Abstract
Background and purpose:
Gallium (Ga) has been shown to be effective in the treatment of disorders associated with accelerated bone loss, including cancer-related hypercalcemia and Paget's disease. These clinical applications suggest that Ga could reduce bone resorption. However, few studies have studied the effects of Ga on osteoclastic resorption. Here, we have explored the effects of Ga on bone cells in vitro.
Experimental approach:
In different osteoclastic models [osteoclasts isolated from long bones of neonatal rabbits (RBC), murine RAW 264.7 cells and human CD14-positive cells], we have performed resorption activity tests, staining for tartrate resistant acid phosphatase (TRAP), real-time polymerase chain reaction analysis, viability and apoptotic assays. We also evaluated the effect of Ga on osteoblasts in terms of proliferation, viability and activity by using an osteoblastic cell line (MC3T3-E1) and primary mouse osteoblasts.
Key results:
Gallium dose-dependently (0–100 µM) inhibited the in vitro resorption activity of RBC and induced a significant decrease in the expression level of transcripts coding for osteoclastic markers in RAW 264.7 cells. Ga also dramatically reduced the formation of TRAP-positive multinucleated cells. Ga down-regulated in a dose-dependant manner the expression of the transcription factor NFATc1. However, Ga did not affect the viability or activity of primary and MC3T3-E1 osteoblasts.
Conclusions and implications:
Gallium exhibits a dose-dependent anti-osteoclastic effect by reducing in vitro osteoclastic resorption, differentiation and formation without negatively affecting osteoblasts. We provide evidence that this inhibitory mechanism involves down-regulation of NFATc1 expression, a master regulator of RANK-induced osteoclastic differentiation.
Keywords: gallium, osteoclast, bone resorption, osteoporosis, osteoblast
Introduction
Osteoclasts are multinucleated cells derived from haematopoietic precursors. They are crucially involved in the resorption of mineralized bone by secreting protons and proteolytic enzymes through their ruffled-border (Blair, 1998). Various bone disorders are characterized by an increased osteoclastic bone resorption that exceeds the bone formation by osteoblasts. This imbalance ultimately leads to skeletal fragility. Osteoporosis is probably one of the most frequent forms of such bone diseases. Osteoporosis has been defined as ‘a systemic disease characterized by low bone mass and micro architectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture’ (Consensus Development Conference, 1993). From 50 years of age onwards, 40% of women are osteoporotic and one in three women from the same age group will suffer osteoporotic fractures (Melton et al., 1992; Klotzbuecher et al., 2000; Lindsay et al., 2001). Eighty percent of these bone fractures affect the proximal femur, vertebral bodies and wrist, and result in prolonged hospitalization, a reduction in the patient's autonomy, a decrease in the quality of life and an increase in mortality rates (Ensrud et al., 2000; van Balen et al., 2001). With the increasing life expectancy of the general population, osteoporotic fractures will undoubtedly be a major public health concern in modern society. In light of these data, the prevention of osteoporotic fractures has become a great challenge, not only from a clinical perspective, but also from a social and economic point of view. To address this issue, a large variety of bone resorption inhibitors have been developed and successfully used to reduce the risk of osteoporosis-associated fractures (Orcel, 2005). Among these resorption inhibitors, bisphosphonates are today one of the preferred therapies for preventing and treating osteoporosis and the molecular mechanisms underlying the effects of these drugs have recently been clarified. Nitrogen-containing bisphosphonates such as alendronate, zoledronate and risedronate, inhibit enzymes of the mevalonate pathway involved in the synthesis of cholesterol. Inhibiting these enzymes leads to a reduction of osteoclast activity and an increase in apoptosis-dependent cell death, thereby resulting in reduced bone resorption (Reszka et al., 1999; Fisher et al., 2000; Rogers et al., 2000). The clinical effectiveness of the bisphosphonates has been extensively demonstrated (McClung, 2000; Bone et al., 2004; Papapoulos et al., 2005) but effective treatment requires total compliance from the patient. However, several major constraints related to administration modalities and adverse events may decrease patient compliance. Some studies have demonstrated an increased risk of fracture as a consequence of poor compliance with treatment (Huybrechts et al., 2006; Rabenda et al., 2008). For example, Siris et al. (2006) showed that patients who took more than 80% of their osteoporosis medications had a 26% reduction in fractures compared with patients with lower long-term compliance. An alternative consisting of a weekly or monthly administration was proposed to improve the compliance and maintenance of therapy (Emkey and Ettinger, 2006). However, despite these strategies, compliance still remains suboptimal (Cramer et al., 2007).
Considering all these data (human cost and effects on the economy), it is evident that the primary aim of osteoporosis therapy is to prevent fracture risk. In this context, we are interested in finding new anti-resorptive compounds. Among these, gallium (Ga) could be a promising candidate, as Ga is used for the treatment of hypercalcemia in cases of malignancy (Warrell et al., 1984; Warrell et al., 1986; Warrell et al., 1988) and Paget's disease (Matkovic et al., 1990; Warrell et al., 1990). In a small trial of myeloma patients treated with chemotherapy, Ga was shown to attenuate the rate of bone loss in patients (Niesvizky, 2003). Moreover, treatment with organic Ga was shown to improve bone content and strength in osteopenic rats (Ma and Fu, 2009). All these data suggest that Ga may have a positive effect on these bone disorders, probably by decreasing the rate of bone resorption.
However, there are only a few studies of the effects of Ga on osteoclastic resorption. For example, Donnelly et al. (1991) demonstrated that bone fragments treated with Ga are less readily resorbed, indicating that Ga treatment seems to confer bone with resistance to resorption. At the dose used for this study, Ga did not modify the morphology or the number of osteoclasts. In contrast to these findings, Blair et al. (1992) showed a cytotoxic effect of Ga on osteoclasts. The anti-osteoclastic effect of Ga still needs to be established, given that the direct effect of Ga on bone cells has only been partially addressed. Similarly, the effect of Ga on osteoblasts needs to be investigated, as a few studies have reported that Ga modified the expression of type-1 collagen and osteocalcin (Bockman et al., 1993; Guidon et al., 1993). However, no study has explored the effect of Ga on osteoblasts in terms of viability, proliferation and enzymatic activity such as alkaline phosphatase (ALP).
Within this context, the first objective of the present work was to explore the in vitro effects of Ga on osteoclasts in terms of resorption activity, differentiation and viability. Our second objective was to determine whether Ga may affect the viability and activity of osteoblastic bone forming cells. Our data have provided new insights into the effects of Ga on both bone resorbing and forming cells.
Methods
Cell culture
All animal handling and surgical procedures were conducted according to European Community guidelines for the care and use of laboratory animals (DE 86/609/CEE) and were approved by the local ethical committee.
To assess the anti-osteoclastic activity of Ga, we used three complementary and well-established osteoclastic models. First, we performed experiments with the unfractioned rabbit bone cell (RBC) model (Guicheux et al., 1998). Neonatal RBC were isolated using a previously reported method (Guicheux et al., 1998). Briefly, after killing 13-day-old rabbits by cervical dislocation, the long bones were isolated and freed from soft tissue, minced with a pair of scissors and agitated in 20 mL a-minimal essential medium (α-MEM) by vortex for 30 s. After sedimentation, the cell suspension was collected (repeated twice). The cell pellet was next washed and re-suspended in a-MEM supplemented with 10% fetal calf serum (FCS), 1% antibiotic mixture (P/S; 100 U·mL−1 penicillin, 100 µg·mL−1 streptomycin) and 1% L-glutamine. Cultures were finally established on glass coverslips and on dentin slices in a humidified, 95% air, 5% CO2 atmosphere at 37°C for 4 days.
Our second osteoclastic model was the murine monocytic RAW 264.7 cell line, from ATCC (Wittrant et al., 2004). Cells were seeded at a density of 104 cells·cm−2 and maintained for 6 days in phenol red-free α-MEM supplemented with 10% FCS, 1% P/S, 1% L-glutamine and RANK-L (50 ng·mL−1). The medium was removed every 2 days. Mature osteoclasts form after 6 days of culture.
