Abstract
Purpose:
This study evaluated the cytotoxicity of five prostaglandin analog ophthalmic solutions on four ocular surface cell lines, ie, Chang (human conjunctiva), SIRC (rabbit cornea), RC-1 (rabbit cornea), and BCE C/D-1b (bovine cornea).
Methods:
Cell viability was measured by neutral red and MTT assays in cells treated for 10, 30, or 60 minutes with various doses of prostaglandins (undiluted, and 2- and 10-fold dilutions). The number of cell lines with viability ≥50% in the presence of selected dilution of the drug (CVS50) was used for comparison. In addition, 24 cell viability comparisons (four cell lines, two assays, and three exposure times) were made between latanoprost (Xalatan®) and each other solution at each dose. A comparison between the newly introduced tafluprost (Tapros®) with 0.01% benzalkonium chloride was also made.
Results:
The order of cell viability determined by CVS50 was Travatan Z® (travoprost with the SofZia system) > Tapros ≥ Travatan® (travoprost) = Xalatan > Rescula® (unoproston). This was consistent with the results of direct comparisons between Xalatan and the other drugs. There was no clear difference in cell viability between Tapros and benzalkonium chloride.
Conclusions:
Use of two assays, multiple cell lines, and various dilutions and exposure times provided a unique evaluation of cytotoxicity among ophthalmic solutions. CVS50 was useful for comparison of the cell viability of the solutions.
Keywords: glaucoma, cornea, cell viability score
Introduction
Prostaglandin analogs (PGs) form a major part of treatment regimens for glaucoma.1 PGs successfully reduce ocular pressure,2–5 with few common side effects including conjunctival hypernemia6–8 and increased iris pigmentation.9–13 Cytopathic evaluations of PGs in conjunctival cells14–18 have indicated that latanoprost (Xalatan®) and travoprost (Travatan®) could be cytoprotective against benzalkonium chloride toxicity via an antioxidative effect.15 Benzalkonium chloride is commonly included as a preservative in ophthalmic solutions,19–21 although Travatan Z® (also travoprost) is preserved with a SofZia system that has proven to be less cytotoxic.21 Among the PGs, Xalatan was introduced early and has become a standard medication. Tafluprost (Tapros®)16,22,23 is a recently introduced PG and the cytotoxicity of preserved tafluprost is untested. We therefore evaluated the cytotoxicity of Tapros compared with that of five other PG ophthalmic agents in four corneoconjunctival cell lines using two cytotoxic assays to compare Xalatan with other available PG drugs, test for intercellular or interassay differences in toxicity, and to determine the optimal dose and exposure time for cytotoxic testing.
Materials and methods
The cell lines used in the present study were SIRC (rabbit corneal epithelium, CCL-60, American Type Culture Collection [ATCC], Manassas, VA), BCE C/D-1b (bovine corneal epithelial cells, JCRB-9129, Health Science Research Resource Bank, Osaka, Japan), RC-1 (rabbit corneal epithelium, JCRB-0246; Health Science Research Resource Bank), and Chang conjunctiva (human conjunctival cells, ATCC CCL-20.2). All cell lines were cultured according to the standard protocols provided by the suppliers.
Approximately 2 ×104 cells were harvested from 96-well culture plates (NUNC® 167008; Thermo Fisher Scientific, Denmark) and incubated for two days. The culture medium was then replaced with undiluted, twofold, or 10-fold dilutions of the different ophthalmic drugs, and the cells were incubated for a further 10, 30, or 60 minutes. After these drug treatments, the culture medium was replaced with fresh media and cells were incubated for a further 48 hours. Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium bromide (MTT) and neutral red assays. The MTT assay is a quantitative colorimetric assay of mitochondrial activity as a measure of cell viability and proliferation; it detects living cells only, and the signal generated is directly proportional to the number of viable cells. In contrast, neutral red staining lysosomes in living cells and the results of the assay are determined spectrophotometrically (Benchmark Microplate Reader; BIO-RAD, Hercules, CA). Cell viability of treated cells is expressed as a percentage of that in control cells (incubated in culture medium alone). All experiments were repeated 8–16 times and results are presented as the mean ± standard deviation (SD). Statistical comparisons were made using the t-test by Microsoft Excel (Microsoft, Tokyo, Japan), and P < 0.05 was determined as significant.
