Abstract
C-reactive protein (CRP) is an inflammatory marker which predicts cardiovascular disease. However, it is not fully understood whether CRP has direct effects on endothelial functions and gene expression. The purpose of current study was to determine the effects and molecular mechanisms of CRP on the expression of plasminogen activator inhibitor-1 (PAI-1) in human endothelial cells. Human coronary artery endothelial cells (HCAEC) were treated with CRP at clinically relevant concentrations for different durations. PAI-1 mRNA, protein and enzyme activities were studied. The effects of CRP on MAPK p38 phosphorylation was also studied by Bio-Plex luminex immunoassay. In addition, other types of human endothelial cells isolated from umbilical vein, skin, and lung microvessels were tested. CRP significantly increased PAI-1 mRNA levels in a time- and concentration-dependent manner. The protein level and enzyme activity of PAI-1 in the supernatant of CRP-treated HCAEC cultures were significantly increased. Anti-CD32 antibody effectively blocked CRP-induced PAI-1 mRNA expression. In addition, CRP significantly increased CD32 mRNA levels and enhanced phosphorylation of MAPK p38. Furthermore, antioxidant curcumin dramatically inhibited CRP-induced PAI-1 mRNA expression. The effect of CRP on PAI-1 expression was also confirmed in other types of human endothelial cells. In conclusion, CRP significantly increased the expression of PAI-1 in HCAEC and other human endothelial cells. CRP also increased its receptor CD32 expression which may further enhance its action. CRP-induced PAI-1 expression may be mediated by oxidative stress and p38 signal pathway as antioxidant effectively blocks the effect of CRP on HCAEC.
Keywords: C-reactive protein, PAI-1, endothelial cells, thrombosis, CD32, curcumin
Introduction
C-reactive protein (CRP) is a prototypical acute-phase reactant protein. Serum CRP is less than 1 µg/ml in healthy people, and however, it may increase as much as 1000-fold within 24 h in response to acute stresses such as inflammation and tissue injury [1]. CRP has been widely used as a biomarker of the presence and severity of inflammatory diseases including cardiovascular disorders [2]. The elevated CRP levels have been demonstrated in myocardial infarction, coronary artery disease, stroke, peripheral arterial disease, metabolic syndrome and type-II diabetes [3–6]. In addition, CRP may have direct biological functions in the vascular system such as altering gene expression and functions of endothelial cells and smooth muscle cells [7–9]. Some of these effects may be mediated through the interaction between CRP and their potential receptors including CD16 and CD32 molecules [10]. However, these studies are very preliminary, and the roles and molecular mechanisms of CRP in endothelial functions such as mediating thrombosis are largely unknown.
Thrombosis factors play a crucial role in the pathogenesis of cardiovascular disease. For instance, plasminogen activator inhibitor–1 (PAI-1) contributes to fibrinolysis by inhibiting tissue plasminogen activator [11]. It is well-known that PAI-1 is an acute phase protein, which was shown originally by Kluft et al [12] and in more detail by Juhan-Vague et al [13]. PAI-1 is synthesized in the liver, endothelial cells, vascular smooth muscle cells, and macrophages. PAI-1 expression is highly regulated by many factors including cytokines, oxidative stress, and cellular signaling molecules such as mitogen-activated protein kinases (MAPKs). The elevated PAI-1 is closely associated with enhanced thrombosis by impairing fibrinolysis [14–18], which has been recognized as an important risk factor for atherosclerotic vascular disease [15]. However, it is not fully understood whether CRP could affect PAI-1 expression in different endothelial cells though a unique molecular mechanism.
The main objective of current study was to determine the effect of CRP on PAI-1 expression in human endothelial cells. The molecule mechanisms including the interaction with CRP receptor CD32, effect of antioxidant, and involvement of MAPKs were investigated. Current study provides a better understanding of biological functions of CRP in the vascular system and may suggest new strategies to control CRP-mediated endothelial dysfunction.
Materials and methods
Chemicals and reagents
Recombinant CRP was purchased from Calbiochem (La Jolla, CA, USA). Purity was confirmed by SDS-PAGE showing single band. We determined the endotoxin level as 0.0005 EU/µg for this CRP preparation by Limulus amebocyte lysate assay. TRI-reagent, monoclonal mouse anti-human β-actin and monoclonal rabbit anti-goat IgG were purchased from Sigma (St. Loius, MO). iQ SYBR Green Supermix kit and iScrip cDNA Synthesis kit were obtained from BioRad Laboratories (Hercules, CA). Goat polyclonal anti-human PAI-1 antibody was obtained from Santa Cruz (Santa Cruz, CA). Spectrolyse PAI-1 kit and IMUBIND tissue PAI-1 ELISA test Kit were obtained from American Diagnostica Inc. (Greenwich, CT). Sheep anti-mouse Ig and ECL plus Western Blotting Detection System were purchased from Amersham (Piscataway, NJ). Mouse anti-human monoclonal CD32 and CD16 were purchased from BD Pharmingen (San Diego, CA).
Cell culture
Human endothelial cells including human coronary artery endothelial cells (HCAEC), human lung microvascular endothelial cells (HMVEC-L), and human umbilical vein endothelial cells (HUVEC) were purchased from Clonetics (Walkersville, MD) at passage 3. Immortalized human dermal microvascular endothelial cells (HMEC) were generously provided by Dr. Wright S. Caughman, Department of Dermatology, Emory University (Atlanta, GA). HCAEC, HUVEC, HMEC and HMVEC-L were cultured in the endothelial cell basal medium-2 (EBM-2) contained with 10% fetal bovine serum and EGM-2 SingleQuots (Invitrogen, Carsbad, CA). All cells were maintained at 37°C in a 5% CO2 humidified milieu. Cells were cultured to 90% confluence and in the starvation medium including EBM-2 supplemented with 1% fetal bovine serum, 0.1% gentamicin sulfate and amphotericin-B, heparin and ascorbic acid for 24 h. Cells were treated by CRP (5, 10 or 25 µg/ml) in the fresh starvation medium at 37°C for 3, 6, 12, 24 or 48 h. Control cells were received the fresh medium without CRP. All experiments were performed in triplet.