Peripheral blood was obtained from human volunteers who were not taking any medication and with their informed consent. Human peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Ficoll gradient (Duplomb et al., 2008). CD14+ cells were magnetically labelled with CD14 Microbeads and positively selected by MACS technology (Miltenyi Biotec, Bergisch Gladbach, Germany). CD14+ cells were seeded in 24-well plates (250 × 103 cells per well) in α-MEM containing 10% FCS and 25 ng·mL−1 human macrophage-colony stimulating factor (hM-CSF). After 3 days of culture, the medium was changed with fresh medium containing 10% FCS, 25 ng·mL−1 hM-CSF, with or without 100 ng·mL−1 human ligand for receptor activator of nuclear factor κB (RANK-L). Cells were maintained for 12 days and the medium was changed every 4 days.
To determine whether Ga could affect the behaviour of osteoblastic cells, we first used one of the most largely described osteoblastic cell lines, MC3T3-E1. MC3T3-E1 cells were routinely grown in α-MEM medium containing 10% FCS, 1% P/S and 1% L-glutamine. Cells were sub-cultured once a week using trypsin/EDTA and maintained at 37°C in a humidified atmosphere of 5% CO2 in air. The medium was completely renewed every 2 days.
The next step was to use primary mouse osteoblasts prepared from the calvaria of 3-day-old mice as previously described (Guicheux et al., 2003). Calvaria were removed from the animals under aseptic conditions and incubated in PBS containing 4 mM EDTA at 37°C for 30 min under continuous agitation. Supernatant was discarded and α-MEM containing 3 mg·mL−1 type-2 collagenase was added. Calvaria were subjected to five sequential enzymatic digestions each conducted at 37°C for 20 min. Cells from fractions 1–2 and 3–5 were pooled, centrifuged and resuspended in α-MEM containing 10% FCS, 1% P/S and 1% L-glutamine. Cells were seeded at a density of 2.104 cells·cm−2 in α-MEM containing 10% FCS, 1% P/S and 1% L-glutamine, at 37°C in a humidified atmosphere of 5% CO2 in air. The medium was completely renewed every 2 days.
Resorption assay
RBC were seeded at a density of 5.106 cells per dentin slice in 24-well plates and were treated with graded concentrations of Ga (0–100 µM) for 4 days. The resorption activity of osteoclasts isolated from total RBC preparations was evaluated by the calculation of resorbed surface area on dentin slices, as described previously (Grimandi et al., 2006). To investigate the anti-resorptive activity of Ga, graded concentrations of Ga (from 0–100 µM) was incubated in 24-multiwell culture plates containing one dentin slice per well. After 4 days of culture, dentin slices were collected, rinsed with sodium hypochlorite and scraped gently using a soft tooth brush. They were observed by scanning electron microscopy (SEM) using backscattered electron modes and a semi-automatic image analyser (LEICA Quantimeter 500, Cambridge, UK). The resorption activity was calculated as the percentage of surface resorption per dentin slice.
Cell viability assay
RBC viability was assessed by using cell tracker green (CTG) staining as previously reported in detail (Magne et al., 2003). Briefly, after the indicated times, RBC established on glass coverslips were treated with 5 µM CTG for 30 min at 37°C and then washed with serum-free medium. Cells were observed using a fluorescent microscope. CTG incorporated into living cells was detected using an isothiocyanate (FITC) fluorescent set characterized by λex= 488 nm; λem= 490–560 nm.
Cell viability was further quantified by the methyl tetrazolium salt (MTS) assay as reported by Magne et al. (2003). The MTS assay is based on mitochondrial NADH/NADPH-dependent dehydrogenase activity, resulting in cellular conversion of the tetrazolium salt MTS into a soluble formazan dye. The medium was aspirated after a given period of time and 100 µL of MTS solution was added to each well. Finally, the colorimetric measurement of formazan dye was made with a spectrophotometer by measuring optical density at 490 nm. Results were expressed as MTS activity relative to that of cells cultured in the presence of vehicle. Furthermore, cellular proliferation was quantified by scoring cells manually after Trypan blue staining. As a positive control of cell death, cells were treated with actinomycin-D (Act-D; 5 µg·mL−1) or its vehicle (DMSO) (Vinatier et al., 2005).
RNA isolation
After the indicated times, total RNA was isolated from cultured cells using TRIzol reagent (Gibco BRL) in accordance with the manufacturer's instructions. Briefly, lysis of the cells in TRIzol was followed by centrifugation at 10 000 g for 15 min, at 4°C in the presence of chloroform. The upper aqueous phase was collected and the RNA was precipitated by addition of isopropanol and centrifugation at 7500 g for 5 min, at 4°C. RNA pellets were washed with cold 75% ethanol, dried, reconstituted in sterile water and quantified by spectrometry.
Real-time polymerase chain reaction (RT-PCR)
RNA samples (1 µg) were treated with DNase I (1 U·µg−1) to remove any contaminating genomic DNA. RNA was then reverse transcribed using AffinityScript QPCR cDNA Synthesis Kit in accordance with manufacturer's protocol, in a total volume of 20 µL. RT products were diluted 10-fold in sterile water. Template cDNAs (5 µL) were subjected to RT-PCR using specific mouse primers (see Table 1). The absence of DNA contamination in RNA preparations was tested by including RNA samples that had not been reverse-transcribed (No Reverse Transcription, NoRT). NoRT and No Template (NTC as DEPC water) controls as well as a positive control for each gene were included in each run. A housekeeping gene (GAPDH) was used as an internal control.
Table 1.
Gene names, sequences of primer pairs, length of PCR products and GeneBank accession numbers used for RT-PCR analysis
| Gene | Primer sequences | Product length | GeneBank accession number |
|---|---|---|---|
| Glyceraldehyde 3 phosphate deshydrogenase (GAPDH) | Forward 5′-gagccaaacgggtcatca-3′ Reverse 5′-catatttctcgtggttcacacc-3′ | 74 bp | NM_32599 |
| Tartrate resistant acid phosphatase (TRAP) ACP5 | Forward 5′-gtgatcaccgcttttggtc-3′ Reverse 5′-ccacccatgaatccatcct-3′ | 88 bp | NM_007388 |
| Cathepsin K (CTK) | Forward 5′-gcctagcgaacagattctcaa-3′ Reverse 5′-cactgggtgtccagcattt-3′ | 105 bp | NM_007802 |
| Calcitonin receptor (CTR) | Forward 5′-ccttccagaggagaagaaacc-3′ Reverse 5′-ggagattccgccttttcac-3′ | 95 bp | NM_007588 |
| Receptor activator of nuclear factor kappa B (RANK) | Forward 5′-cactgaggagaccacccaag-3′ Reverse 5′-tggcagccactactaccaca-3′ | 90 bp | NM_009399 |
| Osteoclastic stimulatory transmembrane protein (OC-STAMP) | Forward 5′-cagccacggaacacctct-3′ Reverse 5′-ggacaggctgggagaagg-3′ | 108 bp | NM_029021 |
| Nuclear factor of antivated T-cell c1 (NFATc1) | Forward 5′-tgaggctggtcttccgagtt-3′ Reverse 5′-cactgggaacactcgatagg-3′ | 91 bp | NM_198429.2 |
| Alkaline phophatase (ALP) | Forward 5′-ggccagctacaccacaaca-3′ Reverse 5′-ctgagcgttggtgttatatgtctt-3′ | 96 bp | NM_007431 |
| Osteocalin (OCN) | Forward 5′-agactccggcgctacctt-3′ Reverse 5′-ctcgtcacaagcagggttaag-3′ | 93 bp | NM_007541 |
| Ostepontin (OPN) | Forward 5′-cccggtgaaagtgactgatt-3′ Reverse 5′-ttcttcagaggacacagcattc-3′ | 142 bp | NM_009263 |
| Osterix (Osx) | Forward 5′-actacccacccttccctcactc-3′ Reverse 5′-ccaccacctagccagttgcc-3′ | 241 bp | NM_130458 |
| Runx2 | Forward 5′-ccacaaggacagagtcagattaca-3′ Reverse 5′-tggctcagataggaggggta-3′ | 92 bp | NM_009820 |
RT-PCR, real-time polymerase chain reaction.
