Abstract
Eating behavior may be affected by dopamine synthesis capacity. In this study, 6-[18F]-fluoro-L-m-tyrosine (FMT) positron emission tomography (PET) uptake in striatal subregions was correlated with BMI (kg/m2) and an estimate of the frequency of prior weight loss attempts in 15 healthy subjects. BMI was negatively correlated with FMT uptake in the dorsal caudate. Although the association between BMI and FMT uptake in the dorsal caudate was not significant upon correction for age and sex, the association fell within the range of a statistical trend. Weight loss attempts divided by years trying was also negatively correlated with FMT uptake in the dorsal putamen (P = .05). These results suggest an association between low dorsal striatal presynaptic dopamine synthesis capacity and overeating behavior.
1. Introduction
Appetite regulation is complex and likely influenced by activity of the midbrain dopaminergic system. Although the hypothalamus integrates intestinal and hormonal signals to regulate appetite and metabolism, the motivation to eat is also regulated by activity of the midbrain dopamine system, which processes cues related to the rewarding properties of palatable food. In fact, hormones implicated in regulating appetitive homeostasis also affect dopamine brain dopamine activity. For example, both leptin, which is released from adipose tissue, and insulin, which is released from the pancreas following feeding, directly inhibit activity of dopamine neurons projecting from ventral tegmental area (VTA) to nucleus accumbens [1].
The striatum is a brain region well known for regulating motivated behavior and is rich in dopaminergic axon terminals. Dopaminergic projections from the midbrain (VTA and substantia nigra) to the striatum are important in the development and potentiation of conditioned reinforcement and habitual appetitive responses in normal behavior and addictive disorders. Aberrant activity in either dorsal or ventral striatum has been reported to contribute to compulsive motivated behavior in the case of both drug and food seeking [1, 2].
Evaluation of brain dopamine function is possible with PET scanning and a number of radiotracers. The ligand 6-[18F]-fluoro-L-m-tyrosine (FMT) is a substrate of aromatic amino acid decarboxylase (AADC), which converts levodopa (L-DOPA) to dopamine. Because the metabolites of FMT, 6-[18F]fluoro-3-hydroxyphenylacetic acid (FPAC) and 6-[18F]fluoro-3-hydroxyphenethylamine (FMA), are trapped in dopaminergic terminals, FMT provides a method to probe the capacity of nigrostriatal neurons to synthesize dopamine. FMT is similar to 6-[18F]fluorodopa (FDOPA), in that both ligands are substrates of AADC. However, unlike FDOPA, FMT lacks the catechol structure and is not a substrate for the peripheral enzyme catechol-O-methyl-transferase (COMT). Therefore, there are no metabolites to contribute to radioactive background signal in the PET image, improving signal to noise and simplifying kinetic models [3].
Human neuroimaging studies show that low D2 receptor availability in the striatum is associated with high body mass index (BMI) [4, 5]. The relationship between dopamine synthesis capacity and BMI or overeating behavior has yet to be explored and was the main purpose of this study.
2. Methods
This study was approved by the University of California at Berkeley Committee for Protection of Human Subjects. Sixteen healthy subjects were recruited through newspaper advertisements and fliers for a study on striatal FMT and aging (age 20–30) [6]. Subjects for the aging study were excluded if they had a history of major neurological disorder, psychiatric disorder, eating disorder, systemic disease, or head injury. Subjects were also excluded if they reported a history of an alcohol or drug abuse problem, if they were regular smokers, or if they drank more than 14 alcoholic beverages per week for men and more than 7 for women. Fifteen of the sixteen subjects in the aging study were willing to answer questions about eating behavior and were included in the analyses in this paper.
Before PET scanning, subjects received 2.5 mg/kg oral carbidopa, a peripheral decarboxylase inhibitor, to increase the availability of FMT in plasma. Subjects were scanned on a Siemens ECAT EXACT HR PET system in 3D acquisition mode approximately 60 minutes (range 45–90 minutes) after carbidopa administration. The instrument has a 3.6 mm in-plane voxel size and 47 parallel imaging planes. First, a 10-minute transmission scan was acquired. Approximately 2.5 mCi (range 2.1–2.5 mCi) of the radiotracer FMT were injected, and immediately afterwards an 89-minute dynamic scan was acquired (4 × 1 minutes, 3 × 2 minutes, 3 × 3 minutes, 14 × 5 minutes).
