Abstract
Background and purpose:
Cholinesterase inhibitors have been widely used for the treatment of patients with dementia. Monitoring of the cholinesterase activity in the blood is used as an indicator of the effect of the cholinesterase inhibitors in the brain. The selective measurement of cholinesterase with low tissue dilution is preferred for accurate monitoring; however, the methods have not been established. Here, we investigated the effect of tissue dilution on the action of cholinesterase inhibitors using a novel radiometric method with selective substrates, N-[14C]methylpiperidin-4-yl acetate ([14C]MP4A) and (R)-N-[14C]methylpiperidin-3-yl butyrate ([14C]MP3B_R), for AChE and butyrylcholinesterase (BChE) respectively.
Experimental approach:
We investigated the kinetics of hydrolysis of [14C]-MP4A and [14C]-MP3B_R by cholinesterases, and evaluated the selectivity of [14C]MP4A and [14C]MP3B_R for human AChE and BChE, respectively, compared with traditional substrates. Then, IC50 values of cholinesterase inhibitors in minimally diluted and highly diluted tissues were measured with [14C]MP4A and [14C]MP3B_R.
Key results:
AChE and BChE activities were selectively measured as the first-order hydrolysis rates of [14C]-MP4A and [14C]MP3B_R respectively. The AChE selectivity of [14C]MP4A was an order of magnitude higher than traditional substrates used for the AChE assay. The IC50 values of specific AChE and BChE inhibitors, donepezil and ethopropazine, in 1.2-fold diluted human whole blood were much higher than those in 120-fold diluted blood. In addition, the IC50 values of donepezil in monkey brain were dramatically decreased as the tissue was diluted.
Conclusions and implications:
This method would effectively monitor the activity of cholinesterase inhibitors used for therapeutics, pesticides and chemical warfare agents.
Keywords: AChE, butyrylcholinesterase, N-methylpiperidin-4-yl acetate, MP4A (R)-N-methylpiperidin-3-yl butyrate, MP3B_R
Introduction
Centrally acting cholinesterase inhibitors have been used for the treatment of dementias such as Alzheimer's disease, and as pesticides and chemical warfare agents. Cholinesterase inhibitors, by blocking the activity of the enzymes, AChE (EC 3.1.1.7) and/or butyrylcholinesterase (BChE, EC 3.1.1.8), elevate the concentration of ACh and activate cholinergic neurones. The decrease in cholinesterase activity induced by the inhibitors in the brain cannot be measured in humans without positron emission tomography; hence, this variable is usually monitored by measuring cholinesterase activity in the blood; this acts as a surrogate marker to predict the clinical effects (Padilla et al., 2007). The inhibition of cholinesterases in the blood is measured by the use of traditional colorimetric (Ellman et al., 1961) and radiometric methods (Lewis and Eldefrawi, 1974) in which acetylthiocholine (ATCh) and [acetyl-3H/14C]-ACh ([3H/14C]ACh) are used as AChE substrates respectively. As ATCh and ACh are not selectively hydrolysed by AChE, but are hydrolysed by BChE, the selective cholinesterase activity is measured by separating red blood cells and plasma, which contain AChE and BChE, respectively (Brimijoin and Hammond, 1988). In particular, cholinesterase activity of red blood cells has been adopted to predict the therapeutic effects of the cholinesterase inhibitors, and to optimize the dose (Sramek and Cutler, 2000). However, selective cholinesterase inhibition in tissues other than blood is hard to measure because separation of AChE and BChE is impossible.
Liquid chromatography with ultraviolet or mass detection is an essential tool for the analysis of the concentration of chemicals in the blood or other tissues. Kosasa et al. (1999) used liquid chromatography to measure the concentration of donepezil in various tissues of rat. However, the concentration of a cholinesterase inhibitor in the blood is not linearly correlated to the inhibitory effect (Rogers et al., 1998; Tiseo et al., 1998); therefore, the inhibitory effect of cholinesterase inhibitors is hard to estimate with these pieces of equipment. In contrast, the decrease in cholinesterase activity in the blood, induced by the inhibitors, is linearly correlated to the extent of cholinesterase inhibition in the brain (Padilla et al., 2007). Furthermore, in order to assess the cholinesterase inhibition accurately, the concentration of any metabolite that possesses an inhibitory effect on cholinesterases should also be measured with liquid chromatography. Indeed, it is important to measure the active metabolite of donepezil, 6-O-desmethyl donepezil, in human plasma (Patel et al., 2008). Hence, by measuring total cholinesterase activity, the actual cholinesterase inhibition can be quantified even if some unknown cholinesterase inhibitors are present in the tissue samples.
Cholinesterase inhibitors used as therapeutics are classified into two types: irreversible, such as rivastigmine, and reversible, such as donepezil. To monitor cholinesterase inhibition in the tissue, minimal dilution of the tissue samples is preferred to avoid reactivation of carbamylated cholinesterases and dilution of the reversible inhibitors (Winteringham and Fowler, 1966; Williams and Casterline, 1969; Nostrandt et al., 1993; Hunter et al., 1997; Padilla et al., 2007). In addition, the IC50 values of inhibitors in highly diluted tissue would be different from those in minimally diluted tissue, because an inhibitor such as donepezil shows non-specific binding to tissue components with a high fraction, and as a result, a small fraction of an inhibitor could interact with cholinesterases in the intact tissue (Shiraishi et al., 2005). Consequently, evaluation of cholinesterases in minimally diluted tissue samples is important in pre-clinical studies. However, a method that allows selective measurement of cholinesterase activity with minimal tissue dilution has not been established.
N-[11C]methylpiperidin-4-yl acetate ([11C]MP4A) and (R)-N-[11C]methylpiperidin-3-yl butyrate ([11C]MP3B_R) are choline ester derivatives developed as probes for in vivo measurement of cerebral AChE and BChE activity, respectively, by positron emission tomography (Iyo et al., 1997; Kikuchi et al., 2001). In particular, [11C]MP4A and its derivative, N-[11C]methylpiperidin-4-yl propionate, have been used to evaluate the pharmacological effect of the AChE specific inhibitor, donepezil, used for the treatment of Alzheimer's disease (Kuhl et al., 2000; Kaasinen et al., 2002; Bohnen et al., 2005; Shiraishi et al., 2005). Cerebral cholinesterase activity has been measured in vivo by positron emission tomography, and in vitro by evaluation of choline ester derivatives, and the metabolism of the compounds in the blood and brain was measured with TLC (Irie et al., 1996; Iyo et al., 1997; Kikuchi et al., 2001; 2004; 2005; Snyder et al., 2001; Shao et al., 2003; Roivainen et al., 2004). Here, by applying this radio-TLC method, we propose a novel assay method for the selective measurement of the activities of AChE and BChE in vitro using 14C-labelled MP4A and MP3B_R, respectively (Figure 1). AChE and BChE activities in minimally diluted human whole blood were measured as the first-order hydrolysis rates of [14C]MP4A and [14C]-MP3B_R, respectively, which were highly selective for their corresponding cholinesterase. Using this novel radiometric method proposed, we demonstrated an effect of the dilution of blood samples on the IC50 values of cholinesterase inhibitors, that is, the IC50 values of donepezil, an AChE specific inhibitor, and ethopropazine, a BChE inhibitor, in the minimally diluted blood samples were much larger than those in the highly diluted tissue samples, as expected. Also, the IC50 values of donepezil in the minimally diluted brain samples were much larger than those in the highly diluted brain samples.
Figure 1.
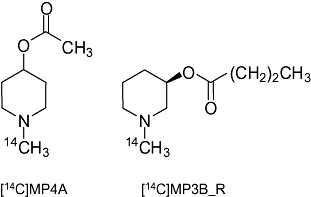
Structures of [14C]MP4A and [14C]MP3B_R.
