Abstract
Objective
To test the hypothesis that rapamycin inhibits induced microvascular hyperpermeability directly in vivo.
Methods
Male golden Syrian hamsters (80–120g) were treated with either rapamycin (at 0.1, 0.5, 2, and 10 mg/kg i.p.) or vehicle at 24 hours and at one hour prior to preparation of the cheek pouch. Caveolin-1 scaffolding (1 mg/kg; positive inhibitory control) was injected i.p. 24 hours prior to the experiment. 10−8 M vascular endothelial growth factor (VEGF) or 10−7 M platelet-activating factor (PAF) were topically applied to the cheek pouch. Microvascular permeability and arteriolar diameter were assessed using integrated optical intensity (IOI) and vascular wall imaging, respectively.
Results
Rapamycin at 0.1 mg/kg and 0.5 mg/kg significantly reduced VEGF-stimulated mean IOI from 63.0±4.2 to 9.7±5.0 (85% reduction, P<0.001) and 3.6±2.7 (95% reduction, P<0.001), respectively. Rapamycin at 2 mg/kg also lowered VEGF-stimulated hyperpermeability (40% reduction, P<0.05). However, 10 mg/kg rapamycin increased VEGF-induced microvascular hyperpermeability. Rapamycin at 0.5 mg/kg attenuated VEGF-induced vasodilation and PAF-induced hyperpermeability, but did not inhibit PAF-induced vasoconstriction.
Conclusions
At therapeutically relevant concentrations, rapamycin inhibits VEGF- and PAF-induced microvascular permeability. This inhibition is 1) a direct effect on the endothelial barrier, and 2) independent of arteriolar vasodilation. Rapamycin at 10 mg/kg stimulates effectors that increase microvascular permeability.
Keywords: Rapamycin, VEGF, PAF, microvascular permeability, arteriolar diameter, mTOR
INTRODUCTION
Rapamycin is a potent inhibitor of endothelial cell and fibroblast proliferation (3, 28). It currently has broad clinical utility, both as a systemic agent and via local delivery to coronary vascular endothelium. It is also being tested in the clinic for efficacy in exudative macular degeneration and diabetic retinopathy, conditions where endothelial cell pathophysiology plays an important role in vision loss (6, 9, 43). Clinical benefits associated with the use of rapamycin are likely due, in part, to the fact that it impacts multiple steps in the angiogenic cascade, including inhibition of endothelial cell responses to pro-angiogenic factors (3, 28).
Vascular endothelial growth factor (VEGF) is a key angiogenic factor involved in a wide variety of biological processes including embryonic development, wound healing, tumor progression, and metastasis (35). VEGF has also emerged as a major mediator of intraocular neovascularization and plays a key role in the etiology of diabetic retinopathy (14), as indicated by the elevated intraocular VEGF levels in diabetic patients suffering from proliferative retinopathy (19). Whether rapamycin inhibits the hyperpermeability associated with VEGF upregulation and angiogenesis is not known. We investigated whether or not rapamycin reduces microvascular permeability under conditions of agonist-induced hyperpermeability. We used the hamster cheek pouch as a model as this tissue is well suited for intravital microscopic evaluation of the microcirculation.
We tested the hypothesis that rapamycin inhibits microvascular permeability directly in vivo. To investigate this hypothesis it was necessary to assess the influence of rapamycin on microvascular flow. To evaluate differentially the impact of rapamycin on microvascular flow and permeability we used VEGF, an agonist that increases both flow and permeability (1), and platelet-activating factor (PAF), an agent that causes vasoconstriction and enhances permeability to macromolecules (11, 12). Our results demonstrate that, at therapeutically relevant concentrations, rapamycin directly inhibits VEGF and PAF induced microvascular permeability.
MATERIALS AND METHODS
The experimental protocols for hamsters were approved by the UMDNJ-New Jersey Medical School’s Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guidelines for the Use of Animals.
Hamster cheek pouch preparation
A total of 42 male golden Syrian hamsters, weighing 80–120g and anesthetized with sodium pentobarbital (50 mg/kg, i.p.) were used in this study. Tracheotomy was performed to ensure clear airway passage. The right jugular vein was cannulated for the administration of supplemental doses of anesthetic. The left hamster cheek pouch was prepared for direct visual observation and intervention (11, 12, 27). After surgical preparation, the hamster was placed on the microscope’s stage. A 1-hour stabilization period ensued, during which the pouch was continuously suffused with a 37 °C bicarbonate buffer solution at a rate of 1 ml/min. The bicarbonate buffer solution was bubbled with a 95% N2 and 5% CO2 gas mixture to maintain the suffusate oxygen tension at approximately 10 mm Hg and pH at 7.35.
Microscopy
Observations were made with an Olympus BH microscope. The recording system comprised an Optronics TEC-470 TV camera (Optronics, Goleta, CA), a Sony monitor, and a MetaMorph image system (Universal Imaging Corporation, Downingtown, PA). This system allowed us to record directly from the microscope to the computer. The MetaMorph program was used for image processing.