Data were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation, an image size of 256 × 256, and 6 iterations with 16 subsets. A Gaussian filter with 6 mm FWHM was applied with scatter correction. The point-spread function used for partial volume correction (PVC) was evaluated from a phantom that had been scanned and reconstructed using this specific scanner. Images were evaluated for patient motion and adequacy of statistical counts. Individual PET frames were realigned using Statistical Parametric Mapping 2 (SPM2).
Magnetic resonance images (MRIs) were obtained using a Varian Unity/Inova whole body 4.0 T scanner (Palo Alto, CA: http://www.varianinc.com/) with a TEM send-and-receive head coil (MR Instruments, Minneapolis, MN; http://www.mrinstruments.com/). MPFLASH T1-weighted volumetric scans were acquired for each subject (TR = 9 msecond, TE = 4.8 msecond, FOV = 22.4 × 22.4 × 19.8 cm, matrix size = 256 × 256 × 128, resolution = 0.875 × 0.875 × 1.54 mm). MRI images were processed by using the Automatic Segmentation Tool (FAST) from version 3.3 of Oxford Centre for Functional MRI of the Brain “(FMRIB) Software Library” (FSL). Nonlinear noise was then reduced using FSL “Smallest Univalue Segment Assimilating Nucleus” (SUSAN). A mask of MRI scan intracranial matter was automatically generated using FSL “Brain Extraction Tool” (BET). Individual intracranial masks were manually refined using “FSL view”. FSL FAST was used to segment the skull-stripped brains into brain matter versus CSF. A binary mask of the brain matter alone was created for use in partial volume correction of the PET data. (For more details about MRI methods and related references please see Braskie et al., 2008 [6].)
Regions of interest (ROIs) (dorsal caudate, dorsal putamen, and ventral striatum) were hand drawn on MRI images using “FSL view”. We used preestablished guidelines with some modification: for ventral striatum we excluded the most posterior 25% of slices on which we had segmented caudate to avoid inclusion of the basal nucleus of Meynert [7]. Good interrater reliability was established on five left ROIs for dorsal caudate (ICC = 0.996), dorsal putamen (ICC = 0.993), and ventral striatum (ICC = 0.967).
MRI images were realigned to summed PET images using SPM2 (Figure 1). Voxelwise Ki values (1/min) were calculated using a graphical analysis for irreversible binding [8]. This approach produces Ki images that are scaled to the volume of distribution of FMT in the reference region and is therefore independent of subject weight and body composition. We used cerebellar grey matter as a reference region. Cerebellar masks were drawn in MRI native space on coronal images and comprised two thirds of the slices posterior to the last slice in which the superior cerebellar peduncles stretched superiorly. FSL FAST was used to segment and exclude major white matter tracts in the cerebellum. Scaled Ki images were created from PET frames corresponding to 24 through 89 minutes.
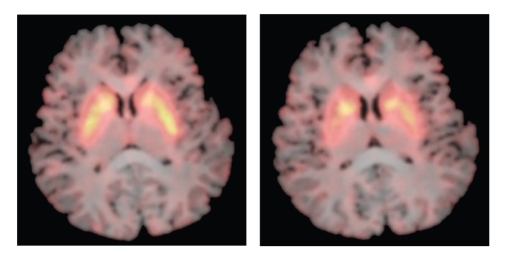
Figure 1.
MRI scan with superimposed FMT PET Ki images showing tracer uptake in caudate and putamen at two different axial slices.
