Abstract
Objective
To evaluate the Health-Related Quality of Life (HRQoL) of American Indians with diabetes, hypertension, or both conditions using the SF36; and to explore how the HRQoL is associated with help seeking among American Indians with and without these chronic conditions.
Methods
We analyzed data obtained from respondents with diabetes and/or hypertension who participated in a large epidemiological study of two culturally distinct American Indian tribes. Comparison data were provided by an age, gender, and tribe matched sample from the same study who did not report either condition.
Results
The respondents with both diabetes and hypertension had the lowest HRQoL on all eight subscales of SF36. Confirmatory factor analysis (CFA) showed that the assumption of equivalent factor loadings for participants with and without diabetes and/or hypertension was not satisfied. Biomedical service use was significantly associated with the SF36 physical health factor in those with hypertension only. Help seeking from traditional healers was significantly negatively related to physical factor scores for all the respondents except those with diabetes only.
Conclusions
Participants with comorbid diabetes and hypertension had worse HRQoL. The relationships between HRQoL and different types of help seeking varied depending on the comorbidity status of the respondents.
Keywords: American Indian, HRQoL, SF36, Diabetes, Hypertension, Help seeking
Introduction
Chronic medical conditions often impact multiple dimensions of health-related quality of life (HRQoL). Diabetes and hypertension are two common chronic conditions in American Indians. These two “silent” conditions frequently coexist, leading to additive increases in the risk of life-threatening cardiovascular disease, which is the leading cause of death among natives [1–3]. Diabetes disproportionately affects American Indians; its prevalence is, on average, 2–3 times greater than that among others in the United State [4, 5]. The prevalence of hypertension among American Indians has typically been similar to or lower than the rates for whites [6], but has increased rapidly in recent years [7, 8]. Further, hypertension is significantly associated with diabetes and is a well-known precursor of many of its microvascular and macrovascular complications. Hence, hypertension in American Indians also deserves rigorous attention because of the high prevalence of diabetes and the high morbidity and mortality attributable to the complications of diabetes, such as retinopathy and end-stage renal disease.
People with diabetes have lower HRQoL scores than people with no chronic illness [9, 10] and those with more severe conditions report significantly lower HRQoL across domains [11–13]. The relationship between hypertension and HRQoL is less clear; most studies show a negative association between HRQoL and hypertension [14–16] but others show no relationship [17, 18]. It is particularly inconsistent for specific domains of the HRQoL. For example, while some studies did not find a significant association between hypertension and Bodily Pain (BP) [19, 20], others did [16, 21, 22]. It was hypothesized that those observations might reflect an association between disease severity and pain, but most of those studies did not measure disease severity [16]. A number of studies have assessed HRQoL among those with diabetes and associated comorbidities [23–29], but only a few of these have focused on diabetes and hypertension, with mixed results. Some studies showed poorer HRQoL among patients with both conditions compared to those with one condition [26–28], while others indicated that the HRQoL was not lower among diabetic patients with comorbid hypertension [25]. Moreover, none of the previous studies investigated the HRQoL with these two chronic conditions in American Indians.
The Medical Outcomes Study 36-item Short Form Health Survey (SF36) [30, 31] is a widely used measurement of HRQoL. Exploratory factor analyses of SF36 data from a national probability sample of the United States confirmed that two dimensions—physical and mental components of HRQoL—efficiently explain the pattern of covariance among eight SF36 subscales. Using factor scores from these analyses, the Physical and Mental Component Summaries (PCS and MCS, respectively) have become common SF36 scale scores [32, 33]. However, in using these component scores, the two-factor structure and factor scores are assumed to be equivalent across samples—an assumption that has not been tested extensively for those with different chronic conditions, particularly among ethnic minorities.
In recent years, low self-reported HRQoL has been shown to be significantly associated with high rates of health services utilization and mortality in Veterans Affairs patients [34]. These findings suggest that PCS and MCS scores can be used to identify patients at higher risk of health care utilization and poor health outcomes, who might be a cost-effective target population for future interventions. However, the relationship between self-reported HRQoL and services use among American Indians remains unexplored. The health service ecology for American Indian populations differs significantly from that of other Americans. The Indian Health Service (IHS) is the primary source of biomedical services in many reservation communities. Yet this agency is dramatically underfunded [35], leaving its contribution to addressing local need unclear. At the same time, studies in rural, reservation, and urban communities have found a high prevalence of use of traditional healing among American Indians [36–40]. Previous studies [36] showed that such practices are often important and independent sources of health care for American Indians. We use the broader concept of “help seeking” to refer to use of either biomedical services or traditional healing in this article.
Using data collected as part of a large community-based service utilization and psychiatric epidemiological study of American Indians, the current study evaluated HRQoL among American Indians with diabetes, hypertension, or both conditions using the subscales of SF36; assessed the factor structure of SF36 and the measurement equivalence of the resulting physical and mental health factors among those with and without these chronic conditions; and explored how measures of HRQoL are associated with different types of help seeking among American Indians with and without diabetes and/or hypertension. We hypothesized that American Indians with comorbid diabetes and hypertension would report a significantly worse HRQoL and higher rates of help seeking.
Methods
Study design and sample
Data for this study were drawn from the American Indian Service Utilization, Psychiatric Epidemiology, Risk and Protective Factors Project (AI-SUPERPFP), a community-based cross-sectional survey conducted in two American Indian reservation communities. The AI-SUPERPFP methods are described in greater detail elsewhere [41] and on the study website (http://www.uchsc.edu/ai/ncaianmhr/research/superpfp.htm). AI-SUPERPFP data were collected between 1997 and 1999 from enrolled members of Southwest (SW) and Northern Plains (NP) tribes who were 15–54 years old and who lived on or within 20 miles of their reservations at the time of sampling (1997). To protect the confidentiality of the participant communities, we use the general descriptors of NP and SW rather than specific tribal names. These two participating tribes are among the larger tribes in the United States and represent both the diversity and common experiences of the American Indian population.
