Abstract
Spinal reflex conditioning changes reflex size, induces spinal cord plasticity, and modifies locomotion. Appropriate reflex conditioning can improve walking in rats after spinal cord injury (SCI). Reflex conditioning offers a new therapeutic strategy for restoring function in people with SCI. This approach can address the specific deficits of individuals with SCI by targeting specific reflex pathways for increased or decreased responsiveness. In addition, once clinically significant regeneration can be achieved, reflex conditioning could provide a means of re-educating the newly (and probably imperfectly) reconnected spinal cord.
Keywords: spinal cord injury, reflex conditioning, plasticity, H-reflex, learning and memory, locomotion, rehabilitation
Over the past 30 years, we have studied spinal cord plasticity produced by reflex conditioning. Most recently, we have begun to explore whether reflex conditioning can be used to improve locomotor function after injury or disease.
This paper reviews the changes within the spinal cord produced by the reflex conditioning, and the supraspinal areas and spinal cord descending pathways involved in this learning process. It also reviews our recent findings that reflex conditioning affects locomotor function, and that appropriate application of reflex conditioning can improve walking in rats with spinal cord injury, and introduces our current effort to use reflex conditioning to improve locomotion in people with spinal cord injuries.
Operant conditioning of spinal cord reflexes
Spinal cord reflexes are simple behaviors produced by CNS pathways within the spinal cord. The spinal stretch reflex (SSR), also called the knee jerk or M1, is the initial response to sudden muscle stretch.1–5 It is the simplest spinal cord reflex, produced largely by a two-neuron, monosynaptic pathway consisting of the Ia afferent neuron from the muscle spindle, its synapse on the alpha motoneuron, and the motoneuron itself. Sudden muscle stretch excites the Ia afferent, which in turn excites motoneurons innervating the same muscle and its synergists, thereby causing muscle contraction that opposes the sudden stretch.
The H-reflex is the electrical analog of the SSR. It is similar to the SSR except that the H-reflex is elicited by direct electrical stimulation of the Ia afferent fiber, and thereby bypasses the muscle spindle. Because the reflex arc is influenced by descending pathways from the brain,3–4 the SSR or the H-reflex can be operantly conditioned. Motivated by a paradigm in which reward depends on reflex size, monkeys, humans, rats, and mice can gradually increase or decrease the SSR or the H-reflex.6–11 This conditioning involves plasticity in the spinal cord itself, since conditioned change remains even after all descending activity is abolished.12
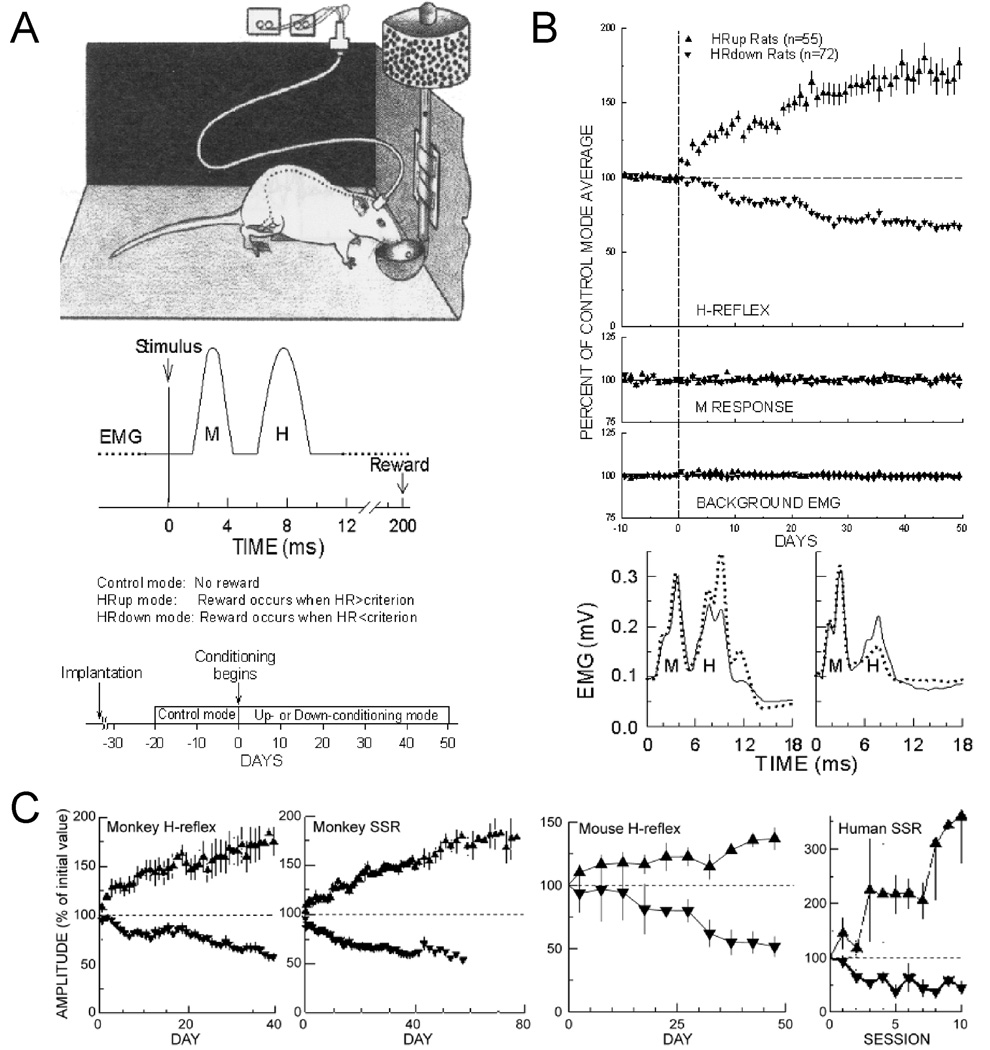
Figure 1 shows the standard conditioning protocol as implemented in the rat. The monkey, human, and mouse H-reflex and SSR conditioning protocols are comparable. Each rat is chronically implanted with fine-wire electromyographic (EMG) recording electrodes in the soleus muscle and a nerve cuff on the posterior tibial nerve (which contains the motoneuron axons that innervate the soleus). The wires connect through a head-mounted tether to a commutator, and then to an amplifier and stimulator. The tether allows the rat to move freely about the cage, and remains connected continuously 24 hr/day. Whenever the ongoing background EMG in soleus remains in a defined range for several seconds, a stimulus through the nerve cuff that is kept just above M response threshold elicits the H-reflex. M response is measured as EMG in the M response interval (typically 2–4 ms after the stimulus). H-reflex size is measured as EMG in the H-reflex interval (typically 6–9 ms after the stimulus). Under the control mode, no reward occurs. Under the up-conditioning mode, a reward (i.e., a food pellet) is delivered if the H-reflex is above a criterion value. Under the down-conditioning mode, a reward is delivered if the H-reflex is below a criterion value. Each animal is first studied under control-mode for 20 days to determine its control H-reflex size, then it is exposed to up- or down-conditioning for 50 days. At the end of conditioning, a reflex size change of at least 20% in the correct direction qualifies as successful conditioning.9,13 Overall, about 80% of animals can be successfully conditioned. In the other 20%, reflex size remains within 20% of its control value.
