Abstract
Objectives
Previous studies have linked alexithymia to an inability to process emotions appropriately. Older persons show changes in emotion processing and have higher alexithymia scores. Because the anterior cingulate cortex (ACC) is one of the regions showing earlier decline in late-life and alexithymia appears to be related to a dysfunction in right hemisphere regions including the ACC subserving affective processes, the present study sought to test the hypothesis that reduced ACC volume accounts for the association between older age and alexithymia.
Design
Correlation analyses between functionally distinct ACC subregions, age and alexithymia features.
Setting
University of Iowa
Participants
24 healthy volunteers aged twenty-four to seventy-nine years.
Measurements
Psychiatric and neuropsychological assessment and assessment of alexithymia using the twenty items Toronto Alexithymia Scale. High-resolution magnetic resonance imaging, and in-house developed methods for ACC parcellation.
Results
Older age directly correlated with higher overall alexithymia, and reduced bilateral rostral and right dorsal ACC grey matter volume. Furthermore, higher alexithymia scores correlated with reduced right rostral ACC volume. This correlation appeared to be influenced primarily by factor 3 of the alexithymia scale measuring diversion of attention to external details in place of internal feelings.
Conclusions
These results suggest that alexithymia in older age may be a result of structural changes in the right rostral ACC.
Keywords: ACC, Alexithymia, Aging, Depression, Emotion
Introduction
In 1931 Kurt Schneider in his treatise Klinische Psychopathologie (1) proposed that an “unerlebbarer Untergrund” (liberally translated: ineffable background) may influence the presentation of new-onset psychopathology. In some cases this background is represented by premorbid brain changes altering the onset and/or presentation of psychiatric disorders. For instance, damage to ventral medial prefrontal cortex and amygdala has been shown to reduce the occurrence of post-traumatic stress disorder in veterans exposed to traumatic events (2). Similarly, ischaemic damage to anterior right hemisphere regions involved in emotion processing may alter the conventional presentation of depression by reducing awareness and/or the ability to report emotional changes (i.e., sadness) (3). For psychiatric disorders with onset in late-life, the background may consist of age-associated alterations in brain function or structure (4).
The term alexithymia (from the Greek a = lack, lexis = word, thymos = emotions) has been used to describe persons who are functionally unaware of their emotions or don't know what they signify (5). People with high levels of alexithymia are noted to rarely express or talk about their emotions or their emotional preferences and often have the tendency of using extra-personal events to explain personal drives and motivations (6–7). Alexithymia is hypothesized to occur in several psychiatric disorders (e.g. depression, anxiety and somatoform disorders) (6). Some degree of alexithymia is considered within the real of normal personality characteristic, but the extent to which alexithymia confers vulnerability to develop psychiatric disorders or contributes to modifying the psychopathology of psychiatric disorders with diverse etiologies is still not well known (6).
Recently there has been a surge in interest in late-life emotion processing in general (8) and in alexithymia in particular (9). Alexithymia has been shown to be more severe in older age (10–12), but these findings are not univocally interpreted (13). While some investigators have considered alexithymia essentially a phenomenon related to poor emotional awareness and a harbinger of poor psychosocial functioning (14), for others its predictive relevance is unclear (13). Studies examining the neuroanatomical bases of alexithymia in older age may offer some clarification. Although not focusing on older age per se, Lane and colleagues (15) suggested that a dysfunction in brain regions subserving emotion processing may be associated with alexithymia (15–16). These regions include areas in the right hemisphere (15,3), which, according to the right hemisphere model of aging (17), may undergo early degeneration compared to homologous left-hemisphere regions. Thus, a more rapid age-related degeneration of the right hemisphere, which has a stronger role in emotion processing (18–19), would explain why alexithymia is relatively more prevalent in older age. Hence confirming an association to brain degeneration may constitute an important step in the definition of the clinical relevance of older age alexithymia.
Among emotion processing structures within the right hemisphere, the anterior cingulate cortex (ACC) is the largest region of the limbic system with functions of mood regulation and facilitation of the conscious awareness of emotions (16,20). The ACC has a strategic position connecting evolutionary older and more recent cortical brain areas where emotions may reach consciousness and become feelings (21). Lesions in the rostral portion of the anterior cingulate have been shown to impair subjective emotional experience (22). Functional imaging studies have shown that subjects with elevated alexithymia display reduced activity in the ACC during exposure to emotional stimuli (23–24). Similarly, reduced regional cerebral blood flow (25–27), glucose metabolism (perhaps consequence of deterioration of neuronal functioning) (28–35), and reduced grey matter volume (36–37,26,38–39) suggest that the ACC undergoes age-related decline. Taken together, these findings suggest that alexithymia in older age may be explained by diminished functioning as a result of decreased volume in the ACC, perhaps more so on the right hemisphere. In addition to having a significant role in emotional awareness (40), the ACC has gained relevance in research on depression (41) and more recently in depression in older age (42). Elderly individuals who have suffered multiple depressive episodes have particularly low ACC activity which has been shown to predict poor response to treatment (43–45). In addition, in older age depression, greater apathy, a psychopathological dimension including lack of insight, flat affect and emotional indifference (46) has been associated with smaller grey matter volumes of the right ACC (47).
No empirical research has been conducted on the neural substrates of alexithymia in older age. The research findings outlined above suggest the hypothesis that age-related reductions in grey matter volume of the ACC predict alexithymia in older age.
The differing functions of the ACC in emotions and cognition are supported by functionally and structurally distinct regions with distinct cytoarchitectural and functional connectivity patterns (48–50). These subregions may undergo differential age-related decline (51). Without precise examination of these sub-regions, meaningful structure-function associations may be missed (48,52–54). Broadly speaking, functional imaging and lesion studies have suggested that dorsal aspects of the ACC subserve motor, attention and cognitive functions (55), whereas more ventral aspects (rostral, and subgenual regions) are involved with emotions and autonomic functions (56–57). We therefore hypothesized that sub-regions of the ACC associated with emotion (e.g, rostral, subgenual) would be likely candidates as substrates for alexithymia in older age. We tested the hypotheses that alexithymia is associated with older age and that emotion-related ACC sub-regions are associated with alexithymia. A competing hypothesis based on prior research positing that poor language abilities may underlie alexithymic features (58–59) was also tested. We predicted a significant inverse association between alexithymia and neuropsychological measures of language, but lack of association between these variables and ACC grey matter volume.