Tribal rolls, the official enumeration of tribal members, were used to define the target populations. Using stratified random sampling procedures [42], participants were selected from the tribal rolls and stratified by tribe, age (15–24, 25–34, 35–44, and 45–54), and gender. Overall, 46.5 and 39.5% of the SW and NP tribal members, respectively, were found to be living on or near their reservations. Of those located and found eligible, 73.7% of the SW tribe and 76.8% of the NP tribe agreed to participate in the study (N = 3,084; 1,638 NP and 1,446 SW), with response rates slightly lower for male tribal members and younger tribal members.
Colorado Multi-Institutional Review Board approval was obtained before data collection along with appropriate tribal approvals. All participants provided informed consent. Interviews were computer assisted and administered by tribal members intensively trained in research and interviewing methods. Extensive quality control procedures verified that all portions of the location, recruitment, and interview procedures were conducted in a standardized, reliable manner.
Here, we identified four groups of respondents, based on self-reported chronic disease status: (1) comorbid diabetes and hypertension (N = 132), (2) diabetes alone (N = 118), (3) hypertension alone (N = 331), and (4) a reference group without diabetes or hypertension. Because the age and gender distributions are quite different between respondents with diabetes and/or hypertension and those without these two chronic conditions, the respondents without diabetes or hypertension were assigned random numbers within tribe, age, and gender blocks. We randomly selected respondents within these blocks to generate a one-to-one matched sample (N = 581) to the respondents with diabetes and/or hypertension.
Measures
Chronic conditions were determined based on self-reports. Respondents were asked, for each of 28 medical conditions, whether they ever had the problem and whether it had ever been diagnosed by a health care professional. Only respondents who indicated that a health care professional had diagnosed their conditions were included in this study.
One question from the SF36 was deleted (“I expect my health to get worse”) from the AI-SUPERPFP SF36 due to cultural considerations. The SF36 measures eight dimensions of health: Physical Functioning (PF), Role-Physical (RP), Bodily Pain (BP), General Health (GH), Vitality (VT), Social Functioning (SF), Role-Emotional (RE), and Mental Health (MH). All subscales were recoded to range from 0 to 100, and most assessed the absence of limitations such that 0 implied extreme limitations and 100 signified no limitations on that particular dimension. Three subscales deviated from this pattern. For the General Health, Vitality, and Mental Health subscales, midrange responses indicated average general health, vitality, or mental health; scores lower or higher than this indicated lower or higher than average health status on these dimensions.
Help seeking for physical health problems was assessed using sets of questions measuring service use, satisfaction with services, and barriers to accessing care in the past year among participants who reported having a physical health problem. The IHS is the primary provider of ambulatory and hospital-based care for physical health as well as psychiatric problems among these two tribes. Such services are provided without cost to users, pursuant to treaty-defined obligations on the part of the Federal government. Examples of other providers of biomedical services were tribally operated facilities and the Veterans Administration [43]. In addition to biomedical services mentioned earlier, American Indian tribes have a rich history of traditional healing through consultation with medicine people and ceremonies designed to intervene in the spiritual world to affect healing. Traditional systems of healing are active in both tribes reported here. Tribal members who participated in focus groups prior to AI-SUPERPFP data collection pointed out that speaking of the use of traditional healing “services” made little sense; rather one approaches a healer to ask for help, and the healer, “patient,” and his or her family come together to seek resolution. Hence, the suggestion was made that this construct was best labeled “help seeking.”
Analyses
The SF Health Outcomes Scoring Software [44] was used to assess item and scale performance as well as to create the eight subscales that were used in the factor analyses. Differences in group characteristics were examined by ANOVA analyses or χ2 analyses. Pairwise comparisons with Bonferroni corrections were performed to compare the difference between each pair of the groups.
To evaluate measurement invariance across groups, multiple group confirmatory factor analyses (CFA) were conducted. Compared to exploratory factor analyses (EFA), CFA methods allow greater flexibility for testing alternative models against one another (e.g., one- vs. two-factor models) and for testing whether the parameters (e.g., factor loadings) are equivalent across groups. For each CFA model, the χ2 statistic, degrees of freedom, comparative fit index (CFI), and root mean square error of approximation (RMSEA) are reported. A CFI over 0.9 [45] and RMSEA under 0.05 [46] indicate adequate fit of the model to the data. To assess relationships between help seeking and HRQoL in different groups while taking potentially different measurement structures of SF36 across groups into consideration, we used structural equation models (SEM) because they allow for simultaneous estimation of measurement structures and regression models. We used Mplus [47] for both CFA and SEM.
Results
Preliminary analyses showed no significant differences between NP and SW tribes in either the measurement structure or the path coefficients in our model (data not shown); hence analyses below were conducted using a combined sample of two tribes.
Univariate analysis
Table 1 shows that the scale scores in all eight dimensions of SF36 were highest among those without diabetes or hypertension. At the other extreme, the respondents with both diabetes and hypertension reported the lowest HRQoL for every dimension. Between those two extremes, those with diabetes alone or hypertension alone reported moderate deficits on the eight dimensions of SF36. The most striking differences between those with both chronic conditions and those without either chronic condition were the differences on Role-Physical (64.2 vs. 86.4) and General Health (50.3 vs. 72.5). The smallest difference was found on Mental Health.
Table 1.
