Abstract
Recent advances in induced pluripotent stem (iPS) cell research significantly changed our perspective on regenerative medicine. Patient specific iPS cells have been derived not only for disease modeling but also as sources for cell replacement therapy. However, there have been insufficient data to prove that iPS cells are functionally equivalent to hES cells or safer than hES cells. There are several important issues which need to be addressed and foremost are the safety and efficacy of human iPS cells from different origins. Human iPS cells have been derived mostly from cells originated from mesoderm, with a few cases from ectoderm. So far there has been no report of endoderm derived human iPS cells, preventing comprehensive comparative investigations on the quality of human iPS cells from different origins.
Here we show for the first time reprogramming of human endoderm derived cells (i.e. primary hepatocytes) to pluripotency. Hepatocyte-derived iPS cells appear indistinguishable from human embryonic stem cells in colony morphology, growth properties, expression of pluripotency-associated transcription factors and surface markers, and differentiation potential in embryoid body formation and teratoma assays. In addition, these cells were able to directly differentiate into definitive endoderm, hepatic progenitors, and mature hepatocytes. The technology to develop endoderm derived human iPS cell lines, together with other established cell lines, will provide a foundation to elucidate the mechanisms of cellular reprogramming and to study the safety and efficacy of differentially originated human iPS cells for cell therapy. For studying liver disease pathogenesis, this technology also provides a potentially more amenable system to generate liver disease specific iPS cells.
Introduction
Recent advances in induced pluripotent stem (iPS) cell research have provided great potential for these somatic cell-derived stem cells as sources for cell replacement therapy and for establishing disease models.1–14 Human iPS cells have been shown to be pluripotent in in vitro differentiation and in vivo teratoma assays, similar to human embryonic stem (hES) cells.9–14 Disease-specific iPS cell lines have been generated from fibroblasts and blood cells and some of the disease features have been recapitulated in tissue culture after directed differentiation of the iPS cells, demonstrating the power of this technology in disease modeling.13, 15 However, several key issues have to be addressed in order for the iPS cells to be used for clinical purposes. First, although pluripotency has been demonstrated, it is premature to claim that iPS cells are functionally equivalent to hES cells. In fact, one study has suggested that iPS cells have distinct protein-coding and microRNA gene expression signatures from ES cells.1 These differences can not be completely explained by the reactivation of transgenes used in the reprogramming process since human iPS cells generated without viral or transgene integration also displayed a different transcriptional signature compared to hES cells.2 Secondly it was demonstrated that human iPS cells retained certain gene expression of the parent cells, suggesting that iPS cells from different origins may possess different capacity to differentiate.2 This issue is important not only for the purposes of generating functional cell types for therapy but also for safety implications. A comprehensive study using various mouse iPS cells has demonstrated that the origin of the iPS cells had a profound influence on the tumor-forming propensities in a cell transplantation therapy model.3 Mouse tail-tip fibroblast-iPS cells (mesoderm origin) showed the highest tumorigenic propensity, whereas gastric epithelial cell- and hepatocyte-iPS cells (both are endoderm) showed lower propensities.3 It is therefore extremely important to establish human iPS cell lines from multiple origins and thoroughly examine the source impact on both the safety issues and their differentiation potentials. In addition, the ability to reprogram human hepatocytes is crucial for developing liver disease models using iPS cells, especially for certain liver diseases carrying acquired somatic mutations which occur only in hepatocytes of patients, but not in other cell types.16–20
In the mouse, iPS cells have been generated from derivatives of all three embryonic germ layers, including mesodermal fibroblasts,6 epithelial cells of endodermal origin7 and ectodermal keratinocytes,8 whereas human iPS cells have been produced mostly from mesoderm (fibroblasts and blood cells) or from ectoderm (keratinocytes and neural stem cells).9–13, 21, 22 Here we show reprogramming of human primary hepatocytes (endoderm) to pluripotency. Hepatocyte-derived iPS cells appear indistinguishable from human embryonic stem cells in colony morphology, growth properties, expression of pluripotency-associated transcription factors and surface markers, and differentiation potential in embryoid body (EB) formation as well as teratoma assays. In addition these cells were able to directly differentiate into definitive endoderm, hepatic progenitors, and mature hepatocytes.
Our study lays the ground work necessary to elucidate the mechanisms of cellular reprogramming and to study the safety and efficacy of differentially originated human iPS cells in cell therapy.
Methods
Cell culture
Primary human hepatocytes were obtained from Lonza plated on collagen 1 and matrigel coated dishes, and cultured in serum containing WEM (Willians' Medium E), Gentamicin, Dexamethasone 10 mM, FBS 5%, L-Glutamine, Hepes 15mM, Insulin 4 mg/ml with 50ng/ml of HGF and EGF. Medium for culturing hES cells and iPS cells is Knockout DMEM supplemented with 20% KOSR, NEAA, 2-ME, GlutaMAX, 6 ng/ml basic fibroblast growth factor (all Invitrogen). hESC lines WA09 (H9) and WA01 (H1) (WiCell) were cultured on irradiated MEF feeder layers in ES medium. This study was done in accordance with Johns Hopkins ESCRO regulations and following a protocol approved by the Johns Hopkins IRB.
