Abstract
Adenosine monophosphate-activated protein kinase (AMPK) senses metabolic stress and integrates diverse physiological signals to restore energy balance. Multiple functions are indicated for AMPK in the CNS. While all neurons sense their own energy status, some integrate neuro-humoral signals to assess organismal energy balance. A variety of disease states may involve AMPK, so determining the underlying mechanisms is important. We review the impact of altered AMPK activity under physiological (hunger, satiety) and pathophysiological (stroke) conditions, as well as therapeutic manipulations of AMPK that may improve energy balance.
Keywords: AMP-activated protein kinase, neurons, C75, energy balance, obesity, stroke
Introduction
AMP-activated protein kinase (AMPK) senses and maintains energy balance in peripheral tissues. When energy is deficient, AMPK activation leads to altered cellular metabolism and gene expression to inhibit anabolic processes, stimulate catabolism, and restore ATP. The CNS integrates diverse central and peripheral signals to maintain homeostasis. CNS AMPK is shown to have important, but complex roles in energy balance. CNS neurons sense their own energy needs, while some also integrate neuro-humoral signals to assess organismal energy balance. In the brain, AMPK is involved in both arenas, coordinating context-specific metabolic responses in many tissues. AMPK plays roles in both physiological (feeding) and pathophysiological (ischemic) states. During the latter, AMPK is highly activated to restore neuronal energy balance, but its over-activation may be deleterious. Here we review AMPK regulation and responses to cellular and organismal energy challenges in the CNS.
AMP-Activated Protein Kinase (AMPK)
AMPK is a serine/threonine kinase with a catalytic α subunit and regulatory β and γ subunits. AMPK not only senses energy status, but also functions at the tissue and organism levels to promote context-specific responses to physiological signals of metabolic status. AMPK modulates many aspects of cellular metabolism (Figure 1A). AMPK was first known to be activated by ATP depletion (increased AMP/ATP ratio) and related stimuli (exercise, starvation, hypoxia, cellular pH and redox status, increased creatine/phosphocreatine ratio). However, AMPK is also activated by certain drugs, hormones, and cellular stressors that do not alter AMP/ATP ratio. Thus, AMPK in various cells and tissues senses both physiological and pathophysiological stimuli (Kahn et al. 2005; Hardie 2007).
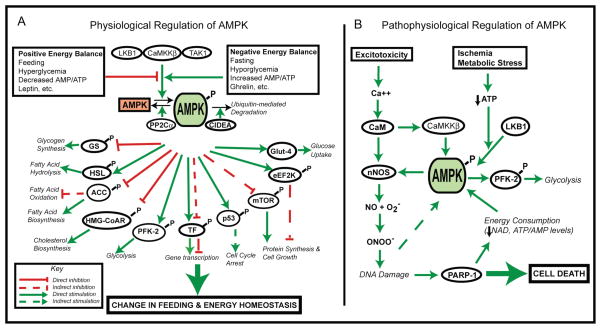
Figure 1.
A) Physiological Regulation of AMPK Signaling. AMPK is activated by physiological signals that indicate negative energy balance (fasting, hypoglycemia, elevated AMP/ATP, or neuropeptides such as ghrelin). In contrast, AMPK is inhibited by signals indicating positive energy balance (feeding, hyperglycemia, decreased AMP/ATP, or adipokines such as leptin). Upon phosphorylation by an upstream kinase (LKB1, CaMKKβ, or TAK-1) AMPK elicits diverse effects on fatty acid, cholesterol, and glucose metabolism, and controls cell cycle progression, protein synthesis, and gene expression. B) Pathophysiological Regulation of AMPK Signaling. AMPK is activated in ischemia, producing excitotoxic, oxidative, and metabolic stresses. Under these conditions, with insufficient energy substrate availability, futile energy-consumptive pathways are over-activated, leading to cellular death. Abbreviations:CaMKKβ (Ca2+/calmodulin-dependent protein kinase β), TAK1 (TGFβ-activated kinase 1), PP2Cα (protein phosphatase-2Cα), GS (glycogen synthase), HSL (hormone-sensitive lipase), ACC (acetyl-CoA carboxylase), HMG-CoAR (3-hydroxy-3-methyl-glutaryl-CoA reductase), PFK-2 (phosphofructokinase-2), TF (Transcription factors), p53 (tumor protein 53), mTOR (mammalian target of rapamycin), eEFK2K (eukaryotic elongation factor-2 kinase), Glut-4 (glucose transporter), nNOS (neuronal nitric oxide synthase), NO (nitric oxide), ONOO− (peroxynitrite), PARP-1 (poly (ADP-ribose) polymerase family member 1).
