Abstract
Purpose of review
The immune modulatory effects of total lymphoid irradiation (TLI) for graft-versus-host disease (GVHD) protection and transplantation tolerance following allogeneic bone marrow and organ transplantation have been studied for years in animal models. In preclinical models nonmyeloablative TLI conditioning alters residual host T cell subsets to favor regulatory natural killer T cells that suppress GVHD and prevent organ allograft rejection. These preclinical models have been recently adapted to human transplantation.
Recent findings
Patients receiving allogeneic hematopoietic cell transplantation for hematological malignancies conditioned with TLI and depletive T cell antibodies showed sustained donor chimerism, a reduced incidence of acute GVHD yet retained graft antitumor activity. As in the preclinical models, nonmyeloablative TLI conditioning significantly altered residual host T cell subsets favoring natural killer T cells, and the low incidence of GVHD was associated with increased IL-4 secretion by chimeric donor T cells. The TLI regimen used in cancer patients was modified to determine conditions for stable mixed chimerism and tolerance induction following combined hematopoietic cell and kidney transplantation.
Summary
This review summarizes the evolution of the preclinical TLI protocols and their recent translation to clinical trials, and discusses the mechanisms involved in protection from GVHD and the induction of tolerance following mixed chimerism.
Keywords: graft-versus-host disease, hematopoietic cell transplantation, transplantation tolerance
Introduction: brief historical perspective of total lymphoid irradiation
Total lymphoid irradiation (TLI) was developed as curative treatment for Hodgkin lymphoma [1] and its administration follows two radio-therapeutic principles: the radiation fields are directed to all major lymph node-bearing areas including the thymus and spleen while shielding the nonlymphoid organs in the head, chest, abdomen and pelvis; and fractionation of the radiation treatment into daily low doses of well tolerated fractions of 80–200 cGy each, results in minimal side effects. The administration of TLI ensures that radiosensitive lymphocytes can be effectively eliminated from the lymphoid compartments while avoiding exposure of other radiosensitive tissue such as bone marrow, intestinal tract and lungs. The use of TLI as an immunemodulating strategy was initially suggested by studies that reported altered T-lymphocyte-mediated immune responses in patients who had received TLI for the treatment of Hodgkin lymphoma [2]. The period of 1976–1978 introduced preclinical models describing TLI as the first nonmyeloablative conditioning regimen that altered the recipient’s immune system and resulted in long-term survival of allogeneic bone marrow and skin grafts [3–5].
The development of chimerism and transplantation tolerance following pretransplantation total lymphoid irradiation in small and large laboratory animals
Slavin et al. [4] showed that 17 doses of TLI (200 cGy per dose) targeted to the lymph nodes, spleen, and thymus of adult BALB/c mice with shielding of the lungs, skull, and long bones was nonmyeloablative and resulted in sustained engraftment of MHC-mismatched bone marrow from C57BL/6 donors. The bone marrow recipients achieved 50–95% donor-type lymphocytes and red blood cells, whereas none developed graft-versus-host disease (GVHD) [4,5]. The addition of relatively few splenocytes to the bone marrow inoculum converted the TLI-treated hosts to complete donor-type chimeras without the development of GVHD by clinical and histologic examination [6]. The ability to successfully achieve long-term chimerism depended on the timing of the bone marrow infusion and on the TLI protocol [7]. When the bone marrow infusion was delayed 1 week after completion of TLI, fewer than 50% of mice achieved chimerism, and no mice developed chimerism if the infusion was delayed 14 days. When the TLI protocol was compacted by administering multiple TLI fractions on the same day the ability to achieve sustained donor cell chimerism was significantly compromised [7].