Retroviral production and Reprogramming of hepatocytes
Retroviruses for the four factors were independently produced after co-transfecting the 293T cell line in 150mm dishes with pMX retroviral vectors expressing Oct4, Sox2, Klf4 or c-Myc (Addgene) and helper plasmids as previously described.13 Viral supernatant was concentrated for ~100 fold by Amicon Ultra Centrifugal Filters (Millipore). Primary hepatocytes were seeded in 6-well tissue culture plates at density of 105 cells/well and cultured for two days before infection. A 1:1:1:1 mix of retroviruses containing Oct4, Sox2, Klf4 and c-Myc was filtered through 0.45 µm cellulose acetate filter before added to hepatocytes (passages 1) at Multiplicity of Infection (MOI) of 10 in the presence of 6 ng/ml polybrene.”
After incubating for 3 days in the WEM, media was replaced with hES medium. After transformed colonies were observed in the reprogramming plates, a reliable pluripotent stem cell marker26, TRA-1-60 antibody (1:200 dilution, Millipore) and Alexa555 conjugated anti-mouse IgM antibody (1:500 dilution, Invitrogen) were added into live cell culture (without fixation) and incubated for 1 hour at 37C, to distinguish the iPS from non-iPS colonies. TRA-1-60 positive colonies appeared in about 6 to 9 days after retroviral transduction, and individual TRA-1-60 positive colonies were picked onto MEF coated plates.
Immunofluorescence and FACS analysis
Cells were fixed with 4% paraformaldehyde. The following antibodies were used: TRA-1-60 (Millipore, 1:100); SSEA-4 (Cell Signaling, 1:200), SSEA-3 (Millipore, 1:200); Tuj1 (Covance, 1:500), α-fetoprotein (Dako, 1:200), SMA (DAKO, 1:100), OCT-4 (Millipore, 1:100), NANOG (BD, 1:200), anti-SSEA-3 488 from eBiosciences; CXCR4 (BioLegend, 1:100), albumin (DAKO, 1:200), AAT (Thermo, 1:200), CyP3A4 (Enzo, 1:200). Secondary antibodies used were all of the Alexa Fluor Series from Invitrogen.
Embryoid body formation and spontaneous differentiation into three germ layer cells
Human ES cells or iPS cells were dissociated by collagenase IV digestion and plated in ultra low attachment plates (Corning) at the density of ~ 1×106 cells/well in the presence of differentiation medium (DMEM supplemented with 20% FBS, L-glutamine, β-mercaptoethanol, and Non-essential amino acids). 50% of the medium was replaced with fresh medium every 2 days. After 7 days the embryoid bodies (EBs) were transferred to 0.1% gelatin-coated culture dishes and cultured for additional 2–3 days before fixation and staining. Antibodies against Tuj1 (Covance, 1:500), α-fetoprotein (Dako, 1:200), or SMA (DAKO, 1:100) were used to detect the spontaneously differentiated cells from EBs.
Teratoma formation
Ten-week-old male NOD/SCID/Il2γC−/− mice (Jackson Laboratories) were anesthetized and ~1 million hHiPS cells, resuspended in 20–40 µl of 50% matrigel, were injected subcutaneously. Mice were euthanized 12 weeks after cell injection and tumors were analyzed following H&E protocol. All animal experiments were conducted following experimental protocols previously approved by Johns Hopkins IACUC.
Differentiation of hES/iPS cells to definitive endoderm
Sixty percent confluent cultures were placed in RPMI medium (supplemented with GlutaMAX and 0.5% defined FBS or 1% KOSR, and 100ng/ml Activin A (R&D Systems) for 5 days. The medium was replaced every other day.
Differentiation of definitive endoderm to hepatic progenitor cells
Day 5 endoderm cultures were passaged with 0.05% trypsin-0.53 mM EDTA and plated on collagen I-coated dishes, in RPMI medium supplemented with GlutaMAX and 2% KOSR, 10 ng/ml FGF-4, and 10 ng/ml HGF. Three days later the cells were switched to minimal MDBK-MM medium (Sigma) supplemented with GlutaMAX and 0.5 mg/ml BSA, 10 ng/ml FGF4, and 10 ng/ml HGF. Day 10 hepatic progenitors were utilized for experiments.
Differentiation of hepatic progenitor cells to mature hepatocytes
Hepatic progenitor cells were switched to complete hepatocyte culture medium (HCM) supplemented with SingleQuots (Lonza) and containing 10 ng/ml FGF-4, 10 ng/ml HGF, 10 ng/ml oncostatin M (R&D Systems), and 10−7 M dexamethasone (Sigma-Aldrich). Differentiation was continued for another 10 days.
Periodic acid-schiff assay for glycogen
Cells were fixed with 4% PFA and stained using a periodic acid-Schiff (PAS) staining system (DAKO). Cells were counterstained using Hematoxylin-QS and mounted with Vectamount AQ (all Vector Laboratories).
Cytochrome P450 Assay
CYP1A2 and CYP3A4 activity was assessed using the pGlo kit (catalog numbers V8771, V8901; Promega) according to manufacturer’s instruction for nonlytic CYP450 activity estimation. hHiPS cell-derived mature hepatocytes were incubated with hepatocyte culture medium supplemented with CYP3A4 or CYP1A2 pGlo substrates. At 4 hours after treatment, 50 uL of culture medium was removed and read on a luminometer (GLOMAX, model 9101-002). CYP activities are expressed as relative light units (RLU/mL) per of media, normalized against percentage of albumin expressing hepatocyte-like cells.
