Abstract
Purpose
Little is known about obesity-related health issues among American Indian and Alaska Native (AIAN) populations.
Approach
A large cohort of AIAN people was assembled to evaluate factors associated with health.
Setting
The study was conducted in Alaska and on the Navajo Nation.
Participants
A total of 11,293 AIAN people were included.
Methods
We present data for body mass index (BMI, kg/m2) and waist circumference (cm) to evaluate obesity-related health factors.
Results
Overall, 32.4% of the population were overweight (BMI 25–29.9 kg/m2), 47.1% were obese (BMI ≥ 30 kg/m2), and 21.4% were very obese (BMI, ≥ 35 kg/m2). A waist circumference greater than 102 cm for men and greater than 88 cm for women was observed for 41.7% of men and 78.3% of women. Obese people were more likely to perceive their health as fair/poor than nonobese participants (prevalence ratio [PR]), 1.91; 95% CI, 1.71–2.14). Participants younger than 30 years were three times more likely to perceive their health as being fair or poor when their BMI results were 35 or greater compared with those whose BMI results were less than 25 kg/m2. A larger BMI was associated with having multiple medical conditions, fewer hours of vigorous activity, and more hours of television watching.
Conclusions
Given the high rates of obesity in AIAN populations and the association of obesity with other health conditions, it is important to reduce obesity among AIAN people.
Keywords: American Indian, Alaska Native, Body Mass Index, Obesity, Physical Activity
INTRODUCTION
The prevalence of obesity has been increasing at alarming rates worldwide.1 Obesity has been associated with type 2 diabetes as well as with other chronic diseases, including certain types of cancer, heart disease, and stroke.1–5 Likewise, reports of greater depression, more injuries from accidents, and economic loss from unemployment linked to obesity magnify the impact of obesity on health.6–10 As such, obesity is a major public health problem and may contribute to health disparities that exist in various populations.
Few studies of obesity among American Indian and Alaska Native (AIAN) populations have been conducted. One study of American Indian children living in the Aberdeen area showed that more than 40% of 5-year-old children were overweight, and 24% were obese.11 Two surveys have examined the extent of obesity in various U.S. populations and have shown significant differences in the prevalence of obesity and overweight individuals among AIAN populations compared with non-Hispanic white populations.12,13 However, these national surveys were limited primarily to samples of American Indian populations living in urban areas, and they included few AIAN people. It is unknown if the extent of the obesity problem extends to AIAN people living in more remote villages and on reservations. Although it has been suggested that developing means to prevent obesity would alleviate health disparities associated with chronic diseases in AIAN populations,13,14 little is known about the extent of the problem in AIAN populations and the impact that obesity has on their health and well-being.
In this paper, we use data from the Alaska and Navajo field centers of the Education and Research Towards Health (EARTH) study to evaluate health, lifestyle, and medical conditions associated with obesity, as indicated by body mass index (BMI) and waist circumference. Data are available from more than 11,000 AIAN people aged 18 years and older and represent the largest study of adult AIAN people conducted. We report the prevalence of obesity in the population, and we evaluate traditional factors that may be associated with BMI and waist circumference in the population as well as the association between obesity, perceived health, and select medical conditions.
METHODS
The EARTH study was initiated in 2001 as a pilot study to explore the feasibility of establishing a cohort of AIAN people. The study methods have been described in detail.15 Data presented in this paper come from 11,243 Navajo and Alaska Native study participants who completed a study visit between March 2004 and October 2007. In Alaska, participants were enrolled in three regions of the state: southcentral, southwestern, and southeastern Alaska; on the Navajo Nation, participants were recruited from the Fort Defiance and Shiprock Health Service Units. Within these regions, we defined urban by using the 2000 U.S. Census definition for an urbanized area that included communities with a population of at least 50,000 people. Tribal partnerships were established, and the study was approved by the Navajo Nation Institutional Review Board (IRB), the Alaska Area IRB, the Indian Health Service National IRB, and the University of Utah IRB. Additionally, regional, local, and village health boards and chapters within local health boards reviewed and approved the study.
Participant eligibility included age of at least 18 years; membership in an American Indian or Alaska Native tribe, as determined by eligibility for health care through the Indian Health Service; currently status as nonpregnant or not receiving chemotherapy; and physical and mental ability to read and understand the consent form. Although study participants represent a sample of convenience rather than a random sample from participating communities, the age distributions were similar to the 2000 census data from which the populations were derived.15
Baseline study visits were conducted in a variety of settings, including stationary locations in the larger population areas, temporary study centers in remote villages, and a mobile van on the Navajo Nation. Clinics were set up to assure participant confidentiality for all study components. The baseline study visit consisted of informed consent, intake questionnaire, medical measurements, an audio computer-assisted self-interview diet histoiy questionnaire, an audio computer-assisted self-interview health and lifestyle questionnaire (HLPA), an exit interview, and individual feedback (i.e., health report to each participant at the conclusion of the study visit). The audio component of the questionnaire could be played in English, Navajo, or Yup'ik languages, depending upon the participant's preference.