Methods
Subjects
Twenty-four persons (9 males and 15 females), aged 24 to 79 years (mean age 53.7, SD=17.1) without present or past history of psychiatric disorders (substance dependence, mood, anxiety, and psychotic disorders) assessed using a modified Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) (60), were recruited for this study. Psychiatric history of first degree relatives was negative and participants were currently not taking any psychotropic drugs. Subjects did not have history of major medical and neurological illness. As part of a comprehensive psychiatric assessment, severity of anxiety and depressive symptoms were assessed with the 28-item Hamilton Depression Rating Scale (HDRS) and the Hamilton Anxiety Rating Scale (HARS) (61–62). As part of a comprehensive neuropsychological assessment (63–64), subjects’ general intellectual abilities were measured using the Weschsler Adult Intelligence Scale Revised (WAIS-R) (65) to derive verbal IQ (VIQ), performance IQ (PIQ) and full-scale IQ (FSIQ). Tasks of verbal ability were constituted from the raw scores of the Token Test (66), the Boston Naming Test (67), and the Controlled Word Association Test (COWA - list generation for letters C+F+L) (68).
Severity of alexithymia was measured using the 20-item Toronto Alexithymia Scale (TAS-20) (69–70). This scale has 3 factor indices for understanding the specific nature of alexithymia:
Factor 1 - difficulty identifying feelings and distinguishing them from the bodily sensations of emotion
Factor 2 - difficulty describing one’s feelings; and
Factor 3 - presence of externally oriented thinking
This study is based on a sample partially overlapping with the sample in described in a previous study of aging, gray matter and blood flow in the ACC (51). The protocol was approved by the University of Iowa Human Subjects Institutional Review Board and written informed consent was obtained for all subjects prior to participation.
MRI acquisition
MR scans were obtained for each subject with a high-resolution T1-weighted three-dimensional spoiled gradient recall acquisition sequence on a 1.5T General Electric Signa scanner (GE Medical Systems, Milwaukee, WI) [TE=5, TR(22)=24, flip angle=40 degrees, NEX=2, FOV=26, matrix=256×192, 1.5-mm slice thickness]. The two-dimensional PD and T2 sequences were acquired as follows: 3.0 or 4.0 mm thick coronal slices TE=36ms (for PD) and 96ms (for T2), TR(22)=3000ms, NEX=1, FOV=26, matrix=256×192. The in-plane resolution is 1.016×1.016 mm for the three modalities.
Image Processing
MR data were visually assessed for quality and movement artifacts and MR scans were repeated if needed. The scans were then processed on Linux workstations with locally developed BRAINS2 software (71). The T1-weighted images were spatially normalized and resampled to 1.0mm3 voxels so that the anterior-posterior axis of the brain was realigned parallel to the ACPC line and the interhemispheric fissure aligned on the other two axes. The T2 and PD-weighted images were aligned to the spatially normalized T1-weighted image (72). The data sets were then segmented using the multispectral data and a discriminate analysis method based on automated training class selection (73). The tissue-classified image was then used to generate a triangle-based iso-surface using a threshold of 130 representing pure gray matter, which corresponds to the parametric center of the cortex (74). This triangulated surface was used as the basis for volume calculation.
ACC Parcellation
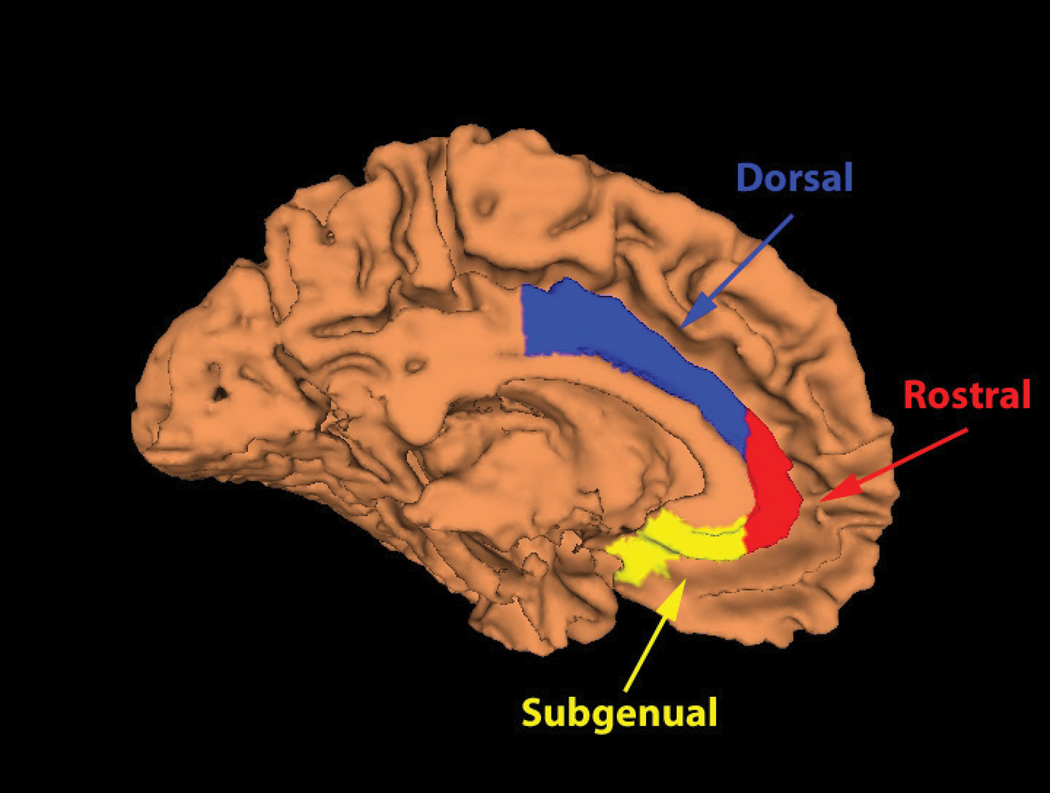
The ACC was manually traced and divided into three sub-regions for the left and right sides of the brain based on methods described in McCormick and colleagues (50) (Figure 1). Subcallosal and subgenual regions were measured as one region to attain a larger area and because the functional difference of these two subregions which are in close proximity is yet unclear. Measurements were performed by one of the authors (A.J.) blind to subjects’ age, sex, and alexithymia. On practice scans (n=10), the inter-rater reliability as determined by intra-class correlation coefficients ranged from 0.81 to 0.96 per region with another senior research assistant involved with the McCormick and colleagues methods paper (50).