In other experiments, skin grafts from C57BL/6 mice were transplanted to TLI-conditioned BALB/c recipients at the same time as the bone marrow infusion. The donor skin grafts were accepted in the mice that developed persistent mixed chimerism [5]. The chimeric mice rapidly rejected third-party skin grafts confirming specific tolerance. TLI-treated mice that received skin grafts but without a donor bone marrow infusion had an average graft survival of around 50 days. Therefore, in this model the attainment of sustained donor hematopoietic cell chimerism was a necessity for donor-specific immune tolerance. In similar experiments with adult rats, persistent mixed chimerism was a requirement for transplantation tolerance to vascularized heart allografts [8].
The rodent TLI protocol needed modifications for the successful adaptation to larger animals because the wide radiation fields and the relatively high total TLI dose resulted in limiting gastrointestinal and bone marrow toxicity [9]. Yet lowering the dose of radiation and narrowing the fields for TLI resulted in allograft rejection due to the persistence of a larger number of conventional host T cells [9]. Thus, it was theorized that the addition of the T-cell-depleting agent, antithymocyte serum (ATS), would compensate for the reduced dose of TLI. Subsequently, it was shown in dogs that reduced-dose TLI combined with six doses of ATS resulted in prolonged mismatched allograft survival [10]. Hosts that accepted the donor allografts rejected third-party grafts, indicating that specific immune tolerance had developed.
Adaptation of nonmyeloablative total lymphoid irradiation and antithymocyte serum conditioning to a post-transplantation tolerance induction protocol in animals
A barrier to the adaptation of tolerance induction protocols to cadaveric organ transplantation in humans is the inability to predict the availability of donor organs. Thus, an entirely post-transplantation regimen is required for clinical adaptability. Accordingly, a post-transplantation TLI-based regimen for tolerance induction was developed. Adult Lewis rats received daily doses of TLI (10 doses × 180 cGy/dose) combined with ATS (five doses) initiated immediately after mismatched heart transplantation. Donor-specific tolerance was achieved if the recipients received same donor bone marrow upon completion of the TLI and ATS (14 days after cardiac transplantation) and had achieved sustained chimerism [11,12]. In this model a brief course of cyclosporine following the administration of TLI and ATS regimen facilitated the attainment of mixed chimerism and prolonged cardiac allograft survival without rejection [13].
Protection against graft-versus-host disease after allogeneic bone marrow transplantation in total lymphoid irradiation-treated hosts
Total lymphoid irradiation alters the host’s immune profile to favor regulatory natural killer T (NKT) cells that suppress GVHD by polarizing donor conventional T cells toward secretion of noninflammatory cytokines such as IL-4.
Predominance of ‘natural suppressor’ natural killer T cells after total lymphoid irradiation
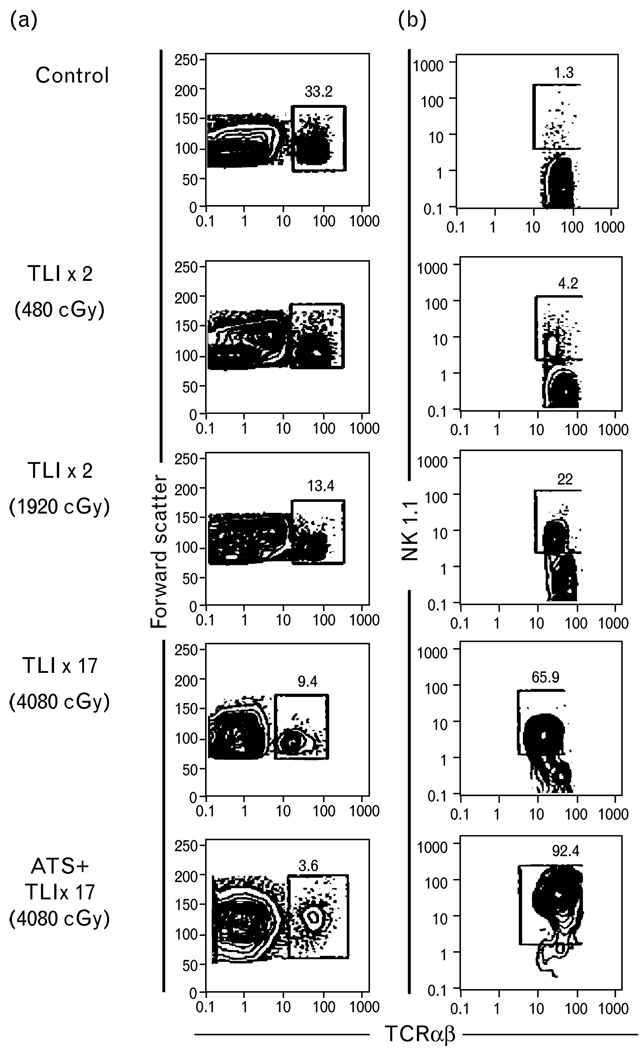