At brain/nonbrain boundaries, partial volume errors occur, and subjects with more brain atrophy, or more extensive brain/cerebrospinal fluid boundaries, will have more apparent PET signal attenuation. This is due to the inherent limitations in spatial resolution of the PET scanner. Although brain atrophy is not typically a concern in younger adults, developmental changes in brain structure render partial volume effects important even in young subjects [9]. To improve the comparability of our measurements across subjects, partial volume correction (PVC) was applied to all our Ki images using a two-compartment method [10]. In this method, the binary mask created from the MRI brain images was convolved with the PET point spread function. The corrected PET value was estimated as the observed PET scan signal divided by the convolved brain matter mask. Partial volume-corrected Ki values (1/minute) are referred to as either “FMT uptake” or “Ki”.
All fifteen subjects filled out a Binge Eating Scale (BES) [11] questionnaire and were asked about number of weight loss attempts in their lives and the number of years they had been trying to lose weight. Reported weight loss attempts were divided by number of years they had been trying, to represent an estimate of the frequency of prior weight loss attempts. Body mass index (BMI) (kg/m2) was obtained on all subjects on the day of their scan.
Because FMT uptake, BMI, and Weight Loss Attempts/Years Trying values were not normally distributed, we used the nonparametric Spearman (rho) correlation coefficient. We averaged left and right hemisphere ROIs because we did not have hypotheses about the unilateral regions of interest, because the regions were correlated with one another, and because we wanted to reduce the number of variables. In this paper, we refer to the averaged left and right hemisphere ROIs as either the ventral striatum, the dorsal putamen, or the dorsal caudate. We used partial Spearman correlation to assess the relationship between FMT uptake and BMI, while controlling for both age and sex [12].
3. Results
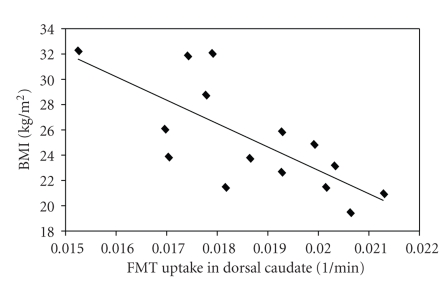
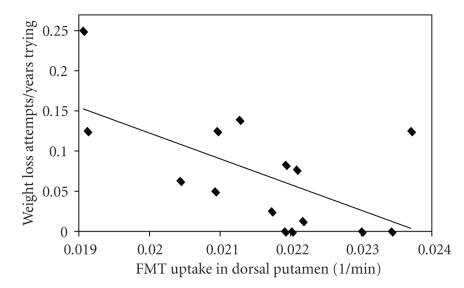
Six men and nine women participated in this study. The mean age was 22.9 years and the mean BMI was 25.3. Three subjects had BMI > 30 kg/m2; three subjects had BMI between 25 kg/m2 and 30 kg/m2. BMI was negatively correlated with dorsal caudate FMT uptake (Table 1, Figure 2). Weight Loss Attempts/Years Trying and dorsal putamen FMT uptake were negatively correlated (P = .05) (Table 1, Figure 3). Only one subject scored in the pathological range (>17) on the BES [13], and this scale was dropped.
Table 1.
Spearman Correlation Coefficients for FMT Uptake versus BMI (kg/m2) and versus Weight Loss Attempts/Years Trying.
| BMI (kg/m2) | Weight loss | |||
|---|---|---|---|---|
| Attempts/Years Trying | ||||
| Region of interest | rho | P | rho | P |
| Dorsal caudate | −0.77 | .001 | −0.37 | .17 |
| Dorsal putamen | −0.39 | .16 | −0.55 | .05 |
| Ventral striatum | −0.13 | .65 | −0.34 | .21 |
Figure 2.
Relationship between body mass index (BMI, kg/m2) and FMT uptake in the dorsal caudate averaged over left and right hemispheres in 15 young healthy individuals. FMT uptake reflects dopamine synthesis capacity and is measured as partial volume corrected influx constant, or Ki in 1/min.
Figure 3.
Relationship between Weight Loss Attempts/Years Trying and FMT uptake in the dorsal putamen averaged over the left and right hemispheres in 15 young healthy individuals. Weight Loss Attempts/Years Trying reflects an estimate of the frequency of prior weight loss attempts. FMT uptake reflects dopamine synthesis capacity and is measured as partial volume corrected influx constant, or Ki in 1/minute.