Several levels of regulation mediate AMPK’s diverse biological effects, including: multiple subunit isoforms, upstream regulation by different metabolites and signaling pathways, and tissue- and context-specific targets. Mammalian isoforms exist for all subunits (α1, α2, beta;1, beta;2, γ1, γ2, γ3) making various heterotrimers possible (Stapleton et al. 1996). Phosphorylation of threonine 172 on AMPKαactivates AMPK. AMP binding to AMPKγmakes AMPKαa better substrate for phosphorylation by upstream kinases and worse substrate for dephosphorylation by protein phosphatase-2Cα (PP2Cα),maximizing AMPK activity (Sanders et al. 2007). Known AMPK kinases include LKB1,Ca2+/calmodulin-dependent protein kinase β(CaMKKβ), and TGF β-activated kinase 1 (TAK1) (Figure 1A) (Hawley et al. 2003; Woods et al. 2003; Woods et al. 2005; Xie et al. 2006). New data show that Cidea protein interacts with AMPKβ to promote ubiquitin-mediated AMPK degradation and down-regulation of AMPK activity (Qi et al. 2008). Thus, various signals (AMP/ATP, intracellular Ca2+, hormones) can modulate AMPK by direct or indirect alterations of its kinase activity.
Activated AMPK inhibits ATP consumption and stimulates ATP generation (Figure 1A). Hormones and pharmacological agents can modulate AMPK activity in muscle, liver, and hypothalamus, promoting fatty acid oxidation, glucose utilization, and controlling food intake (Minokoshi et al. 2002; Andersson et al. 2004; Kim et al. 2004; Minokoshi et al. 2004; Kubota et al. 2007). Activated AMPK inactivates ACC (acetyl-CoA carboxylase) and HMG CoA reductase (3-hydroxy-3-methylglutaryl-coenzyme A), limiting fatty acid and cholesterol synthesis when energy is deficient (Carling et al. 1987; Winder and Hardie 1996). AMPK stimulates catabolism by activating glucose uptake (Glut4 translocation), glycolysis (activating 6-phosphofructo-2-kinase kinase (PFK-2)), glucose oxidation, and fatty acid oxidation (by relieving CPT-1 inhibition by malonyl-CoA) (Winder and Hardie 1996; Merrill et al. 1997; Hayashi et al. 1998; Kurth-Kraczek et al. 1999). Other molecules phosphorylated by AMPK and involved in energy homeostasis include hormone sensitive lipase, glycogen synthase, and PPARγ coactivator-1α (PGC1α) (Hardie 2007). Studies show AMPK linkage to mediators of cell cycle progression (p53), protein synthesis and growth (mammalian target of rapamycin (mTOR); elongation factor 2 kinase (eEF2K), proliferation, and survival (Akt) (Horman et al. 2002; Inoki et al. 2003; Jones et al. 2005; Gwinn et al. 2008). Thus, effects of AMPK activation are cell-type specific and depend on manner of activation.
AMPK Roles in Feeding and Organismal Energy Balance
Peripheral tissue AMPK responds to altered AMP/ATP, nutrients, metabolites and hormones related to body energy status. Recent research confirms these roles in the CNS as well. Hypothalamic AMPK is located in energy-sensing neurons and circuits for body energy homeostasis, and is thus positioned to function as a “master regulator” of organismal energy balance. The growing list of energy-related signals that affect food intake, energy expenditure, and body weight by altering hypothalamic AMPK activity attests to its significance for energy homeostasis.