Height, weight, and waist and hip circumference measurements were taken in duplicate when the participants wore loose clothing without shoes. Weight was recorded in pounds with a Tanita digital scale (BWP800/BWP627A, Tanita Corporation of America Inc., Arlington Hills, Illinois). Standing height was measured in inches with the Road Rod Stadiometer (Seca, Hamburg, Germany). If the two height measurements differed by more than 1.0 inch or if the weight measurements differed by more than 2.0 pounds, measurements were repeated; the average of the last two measurements was used. Waist and hip circumference measurements were recorded to the nearest 0.5 inch by using either the Novel Products Figure Finder tape (Novel Products Inc., Rockton, Illinois) or the Gulick II Plus tape (Country Technology Inc., Gays Mills, Wisconsin) when the participant was standing. Waist circumference was measured at the smallest point between the tenth rib and the iliac crest; hip circumference was measured at the level of maximum protrusion of the gluteal muscles. If the two waist or hip measurements differed by more than 1 inch, a third set of measurements was taken. The average of the closest two measurements was used when three measurements were taken. Quality assurance and quality control procedures were taken to help assure accuracy of measures. As part of the initial training, a detailed manual of procedures was provided to each staff, and the staff members were observed using a checklist for procedures taking five measurement sets on two separate volunteers on two different days by a certified staff member. All measurements had to be within 0.5 inch and were verified by the gold standard certified staff member. On a quarterly basis, a field center quality control supervisor observed one set of body size measurements performed by each staff and measured and compared them to gold standard measurements. If staff failed to pass the ongoing quality control, they had to be recertified to take measurements.
The HLPA included information on a variety of self-reported medical conditions diagnosed by a physician or health care provider prior to the baseline study visit. Medical conditions included in the HLPA that were used to define medical conditions in this study were heart attack, stroke, cancer, diabetes, kidney disease, liver disease, asthma, arthritis, chronic obstructive pulmonary disease (COPD), depression, high blood pressure, high cholesterol, gall bladder disease, and bone fractures after age 18 years.
A detailed physical activity questionnaire was a central component of the HLPA questionnaire and included information on a variety of activities that were performed at moderate and vigorous levels of intensity and the frequency and amount of time that these activities were performed.16 Vigorous physical activities were defined as activities performed at metabolic equivalent values of 6 or more and were assessed as the total hours of vigorous activities performed per week. Vigorous activity was used in these analyses, because it has been shown to be the most valid indicator of activity in this population (Murtaugh, unpublished data, 2008). Additionally, we asked participants to report the amount of time they spent watching television per week as an indictor of physical inactivity.
A 12-item, short-form health survey (SF12)17 was included as part of the HLPA. From the SF12 questionnaire, we assessed the question of perceived health as well as derived summary scores. The perceived health question was “In general would you say your health is excellent, very good, fair, or poor?” The SF12 physical component summary (PCS) and mental component summary (MCS) scores were calculated by using the standard algorithms for the 1998 U.S. population reference group17; with this algorithm, higher scores indicate greater physical and mental functioning.
Other information collected as part of the HLPA were age; marital status; employment status during the past year; education level achieved; cigarette smoking practices; and several questions about affiliation to traditional Native lifestyle and culture, including language usually spoken at home (i.e., native only, both Native and English, or English only) and how much the participants identified with their own tribal traditions (i.e., not at all, a little, some, or a lot).
STATISTICAL METHODS
BMI was calculated by using the formula of kg/m2; cut-points used to analyze BMI were less than 25 kg/m2 for normal weight (includes underweight); 25 to 29.9 kg/m2 for overweight; 30 to 34.9 kg/m2 for class I obesity; and more than 35 kg/m2, which included both class II obesity (BMI 35.0–39.9 kg/m2) and class 111 obesity (BMI ≥ 40 kg/m2).18–20 A waist circumference of greater than 102 cm or 40 inches for men and greater Lhan 88 cm or 35 inches for women was defined as a large waist circumference.21
Associations between BMI and waist circumference and medical conditions were considered in two ways. First, we combined all medical conditions previously stated in the methods and analyzed anthropometric associations between those with two more medical conditions versus less than two medical conditions. We also assessed associations between category of BMI with self-reported diabetes, arthritis, COPD, depression, asthma, high blood pressure, high cholesterol, bone fracture after age 18 years, and gall bladder disease to provide an indication of the broader health conditions associated with BMI. We assessed BMI and waist circumference as categorical variables with perceived health in addition to actual health conditions previously diagnosed by a physician, and we categorized the reported perceived health into three categories of excellent/very good, good, or fair/poor.
SAS statistical program version 9.1 was used for all statistical analyses (SAS Institute, Cary, North Carolina). Chi-square tests were used to test for differences in proportions of variables of interest across categories of BMI and waist circumference. For continuous variables, such as hours of vigorous physical activity performed per week, hours of television watching per week, and physical and mental component scores from the SF12, we assessed the difference in mean levels across BMI and waist circumference categories by using analysis of covariance. In all models, age, gender, and region (i.e., three Alaska locations and Navajo) were adjusted by using the analysis of covariance test for mean levels and the Mantel-Haenzel test for categorical variables. To test for associations between various health conditions and BMI level, we calculated prevalence ratios (PR) by using the proportional hazards SAS program adjusted for age, gender, and region.22 Results include data from the 11,243 AIAN participants enrolled through July 2007. The total number of participants included for each variable varies slightly because of missing values for some variables.