Results
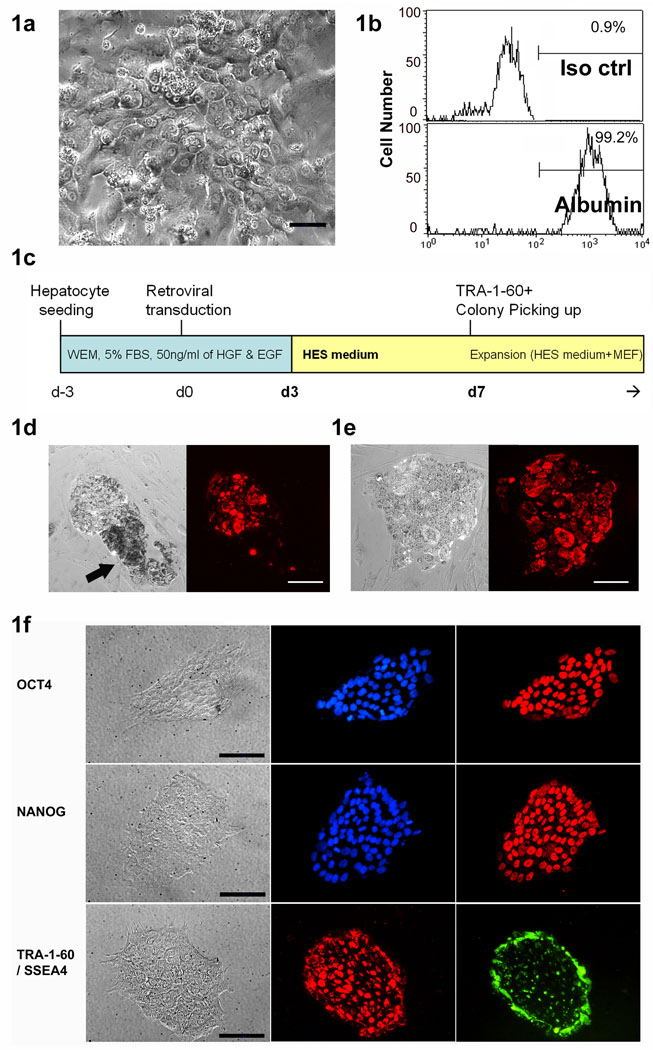
We have previously demonstrated successful generation of human iPS cells from blood cells by retroviral transduction of transcription factors.13 For generation of human endoderm origin iPS cells, human primary hepatocytes were seeded in Williams’ Medium E (WEM) with human HGF and EGF (Fig. 1a). These hepatocytes were over 99% albumin positive before reprogramming (Fig. 1b). Of note, under these conditions, primary human hepatocytes only survive for a short term (about 7 to 10 days) and did not proliferate even with HGF and EGF. After hepatocyte retroviral transduction with Oct4, Sox2, Klf4 and c-Myc (Fig 1c), several hundred (i.e. 100–500) fast-growing small colonies per 105 hepatocytes were observed. By days 6–9 post-infection, at least 30% of these colonies displayed typical human ES cell–like morphology and TRA-1-60 staining positive (Fig. 1d and e). TRA-1-60 antibody live staining was performed and TRA-1-60 positive colonies were picked and seeded onto a layer of irradiated mouse embryonic fibroblasts (MEFs) in hES cell medium.
Figure 1. Human hepatocyte derived iPS (hHiPS) cell colony formation and characterization.
(a) Phase contrast image and (b) albumin expression of primary human hepatocytes before iPS reprogramming. ×200, Scale bars: 50 µm. (c) A diagram of hHiPS generation protocol. (d) Typical example of a small ES cell–like TRA-1-60 (red) positive colony adjacent to a TRA-1-60 negative non-iPS colony (arrow). (e) Typical ES cell–like TRA-1-60 positive iPS cell colony before harvest. ×100, Scale bars: 100 µm. (f) Representative immunofluorescence analysis of one hHiPS cell line (hHiPS10) growing on Matrigel. Clear expression of the ES cell surface antigens SSEA4 and TRA-1-60, the nuclear transcription factors OCT4 and NANOG are observed (×200). Scale bars: 50 µm.
Some of these colonies showed a mosaic pattern of TRA-1-60+ and TRA-1-60− cells (Fig. 1d, arrow indicates a TRA-1-60 negative non-iPS colony adjacent to a TRA-1-60 positive iPS colony). These TRA-1-60 negative colonies were usually darker, thicker and proliferating faster than TRA-1-60 positive colonies during initial days (up to the first 14 days). However the majority of these non-iPS clones were not able to survive for a long time (disappeared within a few passages, data not shown) and in most of cases these non-iPS colonies were easily distinguishable by morphology compared to hES cell–like iPS colonies.