Initial studies in 2004 indicated an important physiological role for hypothalamic AMPK in responses to energy-related signals to the brain, and suggested that AMPK activity could be manipulated pharmacologically to affect food intake and body weight. Andersson et al. showed that administration of the anorexigenic adiposity hormone leptin decreased AMPK activity in rat hypothalamus, whereas the orexigenic stomach-derived hormone ghrelin increased activity. The AMPK activator AICAR (5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside) increased food intake when given i.c.v. into the paraventricular nucleus (PVN) of the hypothalamus (Andersson et al. 2004). Minokoshi and colleagues showed that leptin injections into mediobasal hypothalamus (MBH) decreased AMPK activity in arcuate nucleus (ARC) and PVN. Other anorexigenic signals (insulin, glucose, refeeding) had broader effects, decreasing AMPK activity in samples from ventromedial/dorsomedial and lateral hypothalamus. Food intake and body weight increased after adenoviral induction of constitutively-active AMPK (CA-AMPK) in the MBH, and decreased with dominant-negative AMPK (DN-AMPK) (Minokoshi et al. 2004).
We used C75 (3-carboxy-4-octyl-2-methylenebutyrolactone) for studies of AMPK and feeding behavior. C75 was designed to prevent fatty acyl chain elongation and CPT-1 stimulation (Kuhajda et al. 2000; Thupari et al. 2002; Landree et al. 2004; Nicot et al. 2004). C75 produces hypophagia and weight loss in lean and obese models (Loftus et al. 2000; Kim et al. 2002; Thupari et al. 2004). In vitro, C75 affects neuronal metabolism by increasing ATP (Landree et al. 2004). Our data led us to hypothesize that C75 reduces feeding and body weight by decreasing hypothalamic AMPK activity, and that hypothalamic AMPK is physiologically relevant for feeding control (Kim et al. 2004). Food deprived mice had elevated hypothalamic AMPK activity, reversed by C75. Accordingly, C75 and the AMPK inhibitor compound C (6-[4-(2-Piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo[1,5-a]pyrimidine) given i.c.v. decreased food intake, whereas AICAR increased feeding.
Other reports show that hypothalamic AMPK responds to many peripheral signals of energy status. Signals of energy deficit that enhance hypothalamic AMPK activity to increase feeding include: hypoglycemia (McCrimmon et al. 2004; Han et al. 2005), glucocorticoids (Shimizu et al. 2008), thyroid hormones (Ishii et al. 2008), cannabinoids (Kola et al. 2005), adiponectin (Kubota et al. 2007), ghrelin (Andersson et al. 2004; Kola et al. 2005; Anderson et al. 2008; Lopez et al. 2008), and agouti-related peptide (AgRP) (Minokoshi et al. 2004; Tanaka et al. 2007) (Figure 1A). Signals associated with energy surplus that decrease hypothalamic AMPK activity to inhibit eating include: glucose (Minokoshi et al. 2004), leptin (Andersson et al. 2004; Minokoshi et al. 2004; Namkoong et al. 2005; Gao et al. 2007), insulin (Minokoshi et al. 2004; Namkoong et al. 2005), resistin (Vazquez et al. 2008), alpha-melanocyte-stimulating hormone (Minokoshi et al. 2004; Tanaka et al. 2007), ciliary neurotrophic factor (Steinberg et al. 2006), and glucagon-like-peptide-1 (Seo et al. 2008).
Claret and colleagues recently used genetic knockout mice to examine roles of AMPK in specific ARC neuron subtypes. Mice with AMPKα2 knocked out in ARC POMC-neurons were expected to be lean, but instead developed mild obesity with decreased metabolic rate. Ad libitum feeding was normal, but hyperphagia upon re-feeding was enhanced. Mice without AMPKα2 in AgRP neurons were leaner than wildtype, without obvious effects on energy intake or expenditure, and more hypophagic after melanocortin agonist (Claret et al. 2007). These data indicated importance of AMPK in POMC and AgRP neurons for long-term energy balance. Leptin and insulin are also important for long-term homeostasis, yet knockout neurons retained sensitivity to these hormones, although they lacked normal electrophysiological responses to altered ambient glucose concentration. Authors concluded that AMPK was important for glucose sensing in AgRP and POMC neurons, but not for responses to hormones. These studies did not assess gene expression responses to leptin or insulin. Studies with knockouts yielded complicated results, suggesting that roles of AMPK are complex, or that genetic models display compensatory adjustments obscuring AMPK roles.