RESULTS
Of the study population, 47.1% were obese (BMI ≥ 30), and almost 80% were either overweight or obese (Table 1). The percentage of the study population that was overweight and obese (BMI 30–34.9 kg/m2) increased with age, and obesity was more prevalent among women than men. Southwestern Alaska had the lowest percentage of the population who were obese. Living in a rural versus an urban area was not associated with the prevalence of obesity. Those achieving higher levels of education were less likely to have a BMI of 35 kg/m2 or more. Similar associations were observed for those with a high waist circumference as for those with a high BMI, with the exception og the association with participants' income levels; BMI was associated with income level, whereas waist circumference was not. The Pearson correlation coefficients between BMI and waist circumference and hip circumference measurements were .89 and .90, respectively. The mean waist and hip circumference measurements for men were 100.6 cm (standard error [SE], .23) and 106.0 cm (SE, .19), respectively, and for women were 100.1 cm (SE, .18) and 112.47 cm (SE, .15), respectively.
Table 1.
Description of Population Demographics
| BMI (kg/m2)† |
Waist Circumference (cm)‡ |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall Mean (SE) | <25 Mean (SE) | 25–29.9 Mean (SE) | 30–34.9 Mean (SE) | ≥35 Mean (SE) | p* | Acceptable Mean (SE) | High Mean (SE) | p | |
| BMI, kg/m2 | |||||||||
| Men | 29.0 (0.10) | 22.6 (0.07) | 27.5 (0.06) | 32.2 (0.08) | 39.8 (0.10) | <0.01 | 25.4 (0.08) | 34.1 (0.10) | <0.01 |
| Women | 31.2 (0.08) | 22.4 (0.08) | 27.6 (0.06) | 32.3 (0.06) | 40.0 (0.07) | <0.01 | 23.7 (0.14) | 33.3 (0.07) | <0.01 |
| Waist circumference, cm | |||||||||
| Men | 100.5 (0.23) | 84.9 (0.22) | 97.3 (0.18) | 100.2 (0.23) | 124.7 (0.30) | <0.01 | 90.8 (0.18) | 114.1 (0.22) | <0.01 |
| Women | 100.1 (0.18) | 80.5 (0.23) | 93.1 (0.17) | 103.6 (0.18) | 117.5 (0.19) | <0.01 | 80.5 (0.28) | 105.5 (0.15) | <0.01 |
| Total | 11,243 | 20.5% | 32.4% | 25.7% | 21.4% | 11,236 | 35.4% | 64.6% | |
| Age, y | |||||||||
| <30 | 3249 | 30.1 | 30.5 | 21.3 | 18.0 | <0.01 | 48.2 | 51.8 | <0.01 |
| 30–39 | 2320 | 16.7 | 28.4 | 26.5 | 28.4 | 30.2 | 69.8 | ||
| 40–49 | 2734 | 16.7 | 35.8 | 26.4 | 21.1 | 32.0 | 67.1 | ||
| 50–59 | 1796 | 16.1 | 33.7 | 28.9 | 21.3 | 28.2 | 71.8 | ||
| >60 | 1143 | 16.6 | 36.1 | 30.1 | 17.1 | 27.2 | 72.8 | ||
| Gender | |||||||||
| Men | 4228 | 25.8 | 36.5 | 23.9 | 13.8 | <0.01 | 58.3 | 41.7 | <0.01 |
| Women | 7015 | 17.3 | 30.0 | 26.9 | 25.9 | 21.7 | 78.3 | ||
| Location | |||||||||
| Alaska | 3822 | 26.0 | 31.8 | 22.2 | 20 | <0.01** | 43.8 | 56.2 | <0.01** |
| Southcentrat | 1394 | 22.2 | 32.0 | 23.9 | 22 | <0.01*** | 38.3 | 61.7 | <0.01*** |
| Southeast | 885 | 20.1 | 28.8 | 23.1 | 28 | 40.7 | 59.3 | ||
| Southwest | 1543 | 32.9 | 33.2 | 20.2 | 13.7 | 50.5 | 49.5 | ||
| Navajo | 7421 | 17.6 | 32.8 | 27.6 | 22 | 31.1 | 68.9 | ||
| Urban/airal location | |||||||||
| Urban | 1745 | 21.3 | 32.0 | 24.5 | 22.2 | 0.42 | 36.7 | 63.3 | 0.23 |
| Rural | 9142 | 20.4 | 32.5 | 26.0 | 21.1 | 35.2 | 64.8 | ||
| Income | |||||||||
| ≤$15,000 | 4698 | 21.3 | 31.8 | 25.4 | 21.4 | <0.01 | 37.6 | 62.4 | <0.01 |
| $15,001–35,000 | 2770 | 18.3 | 31.4 | 27.6 | 22.7 | 32.8 | 67.2 | ||
| >$35,000 | 2062 | 16.9 | 34.3 | 25.8 | 22.9 | 34.3 | 65.7 | ||
| Education | |||||||||
| <High school | 2800 | 23 | 31.7 | 25.3 | 20.1 | <0.01 | 37.6 | 62.4 | <0.01 |
| High school or GED | 3746 | 22.5 | 32.0 | 25.4 | 20.2 | 38.5 | 61.5 | ||
| Voc/tech/assoc/col | 4028 | 17.1 | 32.9 | 26.4 | 23.6 | 31.4 | 68.6 | ||
| Bach/Master/PhD | 569 | 18.8 | 35.5 | 26.2 | 19.5 | 33.8 | 66.2 | ||
| Employed within the past year | |||||||||
| Yes | 7023 | 19.9 | 32.9 | 26 | 21.2 | <0.01 | 37.0 | 63.0 | <0.01 |
| No | 3382 | 22.5 | 31.0 | 24.5 | 21.9 | 33.8 | 66.2 | ||
| Retired | 702 | 17.0 | 36.5 | 28.3 | 18.2 | 28.6 | 71.4 | ||
BMI indicates body mass index: SE, standard error; GED, general education degree; voc, vocational; tech, technical; assoc, associate's degree; col, college degree; bach, bachelor's degree; master, master's degree; PhD, doctorate degree.