Figure 1.
A: Soleus H-conditioning protocol in the rat. Soleus EMG is monitored continuously in a rat with chronic EMG electrodes and a tibial nerve cuff. When EMG absolute value is in a defined range for several seconds, nerve cuff stimulation elicits a threshold M response (a direct muscle response45 and an H-reflex. For the first 10–20 days, the rat is exposed to the control mode, in which the H-reflex is simply measured. For the next 40–50 days, it is exposed to the HRup or HRdown mode, in which a food-pellet reward occurs if the H-reflex is above (HRup) or below (HRdown) a criterion. Background EMG and M response are constant throughout. B: Results. Top: Average daily H-reflexes (±SEM) from HRup (▲) and HRdown rats (▼) under control mode (days −10 to 0) and HRup or HRdown mode (days 0–50). In HRup rats, the H-reflex rises gradually to about 175% of control, while in HRdown rats it falls gradually to about 60%. Bottom: Average poststimulus EMG for representative days from an HRup (left) and an HRdown (right) rat under the control mode (solid) and near the end of conditioning (dotted). The H-reflex is much larger after up-conditioning and much smaller after down-conditioning. Background EMG (EMG at 0 time) and M responses are unchanged. C: From left to right: up (▲) and down (▼) conditioning of triceps surae H-reflex in monkeys,7 biceps brachii SSR in monkeys,6 soleus and gastrocnemius H-reflex in mice,10 and biceps brachii SSR in humans.8 Time courses and magnitudes of change are similar to those in the rat.
The top panel of Figure 1B shows average H-reflex size for 127 rats for the final 10 days under the control mode and for 50 days under the up- or down-conditioning mode. The H-reflex does not change under the control mode. It gradually increases during up-conditioning or gradually decreases during down-conditioning, while the M response and background EMG (i.e., EMG at zero time) do not change.
These findings are illustrated in the bottom panel of Figure 1B by examples of post-stimulus EMG from an up-conditioned rat (left) and a down-conditioned rat (right) before conditioning (solid lines) and at the end of conditioning (dashed lines). In these rectified (i.e., absolute value) and averaged traces, the H-reflex is increased at the end of up-conditioning and is decreased after down-conditioning with respect to the control-mode H-reflex without any change in the background EMG level or in the M response.
Figure 1C shows the results of H-reflex conditioning in monkeys, SSR conditioning in monkeys, H-reflex conditioning in mice, and SSR conditioning in humans.6–8,10 In all cases, reflex size gradually increases during up-conditioning, and gradually decreases during down-conditioning. The similarities of the time course and extent of reflex conditioning across species suggest that the phenomenon is similar in these different species.
H-reflex conditioning produces plasticity at multiple sites in the spinal cord
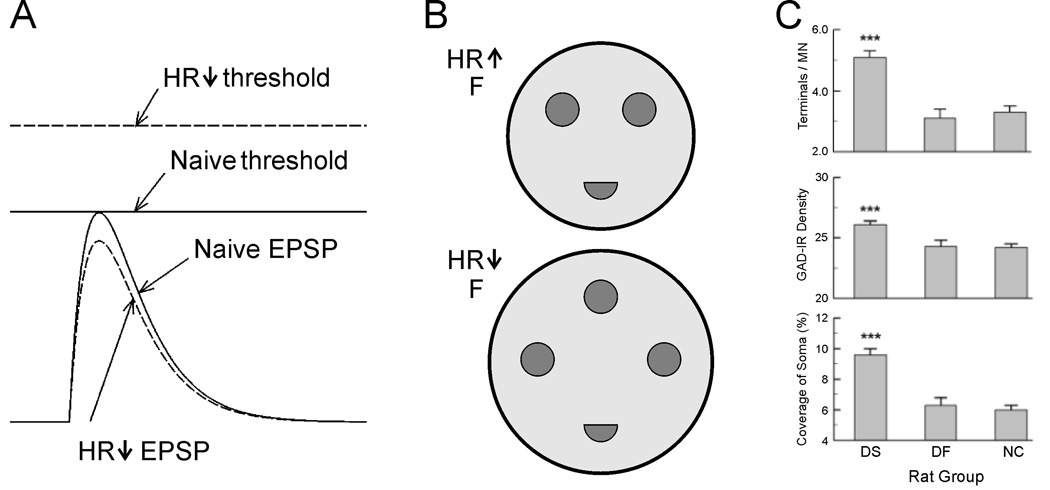
Results from a number of studies revealed that reflex conditioning induces plasticity at multiple sites in the spinal cord.14–16 Intracellular studies in monkeys indicate that down-conditioning and up-conditioning are not mirror images of each other, but rather have different mechanisms.17–18 Down-conditioning appears to be due mainly to change in the motoneuron itself.17,19 It produces a positive shift in motoneuron firing threshold and a modest decrease in Ia EPSP amplitude (Figure 2A). It also decreases motoneuron axonal conduction velocity.17,20 Both the threshold shift and the change in conduction velocity are best explained by a positive shift in sodium channel activation voltage throughout the motoneuron somatic and axonal membrane.19 The change in threshold can largely account for the H-reflex decrease.17,20
Figure 2.