We compared cell viability using the cell viability score. In a previous study,24 we used CVS50 (ie, number of cell lines with viability ≥50% in the presence of a selected dose of the drug) to enable easy comparison of the effects of different drugs, and found it was useful. This concept is similar to that of the MIC50 (ie, the minimum inhibitory concentration of a drug required to inhibit the growth of 50% of organisms). In addition, 24 comparisons of cell viability (four corneal cell lines, two assays, and three exposure times) were also made between Xalatan, a leading PG and serving as the standard, and each other solution at each dose. A comparison between Tapros and 0.01% benzalkonium chloride was also made.
We used the following solutions for cytotoxicity: 0.0015% Tapros (with 0.01% benzalkonium chloride and polysorbate 80), 0.005% Xalatan (with 0.02% benzalkonium chloride), 0.12% unoproston (Rescula®, with 0.005% benzalkonium chloride), 0.004% travoprost (Travatan, with 0.015% benzalkonium chloride), 0.004% travoprost (Travatan Z), and 0.01% benzalkonium chloride.
Results
Figures 1–4 show the cell viability results for four cell lines, and its order determined by CVS50 was Travatan Z ≥ Tapros ≥ Travatan = Xalatan > Rescula (Table 1). Table 2 details the comparison between Xalatan and the other drugs for all cell lines. Based on these results, cell viability was clearly the highest for Travatan Z, with only small changes seen among different dilutions and exposure times. In contrast, it was the lowest for Rescula. CVS50 expressed general trend of graphic presentation and detailed comparison was consistent with CVS50. The comparison between Travatan and Xalatan only exhibited conflicting results in three doses, although those two drugs had the same CVS50.
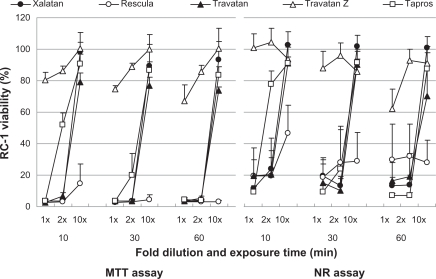
Figure 1.
Effects of prostaglandin analog ophthalmic solutions on the viability of cultured rabbit corneal cells (RC-1) using the neutral red (NR) assay and MTT assay. Data are means ± standard deviations. Note that Travatan Z exhibits the greatest viability and shows minimal differences among exposure times or dilution. The cell viabilities for the other solutions depend on exposure times and dilutions except for Rescula, which shows small cell viabilities even in diluted solutions. These are common findings in all cell lines. Detailed comparisons were made with cell viability score and statistical analysis (Tables 1–3).
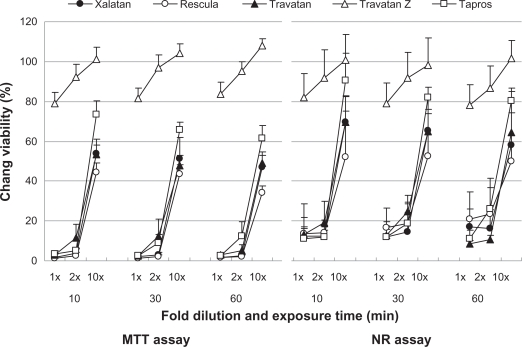
Figure 4.
Effects of prostaglandin analog ophthalmic solutions on the viability of cultured human conjunctival cells (Chang) conjunctiva using the neutral red (NR) assay and MTT assay. Data are means ± standard deviations. Travatan Z exhibited a proliferative effect (cell viability > 100%) with the 10-fold dilution.
Table 1.