Upstream regulators of hypothalamic AMPK activity may be specific to neuronal phenotype. Recent studies by Anderson and colleagues show that the AMPK kinase CaMKKβis enriched in cortex, hippocampus, and MBH (Anderson et al. 2008), regions with high NPY. CaMKKβ (−/−) mice had lower ARC NPY and AgRP, but not POMC, implying CaMKKβinvolvement in NPY production in ARC. CaMKKβ (−/−) mice ate after ghrelin administration, consistent with ghrelin increases of intracellular Ca2+ in ARC NPY neurons. Like NPY knockouts, CaMKKβ (−/−) mice had blunted feeding response to food deprivation. A CaMKK inhibitor decreased eating, and ARC NPY and AgRP, in wildtype but not CaMKKβ (−/−) mice. CaMKKβ (−/−) mice did not respond to ghrelin, but ate in response to 2-deoxyglucose (2-DG), an outcome authors suggested to indicate that 2-DG acted downstream of the ghrelin-Ca2+-CaMMKβ-AMPK pathway in MBH (Anderson et al. 2008). However, glucose-sensing via AMPK can occur in other sites;pAMPK decreased in additional hypothalamic nuclei in response to glucose (Minokoshi et al. 2004), some of which are not rich in CaMKKβ. Other AMPK kinases and upstream modulators await study in the hypothalamus.
Altered fatty acid metabolism appears critical for hypothalamic AMPK to alter feeding behavior in response to some peripheral.signals. Effects on AMPK activity, and manifestations of altered fatty acid metabolism, appear region-specific. Gao et al. showed that leptin-induced decreases in pAMPK, and subsequent decreases in pACC increase metabolic flux through the fatty acid synthetic pathway. This manifests differently in ARC versus PVN. In ARC, the pathway intermediate, malonyl-CoA, is elevated in response to leptin. In PVN, malonyl-CoA level is not increased. Instead the pathway end-product palmitoyl-CoA increases, due to local upregulation of FAS protein. Increased flux through the fatty acid synthetic pathway, as determined by ACC, is required for leptin’s anorexigenic effect. I.c.v. infusion of the ACC inhibitor TOFA (5-tetradecyloxy-2-furoic acid) prevents leptin-induced hypophagia and the elevated ARC malonyl-CoA and PVN palmitoyl-CoA (Gao et al. 2007). Recent studies with ghrelin complement the leptin studies, and again demonstrate anatomical specificity, indicating that VMN AMPK is critical for ghrelin-induced eating through decreased FAS (Lopez et al. 2008).
AMPK Activation During CNS Metabolic Stress
Ischemic brain injury involves a complex sequence of excitotoxic and oxidative events, including: cellular energy depletion, disrupted protein synthesis, membrane depolarization, acidosis, free radical production, DNA damage, apoptosis, and necrosis (Snider et al. 1999; Love 2003) (Figure 1B). Compensatory processes are activated to repair damage and restore normal cell functions (Szabo and Dawson 1998; Ha and Snyder 2000). However, these energy-consuming processes deplete ATP and increase AMP. Over-activation of these pathways might be deleterious to cells (Chan 2001; Du et al. 2003; Ying et al. 2003). Although AMPK activation is an adaptive response to stress in numerous systems, consequences of stress-mediated AMPK activation remain controversial. Some groups show that AMPK activation is neuroprotective. AICAR protects hippocampal neurons against glucose deprivation and glutamate excitotoxicity (Culmsee et al. 2001) and protects astrocytes from ceramide-induced apoptosis (Blazquez et al. 2001). Conversely, other studies suggest that AMPK over-activation is detrimental. AICAR was pro-apoptotic in human neuroblastoma cells(Garcia-Gil et al. 2003), rat hippocampal HN9 cells, and mouse MMIN cells(Pesi et al. 2000). Differences in cell-type and culture conditions might contribute to divergent responses.