FIGURE 1.
Anterior cingulate cortex subregions used for the region of interest analyses. Adapted from McCormick et al., 2006.
Data Analysis
We tested the hypothesis that older age would predict smaller ACC grey matter volumes which, in turn, would predict greater alexithymia, by computing Spearman’s correlations using gender and intracranial volume as covariates. The strength of association among variables was determined using Spearman’s r because this is a non-parametric statistic robust to the effects of outlying values and non-normally distributed variables. Correlations were computed between grey matter volumes of the six ACC sub-regions, TAS-20 total score and 3 factors and age. Two-tailed p-values were set at .05. Alexithymia was also correlated with general intelligence and verbal abilities to address general age-related cognitive influences on emotional awareness and the association between language and alexithymia. Because alexithymia has been associated with generalized negative affectivity (75–76), depression and anxiety scores were correlated with the TAS-20 total score controlling for gender.
Results
Subjects’ mean FSIQ was 114 (SD=15.3) with a VIQ=108 (SD=12.8) and PIQ=119 (SD=14.9). These elevated IQ scores are consistent with the fact that subjects were also highly educated [mean education = 15.5 years (SD=4.2)]. Depressive symptom severity on the HDRS was mild and consistent with a sample screening negative for medical, psychiatric and neurological disorders [mean=3.7 (SD=2.8)]. Subjects also had mild levels of anxiety as measured by the HARS [mean=6.16 (SD=4.6)]. Alexithymia scores were largely within the normative range [Factor 1 mean = 10.7 (SD=4.5), Factor 2 mean=10.2 (SD=3.9), Factor 3 mean=17.8 (SD=4.4)] (58–60).
The mean gray matter volume for total left and right ACC regions as well as subregions are provided in Table 1. The correlations between age and ACC grey matter volume and alexithymia scores are shown in Table 2. Older age was significantly correlated with higher total alexithymia scores [r(22)=−.48, p=.023]. Older age was inversely associated with reduced ACC subregion volume except for the right subgenual region [this finding, which is based on a partially overlapping sample, was reported in (51)]. These associations reached statistical significance for total right grey matter volume [r(22)=− 0.56, p=.007] as well as for right [r(22)=− 0.45, p=.034] and left [r(22)=− 0.48, p=.025] rostral and right dorsal [r(22)=− 0.44, p=.043] sub-regions. Greater alexithymia on the total TAS as well as factor 3 (“externally oriented style of thinking”) of the TAS was significantly correlated to the smaller right rostral sub-region [respectively r(22)=−0.46, p = .031; r(22)=−0.45, p=.035]. All other correlations did not reach statistical significance (Table 3).
Table 1.
Grey matter volumes of the sub-regions of the ACC
| minimum | maximum | mean | SD | |
|---|---|---|---|---|
| Left ACC Volume | 4.385 | 10.741 | 6.935 | 1.771 |
| Right ACC Volume | 4.974 | 10.959 | 7.351 | 1.385 |
| Left Dorsal ACC Volume | 2.558 | 4.979 | 3.574 | 0.674 |
| Left Rostral ACC Volume | 0.422 | 5.281 | 2.284 | 1.220 |
| Left Subgenual ACC Volume | 0.514 | 1.612 | 1.077 | 0.264 |
| Right Dorsal ACC Volume | 2.757 | 4.872 | 3.815 | 0.638 |
| Right Rostral ACC Volume | 1.090 | 5.265 | 2.523 | 0.994 |
| Right Subgenual ACC Volume | 0.622 | 1.454 | 1.013 | 0.230 |
Note: N=24; ACC=anterior cingulate cortex; SD=standard deviation
Table 2.
Age correlations with ACC grey matter volume and alexithymia
| r | p-value | |
|---|---|---|
| ACC grey mattera | ||
| Left ACC | −.31 | .163 |
| Left Dorsal | .03 | .901 |
| Left Rostral | −.48 | .025 |
| Left Subgenual | −.11 | .636 |
| Right ACC | −.56 | .007 |
| Right Dorsal | −.44 | .043 |
| Right Rostral | −.45 | .034 |
| Right Subgenual | −.18 | .425 |
| TAS-20b | ||
| Total score | .48 | .023 |
| Factor 1 | .42 | .051 |
| Factor 2 | .30 | .177 |
| Factor 3 | .32 | .141 |
Note: N=24; ACC=anterior cingulate cortex; L=left; R=right, TAS-20=Toronto Alexithymia Scale-20 items
Spearman’s correlations controlled for total intracranial volume and gender
Spearman’s correlations controlled for gender
Factor 1 = Difficulty identifying feelings and distinguishing them from bodily sensations;
Factor 2 = Difficulty describing one’s feelings;
Factor 3 = Presence of externally oriented thinking.
Table 3.