Real-time PCR was performed in the MX3000pro (Stratagene) using Brilliant SYBR Green QPCR Master Mix (Stratagene) in accordance with the manufacturer's recommendations. The following temperature profile was used: 40 cycles of 30 s at 95°C, 1 min at 60°C and 30 s at 72°C. Amplification curves were analysed using the MxProV3 software (Stratagene). PCR products with Ct over 35 cycles were considered as undetectable (ND). The delta Ct (δCt) (cycle threshold) method was used to calculate relative expression levels. Cycle thresholds were normalized against the housekeeping gene (GAPDH) in order to control for cDNA quantification differences. Results are reported as the fold change in gene expression relative to control conditions. Gene expression was arbitrarily set as 1 in control condition cultures (untreated cultures).
Staining with Hoechst 33342
After the indicated times, cells were fixed for 10 min in 3.7% paraformaldehyde, and washed with PBS. Staining of the cells was carried out with Hoechst 33342 (10 µg·mL−1) for 10 min. After washing of the cells with PBS, the cells were examined with a fluorescent microscope (Axioplan 2, Zeiss, Oberkochen, Germany). Staurosporin (1 µM; 12 h) was used as a positive control of apoptotic cell death. Apoptotic cells were readily recognized, as they had a condensed or fragmented nucleus (Hughes et al., 1995).
Tartrate resistant acid phosphatase (TRAP) staining
At the end of the incubation period, the cells were rinsed gently with pre-warmed PBS, fixed and stained cytochemically in accordance with the manufacturer's instructions to detect the presence of TRAP-positive cells. Briefly, TRAP staining solution [4% solution of 2.5 M acetate buffer (pH 5.2), 12.5 mg·mL−1 naphthol AS-BI phosphoric acid, 0.67 M L(+)-tartrate buffer (pH 5.2) and 15 mg of Fast Garner salt] was freshly prepared and filtered before use. The cells cultured on glass coverslips were fixed in a 4% paraformaldehyde solution and then stained for 10–20 min at 37°C. The stained cells were observed using a light microscope (Axioplan 2, Zeiss, Germany). TRAP-positive multinucleated cells (MNCs) containing at least three nuclei were manually counted as osteoclasts. Results were expressed as the number of TRAP-positive MNCs relative to the number under control conditions.
Alkaline phosphatase (ALP) activity measurement
Cells were treated with 100 µM Ga or 100 ng·mL−1 bone morphogenetic protein-2 (BMP-2; (Guicheux et al., 2003) or their respective vehicles for 48 h. ALP activity was then evaluated by previously described methods (Guicheux et al., 2003). Briefly, cells were washed twice with ice cold PBS and scraped in a 0.2% aqueous solution of Nonidet P-40. Cell suspensions were sonicated on ice for 30 s and centrifuged for 5 min at 4°C. Aliquots of supernatants were subjected to protein assay with the Pierce Coomassie Plus assay reagent (Pierce, Rockford, USA) and to ALP activity measurements. ALP activity was assessed at pH 10.3 in 0.3 M of 2-amino-2methyl-1-propanol containing 1 mM MgCl2. p-Nitrophenyl phosphate (P-NPP; 10 mM) was used as a chromogenic substrate for an optical density measured at 405 nm. ALP activity was corrected for the total protein content and expressed as ALP activity relative to cells cultured in the presence of the vehicle.
Statistical analysis
Each experiment was repeated at least three times with similar results. Results are expressed as mean ± SEM of three independent determinations each performed in triplicate. Means were compared by one way analysis of variance followed by a post hoc test (Fisher's projected least significant difference) with statistical significance set at P < 0.05.
Materials
α-Minimal essential medium (α-MEM), antibiotic mixture (P/S; 100 U·mL−1 penicillin, 100 µg·mL−1 streptomycin), phosphate-buffered saline (PBS), trypsin/EDTA and Trizol reagent were purchased from Invitrogen Corporation (Paisley, UK). Fetal calf serum (FCS), culture plates and plastics were obtained from Dominique Dutscher (Brumath, France). Tartrate-resistant acid phosphatase (TRAP) activity was assayed by a commercially available kit (Sigma, Saint Quentin Fallavier, France). Protein content was determined using the Pierce Coomassie Plus Assay (Pierce, Rockford, IL, USA). Cellular viability was visualized using CTG dye (Invitrogen Corporation) and quantified using an MTS assay (Promega, Charbonnières, France). Staurosporin, actinomycin-D (Act-D; 5 µg·mL−1) and its vehicle (DMSO), EDTA, type-2 collagenase, Ga nitrate, pNPP and Ficoll were purchased from Sigma (Saint Quentin Fallavier, France). BMP-2, hM-CSF and hRANK-L were purchased from Tebu-Bio (Le Perray en Yvelines, France). Apoptosis was detected by the characteristic changes in nuclear morphology following Hoechst 33342 staining (Sigma).
AffinityScript QPCR cDNA Synthesis Kit and Brilliant® SYBR® Green QPCR Master Mix were obtained from Stratagene Europe (Amsterdam Zuidoost, the Netherlands). Oligonucleotide primers were from MWG (Courtaboeuf, France).
Results
Effect of Ga on osteoclastic resorption activity in RBC
To determine whether Ga may be effective in inhibiting osteoclastic resorption, we first investigated the effect of Ga in a well-established osteoclastic model, namely, the unfractioned RBC. The functional activity of osteoclasts was demonstrated by a resorption assay showing numerous typical resorption pits on the surface of dentin slices. In our untreated culture, we observed a high resorption activity on the surface of dentin slices as shown by a high number of large resorption lacunae (Figure 1A). Interestingly, our results showed a significant dose-dependent effect of Ga on the resorption activity (Figure 1B). A marked inhibition was observed with 100 µM of Ga. To determine whether this inhibition of resorption activity by Ga was to cell death, we evaluated the effect of Ga on the viability of unfractioned RBC using CTG staining and MTS conversion activity. Our data (Figure 1C) indicate that Ga, even at the highest concentration used here (100 µM), did not affect CTG staining. In addition, whereas actinomycin D (5 µg·mL−1) induced a dramatic decrease in MTS conversion activity, Ga at concentrations ranging from 2.5 to 100 µM did not significantly affect MTS conversion activity (Figure 1D). All of these results strongly suggest that Ga inhibited the resorption activity of osteoclasts in the RBC model, without affecting RBC viability.
Figure 1.
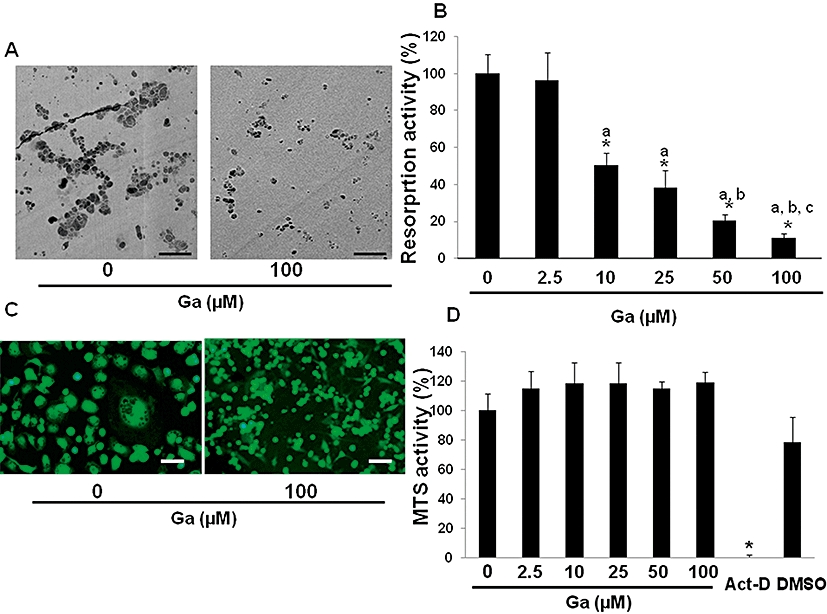
Effect of Ga on unfractioned rabbit bone cells (RBC). Unfractioned RBC were prepared and cultured for 4 days either on dentin slices (A, B, D) or glass coverslips (C) in the presence of Ga at the indicated concentrations. (A) The resorption activity of osteoclasts in the presence or absence of Ga was seen by the formation of typical resorption lacunae observed by scanning electron microscopy with backscattered electrons (bar = 100 µm). (B) Cells were treated with graded concentrations of Ga and quantitative analysis of osteoclastic resorption was performed with a semi automatic image analyser. Osteoclastic resorption is expressed in relative activity compared with untreated cells. *P < 0.05 as compared with untreated cells; a: P < 0.05 as compared with 2.5 µM-treated cells; b: P < 0.05 as compared with 10 µM-treated cells; c: P < 0.05 as compared with 25 µM-treated cells. (C) Cell viability in the presence or absence of Ga was visualized by fluorescent microscopy after CTG staining (bar = 50 µm). (D) Cells were treated with graded concentrations of Ga and MTS activity was evaluated. As a positive control of cell death, cells were treated with actinomycin-D (Act-D; 5 µg·mL−1) or its vehicle (DMSO). Results are expressed as relative MTS activity as compared with untreated cells. *P < 0.05 significantly different from vehicle treated cells. Ga, gallium; MTS, methyl tetrazolium salt.