The link of GVHD protection with NKT cells followed two key observations. First, in the initial studies of allogeneic bone marrow transplantation, hosts conditioned with TLI were protected from GVHD even in the setting of full donor chimerism [4,5]. Second, it was reported that in mouse models of bone marrow transplantation an infusion of NK1.1+ T cells isolated from the normal bone marrow and spleen protected against GVHD [14]. As such, investigators thought to evaluate for alterations in host NKT cells before, during and after TLI treatment [6]. Figure 1 shows flow cytometric analyses of C57BL/6 spleen cells stained for the TCRαβ marker versus forward light scatter (panel a) before and after 2, 8 or 17 irradiation treatments of 240 cGy each targeted to the thymus, spleen, and lymph nodes while shielding the head, lungs, and hind limbs. After 17 treatments of TLI, the mean absolute number of TCRαβ+ T cells decreased over 150-fold [6]. In contrast, the mean absolute number of NK1.1+ TCRαβ+ T cells in the spleen before compared to after TLI administration decreased less than five-fold (panel b). This marked change in the balance of the host T cell subsets in the lymphoid compartment altered the ratio of NK1.1+ to conventional TCRαβ+ T cells from about 1% of all T cells before irradiation to 66% after 17 treatments.
Figure 1. Immunofluorescent staining of C57BL/6 spleen cells.
(a) Immunofluorescent staining of C57BL/6 spleen cells after 2, 8, or 17 doses of TLI or TLI and ATS. Flow cytometric analysis shows light scatter versus TCRαβ or NK1.1 versus TCR αβmarkers. (b) Staining of BALB/c spleen cells for DX5 and TCRαβ markers. Boxes show percentages of TCRαβ cells, and percentage of NK1.1+ or DX5+ cells amongst gated TCRαβ+ cells [6]. ATS, antithymocyteserum; TLI, total lymphoidirradiation.
Additional T cell depletion by the administration of three doses of ATS during TLI further increased the ratio of NK1.1+ T cells to NK1.1− T cells. Almost 93% of all surviving host T cells in the spleen, and marrow of mice given both TLI and ATS marked NK1.1+ (Fig. 1, panel b). This change is likely the result of the resistance of NKT cells to radiation and ATS induced apoptosis due to their increased expression of antiapoptotic genes [15••]. It is noteworthy that the alterations in T cell subsets appear unique to TLI (with or without ATS), because total whole body irradiation (TBI; 450 cGy) does not cause similar phenotypic changes [6] (a single dose of TBI does not induce the profound depletion of host conventional T cells required to increase the ratio of residual NKT cells).
Changes in T cell secretion of cytokines after total lymphoid irradiation
The cytokine milieu of the lymphoid compartment is also unique in TLI-treated mice. Residual host T cells obtained from the spleen of TLI-treated mice and stimulated in vitro with an anti-CD3 monoclonal antibody secreted markedly increased levels of IL-4 and reduced levels of IL-2 and IFN-γ compared to T cells from un-irradiated mice [16,17]. Sorting experiments demonstrated that CD4+ NK1.1+ T cells were the source of IL-4 [6]. The pattern of cytokine secretion in chimeric donor T cells obtained in the weeks to months following allogeneic BMT in TLI-treated hosts also showed polarization toward a Th2 phenotype, with increased secretion of IL-4 as well [16,17].