Cell viability score of the drugs
| Drugs |
Fold dilution and exposure time (min) |
Total of CVS50 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
1 × |
2 × |
10 × |
||||||||
| 10 | 30 | 60 | 10 | 30 | 60 | 10 | 30 | 60 | ||
| Xalatan | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 8 | 7 | 23 |
| Rescula | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 2 | 1 | 7 |
| Travatan | 0 | 0 | 0 | 1 | 0 | 0 | 8 | 7 | 7 | 23 |
| Travatan Z | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 72 |
| Tapros | 0 | 0 | 0 | 6 | 2 | 0 | 8 | 8 | 8 | 32 |
Notes: The full number of assay is eight (four cell lines × two assays for each time and dilution).
Abbreviations: CVS50, number of cell lines with viability ≥50% in the presence of the drug; min, minutes.
Table 2.
Comparison of cell viability between Xalatan and other prostaglandins
| Drugs and comparison results |
Summary of comparisons for each exposure time and fold dilution |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
1 × |
2 × |
10 × |
||||||||
| 10 | 30 | 60 | 10 | 30 | 60 | 10 | 30 | 60 | ||
| Rescula | >Xalatan* | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| NS* | 5 | 6 | 7 | 8 | 8 | 7 | 6 | 2 | 1 | |
| <Xalatan* | 3 | 1 | 1 | 0 | 0 | 1 | 2 | 3 | 7 | |
| Travatan | >Xalatan | 4 | 2 | 2 | 6 | 6 | 3 | 0 | 0 | 0 |
| NS | 4 | 6 | 5 | 2 | 2 | 5 | 4 | 4 | 3 | |
| <Xalatan | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 4 | 5 | |
| Travatan Z | >Xalatan | 8 | 8 | 8 | 8 | 8 | 8 | 4 | 5 | 3 |
| NS | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 2 | 4 | |
| <Xalatan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| Tapros | >Xalatan | 4 | 1 | 0 | 7 | 6 | 3 | 3 | 2 | 2 |
| NS | 4 | 6 | 7 | 1 | 2 | 4 | 4 | 5 | 5 | |
| <Xalatan | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | |
Notes: Each comparison includes eight experiments (two assays for four cell lines);
P < 0.05 (result of t-test).
Abbreviation: NS, not significant.
The comparison of Tapros and 0.01% benzalkonium chloride (Table 3) indicated that cell viability in benzalkonium chloride was always higher with the 10-minute treatments (8/8 experiments), but was not different (3/8) or was lower (5/8) in benzalkonium chloride after a 30-minute exposure. There was no difference in cell viability between Tapros and benzalkonium chloride with the 60-minute exposure.
Table 3.
Comparison of cell viability between undiluted Tapros and 0.01% BAK
| Cell lines |
Exposure time for neutral red assay |
Exposure time for MTT assay |
|||||
|---|---|---|---|---|---|---|---|
| 10 min | 30 min | 60 min | 10 min | 30 min | 60 min | ||
| SIRC | Tapros | 26.1 ± 12.7 | 14.4 ± 8.0 | 9.6 ± 1.4 | 16.7 ± 11.8 | 3.9 ± 1.1* | 4.0 ± 1.3 |
| BAK | 58.2 ± 19.8** | 13.8 ± 7.1 | 8.1 ± 3.0 | 37.5 ± 5.8** | 2.4 ± 0.7 | 3.8 ± 3.4 | |
| RC-1 | Tapros | 9.2 ± 3.6 | 9.1 ± 4.6 | 7.3 ± 1.2 | 3.5 ± 0.2 | 3.5 ± 0.9 | 4.3 ± 1.4 |
| BAK | 30.2 ± 5.6** | 6.8 ± 0.3 | 7.0 ± 0.4 | 26.4 ± 14.8** | 3.7 ± 0.3 | 4.3 ± 0.7 | |
| BCE | Tapros | 18.2 ± 9.2 | 11.5 ± 8.6 | 7.3 ± 2.2 | 11.9 ± 11.1 | 2.4 ± 1.2 | 2.8 ± 1.8 |
| BAK | 49.8 ± 20.8** | 7.4 ± 0.6 | 7.5 ± 0.6 | 33.3 ± 6.9** | 1.5 ± 0.5 | 1.7 ± 0.2 | |
| Chang | Tapros | 11.2 ± 5.9 | 12.0 ± 5.6* | 11.0 ± 4.6 | 3.4 ± 1.8 | 2.6 ± 0.6* | 2.7 ± 1.2 |
| Conjunctiva | BAK | 20.4 ± 3.6** | 7.0 ± 0.7 | 7.5 ± 1.6 | 14.0 ± 3.8** | 2.0 ± 0.2 | 2.1 ± 0.3 |
Notes:
Tapros-treated cells show greater viability (P < 0.05, t-test);
BAK is greater (P < 0.05, t-test).