RNA extraction and quantitative real time PCR
The cells were washed with cold PBS and total RNA was extracted by TRI reagent following manufacturer’s protocol. RNA from each well was resuspended in 20 µl of RNase-free water and the concentration was determined by absorbance at 260-nm wavelength. cDNA was generated by reverse transcription from mRNA using the iScript cDNA Synthesis Kit (Bio-Rad) following the manufacturer’s instructions. Specific primers, including PAI-1, CD32 and CD16 (Table 1), were designed with Beacon designer software (Bio-Rad) and synthesized by Sigma-Genosys (Woodlands, TX). Primers for β-actin are (sense) 5’ CTGGAACGGTGAAGGTGACA 3’; and (antisense) 5’ AAGGGACTTCCTGTAACAATGCA 3’. Quantitative real time PCR was carried by using iQSYBR Green Supermix kit (BioRad Laboratories, Inc., Hercules, CA). The PCR condition was: 95°C for 1.5 min, 40 cycles of 60°C for 1 min, 95°C for 1 min and 55°C for 1 min. Data were presented by the relative amount of mRNA by 2^(−ΔCT). The ΔCT is the discrepancy of threshold cycle between a gene of interest and β–actin. Controls were performed with no RT (mRNA sample only) or no mRNA (water only) to demonstrate the specificity of the primers and the lack of DNA contamination in samples. All data were in triplet.
Table 1.
Design of real time PCR primers
Supernatant PAI-1 protein and activity assay
Supernatant PAI-1 protein levels were measured by using IMUBIND tissue PAI-1 ELISA kit which employs a murine anti-human PAI-1 antibody as the capture antibody. Following kit instructions, the supernatant was incubated in precoated microtest wells and was detected with a secondary biotinylated antibody that recognizes the bound PAI-1 molecules. Adding streptavidin conjugated horseradish peroxidase (HRP) completed the formation of the antibody-enzyme detection complex. The addition of a perborate/3, 3', 3, 5' - tetramethylbenzidine (TMB) substrate, and its subsequent reaction with HRP created a blue colored solution. PAI-1 levels are quantified by measuring solution absorbances at 450 nm and comparing the values with those of a standard curve. All samples were measured in duplicate.
PAI-1 activity in the cell culture supernatant was measured by using Spectrolyse® pL PAI-1 kit following kit instructions. Briefly, 50 µl of sample supernatant was incubated with 50 µl 40 IU/ml tissue plasminogen activator at 25°C for 15 min, in which tissue plasminogen activator reacted with PAI-1 in the supernatant. Then, 100 µl of acetate buffer was added and incubated for 20 min at 37°C. After adding 2 ml H2O, 20 µl of sample was incubated with 200 µl ice cold PAR/pL in a sealed 96 well plate at 37°C for 90 min. The reaction was terminated by adding 50 µl STOP/pL reagent. The absorbance was measured at 405 nm and 492 nm by EL800 Universal Microplate Reader. A result was valid only below 28 U/ml before multiplication with the dilution factor.
Western blot
The cells were washed twice with cold PBS and lysed by gently shaking at 4°C for 1 h in a lysis buffer (25 mM Tris HCl, pH 7.4, 0.15 M NaCl, 1% Triton X-100, and complete protease inhibitor cocktail). Forty µg of protein was loaded and fractioned by 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE), followed by transferring to nitrocellulose membrane. The membrane was blocked by blocking buffer (5% non-fat milk, 10 mmol/L Tris, pH 7.5, 100 mmol/L NaCl, and 0.1% Tween-20) at room temperature for 1 h. PAI-1 was detected by incubating with polyclonal anti-human PAI-1 antibody (1:200) overnight at 4°C. Following three washes (5 min each), the membrane was incubated with secondary antibody (rabbit monoclonal anti-goat Ig, 1:10,000) at room temperature for 30 min. Signal was detected by enhanced chemiluminescence detection kit (ECL plus). Densitometry was performed by Alpha image software (Alpha Innotech).
Bio-Plex luminex immunoassay
HCAEC were treated with 10 µg/ml CRP for 0, 5, 10, 20, 30, and 60 min. The phosphorylated and total proteins were analyzed by Bio-Plex phosphoprotein and total target assays (Bio-Rad). Briefly, the cells were washed with ice-cold PBS twice and were lysed with Bio-Plex Cell Lysis Kit. The antibody coupled beads, which are specific to phosphorylated and total ERK1/2, p38 and JNK, respectively, were added and followed by washing twice. The lysate was incubated for 15–18 h. In the second day, the detection antibodies were incubated for 30 min, and followed by adding streptavidin-PE for 10 min. After the resuspension buffer was added, the phosphoprotein and total protein were analyzed by Luminex 100 instrument and Bio-Plex Manager software.
Statistical analysis
All data were presented as mean ± standard error (SE). The significance was performed by paired Student t-test (one tail) as comparison between treated and control groups. Additionally, analysis of variance (ANOVA) with post-hoc analysis was used to compare multiple groups (Minitab software, Sigma Breakthrough Technologies, Inc., San Marcos, TX). A P<0.05 was considered statistically significant.