Methods
Reagents
ATCh, butyrylthiocholine (BTCh), 1-methyl-4-piperidinol (MP4OH) and (R)-1-methyl-3-piperidinol ((R)-MP3OH) were purchased from Sigma-Aldrich Japan K.K. (Tokyo, Japan). [14C]ACh (2.0 MBq·µmol−1) and [14C]methyl iodide (2.0 MBq·µmol−1) were obtained from American Radiolabeled Chemicals Inc. (St Louis, MO, USA) and GE Healthcare UK Ltd. (Amersham Place, Buckinghamshire, UK) respectively. Other chemicals were of reagent grade or better, and were available commercially.
Preparation of [14C]MP4A and [14C]MP3B_R
Authentic samples and piperidinol esters as precursors for the radiolabelling of MP4A and MP3B_R were prepared using a previously reported method (Kikuchi et al., 2001). Briefly, [14C]methyl iodide in acetone (7.4 MBq in 1 mL) was added to each piperidinol ester (1 mg in 0.5 mL of acetone), and the solution was allowed to stand for 1 h at 50°C. A drop of 4 M HCl/ethyl acetate solution was added to the reaction solution, followed by the evaporation of the solvent under nitrogen gas. The residue was dissolved in 200 µL of methanol, and subjected to TLC with a glass-backed normal phase silica gel plate (silica-gel 60 F254, Merck, Tokyo, Japan) and a mixture of ethyl acetate : iso-propanol : 28% ammonia (15:5:1) as a developing solvent. The radioactive zone corresponding to the authentic compound was collected, and each 14C-labelled piperidinol ester was extracted with a 1:9 mixture of methanol and chloroform, followed by removal of silica gel by filtration. Radiochemical yield based on [14C]methyl iodide was usually more than 70%, and purity was more than 99%. The specific activity of 14C-labelled compounds is the same as that of [14C]methyl iodide (2.0 MBq·µmol−1). The solution was stocked at 4°C after adding a small amount of sodium sulphate. When the purity was less than 98%, the stock solution was concentrated under nitrogen gas, followed by removal of impurities by silica gel column chromatography (ϕ10 × 20 mm, chloroform). Before use in the enzyme assay, a drop of 1 M HCl/ethyl acetate solution was added to each of the [14C]MP4A and [14C]MP3B_R solutions, and the organic solvent was removed under nitrogen gas, followed by the addition of phosphate buffer (0.1 M, pH 7.4).
Hydrolysis rate of cholinesterase substrates in pure human cholinesterases
Purified human AChE (from erythrocytes) and BChE (from serum) were obtained from Sigma-Aldrich Japan K.K. All assays described below were performed in triplicate. The hydrolysis rates of the substrates were measured in serially mixed solutions of human AChE and BChE (AChE/BChE: 100/0, 80/20, 50/50, 20/80, 0/100; % volume) to validate the enzyme selectivity of the substrates and proportionality of the rate to the enzyme concentration. The AChE (78 U·L−1) and BChE (94 U·L−1) solutions were diluted to an appropriate concentration for each experiment, except in that for the estimation of Michaelis–Menten parameters, and the relative activities of AChE and BChE used in each experiment were a constant 1 and 1.2 respectively. One unit represented the amount of AChE and BChE that hydrolysed 1.0 µmol of ATCh and BTCh, respectively, per min at pH 7.4 at 20°C. To calculate enzyme activity (U·L−1), 13 600 M−1·cm−1 of the molar absorption coefficient was used (Ellman et al., 1961).
Hydrolysis rates of ATCh and BTCh in pure human cholinesterases
Hydrolysis rates of ATCh and BTCh in pure human cholinesterases were measured by Ellman's method (Ellman et al., 1961). Three millilitres of the serially mixed enzyme solutions (AChE, 2.6 U·L−1; BChE, 3.1 U·L−1) was placed in cuvettes, and 0.10 mL of 5,5′-dithio-bis(2-nitrobenzoic acid) solution [10 mM in phosphate buffer (0.1 M, pH 7.4)] was added. After standing for 15 min, 10 µL of ATCh and BTCh (75 mM each in phosphate buffer) was added to the cuvettes, and the absorbance of the samples was measured at 412 nm with a double-beam spectrophotometer (U-3900H, Hitachi High-Technologies Co., Tokyo, Japan) at 20°C for 20 min. For reference and measurement of non-enzymatic hydrolysis, phosphate buffer was used instead of substrate solution and enzyme solution respectively.
Hydrolysis rates of [acetyl-1-14C]-ACh in pure human cholinesterases
A radiometric method with [acetyl-1-14C]-ACh to measure the hydrolysis rates in pure human cholinesterases was performed according to a reported procedure (Mizobe and Livett, 1983). Reactions were started by the addition of 50 µL of [14C]ACh (10.7 MBq·mol−1, 2 mM) to 50 µL of enzyme solutions (AChE, 10 U·L−1; BChE, 12 U·L−1), and the mixed solutions were incubated at 19°C for 5–30 min. The reaction was stopped by adding 0.1 mL of stop solution (1 M of chloroacetic acid, 0.5 M of NaOH and 2 M of NaCl, pH 2.5), followed by 4 mL of scintillation cocktail (100 mL of isoamyl alcohol, 4 mg of 2,5-diphenyloxazole, 50 mg of 1,4-bis-2-(5-phenyloxazolyl)-benzene in 900 mL of toluene). The radioactivity was detected with a liquid scintillation counter (LS 6500, Beckman Coulter, Inc., Fullerton, CA, USA).
Kinetics of hydrolysis of [14C]MP4A and [14C]MP3B_R by cholinesterases
When the concentration of the 14C-labelled compounds is much higher than that of cholinesterases, and is much lower than the Km value, the kinetics of the 14C-labelled compounds hydrolysis can be described as follows based on the Michaelis–Menten equation:
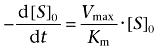 |
(1) |
where Vmax is the maximum velocity, [S]0 is the initial concentration of the 14C-labelled compounds and Km is the Michaelis–Menten constant. This equation is rearranged as follows:
| (2) |
where [S]t is the concentration of the 14C-labelled compounds at a time (t), and then,
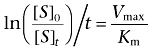 |
(3) |
Because Vmax is a value representative of the turn-over number multiplied by enzyme concentration (kcat[E]), the first-order hydrolysis rate (Vmax/Km) of the 14C-labelled compounds that can be calculated with the reaction rate and reaction time is proportional to the concentration of cholinesterases.
Hydrolysis rates of [14C]MP4A and [14C]MP3B_R in pure human cholinesterases
The hydrolysis rates of [14C]MP4A and [14C]MP3B_R in pure human cholinesterases were measured as follows: 100 µL of enzyme solutions (AChE, 78 U·L−1; BChE, 94 U·L−1) was placed in tubes and pre-incubated at 21°C for 30 min. Each 14C-labelled ester solution (4 kBq in 10 µL of phosphate buffer) was added to initiate the reaction. At a designated interval, 200 µL of EtOH was added to the mixture to stop the reaction, and the solution was centrifuged for 10 min at 5000× g. Then, 5 µL of the supernatant was applied to a silica gel TLC plate, and the TLC plate was developed up to 5 cm above the origin with a mixture of ethyl acetate : iso-propanol : 28% ammonia (15:5:1). The air-dried TLC plate was covered with a 5 µm thick film and placed in a cassette in contact with the imaging phosphor plate for 1 h. The spot of the radioactive ester and alcoholic metabolite was confirmed by co-spotting of the authentic samples and visualization with I2. Radioactivity corresponding to the Rf value of the esters and alcoholic metabolites was quantified using an imaging plate reader (BAS 1800II, Fujifilm Co., Tokyo, Japan). First-order hydrolysis rates for the cholinesterases were calculated using Equation 3. In addition, the first-order kinetics in human whole blood was confirmed experimentally as described below.
