Abstract
Background and purpose:
Compound LASSBio-881 is an orally effective antinociceptive that binds to cannabinoid receptors and is active mainly on the neurogenic component of pain models. We investigated whether transient receptor potential vanilloid subfamily type 1 (TRPV1) channels are involved in the effects of LASSBio-881.
Experimental approach:
Modulation of capsaicin (CAP)- and low pH-induced currents was evaluated in TRPV1-expressing Xenopus oocytes. In vivo effects were evaluated in CAP-induced acute and inflammatory changes in nociception, as well as in partial sciatic ligation-induced thermal hypernociception.
Key results:
LASSBio-881 inhibited TRPV1 currents elicited by CAP with an IC50 of 14 µM, and inhibited proton-gated currents by 70% at 20 µM. Functional interaction with CAP was surmountable. Locally applied LASSBio-881 decreased time spent in CAP-elicited nocifensive behaviour by 30%, and given orally it reduced measures of CAP- or carrageenan-evoked thermal hypernociception by 60 and 40% respectively. In addition, LASSBio-881 decreased the paw withdrawal responses to thermal stimuli of animals with sciatic neuropathy 7–11 days after nerve ligation, at a dose of 300 µmol·kg−1·day−1 p.o. At this dose, hyperthermia was not observed within 4 h following oral administration.
Conclusions and implications:
LASSBio-881 is a TRPV1 antagonist that apparently competes with CAP. Accordingly, LASSBio-881 inhibited nociception in models of acute, inflammatory and neuropathic pain presumed to involve TRPV1 signalling. These in vivo actions were not hindered by hyperthermia, a common side effect of other TRPV1 antagonists. We propose that the antinociceptive properties of LASSBio-881 are due to TRPV1 antagonism, although other molecular interactions may contribute to the effects of this multi-target drug candidate.
Keywords: capsaicin, neuropathic pain, N-acylhydrazone, oocytes, electrophysiology, TRPV1 antagonist
Introduction
The search for novel analgesic drugs has focused on finding new targets other than opioid receptors and COX, which present too many adverse side effects in long-term treatments. Among these new targets is transient receptor potential vanilloid subfamily type 1 (TRPV1), a cation channel expressed in polymodal nociceptors, which is activated by chemical and physical stimuli including capsaicin (CAP), heat, protons and endogenous cannabinoids (Caterina et al., 1997; Zygmunt et al., 1999). TRPV1 became an interesting target when its key involvement was determined not only in acute pain, but also in hypernociceptive states following inflammation (Caterina et al., 2000). More recently, TRPV1 has been linked to neuropathic pain because its blockade inhibits nociception in animal models of nerve lesions (Walker et al., 2003; Honore et al., 2005). Interestingly, in several cases, antinociception is achieved by pharmacological blockade of TRPV1 expressed both centrally in the spinal cord and in the periphery (Jhaveri et al., 2005; Cui et al., 2006).
LASSBio-881 is one of a series of 6-nitro-3,4-methylenedioxyphenyl-N-acylhydrazone compounds derived from safrole (Duarte et al., 2007), structurally related to LASSBio-294 (Gonzalez-Serratos et al., 2001) and to nimesulide. This series was developed with the aim of obtaining a compound with improved analgesic and anti-inflammatory activity relative to LASSBio-294. The presence of a 3,5-di-tert-butyl-4-hydroxyphenyl moiety conferred considerable antioxidant activity to LASSBio-881 and was expected to enhance its efficacy in attenuating deleterious manifestations of both acute and chronic inflammatory responses (Cuzzocrea et al., 2004). However, unlike LASSBio-294 and nimesulide, LASSBio-881 was found to inhibit nociception only in the early neurogenic phase of the formalin test and in the hot plate, with limited anti-inflammatory effects in rodents (Duarte et al., 2007).
The bioisosteric relationships between N-acylhydrazones like LASSBio-881 and unsaturated fatty acids like the endocannabinoid anandamide prompted binding studies that revealed LASSBio-881 as a ligand of type 1 cannabinoid receptors (CB1) (Duarte et al., 2007). It was then postulated that antioxidant and CB1-dependent mechanisms contributed to the antinociceptive effect of this novel compound. However, anandamide can also stimulate TRPV1 (Zygmunt et al., 1999), and ligands of cannabinoid receptors are thought to have the potential to be ligands of the TRPV1 channel as well (Di Marzo et al., 2007). As LASSBio-881 has a 1,3-benzodioxolyl moiety derived from safrole that is present in pungent TRPV1 agonists like piperine (McNamara et al., 2005), we sought to investigate whether this compound affects TRPV1 function.
Several compounds that have been developed targeting TRPV1 to obtain analgesia are in clinical trial, and so far, the main adverse effect these drugs present in humans is hyperthermia (reviewed by Szallasi et al., 2007 and Wong and Gavva, 2009). Here, we showed that LASSBio-881 inhibits TRPV1 channel function like a competitive antagonist, hinders CAP stimulation of TRPV1 in vitro and in vivo and diminishes hypernociceptive responses following inflammation. Interestingly, at a high dose, LASSBio-881 did not affect body temperature regulation within the first 4 h following its oral administration. In addition, LASSBio-881 was found to be effective in a model of neuropathic pain.