Pathways activated in ischemia affect AMPK in astrocytes. In astrocytes high NO levels were shown to inhibit mitochondrial respiration, induce AMPK activity, and promote PFK-2 activation to increase glycolysis dramatically. The ATP thus provided promoted astrocyte survival. Similar response was not observed inneurons, due to low PFK-2 expression (Almeida et al. 2004). The results are consistent with the notion that astrocytes utilizes glycogen for anaerobic ATP generation, an option thought unavailable to ischemic neurons (Pellerin 2005).
Studies utilizing neuronal cultures to study of AMPK and neuronal energy balance must consider that brain glucose concentrations are tightly regulated, ranging from 0.82 mM to 2.4 mM, depending on plasma glucose, brain region, and microdialysis method (Silver and Erecinska 1994; Abi-Saab et al. 2002; de Vries et al. 2003). Neuronal cell culture media are typically 25 mM glucose, far greater than that seen even in plasma during severe hyperglycemia. However, some groups use physiological glucose concentrations for their studies (Abe et al. 2006; Bak et al. 2006; Morgenthaler et al. 2006; Canabal et al. 2007). We established an in vitro culture protocol using physiologically relevant parameters to mimic in vivo conditions, and thus facilitate the study of neuronal responses to altered energy balance (Kleman et al. 2008). In comparing cultures maintained in 3 mM or 25 mM glucose, we showed that neurons maintained in 3 mM glucose responded to metabolic changes in a manner similar to observations in vivo. These results may explain some discordance between in vivo and in vitro studies examining AMPK roles in cell survival and neuroprotection (Culmsee et al. 2005; McCullough et al. 2005).
Despite controversies related to cell-type and experimental conditions, there has been significant progress in clarifying the role of AMPK activation in brain ischemia. We have used in vitro and in vivo models. In hippocampal slice cultures, pAMPK increased robustly up to 6 hours after oxygen and glucose deprivation (OGD) compared with normoxic euglycemic conditions (McCullough et al. 2005). Slices also had increased levels of pACC, indicating physiological consequences. We also used a focal stroke model of transient middle cerebral artery occlusion (MCAO) (Murphy et al. 2003; McCullough et al. 2005). Mice subjected to MCAO had elevated pAMPK persisting well into the reperfusion phase. pAMPK was high in both the ischemic and the non-ischemic hemisphere, suggesting that the metabolic derangements and compensatory responses in one hemisphere resulted in global AMPK activation.
Peroxynitrate (ONOO−) contributes significantly to stroke damage and AMPK activation (Zou et al. 2002). Interestingly, nNOS knockout mice have smaller infarcts than wild-type counterparts, primarily due to decreased ONOO− production (Huang et al. 1994). nNOS(−/−) mice subjected to MCAO did not show the stroke-induced rise in pAMPK seen in wildtype mice (McCullough et al.). These data suggest that the nNOS and ONOO− pathways are important upstream activators of AMPK in vivo.
The consequence of persistent AMPK activation in neurons with compromised energy supply was unknown. We hypothesized that AMP/ATP sensing is important in neuronal responses to ischemia, and treated mice with AMPK inhibitors, then subjected them to MCAO and reperfusion (McCullough et al. 2005). Both compound C and C75 decreased behavioral deficits after stroke, and significantly reduced total infarct volume and pAMPK compared to vehicle control. Furthermore, AMPK inhibition with C75 or compound C provided sustained neuroprotection for days after stroke, even when administration was delayed (Li et al. 2007). In contrast, the AMPK activator AICAR dramatically exacerbated stroke damage.
To determine whether isoforms of the catalytic subunit play different roles in cerebral ischemia, we evaluated stroke outcomes in AMPKα1 and AMPKα2 knockout mice versus littermates (Li et al. 2007). AMPKα2(−/−) had significantly smaller cortical, striatal, and total infarct volumes compared to wildtype, but there were no differences between wildtype and AMPKα1(−/−) mice. In further experiments the effect of compound C was also studied in AMPKα2(−/−) mice. If AMPKα2 mediates toxicity in ischemia, compound C would be expected to be less effective at protecting the knockout mice from stroke damage. Accordingly, compound C did not reduce infarct size in AMPKα2(−/−) mice.