For estimation of Km and Vmax of MP4A and MP3B_R for AChE and BChE, respectively, the initial hydrolysis rates (zero-order rate, µmol·min−1·L−1) at approximately 0.1–10 mM of the substrates were measured using AChE (84 U·L−1) and BChE (33 U·L−1) solutions. The substrate solutions were prepared by the addition of various amounts of the non-radiolabelled esters to the 14C-labelled ester solution (4 kBq in 10 µL of phosphate buffer). The procedure for measuring the hydrolysis rate was the same as that described above. Each value of hydrolysis rate at various concentrations of the substrates was measured in duplicate. The Km and Vmax values were estimated by non-linear regression analysis based on Michaelis–Menten kinetics.
Hydrolysis rate of [14C]MP4A and [14C]MP3B_R in human whole blood
Three healthy human subjects (range: 30–34 years old; male) participated in the present study after giving written informed consent. None of the subjects were taking any medication at the time of the study. The Institutional Review Board of the National Institute of Radiological Sciences in Japan approved this study. Blood samples were collected from the median cubital vein into heparin-coated tubes. Ten microlitres of [14C]MP4A and [14C]MP3B_R (4 kBq each in phosphate buffer) solutions was added to 100 µL of each blood sample, and the reaction was stopped by adding 200 µL of ethanol. The procedure for measuring the hydrolysis rate was the same as that described above. A simultaneous assay for both AChE and BChE activity was performed as follows: 100 µL of a mixed solution of [14C]MP4A and [14C]MP3B_R (40 kBq) was added to 1 mL of blood from an individual (34 years old; male), and the 100 µL reaction mixtures were collected in tubes that contained 200 µL of ethanol, 20, 30, 40, 60 and 90 s after the addition of the substrate mixtures. The procedure for measuring the hydrolysis rate of the substrates was the same as that described above.
IC50 of cholinesterase inhibitors
Theoretical hydrolysis rate of [14C]-MP4A and [14C]-MP3B_R with inhibition
In the same way as described above (Equations 1–3), when a competitive or non-competitive cholinesterase inhibitor exists, the kinetics of hydrolysis of the 14C-labelled compounds can be expected to be as follows:
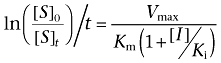 |
(4) |
where [I] is the concentration of an inhibitor, and Ki is the inhibition constant. From this equation, the Ki value of a competitive or non-competitive inhibitor can be calculated with a simple one-parameter model as follows:
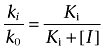 |
(5) |
where ki and k0 represent the first-order hydrolysis rate of the 14C-labelled compounds (ln([S]0/[S]t)/t) with and without an inhibitor, respectively, and [I] represents the concentration of the inhibitor. When the first-order hydrolysis rate of the 14C-labelled compounds is reduced to 50% by the inhibitor, Equation 5 can be described as follows:
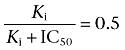 |
(6) |
where IC50 is the concentration of an inhibitor that inhibits the enzyme activity by 50%. From Equation 6, Ki equals IC50, and thus, Equation 5 can be rearranged as follows:
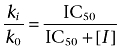 |
(7) |
IC50 of donepezil and ethopropazine in human whole blood
Intact and 100-fold diluted human whole blood samples (100 µL) from a healthy human subject (34 years old; male) were placed into reaction tubes. The blood was diluted with phosphate-buffered saline (0.01 M, pH 7.4). Ten microlitres of donepezil hydrochloride and ethopropazine hydrochloride was added to the blood sample at various concentrations (final concentration, donepezil; 0.001–10 µmol·L−1, ethopropazine; 0.026–260 µmol·L−1). Then, a solution of [14C]MP4A and [14C]MP3B_R [4 kBq in 10 µL phosphate-buffered saline (0.01 M, pH 7.4)] was added to donepezil and ethopropazine samples, respectively, to initiate the reaction. The procedure for measuring the hydrolysis rate of 14C-labelled esters was the same as that described above. The IC50 values were estimated by non-linear regression analysis based on Equation 7.
IC50 of donepezil in homogenates of monkey brain
The postmortem cerebral cortex of a rhesus monkey (Macacca mulatta, male, 23 years old) was homogenized in ice-cold 0.1% Tween 20 containing phosphate buffer (0.1 M, pH 7.4). One hundred microlitres of homogenate samples prepared at concentrations of 245, 101 and 0.776 mg·mL−1 was placed into reaction tubes. Ten microlitres of donepezil hydrochloride solution was added to the homogenate at various concentrations (final concentration, 0.002–20 µg·mL−1). Then, [14C]MP4A solution (4 kBq in 10 µL phosphate buffer) was added to the samples to initiate the reaction. The procedure for measuring the hydrolysis rate of [14C]MP4A was the same as that described above. The IC50 values were estimated by non-linear regression analysis based on Equation 7.
Statistical analysis
The standard errors of Km, Vmax and IC50, estimated by non-linear least squares, were calculated with the variance–covariance matrix (Veng, 1977; Carson, 1986). The effect of tissue dilution on the extent of cholinesterase inhibition by the cholinesterase inhibitors was analysed by two-way repeated-measures anova. The significance level was set at P < 0.05.
Results
Kinetics of hydrolysis of MP4A and MP3B_R mediated by AChE and BChE
The Km and Vmax values of MP4A for AChE and MP3B_R for BChE, respectively, were estimated to confirm whether the first-order hydrolysis of [14C]MP4A and [14C]MP3B_R by corresponding cholinesterases was based on Michaelis–Menten conditions. The concentrations of 14C-labelled substrates, cholinesterases and Km are summarized in Table 1, and the initial hydrolysis rates with various substrate concentrations are presented in Figure 2. The concentrations of 14C-labelled substrates used were much higher than that of cholinesterases, whereas the concentrations of 14C-labelled substrates were lower than the Km values. First-order hydrolysis rates of [14C]MP4A by AChE and [14C]MP3B_R by BChE, which were measured with the same enzyme solution used to obtain an estimation, were consistent with independently estimated Vmax/Km values. These results indicated that [14C]MP4A and [14C]MP3B_R were hydrolysed by corresponding cholinesterases with first-order kinetics based on Michaelis–Menten conditions at the concentration used in this study.
Table 1.
Concentrations and parameters of substrates and cholinesterases
| Substratea(µM) | AChEb(µM) | BChEb(µM) | Km (µM) | Vmax (µmol·min−1·L−1) | Vmax/Km (min−1) | First-order ratec(min−1) | |
|---|---|---|---|---|---|---|---|
| MP4A | 17 | 0.00022 (0.015) | – | 1600 ± 90 | 31 ± 0.58 | 0.019 | 0.019 ± 0.00038 |
| MP3B_R | 17 | – | 0.0014 (0.32) | 260 ± 4.9 | 20 ± 0.099 | 0.077 | 0.080 ± 0.0024 |
The substrate concentrations shown were obtained using 10 µL of solution containing 4 kBq of radiolabelled substrate (2.0 MBq·µmol−1) to 100 µL of enzyme solution.
The concentrations of AChE (84 U·L−1) and BChE (33 U·L−1) used for parameter estimation were calculated using reported turn-over numbers (kcat: AChE, 390 000·min−1; BChE, 24 000·min−1) (Blong et al., 1997; Cohen et al., 2001). In addition, the concentrations of the cholinesterases in the human whole blood were calculated based on the reported AChE and BChE activities (AChE: 19 U·L−1; BChE: 24 U·L−1) measured with ATCh and BTCh, respectively, in 316-fold diluted human whole blood at 37°C (Worek et al., 1999), and are presented in parentheses.