Univariate comparisons of SF36 subscales and service use by diabetes and hypertension status
| Neither diabetes or hypertension (N = 581) | Diabetes only (N = 118) | Hypertension only (N = 331) | Diabetes and hypertension (N = 132) | P value | |
|---|---|---|---|---|---|
| SF36 subscales (mean) | |||||
| 1. Physical functioning (PF) | 85.49†‡ | 80.39‡ | 80.77*‡ | 69.63*£† | <0.0001 |
| 2. Role-physical (RP) | 86.40£†‡ | 74.44* | 78.34*‡ | 64.20*† | <0.0001 |
| 3. Bodily pain (BP) | 79.97£†‡ | 71.13* | 71.01* | 64.20* | <0.0001 |
| 4. General health (GH) | 72.50£†‡ | 62.91*‡ | 63.62*‡ | 50.32*£† | <0.0001 |
| 5. Vitality (VT) | 69.86£†‡ | 63.97* | 63.07* | 61.76* | <0.0001 |
| 6. Social function (SF) | 83.72‡ | 80.98 | 79.29 | 74.53* | 0.0007 |
| 7. Role-emotional (RE) | 89.15‡ | 88.32‡ | 86.58‡ | 78.54*£† | 0.0023 |
| 8. Mental health (MH) | 82.18†‡ | 81.71 | 78.63* | 76.78* | 0.0013 |
| Help seeking (%) | |||||
| Biomedical service use for a physical health problem | 53.11£‡ | 76.07*† | 58.66£‡ | 71.97*† | 0.0003 |
| Traditional healing use for a physical health problem | 17.19 | 23.68 | 19.44 | 23.66 | 0.1995 |
Significantly different from no diabetes and hypertension
Significantly different from diabetes only
Significantly different from hypertension only
Significantly different from diabetes and hypertension
Also shown in Table 1 are the rates of biomedical service use and seeking of traditional healing for a physical health problem in the past year among the four groups. The rates of biomedical service use were significantly higher in those with diabetes only (76%) or comorbid diabetes and hypertension (72%) compared to the respondents in the other two groups (59% for those with hypertension only and 53% for those without either condition). On the other hand, the rates of seeking traditional healing in the past year were not significantly different among the four groups.
Measurement invariance of SF36
Using CFA methods, we compared the model fit for a set of alternative models in all four groups of respondents in this study: a one-factor structure, a two-factor structure in which no subscales loaded on more than one factor (crossloaded), and the more common model in which General Health, Vitality, and Social Functioning were crossloaded for both the physical and mental factors. For all four samples, the two-factor model with crossloadings provided the best fit (data not shown). In Table 2, the typical two-factor model with crossloadings was tested across groups to determine whether the factor loadings and the factor structures could be assumed equivalent across the four groups. The difference in χ2 statistics indicated that significant differences in factor loadings existed across respondents with and without diabetes or hypertension. More specifically, a series of model comparisons showed significant differences in factor loadings of two subscales (Bodily Pain and General Health) across the health condition groups. Moreover, further tests showed that the factor loadings of Bodily Pain and General Health could be assumed equal in the three groups who had diabetes or hypertension or both, but were significantly different in the “neither condition” group.
Table 2.
Multiple group CFA results: tests of measurement equivalence for SF36 across participants with and without diabetes and/or hypertension
| Model | χ2 statistic | df | CFI | RMSEA | Model comparisons | |||
|---|---|---|---|---|---|---|---|---|
| Models compareda | χ2 difference | df | P value | |||||
| 1. All factor loadings constrained equal across groups | 225.78 | 91 | 0.965 | 0.072 | ||||
| 2. All factor loadings free to vary across groups | 181.097 | 64 | 0.970 | 0.080 | 1 vs. 2 | 44.683 | 27 | 0.0176 |
| 3. All factor loadings differ in the “neither condition” group, but equal in the other 3 groups | 198.87 | 82 | 0.970 | 0.070 | 1 vs. 3 | 26.91 | 9 | 0.0014 |
| 2 vs. 3 | 17.773 | 18 | 0.4707 | |||||
| 4. BP loadings differ in the “neither condition” group, but equal in the other 3 groups | 214.067 | 90 | 0.968 | 0.069 | 1 vs. 4 | 11.713 | 1 | 0.0006 |
| 3 vs. 4 | 15.197 | 8 | 0.0454 | |||||
| 5. GH loadings differ in the “neither condition” group, but equal in the other 3 groups | 217.837 | 89 | 0.967 | 0.071 | 1 vs. 5 | 7.943 | 2 | 0.0188 |
| 3 vs. 5 | 18.967 | 7 | 0.0083 | |||||
| 6. BP and GH loadings differ in the “neither” group, but equal in the other 3 groups | 207.168 | 88 | 0.969 | 0.069 | 1 vs. 6 | 18.612 | 3 | 0.0003 |
| 3 vs. 6 | 8.298 | 6 | 0.2171 | |||||
Each model was compared with the null model (model 1) and the best previous model
HRQoL and help seeking
Table 3 shows the fit statistics for the structural equation models testing the relationships of help seeking with the two HRQoL factors; we also assessed whether the two subscales (Bodily Pain and General Health), which showed different factor loadings for the “neither condition” group, were differentially related to help seeking. Model comparisons were performed using χ2 statistics. The results showed the model with biomedical service use effects and traditional healing use effects on both the physical and mental health factors, plus a direct path of biomedical service use on Bodily Pain, adjusted by age, gender, and tribe effects (model 6), provided the best fit. This final model is depicted in Fig. 1.
Table 3.
Structural equation models with partial measurement invariance: comparison of alternative models for help seeking
| Model | χ2 statistic | df | CFI | RMSEA | Model comparisons | |||
|---|---|---|---|---|---|---|---|---|
| Models compareda | χ2 difference | df | P value | |||||
| 1. Without service effects | 775.792 | 288 | 0.883 | 0.077 | ||||
| 2. With biomedical service and traditional healing effects on physical and mental factors | 694.021 | 286 | 0.898 | 0.075 | 1 vs. 2 | 81.771 | 2 | 0.0000 |
| 3. With biomedical service and traditional healing effects on physical and mental factors and GH | 691.495 | 260 | 0.897 | 0.076 | 2 vs. 3 | 2.526 | 26 | 1.0000 |
| 4. With biomedical service and traditional healing effects on physical and mental factors and BP | 655.897 | 260 | 0.905 | 0.073 | 2 vs. 4 | 38.124 | 26 | 0.0590 |
| 5. With biomedical service and traditional healing effects on physical and mental factors, and biomedical service effects on BP | 656.727 | 264 | 0.906 | 0.072 | 2 vs. 5 | 37.294 | 22 | 0.0220 |
| 6. With biomedical service and traditional healing effects on physical and mental factors, biomedical service effects on BP, and adjusted by age, gender, and tribe effects | 422.140 | 204 | 0.946 | 0.061 | 5 vs. 6 | 234.587 | 60 | 0.0000 |
Each model was compared with the best previous model
Fig. 1.