Methods
All drug and molecular target nomenclature conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2009). All procedures involving animals were approved by the Comissão de Ética no Uso de Animais from Universidade Federal do Rio de Janeiro, and were in accordance with international guidelines. Except for the frogs, in experiments with animals, both sexes were used in approximately equal numbers.
Materials
TRPV1 and TRPV1 Δ777-820 clones were a generous gift from Dr David Julius.
Drugs used in this study, except for LASSBio-881, were obtained from the following sources: CAP (Alexis or Sigma), dimethylsulphoxide (DMSO) (Sigma or Vetec), arabic gum (Sigma), carrageenan (Cialgas, Extremoz, RN, Brazil). LASSBio-881 was synthesized as described previously (Duarte et al., 2007).
Preparation of oocytes and microinjections
Adult Xenopus laevis female frogs maintained in 12 h light/dark cycles were anaesthetized by immersion in 0.75 g·L−1 tricaine supplemented with 3 g·L−1 NaHCO3. Stage V and VI oocytes were surgically removed, placed in Barth's saline containing (in mM) 96, NaCl; 2, KCl; 5, MgCl2; 5, HEPES at pH 7.6, and treated with collagenase (type 1, 0.8 mg·mL−1, Worthington, Lakewood, NJ, USA) to remove the follicular membrane. Oocytes were injected by using a nanolitre injector with approximately 2.0 ng of rat TRPV1 or rat TRPV1 Δ777-820 in vitro transcribed RNAs obtained with mMESSAGE mMACHINE T7 (Ambion, Austin, TX, USA). Oocytes were maintained in ND-96 (in mM: 96, NaCl; 2, KCl; 1.8, CaCl2; 1, MgCl2; 5, HEPES) supplemented with 40 µg·mL−1 gentamicin for 5–7 days before analysis.
Oocyte electrophysiology
Oocytes were placed in a small recording chamber and continuously superfused with ND-96 at a flow rate of approximately 1 mL·min−1. For pH 5.5 stimulation, the buffer used was composed of (in mM): 96, NaCl; 2, KCl; 1, MgCl2; 0.1, CaCl2; and 5, sodium acetate. Two electrode voltage clamp recordings were made at −60 mV holding potential and room temperature (20–22°C) using a GeneClamp 500 amplifier (Axon Instruments, Sunnyvale, CA, USA) and MacLab A/D converter with Chart software (AD Instruments, Colorado Springs, CO, USA). Electrodes were pulled on a horizontal puller (P-97, Sutter, Novato, CA, USA), filled with 3 M KCl and used to achieve a final resistance of 0.6–1.2 MΩ. Recordings were digitized at 100 Hz and digitally filtered at 2 Hz (low pass). Oocytes were discarded when the resting membrane potential was above −10 mV, or the baseline current was unstable. Drug stock solutions were made in ethanol or DMSO, and were diluted in ND-96 pH 7.6 just before the experiments. Final ethanol and DMSO concentrations did not exceed 0.1 and 0.2%, respectively, and appropriate controls were tested as indicated. The solutions were exchanged by a programmable solenoid pinch valve controller (AutoMate Scientific Inc., Berkeley, CA, USA), and were generally applied in 30 s pulses. Each pulse of LASSBio-881 in admixture with other agents was immediately preceded by LASSBio-881 alone in the same concentration, to allow drug equilibration.
CAP-induced nociception in mice
The protocol used was adapted from Santos and Calixto (1997). Swiss mice weighing between 18 and 25 g received a subplantar injection of LASSBio-881 (5 nmol per paw in saline with 10% DMSO). Twenty minutes later, a subplantar injection of CAP (488.6 µmol per paw in saline with 10% DMSO) was performed in the same paw. The time the animals spent licking, biting or shaking the paw was recorded with a chronometer for 10 min after CAP administration.
CAP-induced thermal hypernociception in rats
The anti-hypernociceptive activity was investigated using the CAP-induced hypernociceptive test adapted from Mizushima et al. (2005). Wistar rats, deprived of food, weighing from 150 to 200 g, were placed on a hot plate apparatus (Ugo Basile, model-DS 37, Comerio, VA, Italy) set at a temperature of 52 ± 0.1°C to record the basal latency of the withdrawal response. Then, LASSBio-881 was administered orally at 100 µmol·kg−1 in 5% arabic gum solution. After 1 h, 0.1 mL of 5% CAP solution in saline plus 10% DMSO (5 µg per paw) was injected into the animals' right hind footpad. The withdrawal latency of the right hind paw was recorded on the hot plate at 2, 5, 10, 30 and 60 min post-challenge. The basal latencies were found to be 10–16 s. A 21 s cut-off time was established to prevent any injury to the animals' paws.