Assessment of microvascular permeability
Fluorescein isothiocyanate-dextran 70 (FITC-Dx 70; Sigma Chemical Co., St. Louis, MO) served as a tracer for macromolecules. FITC-Dx 70 was administered intravenously as a 100 mg/kg bolus followed by continuous infusion (0.15 mg/kg/min) to maintain a steady plasma concentration. Microvascular transport was assessed by measuring integrated optical intensity (IOI) by computer-assisted digital image analysis (2, 4). Two or three fields were randomly selected in the cheek pouch and recorded on the MetaMorph image system before and after the application of VEGF or PAF in separate experiments. Each field included four to six postcapillary venules ranging from 15 to 25 μm in diameter, which were apart from large veins and were relatively free of capillaries. Images were acquired at 0, 3, 5, 10, 15, 20, 30, 40, 50 and 60 minutes after agonist administration.
Arteriolar diameter measurements
Arteriolar diameter was measured as the width of the epi-illuminated blood column using the MetaMorph image system. Two or three arterioles with diameter of 20–30 μm were studied per animal. Baseline diameter measurements were normalized to a value of one. For each vessel, the experimental diameter was expressed as a ratio of baseline diameter (relative luminal diameter). To compare diameter before and after an agonist application, diameters were measured at the same place in the arterioles of interest.
Rapamycin study groups
Rapamycin (Rapamune oral solution, sirolimus 1 mg/ml, Wyeth, Philadelphia, PA) was given intraperitoneally (i.p.) at 24-hour and at 1-hour prior to starting the experiment. We studied four different doses of rapamycin: 0.1 mg/kg, 0.5 mg/kg, 2 mg/kg, and 10 mg/kg. Rapamycin vehicle (0.5 ml solvent) was applied in the same manner as rapamycin. Because mammalian target of rapamycin (mTOR) interacts with phosphatidylinositol 3-kinase (PI3K)/Akt (13, 24, 34), an important signaling element in the endothelial nitric oxide synthase (eNOS) signaling cascade (8, 29), and because eNOS regulates VEGF-induced hyperpermeability (40), we also evaluated the effects of inhibiting eNOS in our experimental paradigm using intraperitoneal injection of Cavtratin (AP-Cav), a chimeric peptide based on a cellular internalization sequence (antenna pedia) fused to the caveolin-1 scaffolding domain (amino acids 82–101). AP-Cav efficiently enters endothelial cells and selectively inhibits acetylcholine-induced vasodilation and nitric oxide production (5), as well as PAF-induced hyperpermeability (25).
Dose selection for rapamycin was based on rapamycin’s potency in vitro and on dose ranges for systemic delivery in preclinical studies (44). A dose of 1.5 mg/kg/day intraperitoneal delivery showed efficacy in endothelial cell targets in hamsters, while intraperitoneal rapamycin at 2mg/kg/day was an effective dose in a mouse retinal neovascularization study (10, 22). In this study we explored a range of intraperitoneal doses of rapamycin, utilizing doses on the lower and higher ends of what has been used in animal experiments previously (10, 22, 33).
Experimental protocol
Twenty-four hours and one-hour prior to the onset of the experiments, animals received an intraperitoneal injection of either rapamycin (at the specified concentration) or rapamycin vehicle. This dosing regimen was selected so that the cheek pouch would have peak rapamycin tissue levels for each dose at the time of the assessment by intravital microscopy. (44). AP-Cav (generous gift from Dr. William C Sessa, Yale University School of Medicine, New Haven, CT, USA) was injected at 1 mg/kg i.p. 24-hour before the experiment. The dose of AP-Cav was chosen based on its efficacy to inhibit nitric oxide synthesis and PAF-induced hyperpermeability in murine models (5, 25). The volume of study drug injected varied from 10 μl to 1 ml; the injected volume did not induce changes in microvascular dynamics in the hamster cheek pouch. On the day of the experiment, after baseline data collection, VEGF (recombinant human VEGF165; R&D Systems, Minneapolis, MN) or PAF (1-o-alkyl-2-acetyl-sn-3-glycero-phosphoryl-choline; Sigma Chemical Co., St. Louis, MO) was applied topically to the hamster cheek pouch for 3 min via a side port into the suffusate bicarbonate buffer line to achieve the final concentration of 10−8 M, or 10−7 M, respectively. After agonist application, the experiment was continued for an additional 60-min period for the measurements of IOI and arteriolar diameter. The 10−8 M concentration of VEGF was selected because it produces an effective and near maximal increase in macromolecular permeability in vivo in the hamster cheek pouch coincident with vasodilation (1). 10−7 M PAF was selected because this concentration produces an effective and near maximal increase in macromolecular permeability while, in contrast to VEGF, causing vasoconstriction (11, 12). Each hamster received only one application of the test agonist to avoid complications associated with tachyphylaxis (16).