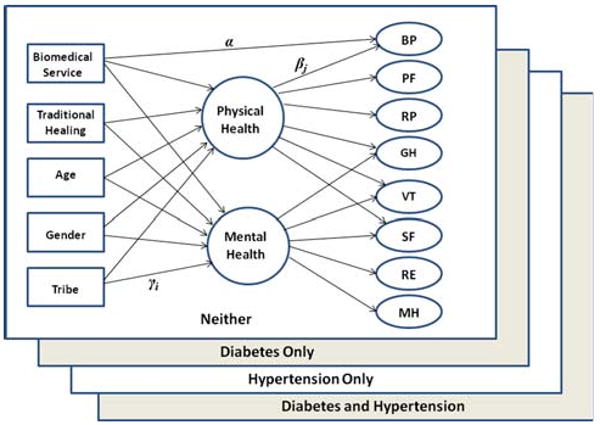
Graphical representation of the final model. βj is the factor loading of confirmatory factor analysis; γi is the estimate of multiple regression of each exogenous variable on latent physical and mental health factors; α is the estimate of direct effect of biomedical service use on Bodily Pain (BP)
Table 4 presents the parameter estimates of the final model for the four groups. Looking first at the factor loadings, as indicated in the multiple group CFA, the standardized loading estimates for Bodily Pain and General Health scales differed across groups. Bodily Pain loaded less strongly on the physical factor for those with diabetes, hypertension, or both compared to those with neither condition. On the other hand, General Health loaded more heavily on the physical factor for those with one or both of these conditions compared to those with neither of these chronic diseases.
Table 4.
Factor loadings and service effects of the final model (model 6 in Table 3)
| SF36 subscales | Group | |||||||
|---|---|---|---|---|---|---|---|---|
| 1. Neither diabetes or hypertension (N = 567) | 2. Diabetes only (N = 114) | 3. Hypertension only (N = 324) | 4. Diabetes and hypertension (N = 130) | |||||
| Physical | Mental | Physical | Mental | Physical | Mental | Physical | Mental | |
| Standardized factor loadings | ||||||||
| 1. Physical functioning (PF) | 0.672 | 0.797 | 0.747 | 0.724 | ||||
| 2. Role-physical (RP) | 0.767 | 0.785 | 0.832 | 0.733 | ||||
| 3. Bodily Pain (BP)a | 0.761 | 0.670 | 0.689 | 0.687 | ||||
| 4. General health (GH)a | 0.346 | 0.423 | 0.585 | 0.193 | 0.562 | 0.201 | 0.565 | 0.226 |
| 5. Vitality (VT) | 0.332 | 0.515 | 0.433 | 0.467 | 0.401 | 0.469 | 0.411 | 0.538 |
| 6. Social function (SF) | 0.455 | 0.351 | 0.577 | 0.310 | 0.553 | 0.323 | 0.510 | 0.333 |
| 7. Role-emotional (RE) | 0.686 | 0.640 | 0.613 | 0.611 | ||||
| 8. Mental health (MH) | 0.801 | 0.840 | 0.807 | 0.810 | ||||
| Correlation between factors | 0.584 | 0.409 | 0.452 | 0.506 | ||||
| Relationships of help seeking variables to SF36 (estimate/SE)b | ||||||||
| Physical/mental factor on biomedical service | −1.789 | −1.597 | −0.597 | −0.231 | −2.999 | −0.213 | 0.075 | −0.045 |
| Physical/mental factor on traditional healing | −3.578 | −2.694 | 0.057 | 0.729 | −2.149 | −1.210 | −3.421 | −0.169 |
| BP on biomedical service | −3.868 | −3.305 | −2.545 | −2.458 | ||||
Factor loadings are bolded to indicate the unstandardized loadings of these two items are assumed to be different between group 1 and groups 2, 3, and 4 (the loadings of these two items are assumed to be equal among groups 2, 3, and 4). The unstandardized loadings of all the other six items are assumed to be equal among all the four groups
Bolded if P < 0.05
Also shown in Table 4, the biomedical service use in the past years was strongly related with the physical factor solely for those who had hypertension only. On the other hand, seeking help from traditional healers was significantly associated with the physical factor for all the respondents, with the exception of those with diabetes only. For the group with neither condition, help seeking from traditional sources was strongly correlated with both lower physical and mental factor scores. However, for those with diabetes only, neither biomedical service use nor traditional healing was significantly associated with worse HRQoL. Finally, even controlling for the Physical and Mental factors, worse Bodily Pain was significantly associated with biomedical service use in the past year in all four groups.
Discussion
As might be expected, but not previously reported for American Indian samples, we found that both diabetes and hypertension were associated with a highly significant impact on HRQoL, reflected by the large effect size from the scale scores across all eight SF36 subscales for the diabetes and hypertension group. This is consistent with previous studies showing that the presence of concurrent diabetes and hypertension was associated with a significant decline in SF36 scores, especially in physical functioning [26, 27]. Another recent study found that the hypertension was not significantly correlated with decreased quality of life [25]; however, that study used another instrument, the Health Utilities Index-III, to measure HRQoL. The greatest mean differences between the comorbid diabetes and hypertension group and the reference group were found on the Role-Physical subscale and the General Health subscale. The relationship of diabetes with the General Health subscale and physical health in general was shown earlier [48], especially among those with diabetes complications [12].