Spinal cord plasticity induced by H-reflex conditioning. A: Motoneurons have more positive firing thresholds and tend to have smaller Ia EPSPs after H-reflex down-conditioning.17 As a result, they are less likely to fire in response to nerve stimulation. B: Schematic showing that down-conditioned monkeys have bigger F terminals than up-conditioned monkeys, and that their F terminals have more active zones.21 C: Soleus motoneurons of successfully down-conditioned rats (DS) have more detectable GAD67-labeled terminals, higher density of GAD immunoreactivity, and larger GAD-terminal coverage of soma than those of naive control rats (NC) or unsuccessful down-conditioned rats (DF). 22
H-reflex conditioning also modifies synaptic terminals on the motoneurons of the H-reflex pathway, and probably changes spinal cord interneurons that convey oligosynaptic group I input to the motoneuron and/or convey descending influence to the motoneuron.15–16 Electron microscopic data show that reflex conditioning affects inhibitory inputs to motoneurons.21 F-terminals (which are typically inhibitory) are bigger and have more active zones after down-conditioning than after up-conditioning (Figure 2B). Recent light microscopic studies using immunostaining revealed that down-conditioning changes GABAergic input to motoneurons (Figure 2C). Soleus motoneurons of successfully down-conditioned rats have a larger number of detectable GAD67-labeled terminals than those of naive control rats or of rats exposed to down-conditioning that failed to reduce their H-reflexes.22 These results imply that down-conditioning of soleus H-reflex strengthens GABAergic input to soleus motoneurons. H-reflex conditioning also affect C terminals (terminals with postsynaptic cisterns) 21 and changes the strength of primary afferent terminal input.17–18
Reflex Conditioning Depends on Supraspinal Descending Influence
The H-reflex conditioning protocol also produces activity-dependent plasticity in the sensorimotor cortex.23 Furthermore, it is clear that mode-appropriate activity in sensorimotor cortex descends in the corticospinal tract (CST) to the spinal cord and induces the spinal cord plasticity directly responsible for H-reflex change.24–27
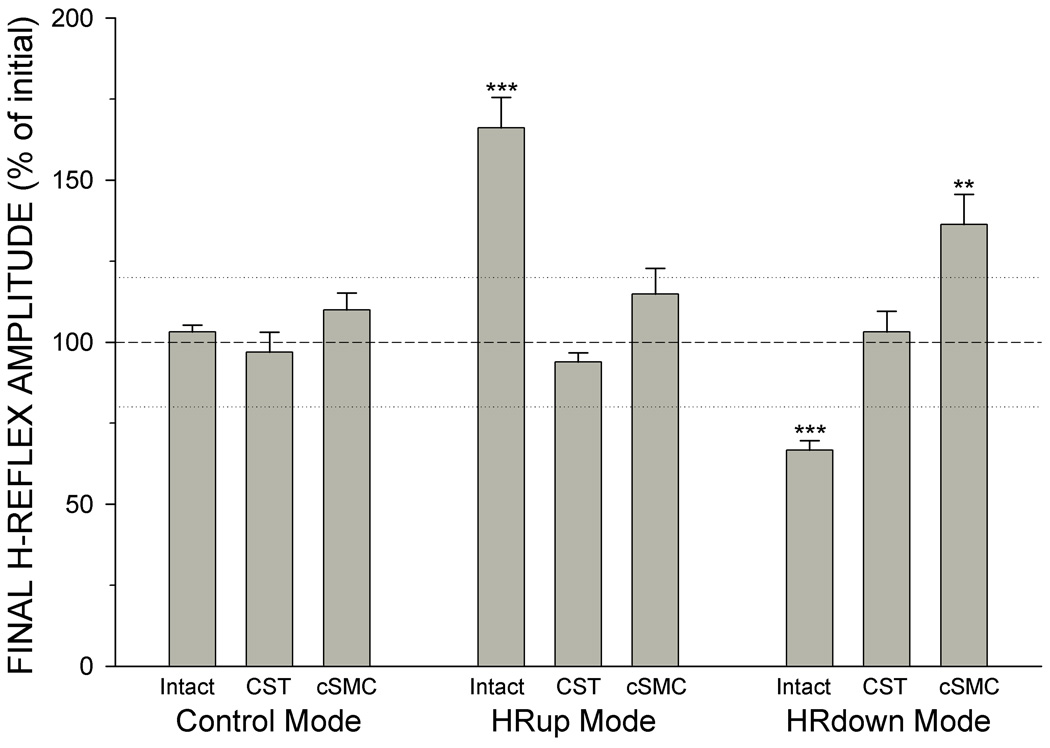
CST transection or ablation of contralateral sensorimotor cortex (cSMC) prevents both down-conditioning and up-conditioning. Figure 3 shows average (±SEM) final H-reflex size (average of final 10 days as % of initial control-mode size) for intact rats (combined data from 9,20,24–25,27–30), CST-transected rats,24–26 and contralateral SMC-ablated rats27 after exposure to continued control mode (i.e., no reward contingency), up-conditioning, or down-conditioning. Continued control-mode exposure has no significant effect on any group. In intact rats, the HRup and HRdown modes have the previously described mode-appropriate effects. In CST-transected rats, up-conditioning and down-conditioning do not change H-reflex size. In cSMC rats, up-conditioning has no significant effect on the H-reflex, while down-conditioning actually increases H-reflex size. The unique role of the CST is emphasized by the observations that transection of the major tracts descending in the lateral column of the spinal cord (i.e., rubrospinal, reticulospinal, and vestibulospinal tracts) or the sensory tracts ascending in the dorsal column does not affect H-reflex conditioning.24–26
Figure 3.
Average (∀SEM) final H-reflex size (average for final 10 days as % of initial size) for intact rats, CST-transected rats, and contralateral SMC-ablated rats after continued-control, HRup, or HRdown mode exposure. Continued control-mode exposure has no significant effect in any group. In intact rats, the HRup and HRdown modes have mode-appropriate effects. In CST rats, the HRup and HRdown modes have no significant effect. In cSMC rats, the HRup mode has no significant effect, while the HRdown mode actually increases H-reflex size. Asterisks indicate significant differences from initial size (***P < 0.001; **P < 0.005 by paired t-test).
The cerebellum also plays an important role in H-reflex conditioning. Down-conditioning does not occur in rats in which the cerebellar output nuclei dentate and interpositus have been ablated.31–32 Descending cerebellar influence on the spinal cord is mediated by projections from the cerebellar output nuclei via the red nucleus.33–34 Transection of the red nucleus projection to the spinal cord (i.e., the rubrospinal tract) by lateral column transection does not impair conditioning.24–26 This indicates that the cerebellar contribution to H-reflex conditioning is mediated through cerebellar output to the cortex rather than directly to the spinal cord.