Carrageenan-induced rat paw oedema and hypernociception assay
Wistar rats (150–200 g), deprived of food, were used. LASSBio-881 was administered orally (300 µmol·kg−1; 0.1 mL·20 g−1) as a suspension in 5% arabic gum in saline (vehicle). Control animals received an equal volume of vehicle. One hour later, the animals were injected with either 0.1 mL of 1% carrageenan solution in saline (1 mg per paw) or sterile saline (NaCl 0.9%), into the subplantar surface of one of the hind paws. The thermal hypernociception was determined using the modified hot plate test (Lavich et al., 2005). Rats were placed individually on a hot plate with the temperature adjusted to 51°C. The latency of the withdrawal response of the left hind paw was determined at 0, 30, 60, 120, 180 and 240 min post-challenge. A 20 s cut-off time was established to prevent any injury to the animals' paws. Hypernociception to heat is defined as a decrease in withdrawal latency, and calculated as follows: Δ paw withdrawal latency (s) = (left paw withdrawal latency at time 0) − (left paw withdrawal latency at the other times).
Neuropathic pain model
The antinociceptive activity of LASSBio-881 was investigated using the partial sciatic ligation model adapted from Seltzer et al. (1990), using adult Swiss mice. Left sciatic nerve ligation was made by tying a knot positioned between the last third or half of the dorsal portion of the nerve, using absorbable 4.0 silk suture. In sham-operated animals, the nerve was exposed without ligation. The withdrawal responses were determined before surgery and after a nociceptive thermal stimulus caused by a radiant heat light source positioned directly on the plantar surface of the left hind paw. LASSBio-881 was administered orally at a dose of 100 µmol·kg−1 once or three times a day starting at day 5 and ending at day 13 post-ligation. The withdrawal latency of the left hind paw was recorded 5, 7, 9, 11 and 13 days after sciatic ligation 1 h after the 12 h 00 min (or only) dose of LASSBio-881 or vehicle (5% arabic gum).
Temperature measurements
The ability of LASSBio-881 to change body temperature was evaluated, according to Ayoub et al. (2004). Adult Swiss mice, weighing between 18 and 25 g, were used in these experiments. The animals had their body temperature measured by using a rectal probe (sensitivity 0.1°C) just before drug administration. Vehicle (5% arabic gum) or LASSBio-881 was given orally at 100 or 300 µmol·kg−1, and new measurements were made at indicated intervals up to 4 h.
Statistical analyses
Peak amplitudes of digitized current traces were measured with the Chart software. Averaged data are reported as mean ± SEM, and differences were tested for significance as indicated in the figure legends. Sigmoidal concentration–response curves were fitted with GraphPad Prism.
Results
LASSBio-881 does not activate TRPV1, even at lower pH
Xenopus oocytes were injected with TRPV1 cRNA, and electrophysiological recordings were performed. During LASSBio-881 (pH 7.6) application, no increase in basal current could be detected (Figure 1A). Application of 20 µM LASSBio-881 in pH 5.5 reduced TRPV1 responses to protons by 71 ± 3.6% (Figure 1B). This prompted us to investigate whether LASSBio-881 had antagonistic properties.
Figure 1.
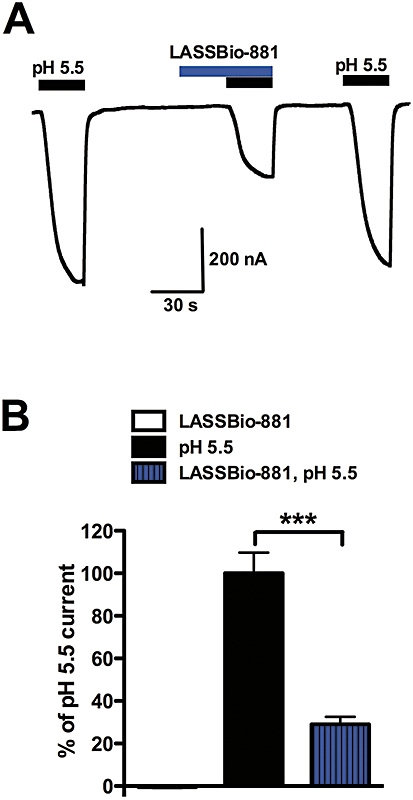
LASSBio-881 does not activate TRPV1 expressed in Xenopus oocytes, but it inhibits pH 5.5-elicited currents. (A) Representative current recording from a TRPV1-expressing oocyte. First, a solution of pH 5.5 was applied, followed by 20 µM LASSBio-881 at pH 7.6 and then at pH 5.5. (B) Quantification of peak currents at pH 5.5 and in LASSBio-881 at pH 7.6 and 5.5, normalized by the response to pH 5.5 applied at the end of recording. Results were analysed with one-way anova, followed by Tukey's post hoc test (***P < 0.001, n= 7).