Collectively, these results suggest that activation of the α2 subunit is detrimental in models of ischemia-reperfusion, that AMPK inhibition is neuroprotective in stroke, and that the neuroprotection correlates with decreased pAMPK. Furthermore, these data suggest that pharmacological improvement of energy balance is a viable approach for stroke intervention.
Targeting AMPK For Therapeutic Interventions
Metabolic syndrome encompasses numerous risk factors leading to diabetes and/or coronary heart disease. By 2030, 324 million people are expected to be diagnosed with type II diabetes (WHO). The position of AMPK at crossroads of energy metabolism makes AMPK an attractive therapeutic target for these metabolic diseases.
Some drugs that activate AMPK in peripheral tissues are approved for treating type-II diabetes and other aspects of metabolic syndrome (for review see (Smiley and Umpierrez 2007)). Perhaps the best known are the biguanide metformin, the thiazolidinedione (TZD) rosiglitazone and metformin-rosiglitazone combination therapy. Metformin inhibits hepatic glucose production, while rosiglitazone increases insulin-dependent glucose uptake in skeletal muscle (Hutchinson et al. 2008). Data suggest that these therapies have additional benefits, including decreased plasma free fatty acids and LDL and increased HDL (Smiley and Umpierrez 2007). Unfortunately, data also indicates that TZDs have a negative impact on bone metabolism, with increased incidence of fractures in women, and increased incidence of myocardial infarction and cardiovascular-related death (Smiley and Umpierrez 2007).
Some data support a role for AMPK in other aspects of metabolic syndrome. Rimonabant, a cannabinoid receptor type 1 (CB1) antagonist, is used in Europe to treat obesity (Hutchinson et al. 2008). Rimonabant is suggested to inhibit the orexigenic effect of ghrelin by preventing ghrelin activation of hypothalamic AMPK (Kola et al. 2008). Resveratol, a naturally occurring polyphenol, has been shown to afford protection to ischemic cardiac cells by inhibiting the build-up of reactive oxygen species. More recently, it has been demonstrated in some cell types that this protection is made possible through the activation of AMPK (Hwang et al. 2008).
Due to rising prevalence of diseases based on energy dysregulation, AMPK remains a popular target for the development of pharmacological modulators. The challenge remains achieving tissue- and context-specific alterations of AMPK activity, given the far-reaching and perhaps undesirable consequences of global AMPK modulation. Targeting fatty acid metabolism provides an example of restricted modulation of AMPK activity, as FAS is expressed in selected tissues, and notably absent from heart and muscle where AMPK inhibition would be deleterious. As our understanding of AMPK regulation expands, we may discover other tissue- and context-specific regulators that may be targeted for the development of new drugs, or alternative therapies for the treatment of obesity and related diseases.