Seventeen (17) TRA-1-60 positive single colonies were picked and passaged onto fresh feeder cells. Most of these subcultures (16 out of 17) expanded and gave rise to stable cell lines with hES cell–like morphology. All the lines could be maintained by standard hES cell culture procedures on both feeder cells and feeder-free matrigel-coated plates (examples are shown in Fig. S1a). When fingerprinting was performed to amplify the microsatellite sequences by PCR, all these iPS cells displayed identical pattern to that of original primary hepatocytes (Fig. S1b). Four human hepatocyte derived iPS (hHiPS) cell lines (hHiPS6, hHiPS10, hHiPS11, and hHiPS14) were selected for further analyses. These lines have been maintained in continuous culture for over 8 months without signs of replicative or karyotypic crisis (Fig. S1c). All hHiPS cells expressed transcriptional regulators and cell-surface markers characteristic of hES cells, including NANOG, OCT4, SSEA4, and TRA-1-60 (Fig. 1f for hHiPS10, and Fig. S2 for hHiPS6, 11 and 14). These cell lines also showed complete loss of hepatocyte-specific markers such as albumin (data not shown). Overall, the expression of stem cell markers in hHiPS cells was indistinguishable from hES cell lines and maintained under the same conditions.
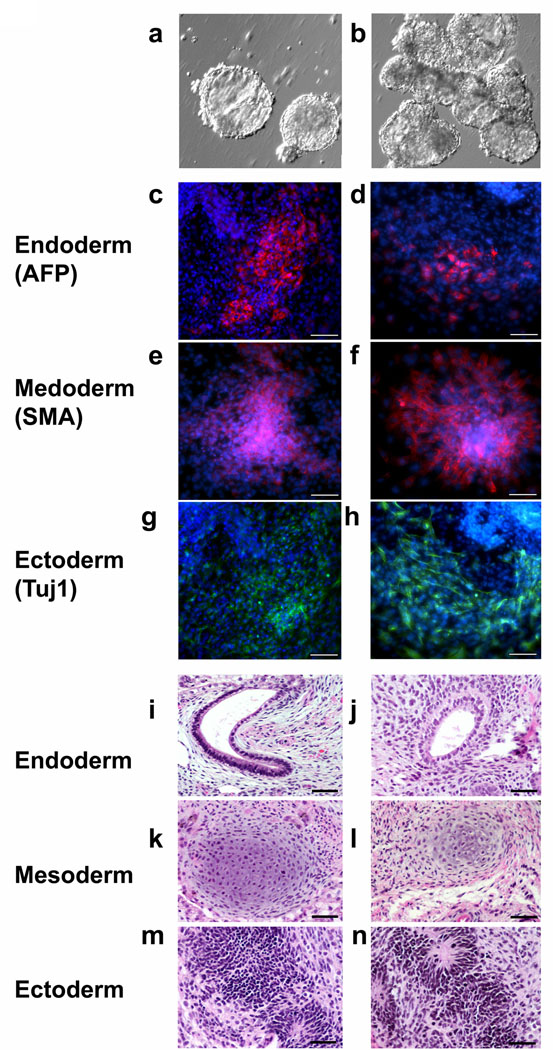
We tested the pluripotency of hHiPS cell lines in assays of embryoid body (EB) formation in vitro and teratoma induction in vivo. All cell lines tested readily differentiated in vitro into EBs (Fig 2a,b) followed by endoderm, mesoderm and ectoderm derivatives that stained positive for α-fetoprotein (AFP), smooth muscle actin (SMA) and TuJ1 immunoreactivity, respectively (Fig. 2c–h). About three months after injection into immunocompromised mice, independent hHiPS cell lines generated complex teratomas (Fig. 2i–n). Structures resembling all three germ layer tissues such as glandular epithelia (endoderm, Fig. 2i and 2j), immature cartilages (mesoderm, Fig. 2k and 2l) and neural rosettes (ectoderm, Fig 2m and 2n) were observed, indicating the pluripotency of these hHiPS cell lines.
Figure 2. hHiPS cells can differentiate into all three primary germ layers in vitro and in vivo.
(a, b) Embryoid bodies derived from hHiPS 10, and 11 lines, respectively. (c–h) In vitro differentiation of hHiPS cells into all three primary germ cell layers. After generation of embryoid bodies hHiPS cells spontaneously differentiated into endoderm (c, d) (α-fetoprotein-positive, red), mesoderm (e, f) (smooth muscle actin-positive, red) and ectoderm (g, h) (TuJ1-positive neuronal cells, green). c, e, g: hHiPS10 and d, f, h: hHiPS11. Blue nuclear staining is DAPI. ×100, Scale bars, 100 µm. (i–n) Spontaneous differentiation into all three germ layers is evident in teratomas. (i, j) endoderm, (k, l) mesoderm, (m, n) ectoderm derived from hHiPS10 and hHiPS14. ×200, Scale bars, 50 µm.
Taken together, these results demonstrated that human endoderm cells (i.e. hepatocytes), like other germ layer derived cells (i.e. fibroblasts or blood cells), can be reprogrammed to pluripotency. Morphologically, nascent hHiPS cell colonies could be identified as early as 6 day post-infection. In contrast, colonies of iPS cells generated by retroviral transduction of human mesenchymal stem cells (MSCs) under the similar experimental condition only appear after day 10 post-infection (Fig. S3) similar to that of blood cells.13 We observed about 50–100 hHiPS cell colonies from ~50,000 viable infected hepatocytes, based on morphological criteria and TRA-1-60 staining, representing an overall reprogramming efficiency close to 0.1–0.2% (n = 3). No significant differences of overall efficiency were observed in the generation of iPS cells from multiple independent human primary hepatocyte cultures.