The first-order hydrolysis rates of substrates in the human pure enzyme solution used for parameter estimation are presented as mean ± SEM of triplicate values.
Figure 2.
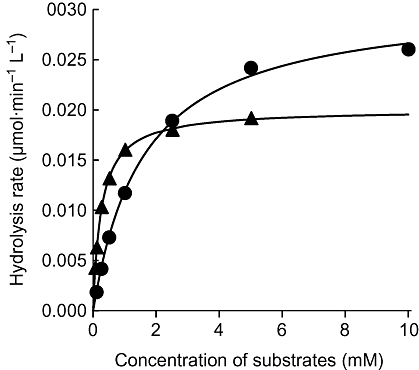
Initial hydrolysis rates of MP4A and MP3B_R by human AChE and BChE, respectively, at various substrate concentrations. Observed value of MP4A (circles) and MP3B_R (triangles), and regression curves based on Michaelis–Menten kinetics are presented. Each value was measured in duplicate.
Hydrolysis rate of cholinesterase substrates in pure enzymes
To investigate the proportion of the hydrolysis rates of substrates that corresponded to the enzyme concentration, and selectivity of each to its corresponding cholinesterase, the hydrolysis rates of the substrates in the serially mixed solution of human AChE and BChE were measured (Table 2). The hydrolysis rates of all substrates were proportional to the enzyme concentration (r2 > 0.99). ATCh and ACh were hydrolysed at a high rate by BChE, whereas the hydrolysis rate of [14C]MP4A by BChE was comparable to the low, non-enzymatic hydrolysis rate. The ratios of hydrolysis rates of ATCh, ACh and [14C]MP4A in the solution of AChE alone to that of BChE alone were 1.5, 5.3 and 40, respectively, indicating that the selectivity of [14C]MP4A for AChE was an order of magnitude higher than those of ACh and ATCh. As for BChE substrates, the hydrolysis rates of BTCh and [14C]MP3B_R by AChE were less than the non-enzymatic hydrolysis rate. The high BChE selectivity of [14C]MP3B_R compared with that of BTCh as reported previously (Kikuchi et al., 2001) was replicated in this study.
Table 2.
Hydrolysis rate of the substrates in a mixture of human AChE and BChE
|
AChE/BChE fractiona |
||||||
|---|---|---|---|---|---|---|
| 100/0 | 80/20 | 50/50 | 20/80 | 0/100 | 0/0b | |
| Traditional substrates | Zero-order hydrolysis rate (µmol·min−1·L−1)c | |||||
| ATCh | 2.5 | 2.3 | 2.0 | 1.8 | 1.6 | 0.0014 |
| ACh | 4.9 | 4.5 | 2.7 | 1.8 | 0.92 | 0.19 |
| BTCh | 0.0037 | 0.68 | 1.6 | 2.5 | 3.0 | 0.094 |
| Current substrates | First-order hydrolysis rate (min−1)c | |||||
| [14C]MP4A | 0.028 | 0.023 | 0.014 | 0.0050 | 0.00070 | 0.00061 |
| [14C]MP3B_R | 0.000095 | 0.038 | 0.094 | 0.15 | 0.19 | 0.00047 |
The fractions are presented as % volume of AChE and BChE solution mixed. The ratio of AChE to BChE activity used was 1:1.2. Each value was measured in triplicate, and the coefficient of variation of each value was less than 0.1% for ATCh and BTCh, less than 1% for [14C]MP4A and [14C]MP3B_R and less than 2% for ACh.
The hydrolysis rates in phosphate buffer (0.1 M, pH 7.4).
Note that the hydrolysis rates are measured as a zero-order rate for traditional substrates, and as a first-order rate for [14C]MP4A and [14C]MP3B_R.
Hydrolysis rate of [14C]MP4A and [14C]MP3B_R in human whole blood
Figure 3 shows the analytical image of radioactivity on a TLC plate developed after the reaction of [14C]MP4A and [14C]MP3B_R in a human blood sample. The radioactivity at the origin was 1.1 ± 0.18% (mean ± SD). When a mixture of [14C]MP4A and [14C]MP3B_R was added to a blood sample to simultaneously measure the activities of ACE and BChE, the radioactivities corresponding to [14C]MP4A, [14C]MP3B_R and their alcoholic metabolites were clearly separated by TLC. The time-dependent hydrolysis of [14C]MP4A and [14C]MP3B_R in human whole blood is shown in Figure 4; [14C]MP4A and [14C]MP3B_R were hydrolysed to 34 and 10%, respectively, of basal levels by 1 min in a first-order manner (r2 > 0.99). The hydrolysis rates of [14C]MP4A and [14C]MP3B_R measured from the simultaneous assay in a human whole blood sample were comparable to those measured from their respective assays ([14C]MP4A, 1.2 ± 0.012 vs. 1.1 ± 0.011 min−1·mL−1; [14C]MP3B_R, 2.5 ± 0.059 vs. 2.9 ± 0.057 min−1·mL−1). The half-lives of [14C]MP4A and [14C]MP3B_R in blood from the three human healthy subjects were 0.74 ± 0.080 and 0.25 ± 0.029 min (mean ± SD) respectively.
Figure 3.
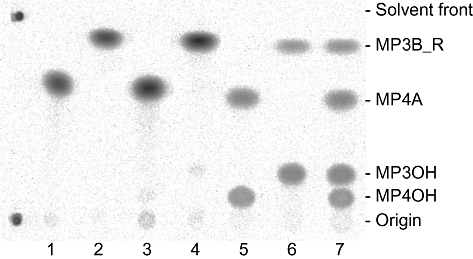
Separation of [14C]MP4A, [14C]MP3B_R and their hydrolysed metabolites by TLC. Images of the radioactivities on a TLC plate before and after incubation of [14C]MP4A and [14C]MP3B_R in human blood are presented. Lanes 1 and 2, purity of [14C]MP4A and [14C]MP3B_R solution added to human blood samples, respectively; lanes 3 and 4, purity of [14C]MP4A and [14C]MP3B_R after a 60 min incubation in phosphate buffer (pH 7.4, 0.1 M), respectively; lanes 5 and 6, purity of [14C]MP4A and [14C]MP3B_R after a 0.5 min incubation in human blood, respectively; lane 7, purity of [14C]MP4A and [14C]MP3B_R after a 0.5 min incubation of the mixture in human blood. MP3OH and MP4OH are the metabolites of MP4A and MP3B_R respectively.
Figure 4.
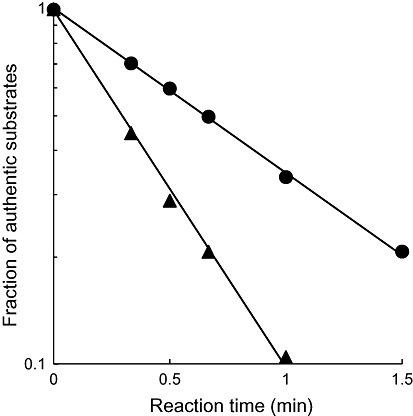
Time-dependent hydrolysis of [14C]MP4A and [14C]MP3B_R in human whole blood. The fractions of authentic substrates were measured in triplicate. The coefficient of variation was less than 2%, and the error bars were smaller than the symbols. Regression curves based on first-order kinetics are presented.