Results
CRP increases PAI-1 mRNA expression in human endothelial cells
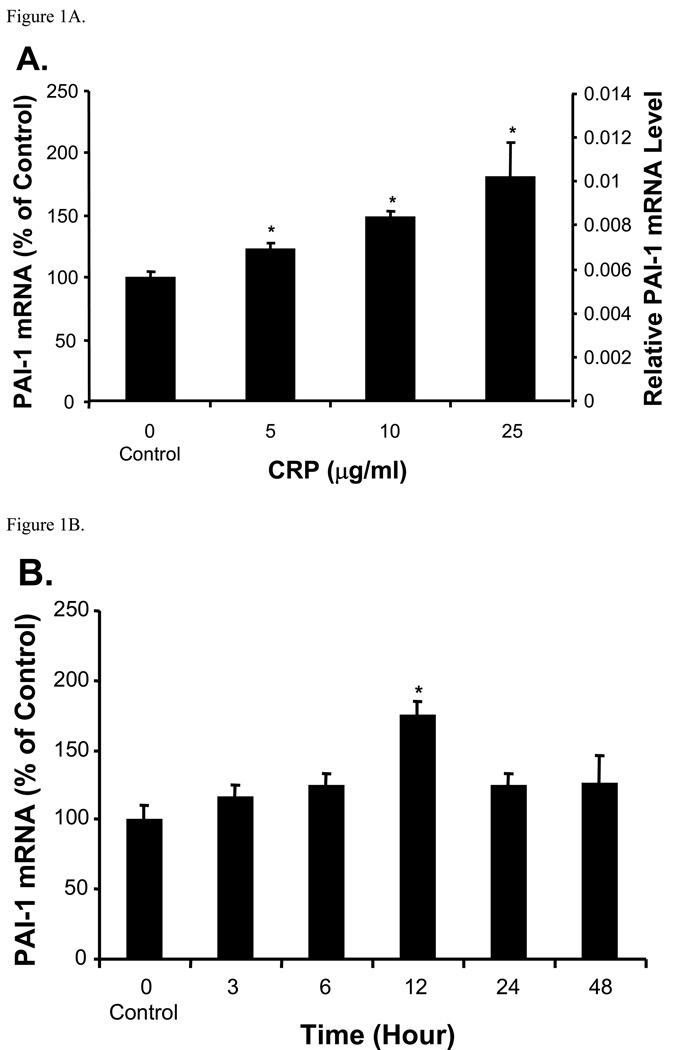
PAI-1 mRNA expression as normalized to β–actin was significantly increased in response to CRP in HCAEC (Fig. 1A). As compared with the control (100%), PAI-1 mRNA levels were increased to 124%, 149%, and 183% at 5, 10, and 25 µg/ml for 12 h, respectively (P<0.05). In HCAEC treated with 10 µg/ml of CRP, PAI-1 mRNA levels were increased to 116%, 126%, and 175% at 3, 6, and 12 h, respectively, compared with untreated controls (P<0.05, Fig. 1B, t-test and ANOVA). However, PAI-1 mRNA levels went down after peaked at 12 h. Moreover, we investigated whether PAI-1 mRNA expression could be up-regulated in other types of human endothelial cells which represent different anatomic sources. Interestingly, PAI-1 mRNA levels were universally increased to 181%, 200%, 156%, and 200% in CRP-treated HCAEC, HUVEC, HMVEC-L, and HMEC, respectively, compared with their untreated controls (P<0.05, Fig. 2). HMEVEC-L had relatively weak response to CRP compared with other types of cells.
Figure 1.
Effect of CRP on PAI-1 mRNA expression in HCAEC. Total mRNA was extracted from HCAEC. PAI-1 mRNA levels were measured by quantitative real time PCR. Data are presented as relative amounts of PAI-1 mRNA normalized to β-actin. (A). Concentration dependent study (12 h treatment). The data are presented in both % of control and relative mRNA levels. PAI-1 mRNA was significantly increased as CRP concentrations increased (*P<0.05, n=3). (B). Time course study. HCAEC were treated with 10 µg/ml CRP for different time points. PAI-1 mRNA levels increased to a peak at 12 h (*P<0.05, n= 3). The data are presented in % of control. t-test and ANOVA.
Figure 2.
Effect of CRP on PAI-1 mRNA expression in different types of human endothelial cells. Different types of human endothelial cells were treated with CRP (10 µg/ml) for 12 h. PAI-1 mRNA levels were determined by real time PCR. CRP significantly increased PAI-1 mRNA in all tested endothelial cells (*P<0.05, n=3). The data are presented in % of control. t-test and ANOVA. HUVEC: Human umbilical venous endothelial cells. HCAEC: Human coronary aorta endothelial cell. HMEC: Human microvascular endothelial cells. HMVEC-L: Human lung microvascular endothelial cells.
CRP increases PAI-1 protein levels and enzyme activities in HCAEC
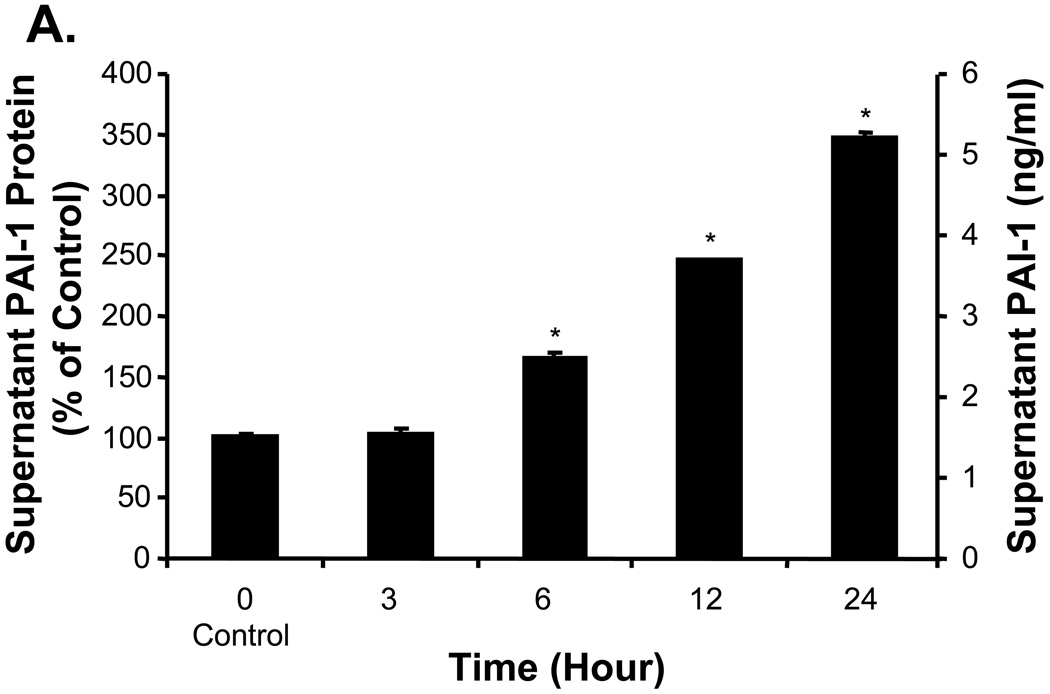
To determine whether CRP could affect PAI-1 expression in the protein level, Western blot was performed. Cellular PAI-1 protein levels were clearly increased in HCAEC exposed to 10 µg/ml CRP (Fig. 3A). The ratio of PAI-1 versus β-actin was dramatically increased to 131%, 137%, and 165% at 6, 12, and 24 h, respectively, compared with the control (P<0.05, Fig. 3B, t-test and ANOVA). In addition, supernatant PAI-1 antigen levels were markedly increased to 166%, 244%, and 344% at 6, 12, and 24 h in cell culture supernatants, respectively (P<0.05, Fig. 4A, t-test and ANOVA). Furthermore, the enzymatic activity of PAI-1 in the cell culture supernatant was also significantly increased to 126%, 143%, 164%, and 196% at 3, 6, 12, and 24 h, respectively, compared with the control (P<0.05, Fig. 4B, t-test and ANOVA).