Acceptable waist circumference is ≤102 cm for men and ≤88 cm for women; values above this are considered high.
The p values are χ2 p values for difference in proportion of categorical variable compared with BMI level and waist circumference level. For continuous variable of BMI and waist circumference, the p value is the difference in means across level of BMI and waist by using analysis of covariance.
This p value is for Alaska and Navajo with BMI categories.
This p value is for difference with Alaska region with BMI categories.
Current cigarette smokers were less likely to be obese than those who had never smoked cigarettes or who were former cigarette smokers (Table 2). Those with higher BMI levels were more likely to report their perceived health as being fair or poor versus excellent or very good among those with a BMI of less than 25 kg/m2. Neither language spoken at home nor identification with tribal traditions was associated with BMI. The SF12 PCS decreased with increasing levels of obesity, which indicated lower levels of physical functioning, whereas the MCS score did not. Study participants with higher BMI levels reported fewer hours of vigorous activity and more hours of watching television. High waist circumference measurements (>102 cm for men and >88 cm for women) were observed in 41.7% of men and in 78.3% of women. Participants whose waist circumferences were considered high were more likely to report their health as fair/poor rather than excellent/very good (Table 2). As with BMI, people who had larger waist circumferences had lower SFI2 PCS scores, were involved in fewer hours of vigorous activity per week, and spent more time watching television or playing video games than people who had smaller waist circumference measurements. These associations were similar for men and women and for location of study visit, with one exception—the association between BMI and smoking was seen only among men.
Table 2.
Associations Among Self-Reported Health and Lifestyle Factors and Body Mass Index and Waist Circumference
| BMI (kg/m2)*,† |
Waist Circumference (cm)‡ |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Overall N | <25 % | 25–29.9 % | 30–34.9 % | ≥35 % | p | Overall N | Acceptable % | High % | p |
| Total | 11,243 | 20.47 | 32.43 | 25.74 | 21.36 | 11,236 | 35.43 | 64.57 | ||
| Cigarette smoking status | ||||||||||
| Never | 7679 | 18.26 | 32.80 | 27.06 | 21.88 | 0.01 | 7676 | 31.51 | 68.49 | 0.02 |
| Former | 664 | 12.50 | 31.63 | 27.71 | 28.16 | 662 | 29.46 | 70.54 | ||
| Current | 2803 | 28.43 | 31.57 | 21.58 | 18.41 | 2801 | 47.63 | 52.37 | ||
| Self-reported health | ||||||||||
| Excellent/very good | 3435 | 26.55 | 36.45 | 23.64 | 13.36 | <0.01 | 3430 | 44.99 | 55.01 | <0.01 |
| Good | 4687 | 19.31 | 32.66 | 26.61 | 21.42 | 4686 | 34.23 | 65.77 | ||
| Fair/poor | 2974 | 15.16 | 27.34 | 26.73 | 30.77 | 2972 | 26.31 | 73.69 | ||
| Language used at home | ||||||||||
| Native | 1159 | 19.84 | 35.63 | 25.11 | 19.41 | 0.87 | 1159 | 38.05 | 61.95 | 0.87 |
| English | 4667 | 22.48 | 30.88 | 24.68 | 21.96 | 4663 | 37.79 | 62.21 | ||
| Both | 5270 | 18.82 | 33.06 | 26.85 | 21.27 | 5267 | 32.79 | 67.21 | ||
| Identify with tribal tradition | ||||||||||
| A lot | 4425 | 19.14 | 34.24 | 25.58 | 21.04 | 0.97 | 4422 | 34.89 | 65.11 | 0.17 |
| Some | 3804 | 20.53 | 32.05 | 26.00 | 21.42 | 3804 | 36.12 | 63.88 | ||
| A little | 2239 | 22.69 | 29.43 | 25.90 | 21.97 | 2234 | 35.63 | 64.37 | ||
| Not at all | 676 | 21.30 | 32.25 | 24.85 | 21.60 | 677 | 34.12 | 65.88 | ||
| N | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | N | Mean (SE) | Mean (SE) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| SF12 PCS† | 11,009 | 50.5 (0.21) | 50.1 (0.18 | 49.5 (0.20) | 47.6 (0.22) | <0.01 | 11,009 | 50.6 ( 0.17) | 48.7 (0.15) | <0.01 |
| SF12 MCS† | 11,009 | 46.3 (0.19) | 46.6 (0.16 | 46.9 (0.18) | 46.4 (0.20) | 0.27 | 11,009 | 46.5 (0.16) | 46.6 (0.14) | <0.01 |
| Vigorous activity | 11,160 | 4.9 (0.22) | 3.8 (0.18 | 3.14 (0.20) | 2.3 (0.22) | <0.01 | 11,168 | 4.6 (0.17) | 2.8 (0.16) | <0.01 |
| TV/video games† | 11,111 | 13.4 (0.30) | 13.2 (0.25) | 14.1 (0.28) | 15.1 (0.30) | <0.01 | 11,119 | 13.1 (0.24) | 14.4 (0.21) | <0.01 |
Analysis was adjusted for age, gender, and region as described in the methods for continuous and categorical variables.