Statistical analysis
Because the baseline remained constant throughout the experiment at values ranging from 3 to 5 IOI units, the baseline was subtracted and the permeability data are presented as net IOI values. All data are presented as mean±SEM. Statistical analysis was performed using a one-way analysis of variance. Significant differences (accepted at P<0.05) were evaluated using the Student-Newman-Keuls test.
RESULTS
Rapamycin and Microvascular Permeability
The time course of the impact of pre-administration of rapamycin on VEGF-stimulated changes in FITC-Dx 70 extravasation (as indicated by IOI) is displayed in Figure 1A. Neither administration of rapamycin vehicle nor administration of rapamycin (all doses) altered IOI. Administration of 10−8 M VEGF significantly elevated IOI of FITC-Dx 70. Pre-treatment with rapamycin at 0.1 mg/kg, 0.5 mg/kg, or 2 mg/kg attenuated the increase in IOI induced by 10−8 M VEGF. In contrast, rapamycin at 10 mg/kg (the highest dose administered) enhanced VEGF-induced hyperpermeability.
Figure 1.
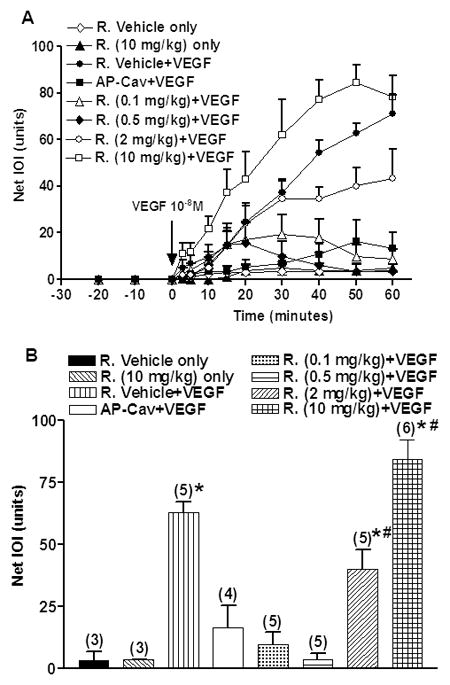
A) Time course of integrated optical intensity (IOI) of fluorescein isothiocyanate-dextran 70 (FITC-Dx 70). After baseline measurements, 10−8 M VEGF was applied at the time indicated by the arrow. Rapamycin or rapamycin vehicle was applied intraperitoneally at 24-hour and again 1-hour before VEGF application. AP-Cav (cavtratin) was applied to block eNOS. Data are mean±SEM. R.: Rapamycin.
B) Effect of Rapamycin on VEGF-induced increase in IOI of FITC-Dx 70 at 50-min after VEGF application. Maximal mean IOI responses are shown for the tested combinations. Data are mean±SEM. Numbers in parentheses show number of animals. *P<0.05 compared with rapamycin vehicle. # P<0.05 compared with rapamycin vehicle+VEGF.
Pretreatment with AP-Cav produced a strong inhibition of VEGF-induced hyperpermeability (Fig. 1A), as evidenced by a slow and small increase in IOI reaching a peak of 16.4±9.0 at 50 min after application of VEGF versus 63.0±4.2 IOI induced by VEGF alone. Figure 1A shows that for the first 5–15 minutes the rate of VEGF-induced increase in IOI in the presence of the lower doses of rapamycin (0.1–2 mg/kg) was comparable to the rate of VEGF-induced rise in IOI in the absence of rapamycin. Because of this delayed function, we analyzed the data at different key time points. The statistical analysis of rapamycin inhibition of VEGF-induced hyperpermeability performed on measurements obtained 50-min after application of VEGF is displayed in Figure 1B. Application of rapamycin at either 0.1 mg/kg or 0.5 mg/kg significantly reduced the VEGF-stimulated mean IOI from 63.0±4.2 to 9.7±5.0 (85% reduction, P<0.001) and 3.6±2.7 (95% reduction, P<0.001), respectively. Rapamycin at 2 mg/kg also lowered VEGF-induced mean IOI to 40.2±7.7 (40% reduction compared to VEGF alone), and this value was significantly different from 10−8 M VEGF (63.0±4.2, P<0.05). 10 mg/kg rapamycin increased VEGF-induced elevation in IOI from 63.0±4.2 to 84.4±7.8 (P<0.05) while rapamycin at 10 mg/kg did not change basal IOI in the absence of VEGF.
Pretreatment with 1 mg/kg AP-Cav significantly reduced the VEGF-induced increase in FITC-Dx 70 IOI from 63.0±4.2 to 16.4±9.0, i.e., 75% reduction compared with VEGF alone (P<0.001; Fig. 1B). The statistical trend at 30-min, 40-min and 60-min after application of VEGF is similar to the trend at the 50-min time point.