Graft-versus-host disease protection after total lymphoid irradiation is dependent on natural killer T cells and IL-4 production
Hosts conditioned with TBI with or without ATS developed acute lethal GVHD and died within weeks [6]. Approximately 1000-fold more donor splenocytes needed to be injected into hosts conditioned with TLI and ATS compared to TBI to induce similar levels of GVHD mortality [18]. Protection against GVHD in the TLI and ATS-treated host was dependent on residual host regulatory NK1.1+ T cells and their ability to produce IL-4 as NKT-cell-deficient CD1−/− and IL-4−/− recipients died from GVHD. Thus, a regimen that maximizes residual host NK1.1+ αβ+ T cells protects from GVHD.
Total lymphoid irradiation/antithymocyte serum-conditioned hosts retain graft antitumor reactions to BCL1 lymphoma
The retention of graft-versus-tumor (GVT) activity in the TLI and ATS transplant model that protects against GVHD remains essential for the successful translation to human cancer patients. In a series of experiments designed to evaluate this question [19], wild-type hosts conditioned with TLI and ATS were injected with BCL1 lymphoma cells without bone marrow transplantation and uniformly died from tumor progression within 3 weeks. Animals that received TLI and ATS followed by allogeneic bone marrow and splenocyte transplantation engrafted with donor cells, did not develop GVHD and demonstrated graft antitumor activity as tumor progression did not occur and the majority of mice survived. Progressive tumor growth was also observed after the transplantation of donor bone marrow cells without splenocytes, or after the transplantation of allogeneic bone marrow and spleen cells from CD8−/−, perforin−/−, or Fas ligand−/− donors. These results indicate that the subset of donor CD8+ T cells that express cell-to-cell contact molecules mediated through perforin and Fas/Fas ligand pathways were required for killing and clearance of the BCL1 lymphoma tumor cells [19]. In control experiments, hosts treated with lethal or sublethal TBI and ATS followed by injection of bone marrow, splenocytes, and BCL1 lymphoma uniformly died by day + 28 from acute GVHD [20]. Thus, the protection from lethal GVHD is dependent upon the presence of regulatory host NKT cells, whereas graft antitumor activity is dependent upon donor CD8+ T cells and their production of perforin and Fas/Fas ligand. These results helped to elucidate the mechanisms of GVHD protection and retention of graft antitumor activity in clinical trials using TLI and antithymocyte globulin (ATG) conditioning.
Clinical studies
The animal-based TLI-conditioning protocols have been adapted to humans for allogeneic hematopoietic cell transplantation (HCT) for cancer patients and tolerance induction following organ transplantation.
Use of total lymphoid irradiation and antithymocyte globulin for hematopoietic cell transplantation in patients with hematolymphoid malignancies
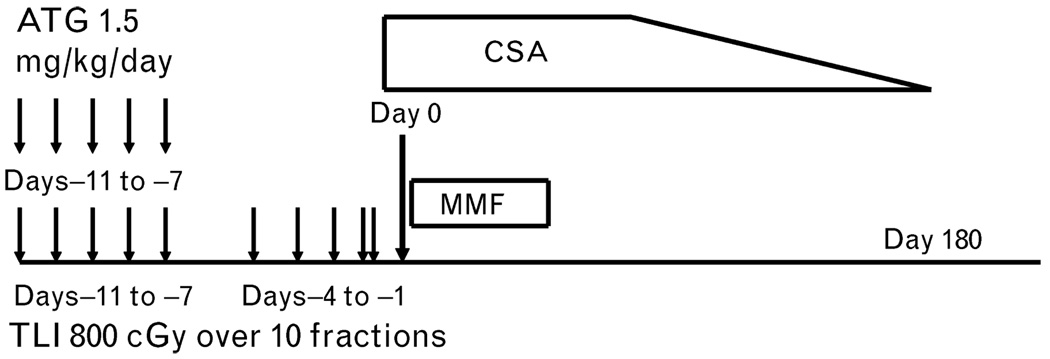