Several recent reports have shown that human fibroblast (mesoderm origin) derived iPS cells could be directed to hepatocytes just like ES cells.14, 23 We tested whether the endodermal origin iPS cells can also be efficiently directed into hepatic cells. We utilized a hepatic differentiation protocol established for hES cells,24 with a slight modification. Our hepatic differentiation protocol is composed of three stages: definitive endoderm induction for 5 days (day 5), hepatic progenitor induction and expansion for another 5 days (day 10) and hepatic maturation for another 10 days (day 20). Cells from these three stages (day 5, day 10, and day 20) were chosen because we observed these time frames are most distinct in terms of their marker expression profile i.e. CXCR4, AFP, or ALB positivity based previously for ES cells (data not shown). With this protocol, hES cells, hHiPS cells and MSC derived iPS (iMSC) cells were able to differentiate into all three stage hepatic cells (Fig 3, 4 and S5).
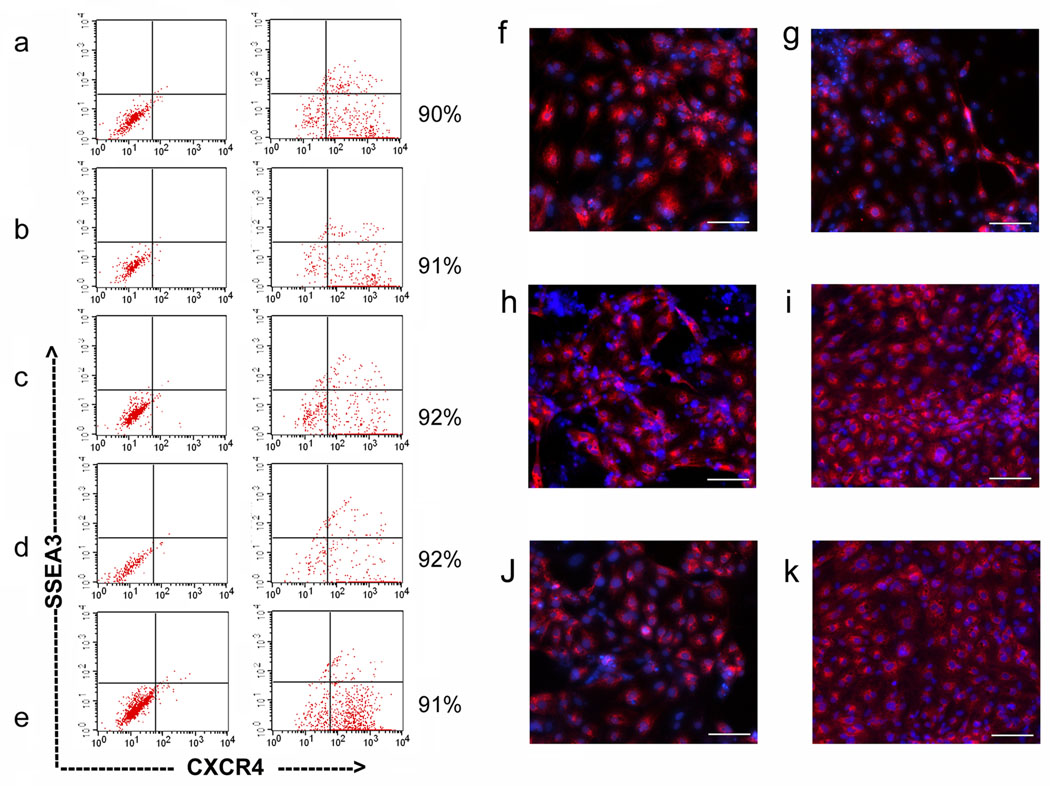
Figure 3. Differentiation of hHiPS cells into DE and hepatic progenitors.
Efficient endoderm induction of human iPS cells and ES cells in the presence of 100 ng/ml Activin A. The FACS analysis showed that at least 90% of the induced cells expressed the definitive endoderm marker CXCR4. H9 (a), H1 (b), and hHiPS6, 10, 11 (c, d, e) at 5 days post-initial Activin A treatment. Immunofluorescence analysis of the expression of AFP in differentiating cells at 10 days post-initial Activin A treatment (f-k, H1, H9, hHiPS6, 10, 11, and 14, respectively). ×100. Scale bars, 100 µm.