The inhibition of VEGF-induced IOI elevation by rapamycin could indirectly result from inhibition of VEGF-induced vasodilation. Because our interest was to test in vivo whether or not rapamycin inhibits microvascular hyperpermeability, we also investigated the inhibitory efficacy of rapamycin on PAF-induced hyperpermeability. In contrast to VEGF, which induces vasodilation, PAF increases postcapillary permeability while eliciting arteriolar constriction in the hamster cheek pouch (11, 12). Thus we were able to compare the permeability inhibitory impact of rapamycin using a permeability-altering agent associated with vasodilation (VEGF) and a permeability-altering agent associated with vasoconstriction (PAF). We chose 0.5 mg/kg rapamycin as the test dose for this assessment because it was the most efficacious dose in blocking VEGF-induced hyperpermeability. Figure 2A shows the time course of the effect of 10−7 M PAF on permeability and the action of 0.5 mg/kg rapamycin on PAF-stimulated changes in FITC-Dx 70 extravasation as indicated by IOI. After a constant baseline IOI value, 10−7 M PAF significantly elevated IOI of FITC-Dx 70. The peak was reached at 20 min after the application of PAF. 0.5 mg/kg rapamycin attenuated the increase of IOI values induced by PAF at 10−7 M without influencing the time course.
Figure 2.
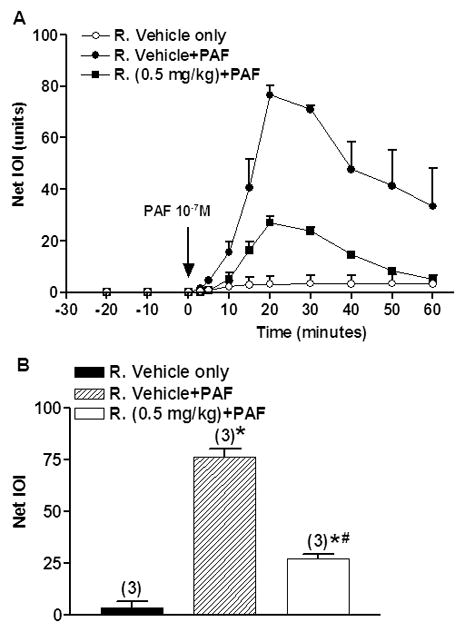
A) Time course of integrated optical intensity (IOI) of fluorescein isothiocyanate-dextran 70 (FITC-Dx 70). After baseline measurements, 10−7 M PAF was applied at the time indicated by the arrow. Rapamycin or rapamycin vehicle was applied intraperitoneally at 24-hour and again 1-hour before PAF application. Data are mean±SEM. R.: Rapamycin.
B) Effect of Rapamycin on PAF-induced increase in IOI of FITC-Dx 70 at 20-min after PAF application. Maximal mean IOI responses are shown. Data are mean±SEM. Numbers in parentheses show number of animals. *P<0.05 compared with rapamycin vehicle. # P<0.05 compared with rapamycin vehicle+PAF. R.: Rapamycin.
Figure 2B shows the statistical analysis of the inhibition of PAF-induced hyperpermeability by administration of 0.5 mg/kg rapamycin. The analysis was performed using the values obtained at 20-min after PAF application. Pretreatment with rapamycin at 0.5 mg/kg significantly reduced the PAF-induced increase in FITC-Dx 70 IOI from 76.5±3.7 to 27.1±2.3 (P<0.05), i.e., 65% reduction compared to PAF alone.
Rapamycin and Arteriolar Diameter
Figure 3A shows the effect of rapamycin on VEGF-stimulated vasodilation. The baseline values of arteriolar diameter did not change significantly as a function of time. The rapamycin vehicle did not cause any significant change of arteriolar diameter. Topical application of 10−8 M VEGF, for 3 min, produced a strong vasodilation. The ratio of experimental to baseline arteriolar diameter gradually increased and achieved its peak at 30 min after application of VEGF; the arteriolar diameter ratio increased from 1.0 to 1.46±0.09 (P<0.05). After the peak, the arteriolar diameter ratio gradually decreased and reached ~80% of baseline value at 50 min after application of VEGF. At 30-min after VEGF application, 0.5 mg/kg rapamycin attenuated the increase in relative luminal diameter induced by VEGF 10−8 M from 1.46±0.09 to 1.09±0.04 (P=0.02; Fig. 3B).
Figure 3.