ACC grey matter volume correlations with alexithymia
| ACC grey matter* | TAS-20 | TAS-20 | TAS-20 | TAS-20 |
|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Total | |
| Total Left ACC | .36 | −.17 | −.03 | −.16 |
| Left Dorsal | −.18 | −.31 | .13 | −.02 |
| Left Rostral | −.38 | −.15 | −.14 | −.23 |
| Left Subgenual | −.37 | .06 | −.09 | −.19 |
| Total Right ACC | −.14 | −.10 | −.24 | −.27 |
| Right Dorsal | .04 | −.04 | −.02 | .02 |
| Right Rostral | −.23 | −.21 | −.45** | −.46** |
| Right Subgenual | −.18 | .08 | .11 | −.10 |
Note: N=24; ACC=anterior cingulate cortex; TAS-20=Toronto Alexithymia Scale-20 items
Correlations controlled for total intracranial volume and gender
p<0.05
Factor 1 = Difficulty identifying feelings and distinguishing them from bodily sensations;
Factor 2 = Difficulty describing one’s feelings;
Factor 3 = Presence of externally oriented thinking.
Severity of depressive symptoms was associated with greater alexithymia [r(22)= 0.43, p=.038]. The affective, cognitive, and vegetative symptom clusters of the HDRS were not significantly associated with the total alexithymia score or with individual factors [all correlations r (22)<.39, ps >.06]. The association with anxiety symptoms did not reach statistical significance [r(22)= 0.27, p>.2]. Depression severity as measured by HDRS was unrelated to ACC sub-regional volumes as well [all correlations r (22)<.38, p>.08].
While there were no significant associations between measures of general intelligence (FSIQ, PIQ, and VIQ), Token Test, Boston Naming Test and alexithymia (all correlations were rs ≤0.2, ps>0.2). The COWA score, which assessed verbal initiation and maintenance of effort was significantly associated with total alexithymia score [r(22)= −0.55, p<.01] and with all three alexithymia factors (rs ranged from .40 to .63, ps<.05). Poorer performance on the COWA did not correlate with older age though [r(22)=−0.23, p>.2]. There were no correlations between the other neurocognitive measures and ACC sub-volumes that approached significance.
In summary, older age was correlated with higher alexithymia and with smaller overall right ACC total grey matter volumes, specifically in rostral and dorsal sub-regions, and in the left rostral sub-region. Greater overall alexithymia and externally oriented style of thinking were associated with smaller right rostral ACC volume. Alexithymia was also associated with lower COWA scores, which are a measure of poor verbal initiation and maintenance of effort indicative of weak executive language abilities, but these measures did not co-vary with age or grey matter volume of the ACC.
Discussion
To our knowledge this is the first study examining the association between alexithymia and grey matter volumes of functionally distinct sub-regions of the ACC as a function of age. There were several important findings in this study. First, we obtained further evidence of a significant association between older age and alexithymia (10–12). Secondly, we demonstrated that age-associated reduction in gray matter volumes occurs in the right ACC, specifically within the dorsal and rostral ACC sub-regions, but also in the left rostral sub-region. Thirdly, we found that among the functionally differentiated ACC sub-regions, deterioration in the rostral sub-region may contribute to greater alexithymic features in older age and perhaps more specifically to externally oriented thinking.
The results of the present study are consistent with the proposed role for the ACC in emotional self-awareness (15) and with functional neuroimaging studies showing younger adults with alexithymic features responding with reduced ACC activity to the exposure to emotional stimuli (23–24,40). As predicted based on these functional imaging studies, we found an age-related reduction in the gray matter of the ACC potentially reflecting a neuro-anatomical substrate for older age alexithymia (10–12).
At the time of this writing, there is only another structural neuroimaging study examining the association between ACC and alexithymia (77). Gündel and colleagues (77) have shown a positive correlation between greater alexithymia scores and surface area of the right ACC gyrus. The discrepancy between their results and our findings is likely a function of the fact that Gündel and colleagues (77) did not focus on age-related alexithymia; consequently the oldest person in their study was 43 years-old. Anatomical/functional associations require population-specific interpretations because of the differing neurobiological mechanisms they may reflect (78). Therefore, because Gündel and colleagues (77) likely did not include individuals with significant age-related neural degeneration, the structure/function correlation in that study of young adults (77) suggests substantially different underlying neurobiological processes compared to the age-related mechanisms in the present study.
Implications for late-life mood disorders
The findings in this study have implications for understanding the link between alexithymia and older age and the impact that alexithymia (and ACC dysfunction) may have on symptom expression in late-life depression (79). Some people may feel incapable of fully explaining their altered emotions or prefer to withhold their depressive symptoms leading to elevated alexithymia scores (80). In agreement with this notion, we found an association between severity of depression (in subjects screening negative for life-time history of mood disorder) and alexithymia. There is a growing realization that underreporting of sad mood complicates diagnosing depression in older persons (81–82) especially in the context of “time constraints, inadequate reimbursement, and lack of support staff” (83), and may result from vascular or degenerative dysfunction in brain regions subserving emotional self-perception (84–85).
This view should be tempered with the possibility that older adults’ perceived stigma for mental illness may reduce their willingness to report sadness (86). The stigma explanation, however, is not consistent with the results of longitudinal research examining subjects first in 1981 and then in 1994 showing that adults 65 years and older were less likely to endorse sadness than younger adults independent of the year of interview (87).
The alexithymia construct may in part overlap with emotional apathy and lack of insight. Partial commonalities are consistent with the lack of insight observed in persons undergoing surgery for arteriovenous malformations of the cingulate gyrus (88), with data showing that ACC activity covaries with the subjective severity of negative cognitions in major depression (89), and with the role of the ACC in monitoring of emotional conflict (90). Lavretsky and colleagues (47) showed that the severity of lack of initiative, interest, and insight, flat affect and emotional indifference were associated with smaller right ACC volume in late-life depression. These findings are consistent with the notion that alexithymic features (e.g., impaired emotional self-perception and underreporting of sad mood and anhedonia) may become more severe in late-life as a result of pathology in the ACC.