Estimation of IC50 for donepezil and ethopropazine in human blood sample
We demonstrated the effect of tissue dilution on the extent of cholinesterase inhibition by donepezil and ethopropazine in a human whole blood sample with the novel radiometric method (Figures 5 and 6). As shown in the TLC image (Figure 5), the generation of the alcoholic metabolites in the blood was almost completely inhibited by specific cholinesterase inhibitors. This indicates that the hydrolysis of [14C]MP4A and [14C]MP3B_R is selectively mediated by corresponding cholinesterases even in the crude tissue samples that contain other esterases. By diluting the blood, the IC50 values of the cholinesterase inhibitors were dramatically decreased (two-way repeated-measures anova, P < 0.05). The IC50 value of donepezil for AChE at blood concentrations of 0.83 mL blood·mL−1 (1.2-fold dilution) was 41 ± 2.2 nM (mean ± SEM), whereas at a concentration of 0.0083 mL blood·mL−1 (120-fold dilution) it was 7.6 ± 0.61. Similarly, the IC50 value of ethopropazine for BChE at the lowest blood sample dilution was 1700 ± 130 nM (mean ± SEM), and the value was much higher than that of the most diluted blood (100 ± 5.2 nM).
Figure 5.
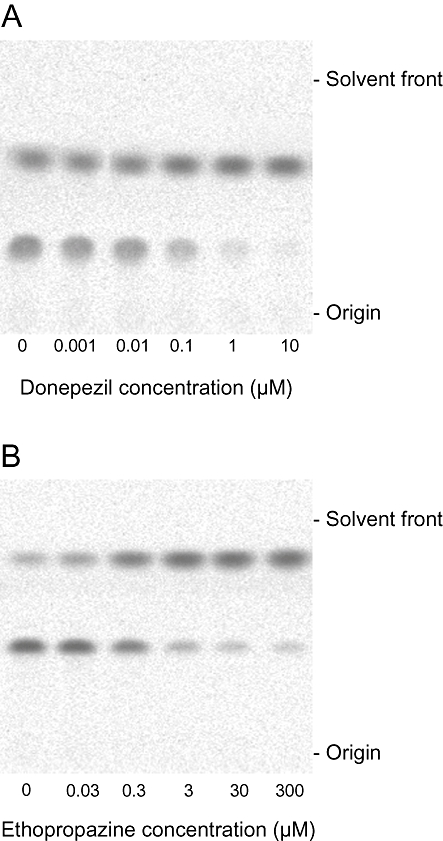
Inhibition of the hydrolysis of [14C]MP4A and [14C]MP3B_R by different concentrations of donepezil (A) and ethopropazine (B), respectively, in 0.83 mL blood·mL−1 of human whole blood. Images of the radioactivities on a TLC plate after incubation of [14C]MP4A (A) and [14C]MP3B_R (B) in human blood with inhibitors are presented.
Figure 6.
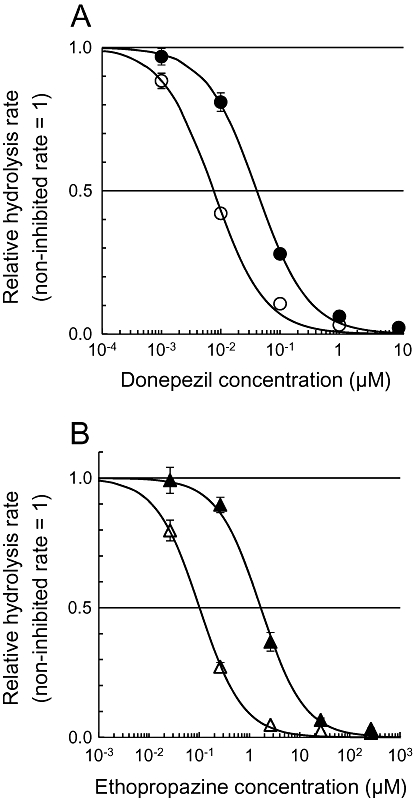
Inhibition of AChE and BChE by donepezil (A) and ethopropazine (B), respectively, in different concentrations of human whole blood (solid symbols, 0.83 mL blood·mL−1; open symbols, 0.0083 mL blood·mL−1). The values are presented as non-inhibited hydrolysis rates of [14C]MP4A (A) and [14C]MP3B_R (B) = 1. Regression curves based on Equation 7 (see Methods section) are presented.
Estimation of IC50 for donepezil in monkey brain sample
We demonstrated the effect of tissue dilution on the extent of AChE inhibition in monkey brain homogenate using donepezil and the novel radiometric method. Unlike donepezil, ethopropazine is not used clinically as a cholinesterase inhibitor, but is used to treat Parkinson's disease because of its anti-cholinergic action (Farquharson and Johnston, 1959). Therefore, ethopropazine was not used in this study. Figure 7 shows the effect of tissue dilution on the extent of AChE inhibition by donepezil in monkey brain tissue. As the tissue was diluted, the IC50 values estimated at brain tissue concentrations of 200, 84 and 0.65 mg·mL−1 decreased dramatically to 390 ± 31, 96 ± 7.3 and 15 ± 0.76 nM (mean ± SEM) respectively.
Figure 7.
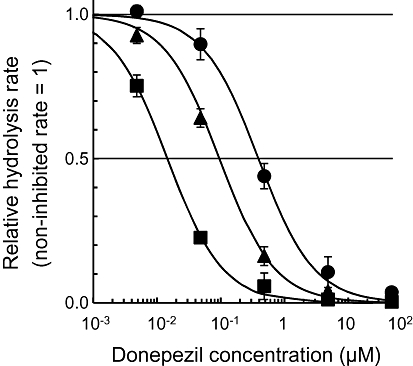
Inhibition of AChE by donepezil in different concentrations of monkey brain homogenate (0.67, 84 and 200 mg·mL−1). The values are presented as a non-inhibited hydrolysis rate of [14C]MP4A = 1. Regression curves based on Equation 7 (see Methods section) are presented.
Discussion and conclusions
In the current study, we first validated the novel radiometric method with [14C]MP4A and [14C]MP3B_R to selectively measure AChE and BChE respectively. Distinguishing points of the novel method from the traditional methods are that the cholinesterase activities (hydrolysis rate) are measured as first-order rates, and the substrates used are highly selective for their corresponding enzymes. As [14C]MP4A and [14C]MP3B_R were hydrolysed selectively with first-order kinetics based on Michaelis–Menten conditions, their hydrolysis rates as AChE and BChE activity could be readily calculated from the purity of [14C]MP4A and [14C]MP3B_R solutions added to the tissue samples and the fraction of residual authentic radioactivity observed after appropriate intervals (Equation 3). In human blood, non-enzymatic hydrolysis would be negligible and unnecessary to measure, because complete hydrolysis would be achieved within a few minutes ([14C]-MP4A, t1/2= 0.74 ± 0.080 min; [14C]MP3B_R, t1/2= 0.25 ± 0.029). From these results and in contrast to traditional methods, it appears that the BChE inhibitor added to tissue samples for the selective AChE assay (Naik et al., 2008) is dispensable. This protocol change is particularly advantageous when specifically measuring the concentration of AChE during the monitoring of anti-AChE therapeutics and exposure assessment, because the tissue samples contain other inhibitory agents.
To measure the hydrolysis rate of [14C]MP4A and [14C]MP3B_R, we applied a simple TLC method to separate [14C]MP4A, [14C]MP3B_R and their metabolites. The compounds were isolated by the development of the TLC for 5 cm with a freshly prepared mixture of ethyl acetate : iso-propanol : 28% ammonia (15:5:1). The separation was poor if concentrated ammonia was not used for the preparation of the developing solution. Although a mixture of dichloromethane, methanol and ammonia can be used as a developing solution, the method with ethyl acetate, isopropanol and ammonia used in this study yielded a better separation between N-[11C]methylpiperidin-4-yl butyrate and its alcoholic metabolite, which is the same metabolite as that of [14C]MP4A (Roivainen et al., 2004). This TLC method only requires [14C]MP4A and [14C]MP3B_R, and does not require specialized equipment or reagents. Moreover, multiple samples can be analysed on a single TLC plate; however, this TLC method would be unsuitable for use with an 3H-labelled compound because of the low β energy of 3H. To measure the hydrolysis rate of an 3H-labelled compound, solid-phase extraction would be applicable. N-[11C]methylpiperidin-4-yl propionate and its alcoholic metabolite were separated by solid-phase extraction (de Groot et al., 2003). In addition, a traditional radiometric procedure (Lewis and Eldefrawi, 1974) can be used for the selective measurement of AChE activity with MP4A instead of [3H/14C]ACh, if the acyl group of MP4A is radiolabelled.