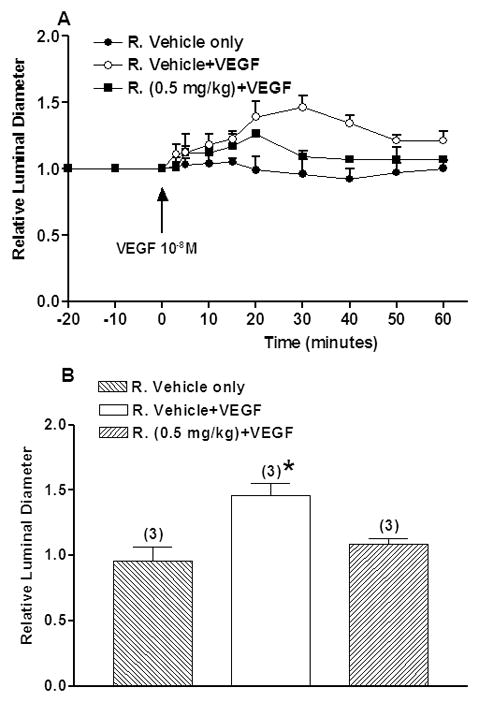
A) Time course of relative luminal diameter. VEGF at 10−8 M was applied at the time indicated by the arrow. Rapamycin or rapamycin vehicle was applied intraperitoneally at 24-hour and again 1-hour before VEGF application. Data are mean±SEM. R.: Rapamycin.
B) Effect of Rapamycin on VEGF-induced arterial vasodilation at 30-min after VEGF application. Maximal mean relative luminal diameter responses are shown. Data are mean±SEM. Numbers in parentheses show number of animals. *P<0.05 compared with rapamycin vehicle.
Figure 4 shows the effect of 0.5 mg/kg rapamycin on PAF-stimulated vasoconstriction. The baseline values of arteriolar diameter did not change significantly in a 30-min period. PAF, applied topically for 3 min at 10−7 M, produced a strong vasoconstriction. The ratio of experimental to baseline arteriolar diameter fell from 1.0 to 0.32±0.02 within 5 min after the topical application of PAF. On removal of PAF and reinstitution of suffusate flow, the arteriolar diameter ratio gradually increased and achieved ~90% of baseline value within 15 min. Pretreatment with 0.5 m g/kg rapamycin did not attenuate the vasoconstrictor action of PAF.
Figure 4.
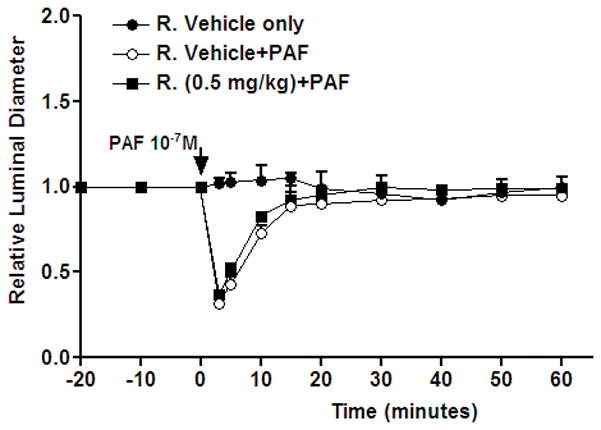
Time course of relative luminal diameter. 10−7M PAF was applied at the time indicated by the arrow. Rapamycin or rapamycin vehicle was applied intraperitoneally at 24-hour and again 1-hour before PAF application. Data are mean±SEM. R.: Rapamycin.
DISCUSSION
Our findings demonstrate that rapamycin has no direct effect on microvascular flow or permeability, and that it influences VEGF-induced microvascular permeability changes in a biphasic manner. At lower doses (0.1 mg/kg, 0.5 mg/kg and 2 mg/kg), rapamycin reduces VEGF-induced hyperpermeability. However, at the highest concentration evaluated (10 mg/kg), rapamycin increases VEGF-induced microvascular hyperpermeability. Importantly, the inhibitory action of rapamycin on postcapillary venular permeability is independent of its action on arterioles because at 0.5 mg/kg rapamycin inhibited PAF-stimulated hyperpermeability but did not inhibit PAF-induced vasoconstriction.