Reflex Conditioning Produces a Complex Pattern of Brain and Spinal Cord Plasticity
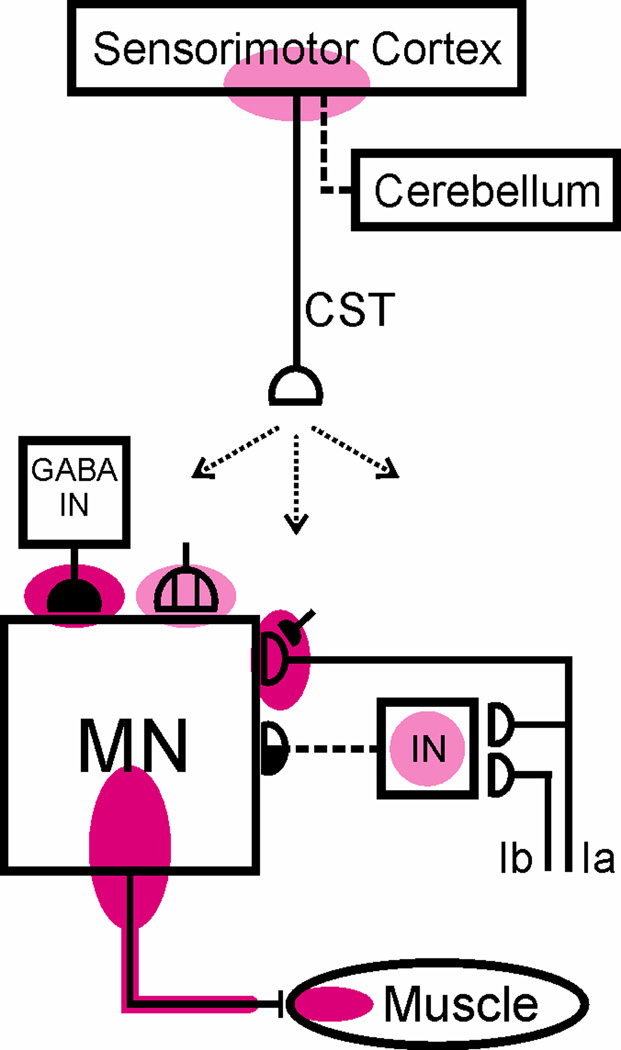
Figure 4 summarizes our current understanding of the plasticity associated with reflex conditioning. Reflex conditioning induces plasticity in the brain (probably in sensorimotor cortex), and the altered activity in sensorimotor cortex projects via the corticospinal tract (CST) to the spinal cord and induces plasticity in the spinal cord. The sites of plasticity within the spinal cord include: the motoneuron, GABAergic terminals and cholinergic C-terminals on the motoneuron, the Ia afferent synaptic connection,17–18 and probably spinal interneurons that participate in oligosynaptic reflex pathways to the motoneuron and/or convey CST influence to the motoneuron.
Figure 4.
Summary of current understanding of spinal and supraspinal plasticity associated with H-reflex conditioning. The shaded ovals indicate the sites of definite (red) or probable (pink) spinal or supraspinal plasticity associated with H-reflex conditioning. Abbreviations: MN, motoneuron; CST, main corticospinal tract; IN, spinal interneuron; and GABA IN, GABAergic interneuron. Open synaptic terminals are excitatory, solid ones are inhibitory, half-open ones could be either, and the subdivided one is a cluster of C terminals. Dashed pathways imply the possibility of intervening spinal interneurons. The monosynaptic and probably oligosynaptic H-reflex pathway from Ia and Ib inputs to the motoneuron is shown. The sites of definite or probable plasticity include: the motoneuron membrane (firing threshold and axonal conduction velocity); motor unit properties; GABAergic terminals and C terminals on the motoneuron; the Ia afferent synaptic connection; interneurons and their terminals conveying oligosynaptic group I inhibition or excitation to the motoneuron; and sensorimotor cortex. The essential roles of the corticospinal tract (originating in sensorimotor cortex) and of cerebellar output to cortex are indicated. The spinal cord plasticity that is directly responsible for H-reflex conditioning appears to be induced and maintained by cortical plasticity that itself depends for its long-term survival on the cerebellum.
Reflex conditioning affects locomotor function in normal animals
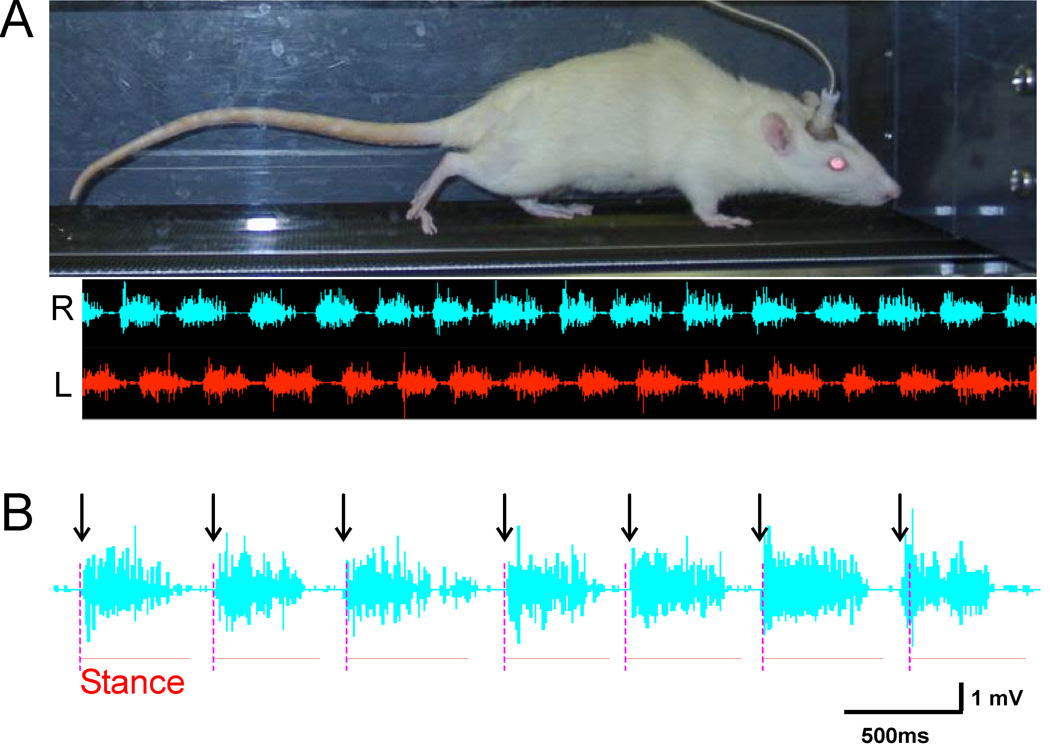
Because spinal motoneurons and interneurons mediate almost all motor behaviors, the plasticity produced by H-reflex conditioning is likely to affect behaviors other than the H-reflex. For example, the primary afferent excitation responsible for the H-reflex plays a major role in locomotion.35–39 To evaluate these wider effects, we assessed the effects of reflex conditioning on treadmill locomotion. Figure 5A shows the alternating right and left soleus EMG activity that occurs during the step-cycle. As Figure 5B shows, the onsets of the right soleus burst (indicated by the arrows) are closely related to the onsets of the right stance phase of locomotion (indicated by the vertical dashed lines).
Figure 5.