In the past, the monitoring of cholinesterase inhibitors was performed by measuring cholinesterase activity in the blood as an indicator of clinical effects (Sramek and Cutler, 2000). Thus, we compared the IC50 values measured in this study with the reported IC50 values. In a clinical study, the concentration of donepezil in the plasma that inhibits 50% of red blood cell AChE activity was reported to be approximately 10–15 ng·mL−1 (24–36 nM) (Rogers et al., 1998; Tiseo et al., 1998). Donepezil inhibits AChE mainly in a non-competitive manner (i.e. IC50= apparent Ki) (Galli et al., 1994; Nochi et al., 1995; Sugimoto et al., 2002); therefore, this value can be directly compared with the IC50 value measured in this study with minimally diluted blood, and our IC50 value (41 nM) was comparable with previously reported values. Similarly, the IC50 value (7.6 nM) we obtained in highly diluted blood was in the range of reported values (6.7–12 nM) that were measured with pure human AChE (Ogura et al., 2000; Camps et al., 2008). Hence, the specific measurement of AChE activity in whole blood using a novel method with [14C]MP4A could be used to monitor the activity of AChE inhibitors. Also, similarly, [14C]MP3B_R could be used for monitoring BChE inhibitors in the blood, because the IC50 value (100 nM) of ethopropazine in highly diluted blood measured in this study was comparable to the previously reported value (Ki: 160 ± 30 nM) measured with human serum BChE (Simeon-Rudolf et al., 2001).
The discrepancy between the IC50 values in minimally diluted blood and in highly diluted blood is probably due to the high non-specific binding of donepezil and ethopropazine to blood components, such as albumin, in the minimally diluted blood. The free fraction of donepezil and ethopropazine in the minimally diluted blood that binds to cholinesterases would be different from that in highly diluted blood. Consequently, the ratio of IC50 values of the cholinesterase inhibitors in the highly diluted blood to those in the minimally diluted blood would differ depending on the non-specific binding fraction in the intact blood. In fact, it was estimated that approximately 81% of donepezil binds to non-specific components in human whole blood [(1–7.6 nM/41 nM) × 100%] in the range of the reported protein binding of donepezil (75–96%) in human plasma (Mihara et al., 1993; Tiseo et al., 1998; Nagy et al., 2004). Similarly, the estimated non-specific binding of ethopropazine to components in human whole blood was 94% [(1–100 nM/1700 nM) × 100%], which is consistent with the recognized high binding of phenothiazine derivatives to protein (Verbeeck et al., 1983; Brocks, 1999; Brocks and Maboudian-Esfahani, 1999).
AChE inhibition by cholinesterase inhibitors in the minimally diluted blood has been selectively measured by separating red blood cells, which mainly contain AChE (Brimijoin and Hammond, 1988). However, this method could not be transferred to the assessment of AChE inhibition in other tissues where AChE cannot be selectively separated. In contrast, our proposed novel method would allow selective assessment of the AChE inhibition in almost all tissues under minimally diluted conditions. In particular, pre-clinical assessment of AChE inhibition in the minimally diluted brain tissue by a centrally acting cholinesterase inhibitor is important to predict therapeutic effects and to optimize the dose, because, as in the blood, the inhibitors non-specifically bind to tissue constituents. Indeed, it was estimated that 99% of donepezil binding was non-specific in monkey brain (Shiraishi et al., 2005). A similar result was observed in this study. In 0.65 mg·mL−1 (1500-fold dilution) of monkey brain homogenate, in which tissue binding is considered negligible, the estimated IC50 for donepezil (15 nM) was almost the same as that for human AChE (12 nM) (Camps et al., 2008). The IC50 value, 390 nM, in 200 mg·mL−1 (fivefold dilution) of monkey brain homogenate is close to the value of 500–700 nM estimated in living monkeys (Shiraishi et al., 2005). From these IC50 values for donepezil in minimally diluted and highly diluted brain tissue, non-specific tissue binding of donepezil was estimated to be 96% [(1–15 nM/390 nM) × 100%], which is consistent with the previously reported value estimated with positron emission tomography (Shiraishi et al., 2005). The distribution volume of donepezil between plasma and brain is between 6 and 8 mL·g−1 in rats (Kosasa et al., 2000). Hence, the results in this study indicate that the non-specific binding of donepezil in the brain is much higher than that in blood. Donepezil binds to albumin and alpha-1-acid glycoprotein in the blood, whereas the constituents to which donepezil binds to in the brain are unknown. The free fraction of donepezil in fivefold diluted brain was estimated to be only 4%, but the value would be increased by the minimal dilution of the intact tissue because the high non-specific binding in monkey brain was readily dissociated by dilution. The non-specific tissue binding of donepezil in 84 mg·mL−1 of monkey brain homogenate was estimated to be 84% [(1–15 nM/95 nM) × 100%], that is, a 2.4-fold dilution of the homogenate from 200 mg·mL−1 was associated with a fourfold increase in the free fraction of donepezil in the brain homogenate. The dissociation of the non-specific binding in the brain would be mainly due to the dilution of the tissue constituents, because the free fraction of donepezil is increased as the tissue is diluted. Therefore, the exact fraction of non-specific binding in intact tissue would be estimated by extrapolation of IC50 values in various concentrations of tissue. However, the destruction of the tissue structure of brain by homogenization may potentially affect the non-specific binding. In such a case, the exact fraction of non-specific binding in intact tissue would not be able to be estimated with the current in vitro method.
It has been reported that the extent of cholinesterase inhibition in red blood cells measured with Ellman's method does not accord with that measured by the traditional radiometric method (Padilla et al., 2007). In addition, the extent of cholinesterase inhibition in red blood cells does not correlate with that measured in the brain with Ellman's method, whereas that in red blood cells was well correlated with that in the brain measured with the traditional radiometric method. Therefore, measurement of the cholinesterase activity of red blood cells with the traditional radiometric method has been used to assess the activity of centrally acting cholinesterase inhibitors. In contrast to Ellman's method, the efficacy of the current method at measuring the decrease in AChE activity induced by donepezil in the blood was comparable to that of the traditional radiometric method, as discussed above. One advantage of the current method over the traditional radiometric method is the selectivity of the substrates used. The specific activity of AChE in the intact tissues cannot be measured with the traditional radiometric method because the radiolabelled ACh is not selective enough for AchE, as shown in Table 2. A method with insufficient AChE selectivity results in an underestimation of AChE inhibition, or prevents the measurement of AChE-specific inhibition by cholinesterase inhibitors. Thus, the inhibition of AChE induced by cholinesterase inhibitors in the blood has been selectively measured by separating red blood cells, which mainly contain AChE (Brimijoin and Hammond, 1988). With our novel method, this separation is not necessary for the measurement of the selective AChE inhibition. Indeed, in this study, we demonstrated that the IC50 for the effect of donepezil on BChE contained in whole blood measured with the current method was comparable to that in red blood cells measured with the traditional radiometric method. The other advantage of the current method over the traditional radiometric method is the simplicity of the procedure. For the measurement of AChE activity in blood, the traditional radiometric method requires the separation of red blood cells from the blood and the preparation of carrier-added radiolabelled ACh, the solution for stopping the reaction, and the organic scintillation cocktail, whereas the current method requires only the preparation of the solutions of radiolabelled compounds.