Because the TLI-based conditioning regimen protected against acute GVHD in preclinical studies, the regimen was adapted for use in clinical studies in cancer patients to determine if it also protected against GVHD following allogeneic HCT [20,21••]. One hundred and eleven patients, 64 with lymphoid and 47 with myeloid malignancies, were given 10 doses of TLI of 80 cGy/dose (total dose 800 cGy) and 5 doses of rabbit ATG (1.5 mg/kg/dose) during a 2-week period as pretransplant conditioning, followed by an infusion of G-CSF ‘mobilized’ human leukocyte antigen (HLA)-matched related (n = 61) or unrelated (n = 50) donor PBMC collections (Fig. 2) [21••]. Mycophenolate mofetil was administered for 1 month after transplantation and cyclosporine was administered with a tapering course for 6 months. Notable features included 34 patients 60 years of age or older, 32 patients at high risk of lymphoma relapse after failing a prior autologous HCT, 85 patients with advanced-stage disease, and 51 patients at high risk of developing GVHD due to lack of availability of an HLA-matched related.
Figure 2. Nonmyeloablative conditioning regimen of total lymphoid irradiation and antithymocyte globulin.
Ten doses of total lymphoid irradiation (TLI) (at 80 cGy each) given over a period of 11 days (as indicated by arrows). Antithymocyte globulin (ATG) (at a dose of 1.5 mg per kilogram of body weight per day) was administered intravenously on days −11 through −7 (as indicated by arrows). An infusion of mobilized human leukocyte antigen (HLA)-matched peripheral blood mononuclear cells from a donor was administered on day 0. Immunosuppressive therapy after transplantation included oral cyclosporine started on day −3, at a dose of 6.25 mg per kilogram twice per day tapered over 6 months, and mycophenolate mofetil, at a dose of 15 mg per kilogram twice a day, started on the first day after transplantation and discontinued on day 28 after transplantation [20].
The TLI-conditioning protocol was well tolerated; all patients received their donor cell infusion as an outpatient and only 30 (27%) patients were re-admitted to hospital during the first 100 days with a median length of stay of 3.5 days [21••]. The probability of acute GVHD (grades II–IV) by day 100 was 2% among related donor transplants and 10% among unrelated donor transplant recipients. Severity of acute GVHD among the entire cohort included three patients with grade II, two with grade III, and one with grade IV. The cumulative risk of extensive chronic GVHD at 3 years among recipients of related and unrelated grafts was 28% [95% confidence interval (CI) 16–39] and 26% (95% CI 13–39), respectively. With a minimum follow-up of 1 year for all patients (range 1–7 years) 69% were on no immunosuppressive therapy. Thus, the TLI and ATG regimen appears to protect against acute GVHD in both the preclinical and clinical studies.
Thirty-four patients with measurable lymphoma at the start of the TLI and ATG regimen achieved durable complete remissions post-transplantation [21••]. It is difficult to discern the antitumor activity contributed by 8 Gy of TLI from alloreactive GVT reactions mediated by donor immune cells except in 20 of the 34 patients who achieved a complete remission had clearing of tumor that was outside the field of the TLI. Fourteen of these patients had bone marrow involvement that cleared after transplantation and 6 patients had resolution of other extranodal sites of disease. Thus, despite a reduction in GVHD risk, GVT reactions appeared maintained.