A: (Top) A rat walks on a treadmill during EMG data collection (top); (Bottom) concurrent right (bottom upper trace) and left (bottom lower trace) soleus EMG activity. B: The relationship between right soleus bursts and the right stance phases of locomotion. The arrows indicate the onsets of the right soleus bursts. The vertical dashed lines indicate the onsets of the right stance phase of locomotion, and the horizontal lines indicate the duration of the right stance phase. The onsets and duration of the right soleus burst are closely related to the onsets and duration of the right stance phase of locomotion.
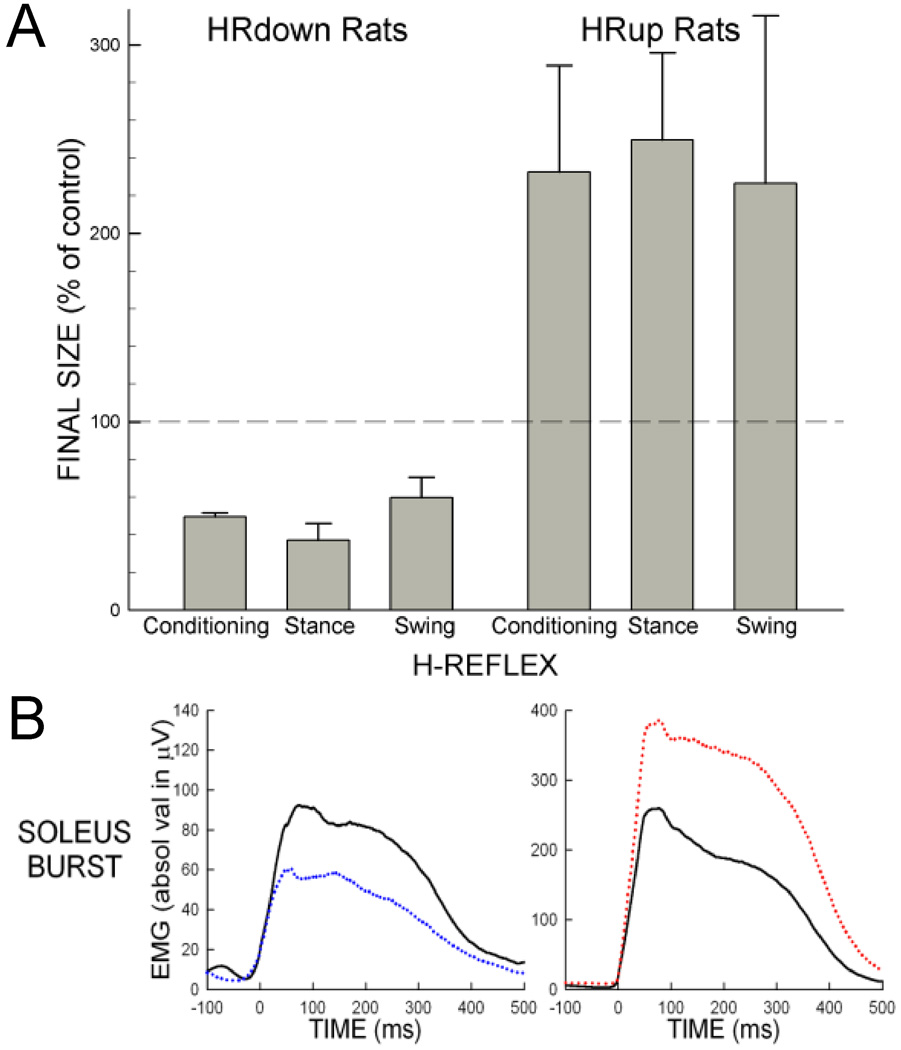
Figure 6 shows the effects of conditioning on H-reflexes elicited during the conditioning protocol and during locomotion in normal rats. In rats in which the soleus H-reflex elicited in the conditioning protocol (i.e., the "conditioning H-reflex") had been decreased by down-conditioning, the H-reflexes elicited in the stance and swing phases of locomotion (i.e., the "locomotor H-reflexes") were also smaller. Similarly, in rats in which the conditioning H-reflex had been increased by up-conditioning, the locomotor H-reflexes were also larger.40 These results indicate that conditioning-induced change in reflex function is also expressed during locomotion.
Figure 6.
A: Effects of conditioning on the conditioning H-reflexes and the locomotor H-reflexes. The average (∀SEM) final values of conditioning, stance, and swing H-reflexes from successful HRdown and HRup rats are shown. The conditioning and locomotor H-reflexes are similarly decreased in HRdown rats and similarly increased in HRup rats. See 40 for detail.
B: Soleus H-reflex conditioning affects soleus activity during locomotion in normal rats. Average right soleus locomotor bursts before (solid) and after (dotted) conditioning from a down-conditioned (left) and an up-conditioned (right) rats. After conditioning, the soleus burst is smaller in the down-conditioned rat and larger in the up-conditioned rat. See 40 for detail.
In these normal rats, right soleus H-reflex conditioning affects the right soleus locomotor burst amplitude.40 Figure 6B shows average rectified right soleus EMG bursts during treadmill walking from a down-conditioned rat (left) and from an up-conditioned rat (right). The right soleus EMG burst decreases after down-conditioning, and increases after up-conditioning. These results indicate that reflex conditioning affects locomotor function. This is not surprising, given that primary afferent excitation is a major contributor to the locomotor burst.35–39 At the same time, however, H-reflex conditioning in these normal rats did not appear to affect step-cycle length, duration or right/left symmetry. This implies that changes also occurred in the locomotor behavior of other muscles that ensured the continued symmetry of the step cycle.41
Reflex conditioning improves walking in spinal cord-injured rats
In normal rats, the hindlimbs exhibit symmetrical muscle activations and limb kinematics during locomotion. Figure 7A illustrates right and left soleus rectified EMG bursts during several steps of treadmill walking, where filled circles mark onsets of right soleus bursts (RBOs), open circles mark onsets of left soleus bursts (LBOs), and dashed vertical lines are the midpoints between RBO onsets. LBO onsets occur at the midpoint between adjacent RBOs. Thus, the times from RBO to LBO and from LBO to RBO are equal (i.e., the gait is symmetrical). In addition, the shapes of the right and left soleus bursts are similar (compare average step cycle bursts shown in Figure 7B).
Figure 7.