LASSBio-881 inhibits CAP currents
Oocytes expressing TRPV1 were challenged with submaximal 1 µM CAP (Figure 2A). Increasing concentrations of co-administered LASSBio-881 inhibited 1 µM CAP-evoked current in a concentration-dependent manner (Figure 2B, IC50= 14.05 µM). A mutant of TRPV1, named TRPV1 Δ777-820, lacks the phosphatidylinositol-4,5-bisphosphate (PIP2) binding domain, which inhibits the channel in normal conditions. This mutated channel, like the native channel after PIP2 hydrolysis, has reduced thresholds for thermal and chemical stimuli (Prescott and Julius, 2003). Therefore, it mimics the TRPV1 receptor in an inflammatory condition, and was used here for this purpose. TRPV1 Δ777-820-expressing oocytes were treated with 50 nM CAP alone or together with increasing concentrations of LASSBio-881. Again, the new compound was able to inhibit the CAP current in a concentration-dependent manner, with an estimated IC50 of 4.95 µM (Figure 2C,D). The finding that LASSBio-881 inhibited CAP currents in both wild-type and mutant TRPV1 suggests that the antagonistic activity of this compound is not affected by PIP2 binding and that it probably does not interact with this region of TRPV1 C-terminus.
Figure 2.
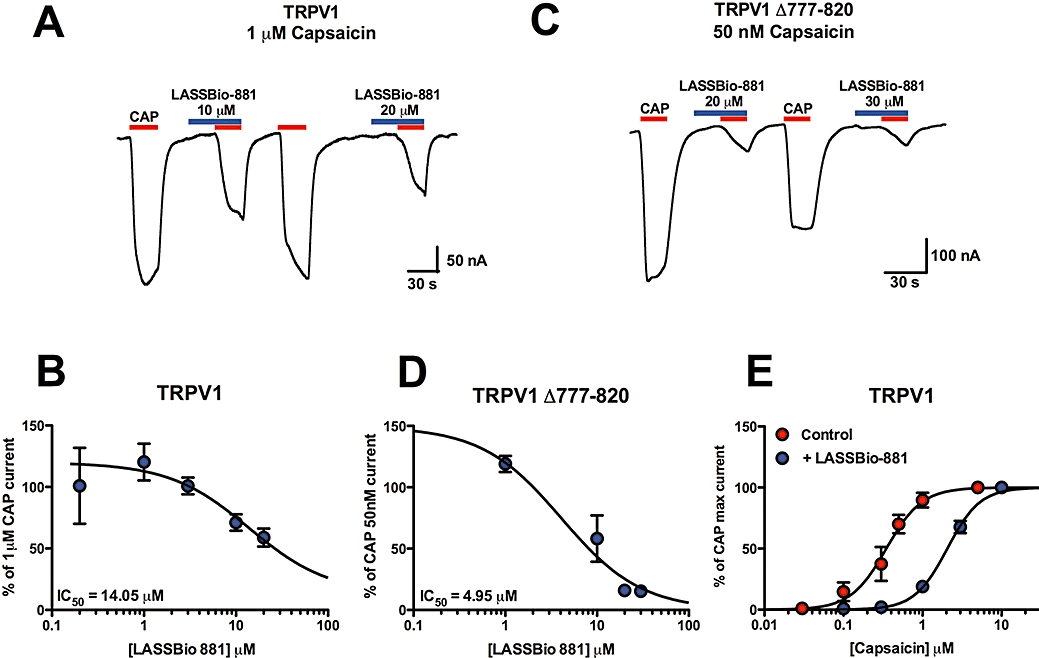
LASSBio-881 inhibition of CAP currents is surmountable in TRPV1-expressing oocytes. (A) Representative recording of LASSBio-881 inhibition of 1 µM CAP currents in TRPV1-expressing oocytes. (B) Concentration–response curve for LASSBio-881 against 1 µM CAP, normalized to 10 µM CAP applied at the end of recording. Estimated IC50 was 14.05 µM (n= 3–7 oocytes per concentration). (C) Representative recording of a TRPV1 Δ777-820-expressing oocyte challenged with 50 nM CAP in the absence or presence of various concentrations of LASSBio-881. (D) Concentration–response curve for LASSBio-881 against 50 nM CAP, normalized to 10 µM CAP applied at the end of recording. Estimated IC50 was 4.95 µM (n= 4–9 oocytes per concentration). (E) Concentration–response curves for TRPV1 currents induced by CAP in the absence and presence of 20 µM LASSBio-881. Estimated EC50 for CAP were 347.8 nM and 2.06 µM, alone and with 20 µM LASSBio-881, respectively (n= 5 oocytes in each curve).
Next, we determined the nature of LASSBio-881 antagonism by treating oocytes with increasing concentrations of CAP with or without 20 µM LASSBio-881. The CAP concentration–response curve shows a shift to the right in the presence of LASSBio-881, and reaches the same maximal effects (Figure 2E). This shows that LASSBio-881-induced inhibition of CAP responses is surmountable, and suggests that LASSBio-881 acts as a competitive antagonist at TRPV1.