When we considered the factor structure of the SF36 subscales, we found that we could not assume measurement equivalence across groups defined by diabetes and hypertension status. Previous work suggested the assumption that the factor scores underlying the published PCS and MCS scores may not be valid in some American Indian populations [49]. Although we did not specifically test that assumption here, the nonequivalence of factor loadings found in the current study again suggests that methods allowing for specification of measurement models within the context of full structural models comprise stronger methods than do the simple use of the PCS and MCS summary scores. We found that, among the respondents with neither diabetes nor hypertension, the factor loadings of General Health for the physical and mental functioning factors were equivalent in magnitude. However, among those with diabetes and/or hypertension, General Health loaded more heavily on the physical factor, indicating that these chronic diseases were associated with stronger links between physical function and general health. On the other hand, Bodily Pain was less related to physical functioning for those with diabetes or hypertension than those with neither, likely reflecting the “silence” of these two conditions. Our findings are consistent with several previous studies evaluating the psychometric assumptions of SF36. Hobart et al. [50] pointed out that when using the SF36 as a health measure in patients with multiple sclerosis, summary scores should be reported with caution, because assumptions for generating two SF36 summary measures were only partially satisfied. The magnitude and pattern of scale to component correlations in patients with multiple sclerosis differ from the general population, indicating that the factor scores used to generate the summary measures are not entirely applicable to people with multiple sclerosis. Taft et al. [51] also concluded that the current PCS/MCS scoring procedure might inaccurately summarize subscale profile scores and should be interpreted with caution and only in combination with subscale scores. Indeed, the creators of the SF36 advise users that the PCS and MCS scores should be used only after careful consideration of the subscale scores [32].
Another important finding was that about 25% of the respondents with diabetes and 40% of the respondents with hypertension only did not use any type of biomedical service in the year prior to interview. In addition, respondents with both diabetes and hypertension did not have a higher rate of biomedical service use compared to those with diabetes only. Theoretically, because of treaties and laws that arose from the government's acquisition of Indian lands, the federal government is obligated to provide health services to the native population, which would mean that native communities enjoy easy access to medical health services. However, in reality, the IHS is underfunded and understaffed [35], resulting in unavailability of care for many American Indians; relatively low rates of biomedical service use in our sample likely reflect this situation. We also found women used biomedical services more frequently than did men, consistent with some previous reports [52, 53]; we found no gender differences in seeking traditional healing though (data not shown). Future studies investigating specific barriers to care among American Indians with chronic conditions (including gender-specific barriers) are urgently needed to improve health care in this unique population.
When assessing the relationships between HRQoL and biomedical service use, Bodily Pain showed the most consistent pattern: even after controlling for the physical and mental health factors, those reporting more Bodily Pain were more likely to seek biomedical services than those in less pain. On the other hand, controlling for Bodily Pain, the full physical factor was only significantly associated with utilization of biomedical service in those with hypertension only. This might be caused by different perceptions of diabetes and hypertension among American Indians. The salience of diabetes is high in American Indian communities, and it is a common knowledge that diabetic patients should visit their biomedical service provider on a regular basis, no matter whether they experienced symptoms or not. However, many people may perceive hypertension as a milder disease, and feel less impetus to see a biomedical service provider until they feel their physical health recently worsened. It is worth noting that in the model without the direct path between Bodily Pain and biomedical service use (data not shown), the effect of biomedical service use on the physical health factor was also significant among those with neither condition, indicating Bodily Pain was the major reason for this group of respondents to use biomedical service.
Meanwhile, seeking help from traditional healers was significantly related to the physical factor in all respondents in this study except those with diabetes only. A previous study has shown that the use of biomedical and traditional services among American Indians varied by problem type with traditional healers providing care for problems affecting the whole person, including emotional problems, rather than specific physical health problems [36]. As an increasingly prevalent disease in American Indian communities, diabetes is usually thought of as a physical health problem that warrants biomedical care. On the other hand, although hypertension is also a physical health problem, it may be perceived as more closely related to psychological and emotional problems, especially life stress [54–56]. Hence, American Indians with hypertension may be more likely to seek help from a traditional healer as their health worsens.
Several limitations exist for this study. First, we relied on self-reports of physician diagnosis of diabetes and hypertension and were unable to confirm these diagnoses through chart reviews or clinical examinations. A previous study comparing self-reported comorbidities and medical chart-based abstraction, however, found that these two methods agreed in more than 90% of the cases for most chronic conditions [57]. Second, we chose to emulate the approach of Ware et al. [58] with factor analyses at the subscale level rather than the item level; a full second-order factor model would likely yield somewhat different results. However, several recent studies investigating the Different Item Functioning (DIF) of SF36 by chronic diseases indicated the presence of DIF related to chronic diseases was minimal and rarely transferred to the subscale level [59, 60]. Third, one of the model fit statistics, RMSEA, of the final model is not optimal (0.061). Although usually only models with a RMSEA under 0.05 are considered good fit, given all the other model fit indices of the final model (CFI, TLI, etc.) indicated reasonable fit, we feel that the fit of this model is adequate. Fourth, subjects aged 55 years and older were not included in the survey. As both diabetes and hypertension are most prevalent in older populations, future studies are needed to determine whether the same findings are obtained in individuals older than age 55. Finally, no data on type of diabetes or severity of illness were collected, and it is possible that our sample include patients with both type 1 and type 2 diabeties. Since type 1 diabetes is mostly seen in younger patients while type 2 diabetes is more prevalent among older populations, our sample could have some patients with type 1 diabetes. However, type 1 diabetes is very rare among American Indians [61], and analyses restricted to participants who were 35 years and older (thus excluding most type 1 diabetic cases) produced similar results (data not shown). Similarly, the lack of severity assessment precludes us investigating the relationship between disease severity and HRQoL and future study with more comprehensive measurements of chronic conditions is warranted.
Nevertheless, this is the first study to compare the HRQoL in a large sample of American Indians with and without diabetes and/or hypertension and to evaluate the measurement equivalence of SF36 scales among those with and without these two chronic conditions. It demonstrates the impact of diabetes on HRQoL among American Indians—a population with very heavy diabetes burden, especially among those with comorbid hypertension. This study also highlighted the relatively low rates of biomedical service use among the American Indians with diabetes and hypertension and revealed interesting relationships between the HRQoL and different types of help seeking among the respondents of this study. Future research needs to examine the causes of the relationships between HRQoL and help seeking among American Indians with different chronic diseases and to investigate barriers to care among those with low HRQoL scores. Additionally to be considered is the extension of such investigations to urban native populations, who comprise about two-thirds of all American Indians and have a very different health care ecology.