Figure 3.
Effect of CRP on cellular PAI-1 protein levels in HCAEC. HCAEC were treated with 10 µg/ml CRP as indicated time points. (A). Cellular protein levels of PAI-1 were determined by Western blot analysis. One representative western blot protein gel is shown. (B). Quantitative measurement of protein levels by densitometry analysis. β-actin was used as a loading control. Ratios of PAI-1/β-actin were significantly increased at different time points (*P<0.05, n=3). The data are presented in both % of control and real PAI-1 / β-actin ratio. t-test and ANOVA.
Figure 4.
Effects of CRP on supernatant PAI-1 levels and enzyme activities in HCAEC. HCAEC were treated with to 10 µg/ml CRP at different time points. PAI-1 proteins and enzyme activities in the supernatant of cell cultures were analyzed. (A). Supernatant PAI-1 levels were determined by IMUBIND PAI-1 tissue ELISA kit and showed a significant increase in different time points (6, 12, and 24 h). (B). Supernatant PAI-1 activity was determined by Spectrolyse (pL) PAI-1 Assay and showed a substantial increase in a time dependent fashion (*P<0.05, n=3). The data are presented in both % of control and real measurement. t-test and ANOVA.
CRP induces CD32 mRNA expression but not CD16 in HCAEC
Since CD32 and CD16 are potential receptors of CRP, we determined whether CD32 and CD16 are expressed in HCAEC and whether CRP could affect the expression of CD32 and CD16 in HCAEC. CD32 mRNA levels were detected in HCAEC by quantitative real time PCR. In response to CRP treatment at 5, 10, and 25 µg/ml, CD32 mRNA was increased to 125%, 286%, and 377%, respectively, compared with the control (P<0.05, Fig. 5, t-test and ANOVA). However, CD16 mRNA was not detected in HCAEC.
Figure 5.
Effect of CRP on CD32 mRNA expression in HCAEC. HCAEC were treated with different concentrations of CRP for 12 h, and CD32 mRNA levels were determined by real time PCR. Data are reported as relative amounts of PAI-1 mRNA normalized to β-actin. PAI-1 mRNA levels were significantly increased in cells treated with 10 and 25 µg/ml of CRP (*P<0.05, n=3). The data are presented in both % of control and relative mRNA levels. t-test and ANOVA.
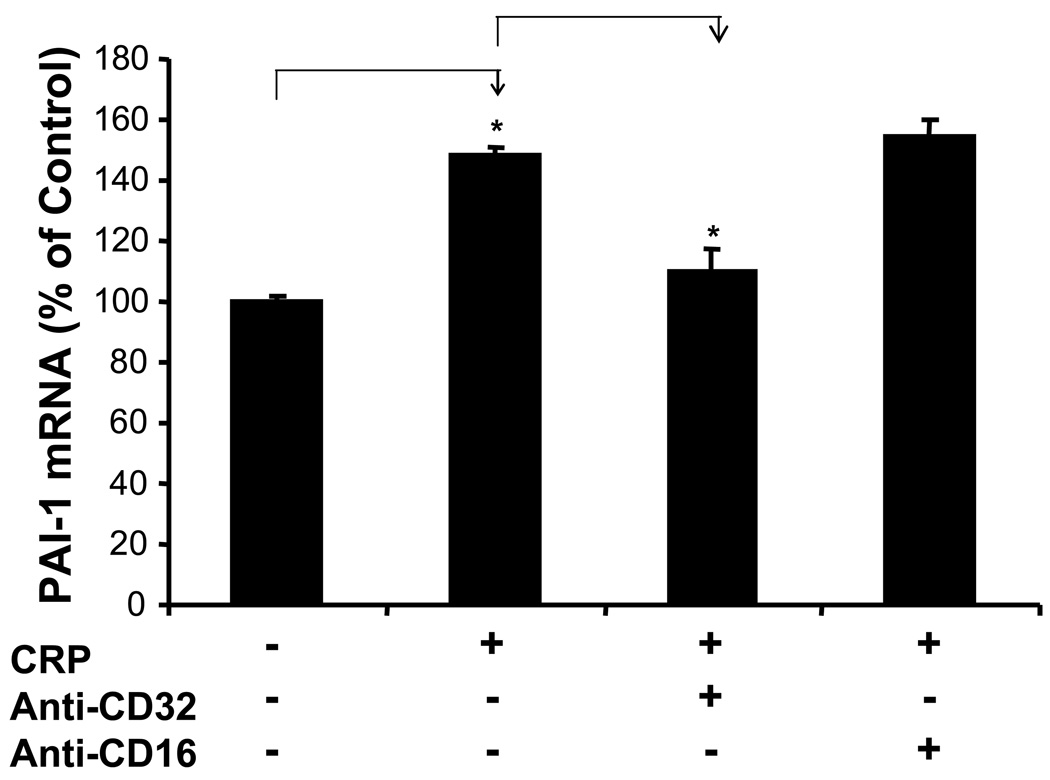
Anti-CD32 antibody blocks CRP-induced PAI-1 mRNA expression in HCAEC
To further identify whether CRP could interact with CD32 for PAI-1 up-regulation, anti-CD32 antibody was used to block the effect of CRP in HCAEC. CRP treatment alone increased PAI-1 mRNA levels to 148% compared with the untreated control, while in the co-cultured group, PAI-1 mRNA levels were reduced to 110% (P<0.05, Fig. 6, t-test). Anti-CD16 antibody had no effect on PAI-1 expression because CD16 was not expressed in HCAEC.