Abbreviations: BAK, benzalkonium chloride; min, minute.
The MTT and neutral red assays produced comparable results and there were no conflicting results in any comparisons. The MTT values were generally less than those obtained with the neutral red assay, especially in undiluted and twofold diluted solutions. There were also no major inconsistencies among results from the four cell lines, as shown in Figures 1–4.
Discussion
Benzalkonium chloride concentration in the various ophthalmic solutions tested in this study increased in the following order: Travatan Z < Rescula < Tapros < Travatan < Xalatan. This increase was inversely in accordance with cell viability, except for Rescula, which was more toxic than Travatan and Tapros with more benzalkonium chloride. Unoproston in Rescula is a metabolized form of natural PG F2 alpha and is different from the other PGs, although this study did not address whether the difference in cytotoxicity is related to this unique structure. The cytotoxicity of preserved tafluprost was first examined in vitro and proved comparable with Xalatan and Travatan. Considering Tapros contains toxic levels of benzalkonium chloride and polysorbate 80, the lower cell viability observed in this study after 10 minutes of exposure compared with benzalkonium chloride alone in all cell lines was not unexpected. Although cell viability in Tapros was greater than or equal to that in 0.01% benzalkonium chloride in some of comparisons, the cytoprotective effect of tafluprost on ocular surface cell lines was unclear in our experiments.
We evaluated cell viability for multiple conditions using two bioassays. The undiluted and twofold diluted solutions seemed suitable for clinically relevant comparisons of cytotoxicity, possibly because these represent levels that are stimulatory immediately after instillation. The 10-fold dilutions were not practical in this experimental setting, but could be useful in testing of long-term cytotoxicity after diluted by tears. The 10-minute exposure time represented actual residence time of the applied solution on an ocular surface and seemed to be the minimum period required for in vitro testing. Our results were similar between the 10- and 30-minute exposures, although the latter was deemed more convincing and clinically applicable. The differences between the drugs reduced in significance after 60 minutes of exposure due to the low viability rates. It should be noted that this experiment would not be correlated with pressure reduction effects that work around the intraocular tissue and last for several hours. As for assays and cells, the MTT assay values were very low in some experiments; however, there were no discrepant comparisons with neutral red assay results. CVS50 was simple and useful to compare cell viability in tested solutions and was confirmed by detailed comparisons. We believe bioassay using multiple cell lines is a contributory method to precise results. CVS50 might be applicable to such toxicity experiments.
This study had some limitations. Although we used two different assays and tested four cell lines, the bioassay only evaluated cytotoxicity, thus the results should be confirmed using a series of methods. Second, toxicity of the PGs for keratoconjunctival cells was not evaluated directly, although the active component and preservative could potentially interact in the commercial eyedrop mixture. Multiple evaluations should be done to investigate the potential side effects of PGs in glaucoma patients further.
Conclusion
Using two cytotoxicity assays on four ocular surface cell lines with a series of dilutions and exposure times of test PG ophthalmic solutions enabled a unique evaluation of cytotoxicity. The results confirmed that Travatan Z without benzalkonium chloride is the least toxic drug among the ocular surface cells tested. The detailed drug comparison included the newly available preserved Tapros and the order of cell viability rate was determined accordingly. Optimal exposure times and dilutions for cytotoxicity testing of ophthalmic solutions were also discussed. CVS50 was useful for comparison of cell viability in the drugs.