Acknowledgments
The study was supported by the following NIH grants: R01 MH48174 (SM Manson and J Beals, PIs) and P01 MH42473 (SM Manson, PI). Manuscript preparation was supported by R01 DA 17803 (J Beals, PI) and by IHS grant HHSI242200400049C. AI-SUPERPFP would not have been possible without the significant contributions of many people. The following interviewers, computer/data management and administrative staff supplied energy and enthusiasm for an often difficult job: Anna E. Barón, Antonita Begay, Amelia T. Begay, Cathy A. E. Bell, Phyllis Brewer, Nelson Chee, Mary Cook, Helen J. Curley, Mary C. Davenport, Rhonda Wiegman Dick, Marvine D. Douville, Pearl Dull Knife, Geneva Emhoolah, Fay Flame, Roslyn Green, Billie K. Greene, Jack Herman, Tamara Holmes, Shelly Hubing, Cameron R. Joe, Louise F. Joe, Cheryl L. Martin, Jeff Miller, Robert H. Moran Jr., Natalie K. Murphy, Melissa Nixon, Ralph L. Roanhorse, Margo Schwab, Jennifer Settlemire, Donna M. Shangreaux, Matilda J. Shorty, Selena S. S. Simmons, Wileen Smith, Tina Standing Soldier, Jennifer Truel, Lori Trullinger, Arnold Tsinajinnie, Jennifer M. Warren, Intriga Wounded Head, Theresa (Dawn) Wright, Jenny J. Yazzie, and Sheila A. Young. We would also like to acknowledge the contributions of the Methods Advisory Group: Margarita Alegria, Evelyn J. Bromet, Dedra Buchwald, Peter Guarnaccia, Steven G. Heeringa, Ronald Kessler, R. Jay Turner, and William A. Vega. Finally, we thank the tribal members who so generously answered all the questions asked of them.
Abbreviations
- HRQoL
Health related quality of life
- SF36
Medical outcomes study 36-item short form health survey
- CFA
Confirmatory factor analysis
- EFA
Exploratory factory analysis
- PCS
Physical component summary
- MCS
Mental component summary
- IHS
Indian health service
- AI-SUPERPFP
American Indian service utilization, psychiatric epidemiology, risk and protective factors project
- SW
Southwest
- NP
Northern plains
- PF
Physical functioning
- RP
Role-physical
- BP
Bodily pain
- GH
General health
- VT
Vitality
- SF
Social functioning
- RE
Role-emotional
- MH
Mental health
- ANOVA
Analysis of variance
- RMSEA
Root mean square error of approximation
Appendix: AI-SUPERPFP team
Cecelia K. Big Crow, Dedra Buchwald, Buck Chambers, Michelle L. Christensen, Denise A. Dillard, Karen DuBray, Paula A. Espinoza, Candace M. Fleming, Ann Wilson Frederick, Joseph Gone, Diana Gurley, Lori L. Jervis, Shirlene M. Jim, Carol E. Kaufman, Ellen M. Keane, Suzell A. Klein, Denise Lee, Monica C. McNulty, Denise L. Middlebrook, Laurie A. Moore, Tilda D. Nez, Ilena M. Norton, Theresa O'Nell, Heather D. Orton, Carlette J. Randall, Angela Sam, James H. Shore, Sylvia G. Simpson, and Lorette Yazzie
Footnotes
Members of the AI-SUPERPFP Team are listed in the appendix.
Contributor Information
Luohua Jiang, Email: Luohua.Jiang@ucdenver.edu, Centers for American Indian and Alaska Native Health, Colorado School of Public Health, University of Colorado, Denver, MS F800, P.O. Box 6508, Aurora, CO 80045-0508, USA.
Janette Beals, Centers for American Indian and Alaska Native Health, Colorado School of Public Health, University of Colorado, Denver, MS F800, P.O. Box 6508, Aurora, CO 80045-0508, USA.
Nancy R. Whitesell, Centers for American Indian and Alaska Native Health, Colorado School of Public Health, University of Colorado, Denver, MS F800, P.O. Box 6508, Aurora, CO 80045-0508, USA
Yvette Roubideaux, Department of Family and Community Medicine, College of Medicine, The University of Arizona, Tucson, AZ, USA.