Figure 6.
Effect of anti-CD32 antibody on CRP-induced PAI-1 mRNA expression in HCAEC. HCAEC were first pretreated with anti-CD32 and anti-CD-16 antibodies for 1 h, and then incubated with 10 µg/ml CRP for additional 12 h. PAI-1 mRNA levels were determined by real time PCR. Anti-CD32 antibody significantly blocked CRP-induced PAI-1 upregulation, while anti-CD16 antibody as a control had no effect on PAI-1 expression (*P<0.05, n=3). The data are presented in % of control. t-test.
Antioxidant curcumin effectively inhibits CRP-induced upregulation of PAI-1 in HCAEC
We investigated whether curcumin could block CRP-induced upregulation of PAI-1 in HCAEC. Cells were pretreated with curcumin (10 µM) for 30 min and then treated with 10 µg/ml CRP for 12 h. Curcumin (10 µM) dramatically reduced PAI-1 mRNA levels by 42% compared with CRP-treated group (P<0.05, Fig. 7, t-test). Curcumin alone had no effect on PAI-1 mRNA levels.
Figure 7.
Effect of curcumin on CRP-induced upregulation of PAI-1 in HCAEC. HCAEC were preincubated with curcumin (10 µM) for 30 min, and then treated with 10 µg/ml CRP for 12 h. PAI-1 mRNA levels were determined by real time PCR. Curcumin significantly reduced CRP-induced PAI-1 expression (*P<0.001, n=3). The data are presented in % of control t-test.
CRP induces phosphorylation of p38 MAPK
It has been reported that p38 MAPK pathway is involved in CRP-induced endothelial cell activation [19]. We investigated whether p38 MAPK could be involved in CRP-treated HCAEC by Bio-Plex phosphoprotein luminex assay. In response to CRP treatment, the ratio of phosphorylated p38 over total p38 at 60 min was substantially increased by 4.36 folds compared with untreated cells (Fig. 8). However, there were no significant changes in phosphorylation of JNK and ERK1/2. Thus, p38 MAPK may be involved in CRP-induced changes in HCAEC, but not JNK and ERK1/2.
Figure 8.
Effect of CRP on MAPK phosphorylation in HCAEC. HCAEC were treated with 10 µg/ml CRP as indicated time points. Phosphorylated and total protein levels of 3 MAPKs were measured by Bio-Plex luminex immunoassay. The ratios of phosphorylated versus total protein of each MAPK were presented. CRP treatment substantially increased p38 phosphorylation, but not ERK1/2 and ERK.
Discussion
In the current study, we have demonstrated that CRP significantly increases PAI-1 expression in HCAEC and several other types of human endothelial cells. CRP receptor CD32 is expressed in HCAEC and mediates CRP-induced PAI-1 expression. Interestingly, CRP increases its receptor CD32 expression which may form a positive feedback mechanism for its action in human endothelial cells. Oxidative stress and p38 MAPK may be involved in CRP-induced PAI-1 expression in HCAEC. These results showed several novel aspects of CRP functions in the vascular system.
PAI-1 is a key regulator of fibrinolysis through inhibiting tissue and/or urokinase plasminogen activator [16]. Our data clearly indicate that CRP upregulates PAI-1 expression in both mRNA and protein levels in HCAEC. PAI-1 was particularly increased in the supernatants of CRP-treated cells. Enzymatic activity of PAI-1 was also confirmed. These findings are particular important because HCAEC used in the current study may be relevant to cardiovascular disease with elevated levels of CRP. The concentrations used in the current study also have clinical relevance. In healthy subjects, serum CRP levels are usually less than 1 µg/ml, while patients with aggressive cardiovascular disease usually have elevated serum CRP levels (5 to 25 µg/ml) [1–6]. We have covered this range of CRP concentrations in the current study. In addition to concentration-dependent studies, CRP at 10 µg/ml was used in the most of experiments. We noticed that the effect of CRP on upregulation of PAI-1 mRNA was peaked at 12 h. The reasons for the loss of CRP activity after 12 hours are unknown. We speculate that CRP could be cleared during this time due to a receptor-mediated clearance process or simple degradation process. Further investigation is warranted to address this interesting issue.