BMI indicates body mass index; SF12 PCS, 12-quesiion short-form survey physical component summary; SF12 MCS, 12-question short-form survey mental component summary; TV, television.
Accepiable waist circumference is ≤102 cm for men and ≤88 cm for women; values above this are considered high.
Although both obese men and women were more likely to perceive their health to be good or fair/poor, this was especially true for men (Table 3). Associations between previously diagnosed medical conditions and obesity were generally stronger among men than women. Meu were 75% more likely to have two or more medical conditions if they had a BMI of 35 kg/m2 or more, whereas women were only 36% more likely to have two or more medical conditions with a BMI of 35 kg/m2 or more. The magnitude of the association between high blood pressure, high cholesterol, prior history of gall bladder disease, arthritis, and type 2 diabetes with obesity was much greater for men than for women. For other health conditions previously diagnosed by a physician, such as asthma, bone fracture after age 18 years, chronic obstructive pulmonary disease, and depression, associations were similar for men and women and were not associated with obesity. Because of the high correlation between high waist circumference and obesity, we only present data for BMI, although similar associations were observed for high waist circumference.
Table 3.
Association Between Body Mass Index and Various Health Conditions by Gender*
| BMI, kg/m2 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men |
Women |
|||||||||||
| 25–29 vs. ≤25 |
30–34.9 vs. ≤25 |
≥35 vs. ≤25 |
25–29 vs. ≤25 |
30–34.9 vs. ≤25 |
≥35 vs. ≤25 |
|||||||
| Factor | PR*,† | 95% CI | PR | 95% CI | PR | 95% CI | PR | 95% CI | PR | 95% CI | PR | 95% CI |
| General health perception | ||||||||||||
| Excellent/very good | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Good | 1.08 | 0.97–1.21 | 1.21 | 1.05–1.40 | 2.09 | 1.65–2.64 | 1.11 | 1.01–1.23 | 1.25 | 1.12–1.40 | 1.44 | 1.27–1.63 |
| Fair/poor | 1.05 | 0.91–1.20 | 1.30 | 1.10–1.53 | 2.88 | 2.27–3.65 | 1.13 | 1.01–1.27 | 1.34 | 1.19–1.52 | 1.70 | 1.50–1.93 |
| Two or more medical condltionst‡ | ||||||||||||
| Yes | 1.08 | 0.96–1.21 | 1.28 | 1.11–1.47 | 1.75 | 1.46–2.09 | 1.10 | 0.99–1.21 | 1.19 | 1.07–1.32 | 1.36 | 1.23–1.51 |
| High blood pressure | ||||||||||||
| Yes | 1.28 | 1.12–1.46 | 1.56 | 1.35–1.82 | 2.57 | 2.13–3.10 | 1.18 | 1.04–1.32 | 1.30 | 1.16–1.46 | 1.47 | 1.31–1.64 |
| High cholesterol | ||||||||||||
| Yes | 1.30 | 1.11–1.52 | 1.59 | 1.33–1.90 | 2.24 | 1.80–2.80 | 1.12 | 0.98–1.27 | 1.17 | 1.02–1.33 | 1.26 | 1.10–1.45 |
| Gall bladder disease | ||||||||||||
| Yes | 1.20 | 0.93–1.55 | 1.34 | 1.01–1.79 | 1.59 | 1.11–2.30 | 1.14 | 1.01–1.29 | 1.23 | 1.09–1.39 | 1.39 | 1.24–1.55 |
| Bone fracture after age 18 y | ||||||||||||
| Yes | 0.95 | 0.85–1.07 | 0.94 | 0.81–1.08 | 0.86 | 0.71–1.04 | 1.03 | 0.92–1.16 | 1.03 | 0.91–1.16 | 1.05 | 0.93–1.19 |
| Arthritis | ||||||||||||
| Yes | 1.03 | 0.88–1.21 | 1.18 | 0.99–1.42 | 1.47 | 1.18–1.84 | 1.02 | 0.90–1.16 | 1.04 | 0.91–1.18 | 1.15 | 1.01–1.30 |
| Asthma | ||||||||||||
| Yes | 0.92 | 0.74–1.13 | 1.19 | 0.97–1.45 | 1.24 | 0.95–1.61 | 1.03 | 0.90–1.19 | 1.12 | 0.98–1.28 | 1.29 | 1.15–1.45 |
| Chronic obstructive pulmonary disease | ||||||||||||
| Yes | 0.95 | 0.73–1.24 | 1.10 | 0.80–1.50 | 1.13 | 0.77–1.64 | 1.08 | 0.88–1.33 | 1.14 | 0.92–1.40 | 1.16 | 0.95–1.42 |
| Depression | ||||||||||||
| Yes | 1.01 | 0.85–1.18 | 1.05 | 0.86–1.28 | 1.17 | 0.92–1.50 | 0.99 | 0.88–1.11 | 1.01 | 0.90–1.14 | 1.05 | 0.94–1.18 |
| Type 2 diabetes | ||||||||||||
| Yes | 1.14 | 0.94–1.37 | 1.34 | 1.09–1.64 | 1.74 | 1.37–2.21 | 1.19 | 1.01–1.40 | 1.28 | 1.11–1.48 | 1.47 | 1.29–1.68 |
BMI indicates body mass index: PR, prevalence ratio; CI, confidence interval.
PRs adjusted for age, region, and smoking status.
Risk of having the condition versus not having it by level of BMI.