Previous studies have found an association between sex and FDOPA uptake [14]. In our sample, men and women differed in FMT uptake in the caudate using a two sample t-test assuming unequal variances (P < .05, two tailed), with a mean FMT uptake of.0194 for women, and.0175 for men. There was not a significant effect of sex on FMT uptake in the dorsal putamen or ventral striatum (rho<|0.4|, P > .15).
We also examined the relationship between FMT uptake and age (years), as previous studies have found a significant relationship between FMT and age [6]. In dorsal caudate, there was a significant correlation between FMT uptake and age (rho = −0.81, P = .0002). In the other regions, there were no significant correlations between age and FMT uptake (rho<|0.4|, p > .15).
To confirm that neither age nor sex was confounding our results, we reexamined the relationship between dorsal caudate FMT uptake and BMI after adjusting for age alone and sex alone. The corrected relationship between dorsal caudate FMT uptake and BMI was still significant (P = .02 after adjusting for age, P = .005 after adjusting for sex). When we examined this relationship adjusting for both age and sex using partial Spearman correlations, we found that the correlation was no longer significant but fell within the range of a statistical trend (P = .07). In addition, when examining males and females separately, the Spearman correlation coefficients for the dorsal caudate FMT uptake versus BMI relationship were −0.49 and −0.48 respectively.
We did not correct for age and sex for the association between Weight Loss Attempts/Years Trying and dorsal putamen Ki because age and sex were not significantly correlated with either variable (rho<|0.4|, P > .15).
4. Discussion
These data suggest that there is an association between low dorsal striatal dopamine synthesis capacity and overeating behavior. With correction for both of the potential confounding effects of age and sex, the correlation between BMI and dorsal caudate FMT uptake was not significant but fell within the range of a statistical trend. In addition, the similar correlation coefficients between FMT uptake and BMI in dorsal caudate for males and females support the notion that these effects are related to BMI rather than sex. Finally, there was a negative correlation between a greater frequency of past dieting attempts and dorsal putamen FMT.
One possible explanation of our findings is that overeating behavior, as indicated by high BMI and higher frequency of weight loss attempts, may downregulate dopamine synthesis, as indicated by FMT uptake. Previous studies have shown that AADC upregulates in response to D1 and D2 receptor antagonist administration [15]. Binge sucrose ingestion and food consumption are known to be associated with dopamine release in the striatum [16, 17]. Increased synaptic dopamine could theoretically downregulate striatal AADC activity and FMT uptake. However, binge sugar consumption has been shown to result in a decrease in D2 receptor density in the dorsal striatum [18]. Such receptor downregulation would be expected to increase AADC activity [15] and result in higher FMT uptake. Thus, it is difficult to explain our findings from this perspective alone.
Another possible explanation is that hormones might contribute to the association between overeating behavior and FMT uptake. Evidence suggests that insulin and leptin, hormones released after ingestion of food, both curb appetite and inhibit dopaminergic VTA neurons [1]. Ghrelin, in contrast, which is released by the stomach as a hunger signal, seems to have a stimulatory effect on VTA dopaminergic neurons [1]. The low FMT uptake in higher weight subjects could simply be related to higher circulating leptin and insulin and lower ghrelin.
Previous studies suggest an overlap between addictive disorders and overeating disorders. Sugar and drugs of abuse can have similar effects on striatal dopamine release and behavior in animal behavioral models [16]. Obese individuals and individuals with Binge Eating Disorder may have similarly impulsive neuropsychological profiles when compared to individuals with addictive disorders [19]. Finally, a low D2 receptor density is also associated with a both higher risk of addictive behavior and overeating behavior [20, 21].