Aging and processing of emotional stimuli
Emotional awareness supports critical skills for successful social interaction. The results of the present study add to the literature on the capacity of older persons to process emotions (91,41,92,16). While early work suggested that older individuals are less emotionally responsive (93–95), more recently it has been suggested that older people demonstrate an increased ability to regulate negative emotions and focus on positive emotions (96–97). The ACC encompasses a significant portion of the medial prefrontal cortex subserving selective attention to subjective emotional states (98,16) and functions as a convergence zone for stimuli carrying social significance (99–100) and for emotional introspection (16,85,101). Therefore, based on neuroanatomy alone, it would be expected that functional decline in frontal limbic structures (31) may lead to both age-related changes in processing of personal emotions and poor perception of emotionally positive and negative stimuli (102–103). Because, however, age-related structural and functional anatomical changes vary greatly among older adults, the capacity of any given older person to process emotionally relevant stimuli is mediated by the dynamic interplay of neurobiological factors affecting frontal limbic regions subserving emotion perception and awareness (84–85,98–99,101) as well as individual psychosocial maturation which continues into late adulthood and leads to improved emotional regulation (104).
Alexithymia and cognition
We did not find a significant association between alexithymia and general intelligence. Subjects in this sample were highly educated and had above average IQ scores. A sample with a wider range of general cognitive abilities may reveal otherwise. While our hypothesis stemmed from a conceptualization of alexithymia as a deficit in emotional awareness (15), alexithymia has also been linked to poor linguistic abilities (58–59). Of three tests of language ability available ( i.e., the Token Test, the Boston Naming Test, and the Controlled Word Association Test), only the measure most influenced by executive functioning (i.e., COWA) was significantly associated with alexithymia. Language abilities were not correlated with age and no correlations between language abilities with ACC volumes were significant. These results signify that while alexithymia may be related to some verbal abilities, this association is not mediated through gray matter volume loss in the ACC.
ACC and its sub-regions
These findings give further validity to the division of the ACC in sub-regions. The results further highlight the vulnerability of some sub-regions to age related deterioration. Similarly, the rostral sub-region of the ACC known to uniquely encompass spindle-shaped neurons which emerge only after birth, have recent evolutionary specialization, and are highly susceptible to environmental stressors (105–106) may be among the earlier structures showing degeneration. The mechanisms leading to smaller rostral ACC volumes in late-life may be either vascular, neurodegenerative, or a combination of the two (51). The vascular hypothesis is supported by age-associated decreases in regional cerebral blood flow (25–26), while the degenerative hypothesis is supported by decreases in glucose metabolism (28–32), perhaps a consequence of neuronal deterioration (33–35). Cross-sectional (36–37,26,38–39) and longitudinal (39) in vivo neuroanatomical studies using magnetic resonance imaging (39) have confirmed that the ACC becomes smaller as a function of aging.
In summary, while the findings of this study should be interpreted cautiously based on sample size and cross-sectional design, they suggest that alexithymia may become more common in late-life as a result of changes in brain regions governing emotions including the right rostral ACC. Alexithymia is a complex psychological construct with multiple determinants that can be found among persons reporting mild depressive symptoms and that may be in part influenced by poor executive language. In our sample however language abilities did not decline with age or were associated with decline in ACC grey matter volumes.
It has been known that older adults are less likely to endorse sadness compared to youth (87). Underreporting sad mood during a depressive episode may constitute one of the reasons why depression in late-life is under-diagnosed (81–82) therefore constituting a significant public health problem (107,83). While stigma for mental illness may complicate recognition of psychiatric disorders in late-life (86), we propose that understanding the neural basis of alexithymia in older age may help uncover the bases of underreported sadness in older persons and therefore complement with a neurobiological explanation the current understanding of poor recognition of depressive disorders that occur in vulnerable people in late-life.
Acknowledgments
This work was supported by the Edward J. Mallinckrodt Jr. and Dana Foundations and NIH sponsored Mentored Research Scholarship (K12) (Dr. Paradiso); and by NIMH research scientist award MH-00163 to Dr. Robinson and NIMH grant MH52879. The authors thank Albert Abreu, Teresa Kopel, Natalie Robinson and Erika Holm, and Steve Ziebel for assistance in this research.