CRP-induced PAI-1 upregulation was also observed in several other types of human endothelial cells isolated from umbilical vein, skin, and lung microvessels. It is well known that endothelial cells from different blood vessels or anatomic locations or organs have distinct and characteristic gene expression profiles and functions although many fundamental functions are the same for all types of endothelial cells [20–22]. For example, the levels of PAI-1 released from HCAEC were 2–4 times higher than those from HUVEC basally and under lipoprotein-stimulated conditions [23]. Clinical significance of these different types of human endothelial cells in response to CRP remains to be determined. In addition, CRP also increases tissue factor, cytokines, and another important thrombotic factor secreted from peripheral monocytes [24] and endothelial cells [25]. In our previous studies, we have demonstrated that CRP decreased the expression of thrombomodulin, endothelial protein C receptor, VEGF receptors and neuropilins in human endothelial cells [26,27]. In addition, we reported that CRP was able to regulate human dendritic cell differentiation in vitro [28]. Our current findings along with our previous investigations and other reports could provide more comprehensive information towards understanding the role and mechanisms of CRP for cardiovascular disease and other systems. Our current study may indicate a direct link between CRP and PAI-1 expression. However, many detail signal transduction pathways involved in CRP action are largely unknown. It is also possible that there are indirect pathways leading to PAI-1 expression in response to CRP treatment such as other cytokines as autocrine or paracrine mechanisms [29]. For examples, CRP could enhance the expression of endothelin-1 and IL-6 [30,31], which could in turn stimulate the PAI-1 production in human endothelial cells [32,33]. We did not investigate these indirect pathways in the current study. It has long been known that TNF-α is able to modulate hemostatic properties through endothelial cells [34,35]. We have previously reported that TNF-α decreases the expression of thrombomodulin and endothelial protein C receptor in human endothelial cells [36]. Moreover, thrombosis was expedited after arterial injury in vivo in CRP transgenic mice [37].
Recent studies in human subjects demonstrated that infusion of recombinant human CRP caused endothelial dysfunction and activation of several inflammation cytokines as well as coagulation including PAI-1 upregulation [28,38]. These studies are of great significance because findings convincingly showed the biomediator roles of CRP in humans. Our in vitro study is complementary to these human studies because we address potential molecular mechanisms of CRP-induced PAI-1 expression in human endothelial cells as well as potential therapeutic strategies for controlling the detrimental effects of CRP. Thus, in vitro investigations on CRP activity and molecular mechanisms are also of clinical significance.
CD32 and CD16 are the high-affinity IgG receptor molecules (FcγR) and could serve as CRP receptors on peripheral blood polymorphonuclear leukocytes and monocytes [10, 39]. CD32 was identified on endothelial cells [19, 39]. In the current study, we clearly demonstrate that CD32, but not CD16, is expressed in HCAEC. CRP-induced PAI-1 upregulation was effectively inhibited by specific antibody against CD32, indicating that CD32 mediates CRP's action in HCAEC. This finding is consistent with our previous results that CRP-induced downregulation of thrombomodulin and endothelial protein C receptor was inhibited by anti-CD32 antibody in human endothelial cells [26]. We also confirmed the anti-CRP antibody could effectively block the effect of CRP on human dendritic cell differentiation [28]. Very interestingly, CRP could upregulate CD32 expression, by which CRP could enhance its biological functions. It could be a positive feedback mechanism for CRP acting on human endothelial cells. It is reported that CD32 expression could be enhanced by many factors such as infection of Chlamydophila pneumoniae, phorbol esters and interferon-gamma [39–42]. Thus, CD32 mediates the biological functions of CRP, thereby contributing to the pathogenesis of cardiovascular disease.
Curcumin, a compound of colorful spice purified from plant Curcumin longa, has anti-oxidation, anti-carcinoma, anti-thrombosis and anti-inflammation properties [43]. Curcumin can counteract the effect of TNF-α on human endothelial cells by inactivation of transcriptional factors NFκB and AP-1 [44,45]. In addition, curcumin effectively inhibited endothelial cell proliferation and the tube formation in vivo [46–48]. In our previous study, we reported that curcumin inhibited both CRP- and TNF-α-induced downregulation of thrombomodulin and endothelial protein C receptor in human endothelial cells [26,36]. Thus, curcumin may have protective functions against vascular thrombosis and vascular disease. In the current study, we further demonstrated that curcumin could effectively block CRP-induced PAI-1 upregulation in HCAEC. Since curcumin is a potent antioxidant, this novel finding indicates that oxidative stress may be involved in CRP-induced PAI-1 upregulation in human endothelial cells. This hypothesis was further supported by our data and other reports that CRP enhanced p38 MAPK activation in human endothelial cells [19]. p38 MAPK is one of oxidation-sensitive signal transduction molecules.
Recombinant human CRP for the current study was purchased from Calbiochem. Previously, we determined the endotoxin level as 0.0005 EU/µg for this CRP preparation by Limulus amebocyte lysate assay [28]. Extensive characterizations were done to rule out any functional contributions of potential contaminations of endotoxin and sodium azide in the CRP preparation. Serial experiments including heat-inactivation, antibody blocking, receptor blocking, endotoxin free CRP control, and endotoxin and sodium azide controls were performed [28]. More recent report also confirmed that effects of CRP were not attributable to endotoxin contamination in human endothelial cells [49].
In conclusion, CRP may stimulate vascular thrombosis through upregulation of PAI-1 and affect other thrombotic molecules in human endothelial cells. The underlying molecular mechanisms of CRP action may be involved in CD32 interaction and upregulation, cellular oxidative stress, and p38 MAPK signal transduction pathway in the vascular system. Current study provides further understanding of relationship between system inflammation and cardiovascular disease. Development of new inhibitors for CRP and its pathway molecules may be considered as one of new therapeutic strategies to prevent or treat cardiovascular disease.
Acknowledgements
This work is partially supported by research grants from the National Institutes of Health (Chen: HL065916, HL072716, EB-002436, and HL083471; Yao: R01 DE15543 and R21 AT003094; and Lin: HL076345) and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine.