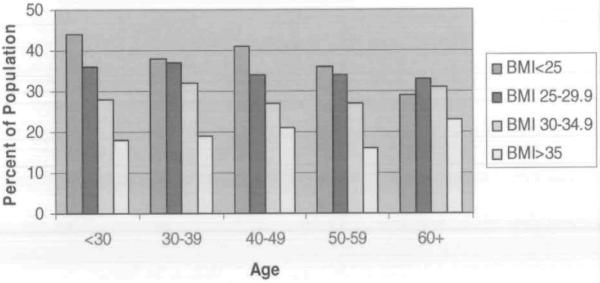
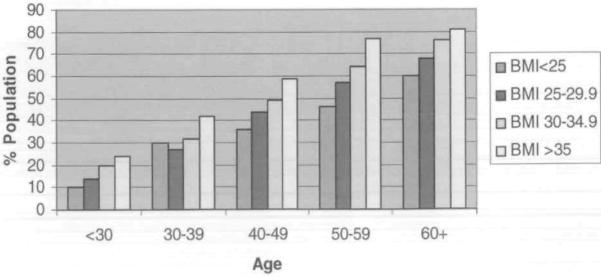
Figure 1 shows the percentage of the population who reported excellent or very good health by BMI level and by age. Individuals younger than 30 years of age were three times as likely to perceive their health as fair/poor versus excellent or good if their BMI was greater than 35 kg/m2 (PR, 3.01; 95% CI, 2.39–3.81). For everyone except for those aged 60 years or older, those who were more obese were less likely to perceive their health to be excellent or very good. The difference across categories was statistically significant for all groups except for those who were 60 years or older. Likewise, the proportion of people reporting two or more medical conditions increased as the BMI category increased across all age groups (Figiue 2).
Figure 1.
Percent of the Population Reporting Excellent/Very Good Health by Body Mass Index Level and Age
Figure 2.
Percent of the Population With Two or More Medical Conditions by Body Mass Index Level and Age
DISCUSSION
We observed that close to 50% of the population were obese and that 80% had a BMI above the normal range of less than 25 kg/m2. Obesity and overweight levels occur at a young age in this population: only 30% of study participants who were younger than 30 years of age had a BMI less than 25 kg/m2, and by 30 to 40 years of age, only 16.7% of participants had a BMI in a normal range. The consequences of obesity at a young age included poorer perceived health as well as more physician diagnosed medical (conditions before age 30 years. Participants younger than 30 years of age were three times as likely to perceive their health as fair/poor compared with people who had normal BMIs. Obese participants younger than, 30 years of age were 70% more likely to have two or more physician-diagnosed medical conditions, despite their younger age. BMI was associated with perceived health, number of medical conditions overall, and with specific medical conditions.
In addition to a high prevalence of overall obesity, the AIAN population included in this study had a high prevalence of central obesity, as indicated by high waist measurements. Overall, 47.1% of men and 78.3% of women had waist circumference measurements greater than 102 cm and 88 cm, respectively; the mean waist circumference for men was 100.6 cm and for women was 100.1 cm. These are much higher than those reported in a summary of 18 populations, in which the mean waist circumference for men between 55 and 64 years of age ranged from 85 to 99 cm and for women ranged from 80 to 96 cm.23 Studies have shown increased risk of several chronic diseases associated with central obesity, including kidney disease, some cancers, heart disease, and respiratory diseases.24–31 Even among people who are obese, central obesity in some populations increases the risk of additional health problems beyond the risk from obesity alone.32 Given both the high rates of obesity and the high waist circumference observed in this population, a greater threat to health may exist than if noncentral obesity were present.
Individuals in our study who were overweight and obese reported more health problems than those who had a BMI less than 25 kg/m2. A report from the National Health and Nutrition Examination Survey (NHANES) showed that, between 1999–2000, 30.6% of U.S. adults had a BMI of 30 kg/m2 or more.33 This figure, although high, is considerably lower than the 47.1% of AIAN participants in this study, despite our population being younger than that in the NHANES. As in the study by McDowell33 of mainly non-Hispanic while people, people with a BMI of more than 30 kg/m2 were more likely to perceive their health as fair/poor than people with a lower BMI.
Assessment of specific medical conditions associated with obesity showed that, among the very obese (i.e., those with a BMI of 35 kg/m2 or more), participants were significantly more likely to report having had physician-diagnosed arthritis, high blood pressure, high cholesterol, gall bladder disease, and type 2 diabetes. It is important to note the gender differences in associations with specific medical conditions, such as having two more medical conditions, high blood pressure, and high cholesterol. The strength of the associations was generally stronger for men than for women for these conditions. Because the population was generally younger than 50 years of age, it is possible that women who are premenopausal and exposed to estrogen are less likely to have some medical conditions despite their obesiiy. Likewise, asthma was only significantly associated with obesity among women. Bone fractures, chronic obstructive pulmonary disease, and depression were not associated with obesity. High BMI has been associated with higher bone mineral density34; thus, not seeing an association is not unexpected. On the other hand, depression has been associated with obesity in large national surveys.35 It is interesting to note that, in one study, perceived weight was associated with depression whereas actual weight was not.35 These results need further exploration and follow-up to obtain a better understanding of the observed associations.
Those who were most obese in this population reported less vigorous activity and more time spent watching television. Other data indicate that ethnic minorities in the United States, includiug American Indians, report less physical activity than non-Hispanic whites.36–38 In other studies of American Indian women,38,39 55% of participants did not participate in the recommended three or more 20-minute sessions of leisure-time physical activity per week. Of these, 50% of participants reported no leisure-iime physical activity at all. Data also have shown that physical activity levels among American Indian populations have substantially decreased over recent decades,40,41 which most likely contributes to the high percentage of the population who are overweight and obese.