REFERENCES
- 1.Schneider K. Klinische Psychopathologie Thieme. Stuttgart: 1931. [Google Scholar]
- 2.Koenigs M, Huey ED, Raymont V, et al. Focal brain damage protects against posttraumatic stress disorder in combat veterans. Nat Neurosci. 2007 doi: 10.1038/nn2032. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paradiso S, Vaidya J, Tranel D, et al. Subsyndromal depressive symptoms following stroke. J Neuropsychiatry Clin Neurosci. 2008;20:52–61. doi: 10.1176/jnp.2008.20.1.52. [DOI] [PubMed] [Google Scholar]
- 4.Mast FW. Impact of cognitive impairment on the phenomenology of geriatric depression. Am J Geriatr Psychiatry. 2005;13:694–700. doi: 10.1176/appi.ajgp.13.8.694. [DOI] [PubMed] [Google Scholar]
- 5.Sifneos PE. Short-term Psychotherapy and Emotional Crisis. Harvard University Press; 1999. [Google Scholar]
- 6.Taylor GJ. Recent developments in alexithymia theory and research. Can J Psychiatry. 2000;45:134–142. doi: 10.1177/070674370004500203. [DOI] [PubMed] [Google Scholar]
- 7.Taylor GJ, Bagby RM, Parker JD. The alexithymia construct. A potential paradigm for psychosomatic medicine. Psychosomatics. 1991;32:153–164. doi: 10.1016/s0033-3182(91)72086-0. [DOI] [PubMed] [Google Scholar]
- 8.Charles ST, Carstensen LL. Emotion regulation and aging. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. [Google Scholar]
- 9.Gunzelmann T, Kupfer J, Brahler E. Alexithymia in the elderly general population. Compr Psychiatry. 2002;43:74–80. doi: 10.1053/comp.2002.29855. [DOI] [PubMed] [Google Scholar]
- 10.Lane RD, Sechrest L, Riedel R. Sociodemographic correlates of alexithymia. Comp Psych. 1998a;38:377–385. doi: 10.1016/s0010-440x(98)90051-7. [DOI] [PubMed] [Google Scholar]
- 11.Mattila AK, Salminen JK, Nummi T, et al. Age is strongly associated with alexithymia in the general population. J Psychosom Res. 2006;61:629–635. doi: 10.1016/j.jpsychores.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Pasini A, Delle Chiaie R, Seripa S, et al. Alexithymia as related to sex, age, and educational level: results of the Toronto Alexithymia Scale in 417 normal subjects. Compr Psychiatry. 1992;33:42–46. doi: 10.1016/0010-440x(92)90078-5. [DOI] [PubMed] [Google Scholar]
- 13.Henry J, Phillips L, Maylor E, et al. A new conceptualization of alexithymia in the general adult population: implications for research involving older adults? J Psychosom Res. 2006;60:535–543. doi: 10.1016/j.jpsychores.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Mattila AK, Ahola K, Honkonen T, et al. Alexithymia and occupational burnout are strongly associated in working population. J Psychosom Res. 2007;62:657–665. doi: 10.1016/j.jpsychores.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Lane RD, Ahern GL, Schwartz GE, et al. Is alexithymia the emotional equivalent of blindsight? Biol Psychiatry. 1997;42:834–844. doi: 10.1016/s0006-3223(97)00050-4. [DOI] [PubMed] [Google Scholar]
- 16.Lane RD, Reiman EM, Axelrod B, et al. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci. 1998b;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- 17.Brown JW, Jaffe J. Hypothesis on cerebral dominance. Neuropsychologia. 1975;13:107–110. doi: 10.1016/0028-3932(75)90054-8. [DOI] [PubMed] [Google Scholar]
- 18.Tabibnia G, Zaidel E. Alexithymia, interhemispheric transfer, and right hemispheric specialization: A critical review. Psychother Psychosom. 2005;74:81–92. doi: 10.1159/000083166. [DOI] [PubMed] [Google Scholar]
- 19.Borod JC, Bloom RL, Brickman AM, Nakhutina L, Curko EA. Emotional processing deficits in individuals with unilateral brain damage. Appl Neuropsychol. 2002;9(1):23–36. doi: 10.1207/S15324826AN0901_4. [DOI] [PubMed] [Google Scholar]
- 20.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 21.Damasio AR. Descartes’ Error: Emotion, Reason, and the Human Brain. New York, NY: Putnam Publishing; 1994. [Google Scholar]
- 22.Hornak J, Bramham J, Rolls ET, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- 23.Huber M, Herholz K, Habedank B, et al. Different patterns of regional brain activation during emotional stimulation in alexithymics in comparisons with normal controls. Psychother Psychosom Med Psychol. 2002;52:469–478. doi: 10.1055/s-2002-35276. [DOI] [PubMed] [Google Scholar]
- 24.Kano M, Fukudo S, Gyoba J, et al. Specific brain processing of facial expression in people with alexithymia: An H2 150-PET study. Brain. 2003;126:1474–1484. doi: 10.1093/brain/awg131. [DOI] [PubMed] [Google Scholar]
- 25.Martin AJ, Friston KJ, Colebatch JG, et al. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991;11:684–689. doi: 10.1038/jcbfm.1991.121. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer CC, Cantwell MN, Greer PJ, et al. Does cerebral blood flow decline in healthy aging? A PET study with partial-volume correction. J Nucl Med. 2000;41:1842–1848. [PubMed] [Google Scholar]
- 27.Schultz SK, O’Leary DS, Boles Ponto LL, et al. Age-related changes in regional cerebral blood flow among young to mid-life adults. Neuroreport. 1999;12:2493–2496. doi: 10.1097/00001756-199908200-00011. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg S, Smith GS, Barnes A, et al. Serotonin modulation of cerebral glucose metabolism in normal aging. Neurobiol Aging. 2004;25:167–174. doi: 10.1016/s0197-4580(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 29.Moeller JR, Ishikawa T, Dhawan V, et al. The metabolic topography of normal aging. J Cereb Blood Flow Metab. 1996;16:385–398. doi: 10.1097/00004647-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Pardo JV, Lee JT, Sheikh SA, et al. Where the brain grows old: decline in anterior cingulate and medial prefrontal function with normal aging. Neuroimage. 2007;35:1231–1237. doi: 10.1016/j.neuroimage.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petit-Taboue MC, Landeau B, Desson JF, et al. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7:176–184. doi: 10.1006/nimg.1997.0318. [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND, Logan J, Fowler JS, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Marchal G, Rioux P, Petit-Taboue MC, et al. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol. 1992;49:1013–1020. doi: 10.1001/archneur.1992.00530340029014. [DOI] [PubMed] [Google Scholar]
