA, Svikis DS, Diclemente C. Motivational enhancement therapy for nicotine dependence in methadone-maintained pregnant women. Psychol Addict Behav. 2004;18:289–292. doi: 10.1037/0893-164X.18.3.289. [DOI] [PubMed] [Google Scholar]
- 5.Kuczkowski KM. The effects of drug abuse on pregnancy. Curr Opin Obstet Gynecol. 2007;19:578–585. doi: 10.1097/GCO.0b013e3282f1bf17. [DOI] [PubMed] [Google Scholar]
- 6.Solomon L, Quinn V. Spontaneous quitting: Self-initiated smoking cessation in early pregnancy. Nicotine Tob Res. 2004;6 (Suppl 2):S203–16. doi: 10.1080/14622200410001669132. [DOI] [PubMed] [Google Scholar]
- 7.Shoptaw S, Rotheram-Fuller E, Yang X, et al. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–28. doi: 10.1046/j.1360-0443.2002.00221.x. discussion 1325. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the national comorbidity survey. II: Cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 9.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: Systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 10.Martin PR, Arria AM, Fischer G, et al. Psychopharmacologic management of opioid-dependent women during pregnancy. Am J Addict. 2009;18:148–156. doi: 10.1080/10550490902772975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blalock JA, Fouladi RT, Wetter DW, Cinciripini PM. Depression in pregnant women seeking smoking cessation treatment. Addict Behav. 2005;30:1195–1208. doi: 10.1016/j.addbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Henry AL, Beach AJ, Stowe ZN, Newport DJ. The fetus and maternal depression: Implications for antenatal treatment guidelines. Clin Obstet Gynecol. 2004;47:535–546. doi: 10.1097/01.grf.0000135341.48747.f9. [DOI] [PubMed] [Google Scholar]
- 13.Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: Impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166:557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ACOG Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, april 2008 (replaces practice bulletin number 87, november 2007). use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001–1020. doi: 10.1097/AOG.0b013e31816fd910. [DOI] [PubMed] [Google Scholar]
- 15.Calderon-Margalit R, Qiu C, Ornoy A, Siscovick DS, Williams MA. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009 doi: 10.1016/j.ajog.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos E, Oraichi D, Rey E, Blais L, Berard A. Prevalence and predictors of antidepressant use in a cohort of pregnant women. BJOG. 2007;114:1055–1064. doi: 10.1111/j.1471-0528.2007.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti F, Romero M, Bonati M, Tognoni G. Use of psychotropic drugs during pregnancy. A report of the international co-operative drug use in pregnancy (DUP) study. collaborative group on drug use in pregnancy (CGDUP) Eur J Clin Pharmacol. 1993;45:495–501. doi: 10.1007/BF00315304. [DOI] [PubMed] [Google Scholar]
- 18.Cohen LS, Nonacs R, Viguera AC, Reminick A. Diagnosis and treatment of depression during pregnancy. CNS Spectr. 2004;9:209–216. doi: 10.1017/s1092852900009007. [DOI] [PubMed] [Google Scholar]
- 19.Howland RH. Categorizing the safety of medications during pregnancy and lactation. J Psychosoc Nurs Ment Health Serv. 2009;47:17–20. doi: 10.3928/02793695-20090401-06. [DOI] [PubMed] [Google Scholar]
- 20.Einarson A, Choi J, Einarson TR, Koren G. Incidence of major malformations in infants following antidepressant exposure in pregnancy: Results of a large prospective cohort study. Can J Psychiatry. 2009;54:242–246. doi: 10.1177/070674370905400405. [DOI] [PubMed] [Google Scholar]
- 21.Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: A report from the american psychiatric association and the american college of obstetricians and gynecologists. Gen Hosp Psychiatry. 2009;31:403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole JA, Modell JG, Haight BR, Cosmatos IS, Stoler JM, Walker AM. Bupropion in pregnancy and the prevalence of congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:474–484. doi: 10.1002/pds.1296. [DOI] [PubMed] [Google Scholar]
- 23.Way CM. Safety of newer antidepressants in pregnancy. Pharmacotherapy. 2007;27:546–552. doi: 10.1592/phco.27.4.546. [DOI] [PubMed] [Google Scholar]
- 24.Chun-Fai-Chan B, Koren G, Fayez I, et al. Pregnancy outcome of women exposed to bupropion during pregnancy: A prospective comparative study. Am J Obstet Gynecol. 2005;192:932–936. doi: 10.1016/j.ajog.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Einarson TR, Einarson A. Newer antidepressants in pregnancy and rates of major malformations: A meta-analysis of prospective comparative studies. Pharmacoepidemiol Drug Saf. 2005;14:823–827. doi: 10.1002/pds.1084. [DOI] [PubMed] [Google Scholar]
- 26.Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: U.S. preventive services task force reaffirmation recommendation statement. Ann Intern Med. 2009;150:551–555. doi: 10.7326/0003-4819-150-8-200904210-00009. [DOI] [PubMed] [Google Scholar]
- 28.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkes S. Bupropion. Drugs Today (Barc) 2006;42:671–681. doi: 10.1358/dot.2006.42.10.1025701. [DOI] [PubMed] [Google Scholar]
- 30.Crawford JT, Tolosa JE, Goldenberg RL. Smoking cessation in pregnancy: Why, how, and what next. Clin Obstet Gynecol. 2008;51:419–435. doi: 10.1097/GRF.0b013e31816fe9e9. [DOI] [PubMed] [Google Scholar]
- 31.Chan B, Einarson A, Koren G. Effectiveness of bupropion for smoking cessation during pregnancy. J Addict Dis. 2005;24:19–23. doi: 10.1300/J069v24n02_02. [DOI] [PubMed] [Google Scholar]
- 32.Rigotti NA, Park ER, Chang Y, Regan S. Smoking cessation medication use among pregnant and postpartum smokers. Obstet Gynecol. 2008;111:348–355. doi: 10.1097/01.AOG.0000297305.54455.2e. [DOI] [PubMed] [Google Scholar]
- 33.Jansson LM, Svikis DS, Velez M, Fitzgerald E, Jones HE. The impact of managed care on drug-dependent pregnant and postpartum women and their children. Subst Use Misuse. 2007;42:961–974. doi: 10.1080/10826080701212451. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Federal Drug Administration. [Accessed October/08, 2009]; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm169986.htm.