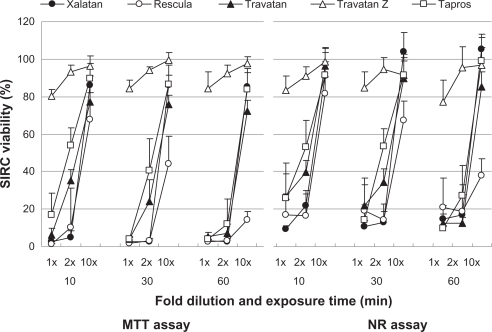
Figure 2.
Effects of prostaglandin analog ophthalmic solutions on the viability of cultured rabbit corneal cells (SIRC) using the neutral red (NR) assay and MTT assay. Data are means ± standard deviations.
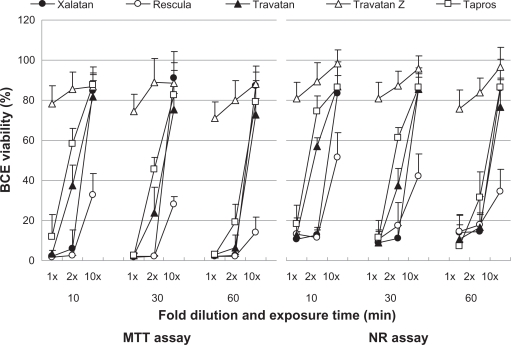
Figure 3.
Effects of prostaglandin analog ophthalmic solutions on the viability of cultured bovine corneal cells (BCE C/D 1-b) using the neutral red (NR) assay and MTT assay. Data are means ± standard deviations.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Camras CB, United States Latanoprost Study Group Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma. A six-month, masked, multicenter trial in the United States. Ophthalmology. 1996;103:138–147. doi: 10.1016/s0161-6420(96)30749-5. [DOI] [PubMed] [Google Scholar]
- 3.Chew PT, Aung T, Aquino MV, et al. EXACT Study Group Intraocular pressure-reducing effects and safety of latanoprost versus timolol in patients with chronic angle-closure glaucoma. Ophthalmology. 2004;111:427–434. doi: 10.1016/j.ophtha.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Sihota R, Saxena R, Agarwal HC, et al. Crossover comparison of timolol and latanoprost in chronic primary angle-closure glaucoma. Arch Ophthalmol. 2004;122:185–189. doi: 10.1001/archopht.122.2.185. [DOI] [PubMed] [Google Scholar]
- 5.Parrish RK, Palmberg P, Sheu WP, XLT Study Group A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: A 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135:688–703. doi: 10.1016/s0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 6.Abelson MB, Mroz M, Rosner SA, et al. Multicenter, open-label evaluation of hyperemia associated with use of bimatoprost in adults with open-angle glaucoma or ocular hypertension. Adv Ther. 2003;20:1–13. doi: 10.1007/BF02850114. [DOI] [PubMed] [Google Scholar]
- 7.Stewart WC, Kolker AE, Stewart JA, et al. Conjunctival hyperemia in healthy subjects after short-term dosing with latanoprost, bimatoprost, and travoprost. Am J Ophthalmol. 2003;135:314–320. doi: 10.1016/s0002-9394(02)01980-3. [DOI] [PubMed] [Google Scholar]
- 8.Feldman RM. Conjunctival hyperemia and the use of topical prostaglandins in glaucoma and ocular hypertension. J Ocul Pharmacol Ther. 2003;19:23–35. doi: 10.1089/108076803762718088. [DOI] [PubMed] [Google Scholar]
- 9.Dutkiewicz R, Albert DM, Levin LA. Effects of latanoprost on tyrosinase activity and mitotic index of cultured melanoma lines. Exp Eye Res. 2000;70:563–569. doi: 10.1006/exer.1999.0819. [DOI] [PubMed] [Google Scholar]
- 10.Hu DN, Stjernschantz J, McCormick SA. Effect of prostaglandins A2, E1, F2α and latanoprost on cultured human iridal melanocytes. Exp Eye Res. 2000;70:113–120. doi: 10.1006/exer.1999.0760. [DOI] [PubMed] [Google Scholar]
- 11.Lindquist NG, Larsson BS, Stjernschantz J. Increased pigmentation of iridial melanocytes in primates induced by a prostaglandin analogue. Exp Eye Res. 1999;69:431–436. doi: 10.1006/exer.1999.0718. [DOI] [PubMed] [Google Scholar]