- 34.Thompson LW. Cerebral blood flow, EEG, and behavior in aging. In: Terry RD, Gershon S, editors. Neurobiology of Aging. New York: Raven Press; 1976. pp. 109–119. [Google Scholar]
- 35.Van Laere KJ, Dierckx RA. Brain perfusion SPECT: age- and sex-related effects correlated with voxel-based morphometric findings in healthy adults. Radiology. 2001;221:810–817. doi: 10.1148/radiol.2213010295. [DOI] [PubMed] [Google Scholar]
- 36.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 37.Grieve SM, Clark CR, Williams LM, et al. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sowell ER, Peterson BS, Thompson PM, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 39.Resnick SM, Pham DL, Kraut MA, et al. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berthoz S, Artiges E, Van De Moortele PF, et al. Effect of impaired recognition and expression of emotions on fronto cingulate cortices: an fMRI study of men with alexithymia. Am J Psychiatry. 2002;159:961–967. doi: 10.1176/appi.ajp.159.6.961. [DOI] [PubMed] [Google Scholar]
- 41.Phillips LH, MacLean RD, Allen R. Age and the understanding of emotions: neuropsychological and sociocognitive perspectives. J Gerontol B Psychol Sci Soc Sci. 2002;57:526–530. doi: 10.1093/geronb/57.6.p526. [DOI] [PubMed] [Google Scholar]
- 42.Alexopoulos GS, Gunning-Dixon FM, Latoussakis V, et al. Anterior cingulated dysfunction in geriatric depression. Int J Geriatr Psychiatry. 2007 doi: 10.1002/gps.1939. pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Microstructural White Matter Abnormalities and Remission of Geriatric Depression. Am J Psychiatry. 2008 Jan 2; doi: 10.1176/appi.ajp.2007.07050744. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 44.Takami H, Okamoto Y, Yamashita H, et al. Attenuated anterior cingulate activation during a verbal fluency task in elderly patients with a history of multiple-episode depression. Am J Geriatr Psychiatry. 2007;15:594–603. doi: 10.1097/01.JGP.0b013e31802ea919. [DOI] [PubMed] [Google Scholar]
- 45.Konarski JZ, Kennedy SH, McIntyre RS, Rafi-Tari S, Soczynska JK, Mayberg HS. Relationship between regional brain metabolism, illness severity and age in depressed subjects. Psychiatry Res. 2007;155:203–210. doi: 10.1016/j.pscychresns.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991 Aug;38(2):143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 47.Lavretsky H, Ballmaier M, Pham D, et al. Neuroanatomical characteristics of geriatric apathy and depression: A magnetic resonance imaging study. Am J Geriatr Psychiatry. 2007;15:386–394. doi: 10.1097/JGP.0b013e3180325a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 49.Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: A brain-mapping meta-analysis. Exp Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- 51.McCormick LM, Ziebell S, Nopoulos P, et al. Anterior cingulate cortex: An MRI-based parcellation method. NeuroImage. 2006;32:1167–1175. doi: 10.1016/j.neuroimage.2006.04.227. [DOI] [PubMed] [Google Scholar]
- 51.Vaidya JG, Paradiso S, Ponto BLL, et al. Aging, gray matter, and blood flow in the anterior cingulate cortex. Neuroimage. 2007;37:1346–1353. doi: 10.1016/j.neuroimage.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 282;1998:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 54.Vogt BA, Nimchinsky ET, Vogt LJ, et al. Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- 55.MacDonald AW, III, Cohen JD, Stenger VA, et al. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 56.Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 57.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Lamberty GJ, Holt CS. Evidence for a verbal deficit in alexithymia. J Neuropsychiatry Clin Neurosci. 1995;7:320–324. doi: 10.1176/jnp.7.3.320. [DOI] [PubMed] [Google Scholar]
- 59.Wood RL, Williams C. Neuropsychological correlates of organic alexithymia. J Int Neuropsychol Soc. 2007;13:471–479. doi: 10.1017/S1355617707070518. [DOI] [PubMed] [Google Scholar]
- 60.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research; 2002. [Google Scholar]
- 61.Hamilton MA. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 62.Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson RG, Paradiso S, Mizrahi R, et al. Neuropsychological correlates of normal variation in emotional response to visual stimuli. J Nerv Ment Dis. 2007;195:112–118. doi: 10.1097/01.nmd.0000254482.44985.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turner B, Paradiso S, Marvel C, et al. The cerebellum and emotional experience. Neuropsychologia. 2007;45:1331–1341. doi: 10.1016/j.neuropsychologia.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weschsler Adult Intelligence Scale Revised: The Psychological Corporation. San Antonio, TX: 1981. [Google Scholar]
- 66.De Renzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- 67.Lezak MD, Howieson DB, Loring DL, et al. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 68.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. Iowa City, Iowa: AJA Associates; 1994. [Google Scholar]
- 69.Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994a;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 70.Bagby RM, Taylor GJ, Parker JD. The Twenty-item Toronto Alexithymia Scale--II. Convergent, discriminant, and concurrent validity. J Psychosom Res. 1994b;38:33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 71.Magnotta VA, Harris G, Andreasen NC, et al. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 72.Woods SW. Regional cerebral blood flow imaging with SPECT in psychiatric disease: Focus on schizophrenia, anxiety disorders, and substance abuse. J Clin Psychiatry. 1992;53:20–25. [PubMed] [Google Scholar]
- 73.Harris G, Andresen NC, Cizadlo T, et al. Improving tissue classification in MRI: A three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 74.Magnotta VA, Heckel D, Andreasen NC, et al. Measurement of brain structures with artificial neural networks: Two- and three-dimensional applications. Radiology. 1999;211:781–790. doi: 10.1148/radiology.211.3.r99ma07781. [DOI] [PubMed] [Google Scholar]