LASSBio-881 inhibits CAP nociceptive responses and thermal hypernociception induced by CAP or carrageenan
Having determined that LASSBio-881 is capable of antagonizing CAP-elicited currents, we proceeded to test whether it could inhibit CAP-triggered nociceptive behaviour. Mice received intraplantar injections of LASSBio-881 or vehicle followed by CAP, and the time spent licking, biting and shaking the paw was measured. The time spent in nocifensive behaviour was significantly reduced (34.5%) in animals that received LASSBio-881 prior to CAP (CAP 57.7 ± 5.5 s; LASSBio-881 + CAP 37.8 ± 6.9 s, n= 9–10 animals per group) (Figure 3A). Therefore, locally applied LASSBio-881 could reduce CAP-induced nociception. The next question was whether LASSBio-881 could also diminish TRPV1-elicited thermal hypernociception. For that purpose, CAP was injected into rats' paws after 1 h of LASSBio-881 administered orally, and the changes in latency of paw withdrawal from a heat source were measured. Throughout the period when changes in withdrawal responses were present, the decrease in latency (Δ) was significantly reduced in LASSBio-881-treated animals when compared to vehicle-treated controls (Figure 3B).
Figure 3.
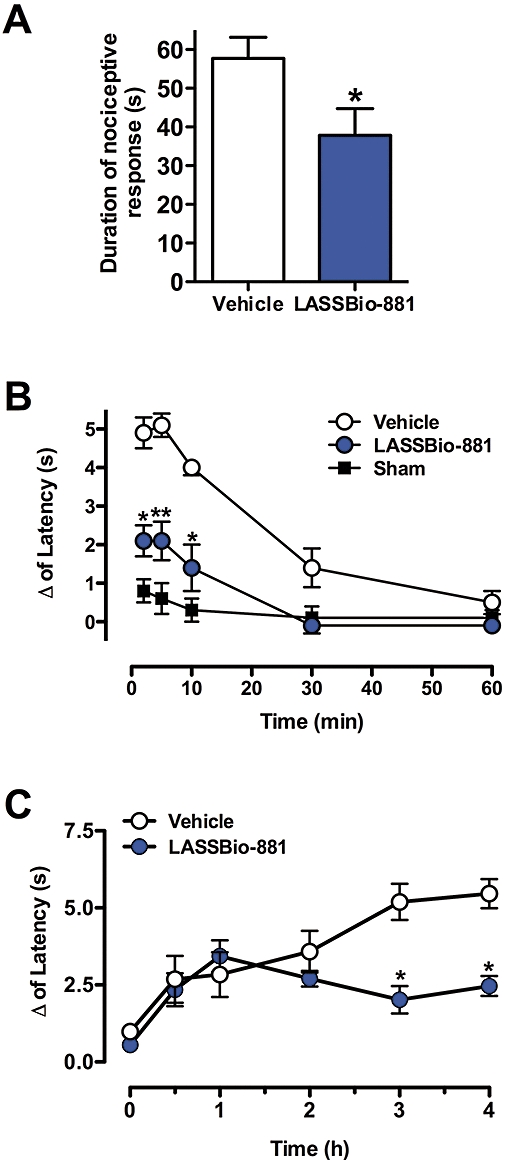
Effect of LASSBio-881 in models of acute nociception and hypernociception caused by CAP and carrageenan. (A) Animals received LASSBio-881 in the paw before an injection of CAP, and the time the animals spent in nocifensive behaviour was measured 10 min after CAP injection. Results are expressed as mean ± SEM (n= 9–10 animals per group, *P < 0.05, Student's t-test when compared to control group which received 10% DMSO). (B) Time-course of the effect of LASSBio-881 (100 µmol·kg−1 p.o.) on CAP-induced thermal hypernociception. Results were analysed by two-way anova, followed by Bonferroni post hoc test (n= 8–16 animals per group; *P < 0.05 and **P < 0.01, when compared to the vehicle group which received 5% arabic gum p.o.). Sham-treated animals received 5% arabic gum p.o., followed by 10% DMSO in the paw. (C) Time-course for the effect of LASSBio-881 (300 µmol·kg−1 p.o.) on carrageenan-induced thermal hypernociception. Results were analysed by two-way anova, followed by Bonferroni post hoc test (n= 8–10 animals per group, *P < 0.05, when compared to the control group (vehicle), which received 5% arabic gum p.o.).
LASSBio-881 proved effective in CAP-induced acute and inflammatory pain, and this can be explained by its antagonist activity at TRPV1. However, we asked ourselves if LASSBio-881 would also diminish the latency of withdrawal in other models of inflammatory hypernociception. Animals received LASSBio-881 or vehicle (p.o.), and 1 h later were injected with 1% carrageenan subplantarly. Figure 3C shows that animals pretreated with LASSBio-881 showed a decreased latency of withdrawal when compared to controls at 3 and 4 h post-challenge. These results show that LASSBio-881 has antinociceptive properties facing different inflammatory nociception models.