Originally identified as a bacterial macrolide with potent antifungal and immunosuppressive activities, rapamycin is a potent inhibitor of the protein kinase mTOR (mammalian target of rapamycin). This effect is mediated by the formation of a complex with the protein FKBP-12, which then binds and inhibits mTOR activity. mTOR is a key regulator of multiple cellular pathways, integrating input from upstream growth factors such as insulin, IGF-1, VEGF and EGF. mTOR also plays an important role as a sensor of cellular nutrient and energy status. Rapamycin has potent activities in animal models of allograft rejection, tumor growth, and tumor associated angiogenesis, with optimal therapeutic effects in the systemic dosing range of 0.1–3 mg/kg (20, 21, 35, 38). However, several studies have indicated that careful dosing of rapamycin is required. For example, immunosuppressive doses (1.5 mg/kg/day) of rapamycin have potent antiangiogenic activities and inhibit tumor growth in mice (21). However, a higher dose (15 mg/kg/day) while delaying tumor development, eventually led to rapid tumor growth and death of the animals (21), while an optimal anti-tumor effect was attained with low continuous dosing of rapamycin, not by attaining high peak concentrations (20). In a separate study, low dose oral rapamycin (0.1 mg/kg) inhibited myointimal hyperplasia in rabbits, whereas the high dose (0.4 mg/kg) was paradoxically less effective (45). In vitro studies have shown that rapamycin inhibition of VEGF stimulated endothelial proliferation and signaling occurs at concentrations as low as 0.1 ng/ml with complete inhibition at 1 ng/ml (21, 35). In the present study, we observed potent inhibitory effects of rapamycin on VEGF and PAF induced hyperpermeability at doses of rapamycin that fall in the range of those reported by others to be optimally effective in the inhibition of angiogenesis, allograft rejection and tumor growth. The opposite effect observed with high dose rapamycin (10 mg/kg) may represent an off-target effect involving different signaling pathways, or could potentially result from activation of an mTORC1 negative feedback loop - e.g. mTORC1 inhibition has been associated with Akt activation through upregulation of platelet derived growth factor receptors or insulin receptor substrate 1(7, 8, 23, 41), or an alteration in the balance of mTORC1/mTORC2 activities. Recent publications have demonstrated that mTOR signals through two distinct complexes, called mTORC1 and mTORC2. mTORC1 is composed of regulatory-associated protein of mTOR (RAPTOR), mLST8 and proline-rich Akt substrate of 40 kDA (PRAS40). mTORC1 function is regulated by the tuberous sclerosis complex 2, which associates with TSC1. TSC2 is a sensor of both PI3K-Akt and RAS-MAPK. The mTORC2 complex plays a role in sensing growth factors, but not nutrients, and this complex consists of mLTS8, rapamycin-insensitive companion of mTOR (RICTOR), SIN1 and protor. Rapamycin is thought to act primarily by inhibition of mTORC1, although at higher concentrations or prolonged incubations it may also inhibit mTORC2 activity (15, 17, 30, 31, 42). Whatever the mechanism of the paradoxical increase in vascular permeability observed with high dose rapamycin, this observation may be of clinical relevance. There have been several case reports of severe limb lymphedema in renal transplant patients treated with rapamycin, while eyelid edema and angioedema have also been observed following prolonged systemic treatment with rapamycin. (18, 32, 39, 46, 47)
Is the reported rapamycin inhibition of VEGF-induced changes in IOI a reflection of inhibition of microvascular permeability or is the inhibition of IOI a reflection of reduction in blood flow? To address this relevant question in vivo in a whole microvascular system, we compared the efficacy of rapamycin in inhibiting hyperpermeability induced by VEGF, an agonist that enhances vasodilation, and inhibition of hyperpermeability induced by PAF, an agonist that enhances vasoconstriction. PAF, especially at 10−7M, is a useful tool for assessing signaling pathways in the hamster cheek pouch microcirculation, because it allows for separation of events on the precapillary (vasoconstriction) and on the postcapillary (permeability) segments. We demonstrated previously that the signaling pathways for enhanced permeability stimulated by PAF involve protein tyrosine kinase (26), protein kinase C (36), phospholipase C (27), and nitric oxide synthase (36, 37, 40). In addition, we showed that the main pathway for PAF-induced vasoconstriction is through activation of phospholipase A2 and production of thromboxane A2 (13). In addition and importantly, we have demonstrated that PAF-induced hyperpermeability is regulated via eNOS (located downstream of PI3K/Akt; ref 36, 37, 40) as is VEGF-induced hyperpermeability (1, 29). Inasmuch as rapamycin blocked PAF-induced hyperpermeability without blocking its vasoconstrictor effect, our experimental data support the concept that rapamycin blockade of VEGF-induced hyperpermeability is independent of its ability to block vasodilation.
Our data provide a basis to better understand the microvascular mechanisms of action of rapamycin. In this context, we conclude 1) rapamycin at concentrations of 0.1 mg/kg, 0.5 mg/kg and 2 mg/kg reduces VEGF-induced hyperpermeability and vasodilation; 2) rapamycin at 10 mg/kg increases VEGF-induced microvascular hyperpermeability compared with VEGF alone; 3) 0.5 mg/kg rapamycin reduces PAF-stimulated hyperpermeability, but does not attenuate the vasoconstrictor action of PAF. Rapamycin may have clinical benefit in the treatment of diseases associated with vascular hyperpermeability. These data supports the clinical evaluation of rapamycin in conditions such as diabetic macular edema or retinal thickening secondary to leakage associated with choroidal neovascular membranes.
Acknowledgments
This work was supported by National Institutes of Health grant 2R01 HL070634 and a grant from MacuSight.