There are many aspects of tribal culture and lifestyle, aud in the EARTH study we used two indicators of traditional Native lifestyle and cultural affiliation, language used at home and identification with tribal traditions. These were of interest in part because of trends in increasing rates of obesity and diabetes that parallel the adoption of a more Western lifestyle by AIAN people. We did not observe an association between the crude indicators of a more traditional lifestyle that were available to us and either BMI or waist circumference measurements. It is possible that our inability to fully capture activity related to traditional lifestyle may have impacted our ability to measure the association between traditional lifestyle and BMI.
This study has limitations. Because of the cross-sectional nature of the study, it is impossible to determine if associations are causal. Additionally, we have measured height, weight, and girth measurements, but we are unable to correlate those measurements to actual body fat. Participants self-reported lifestyle and medical conditions, leaving room for error in reporting that would misclassify individuals in terms of exposures. To obtain a better understanding of obesity in this population, it is necessary to evaluate both genetic and lifestyle factors over time and their interplay in the development of obesity.
The EARTH study is one of only a few studies that has examined obesity and associated factors among AIAN people. The results of this study provide a more complete picture of the degree of obesity and central obesity in this population than has existed to date. It also provides insight into the cross-sectional association of obesity with medical conditions and the impact of obesity on perceived health. This study provides valuable baseline data for future prospective studies and for health promotion efforts in these populations. It is important to identify the factors that lead to obesity in this population and to develop methods to alleviate the obesity problem. These data show the need to start at young ages to prevent obesity, given the number of people younger than 30 years who are obese and the related medical conditions that have developed in those young people.
Acknowledgments
This study was funded by grants CA88958 and CA96095 from the National Cancer Institute. We acknowledge the contributions and support of the Navajo Nation, the Indian Health Service, the Alaska Native Tribal Health Consortium Board of Directors, Southcentral Foundation, Southeast Alaska Regional Health Consortium, the Yukon-Kuskokwim Health Corporation, the Ft. Defiance and Shiprock Health Boards, Tribal Advisory Board members, the staff on the Navajo Nation, and the staff in Alaska. We also acknowledge Roger Edwards, James Bryner, Kelly Cunningham, and Jason Sandidge for computer programming.
Footnotes
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute.
References
- 1.Weight Control and Physical Activity. Vol 6. LARC Press; Lyon, France: 2002. [Google Scholar]
- 2.Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES. Body mass index and colon cancer in a national sample of adult US men and women. Am J Epidemol. 1999;150:390–398. doi: 10.1093/oxfordjournals.aje.a010018. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 5.Colditz GA, Coakley E. Weight, weight gain, activity, and major illnesses: the Nurses' Health Study. Int J Sports Med. 1997 Jul;18(suppl 3):S162–S170. doi: 10.1055/s-2007-972709. [DOI] [PubMed] [Google Scholar]
- 6.Klarenbach S, Padwal R, Chuck A, Jacobs P. Population-based analysis of obesity and workforce participation. Obesity (Silver Spring) 2006;14:920–927. doi: 10.1038/oby.2006.106. [DOI] [PubMed] [Google Scholar]
- 7.Kasen S, Cohen P, Chen H, Must A. Obesity and psychopathology in women: a three decade prospective study. Int J Obes (Lond) 2007;32:558–566. doi: 10.1038/sj.ijo.0803736. [DOI] [PubMed] [Google Scholar]
- 8.Frezza EE, Wachtel MS, Ewing BT. The impact of morbid obesity on the state economy: an initial evaluation. Surg Obes Retal Dis. 2006;2:504–508. doi: 10.1016/j.soard.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein EA, Ruhm CJ, Kosa KM. Economic causes and consequences of obesity. Annu Rev Public Health. 2005;26:239–257. doi: 10.1146/annurev.publhealth.26.021304.144628. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacherjee A, Chau N, Sierra CO, et al. Relationships of job and some individual characteristics to occupational injuries in employed people: a community-based study. J Occup Health. 2003;45:382–391. doi: 10.1539/joh.45.382. [DOI] [PubMed] [Google Scholar]
- 11.Zephier E, Himes JH, Story M, Zhou X. Increasing prevalences of overweight and obesity in Northern Plains American Indian children. Arch Pediatr Adolesc Med. 2006;160:34–39. doi: 10.1001/archpedi.160.1.34. [DOI] [PubMed] [Google Scholar]
- 12.Lucas JW, Schiller JS, Benson V. Summary health statistics for U.S. adults: National Health Interview Survey, 2001. Vital Health Stat 10. 2004;(218):1–134. [PubMed] [Google Scholar]
- 13.Denny CH, Holtzman D, Goins RT, Croft JB. Disparities in chronic disease risk factors and health status between American Indian/Alaska Native and White elders: findings from a telephone survey, 2001 and 2002. Am J Public Health. 2005;95:825–827. doi: 10.2105/AJPH.2004.043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 15.Slattery ML, Schumacher MC, Lanier AP, et al. A prospective cohort of American Indian and Alaska Native people: study design, methods, and implementation. Am J Epidemiol. 2007;166:606–615. doi: 10.1093/aje/kwm109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redwood D, Schumacher MC, Lanier AP, Ferucci E, et al. Physical activity patterns of American Indian and Alaska Native people living in Alaska and the Southwest. Am J Health Pharmol. 2009;23(6):388–395. doi: 10.4278/ajhp.071211130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests ol reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Strawbridge WJ, Wallhagen MI, Shema SJ. New NHLBI clinical guidelines for obesity and overweight: will they promote health? Am J Public Health. 2000;90:340–343. doi: 10.2105/ajph.90.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackwell J. Identification, evaluation, and treatment of overweight and obese adults. J Am Acad Nurse Pract. 2002;14:196–198. doi: 10.1111/j.1745-7599.2002.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 20.NHLBI [Accessed June 2007];Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary, 2007. Available at: http://www.NHLBI.nih.gov.