Effect of Ga on osteoclastic differentiation
To ascertain whether Ga may affect the osteoclastic resorption activity through an inhibitory effect on osteoclastic differentiation, we next embarked on experiments in a well-known murine osteoclastic differentiation model, the RAW 264.7 cell line. Our results (Figure 2A) indicate that when stimulated by RANK-L for 6 days, RAW 264.7 cells support the formation of TRAP-positive multinucleated cells. Moreover, RANK-L treatment significantly up-regulated the expression of transcripts coding for TRAP, cathepsin K (CTK), calcitonin receptor (CTR), receptor activator of nuclear factor kappa B (RANK) and osteoclastic stimulatory transmembrane protein (OC-STAMP). We subsequently sought to determine whether Ga may affect the expression level of these osteoclastic markers in differentiated RAW 264.7 cells. As indicated in Figure 2B, Ga dose-dependently decreased the expression level of TRAP, CTK, CTR, RANK and OC-STAMP. As Ga strongly suppressed the expression of these osteoclastic differentiation markers, we tested the viability of RAW 264.7 cells after incubation with Ga, using the MTS assay on undifferentiated and differentiated RAW 264.7 cells. As indicated in Figure 3A, while Act-D induced a marked decrease in MTS conversion activity in both cells, Ga (100 µM) did not significantly alter MTS activity. To further assess whether Ga may induce apoptotic cell death, we finally carried out a Hoechst 33342 staining on differentiated RAW 264.7 cells (Figure 3B, right panel). Whereas Ga (100 µM) did not induce a noticeable condensation of nuclei, staurosporin triggered a marked apoptotic cell death. All these results suggest that Ga modulated the expression level of osteoclastic markers in RAW 264.7 without affecting cell viability and without inducing cell apoptosis. Finally, to confirm whether these effects of Ga at the transcript level correlated with an alteration in the formation of osteoclast-like cells, we counted the total number of multinucleated TRAP-positive cells treated with increasing doses of Ga in our three models; human CD14-positive cells (Figure 4G–I), RBC (Figure 4A–C) and RAW 264.7 cells (Figure 4D–F). Compared with untreated cultures (Figure 4A, D and G), when cells were treated with Ga (100 µM), there was a marked reduction in the total number of TRAP+ cells in all three models (Figure 4B, E and H) and this reduction was dose-dependent in RBC (Figure 4C), RAW 264.7 (Figure 4F) and CD14+ (Figure 4I) cultures.
Figure 2.
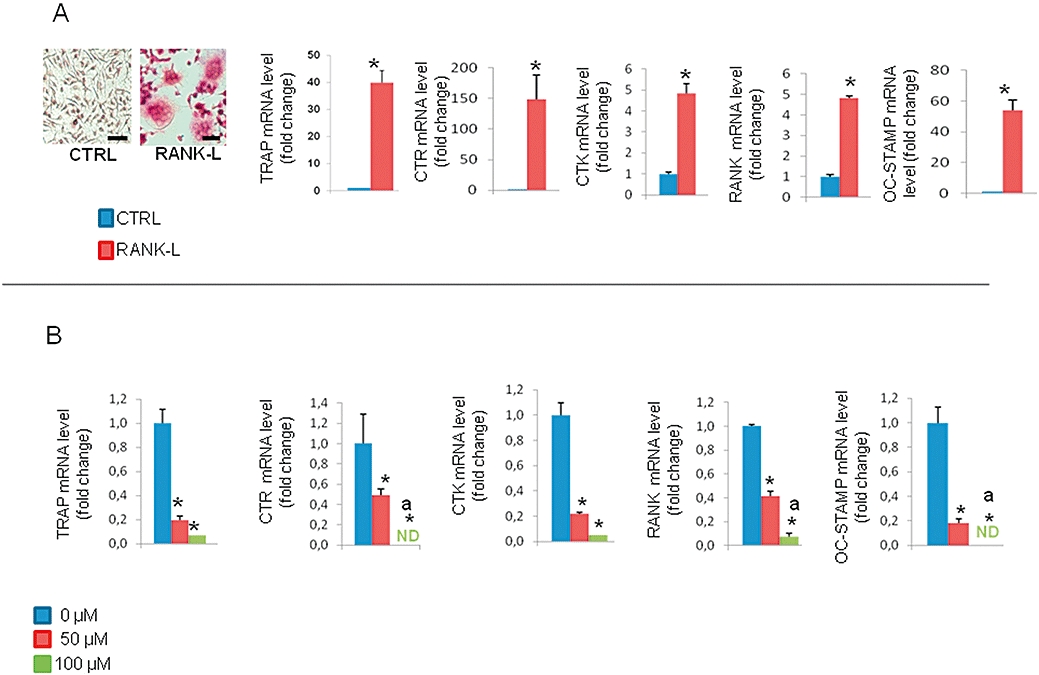
Effects of Ga on RAW 264.7 differentiation. (A) RAW 264.7 cells were established in the presence (RANK-L) or absence (CTRL) of 50 ng·mL−1 of RANK-L for 6 days. Cells were stained for TRAP activity and observed under a light microscope (bar = 50 µm). Quantitative analysis of transcripts was performed by real-time polymerase chain reaction (RT-PCR) using primers specific for TRAP, CTR, CTK, RANK and OC-STAMP. Results are reported as fold change in gene expression relative to untreated cells after normalization against GAPDH. *P < 0.05 significantly different from corresponding untreated cells. (B) RAW 264.7 cells cultured concomitantly with RANK-L (50 ng·mL−1) and different doses of Ga (0–50 µM–100 µM) for 6 days. Quantitative analysis of transcripts was performed by RT-PCR using primers specific for TRAP, CTR, RANK, CTK and OC-STAMP. Results are reported as fold change in gene expression relative to untreated cells after normalization against GAPDH. *P < 0.05 significantly different from corresponding untreated cells; a: P < 0.05 significantly different from 50 µM-treated cells. Ga, gallium; RANK, receptor activator of nuclear factor kappa B; RANK-L, receptor activator of nuclear factor kappa B-Ligand; CTK, cathepsin K; CTR, calcitonin receptor; OC-STAMP, osteoclastic stimulatory transmembrane protein; GAPDH, glyceraldehyde 3 phosphate deshydrogenase; RT-PCR, real-time polymerase chain reaction.
Figure 3.
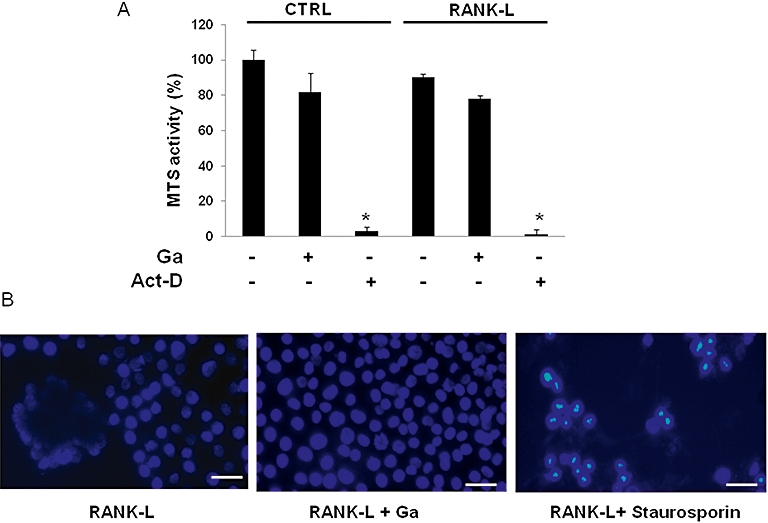
Effect of Ga on RAW 264.7 viability. (A) RAW 264.7 cultured with (RANK-L) or without RANK-L (CTRL) were treated with 100 µM Ga (+) or its vehicle (−) for 6 days. As a positive control for cell death, cells were treated for 48 h with 5 µg·mL−1 Act-D (+) or its vehicle (−). MTS activity was determined and results are expressed as relative MTS activity compared with untreated cells (*P < 0.05 significantly different from corresponding untreated cells). (B) Cells cultured with RANK-L were treated with 100 µM Ga or its vehicle for 6 days. Staurosporin (1 µM; 12 h) was used as a positive control for apoptotic cell death. Chromatin condensation was demonstrated by Hoechst 33342 staining as indicated in the Materials and Methods. Samples were observed with a fluorescent microscope (bar = 30 µm). Ga, gallium; RANK-L, receptor activator of nuclear factor kappa B-Ligand; MTS, methyl tetrazolium salt.