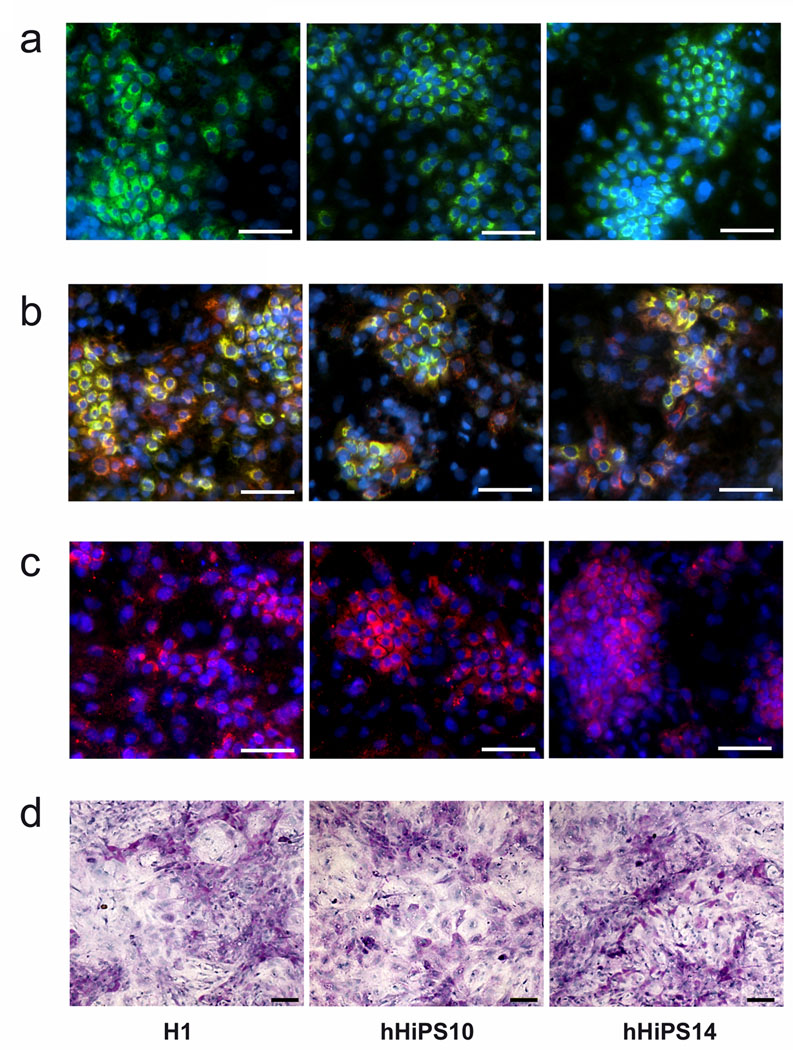
Figure 4. Differentiation of hHiPS cells into mature hepatocytes.
The differentiated cells possessed proteins indicative of functional hepatocytes, including ALB, green (a), ALB (green) + AAT (red) (b), and CYP3A4 (pink) (c) at day 20 after differentiation initiation. ×200, Scale bars, 100 µm. (d) Glycogen storage at day 20. Periodic acid-Schiff assay was performed on differentiating cells at 20 days post-initial Activin A treatment. Nuclei were counterstained with hematoxylin (blue). Glycogen storage is indicated by pink or dark red cytoplasm. ×100, Scale bar = 100 µm.
In the first stage, Activin A efficiently induced the endoderm differentiation of hES and iPS cell lines. After 5 days of Activin A treatment, approximately 90% of the cells in culture expressed the endoderm marker CXCR4 and lost the ES/iPS cell marker SSEA3 (Fig 3a–e, S5a). To induce early hepatic cells or hepatic progenitor cells, hepatocyte growth factor (HGF) and fibroblast growth factor 4 (FGF4) were used. We tested for the expression of AFP, a marker of early hepatic cells, in the differentiated hES cells and hHiPS cells. Approximately 90% of cells were positively stained for AFP in both ES- and iPS derived cells at day 10 (Fig 3f, S5b). No obvious differences were observed between three types of pluripotent stem cells (i.e. hES, hHiPS, and iMSC cells).
At the end of the final differentiation stage, we tested the expression of hepatic markers in the differentiated ES and iPS cells. A majority of the day-20 cells expressed mature hepatic markers including albumin (Fig 4a, S5c), alpha 1-antitrypsin (Fig 4b, S5c) and CYP3A4 (Fig 4c). The efficiency and pattern of iPS cell derived hepatocytes were similar to hES cell derivatives.
We further tested the ability of the differentiating cell populations to store glycogen, another characteristic of functional hepatocytes.24 Cells at day 20 were stained for cytoplasmic glycogen using the Periodic Acid-Schiff staining procedure (Fig 4d). Similar to hES cell-derived cells, the majority of the differentiated hHiPS cells (up to 90%) were stained by PAS (Fig 4d), indicating that they had the capacity to store glycogen. Hence, consistent with the gain of expression of mature hepatic markers, the differentiating cells from hHiPS cells also exhibit a gain of hepatic functionality.