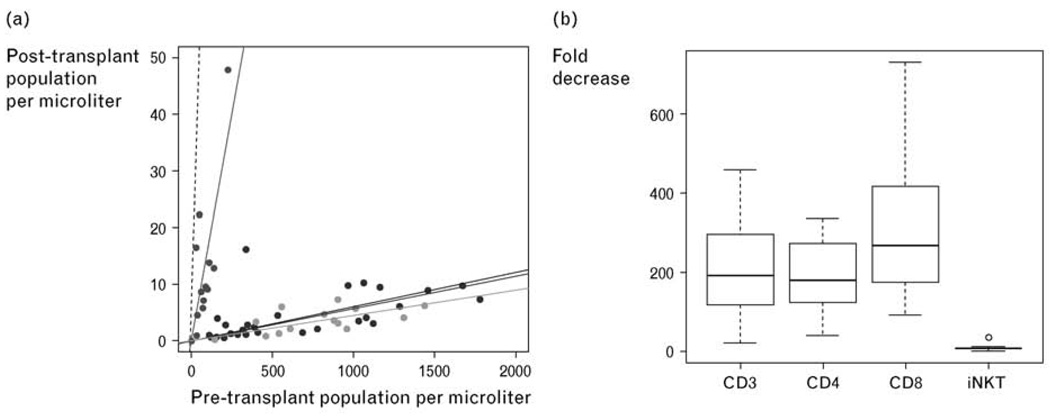
The effect of TLI and ATG on circulating host T cell subsets was monitored for changes in absolute numbers by analysis of peripheral blood samples at 24-h pre-TLI and immediately post-TLI, yet prior to infusion of donor cells (Fig. 3a) [21••]. The median decrease in CD3+, CD4+, and CD8+ T cells was 191-fold, 180-fold, and 268-fold, respectively (Fig. 3b). In contrast, the median decrease (8.6-fold) in invariant NKT cells was significantly less than that observed for conventional T cell subsets (P = 0.001). This resulted in a significant increase in the percentage of NKT cells in the blood amongst gated CD3+ (TCRαβ+) cells, whereas NKT cells accounted for only 0.01% of all CD3+ cells before TLI and ATG administration, 0.5% of T cells expressed the invariant NKT cell TCR Vα24Vβ11 after TLI.
Figure 3. Effect of TLI and ATG on circulating T cell subsets.
Absolute T cell subset population size pre (x-axis) and immediately post-TLI (y-axis) with plotted linear regression (solid lines, constrained through 0,0) for CD3, CD4, CD8, and invariant NKT cell subsets. Dashed line representative of a population with no change (y = x) in population size between pre-TLI and immediately post-TLI (a). Boxplot of fold decrease in CD3, CD4, CD8, and invariant NK T cell populations (median: thick line; quartiles: box; range: whiskers; outliers: circle) (b). ATG, antithymocyte globulin; TLI, total lymphoid irradiation. Reproduced with permission from the American Society of Hematology [21••].
Chimeric donor CD4+ T cells obtained 3–9 months after transplantation showed a marked increase in production of IL-4, compared to the production by CD4+ T cells in samples obtained from normal control patients [20]. Donor CD4+ T cells from patients who underwent conditioning with sublethal TBI, rather than TLI and ATG, did not show an increased production of IL-4. It is likely that the changes in the function of donor T cells reduced the risk of acute GVHD, since donor T cells with a profile of increased Th2 cytokine secretion have a decreased capacity to induce GVHD in rodents [22–24].
Combined human leukocyte antigen-matched living donor kidney and blood hematopoietic progenitor cell transplantation
Immune tolerance in association with persistent mixed hematopoietic cell chimerism can be achieved after combined organ and HCT in mice conditioned with TLI and ATS. The safety and reproducibility of this approach was studied in a cohort of humans with end-stage renal failure who received kidney transplants and an infusion of same donor hematopoietic cells [25••]. TLI was administered in the same manner as it was in cancer patients: 10 doses of 80–120 cGy each to the supradiaphragmatic lymph nodes, thymus, subdiaphragmatic lymph nodes, and spleen during the first 11 days post kidney transplant. ATG (Thymoglobulin, Genzyme) was given intravenously (1.5 mg per kilogram for each of five daily doses) starting with an intraoperative injection. Donor CD34+ selected cells and a defined dose of T cells (1 × 106 per kg recipient weight) were infused intravenously on day 11 in the outpatient clinic in all patients. Posthematopoietic cell infusion immune suppression consisted of short-course mycophenolate mofetil and prednisone and cyclosporine with tapering over 6 months in patients with persistent mixed chimerism.