Spero M. Manson, Centers for American Indian and Alaska Native Health, Colorado School of Public Health, University of Colorado, Denver, MS F800, P.O. Box 6508, Aurora, CO 80045-0508, USA
References
- 1.Lee ET, et al. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45–74 years, 1984–1988. The strong heart study. American Journal of Epidemiology. 1998;147(11):995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- 2.Howard BV, et al. Rising tide of cardiovascular disease in American Indians. The strong heart study. Circulation. 1999;99(18):2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 3.Rhoades DA. Racial misclassification and disparities in cardiovascular disease among American Indians and Alaska Natives. Circulation. 2005;111:1250–1256. doi: 10.1161/01.CIR.0000157735.25005.3F. [DOI] [PubMed] [Google Scholar]
- 4.Acton KJ, et al. Diabetes prevalence among American Indians and Alaska Natives and the overall population—United States, 1994–2002. MMWR Weekly. 2003;52(30):702–704. [PubMed] [Google Scholar]
- 5.Mokdad AH, et al. Diabetes trends among American Indians and Alaska Natives: 1990-1998. Diabetes Care. 2001;24(8):1508–1509. doi: 10.2337/diacare.24.8.1508-a. [DOI] [PubMed] [Google Scholar]
- 6.Howard BV, et al. Hypertension in adult American Indians. The strong heart study. Hypertension. 1996;28(2):256–264. doi: 10.1161/01.hyp.28.2.256. [DOI] [PubMed] [Google Scholar]
- 7.Rhoades DA, Rhoades ER, Welty TK. The rise of cardiovasculare disease. In: Rhoades ER, editor. American Indian health: Innovations in health care, promotion, and policy. Baltimore: The Johns Hopkins University Press; 2000. pp. 151–178. [Google Scholar]
- 8.Rhoades DA, et al. Aging and the prevalence of cardiovascular disease risk factors in older American Indians: The strong heart study. Journal of the American Geriatrics Society. 2007;55(1):87–94. doi: 10.1111/j.1532-5415.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 9.Glasgow RE, et al. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care. 1997;20(4):562–567. doi: 10.2337/diacare.20.4.562. [DOI] [PubMed] [Google Scholar]
- 10.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes/Metabolism Research and Reviews. 1999;15(3):205–218. doi: 10.1002/(SICI)1520-7560(199905/06)15:3<205∷AID-DMRR29>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care. 1994;17(4):267–274. doi: 10.2337/diacare.17.4.267. [DOI] [PubMed] [Google Scholar]
- 12.Ribu L, et al. A comparison of the health-related quality of life in patients with diabetic foot ulcers, with a diabetes group and a nondiabetes group from the general population. Quality of Life Research. 2007;16(2):179–189. doi: 10.1007/s11136-006-0031-y. [DOI] [PubMed] [Google Scholar]
- 13.Happich M, et al. The quality of life and economic burden of neuropathy in diabetic patients in Germany in 2002—results from the diabetic microvascular complications (DIMICO) study. Diabetes Research and Clinical Practice. 2008;81(2):223–230. doi: 10.1016/j.diabres.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Battersby C, et al. Quality of life in treated hypertension: A case–control community based study. Journal of Human Hypertension. 1995;9(12):981–986. [PubMed] [Google Scholar]
- 15.Fernandez-Lopez JA, et al. Study of quality of life on rural hypertensive patients. Comparison with the general population of the same environment. Journal of Clinical Epidemiology. 1994;47(12):1373–1380. doi: 10.1016/0895-4356(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 16.Bardage C, Isacson DG. Hypertension and health-related quality of life. An epidemiological study in Sweden. Journal of Clinical Epidemiology. 2001;54(2):172–181. doi: 10.1016/S0895-4356(00)00293-6. [DOI] [PubMed] [Google Scholar]
- 17.Chatellier G, et al. Symptom prevalence in hypertensive patients. European Heart Journal. 1982;3(Suppl C):45–52. doi: 10.1093/eurheartj/3.suppl_c.45. [DOI] [PubMed] [Google Scholar]
- 18.Kottke TE, et al. The relationship of symptoms and blood pressure in a population sample. International Journal of Epidemiology. 1979;8(4):355–359. doi: 10.1093/ije/8.4.355. [DOI] [PubMed] [Google Scholar]
- 19.Stewart AL, et al. Functional status and well-being of patients with chronic conditions. Results from the medical outcomes study. Journal of the American Medical Association. 1989;262(7):907–913. doi: 10.1001/jama.262.7.907. [DOI] [PubMed] [Google Scholar]
- 20.Fryback DG, et al. The beaver dam health outcomes study: Initial catalog of health-state quality factors. Medical Decision Making. 1993;13(2):89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 21.Kjellgren KI, et al. Perceived symptoms amongst hypertensive patients in routine clinical practice—a population-based study. Journal of Internal Medicine. 1998;244(4):325–332. doi: 10.1046/j.1365-2796.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- 22.Kullman S, Svardsudd K. Differences in perceived symptoms/quality of life in untreated hypertensive and normotensive men. Scandinavian Journal of Primary Health Care. 1990;1:47–53. [PubMed] [Google Scholar]
- 23.Coffey JT, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25(12):2238–2243. doi: 10.2337/diacare.25.12.2238. [DOI] [PubMed] [Google Scholar]
- 24.Maddigan SL, Feeny DH, Johnson JA. Health-related quality of life deficits associated with diabetes and comorbidities in a Canadian National Population Health Survey. Quality of Life Research. 2005;14(5):1311–1320. doi: 10.1007/s11136-004-6640-4. [DOI] [PubMed] [Google Scholar]
- 25.Wexler DJ, et al. Correlates of health-related quality of life in type 2 diabetes. Diabetologia. 2006;49(7):1489–1497. doi: 10.1007/s00125-006-0249-9. [DOI] [PubMed] [Google Scholar]
- 26.Bayliss EA, et al. Predicting declines in physical function in persons with multiple chronic medical conditions: What we can learn from the medical problem list. Health and Quality of Life Outcomes. 2004;2:47. doi: 10.1186/1477-7525-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wee HL, et al. The impact of diabetes mellitus and other chronic medical conditions on health-related quality of life: Is the whole greater than the sum of its parts? Health and Quality of Life Outcomes. 2005;3:2. doi: 10.1186/1477-7525-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oldridge NB, et al. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. Journal of Clinical Epidemiology. 2001;54(9):928–934. doi: 10.1016/S0895-4356(01)00350-X. [DOI] [PubMed] [Google Scholar]