- 12.Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(Suppl 2):S129–S138. doi: 10.1016/s0039-6257(97)80020-3. [DOI] [PubMed] [Google Scholar]
- 13.Liang H, Baudouin C, Pauly A, et al. Conjunctival and corneal reactions in rabbits following short- and repeated exposure to preservative-free tafluprost, commercially available latanoprost and 0.02% benzalkonium chloride. Br J Ophthalmol. 2008;92:1275–1282. doi: 10.1136/bjo.2008.138768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baudouin C, Riancho L, Warnet JM, et al. In vitro studies of antiglaucomatous prostaglandin analogues: Travoprost with and without benzalkonium chloride and preserved latanoprost. Invest Ophthalmol Vis Sci. 2007;48:4123–4128. doi: 10.1167/iovs.07-0266. [DOI] [PubMed] [Google Scholar]
- 15.Guenoun JM, Baudouin C, Rat P, et al. In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:4594–4599. doi: 10.1167/iovs.05-0776. [DOI] [PubMed] [Google Scholar]
- 16.Brasnu E, Brignole-Baudouin F, Riancho L, et al. In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr Eye Res. 2008;33:303–312. doi: 10.1080/02713680801971857. [DOI] [PubMed] [Google Scholar]
- 17.de Saint Jean F, Brignole AF, Bringuier A, et al. Effects of benzalkonium chloride on growth and viability of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40:619–630. [PubMed] [Google Scholar]
- 18.Pisella PJ, Debbasch C, Hamard P, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: An ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45:1360–1368. doi: 10.1167/iovs.03-1067. [DOI] [PubMed] [Google Scholar]
- 19.Pfister RR, Burstein NL. The effects of ophthalmic drugs, vehicles, and preservatives on corneal epithelium: A scanning electron microscope study. Invest Ophthalmol Vis Sci. 1976;15:246–259. [PubMed] [Google Scholar]
- 20.Kahook MY, Noecker RJ. Comparison of corneal and conjunctival changes after dosing of travoprost preserved with SofZia, latanoprost with 0.02% benzalkonium chloride, and preservative-free artificial tears. Cornea. 2008;27:339–343. doi: 10.1097/ICO.0b013e31815cf651. [DOI] [PubMed] [Google Scholar]
- 21.Ayaki M, Yaguchi S, Koide R, et al. Human corneal endothelial toxicity of ophthalmic solution with and without preservatives. Exp Clin Ophthalmol. 2008;36:553–559. doi: 10.1111/j.1442-9071.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 22.Ota T, Aihara M, Saeki T, et al. The IOP-lowering effects and mechanism of action of tafluprost in prostanoid receptor-deficient mice. Br J Ophthalmol. 2007;91:673–676. doi: 10.1136/bjo.2006.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izumi N, Nagaoka T, Sato E, et al. Short-term effects of topical tafluprost on retinal blood flow in cats. J Ocul Pharmacol Ther. 2008;24:521–652. doi: 10.1089/jop.2007.0065. [DOI] [PubMed] [Google Scholar]
- 24.Ayaki M, Iwasawa A, Soda M, et al. Cytotoxicity of five fluoroquinolone and two nonsteroidal anti-inflammatory benzalkonium chloride-free ophthalmic solutions in four corneoconjunctival cell lines. Clin Ophthalmol. 2010 doi: 10.2147/opth.s12452. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]