Figure 4.
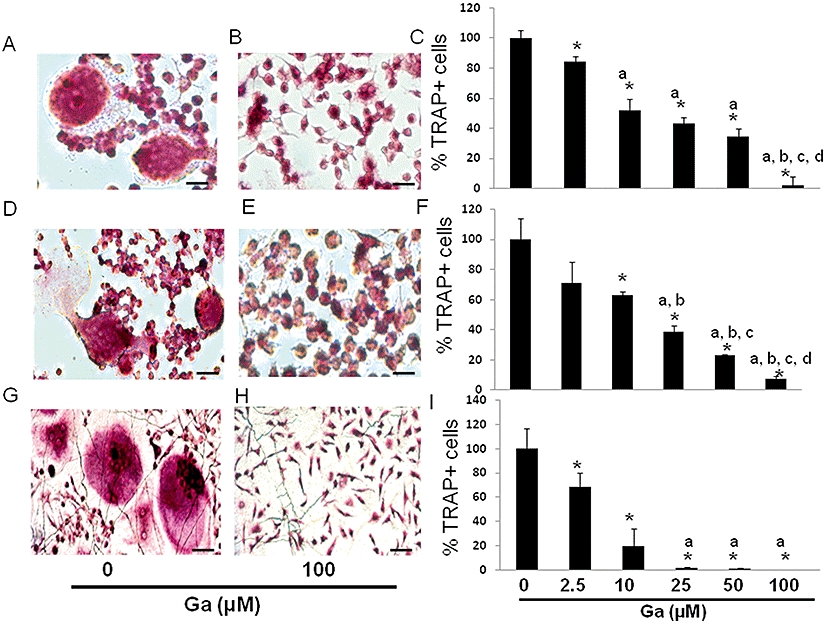
Effect of Ga on the total number of TRAP-positive cells. Unfractioned RBC (A, B, C), RAW 264.7 (D, E, F) and human CD14-positive cells (G, H, I) were established on glass coverslips and treated or not with Ga at the indicated concentrations for 4 days (RBC) and 6 days (RAW 264.7 and CD14+). Cells were stained for TRAP activity and observed under a light microscope (bar = 30 µm). The total numbers of TRAP-positive cells were manually scored (C, F, I). Results were expressed as relative number of TRAP-positive cells compared with untreated cells (*P < 0.05 significantly different from untreated cells; a: P < 0.05 significantly different from 2.5 µM-treated cells; b: P < 0.05 significantly different from 10 µM-treated cells; c: P < 0.05 significantly different from 25 µM-treated cells; d: P < 0.05 significantly different from 50 µM-treated cells). Ga, gallium; TRAP, tartrate resistant acid phosphatase; RBC, rabbit bone cells.
Effect of Ga on the expression of the transcription factor, nuclear factor of activated T-cell c1 (NFATc1)
In an effort to further document the mechanism of Ga-induced inhibition, we tested the effects of Ga on the expression of NFATc1. As shown in Figure 5, in control conditions we observed that RANK-L treatment induced NFATc1 expression, with an early induction measured at 12 h and a sustained activation at 24 h. In the presence of Ga, and for both concentrations used, we observed a dose-dependent down-regulation of the early NFATc1 induction, and a more pronounced inhibition of the sustained activation measured at 24 h.
Figure 5.
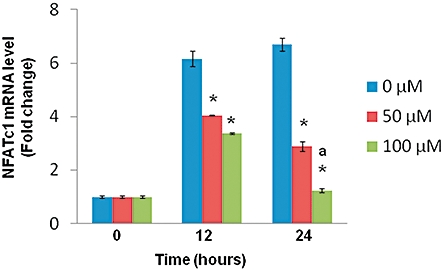
Effect of Ga on the expression of NFATc1. RAW 264.7 cells were cultured concomitantly with RANK-L (50 ng·mL−1) and different doses of Ga (0, 50 µM, 100 µM) for 12 and 24 h. Quantitative analysis of transcripts was performed by RT-PCR using primers specific for NFATc1 as described in the Materials and Methods. Results are reported as fold change in gene expression relative to time zero after normalization against the housekeeping gene GAPDH. *P < 0.05 significantly different from equivalent untreated cells; a: P < 0.05 significantly different from 50 µM-treated cells. Ga, gallium; NFATc1, nuclear factor of activated T cell c1; GAPDH, glyceraldehyde 3 phosphate deshydrogenase.
Effect of Ga on osteoblastic cells
To ascertain whether Ga may affect osteoblastic bone forming cells, we sought to assess the effects of Ga on the viability, proliferation and activity of MC3T3-E1 cells and primary osteoblasts. Based on the dose-dependent effects of Ga on the osteoclast-like cells, we tested Ga at 100 µM for all the subsequent experiments with osteoblasts. Our data indicate that Ga did not affect the MTS conversion activity in both MC3T3-E1 cells (Figure 6A) and primary osteoblasts (Figure 6C). Conversely and as expected, Act-D markedly reduced the MTS conversion activity of both types of cells (Figure 6A and C). To further determine the effects of Ga on the proliferation of osteoblasts, we counted the total number of viable cells after Ga treatments. Our results indicated that Ga did not significantly influence the proliferation of MC3T3-E1 cells (Figure 6B) and primary osteoblasts (Figure 6D).
Figure 6.
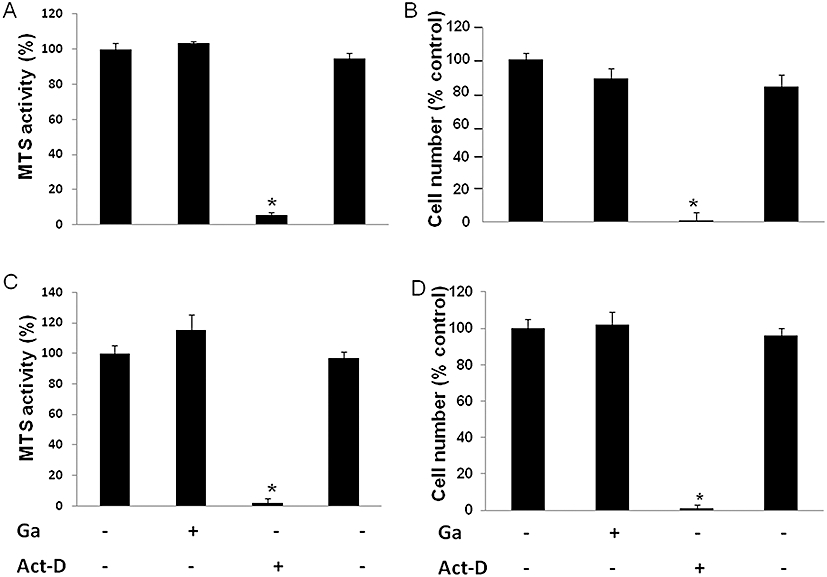
Effect of Ga on osteoblastic viability and proliferation. Murine MC3T3-E1 osteoblastic cells (A, B) and murine primary calvaria-derived osteoblasts (C, D) were cultured as described in the Materials and Methods. After overnight incubation, cells were treated with 100 µM Ga or 5 µg·mL−1 Act-D (+) or their respective vehicles (−) for 48 h. Osteoblastic viability was evaluated by MTS activity. Results are expressed in relative MTS activity compared with untreated cells (*P < 0.05 significantly different from untreated cells) (A, C). Osteoblastic proliferation was quantified by scoring cells manually after Trypan blue staining. Results are expressed as relative cell number as compared with untreated cells (B, D). *P < 0.05 significantly different from corresponding untreated cells. Ga, gallium; MTS, methyl tetrazolium salt.