Highest dose of LASSBio-881 does not cause hyperthermia
The main side effect presented by TRPV1 antagonists undergoing clinical development is hyperthermia. Therefore, we determined whether treatment with a high dose of LASSBio-881 was able to change body temperature. Oral administration of LASSBio-881, 300 µmol·kg−1, did not produce a significant change in temperature at all times measured (Figure 4). As high concentrations of LASSBio-881 are able to inhibit COX (Duarte et al., 2007), this could explain the lack of hyperthermia; hence, a lower dose was also applied to test this hypothesis. Indeed, 100 µmol·kg−1 LASSBio-881 caused mild hyperthermia relative to vehicle, which was statistically significant at 60 and 90 min following its administration. The time-course of LASSBio-881-induced hyperthermia revealed that at 30 min following a 100 µmol·kg−1 oral dose of LASSBio-881, there was a tendency to hyperthermia, although this was not statistically significant. Subsequently, from 60 to 120 min following the administration of LASSBio-881, the animals' temperatures rose and remained approximately 0.5°C above baseline. In the subsequent measurements, their rectal temperatures gradually returned to control levels. The animals' baseline temperatures varied from 35.6 to 37.0°C (36.5 ± 0.1°C, n= 10 for each group).
Figure 4.
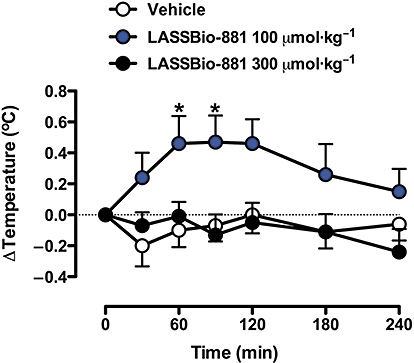
Time-course of LASSBio-881 effects on rectal temperature. Mice had their baseline temperatures measured prior to the administration of LASSBio-881 (time zero); this was then subtracted from subsequent measurements. The animals' baseline temperatures varied from 35.6 to 37.0°C (36.5 ± 0.1°C, n= 10 for each group). Then, the animals received vehicle or LASSBio-881 at 100 or 300 µmol·kg−1 p.o., and new temperature measurements were made at the indicated intervals. Results were analysed with two-way anova RM, followed by Bonferroni's post hoc test (n= 10 for each group, *P < 0.05 when compared to vehicle).
LASSBio-881 reduced neuropathy-induced hypersensitivity
There is considerable evidence suggesting that TRPV1 has an important role in neuropathic pain, and that TRPV1 antagonists can raise pain thresholds. Hence, LASSBio-881 was then given daily (p.o.) to animals from day 5 after a partial nerve injury and the changes in the latency to withdrawal from a radiant heat source were recorded every other day up to day 13. First, the animals were given 100 µmol·kg−1 p.o. every day after day 5. With this dose, the animals demonstrated a significant decrease in Δ only at day 9, and returned to vehicle-treated levels subsequently (Figure 5). Given that 1 h after a single dose of 100 µmol·kg−1, the animals were slightly hyperthermic; this could have caused discomfort and affected the thermal sensitivity test; therefore, another group was treated with a higher daily dose of 300 µmol·kg−1. In order to avoid marked fluctuations in plasma concentration and to possibly achieve a desirable stabilization of body temperature at normal levels as the drug accumulated, the 300 µmol·kg−1 was divided into three equal doses per day. In this way, LASSBio-881 was expected to achieve a larger antinociceptive effect with minimum hyperthermia. Indeed, the animals that received LASSBio-881 had a smaller change in latency at days 7, 9 and 11 post-ligation when compared to the vehicle-treated controls (Figure 5). Subsequently, at day 13, the animals showed a change in latency similar to the vehicle-treated animals. However, by the last day of observation, the animals treated with LASSBio-881 had visibly lost weight and were hyperactive, which made the measurements less reliable.
Figure 5.
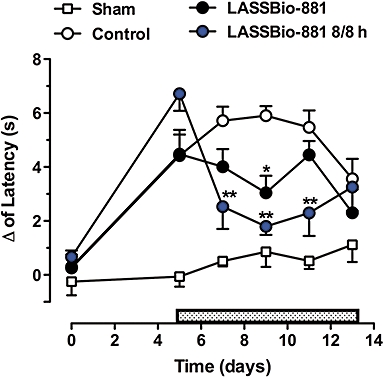
LASSBio-881 is effective at reducing changes in latency in a partial nerve injury model. Mice were subjected to a partial ligation of the sciatic nerve at day zero, and were then treated daily with LASSBio-881 5 days later (day 5; stippled rectangle) at a single daily dose of 100 µmol·kg−1 or three times a day (8/8 h) (p.o.). Sham-operated animals only had the nerve exposed without ligation. The withdrawal responses to a thermal stimulus were determined, and the changes in latency were obtained by comparing paw removal times before and after surgery. Results were analysed with two-way RM anova, followed by Bonferroni post hoc test (n= 10 animals per group, *P < 0.05, **P < 0.01 compared to vehicle-treated animals).
Discussion and conclusions
Here, we report that LASSBio-881, previously described as an antinociceptive compound (Duarte et al., 2007), is a TRPV1 antagonist. The TRPV1 channel has been pointed out as a promising target for new analgesic drugs, because changes in its function and expression underlie painful conditions. TRPV1 expression has been found to be increased in several inflammatory hypernociception models, and in similar conditions in humans (Szallasi et al., 2007). In addition, it has been found that the TRPV1 channel itself shows a decreased threshold for several stimuli in circumstances simulating inflammation, and is itself responsible for thermal hyperalgesia (Caterina et al., 2000; Chuang et al., 2001; Prescott and Julius, 2003). Therefore, molecules targeting this channel are likely to be effective against inflammatory pain.