References
- 1.Aramoto H, Breslin JW, Pappas PJ, Hobson RW, II, Durán WN. Vascular endothelial growth factor stimulates differential signaling pathways in in vivo microcirculation. Am J Physiol. 2004;287:H1590–H1598. doi: 10.1152/ajpheart.00767.2003. [DOI] [PubMed] [Google Scholar]
- 2.Armenante PM, Kim D, Durán WN. Experimental determination of the linear correlation between in vivo TV fluorescence intensity and vascular tissue FITC-Dx concentration. Microvasc Res. 1991;42:198–208. doi: 10.1016/0026-2862(91)90087-r. [DOI] [PubMed] [Google Scholar]
- 3.Baldo P, Cecco S, Giacomin E, Lazzarini R, Ros B, Marastoni S. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr Cancer Drug Targets. 2008;8:647–665. doi: 10.2174/156800908786733513. [DOI] [PubMed] [Google Scholar]
- 4.Bekker AY, Ritter AB, Durán WN. Analysis of microvascular permeability by video-imaging digital processing. Microvasc Res. 1989;38:200–216. doi: 10.1016/0026-2862(89)90028-9. [DOI] [PubMed] [Google Scholar]
- 5.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nature Medicine. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19(6):442–455. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- 7.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cauwels A, Janssen B, Buys E, Sips P, Brouckaert P. Anaphylactic shock depends on Pi3K and eNOS-derived NO. J Clin Invest. 2006;116:2244–2251. doi: 10.1172/JCI25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68(8):1029–1036. doi: 10.2165/00003495-200868080-00002. [DOI] [PubMed] [Google Scholar]
- 10.Dejneka NS, Kuroki AM, Fosnot J, Tang W, Tolentino MJ, Bennett J. Systemic rapamycin inhibits retinal and choroidal neovascularization in mice. Mol Vis. 2004;10:964–972. [PubMed] [Google Scholar]
- 11.Dillon PK, Durán WN. The effect of platelet activating factor on microvascular permselectivity: Dose-response relationships and pathways of action in the hamster cheek pouch microcirculation. Circ Res. 1988;62:732–740. doi: 10.1161/01.res.62.4.732. [DOI] [PubMed] [Google Scholar]
- 12.Dillon PK, Ritter AB, Durán WN. Vasoconstrictor effects of platelet activating factor in the hamster cheek pouch microcirculation: Dose-response relationships and pathways of action. Circ Res. 1988;62:722–731. doi: 10.1161/01.res.62.4.722. [DOI] [PubMed] [Google Scholar]
- 13.Dowling RJ, Pollak M, Sonenberg N. Current status and challenges associated with targeting mTOR for cancer therapy. BioDrugs. 2009;23(2):77–91. doi: 10.2165/00063030-200923020-00002. [DOI] [PubMed] [Google Scholar]
- 14.Duh E, Aiello LP. Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes. 1999;48:1899–1906. doi: 10.2337/diabetes.48.10.1899. [DOI] [PubMed] [Google Scholar]
- 15.Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: signalling inputs, substrates and feedback mechanisms. Cell Signal. 2009;21:827–835. doi: 10.1016/j.cellsig.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Durán WN, Dillon PK. Acute microcirculatory effects of platelet activating factor. J Lipid Mediat. 1990;2:S215–S227. [PubMed] [Google Scholar]
- 17.Foster D, Toschi A. Targeting mTOR with rapamycin: One dose does not fit all. Cell Cycle. 2009;8(7):1026–1029. doi: 10.4161/cc.8.7.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs U, Zittermann A, Berthold HK, Tenderich G, Deyerling KW, Minami K, Koerfer R. Immunosuppressive therapy with everolimus can be associated with potentially life-threatening lingual angioedema. Transplantation. 2005;79:981–983. doi: 10.1097/00007890-200504270-00020. [DOI] [PubMed] [Google Scholar]
- 19.Funatsu H, Yamashita H, Noma H, Shimizu E, Yamashita T, Hori S. Stimulation and inhibition of angiogenesis in diabetic retinopathy. Jpn J Ophthalmol. 2001;45:577–584. doi: 10.1016/s0021-5155(01)00420-8. [DOI] [PubMed] [Google Scholar]
- 20.Guba M, Koehl GE, Neppl E, Doenecke A, Steinbauer M, Schlitt HJ, Jauch KW, Geissler EK. Dosing of rapamycin is critical to achieve an optimal antiangiogenic effect against cancer. Transpl Int. 2005;18:89–94. doi: 10.1111/j.1432-2277.2004.00026.x. [DOI] [PubMed] [Google Scholar]
- 21.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 22.Guba M, Yezhelyev M, Eichhorn M, Schmid G, Ischenko I, Papyan A, Graeb C, Seeliger H, Geissler E, Jauch K, Bruns C. Rapamycin induces tumor-specific thrombosis via tissue factor in the presence of VEGF. Blood. 2005;105:4463–4469. doi: 10.1182/blood-2004-09-3540. [DOI] [PubMed] [Google Scholar]