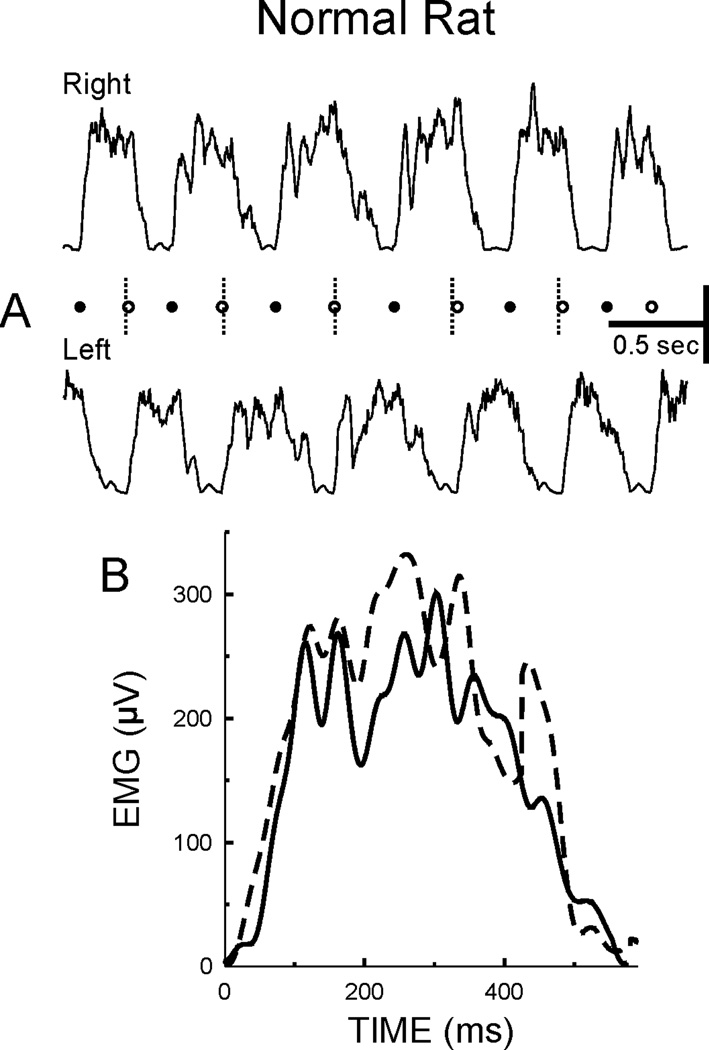
A: Rectified right (upper trace) and left (lower trace) soleus EMG bursts during treadmill walking from a normal rat. Filled circles indicate onsets of right soleus bursts (RBOs) and dashed vertical lines are midpoints between onsets of right soleus bursts. Open circles mark onsets of left soleus bursts (LBOs). LBOs occur on the midpoints between RBOs. Thus, the times from RBO to LBO and from LBO to RBO are equal and the gait is symmetrical. B: Average right (dashed line) and left (solid line) soleus bursts are similar in shape and duration.
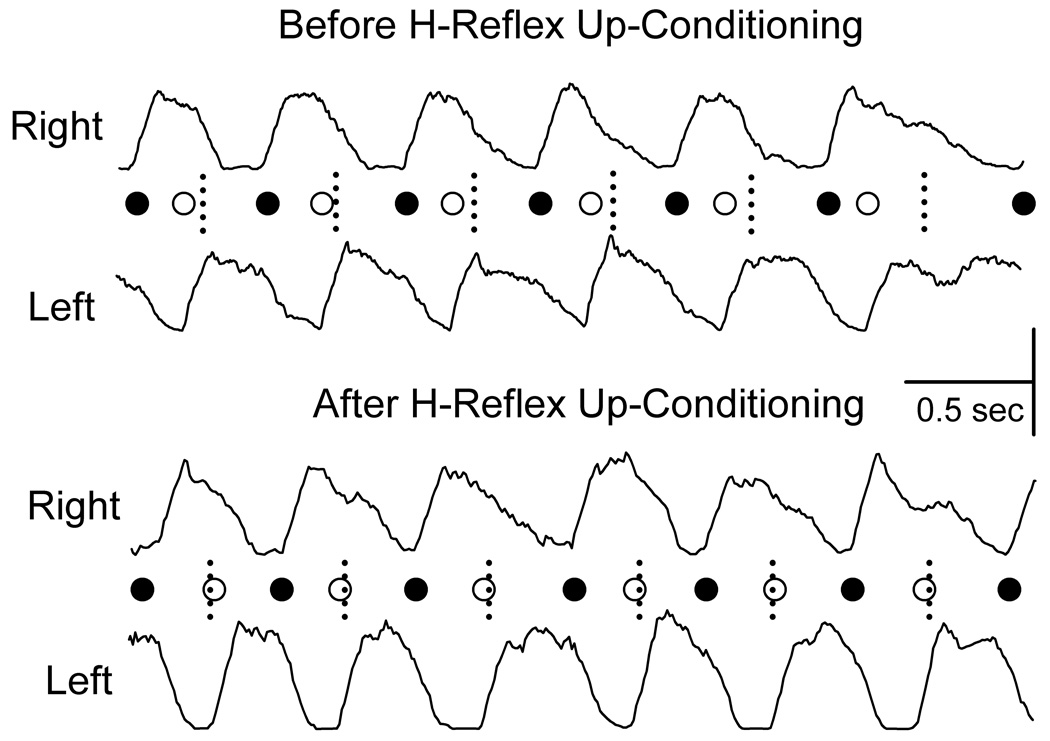
Figure 8 shows the rectified right and left soleus bursts during treadmill walking from a rat with a right lateral column transection at the mid-thoracic level before (top) and after (bottom) H-reflex up-conditioning. In this injured rat before up-conditioning, LBOs occur before the midpoints between RBOs, and the time from RBO to LBO is shorter than that from LBO to RBO. This implies that the gait is asymmetrical, and the rat limps due to inadequate right stance.42 Given that reflex conditioning affects locomotor function (see above), we hypothesized that up-conditioning the right soleus H-reflex could make the gait more symmetrical and thereby improve locomotion. Indeed, up-conditioning the right soleus H-reflex in rats with right lateral column transaction did strengthen the right soleus burst and thereby improve the symmetry of the step cycle.42 Figure 8 bottom illustrates this phenomenon for the same rat after up-conditioning, the right soleus burst is stronger and the LBOs now occur at the midpoints between RBOs (i.e., the previously asymmetrical gait has become symmetrical). Our most recent data show that rats with a right lateral column contusion injury exhibit a locomotor asymmetry similar to that seen in rats with a right lateral column transaction, and preliminary results suggest that up-conditioning of the right soleus H-reflex can reduce that asymmetry (unpublished data).
Figure 8.
Right and left soleus bursts (rectified EMG) from a rat with a right lateral column injury for the first (i.e., before up-conditioning) treadmill session and the second (i.e., after up-conditioning) session. (Horizontal scale bar: 0.5 sec; vertical scale bar: 100 and 150 :V for the right and left bursts, respectively.) Each RBO (●) or LBO (○) is marked. The short vertical dashed lines mark the midpoints between RBOs (i.e., the midpoints of the step-cycles), which is the time when LBOs should occur (as in normal rats). Prior to H-reflex up-conditioning, LBO occurs too early; after up-conditioning, it occurs on time.