Furthermore, we evaluated the effect of Ga on the activity of osteoblasts by measuring the total protein content and the ALP activity in MC3T3-E1 cells (Figure 7A and B) and primary osteoblasts (Figure 7C and D). BMP-2 markedly increased the ALP activity of both cell types without affecting the total protein content (Figure 7C and D). By contrast, Ga failed to affect the total protein content and the ALP activity in both osteoblastic cell types (Figure 7).
Figure 7.
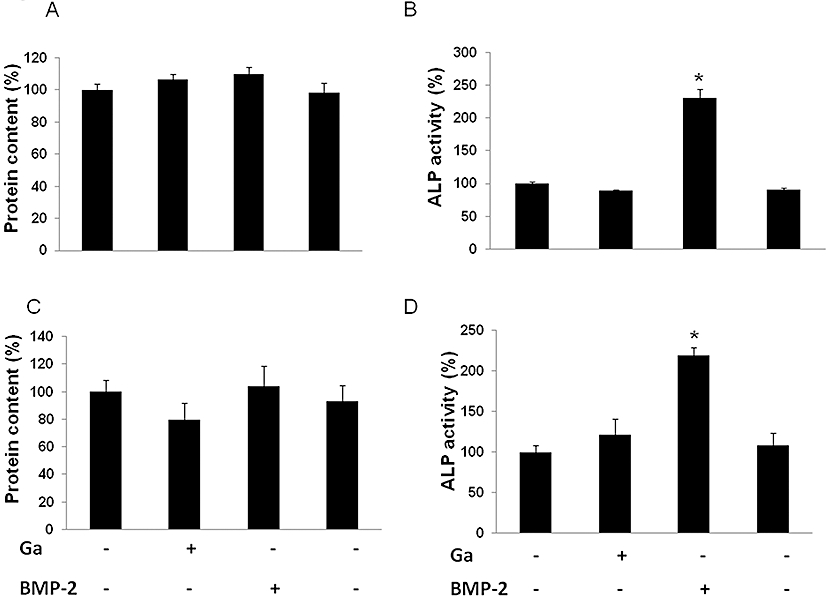
Effect of Ga on alkaline phosphatase (ALP) activity. Murine MC3T3-E1 osteoblastic cells (A, B) and murine primary calvaria-derived osteoblasts (C, D) were cultured for 12 days and 4 days respectively. Then, cells were treated with 100 µM Ga or 100 ng·mL−1 BMP-2 (+) or their respective vehicles (−) for 48 h. Total protein content was determined with a Coomassie assay. Results are expressed as relative protein content as compared with untreated cells (A, C). ALP activity was spectrophotometrically measured. Results are expressed as relative ALP activity as compared with untreated cells (B, D). *P < 0.05 significantly different from corresponding untreated cells. Ga, gallium; BMP-2, bone morphogenetic protein-2.
Finally, we used RT-PCR to study the effect of longer-term treatment of Ga on proliferative osteoblastic cells. The results showed that Ga did not have any negative effect on early and late osteoblastic marker expression (Figure 8).
Figure 8.
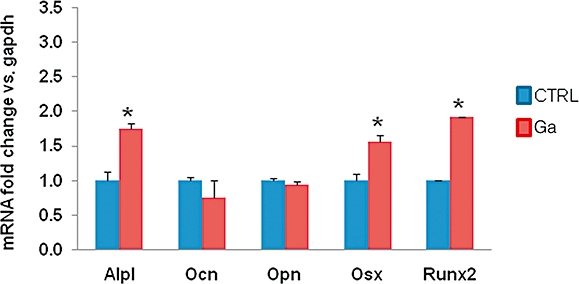
Effect of Ga on the expression of osteoblastic markers. Cultures of murine MC3T3-E1 osteoblastic cells were treated for 8 days with 100 µM Ga. Several markers of osteoblasts (Alp1, alkaline phosphatase, Ocn, osteocalcin; Osx, osterix; Opn, osteopontin; Runx2, Run related transcription factor 2) were measured by RT-PCR. Results are reported as fold change in gene expression relative to untreated cells after normalization against GAPDH. *P < 0.05, significantly different from the equivalent untreated cells. Ga, gallium; RT-PCR, real-time polymerase chain reaction; GAPDH, glyceraldehyde 3 phosphate deshydrogenase.
Discussion
Osteoporosis results in an imbalance of the bone metabolism in favour of osteoclastic resorption. The main complication of osteoporosis is the development of bone fractures, which area serious health problem not only in terms of morbidity and mortality but also from an economic point of view (Ensrud et al., 2000; Lindsay et al., 2001). Among the anti-osteoporotic treatments, the bisphosphonates are probably the most effective in reducing fracture risk. However, some treatment regimens and some adverse effects may decrease the effectiveness of these drugs (Gallagher et al., 2008; Rabenda et al., 2008). In this context, we were interested in finding new anti-resorptive compounds. Given the potential anti-resorptive effects of gallium (Ga) suggested by a few studies (Donnelly et al., 1991; Blair et al., 1992; Ma and Fu, 2009), we focused our efforts on the effect of Ga on osteoclasts.
To address this fundamental issue, we first ascertained whether Ga may affect the most relevant functional parameter of osteoclasts, namely their resorptive activity (Faucheux et al., 2008). For this purpose, we used the unfractioned RBC model as a well-established culture system that supports the activity of mature resorbing osteoclasts (Grimandi et al., 2006). In this model, the functional activity of osteoclasts was evidenced by a resorption assay showing numerous typical resorption pits on the surface of dentin slices. As was previously demonstrated for bisphosphonates in this RBC model (Faucheux et al., 2008), Ga dose-dependently inhibited the resorption activity of osteoclasts. This result seems to confirm the few published reports (Hall and Chambers, 1990; Blair et al., 1992). However, use of the RBC model alone makes it difficult to decide whether the effect of Ga on osteoclasts may be direct or indirect, mediated by osteoblasts or stromal cells present in the RBC model. Nonetheless, at least two mechanisms could contribute to the effect of Ga on the resorption activity of osteoclasts. This effect could be due either to the induction of apoptosis via the inhibition of specific intracellular signalling pathways as reported for the bisphosphonates and the mevalonate pathway (Rogers et al., 1999; 2000;), or to alteration of osteoclast differentiation and formation.
To further address both these issues, we explored the effect of Ga by using the murine RAW 264.7 cell line, a RANKL-dependent osteoclastic differentiation model (Wittrant et al., 2004). Our results demonstrate that Ga significantly affected the RANKL-induced osteoclastic activity of RAW 264.7, as seen by the marked reduction in the expression level of transcripts coding for specific osteoclastic markers (TRAP, CTK, CTR, RANK, OC-STAMP). In our study, the level of CTR was checked as an indicator of the formation/differentiation of osteoclasts as there is no specific effect of calcitonin in our model. To assess whether the RANKL-induced reduction in levels of osteoclastic markers was associated with an alteration in osteoclastic formation, we then demonstrated that Ga dose-dependently affects the total number of TRAP-positive multinucleated cells formed in the RBC model and RAW 264.7 cells. To establish whether this effect of Ga may be translated to a human model of osteoclast-like cells, we finally tested the effect of Ga on the RANKL-induced formation of TRAP-positive cells in a model of human CD14+ cells. Interestingly, Ga down-regulated the formation of osteoclast-like cells in CD14+ cell culture, therefore strengthening the relevance of our data. Viewed together, these data strongly suggest that Ga can affect not only osteoclastic resorption but also the formation and differentiation of osteoclasts. To rule out the possibility that the observed effects of Ga on the activity, differentiation and formation of osteoclasts may be mediated by a cytotoxic effect of Ga, we also tested its effects on cell viability. Based on our results obtained using the MTS assay, CTG and Hoechst 33342 stainings, we failed to detect any effects of Ga on cell viability. All these results point to the hypothesis that Ga affects osteoclasts by specific cellular and molecular mechanisms independent of generally cytotoxic processes.