- 75.Lesser IM. Current concepts in psychiatry: Alexithymia. N Eng J Med. 1985;312:690–692. doi: 10.1056/NEJM198503143121105. [DOI] [PubMed] [Google Scholar]
- 76.Taylor GJ, Bagby RM, Parker JD. The Revised Toronto Alexithymia Scale: Some reliability, validity, and normative data. Psychother Psychosom. 1992;57:34–41. doi: 10.1159/000288571. [DOI] [PubMed] [Google Scholar]
- 77.Gündel H, Lopez-Sala A, Ceballos-Baumann AO, et al. Alexithymia correlates with the size of the right anterior cingulate. Psychosom Med. 2004;66:132–140. doi: 10.1097/01.psy.0000097348.45087.96. [DOI] [PubMed] [Google Scholar]
- 78.Wassink TH, Hazlett HC, Epping EA, et al. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch Gen Psychiatry. 2007;64:709–717. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- 79.Alexopoulos GS, Schultz SK, Lebowitz BD. Late-life depression: A model for medical classification. J Biopsych. 2005;58:283–289. doi: 10.1016/j.biopsych.2005.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Honkalampa K, Hintikka J, Tanskanen A, et al. Depression is strongly associated with alexithymia in the greater population. J Psychosom Res. 2000;48:99–104. doi: 10.1016/s0022-3999(99)00083-5. [DOI] [PubMed] [Google Scholar]
- 81.Gallo JJ, Rabins PV, Lyketsos CG, et al. Depression without sadness: functional outcomes of nondysphoric depression in later life. J Am Geriatr Soc. 1997;45:570–578. doi: 10.1111/j.1532-5415.1997.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 82.Judd LL, Rapaport MH, Paulus MP, et al. Subsyndromal symptomatic depression: a new mood disorder. J Clin Psychiatry. 1994;55:18–28. [PubMed] [Google Scholar]
- 83.Charney DS, Reynolds CF, Lewis L, et al. Depression and Bipolar Support Alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry. 2003;60:664–672. doi: 10.1001/archpsyc.60.7.664. [DOI] [PubMed] [Google Scholar]
- 84.Critchley HD, Wiens S, Rotshtein P, et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 85.Northoff G, Heinzel A, de Greck M, et al. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 86.Sirey JA, Bruce ML, Alexopoulos GS, et al. Perceived Stigma and Patient-Rated Severity of Illness as Predictors of Antidepressant Drug Adherence. Psychiatric Services. 2001;52:1615–1620. doi: 10.1176/appi.ps.52.12.1615. [DOI] [PubMed] [Google Scholar]
- 87.Gallo JJ, Rabins PV, Anthony JC. Sadness in older persons: 13-year follow-up of a community sample in Baltimore, Maryland. Psychol Med. 1999;29:341–350. doi: 10.1017/s0033291798008083. [DOI] [PubMed] [Google Scholar]
- 88.Buklina SB. Clinical-neuroendocrinological syndromes due to lesions of the cingulated gyrus in humans. Neurosci Behav Physiol. 1998;28:601–607. doi: 10.1007/BF02462980. [DOI] [PubMed] [Google Scholar]
- 89.Milak MS, Parsey RV, Keilp J, et al. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry. 2005;62:397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- 90.Etkin A, Egner T, Peraza DM, et al. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 91.Saltzman J, Strauss E, Hunter M, et al. Theory of mind and executive functions in normal human aging and Parkinson's disease. J Int Neuropsychol Soc. 2000;6:781–788. doi: 10.1017/s1355617700677056. [DOI] [PubMed] [Google Scholar]
- 92.Levenson RW, Carstensen LL, Friesen WV, et al. Emotion, physiology, and expression in old age. Psychol Aging. 1991;6:28–35. doi: 10.1037//0882-7974.6.1.28. [DOI] [PubMed] [Google Scholar]
- 93.Cumming E, Henry WE. Growing old: The process of disengagement. New York: Basic Books; 1961. [Google Scholar]
- 94.Botwinick J, Storandt M. Age differences in reaction time as a function of experience, stimulus intensity, and preparatory interval. J Genet Psychol. 1973;123:209–217. doi: 10.1080/00221325.1973.10532679. [DOI] [PubMed] [Google Scholar]
- 95.Rosen JL, Neugarten BL. Ego functions in the middle and later years: a thematic apperception study of normal adults. J Gerontol. 1973;15:62–67. doi: 10.1093/geronj/15.1.62. [DOI] [PubMed] [Google Scholar]
- 96.Magai C. Emotions over the life span. In: Birren J, Schaie KW, editors. Handbook of the psychology of aging. San Diego: Academic Press; 2001. pp. 165–183. [Google Scholar]
- 97.Carstensen LL, Charles ST. Emotion in the second half of life. Curr Dir Psychol Sci. 1999;7:144–149. [Google Scholar]
- 98.Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: An fMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- 99.Damasio AR. On some functions of the human prefrontal cortex. Ann N Y Acad Sci. 1995;769:241–251. doi: 10.1111/j.1749-6632.1995.tb38142.x. [DOI] [PubMed] [Google Scholar]
- 100.Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- 101.Paradiso S, Chemerinski E, Yazici KM, et al. Frontal lobe syndrome reassessed: comparison of patients with lateral or medial frontal brain damage. J Neurol Neurosurg Psychiatry. 1999;67:664–667. doi: 10.1136/jnnp.67.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsai J. Autonomic, Subjective, and Expressive Responses to Emotional Films in Older and Younger Chinese Americans and European Americans. Psychol Aging. 2000;15:684–693. doi: 10.1037//0882-7974.15.4.684. [DOI] [PubMed] [Google Scholar]
- 103.MacPherson SE, Phillips LH, Della Sala S. Age, executive function, and social decision making: a dorsolateral prefrontal theory of cognitive aging. Psychol Aging. 2002;17:598–609. [PubMed] [Google Scholar]
- 104.Isaacowitz DM, Charles ST, Carstensen LL. Emotion and cognition. Mahwah, NJ: Lawrence Erlbaum Associates, Inc.; 2000. [Google Scholar]
- 105.Allman JM, Hakeem A, Erwin JM, et al. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- 106.Nimchinsky EA, Vogt BA, Morrison JH, et al. Spindle neurons of the human anterior cingulate cortex. J Comp Neurol. 1995;335:27–37. doi: 10.1002/cne.903550106. [DOI] [PubMed] [Google Scholar]
- 107.Beekman AT, Geerlings SW, Deeg DJ, et al. The natural history of late-life depression: a 6-year prospective study in the community. Arch Gen Psychiatry. 2002;59:605–611. doi: 10.1001/archpsyc.59.7.605. [DOI] [PubMed] [Google Scholar]