- 29.Otiniano ME, et al. The effect of diabetes combined with stroke on disability, self-rated health, and mortality in older Mexican Americans: Results from the Hispanic EPESE. Archives of Physical Medicine and Rehabilitation. 2003;84(5):725–730. doi: 10.1016/s0003-9993(02)04941-9. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item short form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE., Jr Standards for validating health measures: Definition and content. Journal of Chronic Diseases. 1987;40:473–480. doi: 10.1016/0021-9681(87)90003-8. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: A response. Quality of Life Research. 2001;10(5):405–413. doi: 10.1023/A:1012588218728. discussion 415-20. [DOI] [PubMed] [Google Scholar]
- 33.Peek MK, et al. Reliability and validity of the SF-36 among older Mexican Americans. The Gerontologist. 2004;44(3):418–425. doi: 10.1093/geront/44.3.418. [DOI] [PubMed] [Google Scholar]
- 34.Singh JA, et al. Health-related quality of life, functional impairment, and healthcare utilization by veterans: Veterans' quality of life study. Journal of the American Geriatrics Society. 2005;53(1):108–113. doi: 10.1111/j.1532-5415.2005.53020.x. [DOI] [PubMed] [Google Scholar]
- 35.Manson SM. Behavioral health services for American Indians: Need, use, and barriers to effective care. In: Dixon M, Roubideaux Y, editors. Promises to keep: Public health policy for American Indians and Alaska Natives in the 21st century. Washington, DC: American Public Health Association; 2001. pp. 167–192. [Google Scholar]
- 36.Novins DK, et al. Use of biomedical services and traditional healing options among American Indians: Sociodemographic correlates, spirituality, and ethnic identity. Medical Care. 2004;42(2):670–679. doi: 10.1097/01.mlr.0000129902.29132.a6. [DOI] [PubMed] [Google Scholar]
- 37.Buchwald DS, Beals J, Manson SM. Use of traditional healing among Native Americans in a primary care setting. Medical Care. 2000;38(12):1191–1199. doi: 10.1097/00005650-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Kim C, Kwok YS. Navajo use of native healers. Archives of Internal Medicine. 1998;158(20):2245–2249. doi: 10.1001/archinte.158.20.2245. [DOI] [PubMed] [Google Scholar]
- 39.Guilmet GM. Health care and health care seeking strategies among Puyallup Indians. Culture, Medicine and Psychiatry. 1984;8(4):349–369. doi: 10.1007/BF00114662. [DOI] [PubMed] [Google Scholar]
- 40.Marbella AM, et al. Use of Native American healers among Native American patients in an urban Native American health center. Archives of Family Medicine. 1998;7(2):182–185. doi: 10.1001/archfami.7.2.182. [DOI] [PubMed] [Google Scholar]
- 41.Beals J, et al. Cultural specificity and comparison in psychiatric epidemiology: Walking the tightrope in American Indian research. Culture, Medicine and Psychiatry. 2003;27:259–289. doi: 10.1023/A:1025347130953. [DOI] [PubMed] [Google Scholar]
- 42.Cochran WG. Sampling techniques. Vol. 3. New York: Wiley; 1977. [Google Scholar]
- 43.Rhoades E, editor. American Indian health: Innovations in health care, promotion, and policy. Baltimore, MD: Johns Hopkins University Press; 2000. [Google Scholar]
- 44.Quality Metric Incorporated. SF health outcomes scoring software. Lincoln, RI: Quality Metric Incorporated; 2004. [Google Scholar]
- 45.Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- 46.Wolinsky FD, Stump TE. A measurement model of the medical outcomes study 36-item short-form health survey in a clinical sample of disadvantaged, older, black, and white men and women. Medical Care. 1996;34(6):537–548. doi: 10.1097/00005650-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Muthén LK, Muthén BO. Mplus user's guide. Los Angeles, CA: Muthen & Muthen; 1998-2006. [Google Scholar]
- 48.Alonso J, et al. Health-related quality of life associated with chronic conditions in eight countries: Results from the international quality of life assessment (IQOLA) project. Quality of Life Research. 2004;13(2):283–298. doi: 10.1023/B:QURE.0000018472.46236.05. [DOI] [PubMed] [Google Scholar]
- 49.Beals J, et al. Different factor loadings for SF-36: The strong heart study and the national survey of functional health status. Journal of Clinical Epidemiology. 2006;59:208–215. doi: 10.1016/j.jclinepi.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Hobart J, et al. The SF-36 in multiple sclerosis: Why basic assumptions must be tested. Journal of Neurology, Neurosurgery and Psychiatry. 2001;71(3):363–370. doi: 10.1136/jnnp.71.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taft C, Karlsson J, Sullivan M. Do SF-36 summary component scores accurately summarize subscale scores. Quality of Life Research. 2001;10(5):395–404. doi: 10.1023/A:1012552211996. [DOI] [PubMed] [Google Scholar]
- 52.Keene J, Li X. Age and gender differences in health service utilization. Journal of Public Health (Oxford, England) 2005;27(1):74–79. doi: 10.1093/pubmed/fdh208. [DOI] [PubMed] [Google Scholar]
- 53.Godden S, Pollock AM. How to profile the population's use of health care and social care in one district. Journal of Public Health Medicine. 1998;20(2):175–179. doi: 10.1093/oxfordjournals.pubmed.a024739. [DOI] [PubMed] [Google Scholar]
- 54.Nyklicek I, Vingerhoets JJ, Van Heck GL. Hypertension and objective and self-reported stressor exposure: A review. Journal of Psychosomatic Research. 1996;40(6):585–601. doi: 10.1016/0022-3999(95)00647-8. [DOI] [PubMed] [Google Scholar]
- 55.Perez LH, et al. Relation between overweight, diabetes, stress and hypertension: A case–control study in Yarumal—Antioquia, Colombia. European Journal of Epidemiology. 2001;17(3):275–280. doi: 10.1023/A:1017975925554. [DOI] [PubMed] [Google Scholar]
- 56.Everson SA, et al. Hypertension incidence is predicted by high levels of hopelessness in Finnish men. Hypertension. 2000;35(2):561–567. doi: 10.1161/01.hyp.35.2.561. [DOI] [PubMed] [Google Scholar]
- 57.Katz JN, et al. Can comorbidity be measured by questionnaire rather than medical record review. Medical Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Ware JE. SF-36 physical, mental health summary scales: A user's manual. Boston, MA: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 59.Perkins AJ, et al. Assessment of differential item functioning for demographic comparisons in the MOS SF-36 health survey. Quality of Life Research. 2006;15(3):331–348. doi: 10.1007/s11136-005-1551-6. [DOI] [PubMed] [Google Scholar]
- 60.Yu YF, Yu AP, Ahn J. Investigating differential item functioning by chronic diseases in the SF-36 health survey: A latent trait analysis using MIMIC models. Medical Care. 2007;45(9):851–859. doi: 10.1097/MLR.0b013e318074ce4c. [DOI] [PubMed] [Google Scholar]
- 61.Valway S, et al. Prevalence of diagnosed diabetes among American Indians, Alaska Natives, 1987. Estimates from a national outpatient data base. Diabetes Care. 1993;16(1):271–276. doi: 10.2337/diacare.16.1.271. [DOI] [PubMed] [Google Scholar]