Given the crucial role of the transcription factor NFATc1 for osteoclastogenesis (Takayanagi et al., 2002; Asagiri and Takayanagi, 2007), we decided to investigate the effect of Ga on its expression. Upon RANKL treatment of osteoclastic progenitor cells, and TNF receptor-associated factor 6 (TRAF6) recruitment, nuclear factor kappa B and c-Fos pathways are activated and control the expression of NFATc1. Regulation of NFATc1 expression also involves Ca signalling pathways. The binding of Ca to calmodulin, which is the main Ca binding protein, activates calcineurin. Once activated, this kinase allows the nuclear translocation of NFATc (Soderling and Stull, 2001). Given the chemical similarity of Ca and Ga, it seems reasonable to suggest that Ga could interact with the Ca pathway and affect certain pivotal signalling pathways in the biology of osteoclasts.
In the present report, we provide evidence that Ga dose-dependently decreased the expression of NFATc1 and these preliminary results also suggest that Ga dose-dependently decreased the expression of c-Fos (data not shown). It will be also interesting to explore the effect of Ga on the Ca-calmodulin pathway. One possible mechanism could be competition between Ga and Ca for binding to calmodulin. This explanation would require uptake of Ga by the cell. However, no data exists on mechanisms of Ga uptake by osteoclasts. The effects of Ga could be mediated by a receptor such as the Ca sensing receptor or the transferrin receptor via the Ga-transferrin complex. With a view to validating the hypothesis of a possible interaction of Ga with these receptors, it will be necessary to determine the actual chemical form of Ga in the culture medium (see Benezeth et al., 1997). Finally, the identification of Ga-modulated signalling pathways downstream of these receptors deserves further attention.
Considering the role of osteoblastic cells in the bone remodelling process, we investigated whether Ga could affect osteoblastic behaviour. In the literature, Ga seems to modify the expression of type-1 collagen and osteocalcin in osteoblasts (Bockman et al., 1993; Guidon et al., 1993). However, no study has explored the effect of Ga on osteoblasts in terms of viability, proliferation and relevant enzymic activity such as ALP. We first used a well-known osteoblastic cell line: MC3T3-E1 cells. The MC3T3-E1 cell line is a non-transformed cell line established from newborn mouse calvaria (Sudo et al., 1983). These cells exhibit an osteoblastic phenotype as shown by the expression of ALP activity (Guicheux et al., 2003), the synthesis of extracellular matrix (ECM) components such as osteocalcin and type-1 collagen (Sudo et al., 1983), and their ability to mineralize the ECM (Suzuki et al., 2006). However, as the sensitivity of established cell lines to cytotoxic compounds remains lower than those of primary cells used in culture models, we also embarked on experiments with primary osteoblasts isolated from the calvaria of newborn mice. In both models, Ga failed to affect the viability and activity of osteoblasts.
Finally, to complete our study, we analysed the effect of Ga on the expression of the mainly early and late osteoblastic markers such as osteocalcin. A medium-term treatment (8 days) with Ga failed to affect the expression of these markers. Interestingly, these results are in contradiction with a few published studies that have mainly documented the effect of Ga on osteoblasts after a short-term treatment (Guidon et al., 1993; Jenis et al., 1993).
All these data thereby suggest that despite its marked anti-osteoclastic effects, Ga did not negatively affect the behaviour of osteoblasts in vitro.
In conclusion, our data taken together showed that Ga affected the resorption activity, differentiation and formation of osteoclasts by non-cytotoxic mechanisms. In addition, this compound did not reveal any adverse effects on osteoblastic bone forming cells. This is the first report indicating that the inhibitory effects of Ga on osteoclastogenesis probably involve a reduction in the expression of NFATc1. Further experiments are now required to elucidate the mechanisms of Ga action, for example, determining whether they involve a Ca-dependent pathway. Our results suggest that Ga may be a promising candidate for regulating the excessive resorption activity of osteoclasts as observed in osteoporosis.
Acknowledgments
The authors wish to thank Danielle Quincey, Dominique Heymann, Assem Soueidan, Gaël Grimandi, Pierre Weiss, Paul Pilet and the Graftys company. The authors acknowledge the financial support of the INSERM, the Fondation de l'Avenir pour la Recherche Médicale Appliquée (FARMA), the Société française de rhumatologie (SFR), the Arthritis-Courtin Foundation and the Gabiphoce Project (ANR BioTECS ANR-08-BIOT-008).
Glossary
Abbreviations:
- Act-D
actinomycin-D
- ALP
alkaline phosphatase
- BMP-2
bone morphogenetic protein-2
- BP
bisphosphonate
- Ct
cycle threshold
- CTG
cell tracker green
- CTK
cathepsin K
- CTR
calcitonin receptor
- DMSO
dimethylsulphoxide
- FCS
fetal calf serum
- FITC
fluorescent isothiocyanate
- GAPDH
glyceraldehyde 3 phosphate deshydrogenase
- hM-CSF
human macrophage-colony stimulating factor
- MNCs
multinucleated cells
- MTS
methyl tetrazolium salt
- ND
not detectable
- NFATc1
nuclear factor of activated T cell c1
- NoRT
No Reverse Transcription
- NTC
No Template control
- OC-STAMP
Osteoclastic stimulatory transmembrane protein
- P/S
penicillin/streptomycin
- PBMCs
peripheral blood mononuclear cells
- PBS
phosphate buffered saline
- pNPP
para-nitrophenyl phosphate
- RANK
Receptor activator of nuclear factor kappa B
- RANK-L
Receptor activator of nuclear factor kappa B-Ligand
- RBC
rabbit bone cells
- RT-PCR
real-time polymerase chain reaction
- SEM
scanning electron microscopy
- TRAF-6
TNF receptor-associated factor-six
- TRAP
Tartrate resistant acid phosphatase
Conflicts of interest
The authors declare no conflicts of interest.
References
- Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- van Balen R, Steyerberg EW, Polder JJ, Ribbers TL, Habbema JD, Cools HJ. Hip fracture in elderly patients: outcomes for function, quality of life, and type of residence. Clin Orthop Relat Res. 2001;390:232–243. [PubMed] [Google Scholar]
- Benezeth P, Diakonov II, Pokrovski GS, Dandurand JL, Schott J, Khodakovsky IL. Gallium speciation in aqueous solution. Experimental study and modelling.2. Solubility of alpha-GaOOH in acidic solutions from 150 to 250 degrees C and hydrolysis constants of gallium (III) to 300 degrees C. Geochimica Et Cosmochimica Acta. 1997;61:1345–1357. [Google Scholar]
- Blair HC. How the osteoclast degrades bone. Bioessays. 1998;20:837–846. doi: 10.1002/(SICI)1521-1878(199810)20:10<837::AID-BIES9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Blair HC, Teitelbaum SL, Tan HL, Schlesinger PH. Reversible inhibition of osteoclastic activity by bone-bound gallium (III) J Cell Biochem. 1992;48:401–410. doi: 10.1002/jcb.240480409. [DOI] [PubMed] [Google Scholar]
- Bockman RS, Guidon PT, Jr, Pan LC, Salvatori R, Kawaguchi A. Gallium nitrate increases type I collagen and fibronectin mRNA and collagen protein levels in bone and fibroblast cells. J Cell Biochem. 1993;52:396–403. doi: 10.1002/jcb.240520404. [DOI] [PubMed] [Google Scholar]
- Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- Consensus Development Conference. Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- Donnelly R, Bockman RS, Doty SB, Boskey AL. Bone particles from gallium-treated rats are resistant to resorption in vivo. Bone Miner. 1991;12:167–179. doi: 10.1016/0169-6009(91)90030-4. [DOI] [PubMed] [Google Scholar]