Can reflex conditioning improve walking in people?
Earlier work showed that many people with partial injuries remain capable of SSR conditioning.43 We are now exploring whether H-reflex conditioning can be used as a new strategy to improve locomotor function in people with partial spinal cord injuries. As a first step, we have developed and validated an H-reflex conditioning protocol in humans.11 In this protocol, each subject performs 225 conditioning trials per day, 3 times per week for 8 weeks. Visual feedback after each stimulus shows whether the H-reflex is larger (HRup subjects) or smaller (HRdown subjects) than a criterion value. Soleus background EMG and M-wave size are kept stable throughout. In about 80% of normal subjects, this protocol changes H-reflex size significantly in the correct direction.11 This success rate is similar to that seen in animal studies. With animal protocols, rats and monkeys typically perform several thousand trials per day, 7 days per week, while the human protocol requires only several hundred trials per day, 3 days per week. The fact that this much less demanding protocol is still effective implies that reflex conditioning should be practical for clinical use in patients with spinal cord injuries. We have just begun to test the ability of this protocol to improve locomotion in spinal cord-injured subjects.
Reflex conditioning protocols could become an important new approach to restoring motor function in people with partial spinal cord injuries and other chronic neuromuscular disorders. The recent demonstration that reciprocal inhibition can also be operantly conditioned44 extends the options available for reflex conditioning, and suggests that other pathways might also be changed by conditioning protocols. Reflex conditioning protocols should be especially useful when techniques for achieving significant regeneration become available. At that point, precise methods for re-educating the regenerated spinal cord are likely to become essential components of programs aimed at restoring useful motor function.
Acknowledgments
We thank Ms. Lu Chen and Ms. Rongliao Liu for excellent technical assistance, and Drs. Ann Tennissen, Dennis J. McFarland, and Elizabeth Winter Wolpaw for valuable comments on the manuscript. This work has been supported by the New York State Spinal Cord Injury Trust Fund (XYC), the National Institutes of Health (HD36020(XYC), NS061823(XYC&JRW), and NS22189(JRW)), the Christopher Reeve Paralysis Foundation (XYC), and the International Spinal Research Trust (JRW).
References
- 1.Matthews PBC. Mammalian Muscle Receptors and their Central Actions. Baltimore, MD: Williams & Wilkins; 1972. pp. 319–409. [Google Scholar]
- 2.Lee RG, Tatton WG. Motor responses to sudden limb displacement in primate with specific CNS lesions and in human patients with motor system disorders. Can. J. Neurol. Sci. 1975;2:285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- 3.Burke RE, Rudomin P. Spinal neurons and synapses. In: Brooks VB, editor. Handbook of Physiology. Section I: The Nervous System. Volume I: Cellular Biology of Neurons. Part II. Baltimore, MD: Williams and Wilkins Company; 1977. pp. 877–944. [Google Scholar]
- 4.Baldissera F, Hultborn H, Illert M. Integration in Spinal Neuronal Systems. In: Brooks VB, editor. Handbook of Physiology. Section I: The Nervous System. Volume II: Motor Control, Part I. Baltimore, MD: Williams and Wilkins Company; 1981. pp. 509–595. [Google Scholar]
- 5.Henneman E, Mendell LM. Functional organization of motoneuron pool and inputs. In: Brooks VB, editor. Handbook of Physiology. Section I: The Nervous System. Volume II: Motor Control, Part I. Baltimore, MD: Williams and Wilkins Company; 1981. pp. 423–507. [Google Scholar]
- 6.Wolpaw JR, Braitman DJ, Seegal RF. Adaptive plasticity in the primate spinal stretch reflex: initial development. J. Neurophysiol. 1983;50:1296–1311. doi: 10.1152/jn.1983.50.6.1296. [DOI] [PubMed] [Google Scholar]
- 7.Wolpaw JR. Operant conditioning of primate spinal reflexes: the H-reflex. J. Neurophysiol. 1987;57:443–458. doi: 10.1152/jn.1987.57.2.443. [DOI] [PubMed] [Google Scholar]
- 8.Evatt ML, Wolf SL, Segal RL. Modification of human spinal stretch reflexes: preliminary studies. Neurosci. Lett. 1989;105:350–355. doi: 10.1016/0304-3940(89)90646-0. [DOI] [PubMed] [Google Scholar]
- 9.Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J. Neurophysiol. 1995;73:411–415. doi: 10.1152/jn.1995.73.1.411. [DOI] [PubMed] [Google Scholar]
- 10.Carp JS, Tennissen AM, Chen XY, et al. H-reflex operant conditioning in mice. J. Neurophysiol. 2006;96:1718–1727. doi: 10.1152/jn.00470.2006. [DOI] [PubMed] [Google Scholar]
- 11.Thompson AK, Chen XY, Wolpaw JR. Operant conditioning of soleus H-reflex in humans: short-term and long-term effects. Washington, DC: Society for Neuroscience; 2007. Program No. 404.9. 2007 Abstract Viewer/Itinerary Planner. Online. [Google Scholar]
- 12.Wolpaw JR, Lee CL. Memory traces in primate spinal cord produced by operant conditioning of H-reflex. J. Neurophysiol. 1989;61:563–572. doi: 10.1152/jn.1989.61.3.563. [DOI] [PubMed] [Google Scholar]
- 13.Wolpaw JR, Herchenroder PA, Carp JS. Operant conditioning of the primate H-reflex: factors affecting the magnitude of change. Exp. Brain Res. 1993;97:31–39. doi: 10.1007/BF00228815. [DOI] [PubMed] [Google Scholar]
- 14.Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Ann. Rev. Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- 15.Wolpaw JR. The education and re-education of the spinal cord. Prog. Brain Res. 2006;157:261–280. doi: 10.1016/s0079-6123(06)57017-7. [DOI] [PubMed] [Google Scholar]
- 16.Wolpaw JR, Chen XY. Operant conditioning of reflexes. In: Squire L, Albright T, Bloom F, Gage F, Spitzer N, editors. New Encyclopedia of Neuroscience. Oxford, UK: Elsevier; 2008. In press. [Google Scholar]