FDOPA and FMT are measures of presynaptic dopamine synthesis capacity while the PET tracer [11C]raclopride is a ligand for the D2 receptor. At least four past studies have examined the association between presynaptic dopamine synthesis capacity or dopamine transmission and another form of addictive behavior—severity of alcohol dependence. Consistent with our data, two of these found a negative association between FDOPA uptake and alcohol dependence severity [22, 23]. Another study found a negative association between raclopride displacement in the striatum after stimulant administration (indicating decreased dopamine release) and alcohol dependence [20]. These studies support the notion that there is a low presynaptic dopamine synthesis capacity in subjects with higher severity of addictive behavior. By contrast, only one study showed increased FDOPA uptake in the left putamen and right caudate in alcoholics compared to controls, and this was a study of type I alcoholics, a subtype which may distinguish this population from some of the other studies [2].
Given the similarity between our findings and the majority of the findings of those studies of alcoholic subjects, the association we found in this study between BMI and the estimate of the frequency of prior weight loss attempts and striatal FMT uptake could imply that low presynaptic dopamine synthesis capacity in the dorsal striatum is a marker for poor decision-making and difficulty controlling food intake in response to food cues. Increased craving and food cue presentation are associated with increased fMRI activation and dopamine release in the dorsal striatum [24, 25]. Obesity is also associated with increased dorsal striatal fMRI activation in response to food cues [26]. The dopamine-rich dorsal striatum is critical for the regulation of feeding and addictive behavior and plays an integral role in the habitual or “stimulus-response” component of motivated behavior [1, 27, 28]. In other words, dopamine mediates the ability for environmental cues to trigger learned responses that were previously associated with reward [27, 28]. A dopamine imbalance in the dorsal striatum could predispose to difficulty controlling impulses when exposed to a cue in the environment associated with food reward. A recent study showed that three DRD3 genotypes predicted ADHD symptoms, which inturn was associated with a higher rate of obesity and binge eating symptoms, supporting this hypothetical link between dopaminergic dysfunction and impulsivity in overeaters [29].
Finally, our findings could also indicate that subjects with lower FMT uptake in the dorsal striatum have a greater predisposition to overeat as a result of decreased baseline activation of reward circuitry. Increased fMRI activation, increased regional cerebral blood flow, and increased dopamine release in the dorsal striatum are associated with food administration but appear to decrease as motivation to eat decreases [17, 30, 31]. Moreover, the amount of dopamine released in the dorsal striatum correlates with the degree of experienced pleasure [17]. These studies implicate dorsal striatal dopamine as a mediator of food reward and satiety. Other studies in humans have shown decreased D2 density in the dorsal striatum in obese individuals and hypothesize that low brain dopamine activity leads to overeating in order to compensate for decreased activation of reward circuits [4, 5, 32]. Lower presynaptic dopamine synthesis capacity in the dorsal striatum could similarly predispose an individual to use excessive food in order to stimulate under active reward circuitry and to achieve satiety.
This study was somewhat limited by a relatively small sample size and a narrow range of overeating pathology which makes it difficult to fully examine interactions between dopamine synthesis, overeating, age and sex. Only one subject scored in the pathological range on the BES. Moreover, a partial correlation correcting for two variables with this sample size may have masked true findings. However, one might argue that correcting for age and sex may be too conservative and that BMI may be driving the association between FMT and age or sex, not the other way around. Unadjusted findings were quite strong and still in the range of a trend after adjustment for these potential confounders. These results, along with similar findings using other PET approaches in obesity, and similar findings on the role of dopamine in addictive disorders, suggest a role for dopamine in these behaviors. The association of presynaptic dopamine synthesis capacity with overeating behavior deserves further investigation.
5. Conclusion
In this study, high BMI and difficulty controlling weight were associated with low dorsal striatal FMT uptake, reflective of low dopamine synthesis capacity. These data support the hypothesis that changes in striatal dopamine synthesis are associated with changes in weight and appetitive drive and support other data indicating that striatal dopamine metabolism plays a role in the motivational aspects of food seeking behavior.
Acknowledgment
This research was supported by a Grant from the National Institutes of Health AG027984.