Generally, this novel radiometric method, similar to traditional methods, can be used to measure cholinesterase activity in any tissues, even if the tissue contains any cholinesterase inhibitors. However, there are some limitations to measuring cholinesterase activity in minimally diluted tissue. Firstly, the extremely high AChE activity in the minimally diluted tissue such as striatum cannot be measured with [14C]MP4A because [14C]MP4A is completely hydrolysed within a few seconds in such high AChE-containing regions. Secondly, to measure cholinesterase activity in minimally diluted tissues, the tissues other than the body fluid need to be liquidized enough to mix the substrate solution. As discussed above, the dilution has no small effect on the extent of non-specific binding of cholinesterase inhibitors. In addition, the IC50 values of competitive cholinesterase inhibitors, such as carbamates, obtained with our novel method are theoretically equal to the apparent Ki; therefore, it should be noted that the IC50 values of the competitive inhibitors obtained with our novel method cannot be compared directly with those obtained using traditional methods.
To conclude, although a variety of methods have been developed to assess cholinesterase activity, drug monitoring and the actual exposure assessment of cholinesterase inhibitors are difficult because of insufficient AChE substrate selectivity and high tissue dilution. These problems can be overcome using the novel simple radio-TLC method described in this study, as it uses [14C]MP4A and [14C]MP3B_R for selective and easy measurement of the activities of AChE and BChE, respectively, in minimally diluted samples.
Acknowledgments
The authors thank Eisai Co., Ltd. (Tokyo, Japan) for the generous provision of donepezil. This work was partially funded by Showa Shell Sekiyu Foundation for the Promotion of Environmental Research.
Glossary
Abbreviations:
- ATCh
acetylthiocholine
- BChE
butyrylcholinesterase
- BTCh
butyrylthiocholine
- MP3B_R
(R)-N-methylpiperidin-3-yl butyrate
- MP3OH
N-methyl-3-piperidinol
- MP4A
N-methylpiperidin-4-yl acetate
- MP4OH
N-methyl-4-piperidinol
Conflict of interest
The authors have no conflict of interest with this study.
References
- Blong RM, Bedows E, Lockridge O. Tetramerization domain of human butyrylcholinesterase is at the C-terminus. Biochem J. 1997;327:747–757. doi: 10.1042/bj3270747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Koeppe RA, et al. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:315–319. doi: 10.1136/jnnp.2004.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimijoin S, Hammond P. Butyrylcholinesterase in human brain and acetylcholinesterase in human plasma: trace enzymes measured by two-site immunoassay. J Neurochem. 1988;51:1227–1231. doi: 10.1111/j.1471-4159.1988.tb03091.x. [DOI] [PubMed] [Google Scholar]
- Brocks DR. Anticholinergic drugs used in Parkinson's disease: an overlooked class of drugs from a pharmacokinetic perspective. J Pharm Pharm Sci. 1999;2:39–46. [PubMed] [Google Scholar]
- Brocks DR, Maboudian-Esfahani M. Disposition of ethopropazine enantiomers in the rat: tissue distribution and plasma protein binding. J Pharm Pharm Sci. 1999;2:23–29. [PubMed] [Google Scholar]
- Camps P, Formosa X, Galdeano C, Gómez T, Muñoz-Torrero D, Scarpellini M, et al. Novel donepezil-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. J Med Chem. 2008;51:3588–3598. doi: 10.1021/jm8001313. [DOI] [PubMed] [Google Scholar]
- Carson RE. Parameter estimation in positron emission tomography. In: Phelps M, Mazziotta J, Schelbert H, editors. Positron Emission Tomography and Autoradiography: Principles and Applications for the Brain and Heart. New York: Raven Press; 1986. pp. 347–390. [Google Scholar]
- Cohen O, Kronman C, Chitlaru T, Ordentlich A, Velan B, Shafferman A. Effect of chemical modification of recombinant human acetylcholinesterase by polyethylene glycol on its circulatory longevity. Biochem J. 2001;357:795–802. doi: 10.1042/0264-6021:3570795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Farquharson ME, Johnston RG. Antagonism of the effects of tremorine by tropine derivatives. Br J Pharmacol Chemother. 1959;14:559–566. doi: 10.1111/j.1476-5381.1959.tb00964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A, Mori F, Benini L, Cacciarelli N. Acetylcholinesterase protection and the anti-diisopropylfluorophosphate efficacy of E2020. Eur J Pharmacol. 1994;270:189–193. doi: 10.1016/0926-6917(94)90062-0. [DOI] [PubMed] [Google Scholar]
- de Groot TJ, Verheyen L, Bex M, Detré K, Mortelmans L, Verbruggen A, et al. Optimisation of the synthesis, quality control and metabolic analysis of [11C]PMP for the in vivo evaluation of cholinesterase activity in humans. J Labelled Comp Radiopharm. 2003;46(Suppl.):S203. [Google Scholar]
- Hunter DL, Marshall RS, Padilla S. Automated instrument analysis of cholinesterase activity in tissues from carbamate-treated animals: a cautionary note. Toxicol Methods. 1997;7:43–53. [Google Scholar]
- Irie T, Fukushi K, Namba H, Iyo M, Tamagami H, Nagatsuka S, et al. Brain acetylcholinesterase activity: validation of a PET tracer in a rat model of Alzheimer's disease. J Nucl Med. 1996;37:649–655. [PubMed] [Google Scholar]
- Iyo M, Namba H, Fukushi K, Shinotoh H, Nagatsuka S, Suhara T, et al. Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer's disease. Lancet. 1997;349:1805–1809. doi: 10.1016/S0140-6736(96)09124-6. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Någren K, Järvenpää T, Roivainen A, Yu M, Oikonen V, et al. Regional effects of donepezil and rivastigmine on cortical acetylcholinesterase activity in Alzheimer's disease. J Clin Psychopharmacol. 2002;22:615–620. doi: 10.1097/00004714-200212000-00012. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Fukushi K, Ikota N, Ueda T, Nagatsuka S, Arano Y, et al. Synthesis of piperidinyl and pyrrolidinyl butyrate for potential in vivo measurement of cerebral butyrylcholinesterase activity. J Labelled Comp Radiopharm. 2001;44:31–41. [Google Scholar]
- Kikuchi T, Zhang MR, Ikota N, Fukushi K, Okamura T, Suzuki K, et al. N-[18F]fluoroethylpiperidin-4-ylmethyl butyrate: a novel radiotracer for quantifying brain butyrylcholinesterase activity by positron emission tomography. Bioorg Med Chem Lett. 2004;14:1927–1930. doi: 10.1016/j.bmcl.2004.01.080. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Zhang MR, Ikota N, Fukushi K, Okamura T, Suzuki K, et al. N-[18F]fluoroethylpiperidin-4ylmethyl acetate, a novel lipophilic acetylcholine analogue for PET measurement of brain acetylcholinesterase activity. J Med Chem. 2005;48:2577–2583. doi: 10.1021/jm049100w. [DOI] [PubMed] [Google Scholar]