- 21.Fogli-Cawley JJ, Dwyer JT, Saltzman E, et al. The 2005 Dietary Guidelines for Americans and risk of the metabolic syndrome. Am J Clin Nutr. 2007;86:1193–1201. doi: 10.1093/ajcn/86.4.1193. [DOI] [PubMed] [Google Scholar]
- 22.Thompson ML, Myers JE, Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: what is to be done? Occup Environ Med. 1998;55:272–277. doi: 10.1136/oem.55.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molarius A, Seidell JC, Sans S, Tuomilehto J, Kuulasmaa K. Waist and hip circumferences, and waist-hip ratio in 19 populations of the WHO MONICA Project. Int J Obes Metab Discord. 1999;23:116–125. doi: 10.1038/sj.ijo.0800772. [DOI] [PubMed] [Google Scholar]
- 24.Slattery ML, Sweeney C, Edwards S, et al. Body size, weight change, fat distribution and breast cancer risk in Hispanic and non-Hispanic white women. Breast Cancer Res Treat. 2007;102:85–101. doi: 10.1007/s10549-006-9292-y. [DOI] [PubMed] [Google Scholar]
- 25.Appel SJ, Jones ED, Kennedy-Malone L. Central obesity and the metabolic syndrome: implications for primary care providers. J Am Acad Nurse Pract. 2004;16:335–342. doi: 10.1111/j.1745-7599.2004.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 26.Lin CH, Chou CY, Lin CC, et al. Waist-to-height ratio is the best index of obesity in association with chronic kidney disease. Nutrition. 2007;11–12:788–793. doi: 10.1016/j.nut.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17:319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28:850–856. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 29.Caan BJ, Coates AO, Slattery ML, et al. Body size and the risk of colon cancer in a large case-control study. Int J Obes Relat Metab Disord. 1998;22:178–184. doi: 10.1038/sj.ijo.0800561. [DOI] [PubMed] [Google Scholar]
- 30.Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev. 2003;4:157–173. doi: 10.1046/j.1467-789x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 31.Koziel S, Ulijaszek SJ, Szklarska A, Bielicki T. The effects of fatness and fat distribution on respiratory functions. Ann Hum Biol. 2007;34:123–131. doi: 10.1080/03014460601121795. [DOI] [PubMed] [Google Scholar]
- 32.Lafortuna CL, Agosti F, Proietti M, Adorni F, Sartorio A. The combined effect of adiposity, fat distribution and age on cardiovascular risk factors and motor disability in a cohort of obese women (aged 18–83) J Endocrinol Invest. 2006;29:905–912. doi: 10.1007/BF03349195. [DOI] [PubMed] [Google Scholar]
- 33.McDowell MA, Hughes JP, Borrud LG. Health characteristics of US adults by body mass index category: results from NHANES 1999–2002. Public Health Rep. 2006;121:67–73. doi: 10.1177/003335490612100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin LA, Hawker GA, Peltekova VD, et al. Determinants of peak bone mass: clinical and genetic analyses in a young female Canadian cohort. J Bone Miner Res. 1999;14:633–643. doi: 10.1359/jbmr.1999.14.4.633. [DOI] [PubMed] [Google Scholar]
- 35.Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70:288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 36.Services UDoHaH . Physical Activity and Health: A Report of the Surgeon General. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, Ga: 1996. [Google Scholar]
- 37.Eyler AA, Brownson RC, Donatelle RJ, et al. Physical activity social support and middle- and older-aged minority women: results from a US survey. Soc Sci Med. 1999;49:781–789. doi: 10.1016/s0277-9536(99)00137-9. [DOI] [PubMed] [Google Scholar]
- 38.Brownson RC, Eyler AA, King AC, et al. Patterns and correlates of physical activity among US women 40 years and older. Am J Public Health. 2000;90:264–270. doi: 10.2105/ajph.90.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugarman JR, Warren CW, Oge L, Helgerson SD. Using the Behavioral Risk Factor Surveillance System to monitor year 2000 objectives among American Indians. Public Health Rep. 1992;107:449–456. [PMC free article] [PubMed] [Google Scholar]
- 40.Will JC, Strauss KF, Mendlein JM, et al. Diabetes mellitus among Navajo Indians: findings from the Navajo Health and Nutrition Survey. J Nutr. 1997;127(suppl 10):2106S–2113S. doi: 10.1093/jn/127.10.2106S. [DOI] [PubMed] [Google Scholar]
- 41.Strauss KF, Mokdad A, Ballew C, et al. The health of Navajo women: findings from the Navajo Health and Nutrition Survey, 1991–1992. J Nutr. 1997;127(suppl 10):2128S–2133S. doi: 10.1093/jn/127.10.2128S. [DOI] [PubMed] [Google Scholar]