LASSBio-881 antagonized CAP-elicited currents in both wild-type and mutant TRPV1. This mutant, TRPV1 Δ777-820, lacks the putative PIP2 binding region located in its C-terminus, and was shown to have lower thresholds to protons and high temperatures (Prescott and Julius, 2003). Because LASSBio-881 was effective at inhibiting CAP currents in both wild-type and mutant TRPV1, this property of LASSBio-881 is not affected by PIP2 binding, and means that it is unlikely to be interacting with this region of the TRPV1 C-terminus. As other mediators, such as nerve growth factor and bradykinin, are released during the inflammatory process, they activate phospholipases, which in turn cleave PIP2, thereby releasing the channel from inhibition (Prescott and Julius, 2003). Alternatively, it has been suggested that the mechanism by which these mediators change TRPV1 function operates through phosphorylation of key intracellular residues. Regardless of the mechanism, we were able to show that orally administered LASSBio-881 is effective in preventing thermal hypernociception following subplantar CAP injections. On a different time-scale, LASSBio-881 also diminished carrageenan-evoked thermal hypernociception, also a model where the role of TRPV1 has been determined (Davis et al., 2000). Moreover, acute CAP-elicited nociceptive behaviour was also partially inhibited by LASSBio-881 pretreatment. Thus, these results from heterologous expression of TRPV1 and in vivo experiments suggest that LASSBio-881 is effective at inhibiting TRPV1-evoked nociception in acute models of inflammation via TRPV1 antagonism.
Potential analgesics targeting TRPV1: effective but with substantial side effects
Both agonists and antagonists of TRPV1 are being investigated as analgesics because both can annul TRPV1 function through distinct mechanisms. Repeated or prolonged agonist stimulation of TRPV1 causes desensitization or even loss of the nociceptor, the latter being, except in specific circumstances, an unwanted effect. Furthermore, agonists applied systemically generate a number of other undesirable effects, by triggering autonomic reflexes, such as respiratory depression (Szallasi and Blumberg, 1999). Therefore, CAP analogues and other agonists are being investigated mostly for the treatment of painful conditions by local (topical) application.
We have extended the antinociceptive profiling of LASSBio-881 published by Duarte et al. (2007) to show that oral administration of LASSBio-881 is effective at inhibiting thermal hypernociception and in reducing nociceptive responses following neuropathy. A number of TRPV1 antagonists have been tested in randomized, placebo-controlled trials for inflammatory and neuropathic pain conditions. They have proved to be efficacious systemically and do not cause as many undesirable effects as agonists. However, the main drawback revealed in these trials has been the development of hyperthermia (Wong and Gavva, 2009). With regard to this side effect, we showed that a high dose of LASSBio-881 had no effect on the rectal temperature of the animals 60 min after its oral administration. However, targets other than TRPV1 could compensate for the hyperthermic effects of high doses of LASSBio-881. One possible target is COX-1, where LASSBio-881 has an IC50 of 85 µM, inhibiting about 12% of its activity in vitro at 10 µM (our unpublished data). Indeed, treatment with a lower dose of LASSBio-881 did in fact cause hyperthermia, suggesting that at higher doses COX-1 inhibition might counteract the thermogenesis caused by TRPV1 antagonism.
Activation of CB1 receptors in the hypothalamus is known to induce acute hypothermia (Rawls et al., 2002). Although LASSBio-881 has been shown to displace a CB1 ligand in rat brain homogenates (Duarte et al., 2007), the nature of this interaction has not been established. Any CB1 agonist activity would contribute to the lack of hyperthermia, but would also lead to hypolocomotion and catalepsy, which were never observed in any experiment with LASSBio-881 in mice or rats.
Other possible actions of LASSBio-881 in the pain pathway
Designed as a multi-pharmacophoric molecule, LASSBio-881 has been shown to modulate multiple targets in the pain pathway, which could account for its antinociceptive and modest anti-inflammatory activity. As mentioned, high concentrations of LASSBio-881 can inhibit COX-1 and COX-2 in vitro, with IC50 of 85 and 150 µM, respectively (unpublished data). In the present work, we report that LASSBio-881 can antagonize CAP-evoked TRPV1 currents with an IC50 of 14.05 µM. This indicates that perhaps TRPV1 antagonism is prevalent over COX inhibition in promoting antinociception. However, we cannot rule out the possibility that in vivo the potencies of TRPV1 antagonism and COX inhibition are different from those detected in vitro. An agonist effect at the cannabinoid receptor might also contribute to antinociception of LASSBio-881, as discussed earlier, but there are no indications that this occurs, at least from our in vivo observations. Other targets known to be modulated by TRPV1 antagonists are fatty acid amide hydrolase, an enzyme that metabolizes anandamide, TRPV3, TRPV4, TRPM8 and TRPA1 (Szallasi et al., 2007). The activity of LASSBio-881 on these targets remains to be established.