Multilineage mixed chimerism (levels of donor cells from 5–75%) developed in five of six patients and none developed GVHD [25••]. Persistent (>1 year) mixed chimerism occurred in four patients, all of whom met the monitoring criteria for immune suppression drug withdrawal and had subsequent drug discontinuation. The drugs have been discontinued for 6–35 months without evidence of graft dysfunction as judged by creatinine clearance, urinary protein, and surveillance biopsies. Thus, a protocol that protects against GVHD and can be manipulated to ensure persistent mixed chimerism can be associated with organ allograft tolerance without the need for long-term immune suppression medication.
Conclusion
The preclinical TLI and ATG-conditioning regimen reported to achieve donor cell engraftment without GVHD has been successfully adapted to cancer patients using HLA-matched related and unrelated donors. As in the animal models, the low incidence of acute GVHD is associated with a significant alteration in residual host T cell subsets markedly favoring invariant NKT cells that results in Th2 polarization of chimeric donor T cells; these changes are likely caused by the unique effects of TLI and ATG on host regulatory T cells.
Studies in laboratory animals and in humans show that it is feasible to induce transplantation tolerance in chimeric hosts using post-transplantation TLI conditioning. Our ongoing studies in humans are designed to determine whether sustained mixed chimerism can be achieved in HLA-matched and mismatched hosts by modifications of the TLI and ATG protocol for combined kidney and HCT, and whether sustained chimeras will be free of rejection episodes after complete immunosuppression drug withdrawal.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 526).
- 1.Kaplan HS, Rosenberg SA. Extended field radical radiotherapy in advanced Hodgkin’s disease: short term results of 2 randomized clinical trials. Cancer Res. 1966;26:1268–1276. [PubMed] [Google Scholar]
- 2.Fuks Z, Strober S, Brobrove AM, et al. Long-term effects of radiation of T and B lymphocytes in the peripheral blood of patients with Hodgkin’s disease. J Clin Invest. 1976;58:803–810. doi: 10.1172/JCI108532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slavin S, Strober S, Fuks Z, Kaplan HS. Long-term survival of skin allografts in mice treated with fractionated total lymphoid irradiation. Science. 1976;193:1252–1255. doi: 10.1126/science.785599. [DOI] [PubMed] [Google Scholar]
- 4.Slavin S, Fuks Z, Kaplan HS, Strober S. Transplantation of allogeneic bone marrow without graft-versus-host disease using total lymphoid irradiation. J Exp Med. 1978;147:963–972. doi: 10.1084/jem.147.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146:34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan F, Zeng D, Higuchi M, et al. Predominance of NK1.1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: ‘natural suppressor’ cells. J Immunol. 2001;167:2087–2096. doi: 10.4049/jimmunol.167.4.2087. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb M, Strober S, Kaplan HS. Allogeneic marrow transplantation after total lymphoid irradiation (TLI): effect of dose/fraction, thymic irradiation, delayed marrow infusion, and presensitization. J Immunol. 1979;123:379–383. [PubMed] [Google Scholar]
- 8.Slavin S, Reitz B, Bieber CP, et al. Transplantation tolerance in adult rats using total lymphoid irradiation: permanent survival of skin, heart, and marrow allografts. J Exp Med. 1978;147:700–707. doi: 10.1084/jem.147.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slavin S, Gottlieb M, Strober S, et al. Transplantation of bone marrow in outbred dogs without graft-versus-host disease using total lymphoid irradiation. Transplantation. 1979;27:139–142. [PubMed] [Google Scholar]