- 23.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends in Biochemical Sciences. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Hatakeyama T, Pappas PJ, Hobson RW, II, Boric MP, Sessa WC, Durán WN. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol. 2006;574(Pt 1):275–281. doi: 10.1113/jphysiol.2006.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim D, Durán WN. Platelet-activating factor stimulates protein tyrosine kinase in hamster cheek pouch microcirculation. Am J Physiol. 1995;268:H399–H403. doi: 10.1152/ajpheart.1995.268.1.H399. [DOI] [PubMed] [Google Scholar]
- 27.Kim DD, Ramirez MM, Durán WN. Platelet-activating factor modulates microvascular dynamics through phospholipase C in the hamster cheek pouch. Microvasc Res. 2000;59:7–13. doi: 10.1006/mvre.1999.2195. [DOI] [PubMed] [Google Scholar]
- 28.Konings IR, Verweij J, Wiemer EA, Sleijfer S. The applicability of mTOR inhibition in solid tumors. Curr Cancer Drug Targets. 2009;9:439–450. doi: 10.2174/156800909788166556. [DOI] [PubMed] [Google Scholar]
- 29.Lal BK, Varma S, Pappas PJ, Hobson RW, Durán WN. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric oxide synthase, and MAP kinase pathways. Microvasc Res. 2001;62:252–262. doi: 10.1006/mvre.2001.2338. [DOI] [PubMed] [Google Scholar]
- 30.Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21:656–664. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–2287. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohaupt MG, Vogt B, Frey FJ. Sirolimus-associated eyelid edema in kidney transplant recipients. Transplantation. 2001;72:162–164. doi: 10.1097/00007890-200107150-00031. [DOI] [PubMed] [Google Scholar]
- 33.Napoli KL, Taylor PJ. From Beach to Bedside: History of the development of sirolimus. Ther Drug Monit. 2001;23:559–586. doi: 10.1097/00007691-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10(2):159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramírez MM, Kim DD, Durán WN. Protein kinase C modulates microvascular permeability through nitric oxide synthase. Am J Physiol. 1996;271:H1702–H1705. doi: 10.1152/ajpheart.1996.271.4.H1702. [DOI] [PubMed] [Google Scholar]
- 37.Ramírez MM, Quardt SM, Kim D, Oshiro H, Minnicozzi M, Durán WN. Platelet activating factor modulates microvascular permeability through nitric oxide synthesis. Microvasc Res. 1995;50:223–234. doi: 10.1006/mvre.1995.1055. [DOI] [PubMed] [Google Scholar]
- 38.Rizell M, Lindner P. Inhibition of mTOR suppresses experimental liver tumours. Anticancer Res. 2005;25:789–793. [PubMed] [Google Scholar]
- 39.Romagnoli J, Citterio F, Nanni G, Tondolo V, Castagneto M. Severe limb lymphedema in sirolimus-treated patients. Transplant Proc. 2005;37:834–836. doi: 10.1016/j.transproceed.2004.12.180. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez FA, Rana R, Kim DD, Iwahashi T, Zheng R, Lal BK, Gordon DM, Meininger CJ, Durán WN. Internalization of eNOS and NO delivery to subcellular targets determine agonist-induced hyperpermeability. Proc Natl Acad Sci USA. 2009;106(16):6849–6853. doi: 10.1073/pnas.0812694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the SC/Rheb/mTOR/S6K cassette induces RS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 42.Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid - a competition with rapamycin. Mol Cell Biol. 2009;29:1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277(31):27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- 44.Trepanier DJ, Gallant H, Legatt D, Yatscoff R. Rapamycin: distribution, pharmacokinetics and therapeutic range investigations: an update. Clin Biochem. 1998;31(5):345–351. doi: 10.1016/s0009-9120(98)00048-4. [DOI] [PubMed] [Google Scholar]
- 45.Uchimura N, Perera GB, Fujitani RM, Tobis JM, Ishimaru S, Wilson SE, Gordon IL. Dose-dependent inhibition of myointimal hyperplasia by orally administered rapamycin. Ann Vasc Surg. 2004;18:172–177. doi: 10.1007/s10016-004-0010-0. [DOI] [PubMed] [Google Scholar]
- 46.van Onna M, Geerts A, Van Vlierberghe H, Berrevoet F, de Hemptinne B, Troisi R, Colle I. One-sided limb lymphedema in a liver transplant recipient receiving sirolimus. Acta Gastroenterol Belg. 2007;70:357–359. [PubMed] [Google Scholar]
- 47.Wadei H, Gruber SA, El-Amm JM, Garnick J, West MS, Granger DK, Sillix DH, Migdal SD, Haririan A. Sirolimus-induced angioedema. Am J Transplant. 2004;4:1002–1005. doi: 10.1111/j.1600-6143.2004.00429.x. [DOI] [PubMed] [Google Scholar]