N-acylhydrazones as TRPV1 ligands
TRPV1 antagonists have a typical functional structure, as reviewed by Szallasi et al. (2007). They contain an aromatic ring with an H-bond acceptor or aryl interaction, connected through a linker to another dipole-interacting moiety such as urea and finally joined through another linker to a hydrophobic side chain. All these elements can be readily identified in LASSBio-881, the aromatic ring being a 1,3-benzodioxolyl, dipole-interacting moiety provided by the N-acylhydrazone and the lipophilic side chain represented by 3,5-di-tert-butyl-4-hydroxyphenyl (Duarte et al., 2007). It must be pointed out, however, that this is the first time an N-acylhydrazone with antagonist activity at TRPV1 has been shown to be effective in models of acute, inflammatory and neuropathic nociception, without producing the main side effect of this class of drugs, hyperthermia at higher doses. Further studies with this class of molecules will focus on improving its efficacy and potency.
In conclusion, here we reported a new antinociceptive TRPV1 antagonist designed as a multi-pharmacophoric N-acylhydrazone, which is effective in different pain models. LASSBio-881 treatment does not cause hyperthermia at higher doses, possibly because it inhibits COX. Thus, compounds acting on both TRPV1 and other antinociceptive targets might improve analgesia and minimize the side effects caused by TRPV1 antagonism.
Acknowledgments
The authors wish to thank Dr David Julius for providing TRPV1 and TRPV1 Δ777-820 clones. We are grateful to Dr José Garcia Abreu for providing and caring for the frogs used in this work. This work was supported by Pronex, CNPq and FAPERJ.
Glossary
Abbreviations:
- CAP
capsaicin
- CB1
cannabinoid type 1
- PIP2
phosphatidylinositol-4,5-bisphosphate
- TRP
transient receptor potential
- TRPA1
TRP ankyrin subfamily type 1
- TRPM8
TRP melastatin subfamily type 8
- TRPV1
TRP vanilloid subfamily type 1
- TRPV3
TRP vanilloid subfamily type 3
- TRPV4
TRP vanilloid subfamily type 4
Conflict of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition (2009 revision) Br J Pharmacol. 2009;158:S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub SS, Botting RM, Goorha S, Colville-Nash PR, Willoughby DA, Ballou LR. Acetaminophen-induced hypothermia in mice is mediated by a prostaglandin endoperoxide synthase 1 gene-derived protein. Proc Natl Acad Sci USA. 2004;101:11165–11169. doi: 10.1073/pnas.0404185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Thiemermann C, Salvemini D. Potential therapeutic effect of antioxidant therapy in shock and inflammation. Curr Med Chem. 2004;11:1147–1162. doi: 10.2174/0929867043365396. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L. Endocannabinoids and related compounds: walking back and forth between plant natural products and animal physiology. Chem Biol. 2007;14:741–756. doi: 10.1016/j.chembiol.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Duarte CD, Tributino JL, Lacerda DI, Martins MV, Alexandre-Moreira MS, Dutra F, et al. Synthesis, pharmacological evaluation and electrochemical studies of novel 6-nitro-3,4-methylenedioxyphenyl-N-acylhydrazone derivatives: discovery of LASSBio-881, a new ligand of cannabinoid receptors. Bioorg Med Chem. 2007;15:2421–2433. doi: 10.1016/j.bmc.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos H, Chang R, Pereira EF, Castro NG, Aracava Y, Melo PA, et al. A novel thienylhydrazone, (2-thienylidene)3,4-methylenedioxybenzoylhydrazine, increases inotropism and decreases fatigue of skeletal muscle. J Pharmacol Exp Ther. 2001;299:558–566. [PubMed] [Google Scholar]
- Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314:410–421. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- Jhaveri MD, Elmes SJ, Kendall DA, Chapman V. Inhibition of peripheral vanilloid TRPV1 receptors reduces noxious heat-evoked responses of dorsal horn neurons in naive, carrageenan-inflamed and neuropathic rats. Eur J Neurosci. 2005;22:361–370. doi: 10.1111/j.1460-9568.2005.04227.x. [DOI] [PubMed] [Google Scholar]
- Lavich TR, Cordeiro RS, Silva PM, Martins MA. A novel hot-plate test sensitive to hyperalgesic stimuli and non-opioid analgesics. Braz J Med Biol Res. 2005;38:445–451. doi: 10.1590/s0100-879x2005000300016. [DOI] [PubMed] [Google Scholar]
- McNamara FN, Randall A, Gunthorpe MJ. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1) Br J Pharmacol. 2005;144:781–790. doi: 10.1038/sj.bjp.0706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima T, Obata K, Yamanaka H, Dai Y, Fukuoka T, Tokunaga A, et al. Activation of p38 MAPK in primary afferent neurons by noxious stimulation and its involvement in the development of thermal hyperalgesia. Pain. 2005;113:51–60. doi: 10.1016/j.pain.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J Pharmacol Exp Ther. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- Santos AR, Calixto JB. Ruthenium red and capsazepine antinociceptive effect in formalin and capsaicin models of pain in mice. Neurosci Lett. 1997;235:73–76. doi: 10.1016/s0304-3940(97)00722-2. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, et al. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