References
- Abe T, Takahashi S, Suzuki N. Oxidative metabolism in cultured rat astroglia: effects of reducing the glucose concentration in the culture medium and of Daspartate or potassium stimulation. J Cereb Blood Flow Metab. 2006;26:153–160. doi: 10.1038/sj.jcbfm.9600175. [DOI] [PubMed] [Google Scholar]
- Abi-Saab WM, Maggs DG, Jones T, et al. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab. 2002;22:271–279. doi: 10.1097/00004647-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS. Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J Cereb Blood Flow Metab. 2006;26:1285–1297. doi: 10.1038/sj.jcbfm.9600281. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Geelen MJ, Velasco G, Guzman M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001;489:149–153. doi: 10.1016/s0014-5793(01)02089-0. [DOI] [PubMed] [Google Scholar]
- Canabal DD, Potian JG, Duran RG, McArdle JJ, Routh VH. Hyperglycemia impairs glucose and insulin regulation of nitric oxide production in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R592–600. doi: 10.1152/ajpregu.00207.2007. [DOI] [PubMed] [Google Scholar]
- Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, Blomgren K, Plesnila N. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25:10262–10272. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- Du L, Zhang X, Han YY, Burke NA, et al. Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J Biol Chem. 2003;278:18426–18433. doi: 10.1074/jbc.M301295200. [DOI] [PubMed] [Google Scholar]
- Gao S, Kinzig KP, Aja S, et al. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17358–17363. doi: 10.1073/pnas.0708385104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gil M, Pesi R, Perna S, Allegrini S, Giannecchini M, Camici M, Tozzi MG. 5’-aminoimidazole-4-carboxamide riboside induces apoptosis in human neuroblastoma cells. Neuroscience. 2003;117:811–820. doi: 10.1016/s0306-4522(02)00836-9. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HC, Snyder SH. Poly(ADP-ribose) polymerase-1 in the nervous system. Neurobiol Dis. 2000;7:225–239. doi: 10.1006/nbdi.2000.0324. [DOI] [PubMed] [Google Scholar]
- Han SM, Namkoong C, Jang PG, et al. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia. 2005;48:2170–2178. doi: 10.1007/s00125-005-1913-1. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, et al. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Summers RJ, Bengtsson T. Regulation of AMP-activated protein kinase activity by G-protein coupled receptors: potential utility in treatment of diabetes and heart disease. Pharmacol Ther. 2008;119:291–310. doi: 10.1016/j.pharmthera.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Kwon DY, Park OJ, Kim MS. Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutr. 2008;2:323–326. doi: 10.1007/s12263-007-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kamegai J, Tamura H, Shimizu T, Sugihara H, Oikawa S. Triiodothyronine (T3) stimulates food intake via enhanced hypothalamic AMP-activated kinase activity. Regulatory peptides. 2008;151:164–169. doi: 10.1016/j.regpep.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kim EK, Miller I, Aja S, et al. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem. 2004;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- Kim EK, Miller I, Landree LE, Borisy-Rudin FF, Brown P, Tihan T, Townsend CA, Witters LA, Moran TH, Kuhajda FP, Ronnett GV. Expression of FAS within hypothalamic neurons: a model for decreased food intake after C75 treatment. Am J Physiol Endocrinol Metab. 2002;283:E867–879. doi: 10.1152/ajpendo.00178.2002. [DOI] [PubMed] [Google Scholar]
- Kleman AM, Yuan JY, Aja S, Ronnett GV, Landree LE. Physiological glucose is critical for optimized neuronal viability and AMPK responsiveness in vitro. J Neurosci Methods. 2008;167:292–301. doi: 10.1016/j.jneumeth.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B, Hubina E, Tucci SA, et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5’ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- Landree LE, Hanlon AL, Strong DW, et al. C75, a Fatty Acid Synthase Inhibitor, Modulates AMP-activated Protein Kinase to Alter Neuronal Energy Metabolism. J Biol Chem. 2004;279:3817–3827. doi: 10.1074/jbc.M310991200. [DOI] [PubMed] [Google Scholar]
- Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–2999. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- Lopez M, Lage R, Saha AK, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008;7:389–399. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:267–282. doi: 10.1016/S0278-5846(03)00022-8. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes. 2004;53:1953–1958. doi: 10.2337/diabetes.53.8.1953. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Morgenthaler FD, Kraftsik R, Catsicas S, Magistretti PJ, Chatton JY. Glucose and lactate are equally effective in energizing activity-dependent synaptic vesicle turnover in purified cortical neurons. Neuroscience. 2006;141:157–165. doi: 10.1016/j.neuroscience.2006.03.065. [DOI] [PubMed] [Google Scholar]
- Murphy S, McCullough L, Littleton-Kearney M, Hurn P. Estrogen and selective estrogen receptor modulators: neuroprotection in the Women’s Health Initiative era. Endocrine. 2003;21:17–26. doi: 10.1385/endo:21:1:17. [DOI] [PubMed] [Google Scholar]
- Namkoong C, Kim MS, Jang PG, et al. Enhanced hypothalamic AMP-activated protein kinase activity contributes to hyperphagia in diabetic rats. Diabetes. 2005;54:63–68. doi: 10.2337/diabetes.54.1.63. [DOI] [PubMed] [Google Scholar]
- Nicot C, Napal L, Relat J, Gonzalez S, Llebaria A, Woldegiorgis G, Marrero PF, Haro D. C75 activates malonyl-CoA sensitive and insensitive components of the CPT system. Biochem Biophys Res Commun. 2004;325:660–664. doi: 10.1016/j.bbrc.2004.10.085. [DOI] [PubMed] [Google Scholar]
- Pellerin L. How astrocytes feed hungry neurons. Mol Neurobiol. 2005;32:59–72. doi: 10.1385/MN:32:1:059. [DOI] [PubMed] [Google Scholar]
- Pesi R, Micheli V, Jacomelli G, Peruzzi L, Camici M, Garcia-Gil M, Allegrini S, Tozzi MG. Cytosolic 5’-nucleotidase hyperactivity in erythrocytes of Lesch-Nyhan syndrome patients. Neuroreport. 2000;11:1827–1831. doi: 10.1097/00001756-200006260-00006. [DOI] [PubMed] [Google Scholar]
- Qi J, Gong J, Zhao T, et al. Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. Embo J. 2008;27:1537–1548. doi: 10.1038/emboj.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Ju S, Chung H, Lee D, Park S. Acute effects of glucagon-like peptide-1 on hypothalamic neuropeptide and AMP activated kinase expression in fasted rats. Endocr J. 2008;55:867–874. doi: 10.1507/endocrj.k08e-091. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Arima H, Watanabe M, Goto M, Banno R, Sato I, Ozaki N, Nagasaki H, Oiso Y. Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via adenosine monophosphate-activated protein kinase signaling in the arcuate nucleus of rats. Endocrinology. 2008;149:4544–4553. doi: 10.1210/en.2008-0229. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley D, Umpierrez G. Metformin/rosiglitazone combination pill (Avandamet) for the treatment of patients with Type 2 diabetes. Expert Opin Pharmacother. 2007;8:1353–1364. doi: 10.1517/14656566.8.9.1353. [DOI] [PubMed] [Google Scholar]
- Snider BJ, Gottron FJ, Choi DW. Apoptosis and necrosis in cerebrovascular disease. Ann N Y Acad Sci. 1999;893:243–253. doi: 10.1111/j.1749-6632.1999.tb07829.x. [DOI] [PubMed] [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, et al. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Watt MJ, Fam BC, Proietto J, Andrikopoulos S, Allen AM, Febbraio MA, Kemp BE. Ciliary neurotrophic factor suppresses hypothalamic AMP-kinase signaling in leptin-resistant obese mice. Endocrinology. 2006;147:3906–3914. doi: 10.1210/en.2005-1587. [DOI] [PubMed] [Google Scholar]
- Szabo C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Masuzaki H, Yasue S, et al. Central melanocortin signaling restores skeletal muscle AMP-activated protein kinase phosphorylation in mice fed a high-fat diet. Cell Metab. 2007;5:395–402. doi: 10.1016/j.cmet.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thupari JN, Kim EK, Moran TH, Ronnett GV, Kuhajda FP. Chronic C75 treatment of diet-induced obese mice increases fat oxidation and reduces food intake to reduce adipose mass. Am J Physiol Endocrinol Metab. 2004;287:E97–E104. doi: 10.1152/ajpendo.00261.2003. [DOI] [PubMed] [Google Scholar]
- Vazquez MJ, Gonzalez CR, Varela L, et al. Central resistin regulates hypothalamic and peripheral lipid metabolism in a nutritional-dependent fashion. Endocrinology. 2008;149:4534–4543. doi: 10.1210/en.2007-1708. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, et al. LKB1 Is the Upstream Kinase in the AMP-Activated Protein Kinase Cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Xie M, Zhang D, Dyck JR, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W, Garnier P, Swanson RA. NAD+ repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem Biophys Res Commun. 2003;308:809–813. doi: 10.1016/s0006-291x(03)01483-9. [DOI] [PubMed] [Google Scholar]
- Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002;277:32552–32557. doi: 10.1074/jbc.M204512200. [DOI] [PubMed] [Google Scholar]