Abbreviations
- CRP
C-reactive protein
- PAI-1
plasminogen activator inhibitor-1
- HCAEC
human coronary artery endothelial cell
- MAPK
mitogen-activated protein kinase
- HMVEC-L
human lung microvascular endothelial cell
- HUVEC
human umbilical vein endothelial cell
- HMEC
human dermal microvascular endothelial cell
- EBM-2
endothelial cell basal medium-2
- ELISA
Enzyme-linked immunosorbent assay
References
- 1.Pepys MB. C-reactive protein fifty years on. Lancet. 1981;1:653–657. doi: 10.1016/s0140-6736(81)91565-8. [DOI] [PubMed] [Google Scholar]
- 2.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 4.Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, Pestaner J, Smialek J, Virmani R. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105:2019–2023. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events. An 8-year follow-up of 14719 initially healthy Am women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 6.Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002;23:831–834. doi: 10.1053/euhj.2001.3052. [DOI] [PubMed] [Google Scholar]
- 7.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–1441. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 8.Wang CH, Li SH, Weisel RD, Fedak PW, Dumont AS, Szmitko P, Li RK, Mickle DA, Verma S. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation. 2003;107:1783–1790. doi: 10.1161/01.CIR.0000061916.95736.E5. [DOI] [PubMed] [Google Scholar]
- 9.Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, Waltenberger J, Koenig W, Schmitz G, Hombach V, Torzewski J. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2094–2099. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 10.Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for C-reactive protein on leukocytes is fcgamma receptor II. J Exp Med. 1999;190:585–590. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaughan DE. PAI-1 and vascular disease in diabetes mellitus. In: Johnstone MT, Veves A, editors. Diabetes and Cardiovascular Disease. Totowa, NJ: Humana Press; 2000. pp. 237–247. [Google Scholar]
- 12.de Maat MP, de Bart AC, Hennis BC, Meijer P, Havelaar AC, Mulder PG, Kluft C. Interindividual and intraindividual variability in plasma fibrinogen, TPA antigen, PAI activity, and CRP in healthy, young volunteers and patients with angina pectoris. Arterioscler Thromb Vasc Biol. 1996;16:1156–1162. doi: 10.1161/01.atv.16.9.1156. [DOI] [PubMed] [Google Scholar]
- 13.Juhan-Vague I, Pyke SD, Alessi MC, Jespersen J, Haverkate F, Thompson SG. Fibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. ECAT Study Group. European Concerted Action on Thrombosis and Disabilities. Circulation. 1996;94:2057–2063. doi: 10.1161/01.cir.94.9.2057. [DOI] [PubMed] [Google Scholar]
- 14.Dawson S, Henney A. The status of PAI-1 as a risk factor for arterial and thrombotic disease: a review. Atherosclerosis. 1992;95:105–117. doi: 10.1016/0021-9150(92)90014-8. [DOI] [PubMed] [Google Scholar]
- 15.Hamsten A, de Faire U, Walldius G, Dahlen G, Szamosi A, Landou C, Blomback M, Wiman B. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet. 1997;2:3–9. doi: 10.1016/s0140-6736(87)93050-9. [DOI] [PubMed] [Google Scholar]
- 16.Juhan-Vague I, Alessi MC. Plasminogen activator inhibitor 1 and atherothrombosis. Thromb Haemost. 1993;70:138–143. [PubMed] [Google Scholar]
- 17.Lijnen HR, Collen D. Mechanism of physiological fibrinolysis. Baillieres Clin Hematol. 1995;8:277–290. doi: 10.1016/s0950-3536(05)80268-9. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan DE, Declerck PJ, Van Houtte E, De Mol M, Collen D. Reactivated recombinant plasminogen activator inhibitor-1 (rPAI-1) effectively prevents thrombolysis in vivo. Thromb Haemost. 1992;68:60–63. [PubMed] [Google Scholar]
- 19.Khreiss T, Jozsef L, Potempa LA, Filep JG. Conformational rearrangement in C-reactive protein is required for proinflammatory actions on human endothelial cells. Circulation. 2004;109:2016–2022. doi: 10.1161/01.CIR.0000125527.41598.68. [DOI] [PubMed] [Google Scholar]
- 20.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spanel-Borowski K. Diversity of ultrastructure in different phenotypes of cultured microvessel endothelial cells isolated from bovine corpus luteum. Cell Tissue Res. 1991;266:37–49. doi: 10.1007/BF00678709. [DOI] [PubMed] [Google Scholar]
- 22.Huber SA, Haisch C, Lodge PA. Functional diversity in vascular endothelial cells: role in coxsackievirus tropism. J Virol. 1990;64:4516–4522. doi: 10.1128/jvi.64.9.4516-4522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren S, Lee H, Hu L, Lu L, Shen GX. Impact of diabetes-associated lipoproteins on generation of fibrinolytic regulators from vascular endothelial cells. J Clin Endocrinol Metab. 2002;87:286–291. doi: 10.1210/jcem.87.1.8175. [DOI] [PubMed] [Google Scholar]
- 24.Cermak J, Key NS, Bach RR, Balla J, Jacob HS, Vercellotti GM. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–520. [PubMed] [Google Scholar]
- 25.Napoleone E, Di Santo A, Bastone A, Peri G, Mantovani A, de Gaetano G, Donati MB, Lorenzet R. Long pentraxin PTX3 upregulates tissue factor expression in human endothelial cells: a novel link between vascular inflammation and clotting activation. Arterioscler Thromb Vasc Biol. 2002;22:782–787. doi: 10.1161/01.atv.0000012282.39306.64. [DOI] [PubMed] [Google Scholar]