- 17.Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J. Neurophysiol. 1994;72:431–442. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- 18.Carp JS, Wolpaw JR. Motoneuron properties after operantly conditioned increase in primate H-reflex. J. Neurophysiol. 1995;73:1365–1373. doi: 10.1152/jn.1995.73.4.1365. [DOI] [PubMed] [Google Scholar]
- 19.Halter JA, Carp JS, Wolpaw JR. Operantly conditioned motoneuron plasticity: possible role of sodium channels. J. Neurophysiol. 1995;73:867–871. doi: 10.1152/jn.1995.73.2.867. [DOI] [PubMed] [Google Scholar]
- 20.Carp JS, Chen XY, Sheikh H, et al. Operant conditioning of rat H-reflex affects motoneuron axonal conduction velocity. Exp. Brain Res. 2001;136:269–273. doi: 10.1007/s002210000608. [DOI] [PubMed] [Google Scholar]
- 21.Feng-Chen KC, Wolpaw JR. Operant conditioning of H-reflex changes synaptic terminals on primate motoneurons. Proc. Natl. Acad. Sci. USA. 1996;93:9206–9211. doi: 10.1073/pnas.93.17.9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Pillai S, Wolpaw JR, et al. Motor learning changes GABAergic terminals on spinal motoneurons in normal rats. Eur. J. of Neurosci. 2006;23:141–150. doi: 10.1111/j.1460-9568.2005.04547.x. [DOI] [PubMed] [Google Scholar]
- 23.Wolpaw JR, Chen L, Schalk G, et al. Sensorimotor cortex activity during operant conditioning of H-reflex in rats: initial studies. Washington, DC: Society for Neuroscience; 2006. Program No. 146.1. 2006 Abstract Viewer/Itinerary Planner. Online. [Google Scholar]
- 24.Chen XY, Wolpaw JR. Dorsal column but not lateral column transection prevents down conditioning of H-reflex in rats. J. Neurophysiol. 1997;78:1730–1734. doi: 10.1152/jn.1997.78.3.1730. [DOI] [PubMed] [Google Scholar]
- 25.Chen XY, Wolpaw JR. Probable corticospinal tract control of spinal cord plasticity in rats. J. Neurophysiol. 2002;87:645–652. doi: 10.1152/jn.00391.2001. [DOI] [PubMed] [Google Scholar]
- 26.Chen XY, Carp JS, Chen L, et al. Corticospinal tract transection prevents up-conditioning of H-reflex in rats. Exp. Brain Res. 2002;144:88–94. doi: 10.1007/s00221-002-1026-8. [DOI] [PubMed] [Google Scholar]
- 27.Chen XY, Carp JS, Chen L, et al. Sensorimotor cortex ablation prevents H-reflex up-conditioning and causes a paradoxical response to down-conditioning in rats. J. Neurophysiol. 2006;96:119–127. doi: 10.1152/jn.01271.2005. [DOI] [PubMed] [Google Scholar]
- 28.Chen XY, Wolpaw JR. Reversal of H-reflex operant conditioning in the rat. Exp. Brain Res. 1996;112:58–62. doi: 10.1007/BF00227178. [DOI] [PubMed] [Google Scholar]
- 29.Carp JS, Chen XY, Sheikh H, et al. Motor unit properties after operant conditioning of rat H-reflex. Exp. Brain Res. 2001;140:382–386. doi: 10.1007/s002210100830. [DOI] [PubMed] [Google Scholar]
- 30.Wolpaw JR, Chen XY. Operant conditioning of rat H-reflex: effects on mean latency and duration. Exp. Brain Res. 2001;136:274–279. doi: 10.1007/s002210000609. [DOI] [PubMed] [Google Scholar]
- 31.Chen XY, Wolpaw JR. Ablation of cerebellar nuclei prevents H-reflex down-conditioning in rats. Learn. Mem. 2005;12:248–254. doi: 10.1101/lm.91305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolpaw JR, Chen XY. The cerebellum in maintenance of a motor skill: a hierarchy of brain and spinal cord plasticity underlies H-reflex conditioning. Learn. Mem. 2006;13:208–215. doi: 10.1101/lm.92706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voogd J, Glickstein M. The anatomy of the cerebellum. Trends. Neurosci. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- 34.Voogd J. Cerebellum. In: Paxinos G, editor. The Rat Nervous System. San Diego, CA: Elsevier Academic Press; 2004. pp. 205–242. [Google Scholar]
- 35.Grillner S. Control of locomotion in bipeds, tetrapods and fish. In: Brooks VB, editor. Handbook of Physiology. Section 1, The Nervous System. Volume II, Motor Control. Part I. Baltimore, MD: Williams and Wilkins Company; 1981. pp. 1179–1236. [Google Scholar]
- 36.Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus H-reflex in walking in humans. Exp. Brain Res. 1991;87:679–687. doi: 10.1007/BF00227094. [DOI] [PubMed] [Google Scholar]
- 37.Pearson KG. Common principles of motor control in vertebrates and invertebrates. Ann. Rev. Neurosci. 1993;16:265–297. doi: 10.1146/annurev.ne.16.030193.001405. [DOI] [PubMed] [Google Scholar]
- 38.Bennett DJ, De Serres SJ, Stein RB. Gain of the triceps surae stretch reflex in decerebrate and spinal cats during postural and locomotor activities. J. Physiol. 1996;496:837–850. doi: 10.1113/jphysiol.1996.sp021731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein RB, Misiaszek JE, Pearson KG. Functional role of muscle reflexes for force generation in the decerebrate walking cat. J. Physiol. 2000;525:781–791. doi: 10.1111/j.1469-7793.2000.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Chen XY, Jakeman LB, et al. The interaction of a new motor skill and an old one: H-reflex conditioning and locomotion in rats. J. Neurosci. 2005;25:6898–6906. doi: 10.1523/JNEUROSCI.1684-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Chen XY, Chen Y, et al. Elicitation and conditioning of quadriceps H-reflex in freely moving rats: initial studies. Washington, DC: Society for Neuroscience; 2007. Program No. 404.1. 2007 Abstract Viewer/Itinerary Planner. Online. [Google Scholar]
- 42.Chen Y, Chen XY, Jakeman LB, et al. Operant conditioning of H-reflex improves locomotion after spinal cord injury in rats. J. Neurosci. 2006;26:12537–12543. doi: 10.1523/JNEUROSCI.2198-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segal RL, Wolf SL. Operant conditioning of the biceps brachii spinal stretch reflexes in spinal cord injured patients. Exp. Neurol. 1994;130:202–213. doi: 10.1006/exnr.1994.1199. [DOI] [PubMed] [Google Scholar]
- 44.Chen XY, Chen Y, Chen L, et al. Operant conditioning of reciprocal inhibition in rat soleus muscle. J. Neurophysiol. 2006;96:2144–2150. doi: 10.1152/jn.00253.2006. [DOI] [PubMed] [Google Scholar]
- 45.Brown WF. The Physiological and Technical Basis of Electromyography. Boston, MA: Butterworths; 1984. [Google Scholar]