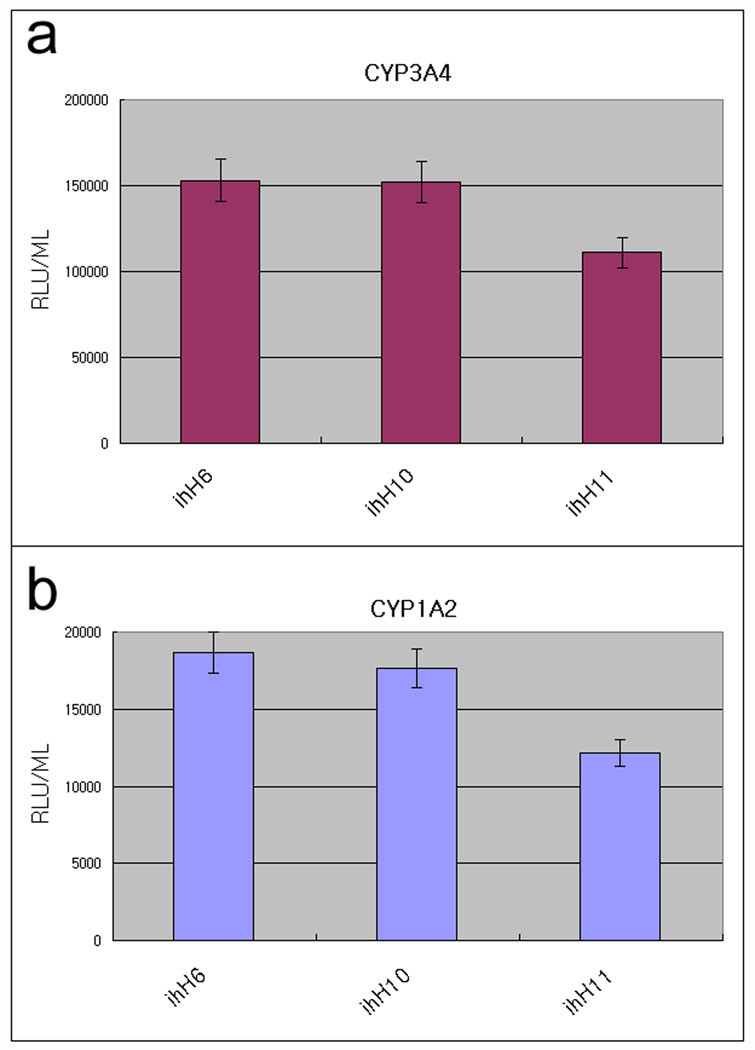
In order to further validate the functionality, multiple hHiPS cell line derived hepatocytes were assessed for their metabolic capabilities (Fig 5). The cytochrome P450 enzymes are critical in drug metabolism and CYP3A4 and CYP1A2 are the key enzymes.14 All three tested hHiPS cell lines (ihH6, 10, and 11) exhibited both CYP450 enzyme activities as assessed by the generation of luminescent metabolites (Fig 5a, b). We observed only slight variation with in these lines. These results suggest that human hepatocyte derived iPS cells are able to differentiate into functional hepatocytes which can be utilized in drug testing and disease modeling.
Figure 5. CYP450 metabolism in hHiPS cell derived mature hepatocytes.
hHiPS cell derived mature hepatocytes display cytochrome P450 metabolism. iPS cell-derived hepatocytes were incubated with hepatocyte culture medium supplemented with CYP3A4 or CYP1A2 pGlo substrates (Promega) as per manufacturer’s instructions. At 4 hours after treatment, 50 uL of culture medium was removed and read on a luminometer (GLOMAX). CYP1A2 and CYP3A4 activity is expressed as relative light units (RLU)/ml of culture medium (n=6). All three hHiPS cell lines (ihH6, 10, 11) exhibited both CYP450 enzyme activities.
Discussion
Here we demonstrate that human endoderm cells (i.e. hepatocytes) can be rapidly reprogrammed to pluripotency. Since iPS cells may retain certain gene expression and/or epigenetic features of their original cell types1–5 and the origin of iPS cells has profound influence on the tumor forming propensity,3 it is important to generate and compare human iPS cells from different origins including endoderm derived cells and systematically compare their safety and differentiation potentials. For the purpose of developing disease models from patient-specific iPS cells to study liver disease mechanisms, it is also crucial to generate hepatocytes-derived iPS cells because certain somatic mutations which play important roles in liver disease progression are acquired only in liver cells and therefore the liver disease features can not be recapitulated by skin fibroblast-derived iPS cells derived from same patients.16–20
Although human hepatocyte reprogramming shares the main features reported for iPS cell generation from other cell types such as fibroblasts, including acquisition of self-renewal ability and pluripotency (Fig 1, 2), the overall pace appears to be faster than that of fibroblast, MSC, or blood cell reprogramming.9–13 Mouse hepatocytes also appear to be more easily reprogrammed than fibroblasts.7 It is conceivable that hepatocytes per se are more amenable to reprogramming, perhaps because, unlike fibroblasts or MSCs, they would not be required to undergo a mesenchymal-to-epithelial transition to give rise to iPS cells. However, we can not rule out the possibility that iPS colonies appear faster because in the case of hepatocytes no cell replating during the early stage of reprogramming is necessary as result of their non-proliferative nature in culture, whereas proliferating MSCs or fibroblasts require culture splitting during the early reprogramming (Fig S3) and thus it takes longer for colonies to emerge.
It also remains to be determined whether the cells that undergo reprogramming are early or mature hepatocytes. Although our hepatocyte source is homogenously albumin positive (see Fig 1b), we also observed that 20–30% of these cells are also AFP positive (Fig S4). Therefore it is possible that the reprogrammed cells are from only the AFP+ALB+ early hepatocytes. It is currently difficult to definitively determine the target cell type because it would require viable cell sorting of the primary hepatocytes but AFP and ALB are the cytoplasmic markers (not surface proteins for viable cell sorting). Moreover, primary hepatocytes would unlikely be healthy enough for reprogramming after the cell sorting and replating processes, considering the fact that primary hepatocytes even without sorting stress can only survive a week in culture.