- 26.Nan B, Yang H, Yan S, Lin PH, Lumsden AB, Yao Q, Chen C. C-reactive protein decreases expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Surgery. 2005;138:212–222. doi: 10.1016/j.surg.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Nan B, Yan S, Li M, Yao Q, Chen C. C-reactive protein decreases expression of VEGF receptors and neuropilins and inhibits VEGF165-induced cell proliferation in human endothelial cells. Biochem Biophys Res Commun. 2005;333:1003–1010. doi: 10.1016/j.bbrc.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Becnel L, Li M, Chen C, Yao Q. C-reactive protein impairs human CD14+ monocyte-derived dendritic cell differentiation, maturation and function. Eur J Immunol. 2006;36:2993–3006. doi: 10.1002/eji.200635207. [DOI] [PubMed] [Google Scholar]
- 29.Ballou SP, Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992;4:361–368. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- 30.Verma S, Wang CH, Weisel RD, Badiwala MV, Li SH, Fedak PW, Li RK, Mickle DA. Hyperglycemia potentiates the proatherogenic effects of C-reactive protein: reversal with rosiglitazone. J Mol Cell Cardiol. 2003;35:417–419. doi: 10.1016/s0022-2828(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 31.Bisoendial RJ, Kastelein JJ, Levels JH, Zwaginga JJ, van den Bogaard B, Reitsma PH, Meijers JC, Hartman D, Levi M, Stroes ES. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res. 2005;96:714–716. doi: 10.1161/01.RES.0000163015.67711.AB. [DOI] [PubMed] [Google Scholar]
- 32.Zidovetzki R, Wang JL, Kim JA, Chen P, Fisher M, Hofman FM. Endothelin-1 enhances plasminogen activator inhibitor-1 production by human brain endothelial cells via protein kinase C-dependent pathway. Arterioscler Thromb Vasc Biol. 1999;19:1768–1775. doi: 10.1161/01.atv.19.7.1768. [DOI] [PubMed] [Google Scholar]
- 33.Rega G, Kaun C, Weiss TW, Demyanets S, Zorn G, Kastl SP, Steiner S, Seidinger D, Kopp CW, Frey M, Roehle R, Maurer G, Huber K, Wojta J. Inflammatory cytokines interleukin-6 and oncostatin m induce plasminogen activator inhibitor-1 in human adipose tissue. Circulation. 2005;111:1938–1945. doi: 10.1161/01.CIR.0000161823.55935.BE. [DOI] [PubMed] [Google Scholar]
- 34.Nawroth PP, Bank I, Handley D, Cassimeris J, Chess L, Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986;163:1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163:740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nan B, Lin P, Lumsden AB, Yao Q, Chen C. Effects of TNF-alpha and curcumin on the expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Thromb Res. 2005;115:417–426. doi: 10.1016/j.thromres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Danenberg HD, Szalai AJ, Swaminathan RV, Peng L, Chen Z, Seifert P, Fay WP, Simon DI, Edelman ER. Increased thrombosis after arterial injury in human C-reactive protein-transgenic mice. Circulation. 2003;108:512–515. doi: 10.1161/01.CIR.0000085568.13915.1E. [DOI] [PubMed] [Google Scholar]
- 38.Bisoendial RJ, Kastelein JJ, Peters SL, Levels JH, Birjmohun R, Rotmans JI, Hartman D, Meijers JC, Levi M, Stroes ES. Effects of CRP infusion on endothelial function and coagulation in normocholesterolemic and hypercholesterolemic subjects. J Lipid Res. 2007;48:952–960. doi: 10.1194/jlr.P600014-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Stein MP, Edberg JC, Kimberly RP, Mangan EK, Bharadwaj D, Mold C, Du Clos TW. C-reactive protein binding to FcγRIIa on human monocytes and neutrophils is allele-specific. J Clin Invest. 2000;105:369–376. doi: 10.1172/JCI7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escribano-Burgos M, Lopez-Farre A, del Mar Gonzalez M, Macaya C, Garcia-Mendez A, Mateos-Caceres PJ, Alonso-Orgaz S, Carrasco C, Rico LA, Porres Cubero JC. Effect of C-reactive protein on Fcgamma receptor II in cultured bovine endothelial cells. Clin Sci (Lond) 2005;108:85–91. doi: 10.1042/CS20040217. [DOI] [PubMed] [Google Scholar]
- 41.Vielma S, Virella G, Gorod A, Lopes-Virella M. Chlamydophila pneumoniae infection of human aortic endothelial cells induces the expression of FC gamma receptor II (FcgammaRII) Clin Immunol. 2002;104:265–273. doi: 10.1006/clim.2002.5237. [DOI] [PubMed] [Google Scholar]
- 42.Alevy YG, Tucker J, Naziruddin B, Mohanakumar T. CD32C (FCγRIIC) mRNA expression and regulation. Mol Immunol. 1999;30:775–782. doi: 10.1016/0161-5890(93)90149-6. [DOI] [PubMed] [Google Scholar]
- 43.Bierhaus A, Zhang Y, Quehenberger P, Luther T, Haase M, Muller M, Mackman N, Ziegler R, Nawroth PP. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost. 1997;77:772–782. [PubMed] [Google Scholar]
- 44.Ishii H, Takada K, Higuchi T, Sugiyama J. Verotoxin-1 induces tissue factor expression in human umbilical vein endothelial cells through activation of NF-kappaB/Rel and AP-1. Thromb Haemost. 2000;84:712–721. [PubMed] [Google Scholar]
- 45.Pendurthi UR, Williams JT, Rao LV. Inhibition of tissue factor gene activation in cultured endothelial cells by curcumin. Suppression of activation of transcription factors Egr-1, AP-1, and NF-kappa B. Arterioscler Thromb Vasc Biol. 1997;17:3406–3413. doi: 10.1161/01.atv.17.12.3406. [DOI] [PubMed] [Google Scholar]
- 46.Singh AK, Sidhu GS, Deepa T, Maheshwari RK. Curcumin inhibits the proliferation and cell cycle progression of human umbilical vein endothelial cell. Cancer Lett. 1996;107:109–115. doi: 10.1016/0304-3835(96)04357-1. [DOI] [PubMed] [Google Scholar]
- 47.Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Flynn E, Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- 48.Thaloor D, Singh AK, Sidhu GS, Prasad PV, Kleinman HK, Maheshwari RK. Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by curcumin. Cell Growth Differ. 1998;9:305–312. [PubMed] [Google Scholar]
- 49.Dasu MR, Devaraj S, Du Clos TW, Jialal I. The biological effects of CRP are not attributable to endotoxin contamination: evidence from TLR4 knockdown human aortic endothelial cells. J Lipid Res. 2007;48:509–512. doi: 10.1194/jlr.C600020-JLR200. [DOI] [PubMed] [Google Scholar]