- Duplomb L, Baud'huin M, Charrier C, Berreur M, Trichet V, Blanchard F, et al. Interleukin-6 inhibits receptor activator of nuclear factor kappaB ligand-induced osteoclastogenesis by diverting cells into the macrophage lineage: key role of Serine727 phosphorylation of signal transducer and activator of transcription 3. Endocrinology. 2008;149:3688–3697. doi: 10.1210/en.2007-1719. [DOI] [PubMed] [Google Scholar]
- Emkey RD, Ettinger M. Improving compliance and persistence with bisphosphonate therapy for osteoporosis. Am J Med. 2006;119:S18–S24. doi: 10.1016/j.amjmed.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Thompson DE, Cauley JA, Nevitt MC, Kado DM, Hochberg MC, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48:241–249. doi: 10.1111/j.1532-5415.2000.tb02641.x. [DOI] [PubMed] [Google Scholar]
- Faucheux C, Verron E, Soueidan A, Josse S, Arshad MD, Janvier P, et al. Controlled release of bisphosphonate from a calcium phosphate biomaterial inhibits osteoclastic resorption in vitro. J Biomed Mater Res. 2008;A:46–56. doi: 10.1002/jbm.a.31989. [DOI] [PubMed] [Google Scholar]
- Fisher JE, Rodan GA, Reszka AA. In vivo effects of bisphosphonates on the osteoclast mevalonate pathway. Endocrinology. 2000;141:4793–4796. doi: 10.1210/endo.141.12.7921. [DOI] [PubMed] [Google Scholar]
- Gallagher AM, Rietbrock S, Olson M, van Staa TP. Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res. 2008;23:1569–1575. doi: 10.1359/jbmr.080510. [DOI] [PubMed] [Google Scholar]
- Grimandi G, Soueidan A, Anjrini AA, Badran Z, Pilet P, Daculsi G, et al. Quantitative and reliable in vitro method combining scanning electron microscopy and image analysis for the screening of osteotropic modulators. Microsc Res Tech. 2006;69:606–612. doi: 10.1002/jemt.20326. [DOI] [PubMed] [Google Scholar]
- Guicheux J, Heymann D, Rousselle AV, Gouin F, Pilet P, Yamada S, et al. Growth hormone stimulatory effects on osteoclastic resorption are partly mediated by insulin-like growth factor I: an in vitro study. Bone. 1998;22:25–31. doi: 10.1016/s8756-3282(97)00224-x. [DOI] [PubMed] [Google Scholar]
- Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003;18:2060–2068. doi: 10.1359/jbmr.2003.18.11.2060. [DOI] [PubMed] [Google Scholar]
- Guidon PT, Jr, Salvatori R, Bockman RS. Gallium nitrate regulates rat osteoblast expression of osteocalcin protein and mRNA levels. J Bone Miner Res. 1993;8:103–112. doi: 10.1002/jbmr.5650080113. [DOI] [PubMed] [Google Scholar]
- Hall TJ, Chambers TJ. Gallium inhibits bone resorption by a direct effect on osteoclasts. Bone Miner. 1990;8:211–216. doi: 10.1016/0169-6009(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38:922–928. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Jenis LG, Waud CE, Stein GS, Lian JB, Baran DT. Effect of gallium nitrate in vitro and in normal rats. J Cell Biochem. 1993;52:330–336. doi: 10.1002/jcb.240520309. [DOI] [PubMed] [Google Scholar]
- Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- Ma Z, Fu Q. Therapeutic effect of organic gallium on ovariectomized osteopenic rats by decreased serum minerals and increased bone mineral content. Biol Trace Elem Res. 2009 doi: 10.1007/s12011-009-8445-3. DOI: 10.1007/s12011-009-8445-3. [DOI] [PubMed] [Google Scholar]
- McClung MR. Bisphosphonates in osteoporosis: recent clinical experience. Expert Opin Pharmacother. 2000;1:225–238. doi: 10.1517/14656566.1.2.225. [DOI] [PubMed] [Google Scholar]
- Magne D, Bluteau G, Faucheux C, Palmer G, Vignes-Colombeix C, Pilet P, et al. Phosphate is a specific signal for ATDC5 chondrocyte maturation and apoptosis-associated mineralization: possible implication of apoptosis in the regulation of endochondral ossification. J Bone Miner Res. 2003;18:1430–1442. doi: 10.1359/jbmr.2003.18.8.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkovic V, Apseloff G, Shepard DR, Gerber N. Use of gallium to treat Paget's disease of bone: a pilot study. Lancet. 1990;335:72–75. doi: 10.1016/0140-6736(90)90540-l. [DOI] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res. 1992;7:1005–1010. doi: 10.1002/jbmr.5650070902. [DOI] [PubMed] [Google Scholar]
- Niesvizky R. Gallium nitrate in multiple myeloma: prolonged survival in a cohort of patients with advanced-stage disease. Semin Oncol. 2003;30:20–24. doi: 10.1016/s0093-7754(03)00172-6. [DOI] [PubMed] [Google Scholar]
- Orcel P. Prevention and treatment of glucocorticoid-induced osteoporosis in 2005. Joint Bone Spine. 2005;72:461–465. doi: 10.1016/j.jbspin.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Papapoulos SE, Quandt SA, Liberman UA, Hochberg MC, Thompson DE. Meta-analysis of the efficacy of alendronate for the prevention of hip fractures in postmenopausal women. Osteoporos Int. 2005;16:468–474. doi: 10.1007/s00198-004-1725-z. [DOI] [PubMed] [Google Scholar]
- Rabenda V, Mertens R, Fabri V, Vanoverloop J, Sumkay F, Vannecke C, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19:811–818. doi: 10.1007/s00198-007-0506-x. [DOI] [PubMed] [Google Scholar]
- Reszka AA, Halasy-Nagy JM, Masarachia PJ, Rodan GA. Bisphosphonates act directly on the osteoclast to induce caspase cleavage of mst1 kinase during apoptosis. A link between inhibition of the mevalonate pathway and regulation of an apoptosis-promoting kinase. J Biol Chem. 1999;274:34967–34973. doi: 10.1074/jbc.274.49.34967. [DOI] [PubMed] [Google Scholar]
- Rogers MJ, Frith JC, Luckman SP, Coxon FP, Benford HL, Monkkonen J, et al. Molecular mechanisms of action of bisphosphonates. Bone. 1999;24:73S–79S. doi: 10.1016/s8756-3282(99)00070-8. [DOI] [PubMed] [Google Scholar]
- Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Stull JT. Structure and regulation of calcium/calmodulin-dependent protein kinases. Chem Rev. 2001;101:2341–2352. doi: 10.1021/cr0002386. [DOI] [PubMed] [Google Scholar]
- Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ghayor C, Guicheux J, Magne D, Quillard S, Kakita A, et al. Enhanced expression of the inorganic phosphate transporter Pit-1 is involved in BMP-2-induced matrix mineralization in osteoblast-like cells. J Bone Miner Res. 2006;21:674–683. doi: 10.1359/jbmr.020603. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- Vinatier C, Magne D, Weiss P, Trojani C, Rochet N, Carle GF, et al. A silanized hydroxypropyl methylcellulose hydrogel for the three-dimensional culture of chondrocytes. Biomaterials. 2005;26:6643–6651. doi: 10.1016/j.biomaterials.2005.04.057. [DOI] [PubMed] [Google Scholar]
- Warrell RP, Jr, Bockman RS, Coonley CJ, Isaacs M, Staszewski H. Gallium nitrate inhibits calcium resorption from bone and is effective treatment for cancer-related hypercalcemia. J Clin Invest. 1984;73:1487–1490. doi: 10.1172/JCI111353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell RP, Jr, Skelos A, Alcock NW, Bockman RS. Gallium nitrate for acute treatment of cancer-related hypercalcemia: clinicopharmacological and dose-response analysis. Cancer Res. 1986;46:4208–4212. [PubMed] [Google Scholar]
- Warrell RP, Jr, Israel R, Frisone M, Snyder T, Gaynor JJ, Bockman RS. Gallium nitrate for acute treatment of cancer-related hypercalcemia. A randomized, double-blind comparison with calcitonin. Ann Intern Med. 1988;108:669–674. doi: 10.7326/0003-4819-108-5-669. [DOI] [PubMed] [Google Scholar]
- Warrell RP, Jr, Bosco B, Weinerman S, Levine B, Lane J, Bockman RS. Gallium nitrate for advanced Paget disease of bone: effectiveness and dose-response analysis. Ann Intern Med. 1990;113:847–851. doi: 10.7326/0003-4819-113-11-847. [DOI] [PubMed] [Google Scholar]
- Wittrant Y, Theoleyre S, Couillaud S, Dunstan C, Heymann D, Redini F. Relevance of an in vitro osteoclastogenesis system to study receptor activator of NF-kB ligand and osteoprotegerin biological activities. Exp Cell Res. 2004;293:292–301. doi: 10.1016/j.yexcr.2003.10.016. [DOI] [PubMed] [Google Scholar]