We also show that human hepatocyte derived iPS (hHiPS) cells can be directly induced to differentiate into endoderm, hepatic progenitors, and mature hepatocytes using a stepwise differentiation method (Fig 3 and 4). The hepatic differentiation efficiency of hHiPS cells is comparable to that of the human ES cell lines. We also observed a similar hepatic differentiation from bone marrow (mesoderm) derived iPS cells (iMSC) in terms of marker expressions based upon immunofluorescence staining (Fig S5). We could not detect a significant enhancement in efficiency of hHiPS cells in either spontaneous (Fig 2) or directed differentiation (Fig 3 and 4) processes. This is not surprising because the differentiation efficiency is already very high in hES cells (more than 90%). A more stringent test of the differentiation ability would be in vivo functionality of the differentiated cells. Significant future studies should examine whether the hepatocytes from hHiPS cells offer any advantages in the in vivo assays over differentiated cells from hES cells or from iPS cells of other origins. Comprehensive studies comparing human iPS cells from all three developmentally distinct germ layers are needed to determine whether the cell origin for reprogramming has a critical influence on functionality or safety of differentiated cells.
An important potential use of human iPS cell-derived hepatic cells is for drug development. Most drugs rely on liver cytochrome P450 activity for detoxification, which cannot be tested in animal liver cells due to species differences.25 In this study, we looked for the expression of CYP in the differentiated cells and found that the hHiPS cell derived hepatic cells expressed Cyp3A4, as detected by immune staining (Fig 4c) in addition to fully differentiated hepatic markers, ALB and AAT expression (Fig. 4a and 4b). The functionality was also confirmed by glycogen storage activity (Fig 4d) and CYP metabolic activities (Fig 5). These data suggest that human iPS cell-derived hepatic cells may be used as a potential cell source for the generation of hepatocytes for drug metabolism analysis.
In conclusion, we have reprogrammed human endoderm derived cells into iPS cells and shown that they can be directly differentiated into hepatic cells. Our reprogramming of primary hepatocytes to pluripotency should provide a valuable experimental model for investigating the bases of cellular reprogramming of other human endoderm cells. Equally important is the generation of liver disease specific iPS cells for studying liver disease pathogenesis, including hepatocellular carcinoma and liver cirrhosis, utilizing available liver tissues which can be obtained after partial hepatectomy or diagnostic liver biopsy. Human hepatocyte derived iPS cells offer the advantages for modeling certain liver diseases associated with acquired somatic mutations that are restricted to liver cells.16–20 The generation of endoderm derived human iPS cells also facilitates the comparative studies to determine the most suitable iPS cells in terms of safety and efficacy for treating particular diseases. We believe these studies would lead to better understanding of pluripotency and reprogramming and would eventually lead to a safer and more potent cell therapy.
Supplementary Material
Acknowledgement
This work was supported in part by NIH grant DK O70971 and Maryland Stem Cell Research Fund grants # 90034362 and #90034927.
References
- 1.Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchetto MCN, Yeo GW, Kainohana O, Marsala M, Gage FH, Muotri AR. Transcriptional Signature and Memory Retention of Human-Induced Pluripotent Stem Cells. PLoS ONE. 2009;4:e7076. doi: 10.1371/journal.pone.0007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 4.Deng J, Shoemaker R, Xie B, Gore A, LeProust EM, Antosiewicz-Bourget J, et al. Targeted bisulfite sequencing reveals changes in DNA methylation associated with nuclear reprogramming. Nat Biotechnol. 2009;27:353–360. doi: 10.1038/nbt.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human iPSCs, embryonic stem cells and fibroblasts. Nat Genet. 2009;41:1350–1353. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 8.Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R, et al. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat. Biotechnol. 2008;26:916–924. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 11.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. USA. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 13.Ye Z, Zhan H, Mali P, Dowey S, Williams DM, Jang YY, et al. Human induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114:5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2009 doi: 10.1002/hep.23335. Published Online: 29 Sep 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha K, Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5:584–595. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006;25:3778–3786. doi: 10.1038/sj.onc.1209547. [DOI] [PubMed] [Google Scholar]
- 18.Bluteau O, Jeannot E, Bioulac-Sage P, Marqués JM, Blanc JF, Bui H, et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312–315. doi: 10.1038/ng1001. [DOI] [PubMed] [Google Scholar]
- 19.Wong CM, Fan ST, Ng IO. Beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–145. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka S, Toh Y, Adachi E, Matsumata T, Mori R, Sugimachi K. Tumor progression in hepatocellular carcinoma may be mediated by p53 mutation. Cancer Res. 1993;53:2884–2887. [PubMed] [Google Scholar]
- 21.Kim JB, Greber B, Araúzo-Bravo MJ, Meyer J, Park KI, Zaehres H, Schöler HR. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–653. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 22.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 23.Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 25.Tuschl G, Lauer B, Mueller SO. Primary hepatocytes as a model to analyze species-specific toxicity and drug metabolism. Expert Opin Drug Metab Toxicol. 2008;4:855–870. doi: 10.1517/17425255.4.7.855. [DOI] [PubMed] [Google Scholar]
- 26.Chan EM, Ratanasirintrawoot S, Park IH, Manos PD, Loh YH, Huo H, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.