References
- 1.Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends in Neurosciences. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Tiihonen J, Vilkman H, Rasanen P, et al. Striatal presynaptic dopamine function in type 1 alcoholics measured with positron emission tomography. Molecular Psychiatry. 1998;3(2):156–161. doi: 10.1038/sj.mp.4000365. [DOI] [PubMed] [Google Scholar]
- 3.Dejesus OT, Endres CJ, Shelton SE, Nickles RJ, Holden JE. Noninvasive assessment of aromatic L-amino acid decarboxylase activity in aging rhesus monkey brain in vivo. Synapse. 2001;39(1):58–63. doi: 10.1002/1098-2396(20010101)39:1<58::AID-SYN8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Haltia LT, Rinne JO, Merisaari H, et al. Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse. 2007;61(9):748–756. doi: 10.1002/syn.20418. [DOI] [PubMed] [Google Scholar]
- 5.Wang G-J, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 6.Braskie MN, Wilcox CE, Landau SM, et al. Relationship of striatal dopamine synthesis capacity to age and cognition. Journal of Neuroscience. 2008;28(52):14320–14328. doi: 10.1523/JNEUROSCI.3729-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mawlawi O, Martinez D, Slifstein M, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow and Metabolism. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. Journal of Cerebral Blood Flow and Metabolism. 1985;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- 9.Bennett CM, Baird AA. Anatomical changes in the emerging adult brain: a voxel-based morphometry study. Human Brain Mapping. 2006;27(9):766–777. doi: 10.1002/hbm.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meltzer CC, Kinahan PE, Greer PJ, et al. Comparative evaluation of MR-based partial-volume correction schemes for PET. Journal of Nuclear Medicine. 1999;40(12):2053–2065. [PubMed] [Google Scholar]
- 11.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addictive Behaviors. 1982;7(1):47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 12.Schemper M. Non-parametric partial association revistited. The Statistitian. 1991;40:73–76. [Google Scholar]
- 13.Marcus MD, Wing RR, Lamparski DM. Binge eating and dietary restraint in obese patients. Addictive Behaviors. 1985;10(2):163–168. doi: 10.1016/0306-4603(85)90022-x. [DOI] [PubMed] [Google Scholar]
- 14.Laakso A, Vilkman H, Bergman J, et al. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biological Psychiatry. 2002;52(7):759–763. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhu M-Y, Juorio AV, Paterson IA, Boulton AA. Regulation of aromatic L-amino acid decarboxylase in rat striatal synaptosomes: effects of dopamine receptor agonists and antagonists. British Journal of Pharmacology. 1994;112(1):23–30. doi: 10.1111/j.1476-5381.1994.tb13023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience and Biobehavioral Reviews. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19(4):1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 18.Colantuoni C, Schwenker J, McCarthy J, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. NeuroReport. 2001;12(16):3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 19.Davis C, Levitan RD, Muglia P, Bewell C, Kennedy JL. Decision-making deficits and overeating: a risk model for obesity. Obesity Research. 2004;12(6):929–935. doi: 10.1038/oby.2004.113. [DOI] [PubMed] [Google Scholar]
- 20.Martinez D, Gil R, Slifstein M, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biological Psychiatry. 2005;58(10):779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Wang G-J, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. Journal of Addictive Diseases. 2004;23(3):39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 22.Heinz A, Siessmeier T, Wrase J, et al. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. American Journal of Psychiatry. 2005;162(8):1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- 23.Volkow ND, Wang G-J, Telang F, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. Journal of Neuroscience. 2007;27(46):12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. NeuroImage. 2004;23(4):1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 25.Volkow ND, Wang G-J, Fowler JS, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44(3):175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 26.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37(2):410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, Wang G-J, Telang F, et al. Cocaine cues and dopamine in dorsal striatumml: mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis C, Patte K, Levitan RD, et al. A psycho-genetic study of associations between the symptoms of binge eating disorder and those of attention deficit (hyperactivity) disorder. Journal of Psychiatric Research. 2009;43(7):687–696. doi: 10.1016/j.jpsychires.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. Journal of Abnormal Psychology. 2008;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 32.Wang G-J, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opinion on Therapeutic Targets. 2002;6(5):601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]