- Kosasa T, Kuriya Y, Matsui K, Yamanishi Y. Inhibitory effects of donepezil hydrochloride (E2020) on cholinesterase activity in brain and peripheral tissues of young and aged rats. Eur J Pharmacol. 1999;386:7–13. doi: 10.1016/s0014-2999(99)00741-4. [DOI] [PubMed] [Google Scholar]
- Kosasa T, Kuriya Y, Matsui K, Yamanishi Y. Inhibitory effect of orally administered donepezil hydrochloride (E2020), a novel treatment for Alzheimer's disease, on cholinesterase activity in rats. Eur J Pharmacol. 2000;389:173–179. doi: 10.1016/s0014-2999(99)00876-6. [DOI] [PubMed] [Google Scholar]
- Kuhl DE, Minoshima S, Frey KA, Foster NL, Kilbourn MR, Koeppe RA. Limited donepezil inhibition of acetylcholinesterase measured with positron emission tomography in living Alzheimer cerebral cortex. Ann Neurol. 2000;48:391–395. [PubMed] [Google Scholar]
- Lewis MK, Eldefrawi ME. A simple, rapid, and quantitative radiometric assay of acetylcholinesterase. Anal Biochem. 1974;57:588–592. doi: 10.1016/0003-2697(74)90114-6. [DOI] [PubMed] [Google Scholar]
- Mihara M, Ohnishi A, Tomono Y, Hasegawa J, Shimamura Y, Yamazaki K, et al. Pharmacokinetics of E2020, a new compound for Alzheimer's disease, in healthy male volunteers. Int J Clin Pharmacol Ther Toxicol. 1993;31:223–229. [PubMed] [Google Scholar]
- Mizobe F, Livett BG. Nicotine stimulates secretion of both catecholamines and acetylcholinesterase from cultured adrenal chromaffin cells. J Neurosci. 1983;3:871–876. doi: 10.1523/JNEUROSCI.03-04-00871.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy CF, Kumar D, Cullen EI, Bolton WK, Marbury TC, Gutierrez MJ, et al. Steady-state pharmacokinetics and safety of donepezil HCl in subjects with moderately impaired renal function. Br J Clin Pharmacol. 2004;58(Suppl.):18–24. doi: 10.1111/j.1365-2125.2004.01803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik RS, Doctor BP, Saxena A. Comparison of methods used for the determination of cholinesterase activity in whole blood. Chem Biol Interact. 2008;175:298–302. doi: 10.1016/j.cbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Nochi S, Asakawa N, Sato T. Kinetic study on the inhibition of acetylcholinesterase by 1-benzyl-4-[(5,6-dimethoxy-1-indanon)-2-yl]methylpiperidine hydrochloride (E2020) Biol Pharm Bull. 1995;18:1145–1147. doi: 10.1248/bpb.18.1145. [DOI] [PubMed] [Google Scholar]
- Nostrandt AC, Duncan JA, Padilla S. A modified spectrophotometric method appropriate for measuring cholinesterase activity in tissue from carbaryl-treated animals. Fundam Appl Toxicol. 1993;21:196–203. doi: 10.1006/faat.1993.1089. [DOI] [PubMed] [Google Scholar]
- Ogura H, Kosasa T, Kuriya Y, Yamanishi Y. Comparison of inhibitory activities of donepezil and other cholinesterase inhibitors on acetylcholinesterase and butyrylcholinesterase in vitro. Methods Find Exp Clin Pharmacol. 2000;22:609–613. doi: 10.1358/mf.2000.22.8.701373. [DOI] [PubMed] [Google Scholar]
- Padilla S, Marshall RS, Hunter DL, Lowit A. Time course of cholinesterase inhibition in adult rats treated acutely with carbaryl, carbofuran, formetanate, methomyl, methiocarb, oxamyl or propoxur. Toxicol Appl Pharmacol. 2007;219:202–209. doi: 10.1016/j.taap.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Patel BN, Sharma N, Sanyal M, Shrivastav PS. Quantitation of donepezil and its active metabolite 6-O-desmethyl donepezil in human plasma by a selective and sensitive liquid chromatography–tandem mass spectrometric method. Anal Chim Acta. 2008;629:145–157. doi: 10.1016/j.aca.2008.09.048. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- Roivainen A, Rinne J, Virta J, Järvenpää T, Salomäki S, Yu M, et al. Biodistribution and blood metabolism of 1-11C-methyl-4-piperidinyl n-butyrate in humans: an imaging agent for in vivo assessment of butyrylcholinesterase activity with PET. J Nucl Med. 2004;45:2032–2039. [PubMed] [Google Scholar]
- Shao X, Butch ER, Kilbourn MR, Snyder SE. N-[18F]fluoroethylpiperidinyl, N-[18F]fluoroethylpiperidinemethyl and N-[18F]fluoroethylpyrrolidinyl esters as radiotracers for acetylcholinesterase. Nucl Med Biol. 2003;30:491–500. doi: 10.1016/s0969-8051(03)00031-3. [DOI] [PubMed] [Google Scholar]
- Shiraishi T, Kikuchi T, Fukushi K, Shinotoh H, Nagatsuka S, Tanaka N, et al. Estimation of plasma IC50 of donepezil hydrochloride for brain acetylcholinesterase inhibition in monkey using N-[11C]methylpiperidin-4-yl acetate ([11C]MP4A) and PET. Neuropsychopharmacology. 2005;30:2154–2161. doi: 10.1038/sj.npp.1300759. [DOI] [PubMed] [Google Scholar]
- Simeon-Rudolf V, Šinko G, Štuglin A, Reiner E. Inhibition of human blood acetylcholinesterase and butyrylcholinesterase by ethopropazine. Croat Chem Acta. 2001;74:173–182. [Google Scholar]
- Snyder SE, Gunupudi N, Sherman PS, Butch ER, Skaddan MB, Kilbourn MR, et al. Radiolabeled cholinesterase substrates: in vitro methods for determining structure–activity relationships and identification of a positron emission tomography radiopharmaceutical for in vivo measurement of butyrylcholinesterase activity. J Cereb Blood Flow Metab. 2001;21:132–143. doi: 10.1097/00004647-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Sramek JJ, Cutler NR. RBC cholinesterase inhibition: a useful surrogate marker for cholinesterase inhibitor activity in Alzheimer disease therapy? Alzheimer Dis Assoc Disord. 2000;14:216–227. doi: 10.1097/00002093-200010000-00006. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Ogura H, Arai Y, Iimura Y, Yamanishi Y. Research and development of donepezil hydrochloride, a new type of acetylcholinesterase inhibitor. Jpn J Pharmacol. 2002;89:7–20. doi: 10.1254/jjp.89.7. [DOI] [PubMed] [Google Scholar]
- Tiseo PJ, Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following evening administration. Br J Clin Pharmacol. 1998;46(Suppl.):13–18. doi: 10.1046/j.1365-2125.1998.0460s1013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veng PP. Curve fitting and modeling in pharmacokinetics and some practical experiences with NONLIN and a new program FUNFIT. J Pharmacokinet Biopharm. 1977;5:513–531. doi: 10.1007/BF01061732. [DOI] [PubMed] [Google Scholar]
- Verbeeck RK, Cardinal JA, Hill AG, Midha KK. Binding of phenothiazine neuroleptics to plasma proteins. Biochem Pharmacol. 1983;32:2565–2570. doi: 10.1016/0006-2952(83)90019-9. [DOI] [PubMed] [Google Scholar]
- Williams CH, Casterline JL., Jr A comparison of two methods for the measurement of erythrocyte cholinesterase inhibition after carbamate administration to rats. Food Cosmet Toxicol. 1969;7:149–151. doi: 10.1016/s0015-6264(69)80297-x. [DOI] [PubMed] [Google Scholar]
- Winteringham FP, Fowler KS. Substrate and dilution effects on the inhibition of acetylcholinesterase by carbamates. Biochem J. 1966;101:127–134. doi: 10.1042/bj1010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta. 1999;288:73–90. doi: 10.1016/s0009-8981(99)00144-8. [DOI] [PubMed] [Google Scholar]