- 10.Strober S, Modry DL, Hoppe RT, et al. Induction of specific unresponsiveness to heart allografts in mongrel dogs treated with total lymphoid irradiation and antithymocyte globulin. J Immunol. 1984;132:1013–1018. [PubMed] [Google Scholar]
- 11.Woodley SL, Gurley KE, Hoffmann SL, et al. Induction of tolerance to heart allografts in rats using posttransplant total lymphoid irradiation and anti-T cell antibodies. Transplantation. 1993;56:1443–1447. doi: 10.1097/00007890-199312000-00032. [DOI] [PubMed] [Google Scholar]
- 12.Zeng D, Ready A, Huie P, et al. Mechanisms of tolerance to rat heart allografts using posttransplant TLI. Changes in cytokine expression. Transplantation. 1996;62:510–517. doi: 10.1097/00007890-199608270-00014. [DOI] [PubMed] [Google Scholar]
- 13.Lan F, Hayamizu K, Strober S. Cyclosporine facilitates chimeric and inhibits nonchimeric tolerance after posttransplant total lymphoid irradiation. Transplantation. 2000;69:649–655. doi: 10.1097/00007890-200002270-00029. [DOI] [PubMed] [Google Scholar]
- 14.Zeng D, Lewis D, Dejbakhsh-Jones S, et al. Bone marrow NK1.1(−) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189:1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yao Z, Liu Y, Jones J, Strober S. Differences in Bcl-2 expression by T-cell subsets alter their balance after in vivo irradiation to favor CD4+Bcl-2hi NKT cells. Eur J Immunol. 2009;39:763–775. doi: 10.1002/eji.200838657. This study is highly significant as it documents that differences in the expression of antiapoptotic genes among T cell subsets account for their differential sensitivity to radiation induced cell death and explains in part the mechanism of enhanced NKT cell survival compared to conventional T cells.
- 16.Field EH, Rouse TM, Gao Q, Chang B. Association between enhanced Th2/Th1 cytokine profile and donor T-cell chimerism following total lymphoid irradiation. Hum Immunol. 1997;52:144–154. doi: 10.1016/S0198-8859(96)00291-1. [DOI] [PubMed] [Google Scholar]
- 17.Rigby SM, Rouse T, Field EH. Total lymphoid irradiation nonmyeloablative preconditioning enriches for IL-4-producing CD4+-TNK cells and skews differentiation of immunocompetent donor CD4+ cells. Blood. 2003;101:2024–2032. doi: 10.1182/blood-2002-05-1513. [DOI] [PubMed] [Google Scholar]
- 18.Lan F, Zeng D, Higuchi M, et al. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: the role of CD1-reactive natural killer T cells. Biol Blood Marrow Transplant. 2003;9:355–363. doi: 10.1016/s1083-8791(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 19.Pillai AB, George TI, Dutt S, et al. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol. 2007;178:6242–6251. doi: 10.4049/jimmunol.178.10.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353:1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 21. Kohrt HE, Turnbull BB, Heydari K, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009 doi: 10.1182/blood-2009-03-211441. [Epub ahead of print] This study is highly significant as it details the clinical outcomes of the first 111 cancer patients treated with TLI and ATG conditioning.
- 22.Fowler DH, Kurasawa K, Smith R, et al. Donor CD4-enriched cells of Th2 cytokine phenotype regulate graft-versus-host disease without impairing allogeneic engraftment in sublethally irradiated mice. Blood. 1994;84:3540–3549. [PubMed] [Google Scholar]
- 23.Fowler DH, Kurasawa K, Husebekk A, et al. Cells of Th2 cytokine phenotype prevent LPS-induced lethality during murine graft-versus-host reaction: regulation of cytokines and CD8+ lymphoid engraftment. J Immunol. 1994;152:1004–1013. [PubMed] [Google Scholar]
- 24.Pan L, Delmonte J, Jr, Jalonen CK, Ferrara JLM. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–4429. [PubMed] [Google Scholar]
- 25. Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. This study is highly significant because it confirms that the establishment of mixed hematopoietic cell chimerism following combined kidney and blood stem cell transplantation is associated with transplantation tolerance.